2. Materials and Methods
Serum samples of healthy donors were collected by employees of the Centers for Hygiene and Epidemiology in the constituent entities of the Russian Federation in October-November 2021 and October-November 2022. Collection regions are shown in
Figure 1. Serum collection and transportation to the FSI SSC WB Vector of Rospotrebnadzor, as well as hemagglutination inhibition tests (HI test) were performed as described in [
4]. Prior to conducting the study, the collected blood serum samples were stored at a temperature not exceeding ‒20°C. The HI test used dry influenza diagnostics, produced by the Diagnostic Drugs Manufacturing Enterprise LLC (St. Petersburg) on the basis of actual vaccine strains. The sera were considered positive on the basis that the reverse antibody titer was equal to or greater than 1:40.
In addition, the blood sera of people, who were in contact with sick and/or dead poultry, as well as residents of regions, located on wild waterfowl migration routes were studied (
Figure 2). Human serum samples were tested by HI and microneutralization (MN) in accordance with [
5]. In HI tests used a virus-containing culture liquid, inactivated with β-propiolactone. Highly pathogenic strains of A/chicken/Astrakhan/32105/2020 (H5N8) and A/Astrakhan/3212/2020 (H5N8), isolated in chickens and humans, respectively, during the zoonotic influenza outbreak in the Astrakhan region in 2020, A/chicken/Khabarovsk/24-12V/2022 (H5N1) and A/chicken/Nghe An/27VTC/2018 (H5N6) highly pathogenic strains, and low pathogenic strains of A/chicken/NghiLoc/222VTC/2019 (H9N2) and A/chicken/Primorsky Krai/03/2018 (H9N2), isolated from samples, collected in Vietnam and Russia, respectively, were used for the preparation of antigens. All strains were isolated in the SEC VB Vector Rospotrebnadzor. In addition, A/Anhui/01/2013(H7N9) virus, kindly provided by the WHO Collaborating Centre in Beijing, China, was also used.
The statistical significance of differences in HI-test titers between groups of patients from the various regions was determined using χ2 criterion. The calculation was carried out using the Statistica 6.0 statistical software package. A value of p < 0.05 was considered significant.
3. Results
In accordance with the antigenic analysis of influenza viruses circulating in the Northern Hemisphere, for the season 2021–2022, WHO recommended to produce a tetravalent vaccine based on chicken embryos using strains antigenically corresponding to A/Victoria/2570/2019 (H1N1)pdm09, A/Cambodia/e0826360/2020 (H3N2), B/Washington/02/2019 (B/Victoria lineage), and B/Phuket/3073/2013 (B/Yamagata lineage) strains. For a trivalent vaccine, it was recommended to use the first three strains of the above.
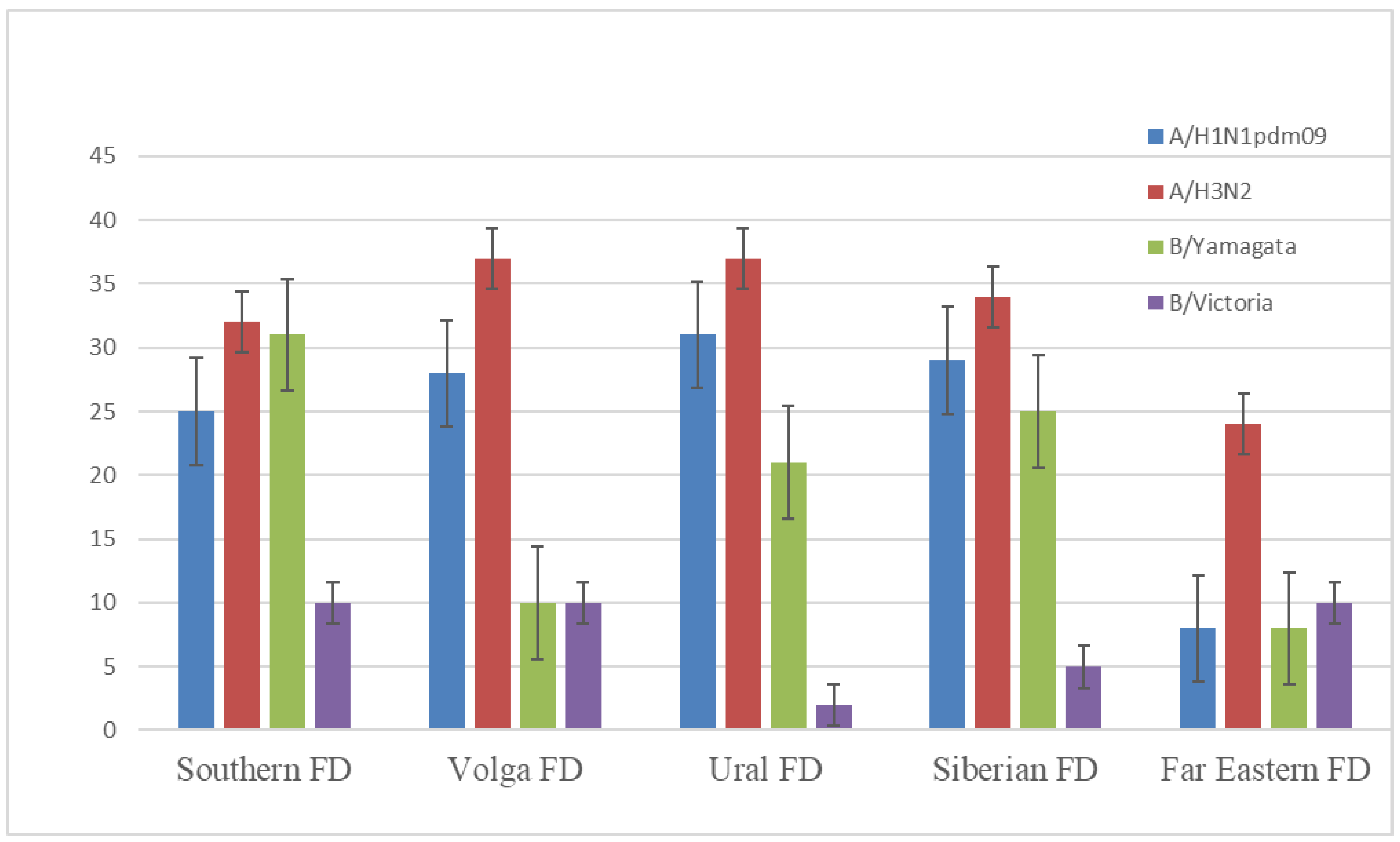
In October–November 2021, prior to the 2021–2022 epidemic season, 1344 blood serum samples were collected in five federal districts: 415, 267, 250, 156, and 256 samples in Siberian, Southern, Far Eastern, Volga, and Ural Federal Districts, respectively. All samples were HI-tested with vaccine influenza viruses. The results of testing are presented in
Figure 3 and
Table 1.
Figure 3 shows that, in October–November 2021, only 8–31, 24–37, and 2–10% of samples in the HI-test were positive with A/Victoria/2570/2019 (H1N1)pdm09, A/Cambodia/e0826360/2020 (H3N2), and B/Washington/02/2019 (B/Victoria lineage) vaccine viruses, respectively. In the
Table 1, you can see the distribution of positive samples in accordance with age and sex of donors. Thus, herd immunity to seasonal influenza by the end of 2021 had significantly decreased when compared to the pre-pandemic seasons. For example, in the 2019–2020 season, the number of influenza A positive samples in all regions of the Russian Federation ranged from 40 to 68%. For the influenza B/Victoria virus, this value comprised 12–46% [
6].
For the season 2022–2023, WHO recommended to produce a tetravalent vaccine based on chicken embryos using strains antigenically corresponding to A/Victoria/2570/2019 (H1N1)pdm09, A/Darwin/9/2021 (H3N2), B/Austria/1359417/2021 (B/Victoria lineage), and B/Phuket/3073/2013 (B/Yamagata lineage) viruses. In 2022–2023, the influenza epidemic in the Russian Federation was little or no different from the pre-Covid period. The outbreak began at the 47–48th weeks of 2022, peaked at 51st week, and ended by mid-April. From the very beginning of the epidemic until mid-January, A/H1N1 pdm09influenza virus significantly prevailed. From the first days of February, it gave way to B/Victoria. Throughout the epidemic, A/H3N2 was lower than 1% of all influenza viruses, circulating in Russia [
7].
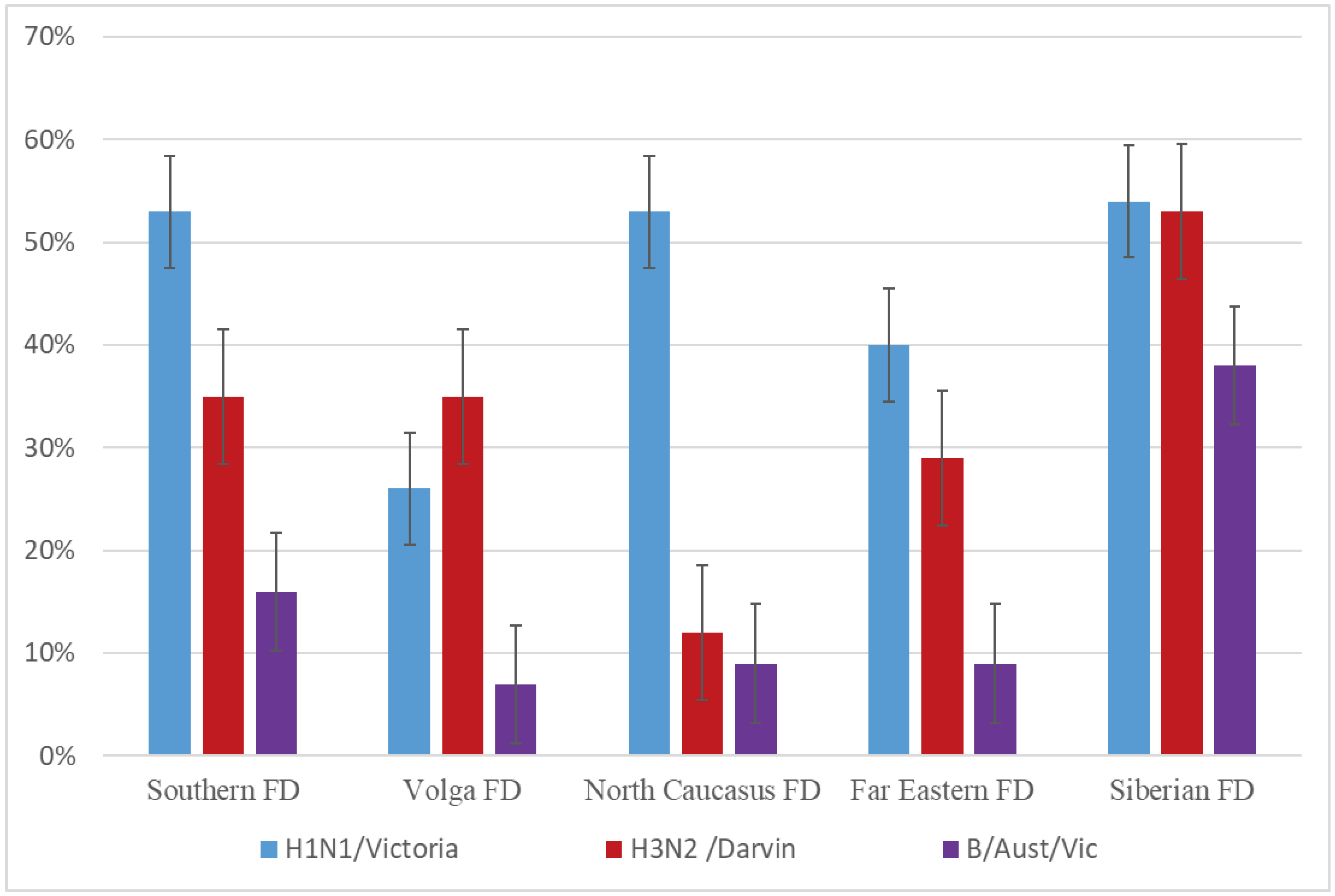
In total, 2216 serum samples were collected just before the epidemic season (October-November 2022). Of these, 44, 37, and 19% were positive for the A/H1N1 pdm09, A/H3N2, and B/Victoria viruses, respectively (
Figure 4).
Table 2 shows the distribution of positive samples depending on the age and gender groups. It can be seen from
Figure 4 that herd immunity was still lower than before the COVID-19 pandemic, despite the fact that 52.8% of the country’s population were vaccinated against influenza in 2022 [
8]. The number of samples negative to influenza A vaccine strains (no significant titers with vaccine strains in HI-tests) was still extremely high, comprising about 40% (
Table 3). In 2021, this value amounted to 55% (
Table 3), while prior to the COVID-19 pandemic, the number of negative samples for influenza A vaccine viruses fluctuated around 25–30% (data not shown).
In 2021, we collected 2564 blood serum samples from people in 29 regions of the Russian Federation who were in contact with poultry. Only single serum samples had significant HI-test titers for A/H5Nx viruses (7 samples, 0.3%). In 2022, 1683 serum samples were collected in 40 regions of the Russian Federation. Of these, 25 samples (1.5%) were positive for influenza A/H5N8 and A/H5N1 viruses. In 2023, 3378 serum samples were collected in 40 regions of the Russian Federation, of which 28 (0.8%) were positive for influenza A/H5N8 and A/H5N1 viruses. All samples which were positive in HI-tests, were negative in MN with a titer below 1:20 (
Table 4).
In 2021, the number of seropositive samples was 13 (0.5%) for the A/H9N2 low-pathogenic influenza virus subtype in contrast to 4.8 (81 out of 1,683 sera) and 1,0 % (33 out of 3,378 samples) in 2022 and 2023, respectively. All serum samples, positive in HI-tests, were additionally tested by MN. As a result, most samples, positive in the HI-test with A/H9N2 virus, were similarly positive in the neutralization test (titers above 1:20) (
Table 4).
None of the serum samples reacted with A(H7N9) virus in HI-tests, even at a 1:10 dilution.
4. Discussion
A high level of herd immunity to vaccine strains of influenza virus is thus ensured by two factors: firstly, the protection of the vaccinated population from the disease, and secondly, the protection of unvaccinated and risk-group population “at the expense of” the vaccinated. The immune response to vaccination in at-risk individuals may be lower than that in healthy individuals of the ages of 18–64. This may influence the effectiveness of vaccines in these population groups. A smaller number of infected people in the environment reduces the probability of people at risk becoming infected. In this regard, WHO recommends at least 50% of the country’s population to be vaccinated and about 75% of people at risk [
9]. Monitoring of herd immunity to vaccine strains of influenza virus allows the burden of the upcoming epidemic and adjusting anti-epidemic measures to be predicted.
In addition, low herd immunity to seasonal influenza increases the risk of the emergence of viral variants with a pandemic potential. Experts believe two main mechanisms to be involved in the emergence of pandemic viruses: reassortment of human and animal influenza virus genomes; and /or gradual adaptation to a new host [
3,
10]. Any mechanism for the emergence of pandemic viral variants includes a pre-pandemic period, necessary for the virus to adapt optimally to humans.
In this connection, since 2005 we have conducted a serum study in people, involved in the breeding and processing of poultry, as well as those living in places where outbreaks of influenza have occurred among poultry or wild waterfowl. In the summer of 2005, a highly pathogenic A(H5N1) influenza virus was first isolated and characterized in Russia. However, in 2005‒2016, no human samples were found to contain an A(H5Nx) virus, and only individual serum samples had significant titers in HI tests and MN with A(H5N1), A(H5N8), A(H5N6), or A(H9N2) viruses. No positive serum for a highly pathogenic A(H7N9) virus was detected during the entire observation period [
5].
In 2016‒2017, a large-scale epizootic was recorded among wild and farm birds in the European part of Russia caused by a highly pathogenic A(H5N8) influenza virus. The largest outbreaks among poultry were recorded at poultry farms and private farmsteads in the Republics of Kalmykia and Tatarstan, as well as in the Moscow, Tula, Nizhny Novgorod, Astrakhan, and Voronezh regions. In each region, blood serum samples were collected from people, who came into contact with sick and dead poultry (farmers or poultry farm employees), to assess the level of antibodies to the highly pathogenic influenza viruses. A total number of 760 paired blood serum samples were HI-tested with A/rook/Chany/32/2015 (H5N1) and A/chicken/Sergiev Posad/38/2017 (H5N8) highly pathogenic influenza viruses. The number of samples that were HI-positive to A(H5N1) and (H5N8) strains comprised 28 and 61, respectively. All HI-positive serum samples were additionally tested by MN. Only 40% of HI-positive samples were shown to be MN positive [
11].
In December 2020, an outbreak of A/H5N8 highly pathogenic avian influenza was recorded at the Vladimir poultry farm in the Astrakhan region. In total, 56 serum samples and 37 nasopharyngeal swabs were collected from 56 farm workers that came in contact with dead poultry or had been at the farm since the outbreak began. Serum samples were re-taken 14 and 44 days after the first collection. According to the district hospital, all persons had no symptoms of the disease within 21 days of follow-up. Serum samples were examined in neutralization tests with MDCK cells and hemagglutination inhibition with horse erythrocytes, as well as using biolayer interferometry (BLI). The presence of specific IgG antibodies to the A/Astrakhan/3212/2020 (H5N8) influenza virus, isolated from a poultry farm employee, was confirmed by biolayer interferometry for all serum samples, obtained on day 14 and day 44 [
12].
In the winter season of 2016‒2017, a highly pathogenic A(H5Nx) avian influenza virus of clade 2.3.4.4 caused one of the largest epizootics in Europe (2781 outbreaks). Even more outbreaks in poultry (3777) and mass deaths of wild birds occurred in 2020‒2021. During this wave, multiple reassortants, involving highly pathogenic A(H5Nx) viruses, appeared and diversified rapidly, resulting in the co-circulation of 19 various genotypes, belonging to five various subtypes (H5N1, H5N3, H5N4, H5N5, and H5N8) [
13]. The global spread of these highly pathogenic viruses and their recombination with various local low pathogenic viruses occur much faster and at higher rates than has been previously observed for other strains of H5, H7, or H9 viruses, ultimately increasing the multiplicity of clade 2.3.4.4 viruses.
WHO experts identify highly pathogenic A/H5Nx and A/H7N9 avian influenza viruses as the most dangerous. Thus, from January 2003 to September 2023, a total of 876 cases of human infection with an A(H5N1) avian influenza virus were registered. Among them, 458 were fatal, resulting in a mortality rate of 53%. Since 2014, WHO had registered a total of 87 laboratory confirmed cases of human infection with an A(H5N6) influenza virus, including 33 deaths (38% mortality rate). Since March 2013, a total of 1568 human cases of A(H7N9) avian influenza had been reported worldwide with a mortality rate of more than 40%. The last case was reported on April 5, 2019. Since December 2015, WHO had reported 90 cases of human infection with A(H9N2) avian influenza, including two fatal cases (both with comorbidities) [
https://www.who.int/data/gho/data/themes/global-influenza-virological-surveillance].
Highly pathogenic A/H5Nx and A/H7N9 avian influenza viruses reassort with the A/H9N2 virus, and can adapt relatively easily to the human host with no immunity to them. Moreover, the low pathogenic A/H9N2 virus also has a pandemic potential due to its circulation among poultry around the world. A small number of mutations in hemagglutinin (T187P + M227L) and PB2 protein (627K) lead to the appearance of mutants that cause the death of mice and infection of guinea pigs in direct contact. [
14].
Thus, it was retrospectively demonstrated that the A/H5N1 virus that caused the outbreak 1997 in Hong Kong received all genes (except for HA and NA) from co-circulating A/H9N2 viruses. Starting from 2013, recombination between G57 A/H9N2 viruses and other circulating subtypes has resulted in the formation of H7N9, H5N1 and H5N6 multiple zoonotic influenza viruses with high mortality rates among people [
15]. Each of these viruses contains six A/H9N2 virus genes. Moreover, several highly pathogenic A/H5Nx viruses contain one or more A/H9N2 genes. In addition to transferring their own genes, A/H9N2 viruses were recorded to receive single or multiple gene combinations from other avian influenza viruses in several cases. For example, the predominant A/H9N2 lineage, circulating in Pakistan and Bangladesh, is known to have received several genes from highly pathogenic A/H7N3 and A/H5N1 viruses [
16]. It appears that the wide spread of A/H9N2 viruses may contribute to the emergence of future pandemic influenza strains.
In this regard, we monitor herd immunity to influenza vaccine viruses, and observe the appearance of influenza viral markers with a potential pandemic (A/H5Nx, A/H7N9, A/H9N2) in human sera during the outbreaks of avian influenza in individual farms, poultry breeding and processing facilities, as well as year-round among people, living in places, where wild waterfowl are concentrated.
We demonstrated that herd immunity to seasonal influenza in Russia after a sharp decrease in the fall of 2020 had not recovered by the autumn of 2022. Although 52.8% of the country’s population was vaccinated in the autumn of 2022, the number of positive serum samples for vaccine strains was lower than on the eve of the epidemic seasons before the COVID-19 pandemic. This may be due to the age distribution of the Russian population: the share of people aged 65 years and older, 15–64 years old, and under 15 is 15.8, 66.5, and 17.7%, respectively. Since the first group is at risk, they should be vaccinated first. However, aging was demonstrated to significantly affect humoral immune responses by marked effects on the formation and function of B-cell [
17]. Therefore, the vaccine developed for the adult population provided no protective antibody titers in the elderly.
In addition to increasing the burden of seasonal epidemics, insufficient herd immunity increases the probability of new influenza viral variants. The greatest probability of the emergence of a new virus is considered to be associated with densely populated geographical regions with a warm climate and large animal populations which can support the replication of several viral subtypes, capable of recombination and/or reassortment [
10]. In the case of the influenza virus, the countries of Southeast Asia are recognized as the hot spots for the emergence of viruses with epidemic and pandemic potentials [
18]. Indeed, we have demonstrated that among 295 serum samples from healthy donors, collected in Vietnam in 2017–2018, 22% were positive in HI-tests and 16% were positive in MN for A/H5Nx viruses [
19]. Since the East Asian-Australasian Flyway connects Vietnam and other Southeast Asian countries with Russia, the variants of the influenza virus with pandemic potential can be assumed to enter the Siberian and Far Eastern regions of Russia by migratory birds and further evolve independently.
In this regard, we conducted seromonitoring of pandemic-potential influenza viruses of A/H5Nx, A/H7N9, and A/H9N2 serotypes. In addition, blood sera of people who were in contact with sick and/or dead poultry, and residents of regions, located on the migration routes of wild waterfowl were studied. In 2021–2023, only 0.85 and 1.78% of samples were positive in HI-tests for A/H5Nx and A/H9N2 influenza viruses, respectively. However, all samples, positive in HI-tests for A/H5, were negative in MN. In Russia, A/H9N2 infections seem to take mainly a mild or asymptomatic course without being transmitted further. This can be explained by the poor (to date) adaptation of A(H9N2) viruses to humans. Nevertheless, HI-positive serum samples, which we collected and analyzed in 2021‒2023, were positive in MN.
In the 2021–2023 studies, no human serum samples, positive for influenza A/H7N9 virus, were detected.
Thus, our data enabled us to establish the absence of the stable circulation of A/H5Nx, A/H7N9, and A/H9N2 influenza viruses currently in Russia. However, we believe that the risk of the pandemic influenza virus emergence can be reduced by 100% vaccination against seasonal influenza in employees of poultry farms and other organizations, directly related to the breeding and processing of poultry.