Submitted:
01 November 2023
Posted:
02 November 2023
Read the latest preprint version here
Abstract
Keywords:
Main Text
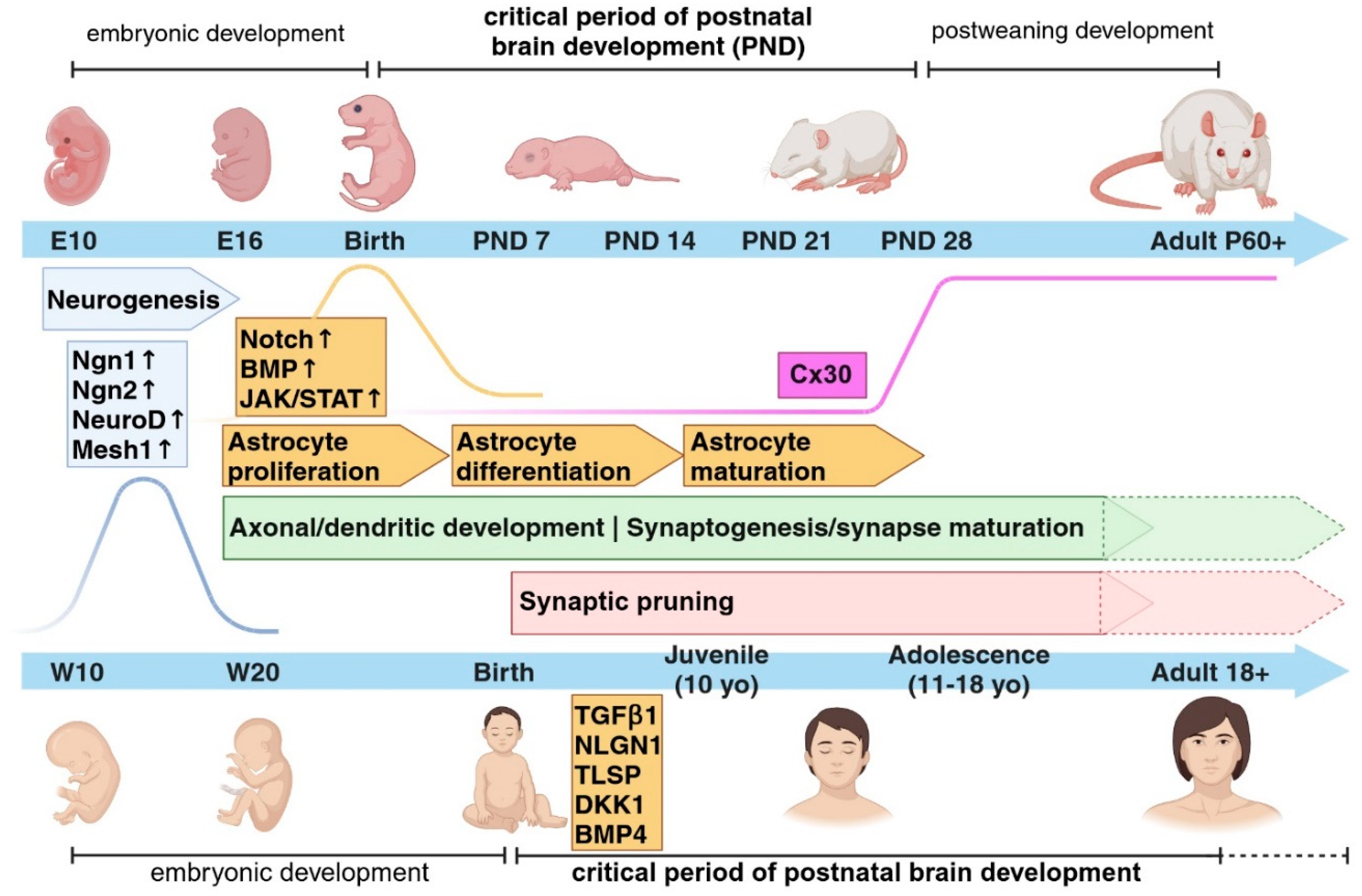
Astrocytes and the Critical Period of Early Postnatal Brain Development
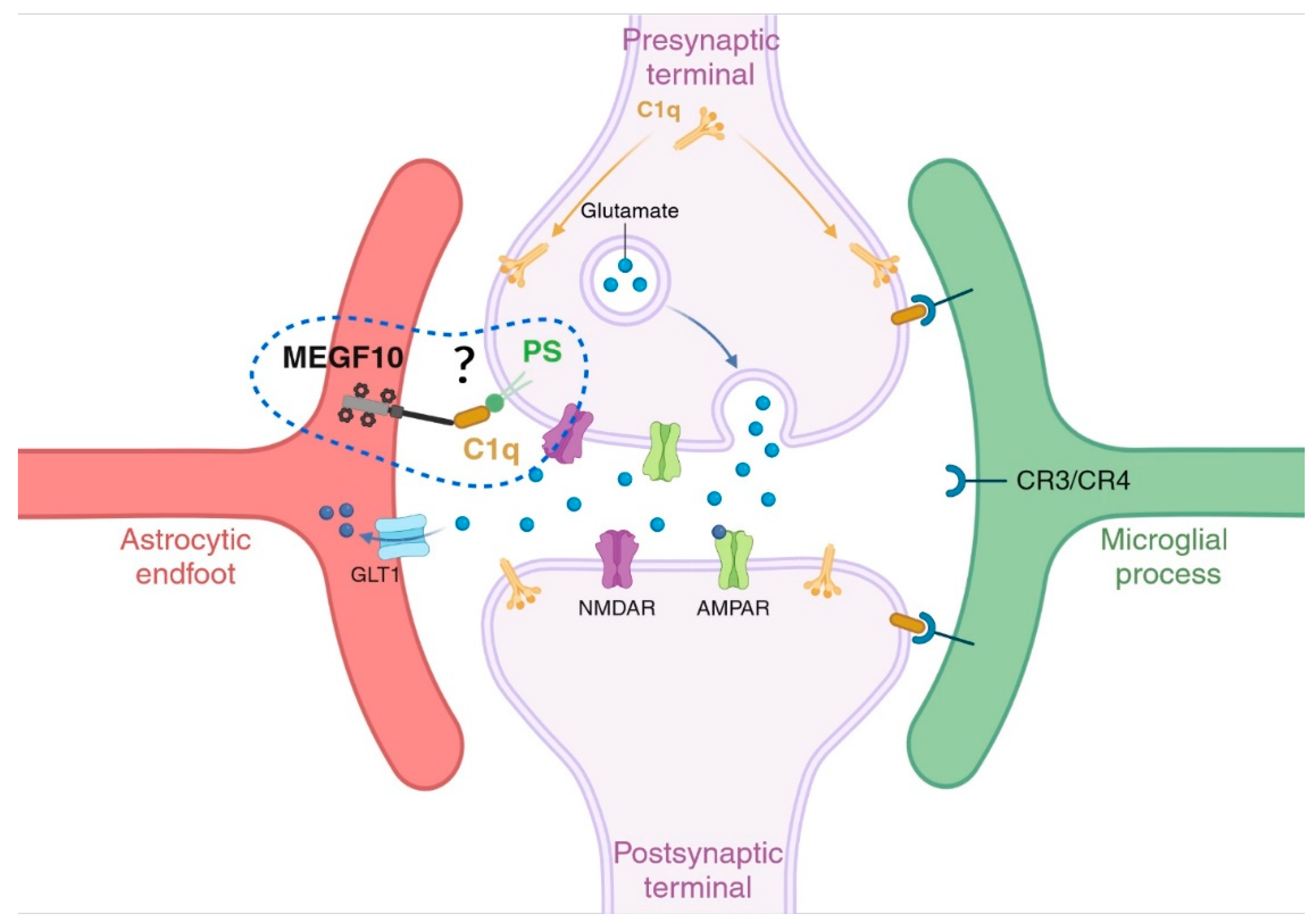
Astrocytes and the Synaptic Compartments: Astrocyte-Mediated Phagocytosis for Neuronal Circuit Refinement
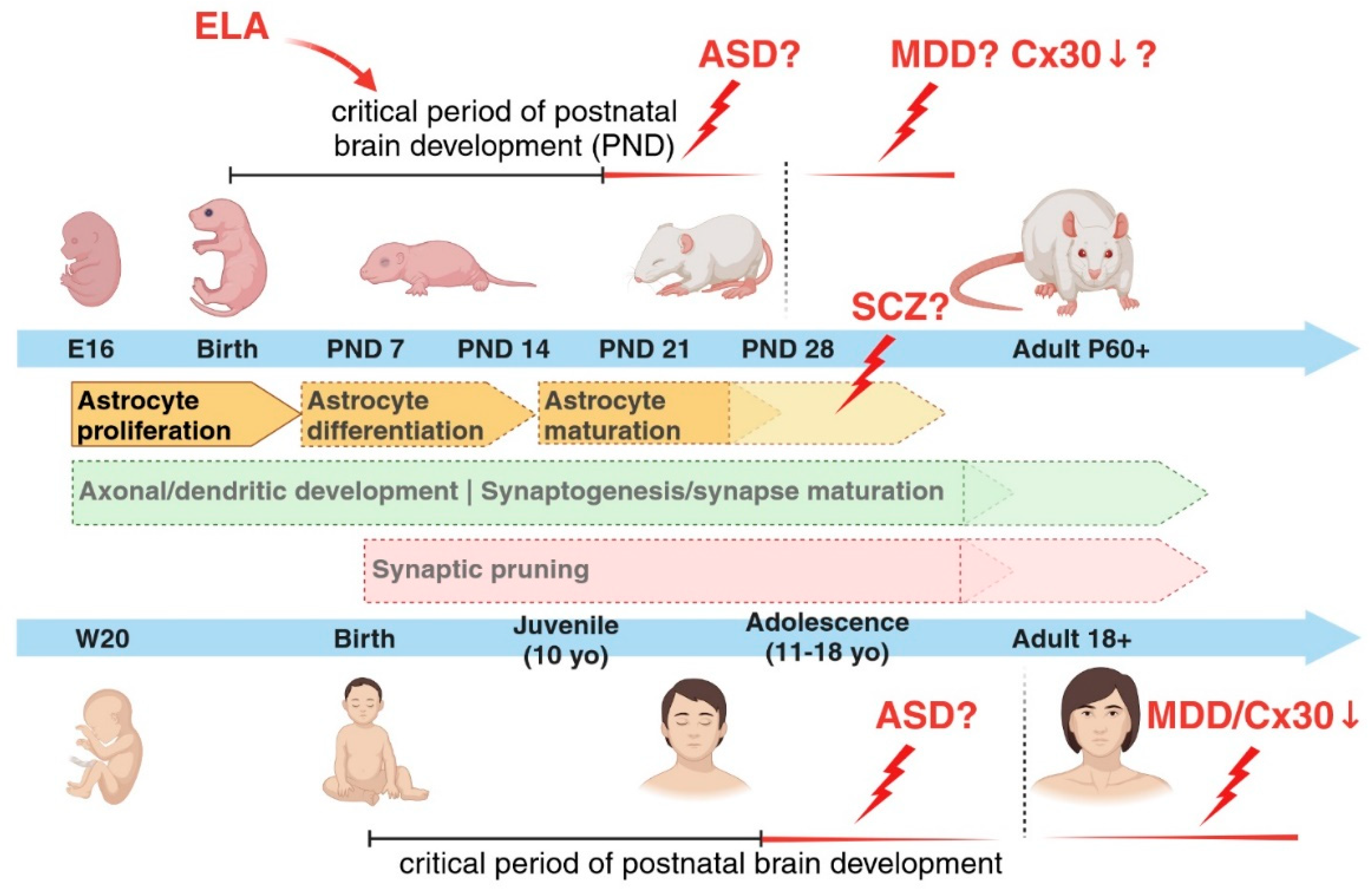
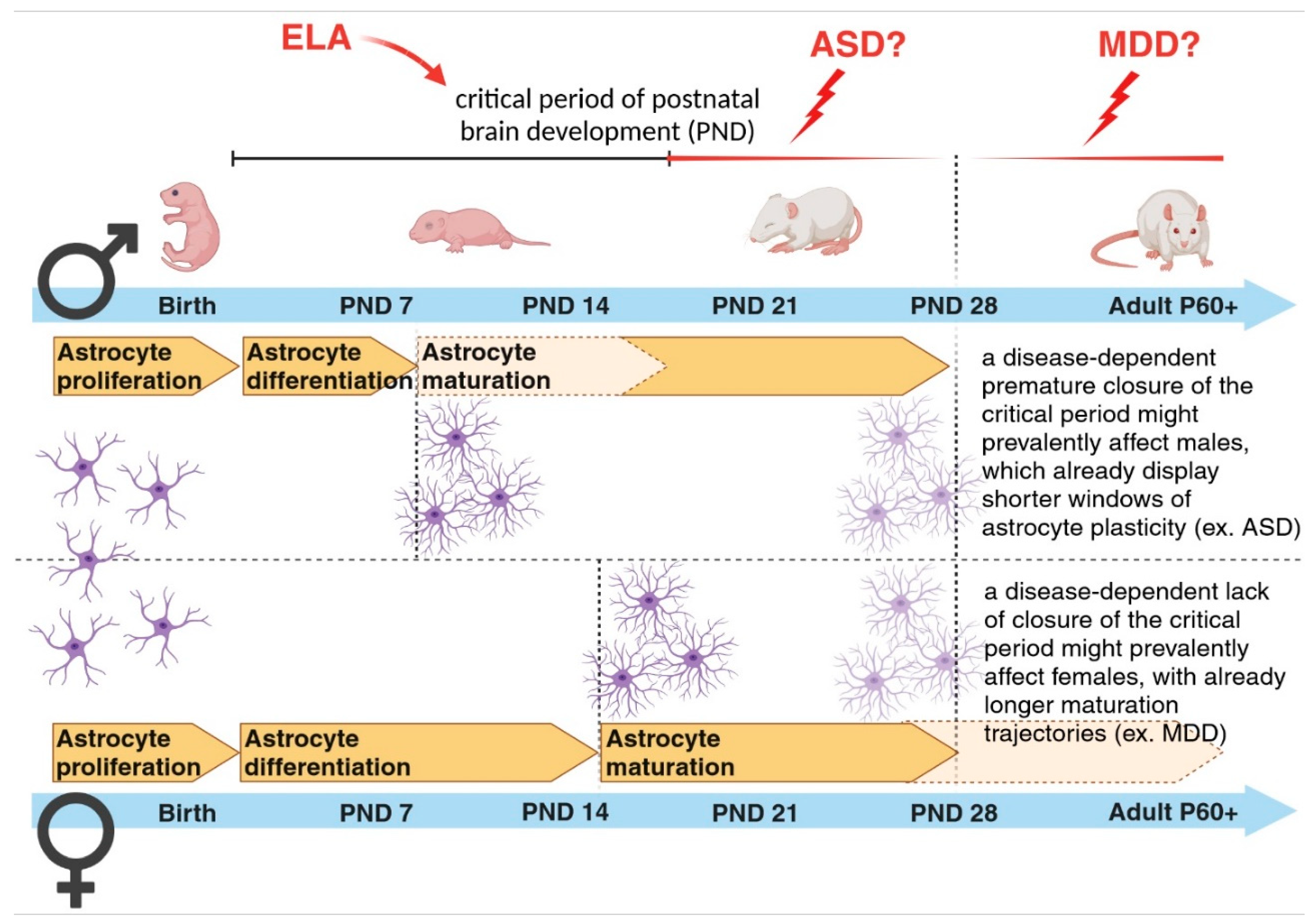
Sex-Dependent Differences in Astrogenesis and in Time Frames of Vulnerability to Perturbations and Disease Onset
Acknowledgments
Conflict of Interest
References
- Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci. 2009 Aug 18;106(33):14108–13. [CrossRef]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015 Jul;18(7):942–52. [CrossRef]
- Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018 Apr;19(4):235–49. [CrossRef]
- Allen NJ, Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017 Nov;96(3):697–708. [CrossRef]
- Bosworth AP, Allen NJ. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol. 2017 Dec;47:38–43. [CrossRef]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J Neurosci. 2004 Mar 3;24(9):2143–55.
- Iram T, Frenkel D. Targeting the Role of Astrocytes in the Progression of Alzheimers Disease. Curr Signal Transduct Ther. 2012 Jan 1;7(1):20–7. [CrossRef]
- Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014 May 8;509(7499):189–94. [CrossRef]
- Cabezas R, Ãvila M, Gonzalez J, El-Bachá RS, Báez E, Garcà a-Segura LM, et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinsonâ€TMs disease. Front Cell Neurosci [Internet]. 2014 Aug 4 [cited 2022 Jan 12];8. Available from: http://journal.frontiersin.org/article/10.3389/fncel.2014.00211/abstract.
- Boulay AC, Saubaméa B, Adam N, Chasseigneaux S, Mazaré N, Gilbert A, et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov. 2017 Dec;3(1):17005. [CrossRef]
- Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013 Dec;61(12):1939–58. [CrossRef]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010 Nov;468(7321):223–31. [CrossRef]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005 Oct;6(10):777–88.
- Takouda J, Katada S, Nakashima K. Emerging mechanisms underlying astrogenesis in the developing mammalian brain. Proc Jpn Acad Ser B. 2017;93(6):386–98. [CrossRef]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007 May 3;54(3):357–69. [CrossRef]
- Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012 Apr;484(7394):376–80. [CrossRef]
- De Zeeuw CI, Hoogland TM. Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Front Cell Neurosci [Internet]. 2015 Jul 2 [cited 2023 Jun 6];9. Available from: http://journal.frontiersin.org/Article/10.3389/fncel.2015.00246/abstract . [CrossRef]
- Güngör Kobat S. Importance of Müller Cells. Beyoglu Eye J [Internet]. 2020 [cited 2023 Jun 6]; Available from: http://beyoglueye.com/jvi.aspx?un=BEJ-28290&volume=.
- Farmer WT, Murai K. Resolving Astrocyte Heterogeneity in the CNS. Front Cell Neurosci. 2017 Sep 27;11:300. [CrossRef]
- Holt MG. Astrocyte heterogeneity and interactions with local neural circuits. Bolaños JP, editor. Essays Biochem. 2023 Mar 3;67(1):93–106. [CrossRef]
- Miller SJ. Astrocyte Heterogeneity in the Adult Central Nervous System. Front Cell Neurosci. 2018 Nov 15;12:401. [CrossRef]
- Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Dev Camb Engl. 2001 Sep;128(18):3585–94. [CrossRef]
- Oliveria JP, Li ZJ. critical role of astrogenesis and neurodevelopment in Fragile X Syndrome and Rett Syndrome. McMaster Univ Med J [Internet]. 2020 Dec 26 [cited 2023 May 31];17(1). Available from: https://journals.mcmaster.ca/mumj/article/view/2338 . [CrossRef]
- Kanski R, Van Strien ME, Van Tijn P, Hol EM. A star is born: new insights into the mechanism of astrogenesis. Cell Mol Life Sci. 2014 Feb;71(3):433–47.
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, et al. Neurogenin Promotes Neurogenesis and Inhibits Glial Differentiation by Independent Mechanisms. Cell. 2001 Feb;104(3):365–76. [CrossRef]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of Gliogenesis in the Central Nervous System by the JAK-STAT Signaling Pathway. Science. 1997 Oct 17;278(5337):477–83. [CrossRef]
- Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH Genes Control the Neuronal versus Glial Fate Decision in Cortical Progenitors. Neuron. 2001 Feb;29(2):401–13. [CrossRef]
- Barnabé-Heider F, Wasylnka JA, Fernandes KJL, Porsche C, Sendtner M, Kaplan DR, et al. Evidence that Embryonic Neurons Regulate the Onset of Cortical Gliogenesis via Cardiotrophin-1. Neuron. 2005 Oct;48(2):253–65. [CrossRef]
- Voss AJ, Lanjewar SN, Sampson MM, King A, Hill EJ, Sing A, et al. Identification of ligand-receptor pairs that drive human astrocyte development. Nat Neurosci. 2023 Aug;26(8):1339–51. [CrossRef]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009 Apr 10;513(5):532–41. [CrossRef]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000 Feb;10(1):138–45. [CrossRef]
- Chugani HT. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prev Med. 1998 Mar;27(2):184–8. [CrossRef]
- Sengpiel F. The critical period. Curr Biol. 2007 Sep;17(17):R742–3.
- Knudsen EI. Sensitive Periods in the Development of the Brain and Behavior. J Cogn Neurosci. 2004 Oct 1;16(8):1412–25. [CrossRef]
- Nelson CA, Gabard-Durnam LJ. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020 Mar;43(3):133–43. [CrossRef]
- Virolainen SJ, VonHandorf A, Viel KCMF, Weirauch MT, Kottyan LC. Gene–environment interactions and their impact on human health. Genes Immun. 2022 Dec 30;24(1):1–11. [CrossRef]
- Milbocker KA, Campbell TS, Collins N, Kim S, Smith IF, Roth TL, et al. Glia-Driven Brain Circuit Refinement Is Altered by Early-Life Adversity: Behavioral Outcomes. Front Behav Neurosci. 2021 Dec 2;15:786234. [CrossRef]
- Juraska JM, Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2017 Jan;1654:87–94. [CrossRef]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997 Oct 20;387(2):167–78.
- Peter R. H. Synaptic density in human frontal cortex — Developmental changes and effects of aging. Brain Res. 1979 Mar;163(2):195–205.
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010 Sep 10;329(5997):1358–61. [CrossRef]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HBM, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci. 2011 Aug 9;108(32):13281–6. [CrossRef]
- Eltokhi A, Janmaat IE, Genedi M, Haarman BCM, Sommer IEC. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J Neurosci Res. 2020 Jul;98(7):1335–69.
- Cardozo PL, De Lima IBQ, Maciel EMA, Silva NC, Dobransky T, Ribeiro FM. Synaptic Elimination in Neurological Disorders. Curr Neuropharmacol. 2019 Oct 2;17(11):1071–95. [CrossRef]
- Zhuang Y, Xu X, Li H, Niu F, Yang M, Ge Q, et al. Megf10-related engulfment of excitatory postsynapses by astrocytes following severe brain injury. CNS Neurosci Ther. 2023 Apr 20;cns.14223. [CrossRef]
- Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, et al. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016 May 11;36(19):5185–92. [CrossRef]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016 May 24;7(1):11475. [CrossRef]
- Honeycutt JA, Demaestri C, Peterzell S, Silveri MM, Cai X, Kulkarni P, et al. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife. 2020 Jan 20;9:e52651.
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Ágústsdóttir A, et al. Fear Erasure in Mice Requires Synergy Between Antidepressant Drugs and Extinction Training. Science. 2011 Dec 23;334(6063):1731–4. [CrossRef]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, F. O’Leary O, et al. The Antidepressant Fluoxetine Restores Plasticity in the Adult Visual Cortex. Science. 2008 Apr 18;320(5874):385–8. [CrossRef]
- Ribot J, Breton R, Calvo CF, Moulard J, Ezan P, Zapata J, et al. Astrocytes close the mouse critical period for visual plasticity. Science. 2021 Jul 2;373(6550):77–81. [CrossRef]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science. 2010 Feb 12;327(5967):863–6. [CrossRef]
- Müller CM, Best J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 1989 Nov;342(6248):427–30. [CrossRef]
- Ghézali G, Calvo CF, Pillet LE, Llense F, Ezan P, Pannasch U, et al. Connexin 30 controls astroglial polarization during postnatal brain development. Development. 2018 Feb 15;145(4):dev155275. [CrossRef]
- Abbink MR, Deijk AF, Heine VM, Verheijen MH, Korosi A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia. 2019 Apr 30;glia.23625. [CrossRef]
- Codeluppi SA, Chatterjee D, Prevot TD, Bansal Y, Misquitta KA, Sibille E, et al. Chronic Stress Alters Astrocyte Morphology in Mouse Prefrontal Cortex. Int J Neuropsychopharmacol. 2021 Oct 23;24(10):842–53. [CrossRef]
- Woodburn SC, Bollinger JL, Wohleb ES. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. 2021 May;14:100312. [CrossRef]
- Dolotov OV, Inozemtseva LS, Myasoedov NF, Grivennikov IA. Stress-Induced Depression and Alzheimer’s Disease: Focus on Astrocytes. Int J Mol Sci. 2022 Apr 30;23(9):4999. [CrossRef]
- Naskar S, Chattarji S. Stress Elicits Contrasting Effects on the Structure and Number of Astrocytes in the Amygdala versus Hippocampus. eNeuro. 2019;6(1):ENEURO.0338-18.2019. [CrossRef]
- Miguel-Hidalgo JJ, Moulana M, Deloach PH, Rajkowska G. Chronic Unpredictable Stress Reduces Immunostaining for Connexins 43 and 30 and Myelin Basic Protein in the Rat Prelimbic and Orbitofrontal Cortices. Chronic Stress. 2018 Jan;2:247054701881418. [CrossRef]
- Rajkowska G, Miguel-Hidalgo JJ. Glial Pathology in Major Depressive Disorder: An Approach to Investigate the Coverage of Blood Vessels by Astrocyte Endfeet in Human Postmortem Brain. In: Di Benedetto B, editor. Astrocytes [Internet]. New York, NY: Springer New York; 2019 [cited 2023 May 9]. p. 247–54. (Methods in Molecular Biology; vol. 1938). Available from: http://link.springer.com/10.1007/978-1-4939-9068-9_17.
- Benedetto B, Rupprecht R. Targeting Glia Cells: Novel Perspectives for the Treatment of Neuropsychiatric Diseases. Curr Neuropharmacol. 2013 Mar 1;11(2):171–85. [CrossRef]
- Roman C, Egert L, Di Benedetto B. Astrocytic-neuronal crosstalk gets jammed: Alternative perspectives on the onset of neuropsychiatric disorders. Eur J Neurosci. 2021 Sep;54(5):5717–29. [CrossRef]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015 Mar;20(3):320–8. [CrossRef]
- Martins-Macedo J, Salgado AJ, Gomes ED, Pinto L. Adult brain cytogenesis in the context of mood disorders: From neurogenesis to the emergent role of gliogenesis. Neurosci Biobehav Rev. 2021 Dec;131:411–28. [CrossRef]
- Feresten AH, Barakauskas V, Ypsilanti A, Barr AM, Beasley CL. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res. 2013 Oct;150(1):252–7. [CrossRef]
- Tarasov VV, Svistunov AA, Chubarev VN, Sologova SS, Mukhortova P, Levushkin D, et al. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front Pharmacol. 2020 Feb 7;10:1612.
- Notter T. Astrocytes in schizophrenia. Brain Neurosci Adv. 2021 Jan;5:239821282110091. [CrossRef]
- Vakilzadeh G, Martinez-Cerdeño V. Pathology and Astrocytes in Autism. Neuropsychiatr Dis Treat. 2023;19:841–50. [CrossRef]
- Allen M, Huang BS, Notaras MJ, Lodhi A, Barrio-Alonso E, Lituma PJ, et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry. 2022 May;27(5):2470–84. [CrossRef]
- Rajkowska G, Miguel-Hidalgo J. Gliogenesis and Glial Pathology in Depression. CNS Neurol Disord - Drug Targets. 2007 Jun 1;6(3):219–33. [CrossRef]
- Belleau EL, Treadway MT, Pizzagalli DA. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol Psychiatry. 2019 Mar;85(6):443–53. [CrossRef]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010 Sep;35(5):337–43. [CrossRef]
- Geng R, Huang X. Identification of major depressive disorder disease-related genes and functional pathways based on system dynamic changes of network connectivity. BMC Med Genomics. 2021 Dec;14(1):55. [CrossRef]
- Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial Plasticity in the Hippocampus is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology. 2006 Aug;31(8):1616–26. [CrossRef]
- Czéh B, Di Benedetto B. Antidepressants act directly on astrocytes: Evidences and functional consequences. Eur Neuropsychopharmacol. 2013 Mar;23(3):171–85.
- Czéh B, Nagy SA. Clinical Findings Documenting Cellular and Molecular Abnormalities of Glia in Depressive Disorders. Front Mol Neurosci. 2018 Feb 27;11:56. [CrossRef]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell. 2012 Aug;150(3):633–46. [CrossRef]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010 Jan 14;463(7278):232–6. [CrossRef]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005 Mar;57(6):577–85. [CrossRef]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010 Aug;121(1–3):125–30. [CrossRef]
- Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2013 May;18(5):557–67. [CrossRef]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: From bow-and-arrow concordances to Star Wars Mx and functional genomics. Am J Med Genet. 2000;97(1):12–7.
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, et al. Cortisol Levels and Risk for Psychosis: Initial Findings from the North American Prodrome Longitudinal Study. Biol Psychiatry. 2013 Sep;74(6):410–7. [CrossRef]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015 Aug 18;5(8):e623–e623. [CrossRef]
- Sheu JR, Hsieh CY, Jayakumar T, Tseng MF, Lee HN, Huang SW, et al. A Critical Period for the Development of Schizophrenia-Like Pathology by Aberrant Postnatal Neurogenesis. Front Neurosci. 2019 Jun 18;13:635.
- De Oliveira Figueiredo EC, Calì C, Petrelli F, Bezzi P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia. 2022 Sep;70(9):1585–604. [CrossRef]
- Russo FB, Freitas BC, Pignatari GC, Fernandes IR, Sebat J, Muotri AR, et al. Modeling the Interplay Between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol Psychiatry. 2018 Apr;83(7):569–78. [CrossRef]
- Berger JM, Rohn TT, Oxford JT. Autism as the Early Closure of a Neuroplastic Critical Period Normally Seen in Adolescence. Biol Syst Open Access [Internet]. 2012 [cited 2023 Jul 23];02(03). Available from: https://www.omicsgroup.org/journals/autism-as-the-early-closure-of-a-neuroplastic-critical-period-normally-seen-in-adolescence-2329-6577-1000118.php?aid=43859.
- Hashimoto Y, Greene C, Munnich A, Campbell M. The CLDN5 gene at the blood-brain barrier in health and disease. Fluids Barriers CNS. 2023 Mar 28;20(1):22. [CrossRef]
- Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, et al. Glial Cell Line-Derived Neurotrophic Factor Induces Barrier Function of Endothelial Cells Forming the Blood–Brain Barrier. Biochem Biophys Res Commun. 1999 Jul;261(1):108–12.
- Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of Blood Vessels by Astrocytic Endfeet Is Reduced in Major Depressive Disorder. Biol Psychiatry. 2013 Apr;73(7):613–21. [CrossRef]
- Lee E, Chung WS. Glial Control of Synapse Number in Healthy and Diseased Brain. Front Cell Neurosci. 2019 Feb 13;13:42. [CrossRef]
- Di Benedetto B, Malik VA, Begum S, Jablonowski L, Gómez-González GB, Neumann ID, et al. Fluoxetine Requires the Endfeet Protein Aquaporin-4 to Enhance Plasticity of Astrocyte Processes. Front Cell Neurosci [Internet]. 2016 Feb 2 [cited 2022 Jan 13];10. Available from: http://journal.frontiersin.org/Article/10.3389/fncel.2016.00008/abstract . [CrossRef]
- Malik VA, Zajicek F, Mittmann LA, Klaus J, Unterseer S, Rajkumar S, et al. GDF15 promotes simultaneous astrocyte remodeling and tight junction strengthening at the blood–brain barrier. J Neurosci Res. 2020 Jul;98(7):1433–56. [CrossRef]
- Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995 Jun;14(6):1181–8. [CrossRef]
- Konishi H, Koizumi S, Kiyama H. Phagocytic astrocytes: Emerging from the shadows of microglia. Glia. 2022 Jun;70(6):1009–26. [CrossRef]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013 Dec 19;504(7480):394–400. [CrossRef]
- Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007 Feb;3(1):63–74. [CrossRef]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping Glial Biology. Neuron. 2003 May;38(4):567–80. [CrossRef]
- Reddien PW, Horvitz HR. THE ENGULFMENT PROCESS OF PROGRAMMED CELL DEATH IN CAENORHABDITIS ELEGANS. Annu Rev Cell Dev Biol. 2004 Nov 1;20(1):193–221.
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007 Dec;131(6):1164–78. [CrossRef]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012 May;74(4):691–705. [CrossRef]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011 Sep 9;333(6048):1456–8. [CrossRef]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci. 2010 Apr 27;107(17):7975–80. [CrossRef]
- Lee JH, Kim J young, Noh S, Lee H, Lee SY, Mun JY, et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature. 2021 Feb 25;590(7847):612–7. [CrossRef]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016 May 6;352(6286):712–6. [CrossRef]
- Dion-Albert L, Bandeira Binder L, Daigle B, Hong-Minh A, Lebel M, Menard C. Sex differences in the blood-brain barrier: implications for mental health. Front Neuroendocrinol. 2022 Mar;100989. [CrossRef]
- Blokland GAM, Grove J, Chen CY, Cotsapas C, Tobet S, Handa R, et al. Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders. Biol Psychiatry. 2022 Jan;91(1):102–17. [CrossRef]
- Riecher-Rössler A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017 Jan;4(1):8–9. [CrossRef]
- 111. Hyer MM, Phillips LL, Neigh GN. Sex Differences in Synaptic Plasticity: Hormones and Beyond. Front Mol Neurosci. 2018 Jul 31;11:266. [CrossRef]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies: Using sex differences in psychopathology to study causal mechanisms. J Child Psychol Psychiatry. 2003 Nov;44(8):1092–115.
- Ruigrok ANV, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014 Feb;39(100):34–50. [CrossRef]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, et al. Maturational Trajectories of Cortical Brain Development through the Pubertal Transition: Unique Species and Sex Differences in the Monkey Revealed through Structural Magnetic Resonance Imaging. Cereb Cortex. 2010 May;20(5):1053–63. [CrossRef]
- Dehorter N, Del Pino I. Shifting Developmental Trajectories During Critical Periods of Brain Formation. Front Cell Neurosci. 2020;14:283. [CrossRef]
- Rurak GM, Simard S, Freitas-Andrade M, Lacoste B, Charih F, Van Geel A, et al. Sex differences in developmental patterns of neocortical astroglia: A mouse translatome database. Cell Rep. 2022 Feb;38(5):110310. [CrossRef]
- Clarkson J, Herbison AE. Hypothalamic control of the male neonatal testosterone surge. Philos Trans R Soc Lond B Biol Sci. 2016 Feb 19;371(1688):20150115. [CrossRef]
- Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol. 2016 Sep;144:5–26. [CrossRef]
- Rurak GM, Woodside B, Aguilar-Valles A, Salmaso N. Astroglial cells as neuroendocrine targets in forebrain development: Implications for sex differences in psychiatric disease. Front Neuroendocrinol. 2021 Jan;60:100897. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




