1. Introduction
Different types of coating have been continuously developed in order to achieve high-performance multifunctional coatings with scratch and abrasion resistance for the surface of various steel substrates for marine applications [
1]. The most important characteristic of the coating is to be environmentally friendly and to achieve multi-functions, e.g., protection, high mechanical properties, fire resistance, and corrosion resistance. In order to obtain these improved properties coatings, as well as protection of materials from hostile environments, optimized epoxy formulations are made incorporating nanoparticles like silica (SiO
2) and zinc oxide (ZnO) [
2]. Epoxy systems tailored with metal-oxide nanoparticles can be used in the automotive and aerospace industries [
3]. Silica nanoparticles are frequently added into the polymer matrices to increase the heat resistance, radiation resistance, mechanical, and electrical properties of the final materials. The nano dimensions of the particles are very important in the properties of the acrylic-based coatings. The nano-silica particles are typically dispersed in the bulk polymer matrices using a suitable dispersing agent.
The epoxy resin has a high bonding strength and superior moisture resistance due to their ability to create chemical bonds or molecular adsorption as well as cross-links with curing agents. Due to their good physical, chemical and mechanical properties, epoxy resin can be used as best candidate for coating matrix [
4]. Incorporating ZnO nanoparticles into epoxy resins can improve the chemical resistance, thermal, mechanical and dielectric properties of composite coatings due to the formation of strong bonds that occur between oxide groups on the ZnO nanoparticles surface and polar groups of epoxy resins [
5]. Dispersion of nanosized particles into epoxy matrix can prevent epoxy disaggregation and lead to obtaining homogeneous coatings [6, 7]. Epoxy resins have disadvantages such as brittleness, low toughness, and poor impact resistance, which limit the wide application.
Several research papers have reported the successful incorporation of ZnO nanoparticles into epoxy resin in order to obtain high-performance composites. Sunny et al. showed that the addition of ZnO NPs to epoxy resin can improves the thermal and mechanical properties of the final composites [
8]. Suntako revealed that the introduction of ZnO NPS enriched the properties of natural rubber such as tensile strength, hardness, and elongation at break [
9]. Ding et al. [
10] demonstrated that the incorporation of ZnO particles having submicron sizes enhanced the tensile and flexural strength of epoxy resin by 41 and 51%, respectively. Thipperudrappa et al. [
11] showed that the ZnO NPs improved the mechanical properties of reinforced epoxy composites (2 wt% of ZnO). Liu et al. [
12] obtained epoxy nano-composites with good thermal and mechanical properties, using hybrids containing ZnO NPs. Samad et al. [
13] reported that the addition of ZnO nanoparticles in epoxy matrix containing epoxy/polyaniline improving its mechanical and thermal properties.
The aim of this study is to report the effect of ZnO nanoparticles on the physico-chemical properties of epoxy resin. The ZnO/epoxy resin composites were synthesized using halloysite nanotubes (HNT) and ZnO nanoparticles (commercial ZnO and ZnO-ODTES). The morphology of these materials was characterized using a Transmission Electron Microscope (TEM) analysis. Chemical composition of the sample was assessed using Fourier Transform Infrared (FTIR) spectroscopy and Energy Dispersive Spectroscopy (EDS). The glass transition temperature was determined using differential scanning calorimeter (DSC). Thermogravimetric analysis (TGA) was used to prove that the ZnO nanoparticles improve the performance of the epoxy resin composites. The nanoindentation technique was used to examine the durability of epoxy resin systems.
2. Experimental
2.1. Materials
Commercial zinc oxide nanopowder (ZnO, ZnO NPs <100 nm, Sigma-Aldrich, Philadelphia, PA, USA), octadecyltriethoxysilane (ODTES, 98%, Alfa Aesar, Karlsruhe, Germany), xylene (Xy, S.C. Chimreactiv S.R.L., Bucharest, Romania), Tetraethyl orthosilicate (TEOS, 98%, Sigma-Aldrich, Philadelphia, PA, USA), methyl isobutyl ketone (MIBK, Sigma-Aldrich, Millipore, Canada), ammonium hydroxide (NH4OH, 32%, Scharlau, Spain), epoxy resin (Resin 530 (component A+hardener B), Nanochem, TUV Austria), natural aluminosilicate clay with a hollow tubular morphology (HNT 100%, NaturalNano Corp, Rochester, NY, USA), were used as purchased.
2.2. Preparation
ZnO nanoparticles (ZnO NPs) modified with ODTES were prepared using the method described in our previous studies [
14]. A small amount of commercial ZnO nanopowder was dispersed in a mixture containing ethanol, demineralized water and ammonium hydroxide solution (32%) and sonicated for one hour in an ultrasonic bath. Tetraethyl orthosilicate (TEOS) and octadecyltriethoxysilane (ODTES) were added dropwise over the formed solution (molar ratio of TEOS/ODTES = 1/1). The resulted solution was magnetically stirred for 6 h, at room temperature (25±2˚C).
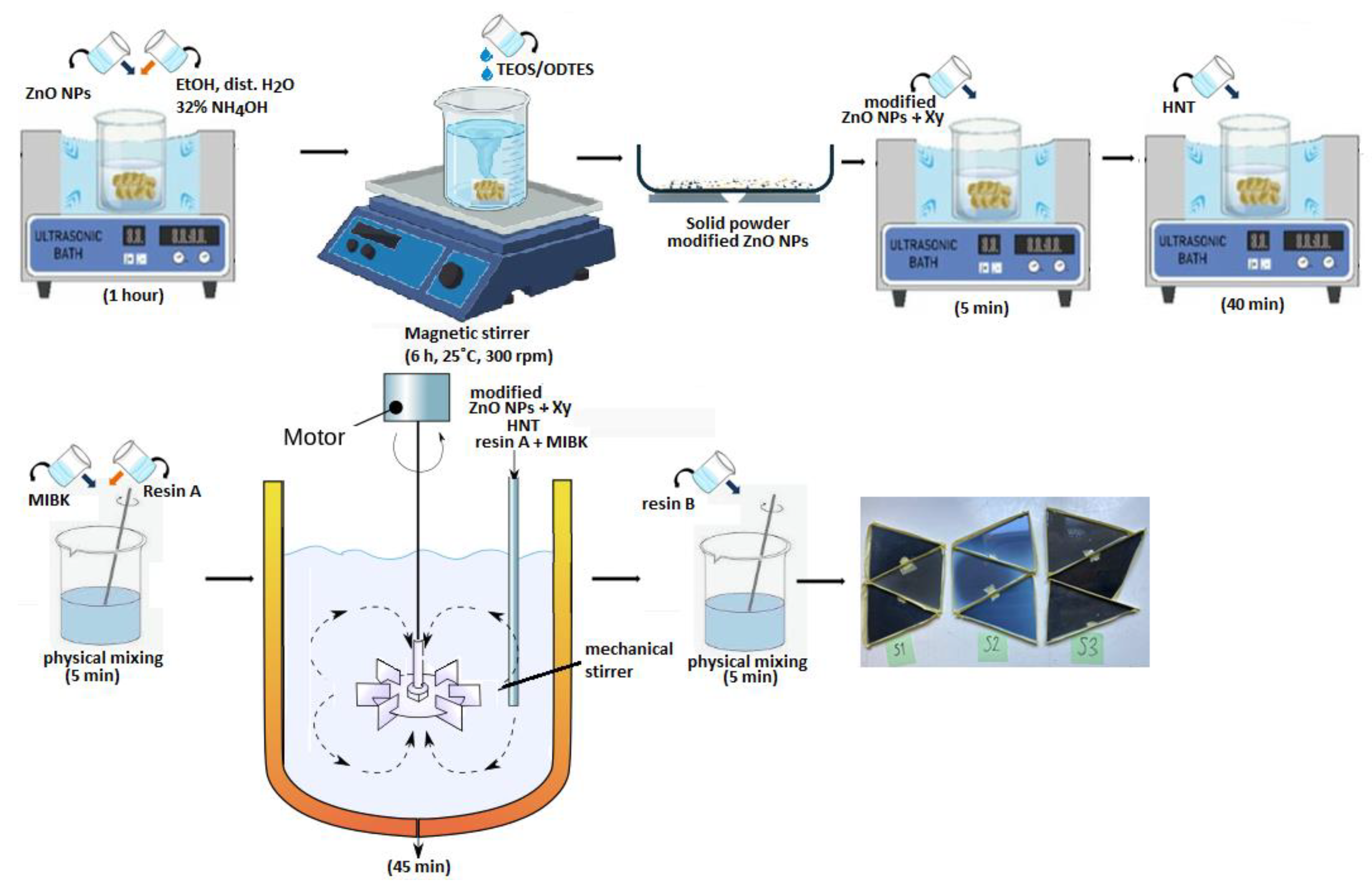
Processing steps for epoxy resin are the following: The modified ZnO with xylene (Xy) was sonicated for 5 minutes in an ultrasonic bath. Aluminosilicate clay with a hollow tubular morphology (HNT) was then added and left to be ultrasonicated for 40 minutes. Methyl isobutyl ketone (MIBK) was poured over component A from the epoxy resin and manually mixed with for 5 minutes. All these components were mixed for 45 minutes with a mechanical stirrer (800 rpm). The mechanical agitator head's shape was specifically designed to prevent bubbles from forming in the material. The last stage involved physically combining resin A with all integrated components and hardener B for five minutes. After the first synthesis, we decreased the amount of resin but maintained the same ratio with the other ingredients, thus the final recipe has the same substances ratio. Three types of modified epoxy resin composites were finally obtained: epoxy resin/HNT/ZnO-ODTES (S1), epoxy resin/HNT/ZnO (S2) and epoxy resin/HNT (S3).
The schematic illustration of synthesis procedure to obtain ZnO/epoxy resin composites is shown in
Figure 1.
2.3. Characterization
FTIR spectra of materials were recorded on a Spectrometer Tensor 37 (Bruker Instrument, Woodstock, NY, USA), in ATR (Attenuated total reflection) mode with a Golden Gate diamond unite (400–4000 cm-1).
Thermal analysis of the synthesized materials (dried under vacuum at 50°C for 24 h) was performed using a Thermogravimetric Analyser TGA Q5000IR (TA Instruments, New Castle, DE, USA) in a nitrogen atmosphere (heating rate of 10˚C/min, at 30–750°C range). Obtained materials (7–9 mg) were analyzed using alumina crucibles.
The Differential Scanning Calorimeter (DSC Q2000, TA Instruments, USA) determines the temperature and heat flow associated with material transitions as a function of time and temperature.
The materials were analyzed through Transmission Electron Microscopy (TEM, model TECNAI F20 G² TWIN Cryo–TEM, FEI Company, Eindhoven, The Netherlands) at 200 kV accelerating voltage. The associated EDX spectra were obtained using the X-MaxN 80T detector (Oxford Instruments, Oxford, UK), installed on the device.
Nanomechanical testing was performed using the Hysitron TriboIndenter Premier (TI) (Premier Hysitron USA) in order to makes nanoscale mechanical characterization simple and consistent.
3. Results and Discussion
3.1. FTIR Spectroscopy
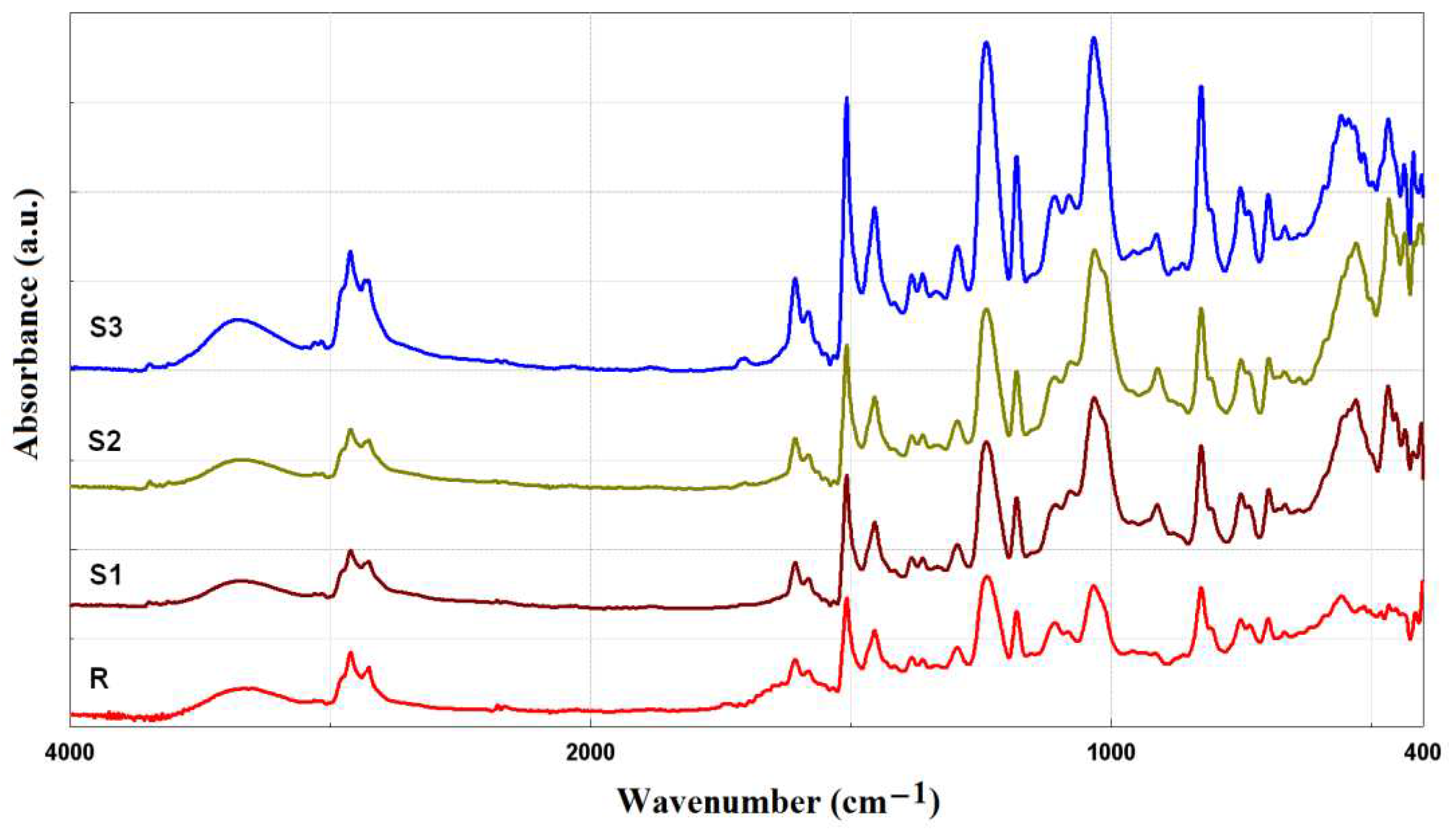
The chemical bonds present in the samples were analyzed using FTIR spectroscopy, and a plot of absorbance versus wave number is shown in
Figure 2. The bands that appeared in the range of 3100–3600 cm
−1 can be assigned to the O–H stretching vibrations [
15]. The peaks at 2922 and 2852 cm
−1 are characteristic of the C–H bonds present in the synthesized materials [
16]. Peaks in the interval of 1580–1610 cm
−1 correspond to primary amine (N–H) [
17]. The peak at 1508 cm
−1 is attributed to N–O stretching vibration indicating the presence of nitro compounds. According to the literature, the C–C and C–O bonds (stretching vibrations) are observed at 1454 cm
−1 and at 1240 cm
−1, respectively [1, 17]. The peaks in the range from 1000 to 1100 cm
−1 correspond to C–N groups. The peak at 826 cm
−1 is associated to the 1,4-substitution of the aromatic ring of the epoxy resin.
In the FTIR spectra of sample S3, a peak at 557cm−1 is revealed and is due to stretching vibrations of the aromatic rings (C–C).
The peak at ~400 cm-1 is notice in the FTIR spectra of samples S1 and S2 confirming the presence of ZnO NPs in the epoxy resin compositions.
3.2. TGA and DSC Analysis
Figure 3 reveals the TGA analysis of the epoxy resin and of ZnO/epoxy resin composites. The decomposition temperature (T
max) and the weight loss (Wt. loss %) of these samples are presented in
Table 2.
From the TGA curves, it can be observed that the decomposition of the modified resins occur around 250°C. A weight loss of 15–20% up to the temperature of 160°C (where a maximum occurs), may be due to the removal by volatilization of some compounds present in the synthesized resins. Since the unmodified epoxy resin (sample R) loses 15% by mass, and the modified epoxy resins composites (samples S1, S2, and S3) lose 20% by mass, it can be concluded that the 5% represent the loss of solvents (xylene and methyl isobutyl ketone) added to the obtained materials (see
Table 2). Unmodified resin (sample R) showed relaxation enthalpy at 40–45°C.
Muhammad et al. [
18] demonstrated that the ZnO NPs improved the stability of epoxy coatings after 90 days of exposure period degradation.
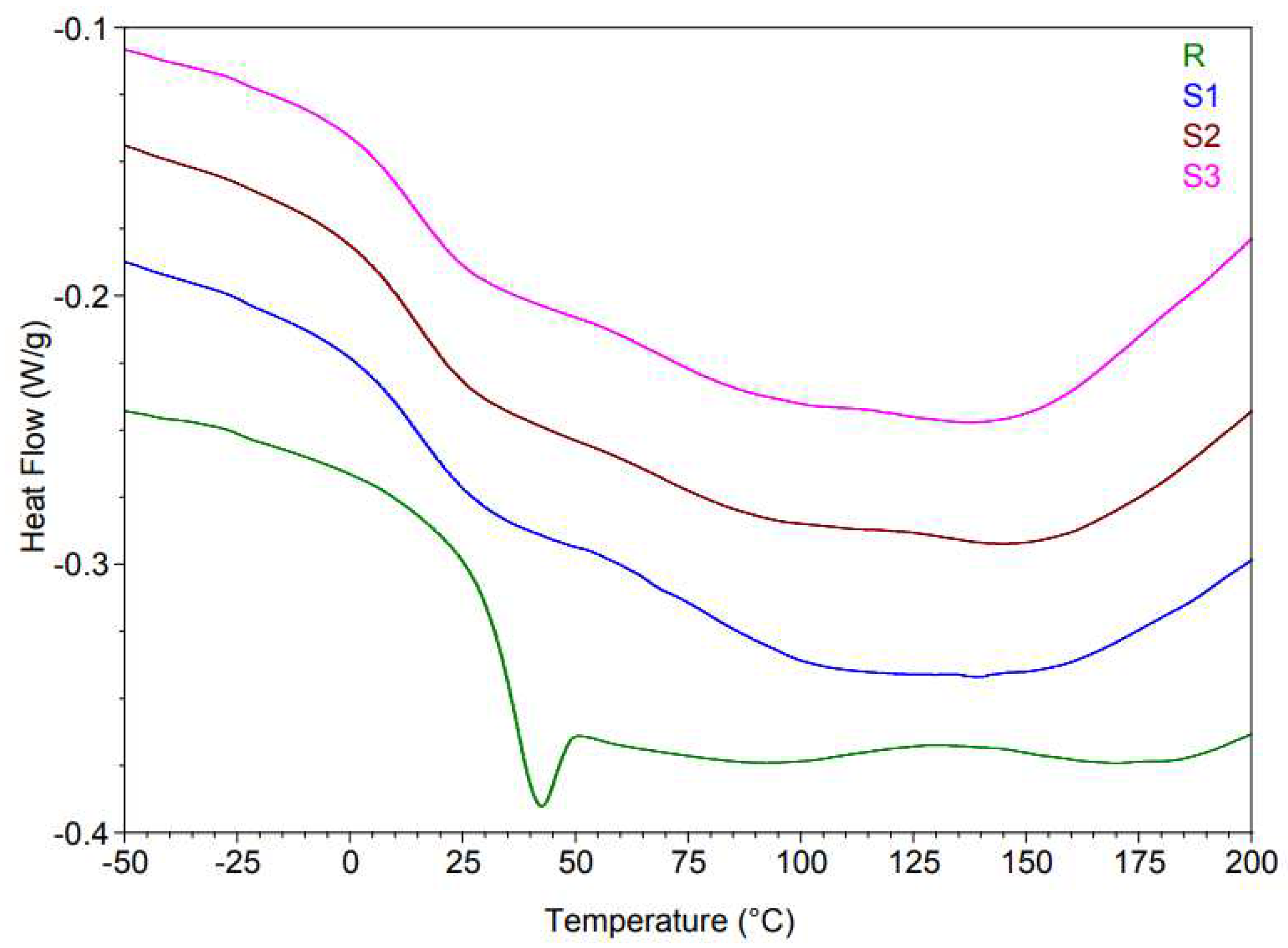
The enthalpy phenomena observed in DSC curves are associated with the mass loss highlighted in TGA analysis, for the 100–250°C range, and may be due to losses of volatile substances (solvent) as well as unreacted reactants.
Xylene (Xy) has a boiling point of 138.5°C, and methyl isobutyl ketone (MIBK) has a boiling point of 117°C. Analyzing the TGA results, it can be observed that the heating behavior of modified resins (samples S1, S2, and S3) is not very different comparing with the unmodified resins (sample R). On the other hand, evaluating the DSC results, the endothermic enthalpy in the range of 75–250°C is due to the elimination of small molecular (volatile) substances, and diluents (
Figure 4). After cooling, modified resins (samples S1, S2, and S3) have a higher mass loss (~14°C), thus a lower
Tg. This can also be seen through an initial optical characterization performed immediately after the resin has cured, at approximately 36 hours. The modified epoxy resins are more flexible and elastic. Modified epoxy resin with ZnO-ODTES (sample S1) has better elasticity than unmodified resin (sample R) and the other modified resins (samples S2 and S3). In all the results presented in the
Table 3, the unmodified epoxy resin is more rigid than the modified epoxy resins.
These results are in agreement with those reported in the literature [19, 20] where it is shown that the addition of ZnO nanoparticles decreases the thermal properties of the materials because ZnO tends to form free oxygen and oxygen vacancies.
3.3. TEM Analysis
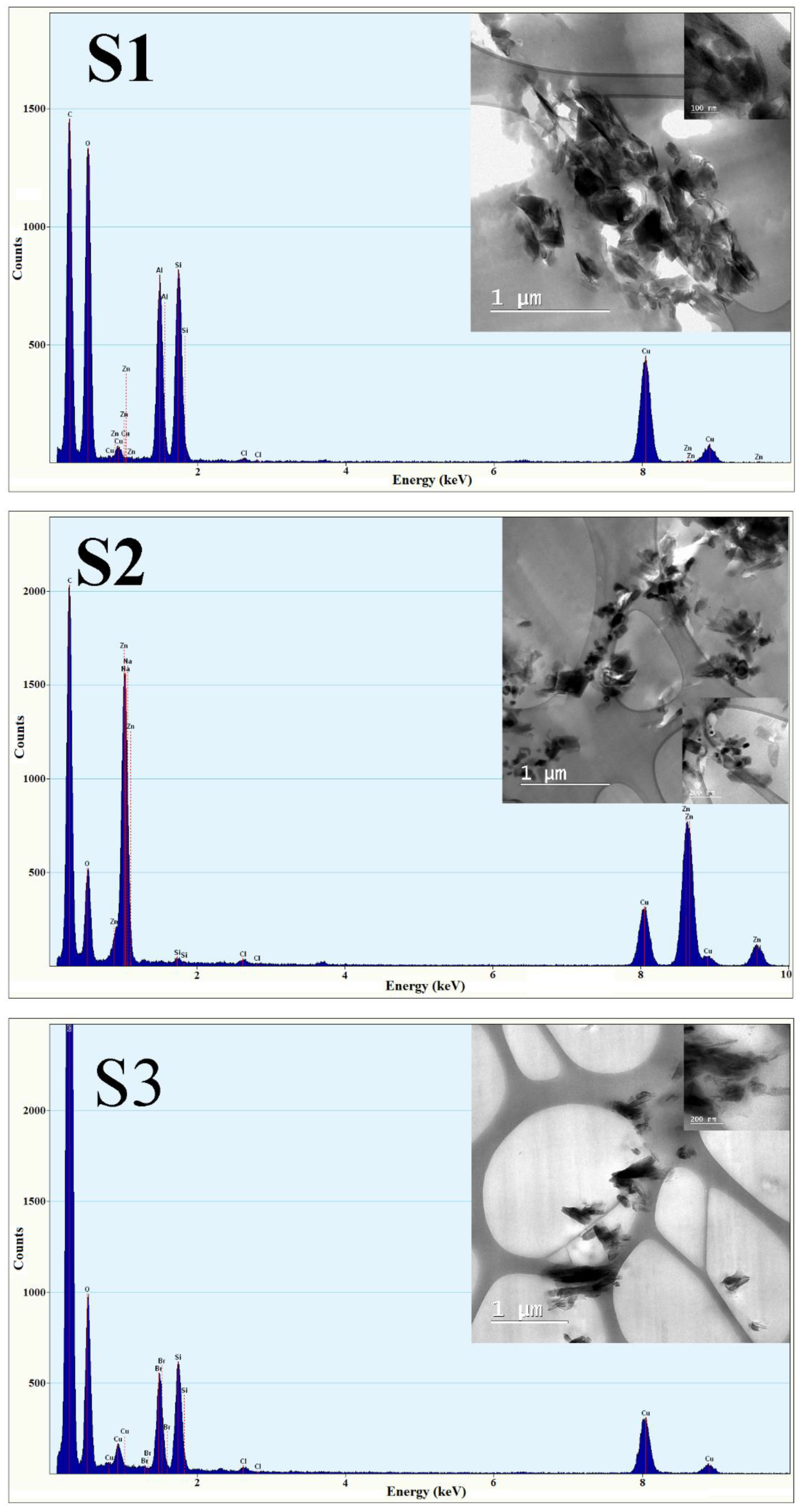
The morphology and elemental analysis of the samples were assessed by Transmission Electron Microscopy (TEM) equipped with energy dispersive X-ray (EDX). The TEM images and EDX analysis are illustrated in
Figure 5.
Through TEM imaging, the presence of HNT nanotubes was observed in all three samples. In samples S1 and S2, the presence of ZnO NPs was also determined. Sample S3 is the one that does not present any kind of ZnO NPs. Moreover, the images reveal that the HNT and used ZnO NPs do not tend to form the agglomerates.
From EDX analysis, the existence of Zn signals could be detected in samples S1 and S2, originating from ZnO NPs utilized in synthesis. Due to the present of Cu lines scan, it can be concluded that the carbon foil on the TEM copper grid may be responsible for the signals.
3.4. Nanoindentation Analysis Method
Nanomechanical characterization was performed on all the prepared coating samples to extract coating hardness and modulus by employing nanoindentation technique.
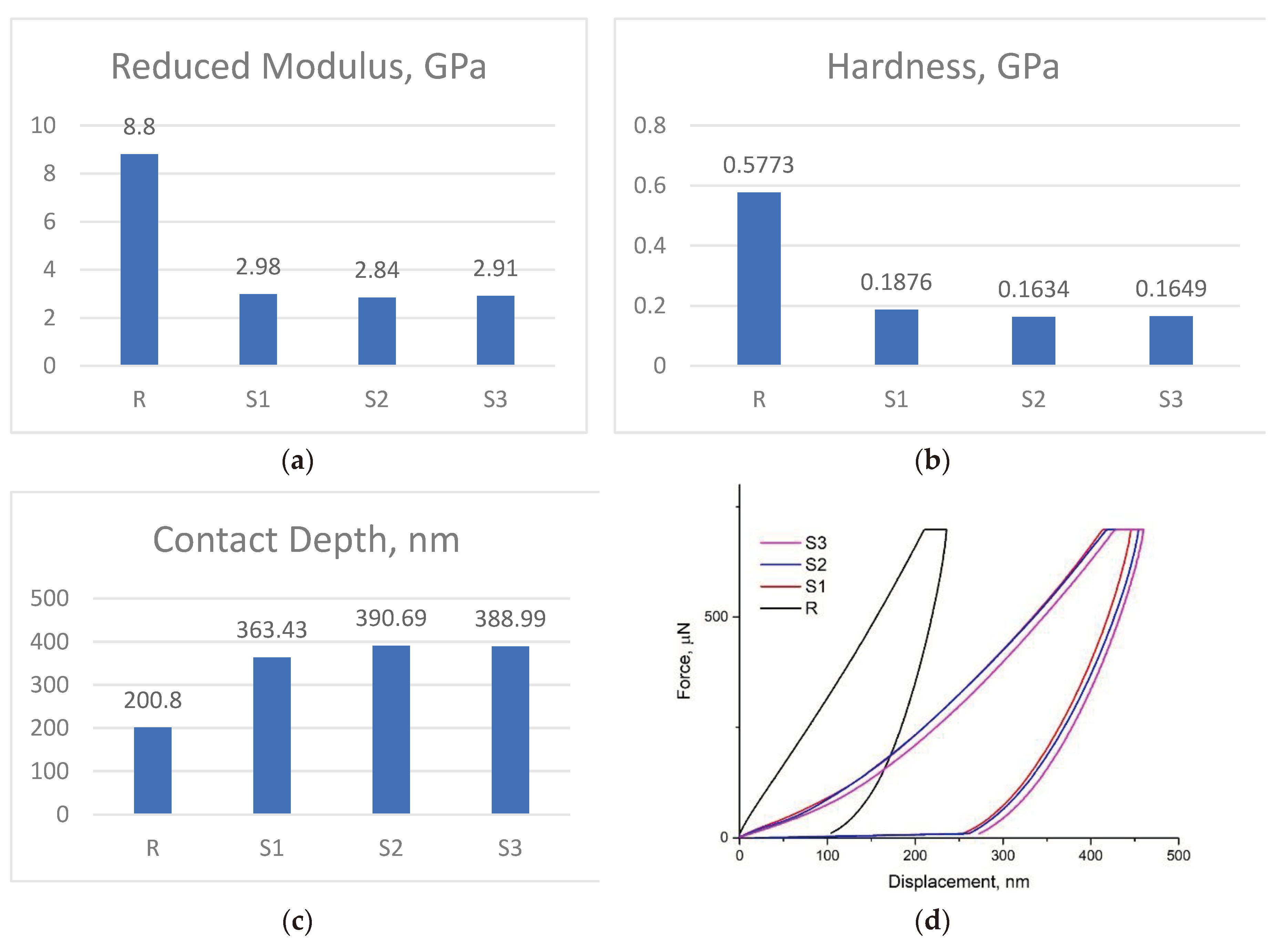
From
Figure 6a,b,c, it can be seen that the modified epoxy resin composites (samples S1, S2 and S3) are much more elastic than unmodified epoxy resin (sample R). The modulus and hardness decrease considerably and the penetration depth increases for the obtained composites. S1 has the highest values for hardness (~0.1876 GPa) and reduced modulus (2.98 GPa), although the difference between the values is quite small, comparing with samples S2 and S3.
From
Figure 6d, it can be observed that the elastic force of the modified epoxy resin composites is much higher compared to unmodified epoxy resin. Sample S1 has the lowest elastic force.
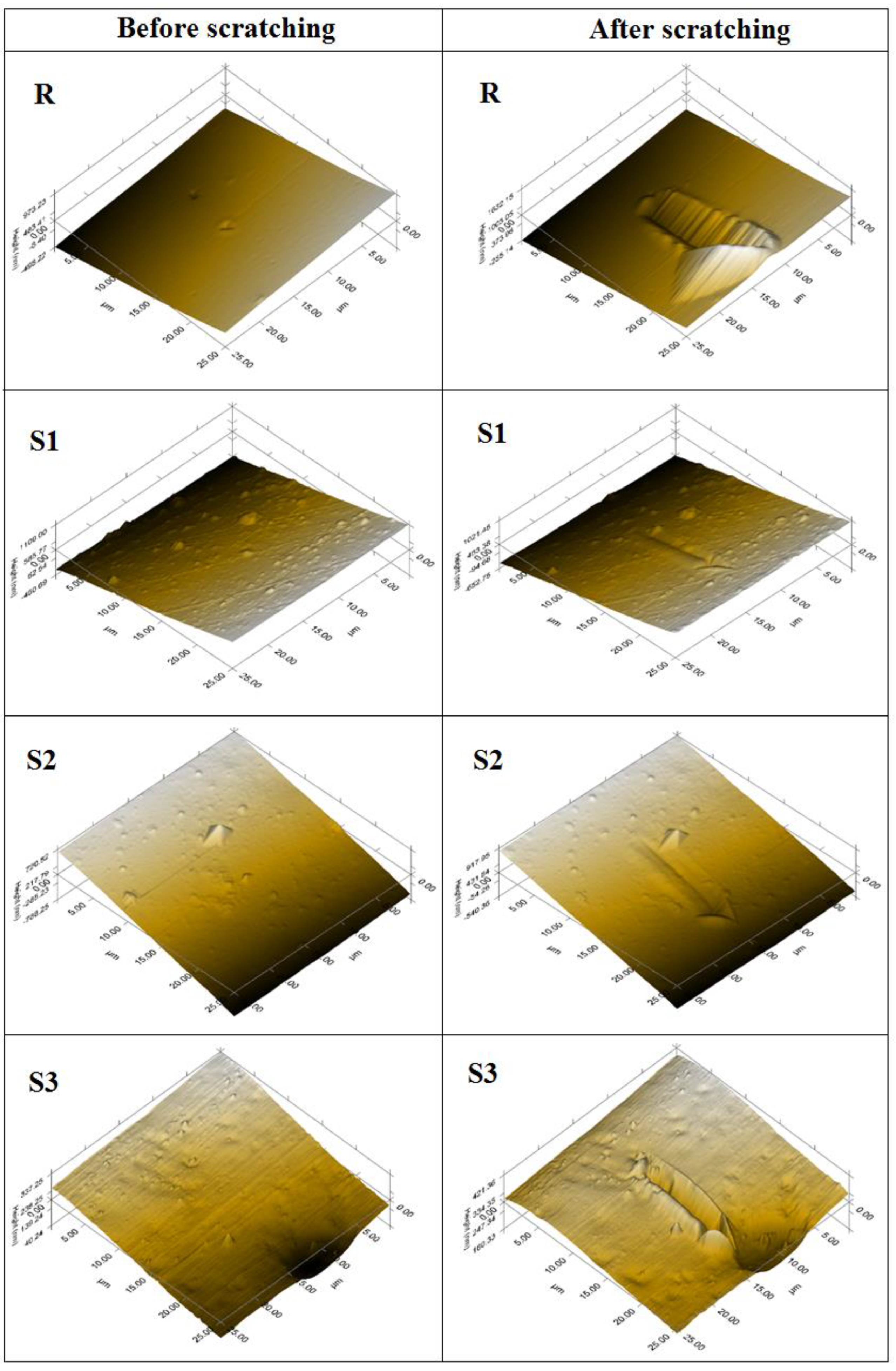
In the present study, hence, after performing the indentations, scanning probe microscopy (SPM) was performed on the samples, as seen in
Figure 7.
Analyzing optical images, it can be seen that final compositions present major differences (aggregates with different shapes) (
Figure 7). Sample S1 shows the most formations and agglomerates, which can be seen both on the surface and inside the sample being semi-transparent. The roughness increases in the case of samples R and S1 as a result of scratching the surface, indicating a harder surface with less elasticity. The SPM images confirm this behavior. In the case of samples S2 and S3, it can be observe a decrease in roughness, probably due to the increased elasticity of the surface. The friction coefficient of modified epoxy resin composites decreases compared to unmodified epoxy resin (0.6) (see
Table 4). This result indicates that a lower coefficient means less wear of the material when it is subjected to various deformation phenomena.
Among all the samples, sample S1 has the best properties because it shows both elastic behavior and surface hardness compared to the other samples. It has a lower friction coefficient (0.4) than sample R but improved values for reduced mode, hardness and penetration depth compared to samples S2 and S3. These characteristics are important for the final applications of the composite, especially in the naval field where the surface is constantly subjected to the forces of friction and impact with water.
Karasinski et al. [
21] demonstrated that the utilization of ZnO NPs actions as a barrier against an external indentation force, leading to superior properties due to excellent charge transferability between matrix and filler.
4. Conclusions
In this study, the effects of ZnO nanoparticles on the chemical, thermal, and mechanical properties of epoxy resin composites were studied. It has been found that the addition of ZnO nanoparticles improved the properties of epoxy resin composites. It was found that addition of ZnO nanoparticles decreases the thermal properties of the materials because ZnO tends to form free oxygen and oxygen vacancies. Nanoindentation results revealed that modified epoxy resin composite with ZnO-ODTES present a good elastic behavior and surface hardness compared to the other samples. The performances of the new ZnO/epoxy resin composites present advantages both in the field of mechanical properties and in the field of surface performances of the coatings. The improvement in mechanical properties of epoxy resins tailored with ZnO nanoparticles brings advantages in reducing the maintenance cycles for surface protection, but also a high reliability of the surfaces subject to friction compared to the unmodified epoxy resin. Increasing the elastic component in the structured material also indirectly contributes to the reliability of the coating and its impact behavior.
Author Contributions
Conceptualization, R.S. and F.O; methodology, S.M. and D.M.S.V.; formal analysis, R.S., R.A.G., T.B., G.M.T. and C.-A.N.; investigation, Z.V., D.M.S.V. and G.M.T.; data curation, S.M., R.A.G., Z.V. and C.-A.N.; writing—original draft preparation, R.S. and F.O.; writing—review and editing, R.S. and T.B.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Research, Innovation, and Digitization through CCCDI—UEFISCDI, project number PN-III-P2-2.1-PTE-2021-0675.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, M.A.; Samad, U.A.; Anis, A.; Alam, M.; Ubaidullah, M.; Al-Zahrani, S.M. Effects of sio2 and zno nanoparticles on epoxy coatings and its performance investigation using thermal and nanoindentation technique. Polymers 2021, 13, 1490. [Google Scholar] [CrossRef] [PubMed]
- Samad, U.A; Alam, M.A.; Anis, A.; Sherif, E.-S.M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of incorporated zno nanoparticles on the corrosion performance of sio2 nanoparticle-based mechanically robust epoxy coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Tehrani-Bagha, A. R. Graphene-based polymer composite films with enhanced mechanical properties and ultra-high in-plane thermal conductivity. Compos. Sci. Technol. 2019, 184, 107797. [Google Scholar] [CrossRef]
- Junid, R.; Siregar, J.P.; Endot, N.A.; Razak, J.A.; Wilkinson, A.N. Optimization of Glass Transition Temperature and Pot Life of Epoxy Blends Using Response Surface Methodology (RSM). Polymers 2021, 13, 3304. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, X.; Wang, J.; Yu, X.; Yang, F.; Yu, Z.; Zhang, H.; Zhao, Y.; Zhang, Y. Mechanical and dielectric properties at liquid nitrogen temperature of epoxy/AlN composites modifed with diferent contents of fexible amine. SN Appl. Sci. 2020, 2, 1. [Google Scholar] [CrossRef]
- Becker, O.; Varley, R.; Simon, G. Morphology, thermal relaxations and mechanical properties of layered silicate nanocomposites based upon high-functionality epoxy resins. Polymer 2002, 43, 4365. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Serra, R.; Poznyak, S.K.; Ferreira, M.G.S. Nanoporous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability. Prog. Org. Coat. 2007, 58, 127. [Google Scholar] [CrossRef]
- Sunny, A.T.; Vijayan, P.P.; Adhikari, R.; Mathew, S.; Thomas, S. Copper oxide nanoparticle in an epoxy network: Microstructure, chain confinement and mechanical behavior. Phys. Chem. Chem. Phys. 2016, 18, 19655–19667. [Google Scholar] [CrossRef] [PubMed]
- Suntako, R. Cure Characteristics and Mechanical Properties of ZnO Nanoparticles as Activator in Unfilled Natural Rubbe. Adv. Mater. Res. 2014, 1044–1045, 23–26. [Google Scholar] [CrossRef]
- Ding, K.H.; Wang, G.L.; Zhang, M. Characterization of mechanical properties of epoxy resin reinforced with submicron-sized ZnO prepared via in situ synthesis method. Mater. Des 2011, 32, 3986–3991. [Google Scholar] [CrossRef]
- Thipperudrappa, S.; Ullal Kini, A.; Hiremath, A. Influence of zinc oxide nanoparticles on the mechanical and thermal responses of glass fiber-reinforced epoxy nanocomposites. Polym. Compos 2020, 41, 174e81. [Google Scholar] [CrossRef]
- Liu, C.; Daneshvar, F.; Hawkins, S.; Kotaki, M.; Sue, H.J. High dielectric constant epoxy nano-composites containing ZnO quantum dots decorated carbon nanotube. J. Appl. Polym. Sci. 2020, 138, e49778. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; Cinteză, L.O. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomater. 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Thakura, S.; Bhattacharyab, R.; Neogia, S.; Neogi, S. Enhancement of Microwave Absorption Properties of Epoxy by Sol–Gel-Synthesised ZnO Nanoparticles. Indian Chem. Eng. 2016, 58, 310–324. [Google Scholar] [CrossRef]
- Dhanapal, D.; Ranjitha, J.; Vijayalakshmi, S.; Sagadevan, S. Fabrication of tetraglycidyl epoxy nano-composites functionalized with amine-terminated zinc oxide with improved mechanical and thermal properties. J. Mater. Res. Technol. 2022, 21, 3947–3960. [Google Scholar] [CrossRef]
- Moussa, S.; Namouchi, F; Guermazi, H. Elaboration, structural and optical investigations of ZnO/epoxy nanocomposites. Eur. Phys. J. Plus 2015, 130, 152. [CrossRef]
- Muhammad, A.N.S.; Farooq, A.; Lodhi, M.; Deen, K. Performance evaluation of epoxy coatings containing different fillers in natural and simulated environmental conditions for corrosion resistance. J. Biomed. Eng. Biosci. 2019, 3, 1. [Google Scholar]
- Hsu, S.C.; Whang, W.T.; Hung, C.H.; Chiang, P.C.; Hsiao, K.N. Effect of the polyimide structure and ZnO concentration on the morphology and characteristics of polyimide/ZnO nanohybrid films. Macromol. Chem. Phys. 2005, 206, 291–298. [Google Scholar] [CrossRef]
- Baghdadi, Y.N.; Youssef, L.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. The effects of modified zinc oxide nanoparticles on the mechanical/thermal properties of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49330. [Google Scholar] [CrossRef]
- Karasinski, E.N.; Da Luz, M.G.; Lepienski, C.M.; Coelho, L.A.F. Nanostructured coating based on epoxy/metal oxides: Kinetic curing and mechanical properties. Thermochim. Acta 2013, 569, 167–176. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).