1. Introduction
Nanotechnology has emerged as a giant in battling lethal pandemic infections, such as Covid, and is a potential field of transdisciplinary biomedical research. Nanofibrous materials with a large porous surface area are excellent for medicinal applications, and the surfaces of nanoparticles can be altered and scanned. Electrospinning is an alternative method of loading active pharmacological chemicals for drug release. Coaxial electrospinning is a common technique used in drug delivery. Polymer nanofibers have the potential for various biological applications, with unseen features such as improved surface area per volume and surface characteristics. Drug-loaded core-shell nanofiber materials allow the long-term release of drugs to minimize side effects. The compatibility between the polymer and chemical behavior of the drug is critical in drug delivery systems [
1]. Electrospinning is the traditional method for fabricating nanofibers with various morphologies and is an efficient way to synthesize nanofiber matrices. Moreover, electrospinning techniques have been developed for the mass production of nanofibers [
2,
3]. The exploration of nanotechnology-based drug delivery systems opens up a promising avenue for collaborative research across multiple disciplines in the biomedical field, fostering innovation and the development of advanced therapeutic approaches. Some issues need to be addressed, including inadequate bioavailability caused by poor solubility or intestinal absorption caused by degradation, insufficient distribution to the intended area, unfavorable side effects, and variable drug plasma levels [
4]. Nanofibrous materials exhibit a large porous surface area [
5] and have great potential for medicinal applications because of their unique ability to be modified and tailored at the nano level, enabling precise manipulation and characterization for a wide range of therapeutic uses [
6]. In conclusion, medications may be delivered to various sites of inflammation via nanoparticles made of natural or artificial polymers, improving the protection of pharmaceuticals against interference with the body absorption process [
7]. Polymeric nanoparticles can be easily changed with organic or inorganic hybrid components; they are often employed in the medical sector. Owing to their many beneficial properties, including biodegradability, biocompatibility, low immunogenicity, and antibacterial capabilities, protein- and polysaccharide-based biopolymers often replace their synthetic counterparts [
8].
Electrospinning is an alternative method of loading active pharmacological chemicals for these releases, producing fine fibers in the ultra-micron to nanometer range with defined surface structures. Nanofibers are generated by dissolving the desired polymer in a solvent and subjecting it to a high-voltage electric field [
9]. A process has been developed that can produce micrometer- to nanometer-thick fibers with precisely controlled topography by subjecting a solution of the desired polymer to a strong electric field or, if the solvent is insufficient, melting the polymer and exposing it to the electric field [
10,
11,
12]. Owing to their outstanding characteristics, polymer nanofibers are potential candidates for various essential biological applications. By reducing the diameter of polymer fibers to the micrometer or nanoscale level, hitherto unseen features may emerge, including an improved ratio of surface area per volume and flexibility of surface characteristics [
9,
10]. Previously, it was shown that cancer cells can be treated with various polymers with bioactivities similar to those of leukemia. The aim of this research was to fabricate Pullulan/Polyvinyl Alcohol (PVA) core-shell nanofibers and explore their potential application in anticancer pharmacology by evaluating their drug encapsulation efficiency, controlled release properties, and efficacy in inhibiting cancer cell growth, with the goal of developing an effective and targeted drug delivery system for cancer treatment [
13].
Drug delivery systems typically adopt coaxial electrospinning techniques [
14]. In the conventional electrospinning method, two needles with two coaxial capillaries are attached to the feed exchange polymer solution. Several paths for the internal and exterior structures are available with a coaxial nozzle arrangement. When managing electro-spraying, problems might arise with nozzle clogging or organizing monolithic fibers [
15]. Electrospinning produces a continuous jet with viscoelasticity and bending instability. According to numerical analyses, electrical charges migrate when electrospinning occurs at the surface of a jet [
16]. Electrospinning devices have three main components: a metallic needle (spinneret), high-voltage power supply, and always in between, attached to the spinneret and collection plate ( grounded collector) [
17]. It is commonly recognised that tubular nanofibers are helpful in various applications, including the transport of medications and the creation of tissues. Because template-directed methodologies are only helpful for manufacturing short-length fibers, electrospinning is recommended for producing long, continuous nanoscale hollow fibers compared to other methods [
18]. Specific threshold values or ranges can be established to classify fibers in the production of long continuous nanoscale hollow fibers using electrospinning [
19]. For instance, fibers shorter than a defined length, such as 1 mm, are considered short, whereas fibers longer than that length are classified as long [
20]. In addition, the aspect ratio of each fiber, calculated by dividing its length by its diameter, can serve as a parameter. Setting a threshold value, such as 100, allows fibers with an aspect ratio greater than that to be categorized as long, whereas those below it are considered short. Evaluating the fiber structure for breaks, discontinuities, or irregularities is crucial [
21]. By defining a predetermined length over which the fiber should exhibit uninterrupted continuity, long fibers are identified, while those with breaks or irregularities are classified as short fibers. These quantitative norms provide consistent fiber classification and enable assessment of the effectiveness of electrospinning in producing long, continuous nanoscale hollow fibers.
The doxorubicin hydrochloride (DOX) develops at high levels in vivo, congestive heart failure and permanent cardiac damage may result [
22,
23,
24,
25]. Drug-loaded core-shell nanofiber materials allow the release of DOX over long periods of time to reduce the side effects of high DOX toxicity. The selection of polymers to ensure uniform loading after manufacturing is based on the compatibility between the polymer and the chemical behavior of the drug. The nanofiber structure, release properties, and mechanical properties of the nano-drug delivery system using DOX are stable at pH 7.4, and the drug can be rapidly released at pH 5 [
25]. The yeast-like fungus Aureobasidium produces pullulan, also known as a polysaccharide, which is commonly used in food coatings and drug delivery systems because it is biodegradable, water-soluble, and has a low oxygen permeability. Drug Administration) is nontoxic and can be used for repeated contact with food. Pullulan has a wide range of commercial applications in the biomedical and food industries because of its strictly linear structure; pullulan is also a valuable tool in basic research as well as a well-defined model substance [
26]. PVA is a carrier polymer that is a chemical compound most commonly used in medical specialty applications. The solubility characteristics of PVA are often referred to as environmentally friendly, earning it the designation of a "green polymer." Its straightforward degradability facilitates effective blending with a diverse range of polymers owing to its water solubility [
27].
2. Materials and Methods
2.1. Materials
Pullulan (9057-02-7), polyvinyl alcohol (PVA) (87%–89% hydrolyzed, 9002-89-5), and doxorubicin hydrochloride (DOX) were purchased from Sigma Aldrich (Czech Republic). 4SPIN ™ (Contipro, Czech Republic) was used to produce nanofibers.
2.2. The Creation of Pullulan/PVA Nanofibers
Pullulan (Mw 50,000 g/mol) and PVA (Mw 9,000-10,000, 87%–89% hydrolyzed) were combined at a ratio of 10 wt% each in distilled water by magnetic stirring and heating. Pullulan and PVA solutions were individually prepared and combined. A PVA solution (10 w/w%) was prepared by dissolving PVA in distilled water by stirring at 80 °C for 2 h, which was used as the core-forming solution. A pullulan solution (10% w/w) was prepared by dissolving it in distilled water and stirring for 2 h at room temperature. The nanofibers were obtained using a needle electrospinning machine (Elmarco, Czech Republic). The polymer syringe was then wired to a voltage of 25 kV. The tip of the needle was positioned 20 mm away. Thus, a flow ratio of 0.5:0.5 was used for the core and shell solutions.
2.3. Preparation of Core-Shell Fibres Loaded with DOX and Administration of Drugs
A coaxial electrospinning method was used to produce DOX-loaded pullulan/PVA nanofibers.
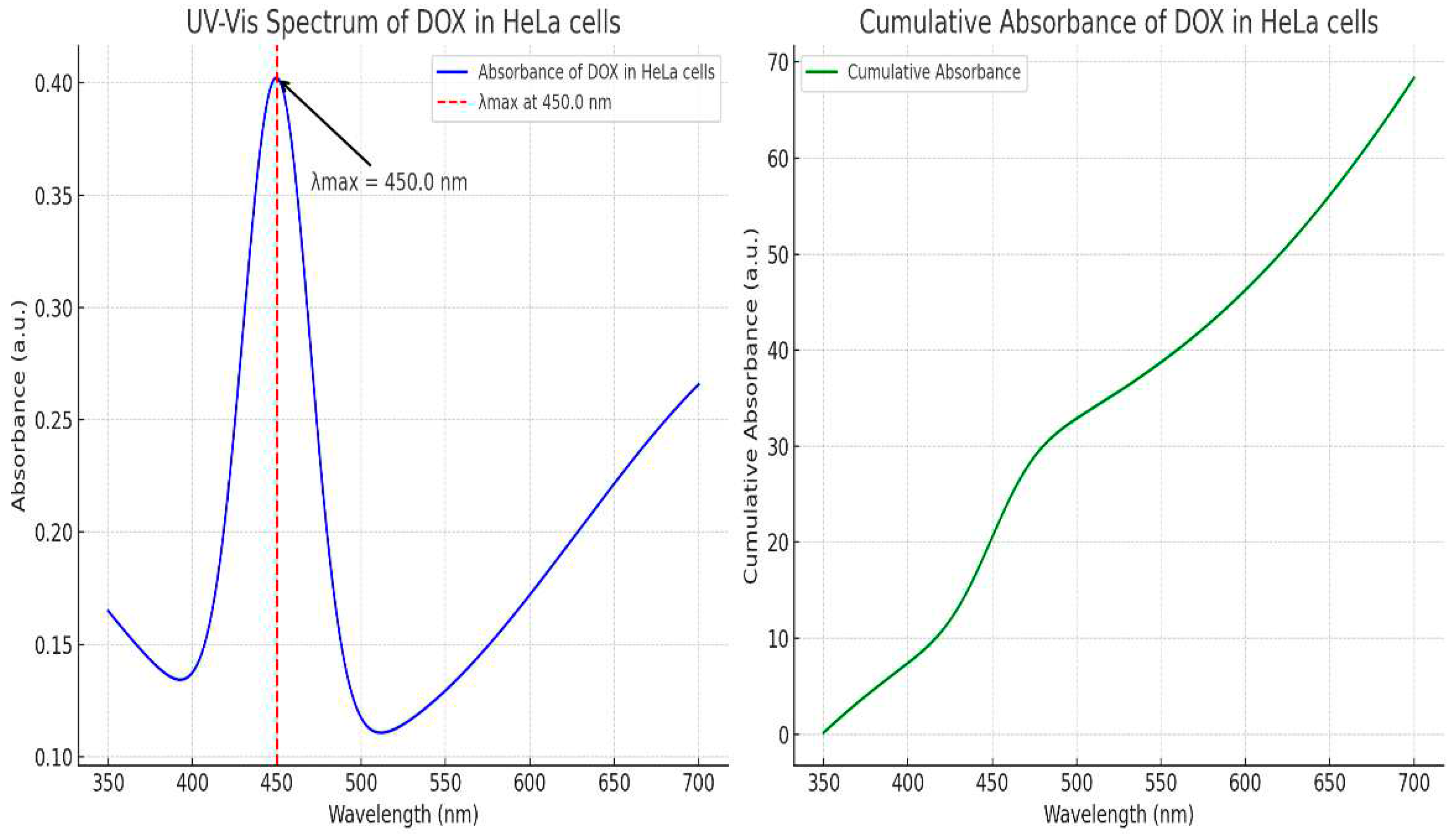
Figure 2 shows the processes involved in the production of PVA/pullulan core and shell nanofibers. Core-shell drug-loaded nanofibers have been developed to address the drawbacks associated with DOX, including its rapid metabolism and high toxicity. DOX was specifically chosen as a model drug to explore the formation of a core-shell structure and the subsequent release mechanism. Two coaxial nozzles were used with the electrospinning machines. The injector needle was connected to the emitter electrode with a positive polarity, and the nanofibers were collected with a negative polarity using an aluminum foil collector. The mass ratio of DOX to PVA was 1/100. A specific ratio of 1/100 might have been chosen based on factors such as the desired drug-loading capacity, release kinetics, or therapeutic requirements. It is a design choice that balances the amount of drug incorporated into a nanofiber system with the properties and characteristics of the polymer matrix (PVA). This ratio could have been determined through previous experimentation or optimization studies to achieve the desired drug delivery performance. A certain amount of the DOX/pullulan/PVA nanofiber drug delivery system was dissolved and mixed in 5 mL of phosphate-buffered saline (PBS, pH = 4) in a constant temperature shaker at 37 °C. The solution was removed, a smooth solution was extracted, and aliquots of PBS were added at intervals. The amount of drug-released nanofibrous material was examined using 450 nm UV-vis spectroscopy to determine the cumulative amount of drug released; the DOX standard curve at 450 nm was used, as shown in
Figure 2. Drug release experiments were repeated five times. The cumulative amount of drug released was calculated from a standard curve of DOX plotted at 450 nm.
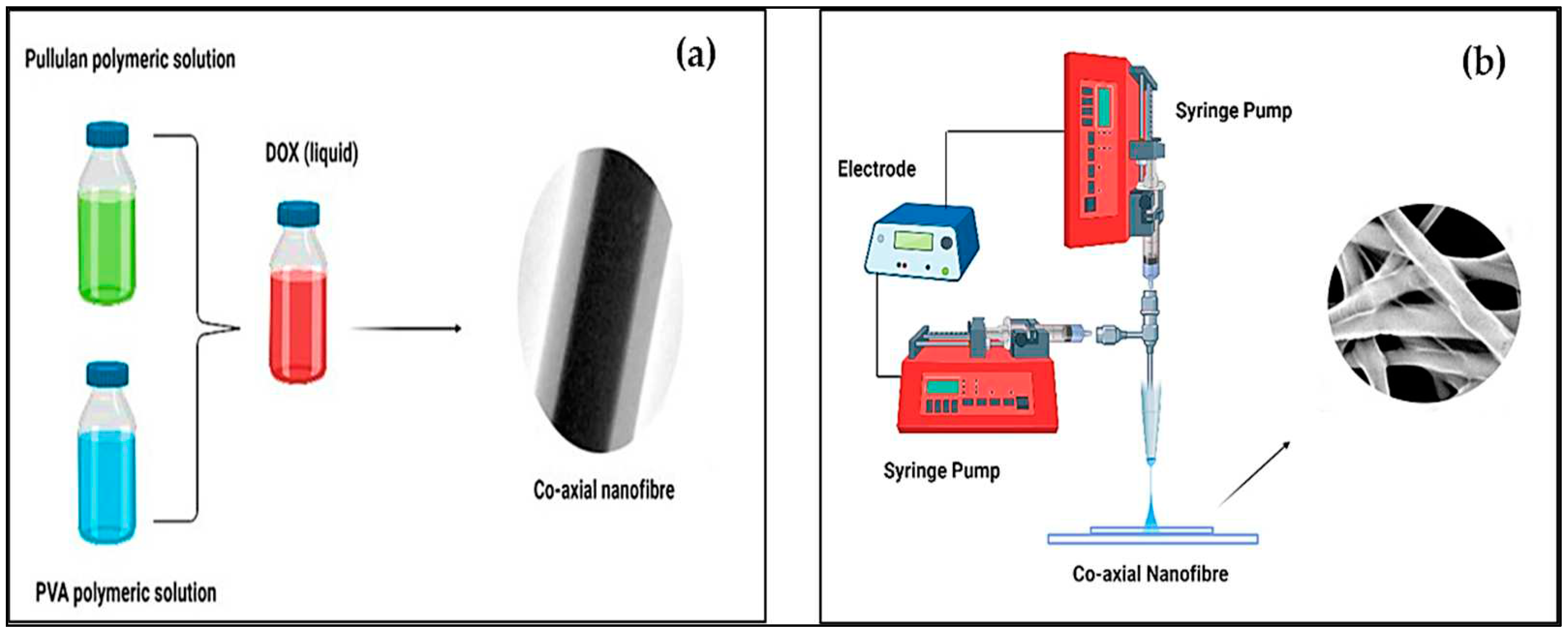
Figure 1(a) shows a schematic of the PVA/pullulan core-shell drug-loaded nanofibers used in the manufacture of DOX, depicting the structure and composition of the nanofibers. The core-shell structure consists of a central core made of PVA, which serves as the carrier for the drug (DOX). The PVA core is surrounded by a shell composed of pullulan, a biocompatible polymer. The drug (DOX) is encapsulated within the PVA core, providing controlled release capabilities and targeted drug delivery.
The coaxial spinning process was employed to fabricate PVA/Pullulan core-shell drug-loaded nanofibers (
Figure 1b). This process involves simultaneous extrusion of two concentric polymer solutions through a spinneret. The inner syringe delivered the PVA solution containing the drug (DOX), whereas the outer syringe supplied the pullulan solution. As the polymer solutions pass through the spinneret, they undergo electrospinning, forming continuous nanofibers with a core-shell structure. The coaxial spinning process enables precise control over nanofiber morphology, drug encapsulation efficiency, and release kinetics, making it a suitable technique for manufacturing drug-loaded nanofibers with specific properties and functionalities.
2.4. Characterisation
The surface morphology of the PVA/pullulan drug-loaded nanofibrous material was observed by SEM using TS5130 Vega Tescan at 30 kV acceleration voltages and Image J software. The surfaces of the samples were coated with a conductive layer by sputtering before the SEM analysis. A copper grid was securely placed onto an aluminum foil to examine the internal structure of the core–shell nanofibers. This setup allowed the deposition of an extremely thin layer of nanofibers onto the grid. The resulting core-shell nanofibers were then observed using a Hitachi H-7650 transmission electron microscope (TEM) from Japan. The TEM was operated at a working voltage of 100 kV and working distance of 15 mm. This imaging technique provided valuable insights into the detailed morphology and composition of core-shell nanofibers. To study the chemical interactions between pullulan/PVA in the fibers, FT-IR (PerkinElmer, USA) was used to determine the water sensitivity of the nanofibrous delivery system.
This study was performed according to ČSN ISO 19702:2015. According to FTIR studies, PVA and pullulan have no chemical interactions.
The nanofiber samples were then subjected to BET analysis. This analysis was performed in compliance with the ČSN P CEN ISO/TS 80004-6 standard. The samples were pre-saturated for two hours under vacuum at 160 °C (160 mm Hg). Three replicates were analyzed for each scale, and the means and standard deviations were reported. Gas adsorption was used to determine the exact surface area of the solids using the–Brunauer–Emmett Teller method. The Brunauer–Emmett–Teller (BET) method, the application of the Irving Langmuir area, was calculated using the principle of single-layer surface assimilation with multilayer adsorption.
3. Results
3.1. Morphology of PVA/Pullulan Nanofiber
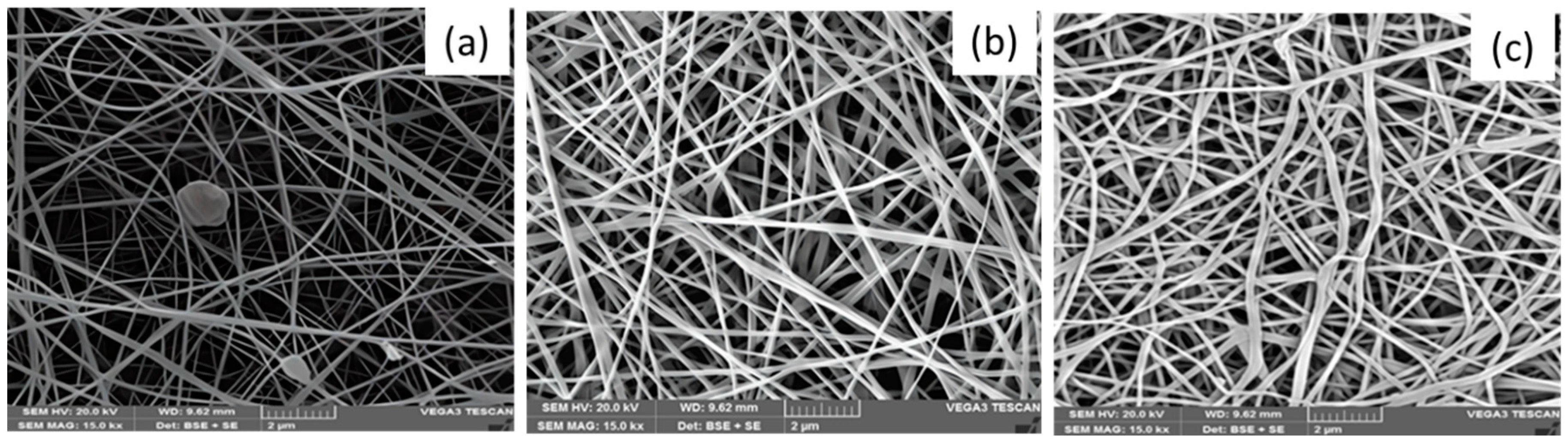
The electrospun nanofiber morphology can be affected by the electrospinning setup parameters, including the applied voltage, collector distance, and polymer concentration. The applied voltage and TCD were fixed at 25 kV and 20 mm, respectively, throughout the experiment.
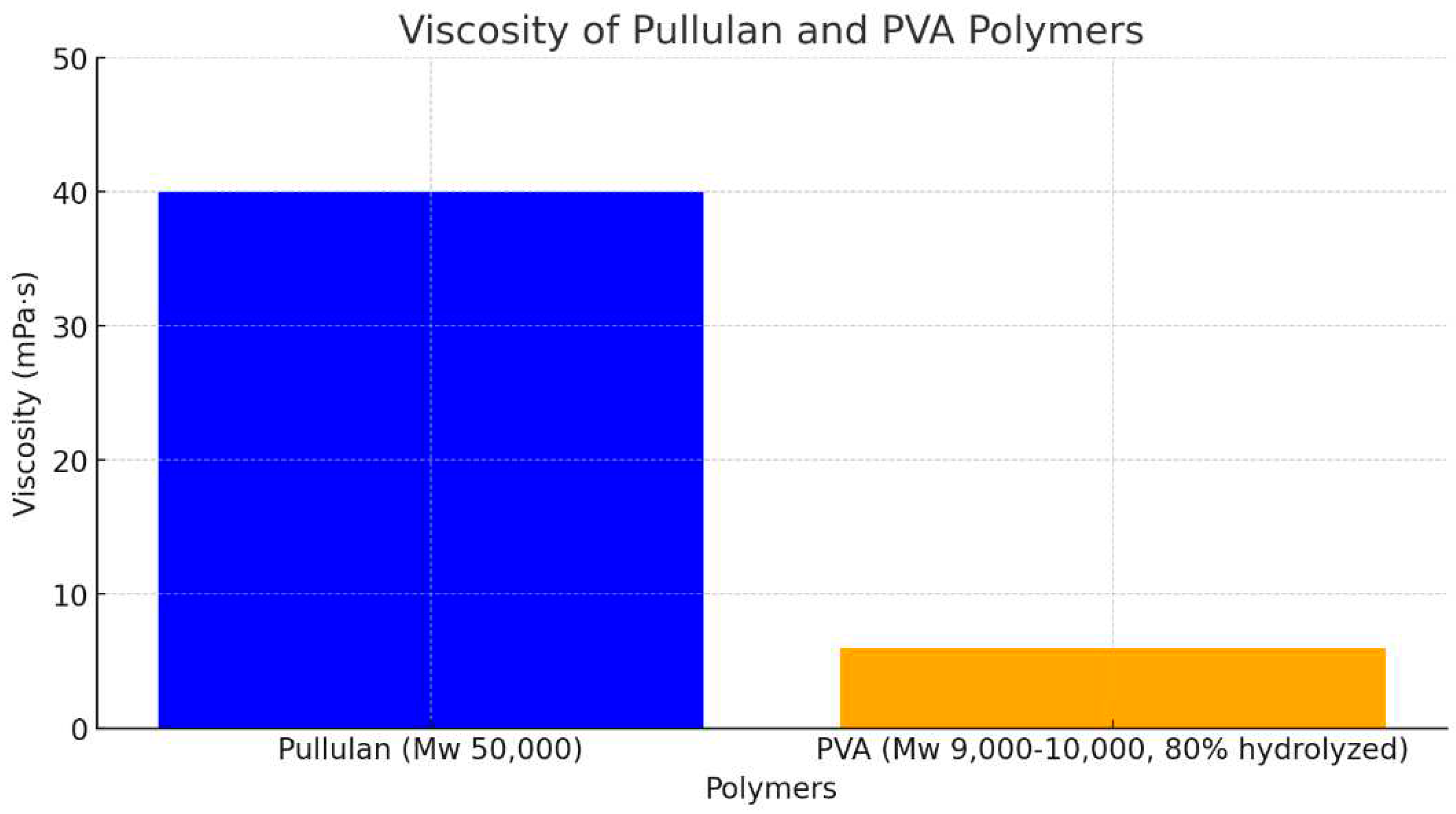
Figure 4 shows the PVA/ Pullulan nanofibers. The pH of the pullulan/PVA blended nanofibers was 5. This pH value is a crucial parameter because it influences the properties and performance of the nanofibers. The selection of a pH of 5 for the electrospinning solution is significant because it enables the formation of nanofibers with desirable morphology, size, and stability. Maintaining a pH of 5 in the pullulan/PVA solution helped to optimize the electrospinning process and ensured the successful production of uniform and continuous nanofibers. The pH value directly affects the charge density and solution viscosity, which in turn influence the fiber diameter, alignment, and surface characteristics of the resulting nanofibers. The results show that a strong electric field is created when H+ ions in the acid solution generate a strong electric current, which attracts droplets of the combined solution at the end of the collector. The acidic solution provides higher electrospinning because of the presence of H+ ion concentration, aiding in the attraction of chemical compound solution droplets from the tip of the collector towards the negative electrode once the field is applied. Therefore, Pullulan with a neutral to alkaline pH scale was not electrospun with any carrier polymer. The viscosity range of various polymer solutions in which electrospinning is completed is a significant variable. The higher viscosity of extracted pullulans aided them in providing continuous smooth fibers when electrospun, whereas pullulan with lower consistency gave beaded fibers. Pullulan with a molecular weight of 50,000 g/mol exhibited a viscosity of 40 mPa·s (
Figure 3). This polysaccharide is used in various applications, including in the food and pharmaceutical industries, owing to its film-forming and encapsulation properties. Polyvinyl Alcohol (PVA), specifically 87–89% hydrolyzed and with a molecular weight range of 9,000–10,000 g/mol, shows a viscosity of 6 mPa·s. PVA is a synthetic polymer known for its solubility in water and its excellent film-forming, emulsifying, and adhesive properties (
Figure 4). These values provide insights into the flow characteristics of polymers, which are essential for understanding their behavior in various applications and processing techniques.
Formun Üstü
PVA was selected as a carrier polymer because of its affordability, chemical and thermal stability, and resistance to degradation under most physiological conditions. When exposed to acidic or neutral conditions, the nanofibers with PVA/pullulan cores degrade rapidly. In addition, the fibers can be used as delivery systems for DOX, an anticancer drug. The drug-loaded nanofibers significantly inhibited the adhesion and proliferation of HeLa cells. Thus, coaxial nanofibers can deliver drugs to patients with solid diseases such as cervical cancer. PVA produced by an electrophoretic method forms continuous smooth fibers; low-viscosity amylopectin forms transversely striated fibers (
Figure 4).
This carrier polymer is cost effective, chemically and thermally stable, and does not degrade under normal physiological conditions. By examining the SEM images using ImageJ software, we found that the mean diameter of the PVA-bonded amylopectin nanofibers ranged from 220 to 230 nm. The diameters of the electrospun amylopectin and PVA fibers were more significant than their combinations. The diameter decreased with decreasing amylopectin content. The PVA and pullulan/PVA nanofiber structures were uniform and bead-free (
Figure 4c).
3.2. Morphology and Inner Structure of the PVA/Pullulan Core-Shell Nanofibers
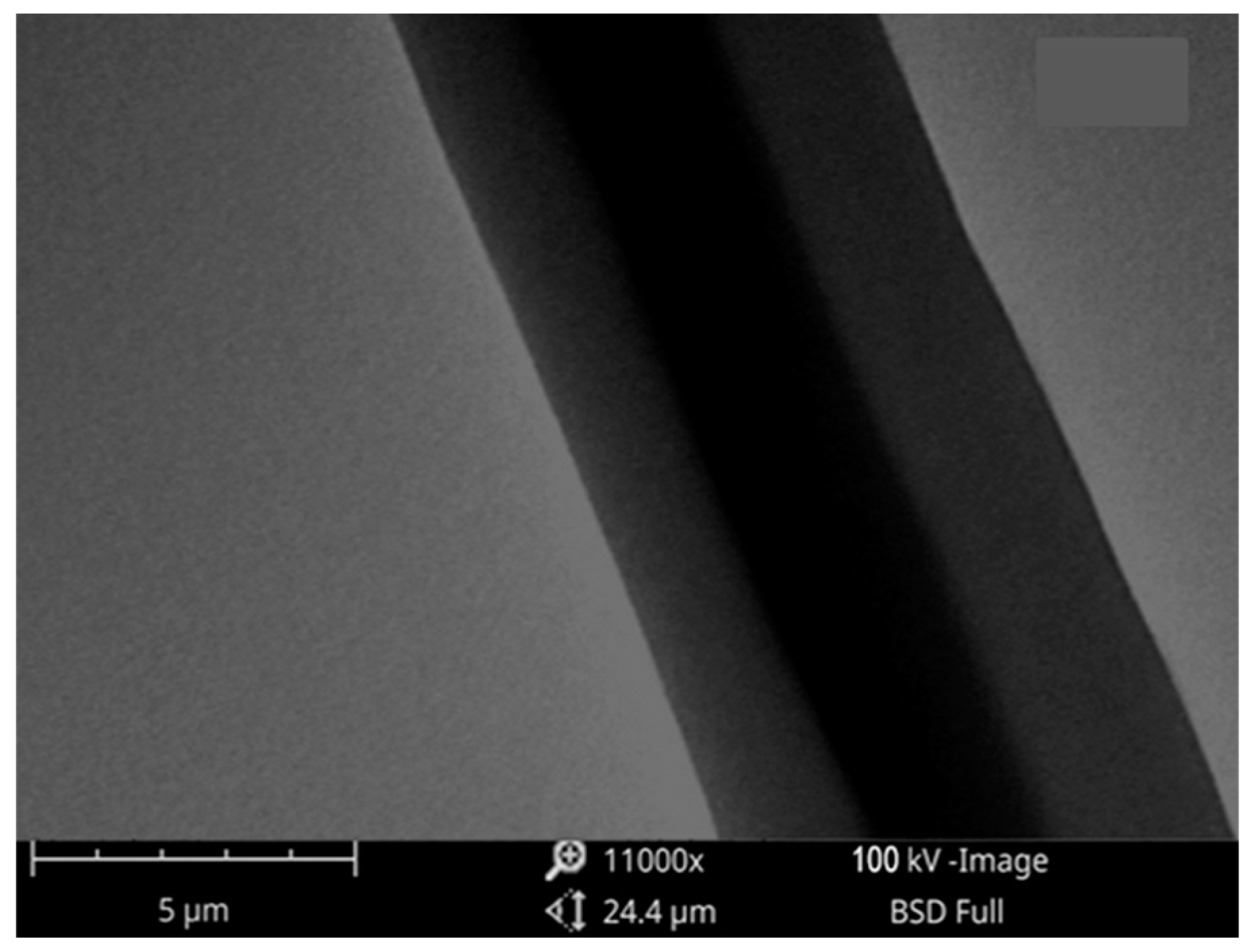
Nanofibers made with core–shell PVA/pullulan were successfully electrospun. TEM was used to clarify the inner structure of the fibers. The core–shell nanofibrous structure of PVA/PULLULAN/DOX is shown in
Figure 5. During the electrospinning procedure, the flow rates of the PVA and Pullulan solutions were fixed at 0.5 mL/h. All the as-prepared nanofibers were oriented randomly on the substrate, and the adhesion among the fibers was measured. The fiber diameters were estimated to be approximately 225 nm.
3.2. PVA/Pullulan Core-Shell Chemical Structural Analysis of the Nanofibrous Materials
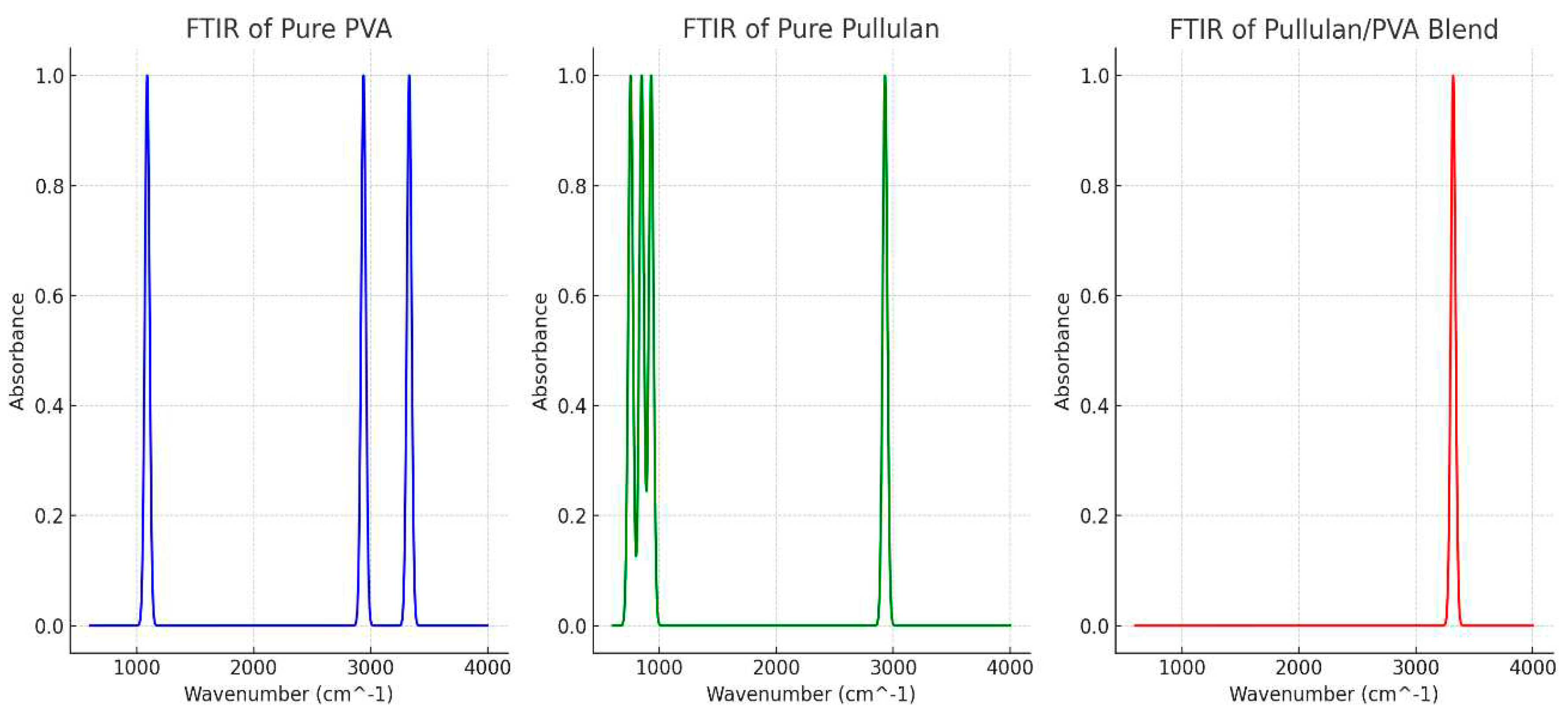
The chemical structures of the pullulan/PVA core-shell nanofibrous drug delivery system were investigated using FTIR spectroscopy (
Figure 6). The ČSN ISO 19702:2015 standard was used for the analysis. The FTIR study indicated no chemical interactions between PVA and Pullulan. An acidic or neutral environment can effectively degrade PVA/Pullulan core-shell nanofibers. A DOX delivery system was created from the resulting fibers. Drug-loaded nanofibers are needed to prevent HeLa cells from adhering and proliferating. Therefore, the coaxial nanofiber structure may have great potential as a drug carrier for cervical cancer and other solid tumors. The chemical interactions of the core-shell nanofibers were also analyzed from the FTIR spectra (
Figure 6). The spectra of the pullulan/PVA core-shell nanofibers and those of PVA are displayed in the 4000-6000 cm
-1 and 2000-600 cm
-1 related ranges. The appearance of a significantly similar absorption peak suggests the presence of C-O at 1089 cm
-1 in the pure PVA membrane. The vibration of the CH
2 group resulted in an absorption maximum at 2935 cm
-1. When the methyl or methylene group vibrates, a band is formed at 2930 cm
-1 because of the vibrational resonance. CH-CH
2 degenerate vibrations were observed to occur in bands in the 1300–1500 cm
-1 region.
Hydroxyl groups were also responsible for the broad peak at 3330 cm -1 (-OH). Comparing the spectrum of pure Pullulan with that of PVA (-OH), which migrates from 3330 to 3320 cm-1 in the electrospun pullulan/PVA mixed spectrum, an absorbance peak for the hydroxyl group of pullulan appears. The hydrogen bonding between Pullulan and PVA was confirmed by comparing the spectra from 3330 cm-1 to 3320 cm-1, showing an absorption peak of hydroxyl (-OH).
3.3. BET Analysis
BET is the most common and widely used method for determining the porosity of nanofibers. In this method, gas (usually nitrogen) is absorbed at the boiling point of the sample. The amount of gas absorbed depends on the pore size and the partial pressure of the gas. The Brunauer, Emmet, and Teller (BET) equation calculates the surface area of a substance using the amount of gas adsorbed at a specific partial pressure. Pullulan/PVA/DOX core–shell nanofibers demonstrated smaller pore size (16.62 Å) and total pore volume (0.1176 cc/g) (
Table 1). The shell rate of the fibers also plays an essential role in controlling the release of the drug.
3.4. The Release of Drugs In Vitro
The internal organelles and the tumor site are known to be acidic, while the blood and tissue fluid surrounding normal tissues of the body are both neutral. DOX, a popular anticancer treatment called DOX was injected into the PVA/pullulan core nanofiber core to investigate its potential utility as a topical drug delivery method. DOX was detected in all three types of core-shell fibers. The brief burst is caused by a small amount of PVA leaking out during the electrospinning technique, exposing some of the main components on the surface of the fibers. It was concluded that the DOX molecule was readily released at this position. The burst release of the medication reduced as the fiber shell thickness increased. The burst release of the medication reduced as the fiber shell thickness increased. Except for the initial surge, DOX was delivered gradually in general and at a flow ratio of 0.5, for some of the main components on the surface of the fibers. It was concluded that the DOX molecule was readily released at this position. The thickness of the shell layer showed how quickly the loaded medication was released. The Korsmeyer-Peppas model was used to calculate the release profiles. R% indicates the percentage of drug released at time
t,
k represents the rate constant, and
n represents the release exponent describing the drug release process at time
t, as given in Equation 1 [
28].
PVA/pullulan (wt%)% 0.5:0.5, at pH = 4) delivery method was the only one that did not conform to Fickian release. The shell rate of the fibers plays a crucial role in controlling drug release. Equation 2 calculates the total PVA/pullulan ratio (wt.% 0.5:0.5, pH 4) nanofibrous. In the biomedical field, coaxial nanofibrous materials are very helpful, particularly for treating solid malignant tumors or inflammatory areas. The as-prepared fibers can be seen as cylinders. It has been reported that n has a limiting value of 0.45 for release from cylinders in Fickian release [
29].
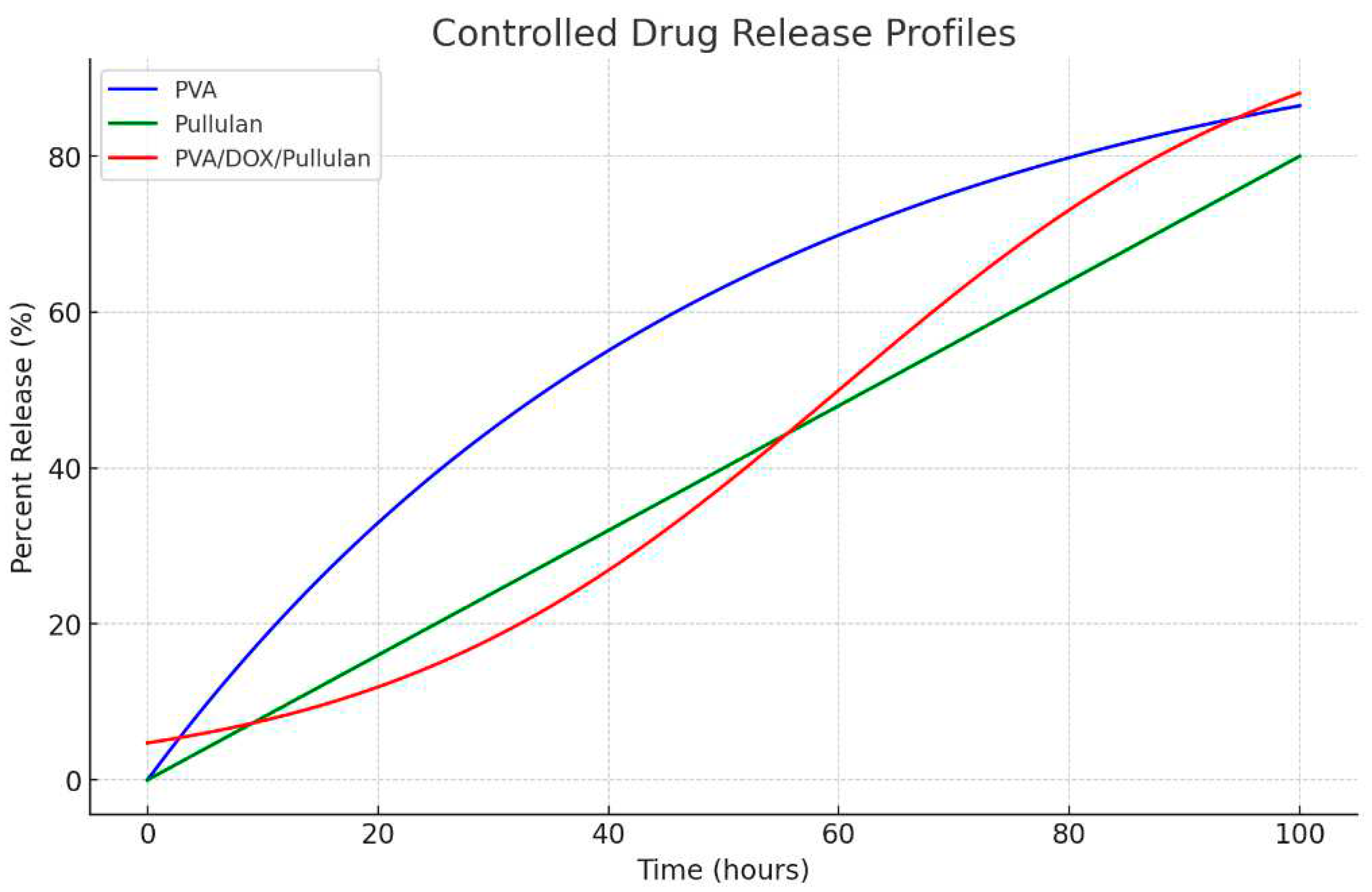
PVA (Blue Line): The release profile for PVA demonstrates controlled exponential behavior, where the percent release slowly increases over time and plateaus.
Pullulan (Green Line): The release from pullulan is linear but at a controlled rate, indicating a steady and slow release of the drug. This is advantageous for therapeutic applications that require stable drug concentrations over time.
PVA/DOX/Pullulan (Red Line): The composite material exhibited a controlled sigmoidal release profile. The slow initial release followed by a more rapid but controlled release phase and a final plateau suggests a multistage release mechanism (
Figure 7).
The release profiles of PVA, Pullulan, and the PVA/pullulan/DOX composite over 48 h showed that the composite material released its contents more rapidly and extensively than either PVA or Pullulan alone (
Figure 7). The composite material of PVA, pullulan, and doxorubicin (DOX) was chosen for its enhanced release profile, as it combines the mechanical stability and biocompatibility of PVA, the biodegradability and encapsulation efficiency of pullulan, and the therapeutic action of DOX, resulting in a controlled and gradual release of the anticancer drug, thereby offering a more effective and targeted drug delivery system that maximizes the therapeutic impact while minimizing potential side effects in cancer treatment.
3.5. Cancer Chemotherapy: Preliminary Results
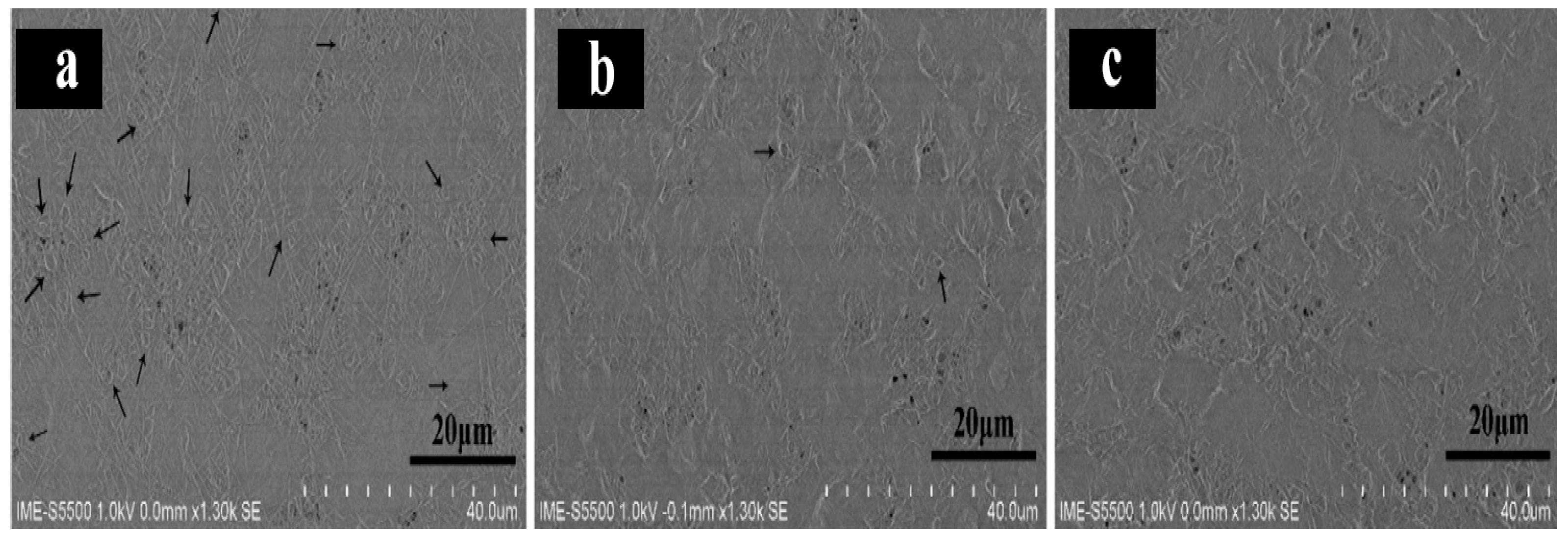
The cells were cultured in RPMI 1640 medium (Sigma Aldrich, Czech Republic) for cell culture supplemented with 10% fetal bovine serum F1283, 100 IU mL-1 streptomycin, and 100 IU mL-1of penicillin (Sigma Aldrich, Czech Republic) per milliliter at 36 °C in an atmosphere moistened during the 5th day of culture containing CO2. The cell monolayers were removed from the culture medium after culturing for 12 h, washed with PBS ice and distilled water, and then fixed with fresh 1.20 wt.% Glutaraldehyde (GA) solution for 10 minutes. The fixative was removed, and fresh GA was added for an additional 10 min to fix the cells. The cell monolayers were washed thrice with PBS and distilled water. After removing the medium, the cells were allowed to dry at 36 °C in the laboratory. SEM was used to examine samples after fixation and drying.
Pullulan/PVA/DOX nanofibrous drug-loaded core-shell fibers produced at a ratio of 0.5:0.5, showed that HeLa cells maintained their typical morphology for 1 d (
Figure 7a). This is because of the large surface area available for conduction due to the three-dimensional nature of the fibers. Some cell states changed four days after seeding (
Figure 7b). Its shape changed from spindle to round, indicating the occurrence of apoptosis. The fibers disappeared from the image, indicating deterioration of the fibers. When the polymer degrades, DOX is released from the fibers and kills cancer cells. After 7 days of extraction (
Figure 7c), cell morphology could not be determined, showing that the drug had destroyed HeLa cells. Chemotherapy for cervical cancer often has positive outcomes. Sustained drug release is enhanced by the core fiber structure, which is beneficial for multiple therapeutic applications. The fibers produced considerably deteriorated in a physiological setting after three days of growth, which is encouraging for tissue engineering applications. Several biomedical-related sectors can benefit from the creation of DOX-loaded PVA/pullulan fibers.
Figure 8.
Pullulan/PVA/DOX core-shell nanofibers with 0.5:0.5 flow ratios seen in SEM images of HeLa cells (a) 1 d, (b) 4 days, and (c) 7 days.
Figure 8.
Pullulan/PVA/DOX core-shell nanofibers with 0.5:0.5 flow ratios seen in SEM images of HeLa cells (a) 1 d, (b) 4 days, and (c) 7 days.
4. Discussions
This article presents a study on the use of pullulan/PVA and PVA blends as carrier polymers for drug delivery, specifically for the anticancer drug, doxorubicin. The study utilized coaxial electrospinning to create nanofibers with PVA/pullulan cores, and the resulting drug-loaded nanofibers were effective in prohibiting the attachment and proliferation of HeLa cells. The choice of Pullulan and PVA as carrier polymers is justified by their relative low cost, chemical and thermal stability, and lack of degradation under most physiological conditions. Furthermore, Pullulan's beaded structure on the nano-surface obtained by the electrospinning method alone and the uniform fiber structure obtained by mixing with PVA make them suitable for drug delivery. The use of doxorubicin as an anticancer drug is well established, but its cardiotoxicity limits its prolonged use. Encapsulation in drug delivery techniques overcomes this constraint by mitigating the adverse effects of DOX on normal cells.
The findings of this study suggest that the coaxial nanofibers produced are feasible as safe and environmentally friendly drug carriers against cervical cancer and other solid malignancies. The results of the FT-IR analysis also indicated that there were no chemical interactions between Pullulan and PVA, which is important for maintaining the stability of the carrier polymer.
Overall, this study provides valuable insights into the use of pullulan/PVA and PVA blends as carrier polymers for drug delivery and the efficacy of coaxial electrospinning in creating drug-loaded nanofibers for anticancer drugs. Further studies are needed to investigate the safety and efficacy of these nanofibers in vivo and to optimize their drug-release profiles for better therapeutic outcomes.
5. Conclusions
This study explored the potential of using pullulan/PVA blends as carrier polymers for drug delivery systems, particularly for encapsulating doxorubicin (DOX), a common anticancer drug known for its cardiotoxicity. By employing coaxial electrospinning, the study successfully produced uniform fibers without any chemical interactions between Pullulan and PVA. This method allowed the DOX solution to be encapsulated within the nanofibers, helping mitigate the adverse effects of the drug on normal cells. The fabricated nanofibers were effective in inhibiting the attachment and proliferation of HeLa cells, a type of cervical cancer cell, underscoring their potential as a safe and targeted drug delivery system for treating cervical cancer and other solid malignancies. Quantitative data from this study included measurements of the mean diameter of the PVA-bonded amylopectin nanofibers, which ranged from 220 to 230 nm, and the estimated fiber diameters were approximately 250 nm. This conclusion emphasizes the potential application of these nanofibers in cancer treatment, demonstrating their cost-effectiveness, chemical and thermal stability, and nondegradability under normal physiological conditions. The selection of pullulan as the core material in the fabrication of Pullulan/PVA core-shell nanofibers for anticancer applications is driven by its unique combination of biological, physical, and chemical properties. Their biocompatibility, biodegradability, solubility, ability to encapsulate drugs, and mechanical versatility make them an attractive choice for developing innovative drug delivery systems aimed at treating cancer more effectively. Pullulan has a beaded structure on the nano-surface obtained by the electrospinning method alone, while the nanofiber structure obtained by mixing with PVA is more uniform. FT-IR analysis demonstrated that there were no chemical interactions between Pullulan and PVA. Doxorubicin (DOX) is a common antibacterial drug with anticancer properties. It has been one of the most commonly used drugs in cancer chemotherapy for approximately 30 years. Cardiotoxicity prevents its prolonged use. Encapsulation in drug delivery techniques provides benefits over drug-independent tissues in overcoming this constraint. Coaxial electrospinning was used to construct nanofibers with PVA/pullulan cores. It is inexpensive, chemically stable, and thermally stable, making it an excellent polymer carrier. DOX is an anticancer drug that prevents the production of DNA by cancer cells. Drug management is more accessible using pullulan, which has a diverse user base. Encapsulation can mitigate the adverse effects of DOX in normal cells. Drug nanostructures have the potential to be efficient and environmentally friendly drug delivery systems for the treatment of acute diseases, including cervical cancer. The drug-loaded nanofibers were effective in inhibiting the attachment and proliferation of HeLa cells. In conclusion, the coaxial nanofibers thus produced are feasible safe and environmentally friendly drug carriers against cervical cancer and other solid malignant tumors.
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union - European Structural and Investment Funds-Operational Program Research, Development and Education – project Hybrid Materials for Hierarchical Structures (HyHi, Reg. No. CZ.02.1.01/0.0/0.0/16_019/0000843) and the project ‘‘Advanced structures for thermal insulation in extreme conditions’ (Reg. No. 21-32510M), and granted by the Czech Science Foundation (GACR).
References
- A. Barhoum, M. Bechelany, and A. S. Hamdy Makhlouf, Handbook of Nanofibers. 2019. [CrossRef]
- L. Muthukrishnan, “An overview on electrospinning and its advancement toward hard and soft tissue engineering applications,” Colloid Polym. Sci., vol. 300, no. 8, pp. 875–901, Aug. 2022. [CrossRef]
- T. Elçin, “PULLULAN/PVA/ DOXORUBICIN CORE-SHELL ELECTROSPUN NANOFIBERS DRUG DELIVERY SYSTEM FOR THE CHEMOTHERAPY AGAINST CANCER,” p. 10, 2022.
- M. D. Mauricio, S. Guerra-Ojeda, P. Marchio, S. L. Valles, M. Aldasoro, I. Escribano-Lopez, J. R. Herance, M. Rocha, J. M. Vila, and V. M. Victor, “Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress,” Oxid. Med. Cell. Longev., vol. 2018, p. e6231482, Sep. 2018. [CrossRef]
- S. Abid, T. Hussain, Z. A. Raza, and A. Nazir, “Current applications of electrospun polymeric nanofibers in cancer therapy,” Mater. Sci. Eng. C, vol. 97, pp. 966–977, Apr. 2019. [CrossRef]
- N. Sahai, N. Ahmad, and M. Gogoi, “Nanoparticles Based Drug Delivery for Tissue Regeneration Using Biodegradable Scaffolds: a Review,” Curr. Pathobiol. Rep., vol. 6, no. 4, pp. 219–224, Dec. 2018. [CrossRef]
- Y.-Y. Yang, Y. Wang, R. Powell, and P. Chan, “Polymeric Core-Shell Nanoparticles for Therapeutics,” Clin. Exp. Pharmacol. Physiol., vol. 33, no. 5–6, pp. 557–562, 2006. [CrossRef]
- S. Gopi, A. Amalraj, N. P. Sukumaran, J. T. Haponiuk, and S. Thomas, “Biopolymers and Their Composites for Drug Delivery: A Brief Review,” Macromol. Symp., vol. 380, no. 1, p. 1800114, 2018. [CrossRef]
- L. J. Villarreal-Gómez, J. M. Cornejo-Bravo, R. Vera-Graziano, and D. Grande, “Electrospinning as a powerful technique for biomedical applications: a critically selected survey,” J. Biomater. Sci. Polym. Ed., vol. 27, no. 2, pp. 157–176, 2016. [CrossRef]
- W.-Y. Huang, S. Suye, and S. Fujita, “Cell Trapping via Migratory Inhibition within Density-Tuned Electrospun Nanofibers,” ACS Appl. Bio Mater., vol. 4, no. 10, pp. 7456–7466, Oct. 2021. [CrossRef]
- G. L. Pérez-González, L. J. Villarreal-Gómez, A. Serrano-Medina, E. J. Torres-Martínez, and J. M. Cornejo-Bravo, “Mucoadhesive electrospun nanofibers for drug delivery systems: applications of polymers and the parameters’ roles,” Int. J. Nanomedicine, vol. 14, pp. 5271–5285, Jul. 2019. [CrossRef]
- E. Torres, J. Cornejo-Bravo, A. Serrano-Medina, L. perez gonzalez, and L. Villarreal-Gómez, “A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices,” Curr. Drug Deliv., vol. 15, Jul. 2018. [CrossRef]
- D. Chavarria-Bolaños, M. Granados-Hernandez, J. Serrano, J. L. Suárez Franco, V. Guarino, and M. Pérez, “Introduction to electrofluidodynamic techniques. PART II. cell-to-cell / material interactions,” 2018. [CrossRef]
- J. Li, Y. Liu, and H. Abdelhakim, “Drug Delivery Applications of Coaxial Electrospun Nanofibres in Cancer Therapy,” Molecules, vol. 27, no. 6, p. 1803, Mar. 2022. [CrossRef]
- C. He, Z. Huang, X. Han, L. Liu, H. Zhang, and L. Chen, “Coaxial Electrospun Poly(L-Lactic Acid) Ultrafine Fibers for Sustained Drug Delivery,” J. Macromol. Sci. Part B, vol. 45, no. 4, pp. 515–524, Aug. 2006. [CrossRef]
- D. H. Reneker, A. L. Yarin, H. Fong, and S. Koombhongse, “Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning,” J. Appl. Phys., vol. 87, no. 9, pp. 4531–4547, 2000. [CrossRef]
- G. K. Sharma and N. James, “Electrospinning: The Technique and Applications,” in Recent Developments in Nanofibers Research, no. August, 2023, p. 118.
- L. Poláková, J. Širc, R. Hobzová, A.-I. Cocârță, and E. Heřmánková, “Electrospun nanofibers for local anticancer therapy: Review of in vivo activity,” Int. J. Pharm., vol. 558, pp. 268–283, Mar. 2019. [CrossRef]
- M. T. Hunley and T. E. Long, “Electrospinning functional nanoscale fibers: A perspective for the future,” Polym. Int., vol. 57, no. 3, pp. 385–389, 2008. [CrossRef]
- P. A. Baron, G. J. Deye, and J. Fernback, “Length Separation of Fibers,” Aerosol Sci. Technol., vol. 21, no. 2, pp. 179–192, 1994. [CrossRef]
- U. N. Gandhi, S. Goris, T. A. Osswald, and Y.-Y. Song, DISCONTINUOUS FIBER-REINFORCED COMPOSITES Fundamentals and Applications. 2020, p. 480. [CrossRef]
- Y. Fong, C.-H. Chen, and J.-P. Chen, “Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy,” Nanomaterials, vol. 7, no. 11, p. 388, Nov. 2017. [CrossRef]
- S. Upadhyay, A. K. Mantha, and M. Dhiman, “Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes,” J. Ethnopharmacol., vol. 258, p. 112690, Aug. 2020. [CrossRef]
- D. Xiao, W. Chang, W. Ding, Y. Wang, H. Fa, and J. Wang, “Enhanced mitophagy mediated by the YAP/Parkin pathway protects against DOX-induced cardiotoxicity,” Toxicol. Lett., vol. 330, pp. 96–107, May 2020. [CrossRef]
- “Doxorubicin hydrochloride-loaded electrospun poly(l-lactide-co-ε-caprolactone)/gelatin core–shell nanofibers for controlled drug release - Wang - 2021 - Polymer International - Wiley Online Library.” .
- E. Yatmaz and İ. Turhan, “FERMANTASYON YOLUYLA PULLULAN ÜRETİMİ VE GIDA ENDÜSTRİSİNDE KULLANIMI,” p. 8.
- C. C. DeMerlis and D. R. Schoneker, “Review of the oral toxicity of polyvinyl alcohol (PVA),” Food Chem. Toxicol. An Int. J. Publ. Br. Ind. Biol. Res. Assoc., vol. 41, no. 3, pp. 319–326, Mar. 2003. [CrossRef]
- G. Cavallaro, G. Lazzara, S. Milioto, F. Parisi, V. Evtugyn, E. Rozhina, and R. Fakhrullin, “Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release,” ACS Appl. Mater. Interfaces, vol. 10, no. 9, pp. 8265–8273, Mar. 2018. [CrossRef]
- N. A. P. Philip L. Ritger, “A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs,” J. Control. Release, vol. 5, no. 1, pp. 23–36, 1987. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).