Submitted:
02 November 2023
Posted:
02 November 2023
You are already at the latest version
Abstract
Keywords:
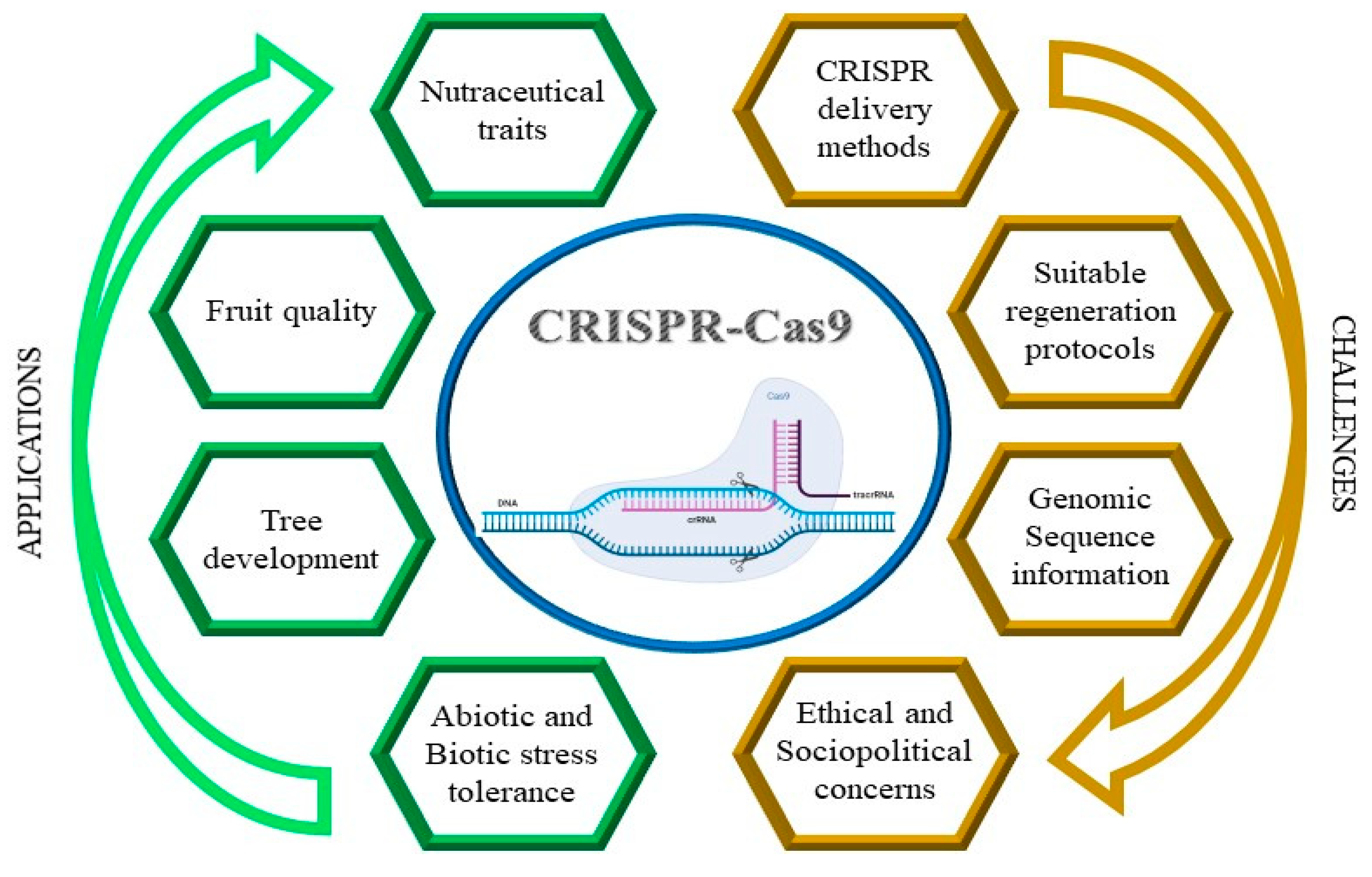
1. Introduction
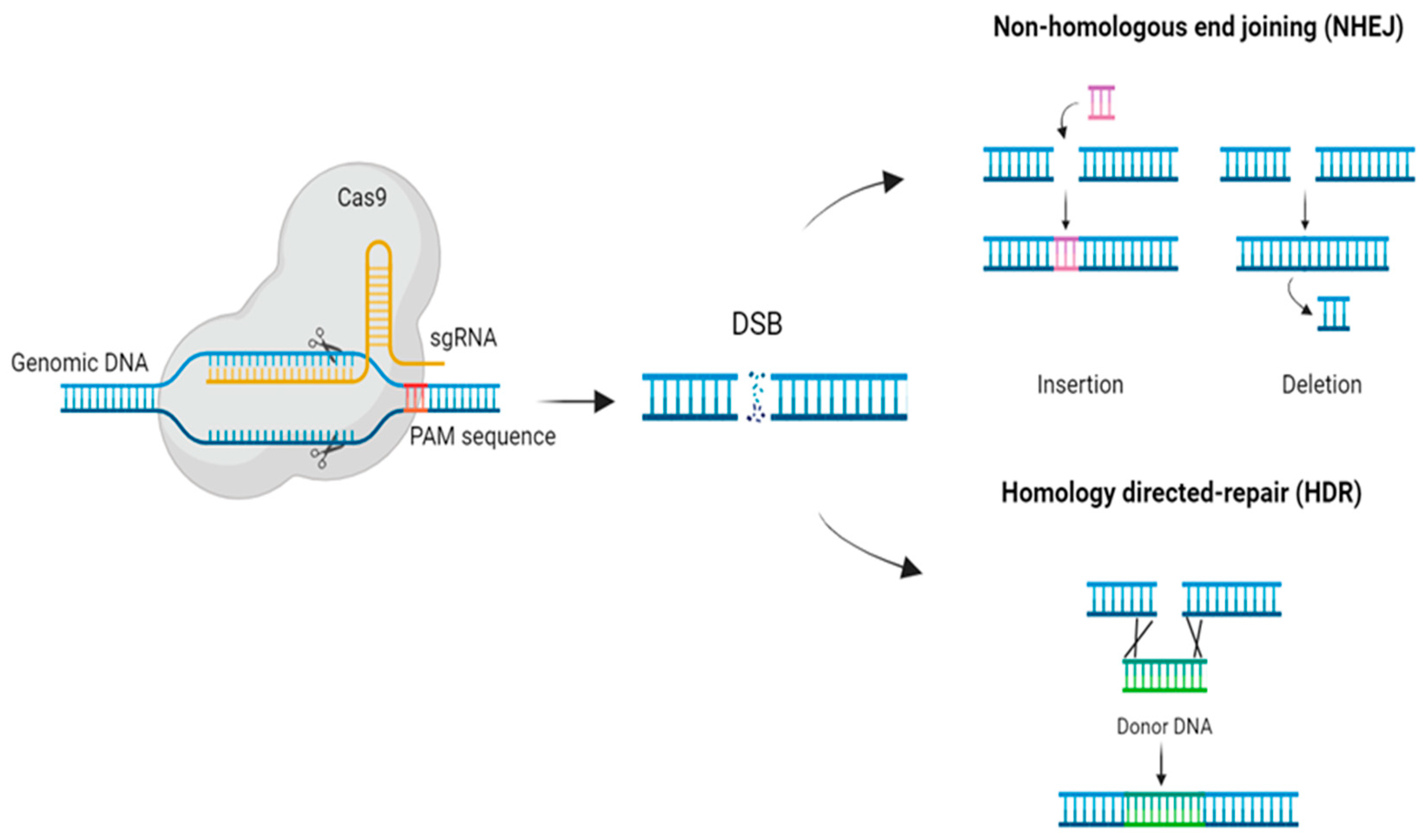
2. Mechanism of CRISPR/Cas System
3. Genetic Transformation Technology in Fruit Trees
4. CRISPR/Cas-Mediated Gene Knock-in and Knock-out in Fruit Trees
4.1. Tree Growth and Development
4.2. Early Flowering
4.3. Fruit Growth and Development
4.4. Shelf-Life and Fruit Ripening
4.5. Fruit color, Flavor, and Bioactive Compounds
4.6. Improving Stress Tolerance in Fruit Trees
5. Off-Target Issue
6. Regulatory Limits of Genome Editing
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.; Korban, S.S. Breeding and Genetics of Disease Resistance in Temperate Fruit Trees: Challenges and New Opportunities. Theor. Appl. Genet. 2022, 135, 3961–3985. [Google Scholar] [CrossRef]
- Savadi, S.; Mangalassery, S.; Sandesh, M.S. Advances in Genomics and Genome Editing for Breeding next Generation of Fruit and Nut Crops. Genomics 2021, 113. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; D, G.P.; Vizzotto, M. Stone Fruits as a Source of Bioactive Compounds. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part II; Silva, L.R., Silva, B., Eds.; Bentham Science: Covilha-Portugal, 2016; pp. 110–142. [Google Scholar]
- Scorza, R.; Callahan, A.; Dardick, C.; Ravelonandro, M.; Polak, J.; Malinowski, T.; Zagrai, I.; Cambra, M.; Kamenova, I. Genetic Engineering of Plum Pox Virus Resistance:‘HoneySweet’Plum—from Concept to Product. Plant Cell, Tissue Organ Cult. 2013, 115, 1–12. [Google Scholar] [CrossRef]
- Alburquerque, N.; Baldacci-Cresp, F.; Baucher, M.; Casacuberta, J M Collonnier, C.; El Jaziri, M.; Nogué, F.; Burgos, L. New Transformation Technologies for Trees. In Biosafety of Forest Transgenic Trees: Improving the Scientific Basis for Safe Tree Development and Implementation of EU Policy Directives; Vettori, C., Gallardo, F., Kazana, V., Häggman, H., Migliacci, F., Pilate, G., Fladung, M., Eds.; Springer: Dordrecht, 2016; pp. 31–66.
- Hanin, M.; Paszkowski, J. Plant Genome Modification by Homologous Recombination. Curr. Opin. Plant Biol. 2003, 6, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wehrkamp-Richter, S.; Degroote, F.; Laffaire, J.B.; Paul, W.; Perez, P.; Picard, G. Characterisation of a New Reporter System Allowing High Throughput in Planta Screening for Recombination Events before and after Controlled DNA Double Strand Break Induction. Plant Physiol. Biochem. 2009, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Puchta, H.; Fauser, F. Synthetic Nucleases for Genome Engineering in Plants: Prospects for a Bright Future. Plant J. 2014, 78, 727–741. [Google Scholar] [CrossRef]
- Abdallah, N.A.; Prakash, C.S.; McHughen, A.G. Genome Editing for Crop Improvement: Challenges and Opportunities. GM Crops Food 2015, 6, 183–205. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Puchta, H. Applying CRISPR/Cas for Genome Engineering in Plants: The Best Is yet to Come. Curr. Opin. Plant Biol. 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Wada, N.; Osakabe, K.; Osakabe, Y. Expanding the Plant Genome Editing Toolbox with Recently Developed CRISPR–Cas Systems. Plant Physiol. 2022, 188, 1825–1837. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision Genome Editing in Plants: State-of-the-Art in CRISPR/Cas9-Based Genome Engineering. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Denes, C.E.; Cole, A.J.; Aksoy, Y.A.; Li, G.; Neely, G.G.; Hesselson, D. Approaches to Enhance Precise Crispr/Cas9-mediated Genome Editing. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A Powerful Tool for Gene Function Study and Crop Improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef]
- Wang, J.Y.; Pausch, P.; Doudna, J.A. Structural Biology of CRISPR–Cas Immunity and Genome Editing Enzymes. Nat. Rev. Microbiol. 2022, 20, 641–656. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46. [Google Scholar] [CrossRef]
- Amitai, G.; Sorek, R. CRISPR-Cas Adaptation: Insights into the Mechanism of Action. Nat. Rev. Microbiol. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Beisel, C.L. The TracrRNA in CRISPR Biology and Technologies. Annu. Rev. Genet. 2021, 55. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR–Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas Guides the Future of Genetic Engineering. Science (80-. ). 2018, 361. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science (80-. ). 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Lipiński, D.; Zeyland, J.; Słomski, R. CRISPR/Cas9 Immune System as a Tool for Genome Engineering. Arch. Immunol. Ther. Exp. (Warsz). 2017, 65. [Google Scholar] [CrossRef]
- Yin, K.; Gao, C.; Qiu, J.L. Progress and Prospects in Plant Genome Editing. Nat. Plants 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, C.; Xiao, D.; Liang, Y.; Wang, Y. Advances and Perspectives of Transgenic Technology and Biotechnological Application in Forest Trees. Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Prieto, H.; Orbovic, V. Agrobacterium-Mediated Transformation of Tree Fruit Crops: Methods, Progress, and Challenges. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Alburquerque, N.; Pérez-Caselles, C.; Faize, L.; Ilardi, V.; Burgos, L. Effective Transfer of Plum Pox Virus Resistance from Transgenic Plum Rootstocks to Apricot Scions. 2023, 1–11. [CrossRef]
- Lobato-Gómez, M.; Hewitt, S.; Capell, T.; Christou, P.; Dhingra, A.; Girón-Calva, P.S. Transgenic and Genome-Edited Fruits: Background, Constraints, Benefits, and Commercial Opportunities. Hortic. Res. 2021, 8. [Google Scholar] [CrossRef]
- Orbovic, V.; Prieto, H. Editorial: New Developments in Agrobacterium Mediated Transformation of Tree Fruit Crops, Volume II. Front. Plant Sci. 2023, 14, 1–2. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Li, Z.; Wang, Y.; Wang, X. Current Progress and Future Prospects for the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Genome Editing Technology in Fruit Tree Breeding. CRC. Crit. Rev. Plant Sci. 2018, 37, 233–258. [Google Scholar] [CrossRef]
- Zhang, X. ming; Wu, Y. fei; Li, Z.; Song, C. bing; Wang, X. ping Advancements in Plant Regeneration and Genetic Transformation of Grapevine (Vitis Spp.). J. Integr. Agric. 2021, 20, 1407–1434. [Google Scholar] [CrossRef]
- Campos, G.; Chialva, C.; Miras, S.; Lijavetzky, D. New Technologies and Strategies for Grapevine Breeding Through Genetic Transformation. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Schröpfer, S.; Lempe, J.; Emeriewen, O.F.; Flachowsky, H. Recent Developments and Strategies for the Application of Agrobacterium-Mediated Transformation of Apple Malus × Domestica Borkh. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- James, D.J.; Passey, A.J.; Barbara, D.J.; Bevan, M. Genetic Transformation of Apple (Malus Pumila Mill.) Using a Disarmed Ti-Binary Vector. Plant Cell Rep. 1989, 7, 658–661. [Google Scholar] [CrossRef]
- Stowe, E.; Dhingra, A. Development of the Arctic® Apple. Plant Breed. Rev. 2021, 44, 273–296. [Google Scholar] [CrossRef]
- Moore, G.A.; Jacono, C.C.; Neidigh, J.L.; Lawrence, S.D.; Cline, K. And Regeneration of Transgenic Plants. 1992, 238–242.
- Cervera, M.; Juárez, J.; Navarro, A.; Pina, J.A.; Durán-Vila, N.; Navarro, L.; Peña, L. Genetic Transformation and Regeneration of Mature Tissues of Woody Fruit Plants Bypassing the Juvenile Stage. Transgenic Res. 1998, 7, 51–59. [Google Scholar] [CrossRef]
- Dutt, M.; Grosser, J.W. Evaluation of Parameters Affecting Agrobacterium-Mediated Transformation of Citrus. Plant Cell. Tissue Organ Cult. 2009, 98, 331–340. [Google Scholar] [CrossRef]
- Peña, L.; Navarro L IV Transgenic Citrus. In Biotechnology in Agriculture and Forestry, Vol. 44. ransgenic trees; Bajaj Y P S, Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1999; pp. 39–54.
- Alquézar, B.; Carmona, L.; Bennici, S.; Peña, L. Engineering of Citrus to Obtain Huanglongbing Resistance. Curr. Opin. Biotechnol. 2021, 70, 196–203. [Google Scholar] [CrossRef]
- Soares, J.M.; Tanwir, S.E.; Grosser, J.W.; Dutt, M. Development of Genetically Modified Citrus Plants for the Control of Citrus Canker and Huanglongbing. Trop. Plant Pathol. 2020, 45, 237–250. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. The Unknown Soldier in Citrus Plants: Polyamines-Based Defensive Mechanisms against Biotic and Abiotic Stresses and Their Relationship with Other Stress-Associated Metabolites. Plant Signal. Behav. 2020, 15. [Google Scholar] [CrossRef]
- Cervera, M.; Esteban, O.; Gil, M.; Gorris, M.T.; Martínez, M.C.; Peña, L.; Cambra, M. Transgenic Expression in Citrus of Single-Chain Antibody Fragments Specific to Citrus Tristeza Virus Confers Virus Resistance. Transgenic Res. 2010, 19, 1001–1015. [Google Scholar] [CrossRef]
- Petri, C.; Burgos, L. Transformation of Fruit Trees. Useful Breeding Tool or Continued Future Prospect? Transgenic Res. 2005, 14. [Google Scholar] [CrossRef]
- Petri, C.; Alburquerque, N.; Faize, M.; Scorza, R.; Dardick, C. Current Achievements and Future Directions in Genetic Engineering of European Plum (Prunus domestica L.). Transgenic Res. 2018, 27, 225–240. [Google Scholar] [CrossRef]
- Urtubia, C.; Devia, J.; Castro, A.; Zamora, P.; Aguirre, C.; Tapia, E.; Barba, P.; Dell’Orto, P.; Moynihan, M.R.; Petri, C.; et al. Agrobacterium-Mediated Genetic Transformation of Prunus salicina. Plant Cell Rep. 2008, 27, 1333–1340. [Google Scholar] [CrossRef]
- Gonzalez-Padilla, I.M.; Webb, K.; Scorza, R. Early Antibiotic Selection and Efficient Rooting and Acclimatization Improve the Production of Transgenic Plum Plants (Prunus domestica L.). Plant Cell Rep. 2003, 22, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Canli, F.A.; Wang, X.; Sibbald, S. Genetic Transformation of Prunus domestica L. Using the Hpt Gene Coding for Hygromycin Resistance as the Selectable Marker. Sci. Hortic. 2009, 119, 339–343. [Google Scholar] [CrossRef]
- Petri, C.; Hily, J.M.; Vann, C.; Dardick, C.; Scorza, R. A High-Throughput Transformation System Allows the Regeneration of Marker-Free Plum Plants (Prunus domestica). Ann. Appl. Biol. 2011, 159, 302–315. [Google Scholar] [CrossRef]
- Wang, H.; Petri, C.; Burgos, L.; Alburquerque, N. Phosphomannose-Isomerase as a Selectable Marker for Transgenic Plum (Prunus Domestica L.). Plant Cell. Tissue Organ Cult. 2013, 113. [Google Scholar] [CrossRef]
- Petri, C.; Ruiz, D.; Faize, M.; Burgos, L.; Alburquerque, N. Prunus Domestica Plum. In Biotechnology of fruit and nut crops; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CABI: Florida, 2020; pp. 512–531. [Google Scholar]
- Ilardi, V.; Tavazza, M. Biotechnological Strategies and Tools for Plum Pox Virus Resistance: Trans-, Intra-, Cis-Genesis, and Beyond. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Scorza, R.; Ravelonandro, M.; Callahan, A.M.; Cordts, J.M.; Fuchs, M.; Dunez, J.; Gonsalves, D. Transgenic Plums (Prunus domestica L.) Express the Plum Pox Virus Coat Protein Gene. Plant Cell Rep. 1994, 14, 18–22. [Google Scholar] [CrossRef]
- Scorza, R.; Callahan, A.; Levy, L.; Damsteegt, V.; Webb, K.; Ravelonandro, M. Post-Transcriptional Gene Silencing in Plum Pox Virus Resistant Transgenic European Plum Containing the Plum Pox Potyvirus Coat Protein Gene. Transgenic Res. 2001, 10, 201–209. [Google Scholar] [CrossRef]
- Scorza, R.; Ravelonandro, M.; Callahan, A.; Zagrai, L.; Polak, J.; Malinowski, T.; Cambra, M.; Levy, L.; Damsteegt, V.; Krška, B.; et al. Honeysweet (C5), the First Genetically Engineered Plum Pox virus-Resistant Plum (Prunus domestica L.) Cultivar. HortScience 2016, 51, 601–603. [Google Scholar] [CrossRef]
- Hily, J.M.; Ravelonandro, M.; Damsteegt, V.D.; Bassett, C.; Petri, C.; Liu, Z.; Scorza, R. Plum Pox Virus Coat Protein Gene Intron-Hairpin-RNA (IhpRNA) Constructs Provide Resistance to Plum Pox Virus in Nicotiana benthamiana and Prunus domestica. J. Am. Soc. Hortic. Sci. 2007, 132, 850–858. [Google Scholar] [CrossRef]
- Di Nicola-Negri, E. Di; Brunetti, A.; Tavazza, M.; Ilardi, V. Hairpin RNA-Mediated Silencing of Plum Pox Virus P1 and HC-Pro Genes for Efficient and Predictable Resistance to the Virus. Transgenic Res. 2005, 14, 989–994. [Google Scholar] [CrossRef]
- Di Nicola-Negri, E.; Tavazza, M.; Salandri, L.; Ilardi, V. Silencing of Plum Pox Virus 5’UTR/P1 Sequence Confers Resistance to a Wide Range of PPV Strains. Plant Cell Rep. 2010, 29, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, S.; Di Nicola-Negri, E.; Gentile, A.; Damiano, C.; Ilardi, V. Production and in Vitro Assessment of Transgenic Plums for Resistance to Plum Pox Virus: A Feasible, Environmental Risk-Free, Cost-Effective Approach. Ann. Appl. Biol. 2012, 161, 293–301. [Google Scholar] [CrossRef]
- García-Almodovar, R.C.; Clemente-Moreno, M.J.; Díaz-Vivancos, P.; Petri, C.; Rubio, M.; Padilla, I.M.G.; Ilardi, V.; Burgos, L. Greenhouse Evaluation Confirms in Vitro Sharka Resistance of Genetically Engineered H-UTR/P1 Plum Plants. Plant Cell. Tissue Organ Cult. 2015, 120, 791–796. [Google Scholar] [CrossRef]
- Alburquerque, N.; Faize, L.; Burgos, L. Silencing of Agrobacterium Tumefaciens Oncogenes Ipt and IaaM Induces Resistance to Crown Gall Disease in Plum but Not in Apricot. Pest Manag. Sci. 2017, 73. [Google Scholar] [CrossRef]
- Faize, M.; Faize, L.; Petri, C.; Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Koussa, T.; Rifai, L.A.; Burgos, L.; Hernandez, J.A. Cu/Zn Superoxide Dismutase and Ascorbate Peroxidase Enhance in Vitro Shoot Multiplication in Transgenic Plum. J. Plant Physiol. 2013, 170, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic Expression of Cytosolic Superoxide Dismutase and Ascorbate Peroxidase Leads to Salt Stress Tolerance in Transgenic Plums. Plant Biotechnol. J. 2013, 11, 976–985. [Google Scholar] [CrossRef]
- Díaz-Vivancos, P.; Faize, L.; Nicolás, E.; Clemente-Moreno, M.J.; Bru-Martinez, R.; Burgos, L.; Hernández, J.A. Transformation of Plum Plants with a Cytosolic Ascorbate Peroxidase Transgene Leads to Enhanced Water Stress Tolerance. Ann. Bot. 2016, 117, 1121–1131. [Google Scholar] [CrossRef]
- Srinivasan, C.; Dardick, C.; Callahan, A.; Scorza, R. Plum (Prunus domestica) Trees Transformed with Poplar FT1 Result in Altered Architecture, Dormancy Requirement, and Continuous Flowering. PLoS One 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Scorza, R.; Dardick, C.D.; Callahan, A.M.; Srinivasan, C.; Delong, T.; Harper, J.; Raines, D.D.; Castro, S. FasTrack’-a Revolutionary Approach to Long-Generation Cycle Specialty Crop Breeding. In Proceedings of the Xth International Symposium Plum & Prune Genetics; 2012; p. Paper No. 101.
- Petri, C.; Wang, H.; Alburquerque, N.; Faize, M.; Burgos, L. Agrobacterium-Mediated Transformation of Apricot (Prunus armeniaca L.) Leaf Explants. Plant Cell Rep. 2008, 27. [Google Scholar] [CrossRef] [PubMed]
- Petri, C.; López-Noguera, S.; Alburquerque, N.; Egea, J.; Burgos, L. An Antibiotic-Based Selection Strategy to Regenerate Transformed Plants from Apricot Leaves with High Efficiency. Plant Sci. 2008, 175. [Google Scholar] [CrossRef]
- Petri, C.; Wang, H.; Burgos, L.; Sánchez-Navarro, J.; Alburquerque, N. Production of Transgenic Apricot Plants from Hypocotyl Segments of Mature Seeds. Sci. Hortic. 2015, 197. [Google Scholar] [CrossRef]
- López-Noguera, S.; Petri, C.; Burgos, L. Combining a Regeneration-Promoting Gene and Site-Specific Recombination Allows a More Efficient Apricot Transformation and the Elimination of Marker Genes. Plant Cell Rep. 2009, 28, 1781–1790. [Google Scholar] [CrossRef]
- Petri, C.; López-Noguera, S.; Wang, H.; García-Almodóvar, C.; Alburquerque, N.; Burgos, L. A Chemical-Inducible Cre-LoxP System Allows for Elimination of Selection Marker Genes in Transgenic Apricot. Plant Cell. Tissue Organ Cult. 2012, 110, 337–346. [Google Scholar] [CrossRef]
- Alburquerque, N.; Ruiz, D.; Burgos, L.; Petri, C. Prunus armeniaca Apricot. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CABI: Florida, 2020; pp. 496–511. [Google Scholar]
- Laimer da Câmara Machado, M.; da Câmara Machado, A.; Hanzer, V.; Weiss, H.; Regner, F.; Steinkeliner, H.; Mattanovich, D.; Plail, R.; Knapp, E.; Kalthoff, B.; et al. Regeneration of Transgenic Plants of Prunus armeniaca Containing the Coat Protein Gene of Plum Pox Virus. Plant Cell Rep. 1992, 11, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Smigocki, A.C.; Hammerschlag, F.A. Regeneration of Plants from Peach Embryo Cells Infected with a Shooty Mutant Strain of Agrobacterium. J. Am. Soc. Hortic. Sci. 1991, 116, 1092–1097. [Google Scholar] [CrossRef]
- Ricci, A.; Sabbadini, S.; Prieto, H.; Padilla, I.M.G.; Dardick, C.; Li, Z.; Scorza, R.; Limera, C.; Mezzetti, B.; Perez-Jimenez, M.; et al. Genetic Transformation in Peach (Prunus persica l.): Challenges and Ways Forward. Plants 2020, 9, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Fitch, M.M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L.; Sanford, J.C. Virus Resistant Papaya Plants Derived from Tissues Bombarded with the Coat Protein Gene of Papaya Ringspot Virus. Bio/Technology 1992, 10, 1466–1472. [Google Scholar] [CrossRef]
- Gouthu, S.; Mandelli, C.; Eubanks, B.A.; Deluc, L.G. Transgene-Free Genome Editing and RNAi Ectopic Application in Fruit Trees: Potential and Limitations. Front. Plant Sci. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Fernandez i Marti, A.F.; Dodd, R.S. Using CRISPR as a Gene Editing Tool for Validating Adaptive Gene Function in Tree Landscape Genomics. Front. Ecol. Evol. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Nishitani, C.; Hirai, N.; Komori, S.; Wada, M.; Okada, K.; Osakabe, K.; Yamamoto, T.; Osakabe, Y. Efficient Genome Editing in Apple Using a CRISPR/Cas9 System. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Charrier, A.; Vergne, E.; Dousset, N.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient Targeted Mutagenesis in Apple and First Time Edition of Pear Using the CRISPR-Cas9 System. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Piazza, S.; Pompili, V.; Salvagnin, U.; Cestaro, A.; Moffa, L.; Vittani, L.; Moser, C.; Malnoy, M. Strategies to Produce T-DNA Free CRISPRed Fruit Trees via Agrobacterium tumefaciens Stable Gene Transfer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Malabarba, J.; Chevreau, E.; Dousset, N.; Veillet, F.; Moizan, J.; Vergne, E. New Strategies to Overcome Present CRISPR/CAS9 Limitations in Apple and Pear: Efficient Dechimerization and Base Editing. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Zhou, H.; Bai, S.; Wang, N.; Sun, X.; Zhang, Y.; Zhu, J.; Dong, C. CRISPR/Cas9-Mediated Mutagenesis of MdCNGC2 in Apple Callus and VIGS-Mediated Silencing of MdCNGC2 in Fruits Improve Resistance to Botryosphaeria Dothidea. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, P.; Bozorov, T.A.; Zhang, D. Application of CRISPR/Cas9 Technology in Wild Apple (Malus sieverii) for Paired Sites Gene Editing. Plant Methods 2021, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Wang, Y.; Xu, J.; Jiang, S.; Zhang, Y. MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Kong, K.; Han, J.; Song, S.; Bai, T.; Song, C.; Wang, M.; Yan, Z.; Zhang, H.; Zhang, R.; et al. Field Detection of Multiple RNA Viruses/Viroids in Apple Using a CRISPR/Cas12a-Based Visual Assay. Plant Biotechnol. J. 2021, 19. [Google Scholar] [CrossRef]
- Kaur, N.; Alok, A.; Shivani; Kaur, N.; Pandey, P.; Awasthi, P.; Tiwari, S. CRISPR/Cas9-Mediated Efficient Editing in Phytoene Desaturase (PDS) Demonstrates Precise Manipulation in Banana Cv. Rasthali Genome. Funct. Integr. Genomics 2018, 18. [Google Scholar] [CrossRef]
- Naim, F.; Dugdale, B.; Kleidon, J.; Brinin, A.; Shand, K.; Waterhouse, P.; Dale, J. Gene Editing the Phytoene Desaturase Alleles of Cavendish Banana Using CRISPR/Cas9. Transgenic Res. 2018, 27, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ntui, V.O.; Tripathi, J.N.; Tripathi, L. Robust CRISPR/Cas9 Mediated Genome Editing Tool for Banana and Plantain (Musa Spp.). Curr. Plant Biol. 2020, 21. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Ntui, V.O.; Ron, M.; Muiruri, S.K.; Britt, A.; Tripathi, L. CRISPR/Cas9 Editing of Endogenous Banana Streak Virus in the B Genome of Musa Spp. Overcomes a Major Challenge in Banana Breeding. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F.; et al. Using CRISPR/Cas9 Genome Editing System to Create MaGA20ox2 Gene-Modified Semi-Dwarf Banana. Plant Biotechnol. J. 2020, 18. [Google Scholar] [CrossRef]
- Kaur, N.N.; Alok, A.; Shivani; Kumar, P.; Kaur, N.N.; Awasthi, P.; Chaturvedi, S.; Pandey, P.; Pandey, A.K.A.; Pandey, A.K.A.; et al. CRISPR/Cas9 Directed Editing of Lycopene Epsilon-Cyclase Modulates Metabolic Flux for β-Carotene Biosynthesis in Banana Fruit. Metab. Eng. 2020, 59, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sheng, O.; Deng, G.; He, W.; Dong, T.; Yang, Q.; Dou, T.; Li, C.; Gao, H.; Liu, S.; et al. CRISPR/Cas9-Mediated Genome Editing of MaACO1 (Aminocyclopropane-1-Carboxylate Oxidase 1) Promotes the Shelf Life of Banana Fruit. Plant Biotechnol. J. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma Cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Nian, W. Targeted Genome Editing of Sweet Orange Using Cas9/SgRNA. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Jia, H.; Xu, J.; Orbović, V.; Zhang, Y.; Wang, N. Editing Citrus Genome via SaCas9/SgRNA System. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome Editing of the Disease Susceptibility Gene CsLOB1 in Citrus Confers Resistance to Citrus Canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering Canker-Resistant Plants through CRISPR/Cas9-Targeted Editing of the Susceptibility Gene CsLOB1 Promoter in Citrus. Plant Biotechnol. J. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Orbović, V.; Wang, N. CRISPR-LbCas12a-Mediated Modification of Citrus. Plant Biotechnol. J. 2019, 17. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Peng, A.; Xie, Z.; He, Y.; Zou, X. CRISPR/Cas9-Mediated Editing of CsWRKY22 Reduces Susceptibility to Xanthomonas Citri Subsp. Citri in Wanjincheng Orange (Citrus sinensis (L.) Osbeck). Plant Biotechnol. Rep. 2019, 13, 501–510. [Google Scholar] [CrossRef]
- Jia, H.; Wang, N. Generation of Homozygous Canker-Resistant Citrus in the T0 Generation Using CRISPR-SpCas9p. Plant Biotechnol. J. 2020, 18. [Google Scholar] [CrossRef]
- Dutt, M.; Mou, Z.; Zhang, X.; Tanwir, S.E.; Grosser, J.W. Efficient CRISPR/Cas9 Genome Editing with Citrus Embryogenic Cell Cultures. BMC Biotechnol. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Wang, N. Highly Efficient Generation of Canker-Resistant Sweet Orange Enabled by an Improved CRISPR/Cas9 System. Front. Plant Sci. 2022, 12. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Chardonnay (Vitis Vinifera L.). Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-Mediated Targeted Mutagenesis in Grape. PLoS One 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef]
- Ren, F.; Ren, C.; Zhang, Z.; Duan, W.; Lecourieux, D.; Li, S.; Liang, Z. Efficiency Optimization of CRISPR/CAS9-Mediated Targeted Mutagenesis in Grape. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Li, M.Y.; Jiao, Y.T.; Wang, Y.T.; Zhang, N.; Wang, B.B.; Liu, R.Q.; Yin, X.; Xu, Y.; Liu, G.T. CRISPR/Cas9-Mediated VvPR4b Editing Decreases Downy Mildew Resistance in Grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7. [Google Scholar] [CrossRef]
- Sunitha, S.; Rock, C.D. CRISPR/Cas9-Mediated Targeted Mutagenesis of TAS4 and MYBA7 Loci in Grapevine Rootstock 101-14. Transgenic Res. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.Y.; Guo, Y.; Cheng, Y.; Hu, Y.; Xiao, S.; Wang, Y.; Wen, Y.Q. CRISPR/Cas9-Mediated Mutagenesis of VvMLO3 Results in Enhanced Resistance to Powdery Mildew in Grapevine (Vitis vinifera). Hortic. Res. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Liu, Y.; Guo, Y.; Duan, W.; Fan, P.; Li, S.; Liang, Z. Optimizing the CRISPR/Cas9 System for Genome Editing in Grape by Using Grape Promoters. Hortic. Res. 2021, 8. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.M.; Gleave, A.P.; Allan, A.C. Mutagenesis of Kiwifruit CENTRORADIALIS-like Genes Transforms a Climbing Woody Perennial with Long Juvenility and Axillary Flowering into a Compact Plant with Rapid Terminal Flowering. Plant Biotechnol. J. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Li, D.; Zhang, Q.; Li, L.; Zhong, C.; Liu, Y.; Huang, H. Optimized Paired-sgRNA/Cas9 Cloning and Expression Cassette Triggers High-efficiency Multiplex Genome Editing in Kiwifruit. Plant Biotechnol. J. 2018, 16, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Herath, D.; Voogd, C.; Mayo-Smith, M.; Yang, B.; Allan, A.C.; Putterill, J.; Varkonyi-Gasic, E. CRISPR-Cas9-Mediated Mutagenesis of Kiwifruit BFT Genes Results in an Evergrowing but Not Early Flowering Phenotype. Plant Biotechnol. J. 2022, 20, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Herrera, A.; Figueroa-Yáñez, L.; Castaño, E.; Santamaría, J.; Pereira-Santana, A.; Espadas-Alcocer, J.; Sánchez-Teyer, F.; Espadas-Gil, F.; Alcaraz, L.D.; López-Gómez, R.; et al. A Novel Dreb2-Type Gene from Carica Papaya Confers Tolerance under Abiotic Stress. Plant Cell. Tissue Organ Cult. 2016, 125. [Google Scholar] [CrossRef]
- Gumtow, R.; Wu, D.; Uchida, J.; Tian, M. A Phytophthora Palmivora Extracellular Cystatin-like Protease Inhibitor Targets Papain to Contribute to Virulence on Papaya. Mol. Plant-Microbe Interact. 2018, 31, 363–373. [Google Scholar] [CrossRef]
- Pettongkhao, S.; Navet, N.; Schornack, S.; Tian, M.; Churngchow, N. A Secreted Protein of 15 KDa Plays an Important Role in Phytophthora Palmivora Development and Pathogenicity. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Pang, H.G.; Yan, Q.; Zhao, S.; He, F.; Xu, J.F.; Qi, B.X.; Zhang, Y. Knockout of the S-Acyltransferase Gene, Pbpat14, Confers the Dwarf Yellowing Phenotype in First Generation Pear by ABA Accumulation. Int. J. Mol. Sci. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Kanchiswamy, C.N. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, H.; Liu, J.; Yang, Q.; Shao, X.; Bi, F.; Hu, C.; Huo, H.; Chen, K.; Yi, G. Establishment of a PEG-Mediated Protoplast Transformation System Based on DNA and CRISPR/Cas9 Ribonucleoprotein Complexes for Banana. BMC Plant Biol. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Pavese, V.; Moglia, A.; Abbà, S.; Milani, A.M.; Marinoni, D.T.; Corredoira, E.; Martinez, M.T.; Botta, R. First Report on Genome Editing via Ribonucleoprotein (RNP) in Castanea Sativa Mill. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Scintilla, S.; Salvagnin, U.; Giacomelli, L.; Zeilmaker, T.; Malnoy, M.A.; Rouppe van der Voort, J.; Moser, C. Regeneration of Non-Chimeric Plants from DNA-Free Edited Grapevine Protoplasts. Front. Plant Sci. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Bertini, E.; D’Incà, E.; Fasoli, M.; Zenoni, S. DNA-Free Genome Editing in Grapevine Using CRISPR/Cas9 Ribonucleoprotein Complexes Followed by Protoplast Regeneration. Hortic. Res. 2023, 10. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Kaur, P.; Stanton, D.; Grosser, J.W.; Dutt, M. A Cationic Lipid Mediated CRISPR/Cas9 Technique for the Production of Stable Genome Edited Citrus Plants. Plant Methods 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Fang, H.; Roberts, N.; Zhang, L.; Vakulskas, C.A.; Niedz, R.P.; Culver, J.N.; Qi, Y. Highly Efficient Genome Editing in Plant Protoplasts by Ribonucleoprotein Delivery of CRISPR-Cas12a Nucleases. Front. Genome Ed. 2022, 4, 1–11. [Google Scholar] [CrossRef]
- Pak, S.; Li, C. Progress and Challenges in Applying CRISPR/Cas Techniques to the Genome Editing of Trees. For. Res. 2022, 2, 0–0. [Google Scholar] [CrossRef]
- Min, T.; Hwarari, D.; Li, D.; Movahedi, A.; Yang, L. CRISPR-Based Genome Editing and Its Applications in Woody Plants. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Donadio, L.C.; Lederman, I.E.; Roberto, S.R.; Stucchi, E.S. Dwarfing-Canopy and Rootstock Cultivars for Fruit Trees. Rev. Bras. Frutic. 2019, 41. [Google Scholar] [CrossRef]
- Dongariyal, A.; Chandra, A.K.; Dongriyal, A.; Kumar, A.; Sharma, P. Tending Genome Editing via CRISPR/Cas9-Induced Mutagenesis: Opportunity and Challenges for Yield, Quality and Nutritional Improvement of Fruit Crops. Sci. Hortic. 2023, 311. [Google Scholar] [CrossRef]
- Rudikovskii, A. V.; Stolbicova, A. V.; Rudikovskaya, E.G.; Dudareva, L. V. Role of Phytohormones in the Formation of Dwarf and Tall Siberian Crabapple (Malus Bаccata l. Borkh.). Zemdirbyste 2019, 106. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Tan, B.; Jiang, Y.; Zheng, X.; Ye, X.; Guo, Z.; Xiong, T.; Wang, W.; Li, J.; et al. A Single Nucleotide Mutation in GID1c Disrupts Its Interaction with DELLA1 and Causes a GA-Insensitive Dwarf Phenotype in Peach. Plant Biotechnol. J. 2019, 17, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation Effect on IPT5b Gene Expression Determines Cytokinin Biosynthesis in Apple Rootstock. Biochem. Biophys. Res. Commun. 2017, 482. [Google Scholar] [CrossRef]
- Sattar, M.N.; Iqbal, Z.; Al-Khayri, J.M.; Jain, S.M. Induced Genetic Variations in Fruit Trees Using New Breeding Tools: Food Security and Climate Resilience. Plants 2021, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Gong, X.; Li, M.; Li, C.; Sun, T.; Ma, F. Overexpression of a Novel Apple NAC Transcription Factor Gene, MdNAC1, Confers the Dwarf Phenotype in Transgenic Apple (Malus domestica). Genes 2018, 9. [Google Scholar] [CrossRef]
- Jia, P.; Xing, L.; Zhang, C.; Chen, H.; Li, Y.; Zhang, D.; Ma, J.; Zhao, C.; Han, M.; Ren, X.; et al. MdKNOX15, a Class I Knotted-like Transcription Factor of Apple, Controls Flowering and Plant Height by Regulating GA Levels through Promoting the MdGA2ox7 Transcription. Environ. Exp. Bot. 2021, 185. [Google Scholar] [CrossRef]
- Ren, C.; Guo, Y.; Kong, J.; Lecourieux, F.; Dai, Z.; Li, S.; Liang, Z. Knockout of VvCCD8 Gene in Grapevine Affects Shoot Branching. BMC Plant Biol. 2020, 20. [Google Scholar] [CrossRef]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis Dnd1 “Defense, No Death” Gene Encodes a Mutated Cyclic Nucleotide-Gated Ion Channel. Proc. Natl. Acad. Sci. U. S. A. 2000, 97. [Google Scholar] [CrossRef]
- Ma, W.; Smigel, A.; Walker, R.K.; Moeder, W.; Yoshioka, K.; Berkowitz, G.A. Leaf Senescence Signaling: The Ca2+-Conducting Arabidopsis Cyclic Nucleotide Gated Channel2 Acts through Nitric Oxide to Repress Senescence Programming. Plant Physiol. 2010, 154. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.U. Cyclic Nucleotide-Gated Channels: Essential Signaling Components in Plants for Fertilization and Immunity Responses. In Plant Signaling: Understanding the Molecular Crosstalk; 2014; Vol. 9788132215.
- Corte, L.E.D.; Mahmoud, L.M.; Moraes, T.S.; Mou, Z.; Grosser, J.W.; Dutt, M. Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants 2019, 8. [Google Scholar]
- Pillitteri, L.J.; Lovatt, C.J.; Walling, L.L. Isolation and Characterization of a Terminal Flower Homolog and Its Correlation with Juvenility in Citrus. Plant Physiol. 2004, 135. [Google Scholar] [CrossRef] [PubMed]
- Eshed, Y.; Lippman, Z.B. Revolutions in Agriculture Chart a Course for Targeted Breeding of Old and New Crops. Science (80-. ). 2019; 366. [Google Scholar]
- Endo, T.; Shimada, T.; Fujii, H.; Kobayashi, Y.; Araki, T.; Omura, M. Ectopic Expression of an FT Homolog from Citrus Confers an Early Flowering Phenotype on Trifoliate Orange (Poncirus trifoliata L. Raf.). Transgenic Res. 2005, 14. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, K.; Agüero, J.; Vives, M.C.; Aleza, P.; Pina, J.A.; Moreno, P.; Navarro, L.; Guerri, J. Precocious Flowering of Juvenile Citrus Induced by a Viral Vector Based on Citrus Leaf Blotch Virus: A New Tool for Genetics and Breeding. Plant Biotechnol. J. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Callahan, A.M.; Srinivasan, C.; Dardick, C.; Scorza, R. Rapid Cycle Breeding: Application of Transgenic Early Flowering for Perennial Trees. In Plant Breeding Reviews; 2016; Vol. 40.
- Prudencio, A.S.; Devin, S.R.; Mahdavi, S.M.E.; Martínez-García, P.J.; Salazar, J.A.; Martínez-Gómez, P. Spontaneous, Artificial, and Genome Editing-Mediated Mutations in Prunus. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sapkota, M.; van der Knaap, E. Perspectives of CRISPR/Cas-Mediated Cis-Engineering in Horticulture: Unlocking the Neglected Potential for Crop Improvement. Hortic. Res. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Alonge, M.; Wang, X.; Benoit, M.; Soyk, S.; Pereira, L.; Zhang, L.; Suresh, H.; Ramakrishnan, S.; Maumus, F.; Ciren, D.; et al. Major Impacts of Widespread Structural Variation on Gene Expression and Crop Improvement in Tomato. Cell 2020, 182. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Q.; Snouffer, A.; Zhang, B.; Rodríguez, G.R.; van der Knaap, E. Increasing Fruit Weight by Editing a Cis-Regulatory Element in Tomato KLUH Promoter Using CRISPR/Cas9. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Zapata, P.; Martínez-García, P.J.; Martínez-Gómez, P. Whole Transcriptome Analyses of Apricots and Japanese Plum Fruits after 1-MCP (Ethylene-Inhibitor) and Ethrel (Ethylene-Precursor) Treatments Reveal New Insights into the Physiology of the Ripening Process. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Hu, H.; Scheben, A.; Verpaalen, B.; Tirnaz, S.; Bayer, P.E.; Hodel, R.G.J.; Batley, J.; Soltis, D.E.; Soltis, P.S.; Edwards, D. Amborella Gene Presence/Absence Variation Is Associated with Abiotic Stress Responses That May Contribute to Environmental Adaptation. New Phytol. 2022, 233. [Google Scholar] [CrossRef]
- Fizikova, A.; Tikhonova, N.; Ukhatova, Y.; Ivanov, R.; Khlestkina, E. Applications of CRISPR/Cas9 System in Vegetatively Propagated Fruit and Berry Crops. Agronomy 2021, 11. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Holt, N.E.; Kaligotla, S.; Fuciman, M.; Cazzaniga, S.; Carbonera, D.; Frank, H.A.; Alric, J.; Bassi, R. Zeaxanthin Protects Plant Photosynthesis by Modulating Chlorophyll Triplet Yield in Specific Light-Harvesting Antenna Subunits. J. Biol. Chem. 2012, 287. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wu, S.; Tian, L. Effective Genome Editing and Identification of a Regiospecific Gallic Acid 4-O-Glycosyltransferase in Pomegranate (Punica granatum L.). Hortic. Res. 2019, 6. [Google Scholar] [CrossRef]
- Goulet, C.; Kamiyoshihara, Y.; Lam, N.B.; Richard, T.; Taylor, M.G.; Tieman, D.M.; Klee, H.J. Divergence in the Enzymatic Activities of a Tomato and Solanum Pennellii Alcohol Acyltransferase Impacts Fruit Volatile Ester Composition. Mol. Plant 2015, 8. [Google Scholar] [CrossRef]
- Cao, X.; Wei, C.; Duan, W.; Gao, Y.; Kuang, J.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Transcriptional and Epigenetic Analysis Reveals That NAC Transcription Factors Regulate Fruit Flavor Ester Biosynthesis. Plant J. 2021, 106. [Google Scholar] [CrossRef]
- Zaidi, S.M.S.; Saud, A. Future of US-China Relations: Conflict, Competition or Cooperation? Asian Soc. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Joshi, R.K.; Bharat, S.S.; Mishra, R. Engineering Drought Tolerance in Plants through CRISPR/Cas Genome Editing. 3 Biotech 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhao, X.; Zhao, Q.; Wang, G.; Li, Y.; Hao, Y. MdDREB2A in Apple Is Involved in the Regulation of Multiple Abiotic Stress Responses. Hortic. Plant J. 2021, 7. [Google Scholar] [CrossRef]
- Liao, X.; Guo, X.; Wang, Q.; Wang, Y.; Zhao, D.; Yao, L.; Wang, S.; Liu, G.; Li, T. Overexpression of MsDREB6.2 Results in Cytokinin-Deficient Developmental Phenotypes and Enhances Drought Tolerance in Transgenic Apple Plants. Plant J. 2017, 89, 510–526. [Google Scholar] [CrossRef]
- Jin, W.; Dong, J.; Hu, Y.; Lin, Z.; Xu, E.; Han, Z. Improved Cold-Resistant Performance in Transgenic Grape (Vitis Vinifera L.) Overexpressing Cold-Inducible Transcription Factors AtDREB1b. HortScience 2009, 44. [Google Scholar] [CrossRef]
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Leonhardt, N.; Dellaporta, S.L.; Tonelli, C. A Guard-Cell-Specific MYB Transcription Factor Regulates Stomatal Movements and Plant Drought Tolerance. Curr. Biol. 2005, 15. [Google Scholar] [CrossRef]
- Wen, X.P.; Pang, X.M.; Matsuda, N.; Kita, M.; Inoue, H.; Hao, Y.J.; Honda, C.; Moriguchi, T. Over-Expression of the Apple Spermidine Synthase Gene in Pear Confers Multiple Abiotic Stress Tolerance by Altering Polyamine Titers. Transgenic Res. 2008, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, T.; Li, T.; Wang, M.; Yang, G.; He, G. A CBL-Interacting Protein Kinase TaCIPK2 Confers Drought Tolerance in Transgenic Tobacco Plants through Regulating the Stomatal Movement. PLoS One 2016, 11. [Google Scholar] [CrossRef]
- Kaur, N.; Awasthi, P.; Tiwari, S. Fruit Crops Improvement Using CRISPR/Cas9 System; Elsevier Inc., 2020; ISBN 9780128181409.
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic Selection in the Era of Next Generation Sequencing for Complex Traits in Plant Breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Devin, S.R.; Prudencio, Á.S.; Mahdavi, S.M.E.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Orchard Management and Incorporation of Biochemical and Molecular Strategies for Improving Drought Tolerance in Fruit Tree Crops. Plants 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, X.; Dong, X. Generation of Broad-Spectrum Disease Resistance by Everexpression of an Essential Regulatory Gene in Systemic Acquired Resistance. Proc. Natl. Acad. Sci. U. S. A. 1998, 95. [Google Scholar] [CrossRef]
- Le Henanff, G.; Farine, S.; Kieffer-Mazet, F.; Miclot, A.S.; Heitz, T.; Mestre, P.; Bertsch, C.; Chong, J. Vitis Vinifera VvNPR1.1 Is the Functional Ortholog of AtNPR1 and Its Overexpression in Grapevine Triggers Constitutive Activation of PR Genes and Enhanced Resistance to Powdery Mildew. Planta 2011, 234. [Google Scholar] [CrossRef]
- Malnoy, M.; Jin, Q.; Borejsza-Wysocka, E.E.; He, S.Y.; Aldwinckle, H.S. Overexpression of the Apple MpNPR1 Gene Confers Increased Disease Resistance in Malus x domestica. Mol. Plant-Microbe Interact. 2007, 20. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. Mlo-Based Resistance: An Apparently Universal “Weapon” to Defeat Powdery Mildew Disease. Mol. Plant-Microbe Interact. 2017, 30.
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of Broad Virus Resistance in Non-Transgenic Cucumber Using CRISPR/Cas9 Technology. Mol. Plant Pathol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Genome Engineering for Crop Improvement and Future Agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally Engineered Cas9 Nucleases with Improved Specificity. Science (80-. ). 2016, 351. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas Nuclease Specificity Using Truncated Guide RNAs. Nat. Biotechnol. 2014, 32. [Google Scholar] [CrossRef]
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 Genome Editing to Modify Abiotic Stress Responses in Plants. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.; Schiml, S.; Fauser, F.; Puchta, H. Highly Efficient Heritable Plant Genome Engineering Using Cas9 Orthologues from Streptococcus Thermophilus and Staphylococcus Aureus. Plant J. 2015, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, H.; Li, T.; Chen, K.; Qiu, J.L.; Gao, C. Perfectly Matched 20-Nucleotide Guide RNA Sequences Enable Robust Genome Editing Using High-Fidelity SpCas9 Nucleases. Genome Biol. 2017, 18. [Google Scholar] [CrossRef]
- Mikami, M.; Toki, S.; Endo, M. Precision Targeted Mutagenesis via Cas9 Paired Nickases in Rice. Plant Cell Physiol. 2016, 57. [Google Scholar] [CrossRef]
- Spök, A.; Sprink, T.; Allan, A.C.; Yamaguchi, T.; Dayé, C. Towards Social Acceptability of Genome-Edited Plants in Industrialised Countries? Emerging Evidence from Europe, United States, Canada, Australia, New Zealand, and Japan. Front. Genome Ed. 2022, 4, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Sprink, T.; Wilhelm, R.; Hartung, F. Genome Editing around the Globe: An Update on Policies and Perceptions. Plant Physiol. 2022, 190, 1579–1587. [Google Scholar] [CrossRef]
- Eriksson, D.; Kershen, D.; Nepomuceno, A.; Pogson, B.J.; Prieto, H.; Purnhagen, K.; Smyth, S.; Wesseler, J.; Whelan, A. A Comparison of the EU Regulatory Approach to Directed Mutagenesis with That of Other Jurisdictions, Consequences for International Trade and Potential Steps Forward. New Phytol. 2019, 222, 1673–1684. [Google Scholar] [CrossRef]
- Van Der Meer, P.; Angenon, G.; Bergmans, H.; Buhk, H.J.; Callebaut, S.; Chamon, M.; Eriksson, D.; Gheysen, G.; Harwood, W.; Hundleby, P.; et al. The Status under EU Law of Organisms Developed through Novel Genomic Techniques. Eur. J. Risk Regul. 2023, 14, 93–112. [Google Scholar] [CrossRef]
- Hu, J.; Gao, C. CRISPR-Edited Plants by Grafting. Nat. Biotechnol. 2023, 41, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 6–10. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome Editing in Maize Directed by CRISPR-Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.W.; Kim, J.; Kwon, S. Il; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-Free Genome Editing in Plants with Preassembled CRISPR-Cas9 Ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Turesson, H.; Nicolia, A.; Fält, A.S.; Samuelsson, M.; Hofvander, P. Efficient Targeted Multiallelic Mutagenesis in Tetraploid Potato (Solanum tuberosum) by Transient CRISPR-Cas9 Expression in Protoplasts. Plant Cell Rep. 2017, 36, 117–128. [Google Scholar] [CrossRef]
| CRISPR-Cas delivery | Fruit trees | Target gene edited | Improved Traits | References |
|---|---|---|---|---|
| A. tumefaciens-mediated | Apple | PDS | Enhanced biosynthesis of carotenoid | [80] |
| PDS and TFL1 | Albino phenotype and early flowering | [81] | ||
| MdDIPM1 and MdDIPM4 | Fire blight resistance | [82] | ||
| ALS | Chlorsulfuron resistance | [83] | ||
| CNGC2 | B. dothidea resistance | [84] | ||
| MsPDS | Albino phenotype | [85] | ||
| MdMKK9 | Increased anthocyanin content | [86] | ||
| Detection viruses and viroids CRISPR | [87] | |||
| Banana | RAS-PDS1 and 2 | Albino phenotype | [88] | |
| PDS | Albino phenotype and dwarfing | [89,90] | ||
| eBSV | Control of virus pathogenesis | [91] | ||
| MaGA20ox2 | Dwarf phenotype | [92]) | ||
| LCYε | Carotene biosynthesis | [93] | ||
| MaACO1 | Fruit ripening | [94] | ||
| Cacao | TcNPR3 | Phytophthora tropicalis resistance | [95] | |
| Citrus | CsPDS | Method optimization | [96] | |
| Cs7g03360 | Phenotypic changes in Carrizo leaf | [97] | ||
| CsLOB1 | Citrus canker resistance | [98] | ||
| CsLOB1 promoter | Citrus canker resistance | [99] | ||
| PDS and CsLOB1 | Albino phenotype and canker resistance | [100] | ||
| CsWRKY22 | Citrus canker resistance | [101] | ||
| CsLOB1 | Citrus canker resistance | [102] | ||
| pC-PDS1 and Pc-PDS2 | Chlorophyll and carotenoid content | [103] | ||
| CsLOB1 | Citrus canker resistance | [104] |
| CRISPR-Cas delivery | Fruit trees | Target gene edited | Improved Traits | References |
|---|---|---|---|---|
| Grape | IdnDH | High tartaric acid biosynthesis | [105] | |
| VvPDS | Increased carotenoid biosynthesis | [106] | ||
| VvWRKY52 | Botrytis cinerea resistance | [107] | ||
| VvPDS | Albino phenotype | [108] | ||
| VvMLO7 | Powdery mildew resistance | [82] | ||
| VvPR4b | Downy mildew resistance | [109] | ||
| MYBA5/6/7 and TAS4a/b | Anthocyanin accumulation | [110] | ||
| VvMLO3 and VvMLO4 | Powdery mildew resistance | [111] | ||
| TMT1 and TMT2 | Reduced sugar accumulation | [112] | ||
| Kiwifruit | CEN4 and CEN | Terminal flower and fruit | [113] | |
| AcPDS | Albino phenotype (leaves) | [114] | ||
| AcBFT2 | Reduced dormancy and early bud break | [115] | ||
| Papaya | CpDreb2 | Gene disruption for water stress | [116] | |
| PpalEPIC8 | Phytophthora palmivora resistance | [117] | ||
| Ppal15kDa | Phytophthora palmivora resistance | [118] | ||
| Pear | TFL1 | Early flowering | [81] | |
| PbPAT14 | Dwarf yellowing phenotype | [119] | ||
| PDS and ALS | Albino phenotype and chlorsulfuron resistance | [83] | ||
| PEG-meditated | Apple | DIPM-1, -2 and -4 | Fire blight resistance | [120] |
| Banana | PDS | Method optimization | [121] | |
| Chestnut | PDS | Method optimization | [122] | |
| Grape | MLO7 | Enhanced biotic resistance against powdery mildew | [120] | |
| DMR6 and MLO6 | Downy- and powdery-mildew resistance | [123] | ||
| GFP | Method optimization | [124] | ||
| Orange | CsNPR3 | Induced biotic stress tolerance | [125] | |
| PH5 | Method optimization | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





