Submitted:
01 November 2023
Posted:
02 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Study Endpoints
2.4. Statistical Analyses
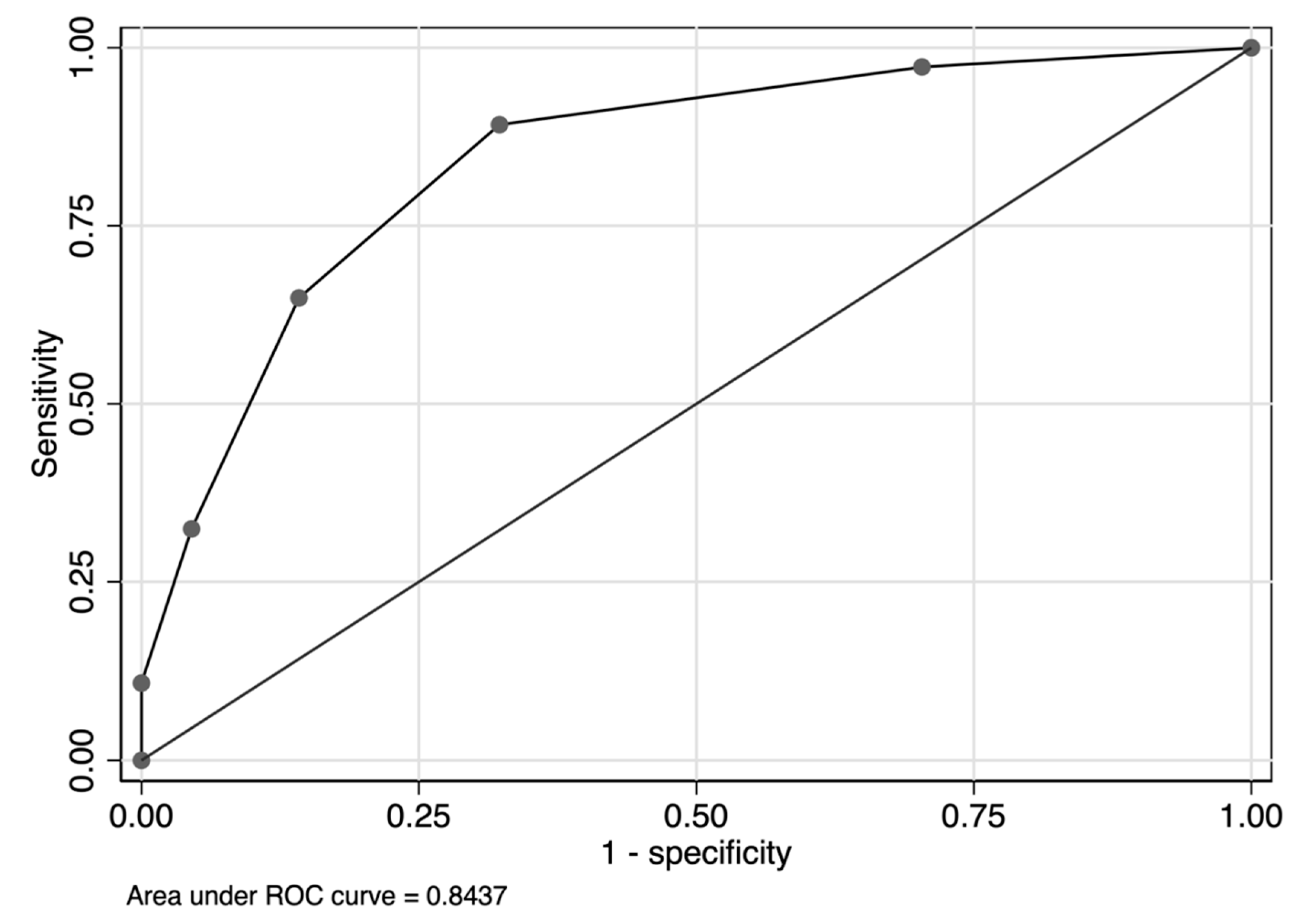
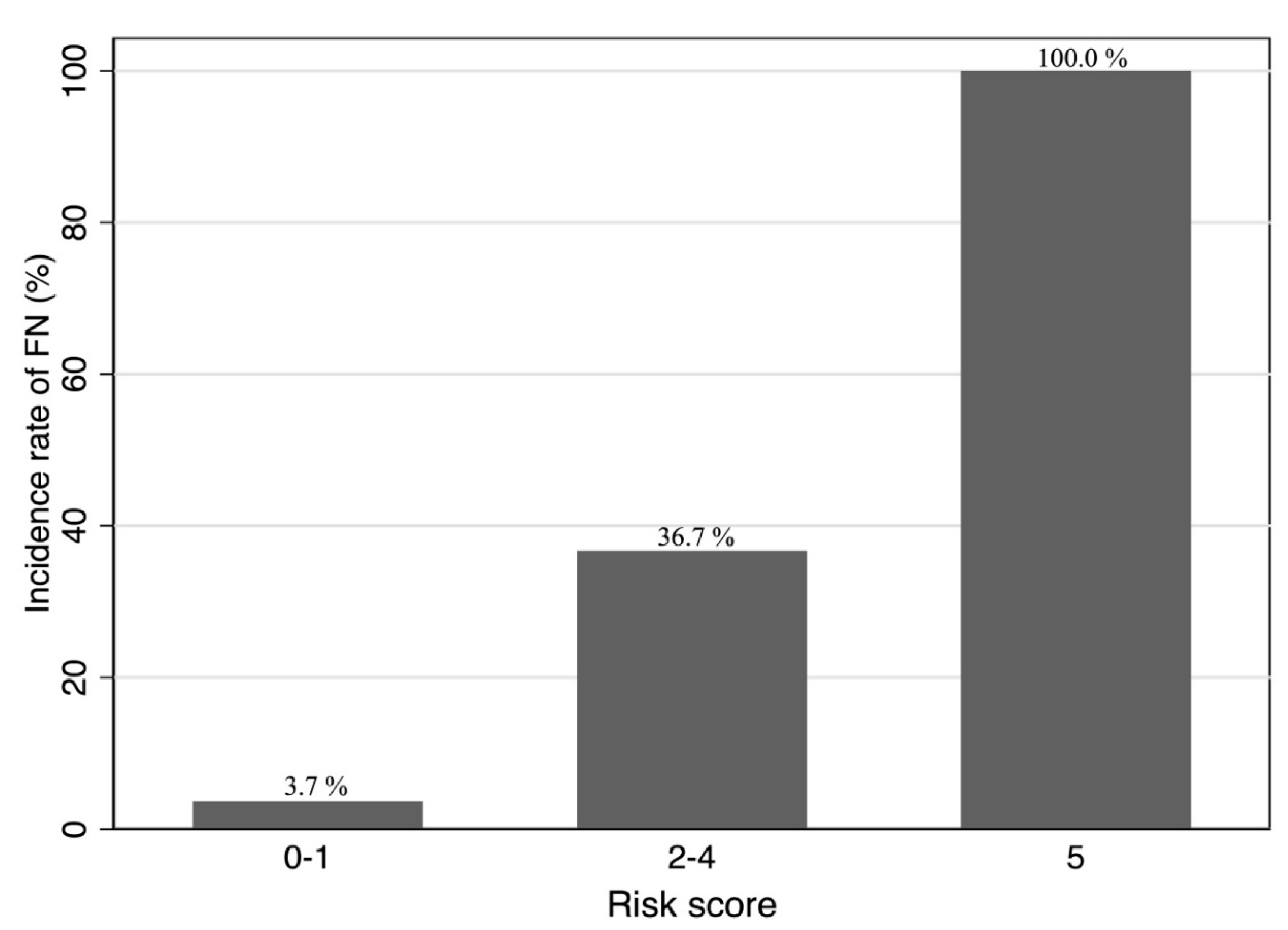
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Pettengell, R.; Schwenkglenks, M.; Leonard, R.; Bosly, A.; Paridaens, R.; Constenla, M.; Szucs, T.D.; Jackisch, C.; Impact of Neutropenia in Chemotherapy-European Study Group (INC-EU). Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008, 16, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Weller, E.A.; Habermann, T.M.; Li, S.; Fisher, R.I.; Cheson, B.D.; Peterson, B.A. Patterns of growth factor usage and febrile neutropenia among older patients with diffuse large B-cell non-Hodgkin lymphoma treated with CHOP or R-CHOP: the Intergroup experience (CALGB 9793; ECOG-SWOG 4494). Leuk Lymphoma. 2017, 58, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.S.; Bohlius, J.; Cameron, D.A.; Dal Lago, L.; Donnelly, J.P.; Kearney, N.; Lyman, G.H.; Pettengell, R.; Tjan-Heijnen, V.C.; Walewski, J.; et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011, 47, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, U.; Trněný, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017, 35, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, G.S.; Chiappella, A.; Gascoyne, R.D.; Scott, D.W.; Zhang, Q.; Jurczak, W.; Özcan, M.; Hong, X.; Zhu, J.; Jin, J.; et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with abc-type diffuse large B-cell lymphoma. J Clin Oncol. 2021, 39, 1317–1328. [Google Scholar] [CrossRef]
- Younes, A.; Sehn, L.H.; Johnson, P.; Zinzani, P.L.; Hong, X.; Zhu, J.; Patti, C.; Belada, D.; Samoilova, O.; Suh, C.; et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019, 37, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Delgado, D.J. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003, 98, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Morrison, V.A.; Dale, D.C.; Crawford, J.; Delgado, D.J.; Fridman, M.; OPPS Working Group; ANC Study Group. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003, 44, 2069–2076. [Google Scholar] [CrossRef]
- Rabinowitz, A.P.; Weiner, N.J.; Tronic, B.S.; Fridman, M.; Liberman, R.F.; Delgado, D.J. Severe neutropenia in CHOP occurs most frequently in cycle 1: a predictive model. Leuk Lymphoma. 2006, 47, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis I. Background, goals, and general strategy. J Clin Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Jeong, S.H.; Ahn, M.S.; Lee, H.W.; Kang, S.Y.; Choi, J.H.; Jin, U.R.; Park, J.S. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. J Korean Med Sci. 2014, 29, 1493–1500. [Google Scholar] [CrossRef]
- Irvine, K.M.; Ratnasekera, I.; Powell, E.E.; Hume, D.A. Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Moretto, F.; Catherine, F.X.; Esteve, C.; Blot, M.; Piroth, L. Isolated anti-HBc: significance and management. J Clin Med. 2020, 9, 202. [Google Scholar] [CrossRef]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; García-Buey, L.; Arranz, R.; Blas, C.; Pinilla, I.; Khorrami, S.; Acevedo, A.; Borque, M.J.; Pajares, J.M.; Fernández-Rañada, J.M.; et al. The prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Eur J Gastroenterol Hepatol. 2004, 16, 135–138. [Google Scholar] [CrossRef]
- Dal Maso, L.; Franceschi, S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006, 15, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, F.; Peng, L.; Shen, L.; Zhao, P.; Ni, B.; Hou, J.; Huang, H. Distinct clinical features and prognostic factors of hepatitis C virus-associated non-Hodgkin’s lymphoma: a systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 524. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Mele, A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011, 117, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Peveling-Oberhag, J.; Arcaini, L.; Hansmann, M.L.; Zeuzem, S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013, 59, 169–177. [Google Scholar] [CrossRef]
- Ferri, C.; Sebastiani, M.; Giuggioli, D.; Colaci, M.; Fallahi, P.; Piluso, A.; Antonelli, A.; Zignego, A.L. Hepatitis C virus syndrome: a constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World J Hepatol. 2015, 7, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Vokes, E.E.; Weichselbaum, R.R.; Lippman, S.M.; Hong, W.K. Head and neck cancer. N Engl J Med. 1993, 328, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Tsurumi, H.; Goto, H.; Shimomura, Y.I.; Kasahara, S.; Hara, T.; Yasuda, I.; Shimizu, M.; Murakami, N.; Yoshikawa, T.; et al. Serum soluble interleukin-2 receptor (sIL-2R) level is associated with the outcome of patients with diffuse large B cell lymphoma treated with R-CHOP regimens. Ann Hematol. 2012, 91, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Umino, K.; Fujiwara, S.I.; Minakata, D.; Yamamoto, C.; Meguro, A.; Matsuyama, T.; Sato, K.; Ohmine, K.; Izumi, T.; Muroi, K.; et al. Prognostic impact of serum soluble interleukin-2 receptor level at diagnosis in elderly patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Lymphoma. 2019, 60, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Nam, J.; Chung, J.S.; Jeon, B.E.; Lee, J.H.; Jo, J.C.; Kim, S.W.; Shin, H.J. Predictive parameters of febrile neutropenia and clinical significance of G-CSF receptor signaling pathway in the development of neutropenia during R-CHOP chemotherapy with prophylactic pegfilgrastim in patients with diffuse large B-cell lymphoma. Cancer Res Treat. 2022, 54, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.L.; Vlekova, V.; Le Deist, F.; Blanche, S.; Donadieu, J.; De Saint-Basile, G.; Durandy, A.; Griscelli, C.; Fischer, A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993, 123, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Savino, W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002, 56 Supplement 3, S46–S49. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Merayo-Chalico, J.; Gómez-Martín, D.; Piñeirúa-Menéndez, A.; Santana-de Anda, K.; Alcocer-Varela, J. Lymphopenia as risk factor for development of severe infections in patients with systemic lupus erythematosus: a case-control study. Q J M. 2013, 106, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, D.S.; Esmadi, M.; Steinmann, W.C. Idiopathic CD4 Lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013, 3, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Helby, J.; Nordestgaard, B.G.; Birgens, H.; Bojesen, S.E. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLOS Med. 2018, 15, e1002685. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Borg, C.; Bachelot, T.; Sebban, C.; Philip, I.; Clapisson, G.; Le Cesne, A.; Biron, P.; Chauvin, F.; Blay, J.Y.; et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003, 88, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Borg, C.; Ray-Coquard, I.; Philip, I.; Clapisson, G.; Bendriss-Vermare, N.; Menetrier-Caux, C.; Sebban, C.; Biron, P.; Blay, J.Y. CD4 Lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer. 2004, 101, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Nordvig, J.; Aagaard, T.; Daugaard, G.; Brown, P.; Sengeløv, H.; Lundgren, J.; Helleberg, M. Febrile neutropenia and long-term risk of infection among patients treated with chemotherapy for malignant diseases. Open Forum Infect Dis. 2018, 5, ofy255. [Google Scholar] [CrossRef] [PubMed]
- Case, D.C., Jr.; Desch, C.E.; Kalman, L.A.; Vongkovit, P.; Mena, R.R.; Fridman, M.; Allen, B. Community-based trial of R-CHOP and maintenance rituximab for intermediate- or high-grade non-Hodgkin lymphoma with first-cycle filgrastim for older patients. Clin Lymphoma Myeloma. 2007, 7, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Lathia, N.; Isogai, P.K.; De Angelis, C.; Smith, T.J.; Cheung, M.; Mittmann, N.; Hoch, J.S.; Walker, S. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against febrile neutropenia in lymphoma patients. J Natl Cancer Inst. 2013, 105, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Salar, A.; Haioun, C.; Rossi, F.G.; Duehrsen, U.; Pettengell, R.; Johnsen, H.E.; Jaeger, U.; Verhoef, G.; Schwenkglenks, M.; Bacon, P.; et al. The need for improved neutropenia risk assessment in DLBCL patients receiving R-CHOP-21: findings from clinical practice. Leuk Res. 2012, 36, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Pettengell, R.; Bosly, A.; Szucs, T.D.; Jackisch, C.; Leonard, R.; Paridaens, R.; Constenla, M.; Schwenkglenks, M.; Impact of Neutropenia in Chemotherapy-European Study Group (INC-EU). Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European neutropenia Study. Br J Haematol. 2009, 144, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
| Total DLBCL | R-CHOP-like | Salvage therapy | |
| n = 246 | n = 194 | n = 52 | |
| Age, years | 72.0 (63.0–79.0) | 73.0 (63.0–80.0) | 69.5 (61.5–75.5) |
| Sex | |||
| Female | 146 (59%) | 109 (56%) | 37 (71%) |
| Male | 100 (41%) | 85 (44%) | 15 (29%) |
| Comorbidity | |||
| Diabetes | 36 (15%) | 32 (16%) | 4 (8%) |
| Chronic kidney disease | 14 (6%) | 10 (5%) | 4 (8%) |
| Cardiac disease | 38 (15%) | 33 (17%) | 5 (10%) |
| Pulmonary disease | 5 (2%) | 4 (2%) | 1 (2%) |
| Malignancy | 31 (13%) | 24 (12%) | 7 (13%) |
| Ann Arbor stage | |||
| I | 39 (16%) | 38 (20%) | 1 (2%) |
| II | 55 (22%) | 48 (25%) | 7 (13%) |
| III | 44 (18%) | 34 (18%) | 10 (19%) |
| IV | 108 (44%) | 74 (38%) | 34 (65%) |
| Bone marrow infiltration | |||
| Yes | 44 (18%) | 30 (15%) | 14 (27%) |
| No | 202 (82%) | 164 (85%) | 38 (73%) |
| Extranodal involvement | |||
| Yes | 140 (57%) | 102 (53%) | 38 (73%) |
| No | 106 (43%) | 92 (47%) | 14 (27%) |
| Baseline laboratory data | |||
| eGFR < 60 mL/min/1.73 m2 | 61 (25%) | 52 (27%) | 9 (17%) |
| T-Bil > 1.0 g/dL | 35 (15%) | 27 (14%) | 8 (16%) |
| Albumin < 3.5 g/dL | 87 (36%) | 71 (37%) | 16 (31%) |
| LD > 222 IU/L | 147 (60%) | 109 (57%) | 38 (73%) |
| CRP > 10 mg/dL | 13 (6%) | 10 (6%) | 3 (6%) |
| sIL2R > 2000 U/mL | 97 (40%) | 72 (37%) | 25 (48%) |
| WBC < 3.5 × 109 /L | 29 (12%) | 22 (11%) | 7 (13%) |
| ANC < 1.5 × 109 /L | 9 (4%) | 8 (4%) | 1 (2%) |
| ALC < 0.7 × 109 /L | 54 (22%) | 42 (22%) | 12 (23%) |
| Hemoglobin < 12.0 g/dL | 114 (46%) | 92 (47%) | 22 (42%) |
| Platelet count < 100 × 109 /L | 32 (13%) | 24 (12%) | 8 (15%) |
| Hepatitis viral status | |||
| Viral hepatitis | 50 (20%) | 40 (21%) | 10 (19%) |
| HCV | 15 (6%) | 13 (7%) | 2 (4%) |
| HBV | 43 (17%) | 33 (17%) | 10 (19%) |
| HBsAg + | 5 (2%) | 3 (2%) | 2 (4%) |
| anti-HBs + | 27 (11%) | 21 (11%) | 6 (12%) |
| anti-HBc + | 34 (14%) | 28 (14%) | 6 (12%) |
| Developed FN during the first therapy cycle |
Did not develop FN during the first therapy cycle |
p-value | |
| n = 37 | n = 157 | ||
| Age ≥ 65 years | 26 (70%) | 112 (71%) | 0.90 |
| Sex: male | 21 (57%) | 88 (56%) | 0.94 |
| Comorbidity | |||
| Diabetes | 4 (11%) | 28 (18%) | 0.30 |
| Chronic kidney disease | 2 (5%) | 8 (5%) | 0.94 |
| Cardiac disease | 7 (19%) | 26 (17%) | 0.73 |
| Pulmonary disease | 2 (5%) | 2 (1%) | 0.11 |
| Malignancy | 6 (16%) | 18 (11%) | 0.43 |
| Baseline laboratory data | |||
| WBC < 3.5 × 109 /L | 5 (14%) | 17 (11%) | 0.64 |
| Hemoglobin < 12.0 g/dL | 20 (54%) | 72 (46%) | 0.37 |
| Platelet < 100 × 109 /L | 11 (30%) | 13 (8%) | < 0.001 |
| ANC < 1.5 × 109 /L | 2 (5%) | 6 (4%) | 0.67 |
| ALC < 0.7 × 109 /L | 20 (54%) | 22 (14%) | < 0.001 |
| T-Bil > 1.0 g/dL | 6 (16%) | 21 (14%) | 0.73 |
| Albumin < 3.5 g/dL | 22 (63%) | 49 (31%) | < 0.001 |
| LD > 222 IU/L | 27 (75%) | 82 (53%) | 0.014 |
| CRP > 10 mg/dL | 5 (14%) | 5 (4%) | 0.02 |
| eGFR < 60 mL/min/1.73 m2 |
13 (35%) | 39 (25%) | 0.2 |
| sIL2R > 2000 U/mL | 26 (70%) | 46 (29%) | < 0.001 |
| Ann Arbor stage: Advanced (III–IV) |
30 (83%) | 78 (50%) | < 0.001 |
| Extranodal involvement | 27 (73%) | 75 (48%) | 0.006 |
| Bone marrow infiltration | 12 (32%) | 18 (11%) | 0.002 |
| Hepatitis viral status | |||
| Viral hepatitis | 16 (43%) | 24 (15%) | < 0.001 |
| HCV | 8 (22%) | 5 (3%) | < 0.001 |
| HBV | 11 (30%) | 22 (14%) | 0.022 |
| HBsAg + | 1 (3%) | 2 (1%) | 0.53 |
| anti-HBs + | 6 (16%) | 15 (10%) | 0.24 |
| anti-HBc + | 11 (30%) | 17 (11%) | 0.003 |
| n = 191 | ||||
| Odds ratio [95% CI] | β coefficient [95% CI] | Score | p-value | |
| ALC < 0.7 × 109 /L | 6.32 [2.51–15.92] | 1.84 [0.92–2.77] | 2 | < 0.001 |
| sIL2R > 2000 U/mL | 3.05 [1.21–7.67] | 1.12 [0.19–2.04] | 1 | 0.018 |
| Extranodal involvement | 2.94 [1.08–7.99] | 1.08 [0.08–2.08] | 1 | 0.034 |
| Viral hepatitis | 4.85 [1.85–12.74] | 1.58 [0.61–2.54] | 1 | 0.001 |
| n = 191 | |||
| Logistic regression β coefficient [95% CI] |
Bootstrapped β coefficient [95% CI] |
Score | |
| ALC < 0.7 × 109 /L | 1.84 [0.92–2.77] | 1.97 [0.81–2.88] | 2 |
| sIL2R > 2000 U/mL | 1.12 [0.19–2.04] | 1.17 [0.09–2.14] | 1 |
| Extranodal involvement | 1.08 [0.08–2.08] | 1.18 [0.02–2.14] | 1 |
| Viral hepatitis | 1.58 [0.61–2.54] | 1.68 [0.55–2.61] | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





