1. Introduction
Endothelial dysfunction (ED) corresponds to a decreased release of vasodilator factor by endothelial cells, including the nitric oxide (NO) and is considered a predictor for the development of cardiovascular diseases1, besides being present in its progression2,3. In addition, ED occurs with aging even in healthy adults4,5. The blood vessels walls become thicker and stiffer with age and occurs a deterioration in certain processes such as regulation of vascular tonus and loss of endothelium-dependent factors that induces vasodilation, including NO3,6.
Some studies have shown that vascular relaxation in aorta from renal hypertensive rats is compromised due to many factors, among them is the increase in the generation of superoxide anion (O2•-)7-9. The superoxide anions at high concentrations react with NO, decreasing their availability, and leads to the formation of peroxonitrite, a potent oxidant10. In this context, a compound with antioxidant properties that removes superoxide anions would be a good alternative to improve endothelial function.
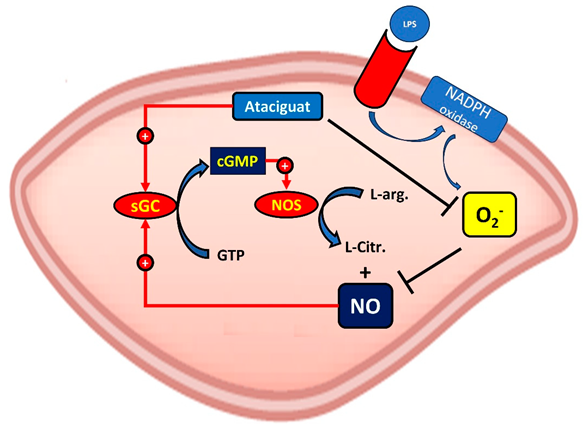
Direct activation of soluble guanylate cyclase (sGC) has been considered as a promising approach for various cardiovascular disorders associated with endothelial dysfunction. Ataciguat (HMR 1766) bind to sGC when the iron is in the ferrous heme (Fe2+), ferric heme (Fe3+) state or even without this grouping11, 12. This compound is being proposed for the treatment of cardiovascular diseases associated with oxidative stress and pulmonary hypertension13. Since the first publication in 2004, only a few studies have been published investigating the effects of HMR-1766. Among them, in a rat model with congestive heart failure, chronic treatment with ataciguat induced improvement of vascular function, NO sensitivity and reduced platelet activation13.
In this context, the aim of this study is to investigate whether the sGC activator ataciguat can revert the endothelial dysfunction induced by angiotensin II by superoxide anion inactivation.
2. Materials and Methods
2.1. Experimental animals
Male wistar rats were used weighing 180- 200 g. Animals were maintained on a light-dark cycle with free access to both food (standard rat chow) and water. Renovascular hypertension was induced in rats using the 2K-1C model proposed by Goldblatt et al. (1934), for small animals, where only one renal artery is constricted to reduce chronic renal perfusion. Animals were anesthetized with tribromoethanol (2.5 mg/kg, i.p.) and after a midline laparotomy a silver clip with an internal diameter of 0.20 mm was placed around the left renal artery. Normotensive two-kidney rats (2K) were only submitted to laparotomy. The systolic blood pressure (SBP) was measured by an indirect tail-cuff method (MLT125R pulse transducer/pressure cuff coupled to the PowerLab 4/S analog-to-digital converter; AD Instruments Pty Ltd., Castle Hill, Australia) weekly in non-anesthetized animals. Rats were considered hypertensive when the SBP was higher than 160 mmHg, six weeks after surgery. All procedures were in accordance with the Animal Care and Use Committee of the Federal University of São Carlos, and was approved by this committee (CEUA nº 1626101216).
2.1. Vascular Reactivity Studies
Male Wistar rats (180 - 200 g), hypertensive and normotensive, were anesthetized with isoflurane and after euthanized by decapitation and the thoracic aortas were isolated. Aortic rings, 3 mm in length, were placed in bath chambers (5 mL) for isolated organs containing Krebs solution at 37˚C, continuously bubbled with 95% O2 and 5% CO2, pH 7.4, in an isometric myograph (Mulvany-Halpern-model 610 DMT-USA, Marietta, GA) and recorded by a PowerLab8/SP data acquisi tion system (ADInstruments Pty Ltd., Colorado Springs, CO).
The aortic rings were submitted to a tension of 1.5 g, which was readjusted every 15 min throughout a 60-min equilibration period before the addition of the given drug. Endothelial integrity was assessed by the degree of relaxation induced by 1 μM of acetylcholine after contraction of the aortic ring by phenylephrine (0.1 μM). The ring was discarded when the acetylcholine relaxation was less than 60% in 2K-1C and 80% in 2K rat aortas. The aortic rings of 2K and 2K-1C were treated for 30 min with ataciguat (at concentration of 0.1 mM) or PBS (control). After incubation, the aortic rings were washed 3 times to remove the drug, and contracted with phenylephrine (0.1 μM) and concentration-effect curves were made for acetylcholine. Each experiment was performed on rings prepared from different animal.
2.3. Cell culture
Immortalized human umbilical endothelial cells (HUVEC) were grown in DMEM (Inlab) supplemented with 10% of fetal calf serum, antibiotics and antimycotics. Cultures were maintained at 37±2 ºC in 5% CO2 atmosphere. The cells in confluence of 80 to 90% were trypsinised, centrifuged at 1200 rpm during 5 minutes, and plated in 96-well plates (TPP).
2.4. Nitric Oxide (NO) Measurements
HUVEC were placed in 96 well plates at the concentration of 5x10⁴ cells per well. The plates were incubated for 24 hours in humidified incubator containing 5% CO2, 37°C. After 24 hours, the treatment was removed and the plates were gently washed with Phosphate Buffer Saline (PBS). The detection of intracellular NO was performed by incubation with selective fluorescent probe 4,5-diaminofluorescein (DAF-2T - 10 μM) during 30 minutes, to react with dinitrogen trioxide (N2O3) (oxidation product of NO) and produces the fluorescent compound DAF-2T14. The reading was held in SpectraMax GeminiXS fluorometer (Molecular Devices) at 435 nm excitation and 538 nm emission wavelength pair, respectively.
2.4. Measurement of general reactive oxygen species (ROS) production
HUVEC were placed in 96 well plates at the concentration of 5x10⁴ cells per well. The plates were incubated for 24 hours in humidified incubator containing 5% CO2, 37°C. After 24 hours, the treatment was removed and the plates were gently washed with Phosphate Buffer Saline (PBS). Cells were treated with atacicguat 0.1; 1 or 10 µM during 30 min followed by treatment with angiotensin II (Ang II) 0.1 μM for 30 min. The detection of intracellular superoxide radical (O2•-) was performed with 50 μM Dihydroethidium (DHE) added to the samples and the reads were conducted after 20 min. The increase in fluorescence intensity was monitored with a fluorescence microplate reader (SpectraMaxGeminiXS, Molecular Devices) at 510 nm and 595 excitation wavelength pair.
Detection of ROS was also performed in the presence of hydroxocobalamin (Hcb), a nitric oxide scavenger, to evaluate if the reduction of superoxide anions is not due to reaction with NO forming peroxynitrite. In addition, we have performed this experiment in the presence of sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), to verify if ataciguat effect on ROS concentration is due to intracellular cGMP accumulation. Cells were treated with ataciguat 10 µM, ODQ, and Hcb during 30 min followed by treatment with Ang II 0.1 μM for 30 min. The detection of intracellular superoxide radical (O2•-) was performed with 50 μM Dihydroethidium (DHE) added to the samples and the reads were conducted after 20 min. The increase in fluorescence intensity was monitored with a fluorescence microplate reader (SpectraMaxGeminiXS, Molecular Devices) at 510 nm and 595 excitation wavelength pair.
2.5. Measurement of superoxide anion production
This experiment was performed using the lucigenin probe (5µM), which is specific for the detection of superoxide anion. HUVEC were seeded in 96 well plates at a concentration of 5× 104 cells per well and maintained at 37 °C in humidified incubator containing 5% CO2 per 24 h. Cells were treated with ataciguat 0.1; 1 or 10 µM and lucigenin during 30 min followed by treatment with Ang II 0.1 μM for 30 min. The increase in fluorescence intensity was monitored with a fluorescence microplate reader (SpectraMaxGeminiXS, Molecular Devices) at 510 nm and 595 excitation wavelength pair.
2.6. Statistical analysis
Statistical analysis of the results was performed using GraphPad Prism version 3.0. Statistical significance was tested by ANOVA one way (post hoc test: Newman–Keuls). Data are expressed as mean ± S.E.M. In each set of experiments, n indicates the number of rats studied. Values of p < 0.05 were considered significant.
3. Results
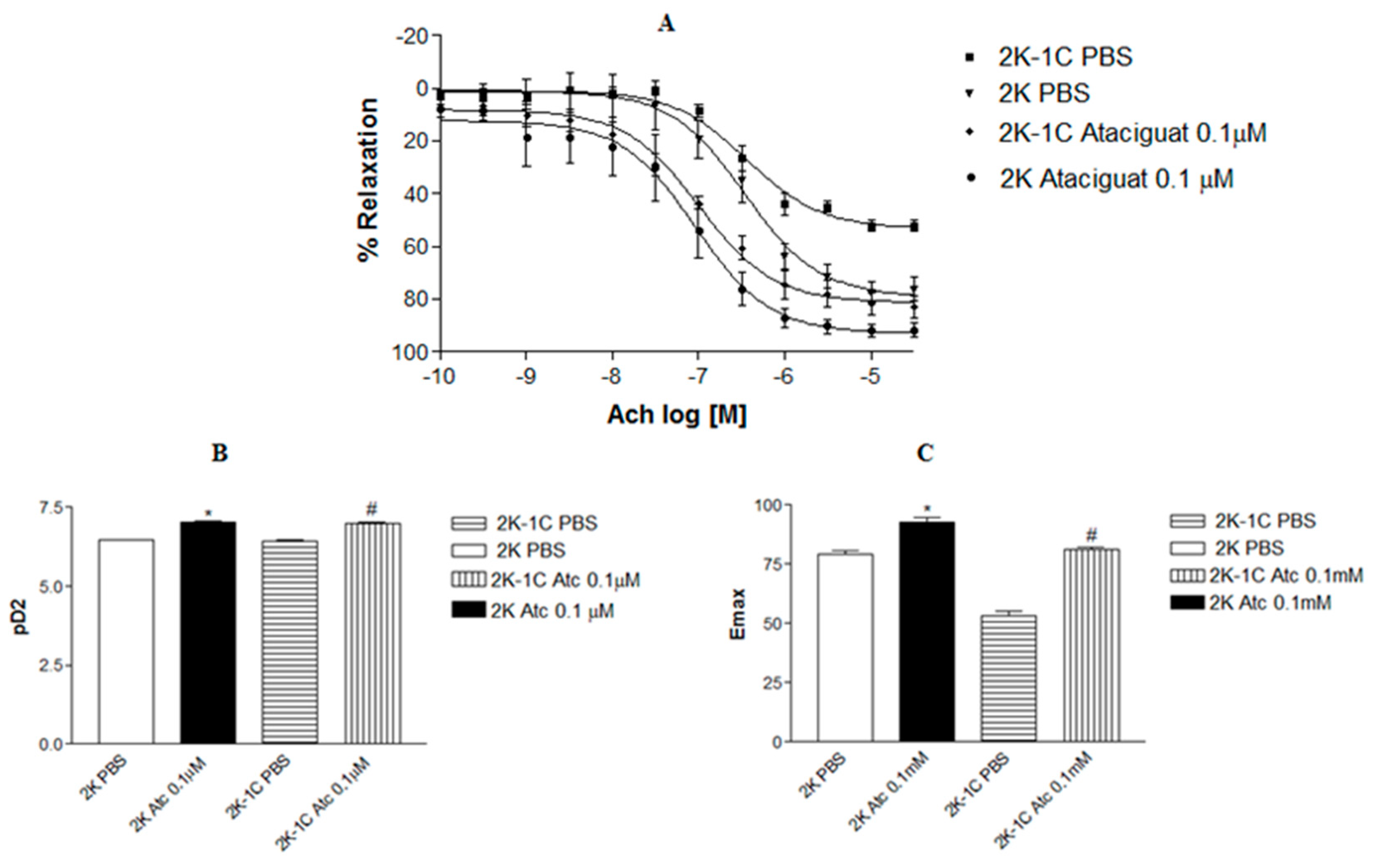
Treatment of aortic rings from normotensive and hypertensive rats with ataciguat improved endothelium-dependent relaxation induced by acetylcholine, as can be verified in
Figure 1A. The incubation of aortic rings with 0.1 µM ataciguat improved the endothelium-dependent relaxation induced by acetylcholine in 2K-1C (pD2: 6.99 ± 0.08, n=6) compared to 2K-1C aortic rings treated with PBS (pD2: 6.43 ± 0.07, n=6, p<0.05). A similar result was verified in aortic rings from normotensive rats. The treatment with ataciguat 0.1 µM in 2K (pD2: 7.04 ± 0.13, n=6) rats improved endothelium-dependent relaxation induced by acetylcholine compared to the 2K ring treated with PBS (pD2: 6.59 ± 0.07, n=6). In comparison with normotensive rats without treatment with ataciguat, the aortic rings from hypertensive rats treated with ataciguat were better.
Figure 1B presents the difference in the potency (pD2) of acetylcholine in inducing relaxation in aortas with and without ataciguat treatment. Also, the maximum relaxant effect (Emax) was improved by treatment at ataciguat in the 2K-1C aorta (Emax: 81.0±1.0; n=6), and 2K aorta (Emax: 92.98 ± 1.83; n=6), compared to control (Emax 2K-1C: 52.14 ± 2.16, n=6; and Emax 2K: 76.07 ± 4.35, n=6, p<0.05) as can be seen in
Figure 1C.
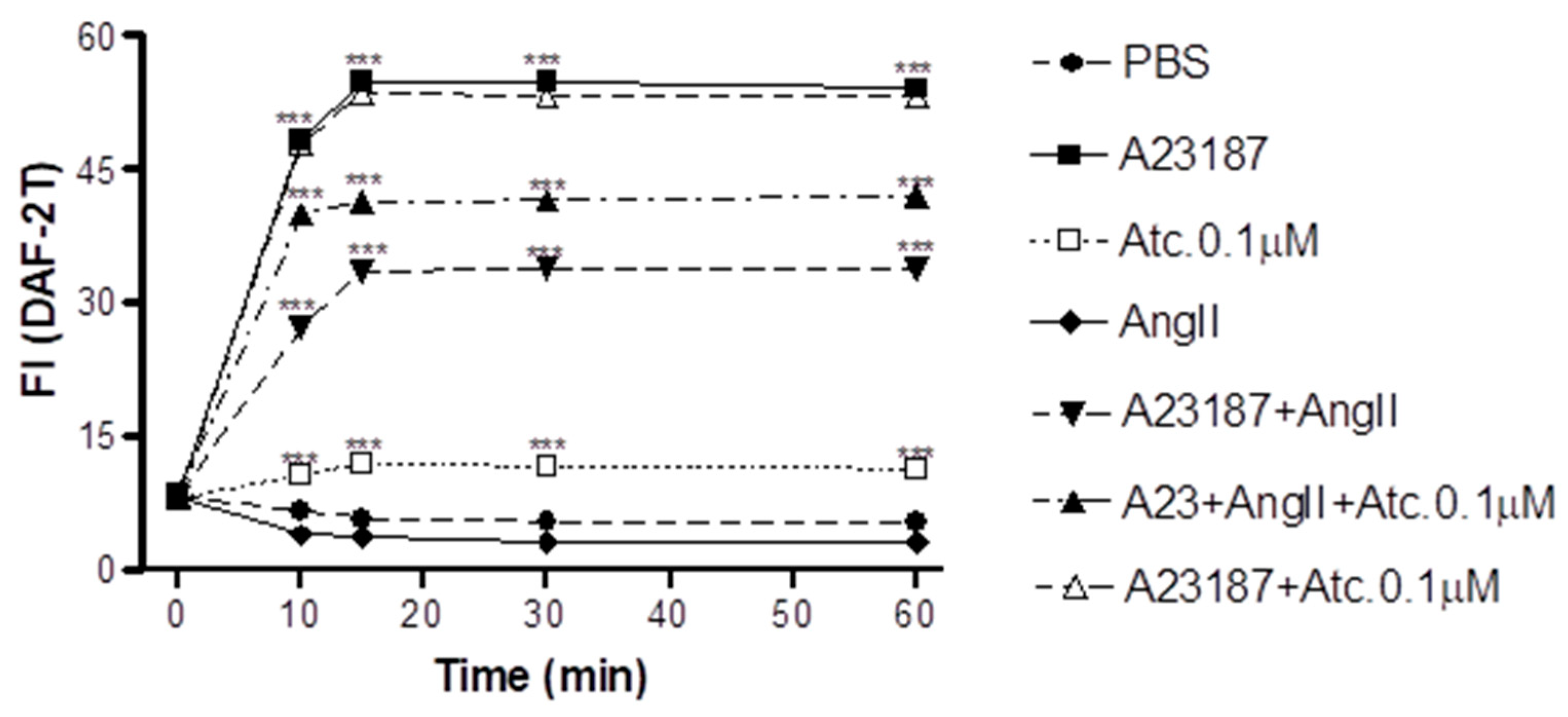
To verify if the improvement in aortic ring relaxation in hypertensive rats treated with ataciguat is induced by NO production, we performed the NO quantification by fluorescence intensity measurement (FI).
As can be verified in
Figure 2, our results indicate that ataciguat induces NO production in endothelial cells (ataciguat 60 min: 11.34±0.34 FI: n=5) compared to control (PBS 60 min: 5.29±0.36 FI; n=5, p<0.001). After treatment, the NO production reached a stabilization after 15 minutes for all performed protocols, including ataciguat (15 min: 11.94±0.33 FI; n=5; 30 min: 11.64±0.56 FI, n=5 and 60 min: 11.34±0,34 FI, n=6). Ataciguat induced increase on NO concentration (ataciguar 0.1µM 60: 11.34 ± 0.34 FI, n=5), compared to control (PBS 60: 5.29± 0.36 FI, n=5, p<0.001). As a positive control, we have used A23187 to induces NO production (A23187 10 min: 48.31±0.61, n= 5; 15 min: 54.75±0.54, n=5; 30 min: 54.75±0.55, n=5 and 60 min: 54.08±0.52, n=5, p<0.001. To induces the NO degradation by superoxide, we have performed experiments in the presence of Angiotensin II (Ang. II), and we have verified that Ang II decreased the NO production induced by A23187 (Ang. II + A23187: 10 min: 27.27±0.67, n=5; 15 min: 33.54±0.65, n=5; 30 min: 33.80±0.52, n=5 and 60 min: 33.93±0.68, n=5), compared to A23178 (p<0.001). Ataciguat avoided part of NO degradation induced by Angiotensin II (A23187 + Ang. II + Ataciguat: 10 min: 39.99±0.52; 15 min: 41.32±0.45; 30 min: 41.51±0.43 and 60 min: 41.98±0.44), compared to A23187 + Angiotensin II (p<0.001). No additive production of NO was verified to A23187 in the presence of ataciguat (A23187 + Atciguat: 10 min: 47.90±0.66; 15 min: 53.56±0.44; 30 min: 53.23±0.46 and 60 min 53.12±0.44, p<0.001).
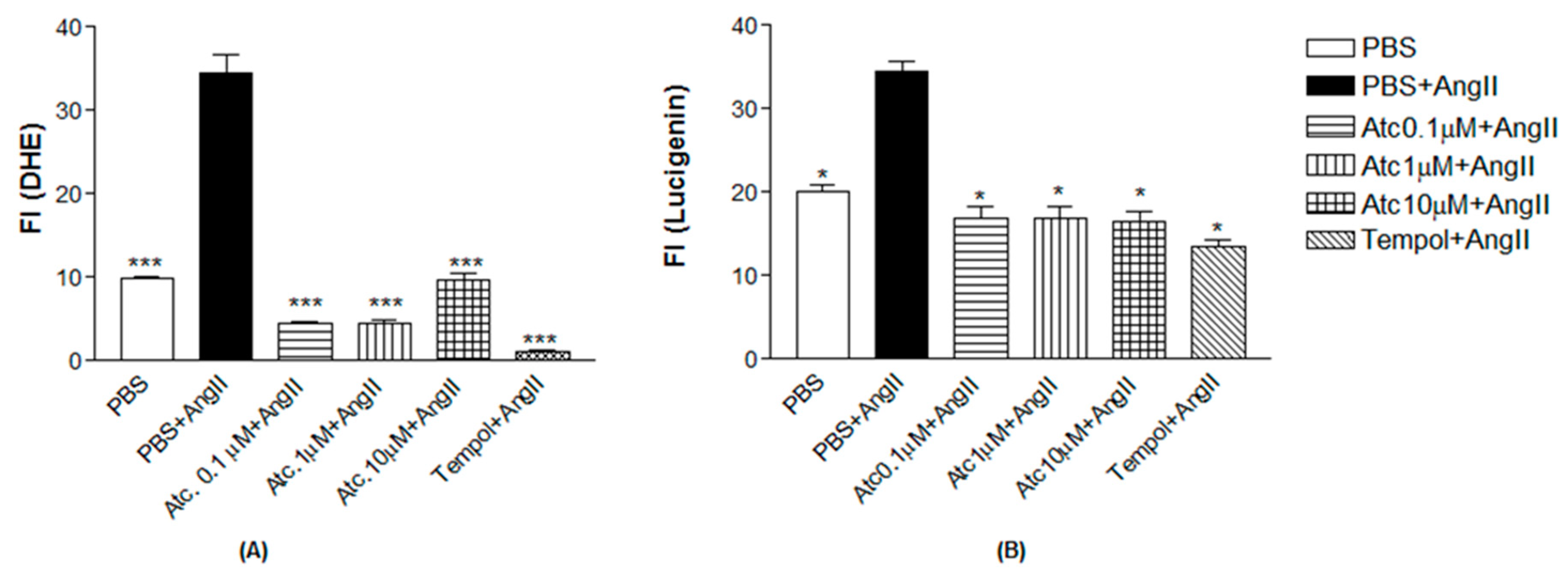
To verify if the improvement in aortic rings relaxation in hypertensive rats treated with ataciguat is due to decrease reactive oxygen species, we performed the ROS quantification by fluorescence intensity measurement (FI), by using a non-selective ROS probe (DHE) and a selective superoxide probe (lucigenin).
As a positive control to induce superoxide production, we have used Angiotensin II. As can be seen in
Figure 3, Ang II-induced ROS and superoxide production in endothelial cells (DHE: 34.51±2.20; Lucigenin: 34.47±1.22), compared to control (DHE: 9.97±0.19; Lucigenin: 20.10±0.70, n=5, p< 0.05). The treatment with ataciguat decreased ROS and superoxide formation, induced by Angiotensin II, verified to all concentration verified to ataciguat, including 0.1 uM (DHE: 4.53±0.20; Lucigenin: 16.97±1.24, n=5, p<0.05), 1 uM (DHE: 4.59±0.24; Lucigenin: 16.97±1.24, n=5, p< 0.05) and 10 uM (DHE: 9.60±0.93; Lucigenin: 16.58±1.03, n=5, p< 0.05). We have used tempol as an antioxidant positive control. The treatment with tempol decreased ROS and superoxide formation, induced by Angiotensin II (DHE: 1.07±0.25; Lucigenin: 13.57±0.68), compared to Angiotensin II (DHE: 34.51±2.20); Lucigenin: 34.47±1.22, n=5, p< 0.05).
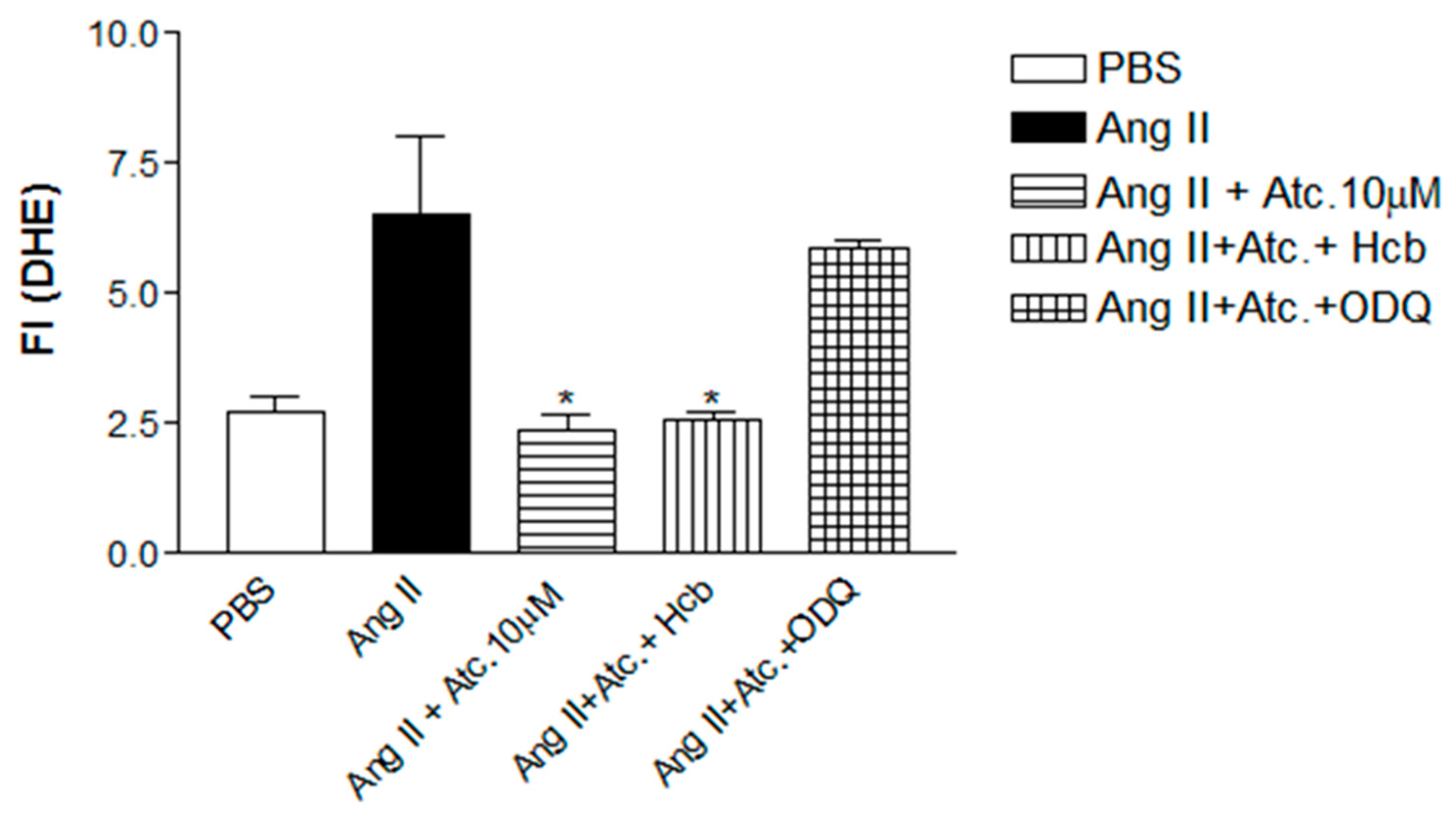
To investigate if the ataciguat effect on reducing ROS and superoxide is due to sGC activation and or NO formation, we have performed experiments in the presence of hydroxicobalamin (NO scavenger) or ODQ (sGC inhibitor). In the presence of hydroxicobalamin no alteration of the ataciguat effect was verified (Ang. II + Ataciguat + Hcb: 2.56±0.16, n=5, p< 0.05), indicating that the ROS decreasing induced by ataciguat is not due to NO production. However, the sGC inhibition (ODQ) abolished the ataciguat effect (Ang. II + Ataciguat + ODQ: 5.86±0.14, n=5, p< 0.05), indicating that the ataciguat effect is involved with sGC.
Figure 1.
Concentration-effect curves to acetylcholine (Ach) in the aortic rings with intact endothelium (to Ach), contracted with phenylephrine from 2K and 2K-1C rats. A. Values are means ± S.E.M. They are expressed as percentage of relaxation on preparations obtained from different animals 2K-1C and 2K.B. Bars represent the potency (pD2) of Acetylcholine obtained from concentration-effect curves of different 2K-1C and 2K animals. * indicates difference (p < 0.01) on pD2 from 2K Atc 0.1µM (n =6) vs. 2K PBS (n = 6). # indicates difference (p < 0.01) on pD2 from 2K-1C Atc 0.1µM (n =6) vs. 2K 1CPBS (n = 6). C. Bars represent the Maximum efect (Emax) of Acetylcholine obtained from concentration-effect curves of different 2K-1C and 2K animals. * indicates difference (p < 0.01) on Emax from 2K Atc 0.1µM (n =6) vs. 2K PBS (n = 6). # indicates difference (p < 0.01) on Emax from 2K-1C Atc 0.1µM (n =6) vs. 2K-1C PBS (n = 6).
Figure 1.
Concentration-effect curves to acetylcholine (Ach) in the aortic rings with intact endothelium (to Ach), contracted with phenylephrine from 2K and 2K-1C rats. A. Values are means ± S.E.M. They are expressed as percentage of relaxation on preparations obtained from different animals 2K-1C and 2K.B. Bars represent the potency (pD2) of Acetylcholine obtained from concentration-effect curves of different 2K-1C and 2K animals. * indicates difference (p < 0.01) on pD2 from 2K Atc 0.1µM (n =6) vs. 2K PBS (n = 6). # indicates difference (p < 0.01) on pD2 from 2K-1C Atc 0.1µM (n =6) vs. 2K 1CPBS (n = 6). C. Bars represent the Maximum efect (Emax) of Acetylcholine obtained from concentration-effect curves of different 2K-1C and 2K animals. * indicates difference (p < 0.01) on Emax from 2K Atc 0.1µM (n =6) vs. 2K PBS (n = 6). # indicates difference (p < 0.01) on Emax from 2K-1C Atc 0.1µM (n =6) vs. 2K-1C PBS (n = 6).
Figure 2.
Intracellular NO quantification in HUVEC - Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with PBS, A23187, Ataciguat and Aniotensina II *** Indicates statistical difference between A23187+ Ang II vs PBS; A23187+AngII+Atc 0.1µM vs PBS; Ataciguat 0.1 µM vs PBS; A23187 vs PBS (p <0.001).
Figure 2.
Intracellular NO quantification in HUVEC - Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with PBS, A23187, Ataciguat and Aniotensina II *** Indicates statistical difference between A23187+ Ang II vs PBS; A23187+AngII+Atc 0.1µM vs PBS; Ataciguat 0.1 µM vs PBS; A23187 vs PBS (p <0.001).
Figure 3.
A. Quantification of Reactivity Oxygen Species (ROS) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Tempol and Angiotensin II. *** Indicates difference between Ataciguat 0.1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II and Tempol + Angiotensin II vs PBS + Angiotensin II(p <0.001). B. Quantification of superoxide anions (O2-) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Tempol and Angiotensin II. *Indicates difference between PBS + Angiotensina II vs PBS; Ataciguat 0.1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II and Tempol + Angiotensin II vs PBS + Angiotensin II (p <0.05).
Figure 3.
A. Quantification of Reactivity Oxygen Species (ROS) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Tempol and Angiotensin II. *** Indicates difference between Ataciguat 0.1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II and Tempol + Angiotensin II vs PBS + Angiotensin II(p <0.001). B. Quantification of superoxide anions (O2-) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Tempol and Angiotensin II. *Indicates difference between PBS + Angiotensina II vs PBS; Ataciguat 0.1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 1 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II and Tempol + Angiotensin II vs PBS + Angiotensin II (p <0.05).
Figure 4.
Quantification of Reactivity Oxygen Species (ROS) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Hidroxycobalamin, ODQ and Angiotensin II. *Indicates difference between Ataciguat 10 µM + Angiotensin II vs Angiotensin II; Hoob + Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + ODQ + Angiotensin II vs PBS + Angiotensin II (p <0.05).
Figure 4.
Quantification of Reactivity Oxygen Species (ROS) in HUVEC. Values are means ± E.P.M fluorescence intensity obtained on HUVEC cells after 30 minutes of treatment with Ataciguat, Hidroxycobalamin, ODQ and Angiotensin II. *Indicates difference between Ataciguat 10 µM + Angiotensin II vs Angiotensin II; Hoob + Ataciguat 10 µM + Angiotensin II vs PBS + Angiotensin II; Ataciguat 10 µM + ODQ + Angiotensin II vs PBS + Angiotensin II (p <0.05).
4. Discussion
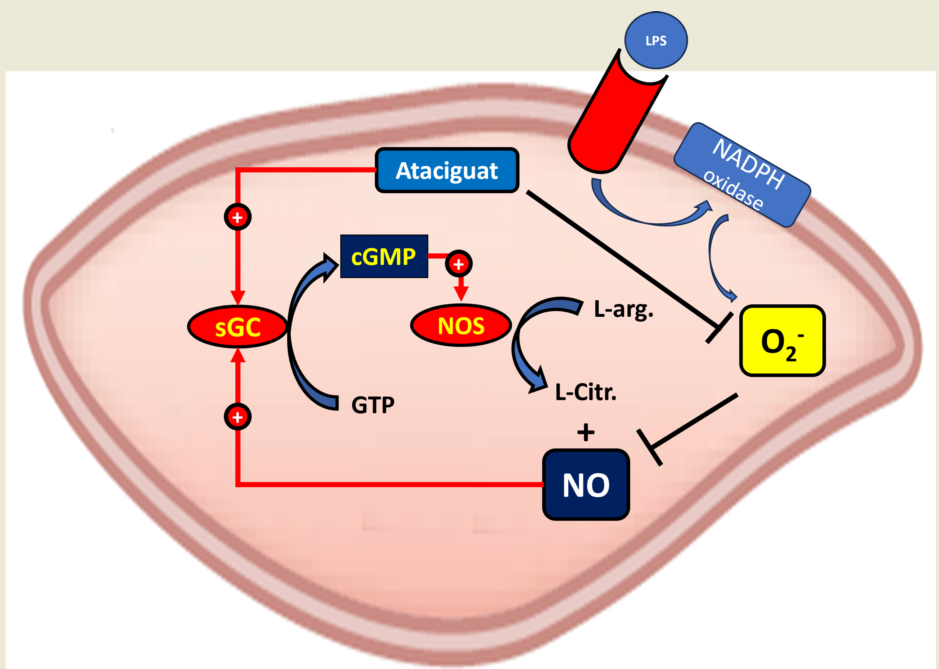
The main finding of this study was that ataciguat is able to improve the endothelial function in vessels with and without endothelial dysfunction by superoxide anion inactivation.
To avoid the direct vasodilation induced by ataciguat, we have incubated aortic rigs with a concentration that does not induce vasodilation, as we verified previously15. Low concentration of ataciguat was able to improve the endothelium dependent relaxation, in isolated aortic rings with and without endothelial dysfunction. In rats with cardiac heart failure, the chronic treatment with ataciguat improved the relaxation endothelium dependent (induced by acetylcholine) and non-endothelium dependent (induced by NO donor) in aortic rings13. These effects were attributed to lower concentration of ROS, in aortic rings, measured after chronic ataciguat treatment13. High concentration of superoxide anion is detected in aortic rings from rats with renovascular hypertension (2K-1C)7, contributing to endothelial dysfunction16,17. Thus, the improvement on relaxation endothelium dependent verified in this study, by in vitro treatment with ataciguat, can be attributed to reduction on superoxide anion in aortic rings.
Increased ROS formation leads to reduced NO bioavailability and is modulated by various endogenous neuro-human systems18. The NO requires the iron heme group of sGC is in the ferrous state (Fe2+) for activation. However, in endothelial dysfunction, the sGC iron is in its oxidized state (Fe3+) or even absent19. Thus, increased oxidative stress decreases expression and impairs NO-induced activation of heme-containing sGC, rendering vasodilatory therapy with NO donors or eNOS-stimulating compounds less effective.
To evaluate whether the improvement in endothelial function induced by ataciguat occurred due to a decrease in ROS formation, the experiment with human umbilical vein endothelial cells (HUVEC) was performed using the DHE probe. An increase in fluorescence intensity was observed when Angiotensin II was added, indicating an increase in ROS production. Our results are in agreement with previous publication that demonstrated increased ROS production in cells stimulated with Ang II17. Treatment with different concentrations of ataciguat (0.1, 1 and 10µM) decreased the intensity of the DHE fluorescence on Ang II stimulated cells, with results similar to the treatment with tempol, a SOD mimetic. This result shows that ataciguat decreases ROS concentration in HUVECS.
To evaluate whether the reactive oxygen species being formed by Ang II stimulation in HUVECS is the superoxide anion (O2•-) the lucigenin probe was used20. Our results showed that after the treatment of HUVECS with angiotensin II, there was an increase in lucigenin fluorescence, suggesting that ROS being formed is superoxide anion (O2•-). By using lucigenin, we have verified similar results obtained by DHE probe, indicating that ataciguat is inducing a decrease in superoxide anion.
Oxidative stress is characterized by increased ROS associated with decreased antioxidant capacity. ROS, mainly superoxide radical (O2•-), react with nitric oxide (NO), forming peroxynitrite (ONOO-), which is highly oxidant capable of causing the nitration of proteins and inducing lipid peroxidation21. To evaluate whether the decrease of ROS in endothelial cells by treatment with ataciguat does not occur due to reaction with NO, an experiment was performed using hydrocobalbalamin (Hcb), which is an NO scavenger. It was observed that in the presence of Hcb, there was a decrease in ROS production similar to ataciguat treatment, indicating that the ROS decreasing induced by ataciguat is not due to NO production.
In order to investigate if the ROS decrease induced by ataciguat is dependent of sGC stimulation, we have used a sGC inhibitor (ODQ). The ODQ abolished the ataciguat effect, indicating that ataciguat effect is depedent of sGC stimulation. The ODQ mechanism of sGC inhibition is due to oxidation of the sGC heme22. Considering that ataciguat is able to activate the sGC when the iron is in the ferrous heme (Fe2+), ferric heme (Fe3+) state or even without this grouping11, our results suggest that the reduction on superoxide anion induced by ataciguat is dependent of sGC ferrous heme (Fe2+). Also, this effect is not due sGC nitric oxide stimulation, once NO scavenger did not abolished this effect.
In addition, we performed experiments in HUVECs by stimulating the NO production (A23187) and superoxide anion generation (Angiotensin II). In these experiments, we have verified that ataciguat is able to avoid the NO degradation induced by angiotensin II, as well to induce the NO production by endothelial cells. Thus, these results are corroborating with ROS and superoxide detection, indicating that ataciguat is able to decrease ROS effect. These results are in accordance with literature, that shown that ataciguat is a good stimulus for the cGMP production in cells exposed to oxidative stress23.
5. Conclusion
Taken together, our results suggest that the activation of sGC by ataciguat can improve endothelial function by inactivation of superoxide anion, as suggested at central figure.
Acknowledgments
This work was supported by grants from São Paulo Research Foundation (FAPESP grants: 2012/24477-8, 2022/01093-1) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES).
References
- Shechter, M., Issachar, A., Marai, I., Koren-Morag, N., Freinark, D., Shahar, Y., et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int. J. Cardiol. 2009, 134, 52–58. [CrossRef] [PubMed]
- Chong, A.Y., Blann, A.D., Patel, J., Freestone, B., Hughes, E., Lip, G.Y. Endothelial dysfunction and damage in congestive heart failure relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation 2004, 110, 1794–1798.
- Gerhard, M., Roddy, M.A., Creager, S.J., Creager, M.A. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 1996, 27, 849–853. [CrossRef] [PubMed]
- Mazzoccoli, G., Fontana, A., Grilli, M., Dagostino, M.P., Copetti, M., Pellegrini, F., et al. Idiopathic deep venous thrombosis and arterial endothelial dysfunction in the elderly. Age 2012, 34, 751–760. [CrossRef] [PubMed]
- Yavuz, B., Yavuz, B., Sener, D.D., Cankurtaran, M., Halil, M., Ulger, Z., et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology 2008, 54, 153–156. [CrossRef]
- Zeiher, A.M., Drexler, H., Saurbier, B., Just, H. Endothelium-mediated coronary blood flow modulation in humans Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Invest. 1993, 92, 652–662. [CrossRef]
- Rodrigues, G.J., Lunardi, C.N., Lima, R.G., Santos, C.X., Laurindo, F.R., Da Silva, R.S.,Bendhack, L.M. Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide 2008, 18, 176–183. [CrossRef] [PubMed]
- Harrison, D.G., Ohara, Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: implications for impaired vasomotion. Am. J. Cardiol. 1995, 75, 75B–81B. [CrossRef]
- Heitzer, T., Wenze, U., Hink, U., Krollner, D., Skatchkov, M., Stahl, R.A., MacHarzina, R.,Bräsen, J.H., Meinertz, T., Münzel, T. Increased NAD(P)H oxidase mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase. C. Kidney Int. 1999, 55, 252–260.
- Ischiropoulos, H., Al-Mehdi, A.B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995, 364, 279–282. [CrossRef]
- Stasch, J.P; Schmidt, P; Alonso-Alija, C.; et al. NO- and haemindependent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002, 136, 773–783. [CrossRef] [PubMed]
- Surmeli, N.B.; Marletta, M.A. Insight into the rescue of oxidized soluble guanylate cyclase by the activator cinaciguat. Chembiochem 2012, 7, 977–981.
- Schäfer, A.; Fraccarollo, D.; Werner, L.; Bauersachs, J. Guanylyl cyclase activator ataciguat improves vascular function and reduces platelet activation in heart failure. Pharmacological Research 2010, 62, 432–8. [CrossRef] [PubMed]
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofuoresceins. FEBS Letters 1998, 42, 263–266.
- Martinelli AM, Rodrigues CNDS, Moraes TF, Rodrigues GJ. In Endothelial Cells, the Activation or Stimulation of Soluble Guanylyl Cyclase Induces the Nitric Oxide Production by a Mechanism Dependent of Nitric Oxide Synthase Activation. J Pharm Pharm Sci. 2018, 21, 38–45. [CrossRef] [PubMed]
- Oishi J.C, Buzinari, T.C, Pestana, C.R, De Moraes, T.F, Vatanabe, I.P, Wink, da Silva Jr. RS, Bendhack, L.M, Rodrigues, G.J. In vitro Treatment with cis-[Ru(H-dcbpy-)2(Cl)(NO)] Improves the Endothelial Function in Aortic Rings with Endothelial Dysfunction. J Pharm Pharm Sci. 2015, 18, 696–704. [CrossRef] [PubMed]
- Buzinari, TC, Oishi, JC, De Moraes, TF, Vatanabe, IP, Selistre-de-Araújo, HS, Pestana CR, Rodrigues GJ. Treatment with sodium nitroprusside improves the endothelial function in aortic rings with endothelial dysfunction. Eur J Pharm Sci. 2017, 105, 144–149. [CrossRef]
- Bauersachs J, Schäfer A. Endothelial dysfunction in heart failure: mechanisms and therapeutic approaches. Curr Vasc Pharmacol 2004, 2, 115–24. [CrossRef] [PubMed]
- Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, AK HS, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 2006, 116, 2552–61. [CrossRef]
- Trush, M.A. et al. Validation of Lucigenin (Bis-N-methylacridinium) as an Chemilumigenic Probe for Detecting Superoxide Anion Radical Production by Enzymatic and Cellular Systems. The Journal Biological Chemistry. 1998, 273, 2015–2023. [CrossRef]
- Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Microbiol Lett 1994, 341, 65–68. [CrossRef] [PubMed]
- Zhao, Y., Brandish, P.E., Di Valentin, M., Johanner, P.M., Babcock, G.T., Marletta, M.A. Inhibition of Soluble Guanylate Cyclase by ODQ. Biochemistry 2000, 39, 10848–10854.
- Zhou, Z., Pyriochou, A., Kotanidou, A., et al. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. American Journal of Physiology.Heart and Circulatory Physiology 2008, 295, 1763–1771. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).