Submitted:
01 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents and materials
2.2. Synthesis of compounds 7, 9, 11 and 12
2.3. Collection of C. reticulata 'Chachi' and C. reticulata 'Dahongpao'
2.4. Samples extraction
2.5. Preparation of standard solutions
2.6. LC-HR-MS analysis
2.7. LC-ESI-CID-MS analysis
3. Results
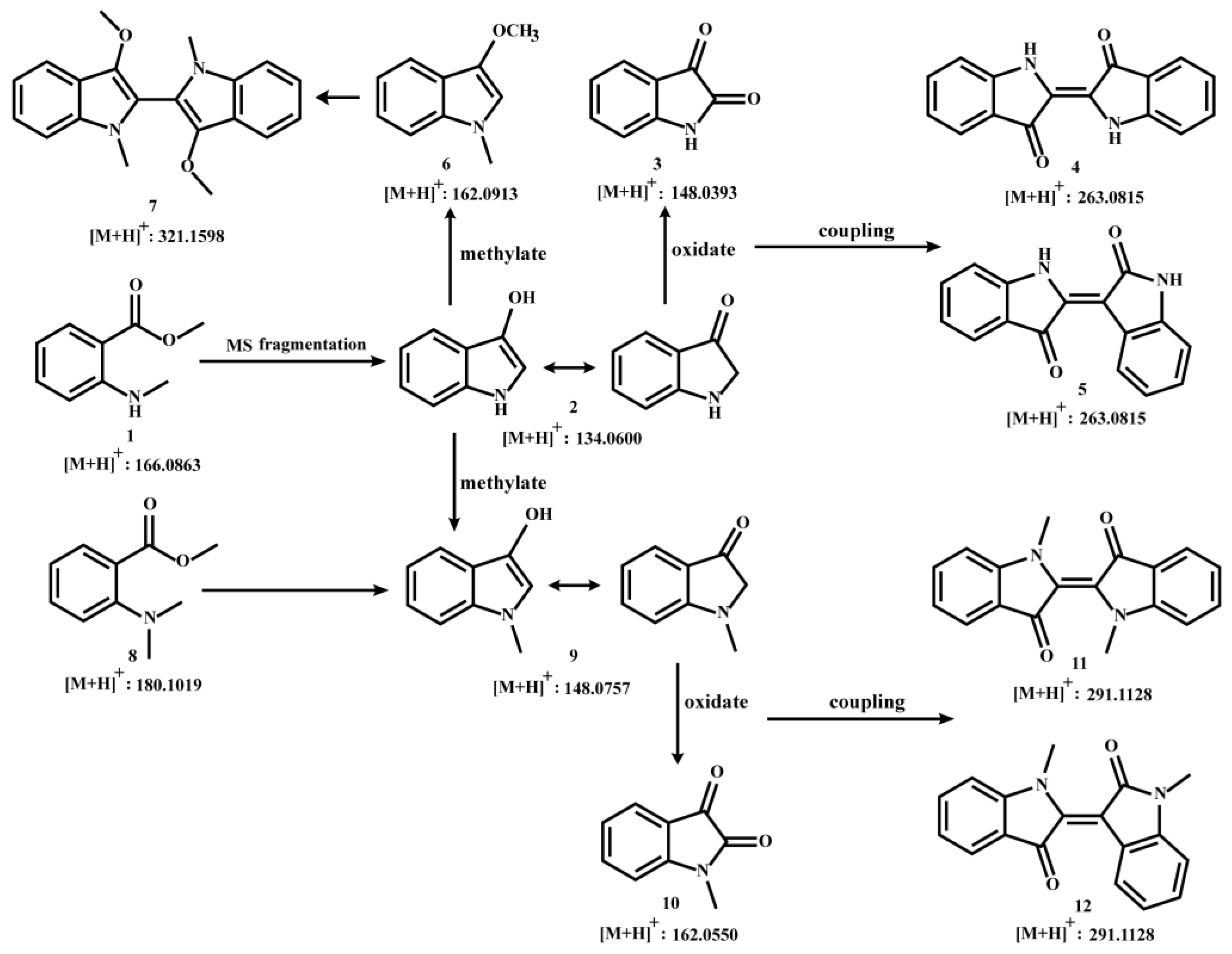
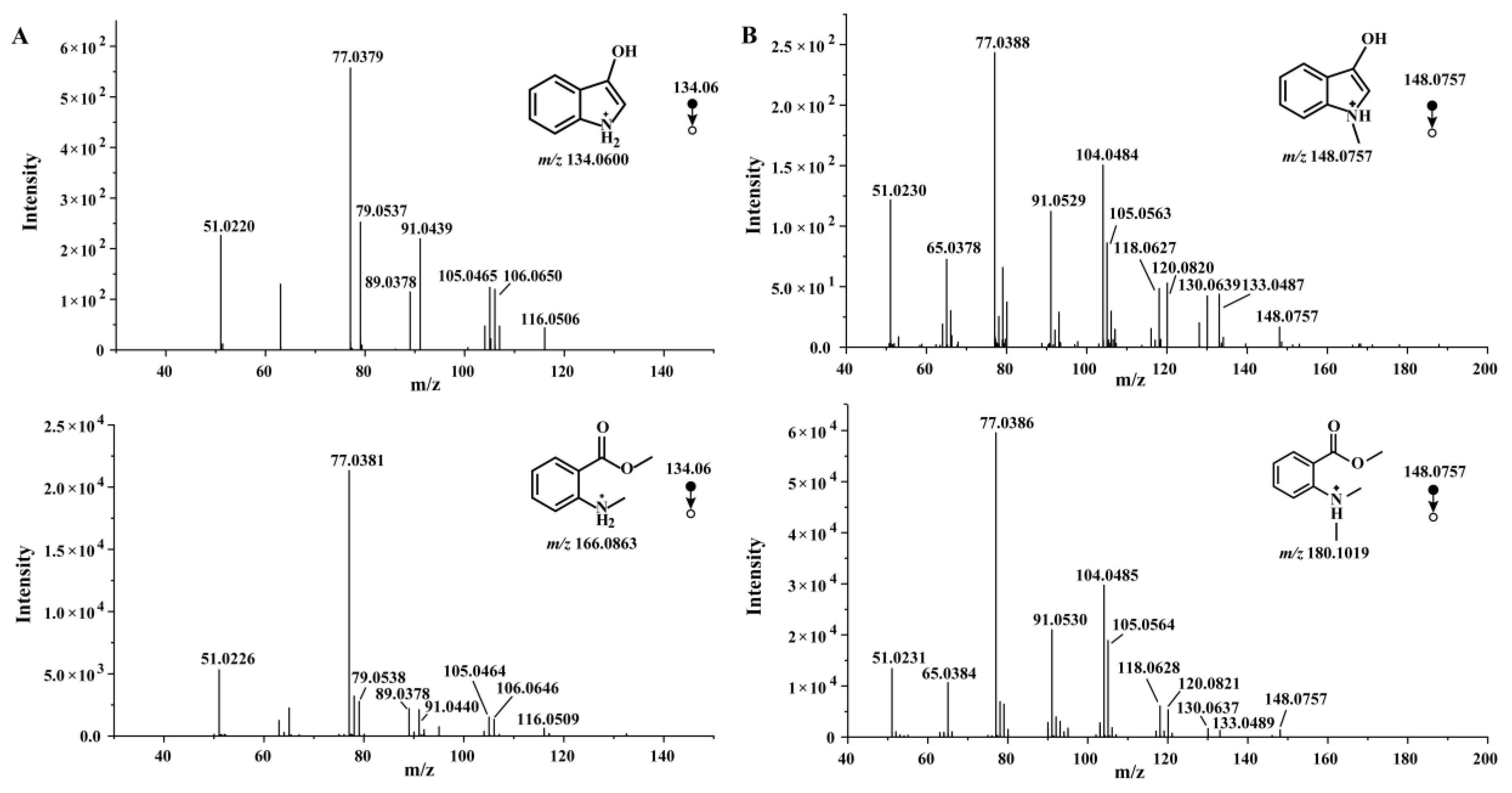
3.1. Prediction of indole derivatives based on rearrangement ions and metabolic pathways
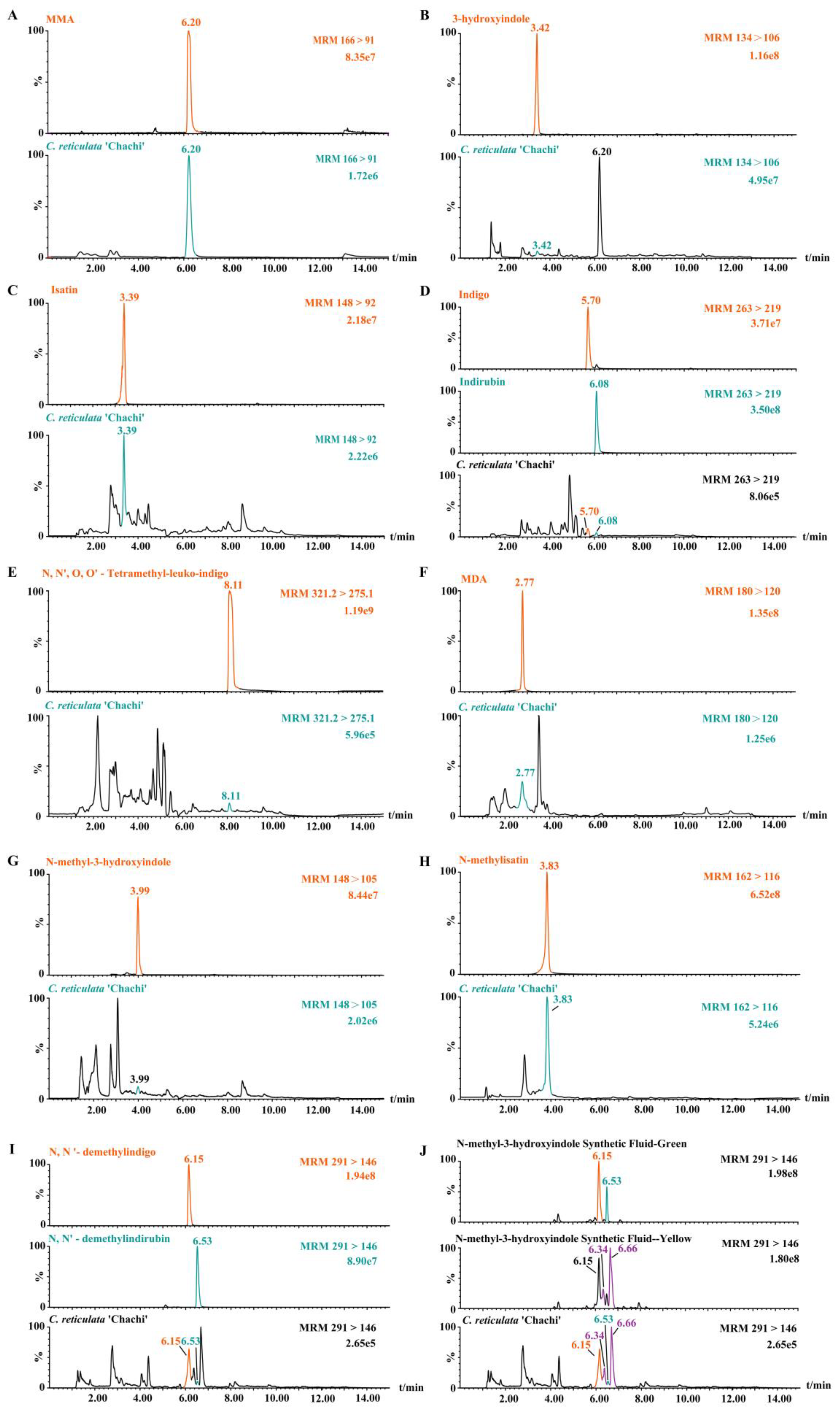
3.2. Feasibility verification of predicted compounds by the reference substance
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onorato, J.; Henion, J.D. Evaluation of Triterpene Glycoside Estrogenic Activity Using LC/MS and Immunoaffinity Extraction. Anal. Chem. 2001, 73, 4704–4710. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; de Figueiredo, M.; Strassel, O.; Boccard, J.; Vuilleumier, N.; Jaques, D.; Ponte, B.; Rudaz, S. Multitargeted Internal Calibration for the Quantification of Chronic Kidney Disease-Related Endogenous Metabolites Using Liquid Chromatography–Mass Spectrometry. Anal. Chem. 2023, 95, 13546–13554. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Zhang, M.; Hu, Y.; Ye, Z. Profiling of naturally occurring proanthocyanidins and other phenolic compounds in a diverse peach germplasm by LC-MS/MS. Food Chem. 2023, 403, 134471. [Google Scholar] [CrossRef]
- Xue, G.; Su, S.; Yan, P.; Shang, J.; Wang, J.; Yan, C.; Li, J.; Wang, Q.; Xiong, X.; Xu, H. Integrative analyses of widely targeted metabolomic profiling and derivatization-based LC-MS/MS reveals metabolic changes of Zingiberis Rhizoma and its processed products. Food Chem. 2022, 389, 133068. [Google Scholar] [CrossRef]
- Custodio-Mendoza, J.A.; Sendón, R.; de Quirós, A.R.-B.; Lorenzo, R.A.; Carro, A.M. Development of a QuEChERS method for simultaneous analysis of 3-Monochloropropane-1,2-diol monoesters and Glycidyl esters in edible oils and margarine by LC-APCI-MS/MS. Analytica Chimica Acta 2023, 1239, 340712. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics--the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Wang, L.; Xing, X.; Chen, L.; Yang, L.; Su, X.; Rabitz, H.; Lu, W.; Rabinowitz, J.D. Peak Annotation and Verification Engine for Untargeted LC–MS Metabolomics. Anal. Chem. 2019, 91, 1838–1846. [Google Scholar] [CrossRef]
- Qiu, J.; Li, T.; Zhu, Z.-J. Multi-dimensional characterization and identification of sterols in untargeted LC-MS analysis using all ion fragmentation technology. Analytica Chimica Acta 2021, 1142, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, H.-Y.; Wu, W.-S.; Hsu, J.-Y.; Chang, C.-W.; Lee, Y.-H.; Liao, P.-C. Identification of Xenobiotic Biotransformation Products Using Mass Spectrometry-Based Metabolomics Integrated with a Structural Elucidation Strategy by Assembling Fragment Signatures. Anal. Chem. 2023, 95, 14279–14287. [Google Scholar] [CrossRef]
- Dudley, E.; Yousef, M.; Wang, Y.; Griffiths, W.J. Chapter 2 - Targeted metabolomics and mass spectrometry. In Adv. Protein Chem. Struct. Biol.; Donev, R., Ed.; Academic Press, 2010; Volume 80, pp. 45–83. [Google Scholar]
- Kell, D.B. Metabolomics and systems biology: making sense of the soup. Curr. Opin. Microbiol. 2004, 7, 296–307. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, B.; Zhang, F.; Yang, Y.; Shen, X.; Li, Z.; Zhao, W.; Zhang, Y.; Deng, K.; Rong, Z.; et al. Development of a Correlative Strategy To Discover Colorectal Tumor Tissue Derived Metabolite Biomarkers in Plasma Using Untargeted Metabolomics. Anal. Chem. 2019, 91, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Chen, Y.-Y.; Wu, X.-Z.; Bai, P.-R.; An, N.; Liu, X.-L.; Zhu, Q.-F.; Feng, Y.-Q. Uncovering the Carboxylated Metabolome in Gut Microbiota–Host Co-metabolism: A Chemical Derivatization-Molecular Networking Approach. Anal. Chem. 2023, 95, 11550–11557. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cai, J.; Kong, H.; Wu, M.; Hua, R.; Zhao, M.; Liu, J.; Xu, G. Analysis of Cigarette Smoke Condensates by Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry I Acidic Fraction. Anal. Chem. 2003, 75, 4441–4451. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, M.; Kong, H.; Cai, J.; Wu, J.; Wu, M.; Hua, R.; Liu, J.; Xu, G. Characterization of complex hydrocarbons in cigarette smoke condensate by gas chromatography–mass spectrometry and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2004, 1043, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, J.; Le, W.; Wu, G.; Zhang, W. Improving the Data Quality of Untargeted Metabolomics through a Targeted Data-Dependent Acquisition Based on an Inclusion List of Differential and Preidentified Ions. Anal. Chem. 2023, 95, 12964–12973. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Zhou, B.-W.; Cheng, J.; Zhang, F.; Zhang, J.; Zhang, L.; Guo, Y.-L. Mass Spectrometry-Based Discovery of New Chemical Scaffold Rearrangement Ions: Aza-biphenylene as a Novel Potent Biradical Agent in Cancer Chemotherapy. Anal. Chem. 2020, 92, 14517–14527. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, S.; Zhang, X.; Wang, H.; Zhang, F.; Hou, Y.; Su, Y.; Guo, Y. An unexpected acid-catalyzed decomposition reaction of cilnidipine and pranidipine to the decarboxylative bridged tricyclic products via cascade rearrangements. Org. Chem. Front. 2017, 4, 2163–2166. [Google Scholar] [CrossRef]
- Lu, H.; Yin, Y.; Sun, J.; Li, W.; Shen, X.; Feng, X.; Ouyang, J.; Na, N. Accelerated plasma degradation of organic pollutants in milliseconds and examinations by mass spectrometry. Chin. Chem. Lett. 2021, 32, 3457–3462. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Fu, M.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; An, K.; Xiao, G. Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017, 230, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Wu, X.; Li, M.-M.; Li, G.-Q.; Yang, Y.-T.; Luo, H.-J.; Huang, W.-H.; Chung, H.Y.; Ye, W.-C.; Wang, G.-C.; et al. Antiviral Activity of Polymethoxylated Flavones from “Guangchenpi”, the Edible and Medicinal Pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food. Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zou, B.; An, K.; Yu, Y.; Tang, D.; Wu, J.; Xu, Y.; Ti, H. Anti-asthmatic activity of alkaloid compounds from Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’). Food Funct. 2019, 10, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, X.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Jiang, Y.; Wen, L.; Yang, B. Characterization of polysaccharide structure in Citrus reticulate ‘Chachi’ peel during storage and their bioactivity. Carbohydr. Res. 2021, 508, 108398. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Xu, Y.; Tu, H.-S.; Zhang, L.; Liao, B.-s.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; et al. Guang Chen Pi (the pericarp of Citrus reticulata Blanco's cultivars ‘Chachi’) inhibits macrophage-derived foam cell formation. J. Ethnopharmacol. 2022, 293, 115328. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Gao, Y.; Han, Y.; Wu, X. Path analysis of non-enzymatic browning in Dongbei Suancai during storage caused by different fermentation conditions. Food Chem. 2021, 335, 127620. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-N.; Xie, S.-M.; Dai, Y.-T. Study on the change of compositions and Maillard Browning Reaction in Guang Citri Reticulatae Pericarpium during ageing. Journal of Guangdong Pharmaceutical University 2023, 39, 73–81. [Google Scholar]
- Li, S.-Z.; Guan, X.-M.; Gao, Z.; Lan, H.-C.; Yin, Q.; Chu, C.; Yang, D.-P.; Liu, E.H.; Zhou, P. A simple method to discriminate Guangchenpi and Chenpi by high-performance thin-layer chromatography and high-performance liquid chromatography based on analysis of dimethyl anthranilate. J. Chromatogr. B 2019, 1126-1127, 121736. [Google Scholar] [CrossRef]
- Chao, Y.; Tan, E.y.; Ma, S.; Chen, B.; Liu, M.; Wang, K.; Yang, W.; Wei, M.; Zheng, G. Dynamic variation of the phytochemical and volatile compounds in the pericarp of Citrus reticulata ‘‘Chachi’’ (Rutaceae) during 2 years of storage. J. Food Sci. 2022, 87, 153–164. [Google Scholar] [CrossRef]
- Feng, J.; Huang, D.; Yang, Y.; Chen, J.; Qiu, S.; Lv, Z.; Ma, X.; Li, Y.; Li, R.; Xiao, Y.; et al. Isatis indigotica: from (ethno) botany, biochemistry to synthetic biology. Mol Horticulture 2021, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.G.; Campeol, E.; Tozzi, S.; Gilbert, K.G.; Cooke, D.T.; John, P. A New HPLC-ELSD Method To Quantify Indican in Polygonum tinctorium L. and To Evaluate β-Glucosidase Hydrolysis of Indican for Indigo Production. Biotechnol. Progr. 2003, 19, 1792–1797. [Google Scholar] [CrossRef]
- Gilbert, K.G.; Maule, H.G.; Rudolph, B.; Lewis, M.; Vandenburg, H.; Sales, E.; Tozzi, S.; Cooke, D.T. Quantitative Analysis of Indigo and Indigo Precursors in Leaves of Isatis spp. and Polygonum tinctorium. Biotechnol. Progr. 2004, 20, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.-O.; Marcelo, P.; Gontier, E.; Dauwe, R. Metabolic markers for the yield of lipophilic indole alkaloids in dried woad leaves (Isatis tinctoria L.). Phytochemistry 2019, 163, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Oberthür, C.; Graf, H.; Hamburger, M. The content of indigo precursors in Isatis tinctoria leaves--a comparative study of selected accessions and post-harvest treatments. Phytochemistry 2004, 65, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Chen, M.-H.; Wang, X.-L.; Guo, Q.-L.; Zhu, C.-G.; Lin, S.; Xu, C.-B.; Jiang, Y.-P.; Li, Y.-H.; Jiang, J.-D.; et al. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin. Chem. Lett. 2015, 26, 931–936. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Li, Q.; Zhou, X.; Gao, S.; Chen, R.; Sun, L.; Zhang, L.; Chen, W. Biosynthesis of the active compounds of Isatis indigoticabased on transcriptome sequencing and metabolites profiling. BMC Genomics 2013, 14, 857. [Google Scholar] [CrossRef]
- Maier, W.; Schumann, B.; Gröger, D. Biosynthesis of indoxyl derivatives in Isatis tinctoria and Polygonum tinctorium. Phytochemistry 1990, 29, 817–819. [Google Scholar] [CrossRef]
- Xia, Z.-Q.; Zenk, M.H. Biosynthesis of indigo precursors in higher plants. Phytochemistry 1992, 31, 2695–2697. [Google Scholar] [CrossRef]
- Zou, P.; Koh, H.L. Determination of indican, isatin, indirubin and indigotin in Isatis indigotica by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1239–1246. [Google Scholar] [CrossRef]
- Inoue, S.; Morita, R.; Minami, Y. An indigo-producing plant, Polygonum tinctorium, possesses a flavin-containing monooxygenase capable of oxidizing indole. Biochem. Biophys. Res. Commun. 2021, 534, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kanafuji, T.; Miura, K. Purification and Characterization of a β-Glucosidase from Polygonum tinctorium, Which Catalyzes Preferentially the Hydrolysis of Indican. Biosci., Biotechnol., Biochem. 1996, 60, 147–149. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Xie, J.; Tian, S.; Liu, J.; Huang, S.; Yang, M.; Yang, X.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Combination Therapy with Indigo and Indirubin for Ulcerative Colitis via Reinforcing Intestinal Barrier Function. Oxid. Med. Cell. Longev. 2023, 2023, 2894695. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Peng, R.; Min, Q.; Hui, S.; Chen, X.; Yang, G.; Qin, S. Bisindole natural products: A vital source for the development of new anticancer drugs. Eur. J. Med. Chem. 2022, 243, 114748. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Wang, T.; Li, T.; Zhang, A.; Cai, M.; Zhao, G.; Fu, Q.; Wang, Q.; Liu, X.; Hou, M. Indirubin regulates MPL and TNF expression in peripheral blood mononuclear cells from patients with primary immune thrombocytopenia. Exp. Hematol. 2019, 73, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wei, H.; Duan, Y.; Ning, H.; Yu, J.; Zhu, Y.; Hou, W.; Li, Y. An Improved Synthesis of the Triethylene Glycol-Substituted 4-(N-Methyl-N-Boc-Amino)Styrylpyridine. ACS Omega 2020, 5, 19446–19452. [Google Scholar] [CrossRef] [PubMed]
- Banjare, S.K.; Nanda, T.; Ravikumar, P.C. Cobalt-Catalyzed Regioselective Direct C-4 Alkenylation of 3-Acetylindole with Michael Acceptors Using a Weakly Coordinating Functional Group. Org. Lett. 2019, 21, 8138–8143. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Yi, H.; Wu, P.; Liu, J.; Lei, A. Pd-Catalyzed Direct and Selective C-H Functionalization: C3-Acetoxylation of Indoles. Chemistry – A European Journal 2011, 17, 2353–2357. [Google Scholar] [CrossRef]
- McCosker, P.M.; Butler, N.M.; Shakoori, A.; Volland, M.K.; Perry, M.J.; Mullen, J.W.; Willis, A.C.; Clark, T.; Bremner, J.B.; Guldi, D.M.; et al. The Cascade Reactions of Indigo with Propargyl Substrates for Heterocyclic and Photophysical Diversity. Chemistry – A European Journal 2021, 27, 3708–3721. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Bonasera, A.; Hristov, L.; Garmshausen, Y.; Schmidt, B.M.; Jacquemin, D.; Hecht, S. N,N′-Disubstituted Indigos as Readily Available Red-Light Photoswitches with Tunable Thermal Half-Lives. J. Am. Chem. Soc. 2017, 139, 15205–15211. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules (Basel, Switzerland) 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Rahikainen, M.; Alegre, S.; Trotta, A.; Pascual, J.; Kangasjärvi, S. Trans-methylation reactions in plants: focus on the activated methyl cycle. Physiol. Plant. 2018, 162, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Liscombe, D.K.; Facchini, P.J. Evolutionary and cellular webs in benzylisoquinoline alkaloid biosynthesis. Curr. Opin. Biotechnol. 2008, 19, 173–180. [Google Scholar] [CrossRef]
- Menéndez-Perdomo, I.M.; Facchini, P.J. Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera). J. Biol. Chem. 2020, 295, 1598–1612. [Google Scholar] [CrossRef]
| Substrates | Reaction | Products | Structure | Retention time/min | [M+H] + | Secondary fragment ions (m/z) |
|
|---|---|---|---|---|---|---|---|
| Theoretical mass (m/z) |
Experimental mass (m/z) |
||||||
| 3-hydroxyindole | oxidation | Isatin |  |
3.39 | 148.0393 | 148.0393 | 65.0, 77.0, 92.0, 102.0, 120.0, 130.0 |
| Indigo | 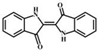 |
5.70 | 263.0815 | 263.0817 | 132.0, 206.0, 180.0, 190.0, 235.0, 219.0 | ||
| Indirubin | 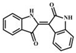 |
6.08 | 263.0815 | 263.0817 | 190.0, 219.0, 165.0, 132.0, 235.0, 206.0 | ||
| methylation | N, N', O, O'-Tetramethyl-leuko-indigo | 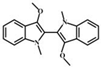 |
8.11 | 321.1598 | 321.1592 | 306.1, 291.1, 275.1, 247.1, 175.1, 160.1 | |
| N-methyl-3-hydroxyindole | oxidation | N-methylisatin | 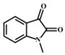 |
3.83 | 162.0550 | 162.0551 | 116.0, 106.0, 79.0, 65.0, 77.0, 134.0, 144.0 |
| N, N'-demethylindigo | 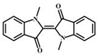 |
6.15 | 291.1128 | 291.1128 | 132.0, 276.0, 146.1, 247.1, 120.1, 172.1 | ||
| N, N'-demethylindirubin | 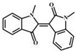 |
6.53 | 291.1128 | 291.1128 | 247.1, 276.1, 146.1, 132.0, 263.1, 158.1 | ||
| Samples Number | Aging Time | MMA | 3-hydroxyindole | isatin | indigo | indirubin | N, N', O, O'-Tetramethyl-leuko-indigo | MDA | N-methyl-3-hydroxyindole | N-methylisatin | N, N'-demethylindigo | N, N'-demethylindirubin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | +++ | − | ++ | + | + | + | ++ | ++ | ++ | + | + |
| 2 | 2 | +++ | ++ | ++ | + | + | − | ++ | ++ | − | + | + |
| 3 | 2 | +++ | − | ++ | ++ | + | + | ++ | − | ++ | + | + |
| 4 | 3 | +++ | ++ | ++ | + | + | ++ | ++ | ++ | − | + | + |
| 5 | 3 | +++ | − | ++ | + | + | − | ++ | +++ | ++ | − | + |
| 6 | 3 | +++ | ++ | ++ | + | + | + | ++ | ++ | ++ | + | − |
| 7 | 5 | +++ | ++ | ++ | + | + | + | ++ | ++ | ++ | + | + |
| 8 | 5 | +++ | ++ | ++ | + | + | + | ++ | ++ | ++ | + | + |
| 9 | 6 | +++ | ++ | ++ | + | + | + | ++ | ++ | ++ | + | + |
| 10 | 9 | +++ | ++ | ++ | + | + | + | ++ | ++ | ++ | + | + |
| 11 | 10 | +++ | ++ | ++ | + | + | − | ++ | ++ | ++ | ++ | + |
| 12 | 10 | +++ | ++ | ++ | + | + | + | ++ | ++ | − | − | + |
| 13 | >10 | +++ | ++ | ++ | + | + | + | ++ | ++ | − | + | + |
| 14 | >10 | +++ | ++ | ++ | + | + | + | ++ | ++ | − | − | + |
| 15 | >10 | +++ | ++ | ++ | + | + | − | ++ | +++ | ++ | + | + |
| 16 | >10 | +++ | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | + | + |
| 17 | >10 | +++ | − | ++ | + | + | + | +++ | ++ | ++ | + | − |
| 1' | 1 | − | − | ++ | − | + | − | ++ | ++ | ++ | − | − |
| 2' | 2 | − | − | ++ | − | + | − | ++ | ++ | ++ | − | − |
| 3' | 3 | − | − | ++ | − | + | − | ++ | ++ | ++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





