Submitted:
03 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials & Methods
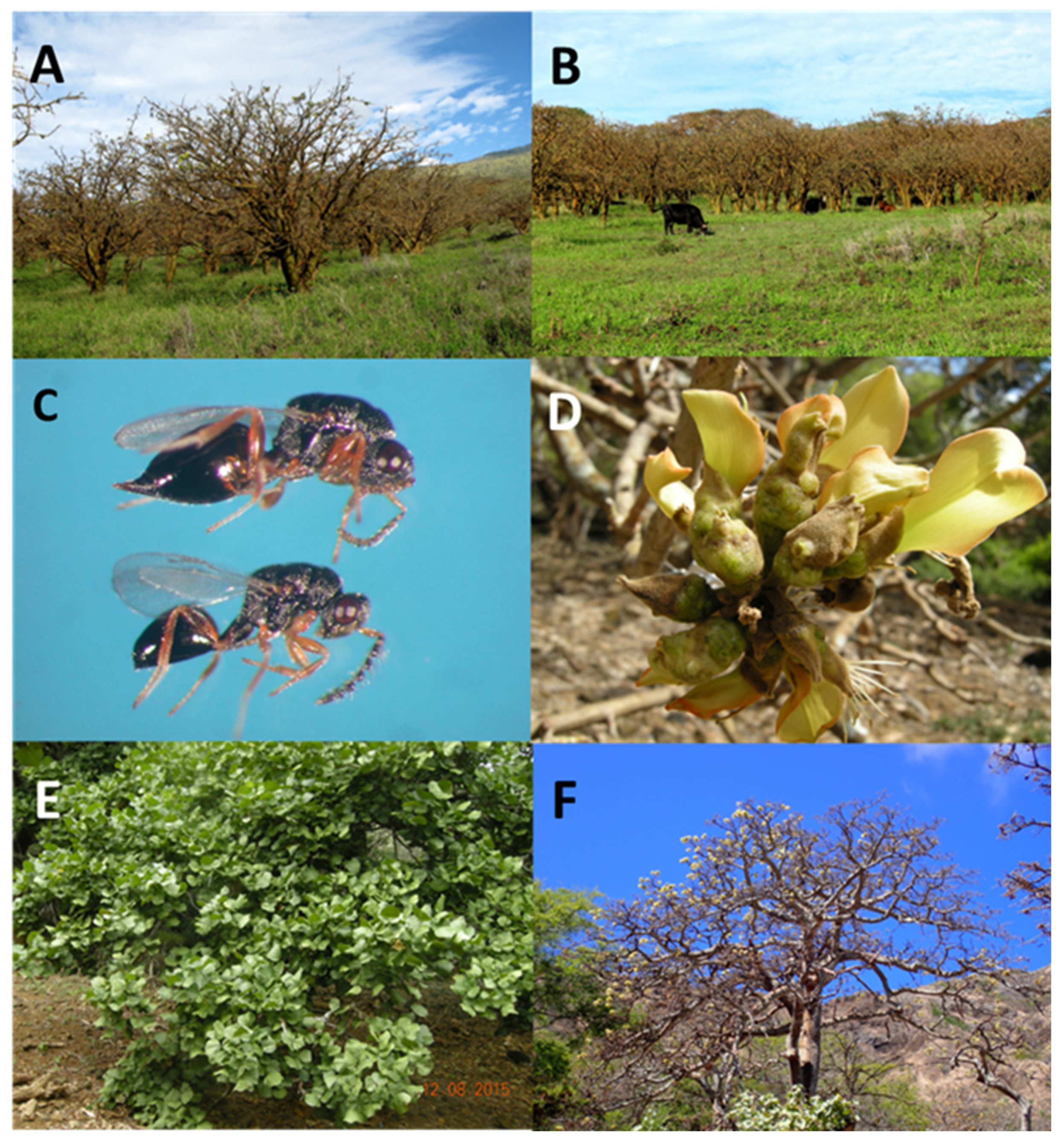
2.1. Plant propagation
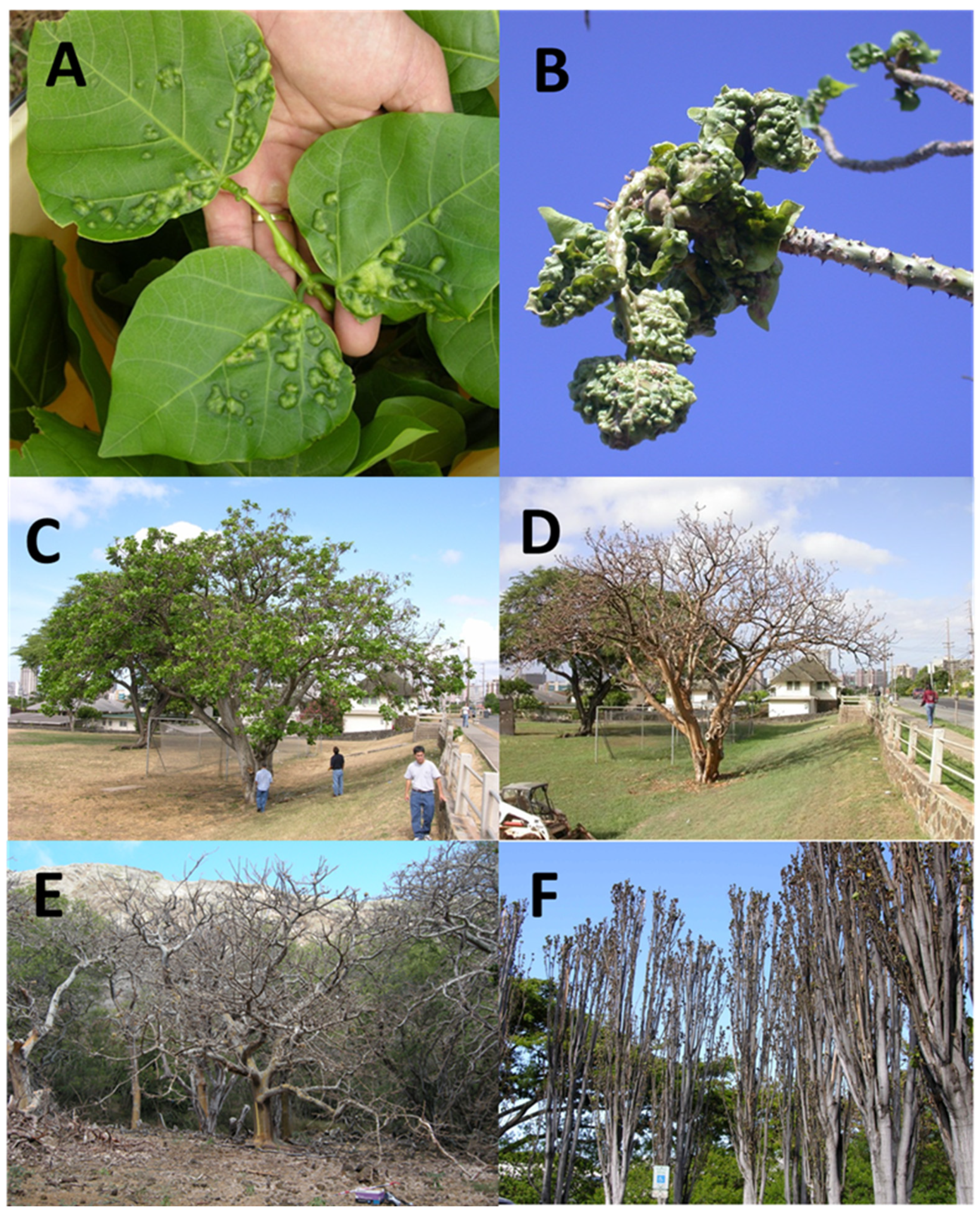
2.2. EGW propagation
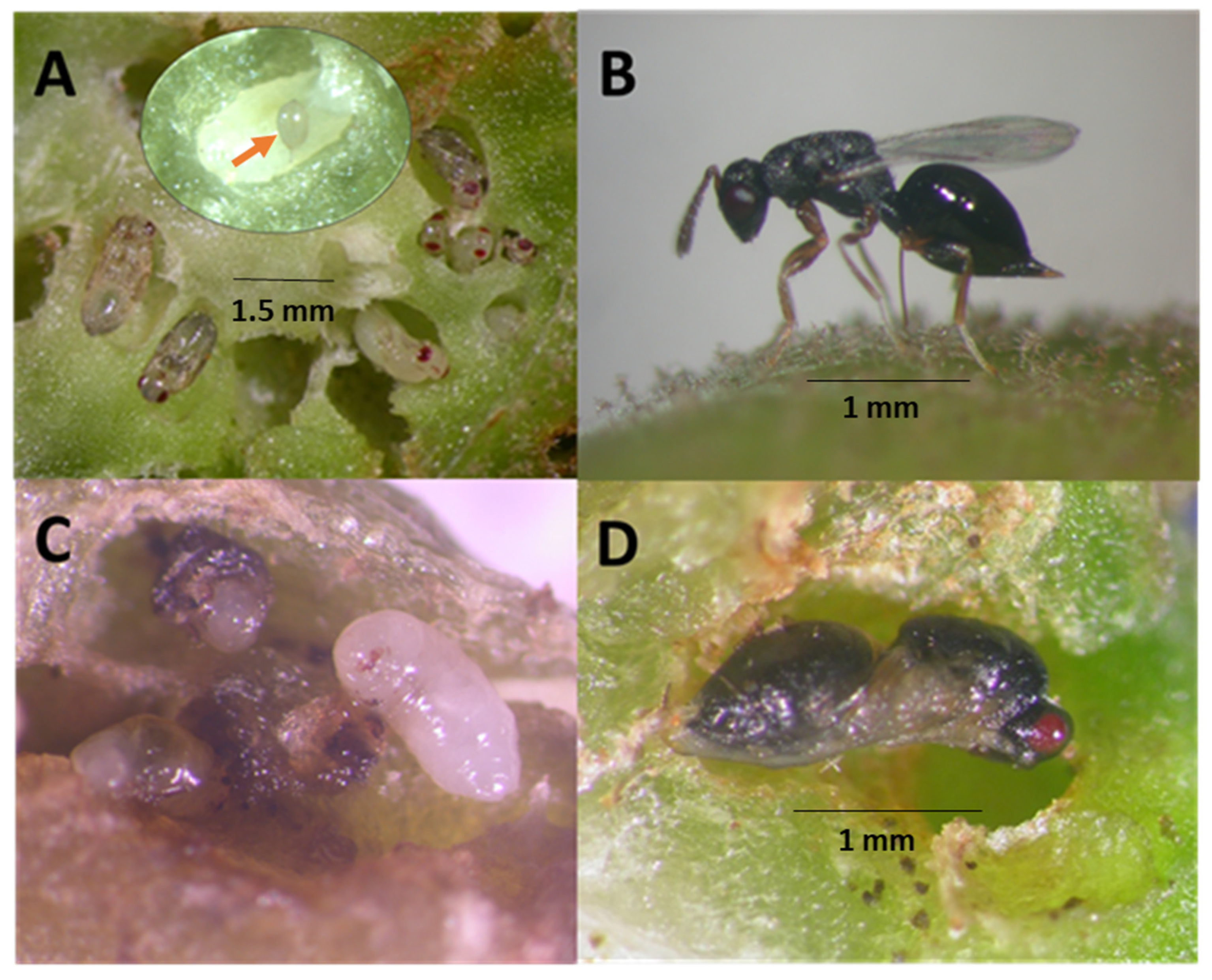
2.3. Origin of the parasitoid colony.
2.4. Eurytoma erythrinae propagation
2.5. Life history study
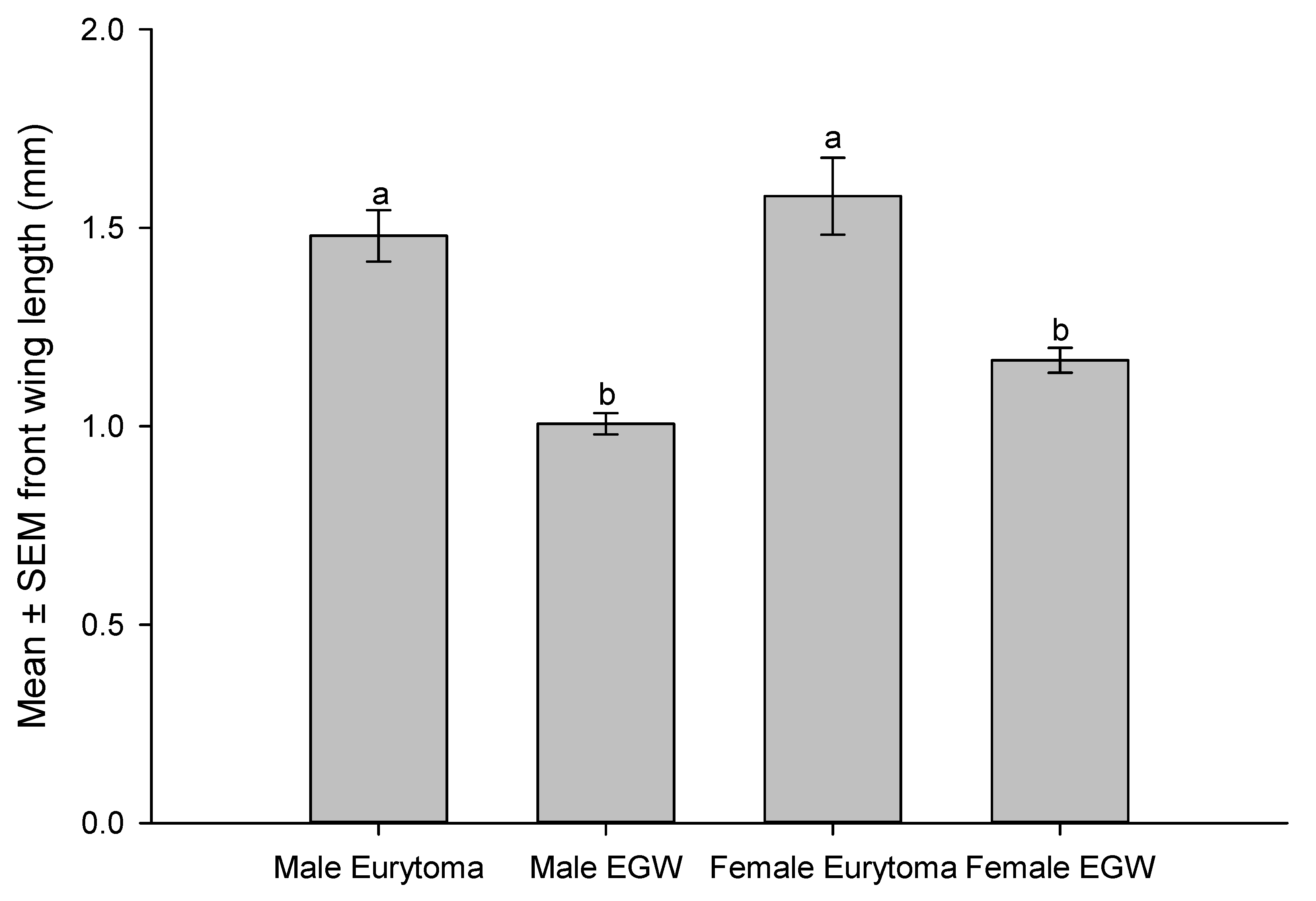
2.6. Longevity study and size of wasps
2.7. Fecundity
2.8. Host specificity testing
2.9. Colonization records on the islands.
2.10. Statistical analysis and vouchers
3. Results
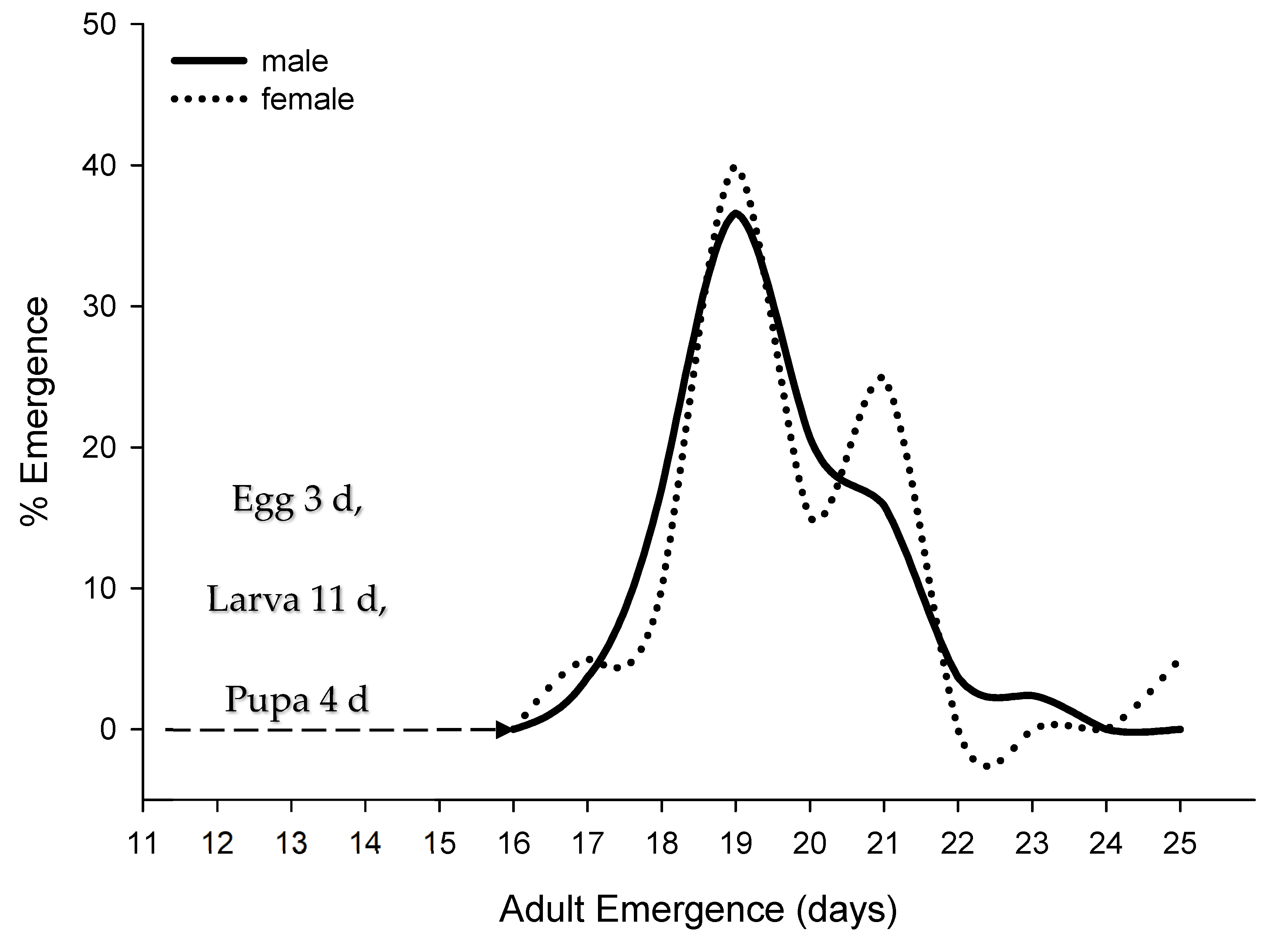
3.1. Life history
3.2. Longevity
3.3. Reproductive attributes
3.4. Host specificity testing
3.5. Records of field releases
4. Discussion
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Kim, I. K.; Delvare, G.; LaSalle, J. A new species of Quadrastichus (Hymenoptera: Eulophidae): A gall-inducing pest on Erythrina spp. (Fabaceae). Journal of Hymenoptera Research. 2004, 13(2), 243–249.
- Yang, M.; Tung, G.; LaSalle, J.; Wu, M. Outbreak of erythrina gall wasp on Erythrina spp. (Fabaceae) in Taiwan. Plant Protection Bulletin. 2004, 46, 391–396.
- Heu, R.; Tsuda, D.; Nagamine, W.; Yalemar, J.; Suh, T. Erythrina Gall Wasp, Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae). Hawaii Department of Agriculture, New Pest Advisory, 2006, No. 05-03. http://www.hawaiiag.org/hdoa/npa/npa05-03-EGW.pdf [online date accessed, October 26, 2007].
- Nami, U.; Takumi, U.; Yukawa, J. Detection of an invasive gall-inducing pest, Quadrastichus erythrinae (Hymenoptera: Eulophidae), causing damage to Erythrina variegata L. (Fabaceae) in Okinawa Prefecture, Japan. Entomological Science. 2007, 10(2), 209–212. [CrossRef]
- Rubinoff, D.; Holland, B.S.; Shibata, A.; Messing, R.H.; Wright, M.G. Rapid invasion despite lack of genetic variation in the Erythrina gall wasp (Quadrastichus erythrinae Kim). Pacific Science. 2010, 64: 23–31. [CrossRef]
- Wiley, J.; Skelley, P. Erythrina gall wasp, Quadrastichus erythrinae Kim, in Florida. Pest Alert, Florida Department of Agriculture and Consumer Services. October 25, 2006. http://www.doacs.state.fl.us/pi/enpp/ento/gallwasp.html.
- Rogelio, Enrique Palacios; Torres, Jorge Malpica Pita; Aldo, G. Bustamante-Ortiz; Jorge Valdez-Carrasco; Amadeo Santos-Chávez, Ricardo Vega Muñoz; Heike, Vibrans Lindemann. “The Invasive Gall Wasp Quadrastichus erythrinae Kim in Mexico,” Southwestern Entomologist. 2017, 42(4), 1099-1102. [CrossRef]
- Medianero, E.; Zachrisson, B. Erythrina gall wasp, Quadrastichus erythrinae Kim, 2004 (Hymenoptera: Eulophidae: Tetrastichinae): a new pest in Central America. BioInvasions Records. 2019, 8(2): 452–456, . [CrossRef]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Erythrina, in Manual of the flowering plants of Hawaii. University of Hawaii Press and Bishop Museum Press.1990, 2 volumes. pp. 671-672.
- POWO. “Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://www.plantsoftheworldonline.org/ 2023, (online date accessed 22 October 2023).
- Neal, M.C. In Gardens of Hawaii. Bishop Museum Press. Honolulu, Hawaii. 1965, 924 pp.
- Hollier, D. The Seed Savers. Hana Hou! The Magazine of Hawaiian Airlines. February/March 2007, v.10 no.1: 74-84.
- Dingeman, R. Victory at hand in war against invasive, tree-killing gall wasp. The Honolulu Advertiser, March 26, 2010.
- Rotar, P.P.; Joy, R.J.; Weissich, P.R. “Tropic Coral” tall erythrina. Univ. Haw. CTAHR Res. 1986, Ext. Ser. 072.
- Messing, R.H.; Noser, S.; Hunkeler, J. Using host plant relationships to help determine origins of the invasive Erythrina gall wasp Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae. Biol. Invasions. 2009, 11:2233-2241. [CrossRef]
- Jiao, Yi; Chen, Zhi-Lin; Yu, Dao-Jian; Kang, Lin; Yang, Wei-Dong; Chen, Zhi-Nan; Chen, Xiao-Ying. Bionomics of the erythrina gall wasp, Quadristichus erythrinae Kim (Hymenoptera: Eulophidae), Acta Entomol. Sinica. 2007, 50(1): 46-50.
- Doccola, Joseph J.; Smith, Sheri L.; Strom, Brian L.; Medeiros, Arthur C.; Allmen, Erica von. Systemically Applied Insecticides for Treatment of Erythrina Gall Wasp, Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae). Arboriculture & Urban Forestry. 2009, 35(4): 173–181.
- Xu, T.; Jacobsen, C.M.; Hara, A.H.; Li, J.; Li, Q.X. Efficacy of systemic insecticides on the gall wasp Quadrastichus erythrinae in wiliwili trees (Erythrina spp.). Pest Manag. Sci. 2009, 65(2):163-9; PMID: 18833544. [CrossRef]
- Brannon, J. Dying trees cost $1M a year. The Honolulu Advertiser. 2007, (online date accessed 21 October 2023). http://the.honoluluadvertiser.com/article/2007/Mar/23/ln/FP703230359.html.
- Gates, M.; Delvare, G. A new species of Eurytoma (Hymenoptera: Eurytomidae) attacking Quadrastichus spp. (Hymenoptera: Eulophidae) galling Erythrina spp. (Fabaceae), with a summary of African Eurytoma biology and species checklist. Zootaxa. 2008, 1751: 1-24.
- 21. Ohl, Michael; Thiele, Kathrin. Estimating body size in apoid wasps: the significance of linear variables in a morphologically diverse taxon (Hymenoptera, Apoidea). 2007, (83) 2, 110 – 124. [CrossRef]
- Yeager, J. F. Stimulation of isolated heart roach. J. Agric. Res. 1939, 59: 121-137.
- Nishida, G.M. Hawaiian Terrestrial Arthropod Checklist. Fourth Edition. Bishop Museum Technical Bulletin. 2002, 22, 313 pp.
- Perkins, R.C.L.; Forel, A. Fauna Hawaiiensis, vol. 1, part 1. Hymenoptera Aculeata. D. Sharp, editor. Cambridge University Press, London. 1899.
- Yoshimoto, C. M. Synopsis of Hawaiian Eulophidae including Aphelininae (Hym.: Chalcidoidea). Pacific Insects. 1965, 7:665 – 699.
- Boucek, Z. Australasian Chalcidoidea (Hymenoptera): A biosystematic revision of genera of fourteen families, with reclassification of species. 1988, 832 pp. CAB International, UK.
- JMP®, Version <11>. 2023, SAS Institute Inc., Cary, NC, 1989–2023.
- Lotfalizadeh, H.; Delvare, G.; Rasplus, J-Y. Phylogenetic analysis of Eurytominae (Chalcidoidea: Eurytomidae) based on morphological characters. Zoological Journal of the Linnean Society. 2007, 151: 441–510. [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database. World Wide Web electronic publication. 2020, http://www.nhm.ac.uk/chalcidoids. (online date accessed 10 October 2020).
- Gómez, J. F.; Nieves-Aldrey, J. L.; Hernández, Nieves M.; Stone, G. N. Comparative morphology and biology of terminal-instar larvae of some Eurytoma (Hymenoptera, Eurytomidae) species parasitoids of gall wasps (Hymenoptera, Cynipidae) in western Europe. Zoosystema. 2011, 33 (3): 287-323. [CrossRef]
- Vårdal, H.; Gómez, J. F.; Nieves-Aldrey, J. L. Ovarian egg morphology in chalcidoid wasps (Hymenoptera: Chalcidoidea) parasitizing gall wasps (Hymenoptera: Cynipidae). Graellsia. 2016, 72(1): e044 ISSN-L: 0367-5041. [CrossRef]
- Heimpel, George E.; Rosenheim, Jay A.; Kattari, David. Adult feeding and lifetime reproductive success in the parasitoid Aphytis Melinus. Entomologia Experimentalis et Applicata. 1997, 83: 305–315. [CrossRef]
- Boulton, R.A; Shuker, D.M. A sex allocation cost to polyandry in a parasitoid wasp. Biol Lett. 2015, 11(6):20150205. PMID: 26041866; PMCID: PMC4528469. [CrossRef]
- Prinsloo, G. L.; Kelly, J.A. The tetrastichine wasps (Hymenoptera: Chalcidoidea: Eulophidae) associated with galls on Erythrina species (Fabaceae) in South Africa, with the description of five new species. Zootaxa. 2009, 2083: 27–45.
- Lin, S.F.; Tung, G.S.; Yang, M.M. The Erythrina Gall Wasp Quadrastichus erythrinae (Insecta: Hymenoptera: Eulophidae): Invasion History, Ecology, Infestation and Management. Forests. 2021, 12, 948. [CrossRef]
- Lin, Sheng-Feng; Tung, Gene-Sheng; Yang, Man-Miao. Out of Africa: Origin of the Erythrina Gall Wasp Quadrastichus erythrinae (Hymenoptera: Chalcidoidea: Eulophidae). Formosan Entomol. 2021, 41: 26-36. [CrossRef]
- Day, M.D.; Cock, M.J.W.; Conant, P.; Cooke, B.; Furlong, M.J.; Paynter., Q.; Ramadan, M.M.; Wright, M.G. Biological control successes and failures: Oceania region. In Mason, P. Biological Control: Global impacts, challenges and future directions of pest management. CSIRO Publishing. Australia. 2021, pp. 342-376.
- Ramadan, M., M.; Kaufman, L.V.; Wright, M., G. Insect and weed biological control in Hawaii: Recent case studies and trends. Biol. Control. 2023, 179, 105170. 397 . [CrossRef]
- Kaufman, L.V.; Yalemar, J.; Wright, M.G. Classical biological control of the erythrina gall wasp, Quadrastichus erythrinae, in Hawaii: Conserving an endangered habitat. Biol. Control. 2020, 142, 104161. [CrossRef]
- Bell, Rayna C.; Belmaker, Amos; Couch, Courtney S.; Marchetto, Katherine M.; Simonis, Joseph L.; Quinn Thomas, R.; Sparks, Jed P. “Effectiveness of Erythrina gall wasp biocontrol and implications for the recovery of threatened Wiliwili trees (Fabaceae: Erythrina sandwicensis),” The Journal of the Torrey Botanical Society. 2013, 140(2), 215-224. [CrossRef]
- U.S. Department of Agriculture Forest Service Forest Health Protection, R-5 and Southern Research Station 2007. Early Detection Pest Advisory: Identifying and managing the Erythrina Gall Wasp. Science Update SRS-012. United States Department of Agriculture, Southern Research Station. Brochure. 2007. https://www.fs.usda.gov/research/treesearch/27989 (online date accessed 25 October 2023).
- LaSalle, John; Ramadan, Mohsen; Bernarr, Kumashiro R. A new parasitoid of the Erythrina Gall Wasp, Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae). Zootaxa. 2009, 2083: 19-26. [CrossRef]
| Order and Family |
Gall-former (Scientific and Common name) |
Gall-former status and source |
Host plant (Scientific, Common name), Infested plant part used for testing |
| Hymenoptera: Agaonidae |
Josephiella microcarpae Beardsley & Rasplus, Banyan gall wasp |
Immigrant, Field-collected, Honolulu, Oahu |
Ficus microcarpa Chinese banyan, cuttings |
| Hemiptera: Eriococcidae |
Tectococcus ovatus Hempel biocontrol agent |
Biocontrol agent, Lab-reared, USFS, HVNP and HDOA |
Psidium cattleianum strawberry guava, whole plants |
| Hymenoptera: Eulophidae |
Ophelimus sp. Eucalyptus gall wasp |
Immigrant, Field-collected, Camp Maluhia, Maui Island |
Eucalyptus sp. Eucalyptus, Cuttings |
| Hemiptera: Psyllidae |
Trioza sp. Ohia psyllid |
Endemic, Field-collected, Aiea and Manoa, Oahu Island |
Metrosideros polymorpha Ohia, Cuttings |
| Diptera: Tephritidae |
Eutreta xanthochaeta Aldrich Lantana gall fly |
Biocontrol agent, Field-collected, Hauula, Oahu Island and lab-reared HDOA |
Lantana camara Lantana , whole plants |
| Diptera: Tephritidae |
Procecidochares alani Steyskal Hamakua pamakani gall fly |
Biocontrol agent, Field-collected, Nuuanu, Oahu Island and lab-reared HDOA |
Ageratina riparia Hamakua pamakani, whole plants |
| Diptera: Tephritidae |
Procecidochares utilis Stone Maui pamakani gall fly |
Biocontrol agent, Lab-reared, UH- Manoa and lab-reared HDOA |
Ageratina adenophora Maui pamakani, whole plants |
| Parasitoid and host | N |
Longevity (days) Mean ± SEM |
Body lengtha (mm) Mean ± SEM |
|
| Laboratory colonya | Wild waspsb | |||
| Female Eurytoma | 39 | 40.4 ± 2.2 | 2.1 ± 0.05 | 2.2 ± 0.08 a |
| Male Eurytoma | 92 | 20.5 ± 1.1 | 1.5 ± 0.04 | 2.04 ± 0.07 a |
| Mean comparison | T58 = 8.12, P = 0.0001 | T128 = 9.20, P = 0.0001 | ||
| Female EGW | 100 | 5.9 ± 0.3 | - | 1.6 ± 0.03 b |
| Male EGW | 100 | 7.8 ± 0.3 | - | 1.45 ± 0.07 b |
| Mean comparisons | T197 = 4.106, P = 0.0001 | - | F3,44 = 25.55, P <0.0001 | |
| Reproductive parameter | Mean ± SEM | Range | unit |
| Oviposition period a | 37.7 ± 6.7 | 31 – 51 | days |
| Post-oviposition period | 13.7 ± 5.4 | 3 – 20 | days |
| Mature ovarian eggs at death | 2.3 ± 1.5 | 0 – 5 | number eggs |
| Female longevity | 51.3 ± 1.5 | 49 – 54 | days |
| Male longevity (n=15) b | 24.6 ± 2.7 | 3 – 42 | days |
| Female progeny | 40.0 ± 15.3 | 20 – 70 | adult |
| Male progeny | 125.3 ± 41.8 | 82 – 209 | adult |
| Total progeny per female | 165.3 ± 39.3 | 105 – 239 | offspring |
| Daily progeny | 4.3 ± 0.5 | 3.4 – 4.9 | adult |
| Sex ratio (% females) | 25.9 ± 10.3 | 12.6 – 46.1 | % female |
| Sex ratio (% males) | 74.1 ± 10.3 | 53.9 – 87.4 | % male |
| Female lifecycle (n=120) | 18.4 ± 0.1 | 15 – 24 | days |
| Male lifecycle (n=376) | 18.2 ± 0.1 | 15 – 26 | days |
| Galled plant infested with insect |
Frequency of visits by E. erythrinae (mean ± SEM) |
E. erythrinae adult emergence (mean ± SEM) |
| Josephiella microcarpae | 0 b | 0 b |
| Q. erythrinae (control) | 7.6 ± 2.6 a | 25.0 ± 9.6 a |
| Tectococcus ovatus | 0.2 ± 0.2 b | 0 b |
| Q. erythrinae (control) | 10.6 ± 3.4 a | 62.2 ± 17.9 a |
| Ophelimus sp. | 1.0 ± 1.0 b | 0 b |
| Q. erythrinae (control) | 7.2 ± 1.4 a | 19.8 ± 3.1 a |
| Trioza sp. | 0 b | 0 b |
| Q. erythrinae (control) | 7.6 ± 1.2 a | 25.4 ± 9.8 a |
| Eutreta xanthochaeta | 0.8 ± 0.3 b | 0 b |
| Q. erythrinae (control) | 6.0 ± 0.7 a | 14.8 ± 5.8 a |
| Procecidochares alani | 0 b | 0 b |
| Q. erythrinae (control) | 7.0 ± 1.8 a | 14.5 ± 5.4 a |
| Procecidochares utilis | 0.2 ± 0.2 b | 0 b |
| Q. erythrinae (control) | 9.8 ± 3.2 a | 17.0 ± 6.0 a |
| Island |
Erythrina species infested by the gall wasp |
N2 | Numbers of Eurytoma released. |
Release time (month/day/year) |
Dates of recovery (month/day/year) |
| Oahu | Erythrina sandwicensis O.Deg. | 1070 | 11/25/2008 – 8/23/2010 | 2/6/2009 – 8/23/20103 | |
| Erythrina crista-galli L. | 470 | ||||
| Erythrina variegata L.1 | 130 | ||||
| Sub-total and % of total |
38 | 1670 (41.8%) | |||
| Maui | Erythrina sandwicensis | 380 | 12/17/2008 – 12/23/2009 | 2/25/09 – 1/21/2010 | |
| Erythrina crista-galli | 60 | ||||
| Sub-total % of total |
10 | 440 (11.0%) | |||
| Hawaii | Erythrina sandwicensis. | 688 | 12/1/2008 – 1/27/2010 | 1/14/2009 – 1/27/20103 | |
| Erythrina crista-galli | 30 | ||||
| Erythrina variegata1 | 330 | ||||
| Sub-total and % of total |
20 | 1048 (26.2%) | |||
| Kauai | Erythrina sandwicensis | 240 | 12/4/2008 | 3/3/2009 – 6/30/2010 | |
| Erythrina variegata | 30 | ||||
| Sub-total and % of total |
5 | 270 (6.7%) | |||
| Molokai | Erythrina sandwicensis | 390 | 4/14/2009 – 6/9/2009 | 2/10/2010 – 3/24/2010 | |
| Erythrina crista-galli | 60 | ||||
| Erythrina variegata 1 | 30 | ||||
| Sub-total and % of total |
9 | 480 (12.0%) | |||
| Lanai total and % of total |
Erythrina sandwicensis | 2 | 90 (2.2%) |
5/12/2009 | 2/17/2010 – 4/7/2010 |
| Total released on all islands | 84 | 3998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




