Submitted:
03 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Aim
Materials and methods
Design
DOAC consumption and expenditure (Pharmacoepidemiology of DOAC in Russia in COVID-19 pandemic)
Consumption of apixaban (Eliquis) in the Russian Federation, National consumption: Example
CPG recommended consumption:
Consumption of apixaban (Eliquis) in the Russian Federation for 2022 (January-August). Example
Eligibility criteria for inclusion of CPGs and Clinical trials
Search methods for identification of CPGs and clinical trials
- Official collection of clinical guidelines of the Ministry of Health (https://cr.minzdrav.gov.ru/; last searched 01 September 2023;
- Russian Databases: eLIBRARY.RU (www.elibrary.ru; from 2020 to 2023; last searched 01 September 2023;
- National Library of Medicine (National Centre for Biotechnology Information, NCBI, https://pubmed.ncbi.nlm.nih.gov; from 2020 to 2023; last searched 01 September 2023;
- Google scholar (https://scholar.google.ru; from 2020 to 2023; last searched 01 September 2023;
- US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; from 2020 to 2023; last searched 01 September 2023; Appendix 5);
- the Cochrane Library (https://www.cochranelibrary.com; from 2020 to 2023; last searched 01 September 2023;
Description of the databases and websites used for searched, selection and analysis of clinical practice guidelines and clinical trials
- 1.
- The official collection of clinical practice guidelines of the Ministry of Health (MoH), named Rubricator
- 2.
- eLIBRARY.RU
- 3.
- National Library of Medicine (National Centre for Biotechnology Information, NCBI, https://pubmed.ncbi.nlm.nih.gov);
- 4.
- Google scholar (https://scholar.google.ru);
- 5.
- US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
- 6.
- The Cochrane Library (https://www.cochranelibrary.com
The official collection of clinical practice guidelines of the Ministry of Health (MoH), named Rubricator
Results of the searches
CPG assessment with AGREE II instrument
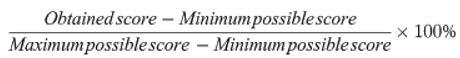
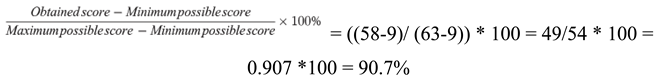
Results
DOAC pharmacoepidemiology
| Name of the drug | DDD (WHO), mg | 2020 | 2021 | 2022 |
|---|---|---|---|---|
| Apixaban | 10 | 26.59 | 15.75 | 10.67 |
| Rivaroxaban | 20 | 26.59 | 7.87 | 5.48 |
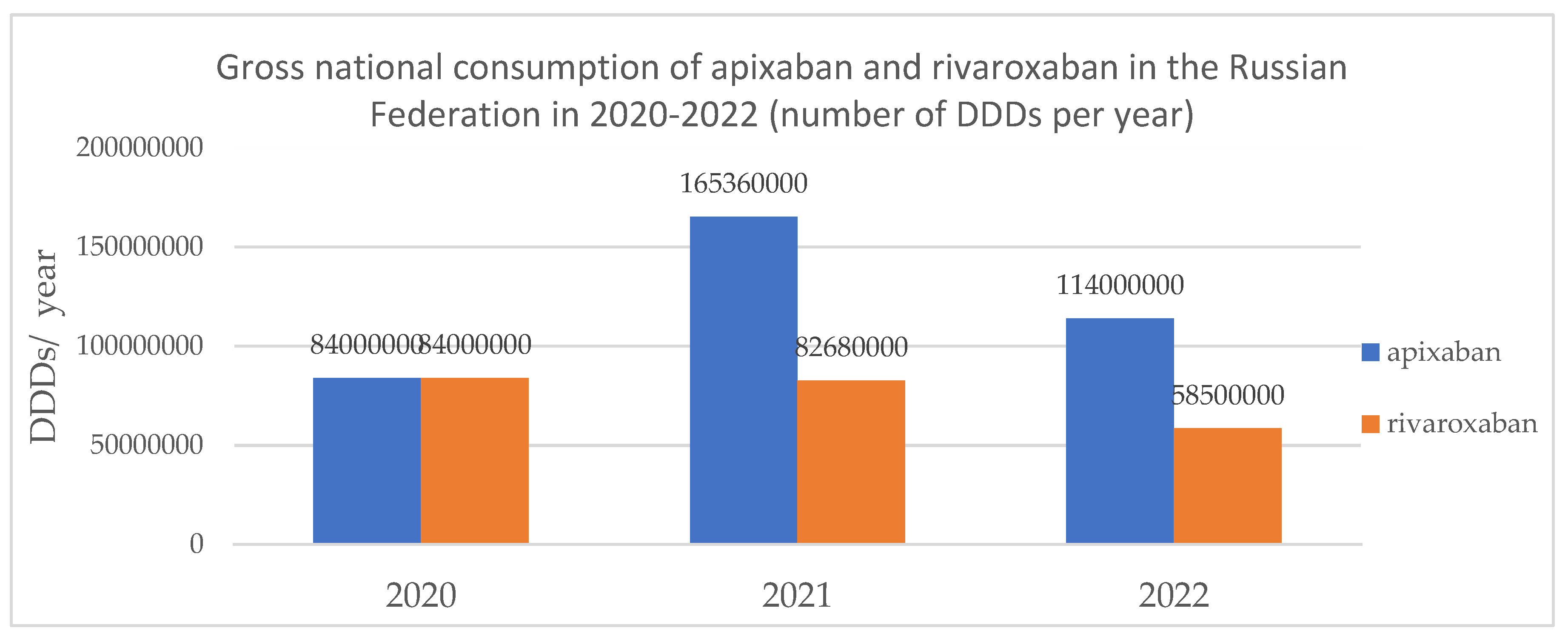
| Name of the drug | DDD (according to Russian clinical guidelines) | 2020 | 2021 | 2022 |
|---|---|---|---|---|
| Apixaban | 15 DDD (30 days) | 5 600 000 | 11 024 000 | 7 600 000 |
| 22,5DDD (45 days) | 3 733 333 | 7 349 333 | 5 066 667 | |
| Rivaroxaban | 15 DDD (30 days) | 5 600 000 | 5 512 000 | 3 900 000 |
| 22,5 DDD (45 days) | 3 733 333 | 3 674 667 | 2 600 000 |
| 2020 | 2021 | 2022 | |
|---|---|---|---|
| COVID-19 patients | 3 159 297 | 10 499 982 | 10 684 204 |
| 30 days | |||
| Total consumption of Rivaroxaban and Apixaban (patients) | 11 200 000 | 16 536 000 | 11 500 000 |
| 45 days | |||
| Total consumption of Rivaroxaban and Apixaban (patients) | 7 466 666 | 11 023 999 | 7 666 667 |
Results of CPGs assessment domain scores
Total sum of assessment scores
Comparison with U.S. data in consumption and sales of DOACs
Discussion


Study limitations
Conclusions:
List of abbreviation
| AGREE II | Appraisal of Guidelines for REsearch & Evaluation |
| ATC | Anatomical Therapeutic Chemical code |
| CDSR | Cochrane Database of Systematic Reviews |
| CONSORT | Consolidated Standards of Reporting Trials |
| COVID-19 | COronaVIrus Disease 2019 |
| CPG | clinical practice guidelines |
| DDD | Defined Daily Dose |
| DOAC | direct oral anticoagulants |
| DVT/ PE | deep vein thrombosis/ pulmonary embolism |
| FAR | The All-Russia Public Organization «Federation of Anaesthesiologists and Reanimatologists» |
| ICD-10 | International Classification of Diseases 10 |
| LMWH | Low molecular weight heparin |
| MGNOT (in Russ) | Moscow City Scientific Society of Physicians |
| MoH | Ministry of Health of the Russian Federation |
| NIIOZMM (in Russ) | Research Institute for Healthcare Organization and Medical Management of Moscow Healthcare Department |
| PE | pulmonary embolism |
| PMC | Pubmed Central |
| ROPNIZ (in Russ) | Russian Society for the Prevention of Non-communicable Diseases |
| RSC | Russian Society of Cardiology |
| RSCI | Russian Scientific Citation Index |
| VTE | venous thromboembolism |
| WHO | World health organization |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schutgens RE. DOAC in COVID-19: Yes or No? Hemasphere. 2020 Dec 29;5(1):e526. PMID: 33403357; PMCID: PMC7773328. [CrossRef]
- Liao, SC., Shao, SC., Chen, YT. et al. Incidence and mortality of pulmonary embolism in COVID-19: a systematic review and meta-analysis. Crit Care 24, 464 (2020). [CrossRef]
- Roguljić H, Arambašić J, Ninčević V, Kuna L, Šesto I, Tabll A, Smolić R, Včev A, Primorac D, Wu GY, Smolić M. The role of direct oral anticoagulants in the era of COVID-19: are antiviral therapy and pharmacogenetics limiting factors? Croat Med J. 2022 Jun 22;63(3):287-294. PMID: 35722697; PMCID: PMC9284020. [CrossRef]
- Santos BC, Flumignan RL, Civile VT, Atallah ÁN, Nakano LC. Prophylactic anticoagulants for non-hospitalised people with COVID-19. Cochrane Database Syst Rev. 2023 Aug 16;8(8):CD015102. PMID: 37591523; PMCID: PMC10428666. [CrossRef]
- Flumignan RL, Civile VT, Tinôco JDS, Pascoal PI, Areias LL, Matar CF, Tendal B, Trevisani VF, Atallah ÁN, Nakano LC. Anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2022 Mar 4;3(3):CD013739. PMID: 35244208; PMCID: PMC8895460. [CrossRef]
- Siegal DM. What we have learned about direct oral anticoagulant reversal. Hematology Am Soc Hematol Educ Program. 2019 Dec 6;2019(1):198-203. PMID: 31808835; PMCID: PMC6913453. [CrossRef]
- Nakamura J, Tsujino I, Yachi S, Takeyama M, Nishimoto Y, Konno S, Yamamoto N, Nakata H, Ikeda S, Umetsu M, Aikawa S, Hayashi H, Satokawa H, Okuno Y, Iwata E, Ogihara Y, Ikeda N, Kondo A, Iwai T, Yamada N, Ogawa T, Kobayashi T, Mo M, Yamashita Y; CLOT-COVID Study Investigators. Incidence, risk factors, and clinical impact of major bleeding in hospitalized patients with COVID-19: a sub-analysis of the CLOT-COVID Study. Thromb J. 2022 Sep 20;20(1):53. PMID: 36127738; PMCID: PMC9485792. [CrossRef]
- Popa P, Iordache S, Florescu DN, Iovanescu VF, Vieru A, Barbu V, Bezna MC, Alexandru DO, Ungureanu BS, Cazacu SM. Mortality Rate in Upper Gastrointestinal Bleeding Associated with Anti-Thrombotic Therapy Before and During Covid-19 Pandemic. J Multidiscip Healthc. 2022 Nov 18;15:2679-2692. PMID: 36425876; PMCID: PMC9680964. [CrossRef]
- Davtyan P.A., Gumerov R.M., Zagidullin S.Z., Samorodov A.V., Cai B., Zagidullin N.S. Is anticoagulant therapy necessary after hospitalization with COVID-19 pneumonia? Russian Journal of Cardiology. 2021;26(4S):4652. (In Russ.). [CrossRef]
- Tian Y, Pan T, Wen X, Ao G, Ma Y, Liu X, Liu R, Ran H. Efficacy and Safety of Direct Oral Anticoagulants Compared With Heparin for Preventing Thromboembolism in Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296231164355. PMID: 37131319; PMCID: PMC10170187. [CrossRef]
- Toubasi AA. Effect on Morbidity and Mortality of Direct Oral Anticoagulants in Patients With COVID-19. Am J Cardiol. 2022 May 15;171:174-177. Epub 2022 Mar 12. PMID: 35292148; PMCID: PMC8917387. [CrossRef]
- https://delprof.ru/press-center/open-analytics/farmatsevticheskiy-rynok-rossii-itogi-2021-goda-i-sobytiya-2022-goda/.
- Zhuravleva M.V., Chulanov V.P., Gagarina Yu.V., Shabalina E.A. Pharmacoeconomic analysis of tixagevimab and cilgavimab combination for COVID-19 therapy. FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology. 2023;16(2):149-161. (In Russ.). [CrossRef]
- Wang T, Tan JB, Liu XL, Zhao I. Barriers and enablers to implementing clinical practice guidelines in primary care: an overview of systematic reviews. BMJ Open. 2023 Jan 6;13(1):e062158. PMID: 36609329; PMCID: PMC9827241. [CrossRef]
- https://www.whocc.no/atc_ddd_index/.
- AlphaRM Pharmaceutical sales in Russia https://pharmvestnik.ru/content/news/Aptechnye-prodaji-lekarstv-ushedshego-goda-pobili-rekord-2020-goda.html.
- Annual report (2022) of DSM group https://dsm.ru/docs/analytics/Annual_report_2023_rus.pdf.
- Report on the current situation in the region with coronavirus. Communication Center for the Economy of the Russian Federation, 01/11/2022.
- https://who.maps.arcgis.com/apps/dashboards/a19d5d1f86ee4d99b013eed5f637232d.
- https://covid19.rosminzdrav.ru/.
- Lienhard DA, Kisser LV, Ziganshina LE. Assessing methodological quality of Russian clinical practice guidelines and introducing AGREE II instrument in Russia. PLoS One. 2018 Sep 11;13(9):e0203328. PMID: 30204760; PMCID: PMC6133363. [CrossRef]
- Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022 Dec;10(12):1169-1177. Epub 2022 Oct 10. PMID: 36228641; PMCID: PMC9635892. [CrossRef]
- Shlyakhto E.V., Konradi A.O., Arutyunov G.P., et al. Guidelines for the diagnosis and treatment of circulatory diseases in the context of the COVID-19 pandemic. Russian Journal of Cardiology. 2020;25(3):3801. (In Russ.). [CrossRef]
- Zabolotskikh I.B., Kirov M.Yu., Lebedinskii K.M., et al. Anesthesia and intensive care for patients with COVID-19. Russian Federation of anesthesiologists and reanimatologists guidelines. Annals of Critical Care. 2021; S1:9–143. (In Russ.). [CrossRef]
- Drapkina O. M., Drozdova L. Y., Avdeev S. N., et al. The outpatient medical care in patients with chronic diseases under dispensary supervision in the conditions of the COVID-19 pandemic. Temporary guidelines. Version 2. Cardiovascular Therapy and Prevention. 2021;20(8):3172. (In Russ.). [CrossRef]
- Livzan M. A., Drapkina O. M., Nikolaev N. A., et al. Algorithms for adult outpatient care of coronavirus disease 2019 (COVID-19) and its assumption. Cardiovascular Therapy and Prevention. 2021;20(4):2916. (In Russ.). [CrossRef]
- Clinical protocol for the treatment of patients with new coronavirus infection (COVID-19), undergoing inpatient treatment in medical organizations of the healthcare state system of Moscow / M. B. Antsiferov, L. S. Aronov, A. S. Belevsky, et al. Ed. by A. I. Khripun. – M.: GBU “NIIOZMM DZM”, 2020. – 28 p.: ill.
- Recommendations for the outpatient management of COVID-19 patients with the acute infection and POST-COVID syndrome (Issued by the Moscow City Scientific Society of Physicians). Ed. By prof. Vorobyev P.A. Problems of standardization in healthcare. 2021; 7-8: 3-96. [CrossRef]
- Temporary methodological recommendations for prevention, diagnostics and treatment of new coronavirus infections (COVID-19). version 17 (14.12.2022). Ministry of Health of the Russian Federation.
- Tichy EM, Hoffman JM, Suda KJ, Rim MH, Tadrous M, Cuellar S, Clark JS, Wiest MD, Matusiak LM, Schumock GT. National trends in prescription drug expenditures and projections for 2021. Am J Health Syst Pharm. 2021 Jul 9;78(14):1294-1308. PMID: 33880494; PMCID: PMC8365501. [CrossRef]
- Tichy EM, Hoffman JM, Suda KJ, Rim MH, Tadrous M, Cuellar S, Clark JS, Ward J, Schumock GT. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022 Jul 8;79(14):1158-1172. PMID: 35385103; PMCID: PMC9383648. [CrossRef]
- Petrukhina I.K., Lebedev P.A., Ryazanova T.K., Blinkova P.R., Paranina E.V. Consumption of cardiovascular and antithrombotic drugs during the spread of coronavirus infection in retail sector of the Samara region pharmaceutical market. Cardiosomatics. 2021;12(4):219–226. [CrossRef]
- Dawwas GK, Leonard CE, Lewis JD, Cuker A. Risk for Recurrent Venous Thromboembolism and Bleeding With Apixaban Compared With Rivaroxaban: An Analysis of Real-World Data. Ann Intern Med. 2022 Jan;175(1):20-28. Epub 2021 Dec 7. Erratum in: Ann Intern Med. 2022 Nov;175(11):1627-1628. [CrossRef] [PubMed]
| Year | Population of the Russian Federation | Incidence of COVID-19 in the Russian Federation, people |
|---|---|---|
| 2020 | 144 100 000 people | 3 159 297 people |
| 2021 October- November | 143 400 000 people | 10 499 982 people |
| 2022 (01.01.2022) | 146 980 000 people | 10 684 204 people (10.01.22) |
| 2023 (01.01.2023) |
146 447 000 people | 4 718 854 people |
| Total | 23 078 812 people (confirmed cases) |
| Condition | Organization | Year | Organization abbreviation with expansion |
|---|---|---|---|
| COVID-19 | Russian Society of Cardiology | 2020 | RSC 2020 (Shlyakhto E.V.) [23] |
| COVID-19 | The All-Russia Public Organization «Federation of Anaesthesiologists and Reanimatologists» | 2020 | FAR 2020 (Zabolotskikh I.B.) [24] |
| COVID-19 | Russian Society for the Prevention of Non-communicable Diseases | 2021 | ROPNIZ 2021(Drapkina O.M.) [25] |
| COVID-19 | Russian Society for the Prevention of Non-communicable Diseases | 2021 | ROPNIZ 2021 (Livzan M.A.) [26] |
| COVID-19 | Research Institute for Healthcare Organization and Medical Management of Moscow Healthcare Department | 2021 | NIIOZMM 2021 (Khripun A.I.) [27] |
| COVID-19 | Moscow City Scientific Society of Physicians | 2021 | MGNOT 2021 (Vorobyev P.A.) [28] |
| COVID-19 | Ministry of Health of the Russian Federation | 2022 | MoH 2022 [29] |
| SCOPE AND PURPOSE | Е1 | Е2 | Е3 | Total (n) | Total (%) |
|---|---|---|---|---|---|
| 16 | 21 | 21 | 58 | 90.7% | |
| The overall objective(s) of the guideline is (are) specifically described. | 6 | 7 | 7 | 20 | |
| The health question(s) covered by the guideline is (are) specifically described. | 5 | 7 | 7 | 19 | |
| The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described. |
5 | 7 | 7 | 19 |
| № | DOAC | DDD/ course of treatment or prophylaxis /patient | CPG |
|---|---|---|---|
| 1. | Rivaroxaban | 15 DDDs (30 days of course); 22,5 DDDs (45 days of course) |
MoH 2022; ROPNIZ 2021 (Drapkina); ROPNIZ 2021 (Livzan) |
| 2. | Apixaban | 15 (30 days of course); 22,5 (45 days of course) |
MoH 2022; ROPNIZ 2021 (Drapkina); ROPNIZ 2021 (Livzan) |
| 3. | Dabigatran etexilate | 22 (30 days of course); 15 (with creatinine clearance 30-49 ml/min) |
MoH 2022; ROPNIZ 2021 (Livzan) |
| 4. | Rivaroxaban | n/a | NIIOZMM 2021, MGNOT 2020, FAR 2020 |
| 5. | Apixaban | n/a | |
| 6. | Dabigatran etexilate | n/a |
| Name of the drug | 2020 (DDDs / year) | 2021 (DDDs / year) | 2022 (DDDs / year) |
|---|---|---|---|
| Apixaban | 84 000 000 DDD | 165 360 000 DDD | 114 000 000 DDD |
| Rivaroxaban | 84 000 000 DDD | 82 680 000 DDD | 58 500 000 DDD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





