1. Introduction
Infectious diseases, preterm birth complications, and birth trauma are the leading causes of death in children under the age of 5 [
1]. Due to the immaturity of the immune system, children are particularly prone to infectious diseases and their poor outcomes. Research suggests that over 60% of child deaths may be preventable with correct actions with both maternal and childhood vaccinations playing a huge role in infectious disease prevention [
2,
3]. Country-wide vaccination programs provide access to immunizations for all children and high immunization rates which create herd immunity and the possibility of eradication of some infectious diseases [
4,
5]. Recently, however, vaccine hesitancy is believed to be the biggest threat to the vaccination programs which is reflected in decreasing immunization rates and an increasing number of reported cases of infections such as measles or pertussis [
6]. The responsibility lies both on governments and on physicians to implement strategies for educating parents about vaccines and the immune system, providing reliable information, and dispelling doubts which all play a huge role in combating vaccine hesitancy among parents [
7].
2. Materials and Methods
Research for this paper was conducted through PUBmed searches in English concerning the topics of immunology, childhood vaccinations and vaccine-preventable infectious diseases. It also involved Google searches both in English and Polish and sourcing information from World Health Organization websites. Information concerning the epidemic situation in Poland and law regulations was sourced from the official websites of the Polish government, Sanitary Inspectorate, and Statistical Office.
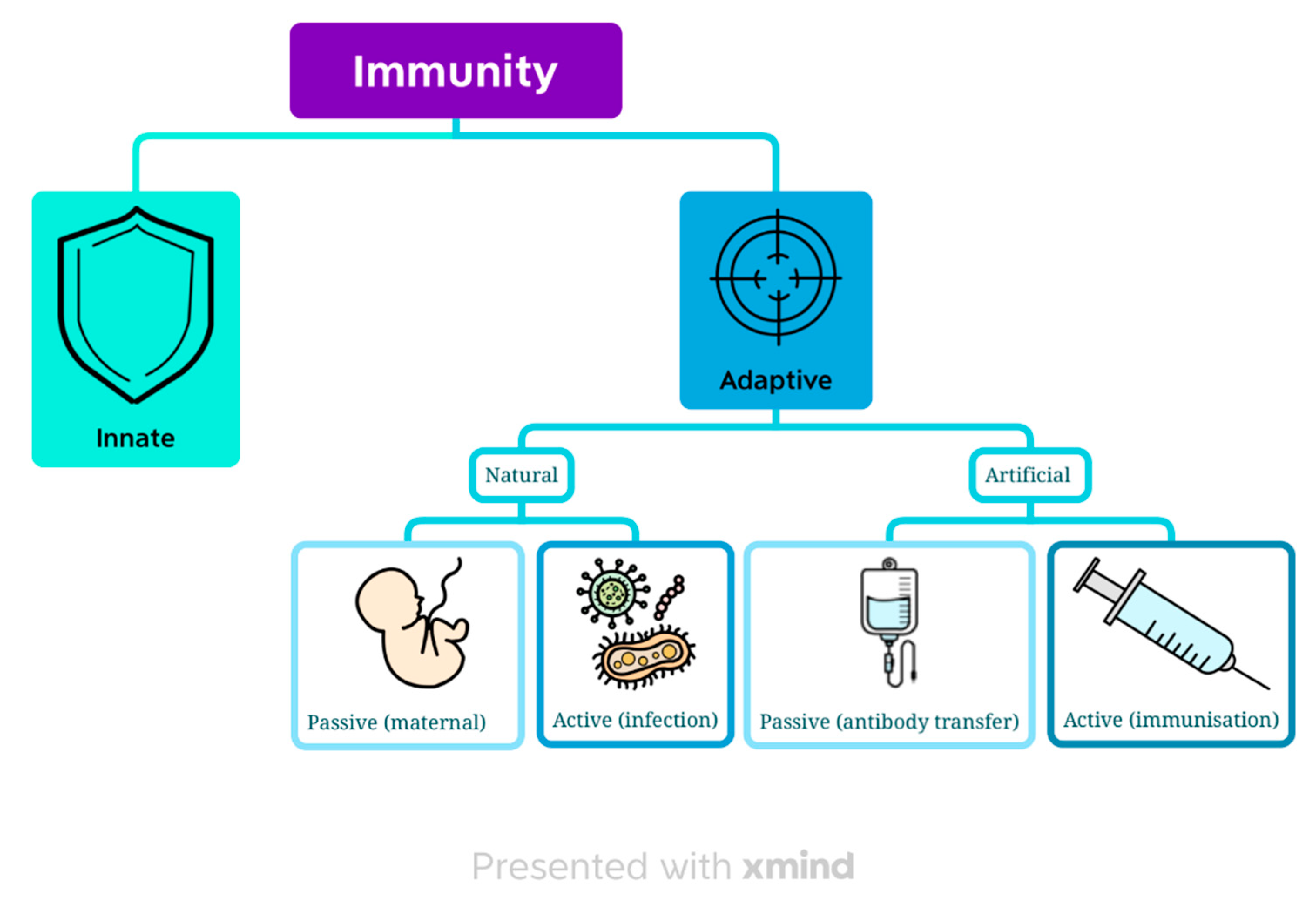
3. Immunity
The main objective of the immune system is to provide a defensive reaction against pathogenic microbes without destroying the body’s own tissues in the process [
8]. We can divide immunity based on two criteria: the time and how it is acquired into innate and adaptive, natural or artificial (
Figure 1).
3.1. Innate immunity
Innate immunity is present in the body from birth. It focuses on recognizing the damage to cells or a pathogen as foreign through DAMPs (Damage-Associated Molecular Patterns) or PAMPs (Pathogen-Associated Molecular Patterns) [
9]. This type of immunity is not antigen-specific [
8] but is able to distinguish foreign particles from self [
10]. This response can be initiated by any cell in the body and happens quickly but does not provide an immunity memory. It does not involve T- and B-lymphocytes, so it falls into the non-cell mediated immunity category [
9]. Moreover, it only recognizes extracellular pathogens so is predominantly targeted towards eradicating bacteria [
10].
3.2. Adaptive immunity
The main characteristic differentiating adaptive immunity from innate is its antigen-specificity. It can be divided into two main types based on which immunity cells are activated. Humoral-mediated immunity depends on B cells and their ability to produce antibodies. It targets mainly extracellular bacteria which are neutralized by antibodies in the blood or on the surface of mucosas. Intracellular bacteria are immune to antibodies as they cannot penetrate inside a cell. Those bacteria together with fungi are targeted by cell-mediated immunity with T-cells involved. In viral infections, both types of immunity are activated [
8,
11].
3.2.1. Natural adaptive immunity
Natural adaptive immunity can be divided into active and passive.
Active natural adaptive immunity is acquired through infection, either symptomatic or asymptomatic [
8]. The difference in immune responses to different microorganisms results in immunization of varying quality. Long-term and highly antigen-specific immunity results from an infection caused by an extracellular bacteria or a virus with a low mutability by activating humoral-mediated immunity [
8,
12]. The primary immune response is not immediate after the first contact with the antigen as it takes a couple of weeks to develop IgM antibodies and generate memory T and B cells. During the next exposure, those cells induce a secondary response, producing the IgG antibodies way quicker than during a primary response [
13].
Passive natural adaptive immunity refers to a transfer of ready antibodies from mother to baby through the placenta or breast milk. IgG immunoglobulins are mainly transferred through the placenta whereas breast milk is rich in IgA antibodies [
14]. Therefore a baby cannot gain natural adaptive passive immunity towards pathogens that trigger only cell-mediated immunity, as those cells cannot pass through the placenta [
8].
3.2.2. Artificial adaptive immunity
Active artificial adaptive immunity aims to emulate a natural immune response without the actual infection [
8] by targeting humoral immunity [
15]. Vaccines are medical products containing immunogenic antigens used to provoke a primary immune reaction and production of immunity memory cells [
16] and are the most reliable and effective way of infectious disease prevention [
17,
18]. The aim of popular vaccination programs is to keep a high vaccination coverage rate in the population, create herd immunity, and hopefully completely eradicate the disease [
8]. Herd immunity occurs when an adequate proportion of the population is immunized and is a sufficient way to prevent infectious diseases in people who cannot be vaccinated [
19,
20].
Passive artificial adaptive immunity is a short-term immunization acquired through an injection of antibodies. It is used in case of already active infection as it works immediately [
8]. It is also independent of the patient’s own immune system and therefore fit for immunocompromised patients or those with vaccine contraindications [
14,
15].
4. A brief history of vaccinations
The most important infectious diseases concerning humans are relatively young and emerged around 11000 years ago following the establishment of agriculture. Dense populations created perfect conditions for these infections to spread which was impossible before that point. Most of these diseases were originally only present in animals and then evolved to affect humans [
21,
22]. The diseases that have appeared repeatedly in historians’ reports are bubonic plague, cholera, smallpox, and influenza [
23]. Attempts to avoid those diseases have been reported as early as the 15th century and involved variolation. The process in which a healthy person was intentionally exposed to pus or scab from smallpox blister in hopes of surviving the more mellow case of smallpox and acquiring lifelong immunity to it [
24,
25]. A modern history of vaccinations began in 1796 with Edward Jenner in the 18th century with the same concept. He successfully immunized an 8-year-old boy against smallpox using pus from the hand of the milkmaid sick with cowpox [
24,
26]. The 19th century brought a breakthrough with the development of vaccines against diphtheria, rabies, and typhoid. In 1918 and 1919 Spanish flu killed nearly 50 thousand people worldwide so creating an influenza vaccine became a priority. It was developed and approved for use in 1945. The Polio vaccine was developed a decade later. In 1967 WHO announced an Intensified Smallpox Eradication Programme [
18]. In 1980 WHO declared smallpox eradicated. The second half of the 20th century brought us the majority of vaccines we use today: against hepatitis B, rubella, mumps, measles, pneumococcal pneumonia, Haemophilus influenzae type B. In 1988 WHO launched a Global Polio Eradication Initiative and in 2003 polio was completely eradicated in the Americas and Europe remaining endemic in 6 countries [
24]. In 1999 the first rotavirus vaccine was invented but unfortunately withdrawn from use a year later because of severe side effects. A safer version was introduced in 2006 [
27]. The 21st century brought the first vaccine against cancer with an HPV vaccine approved for use in 2006. In January 2020 WHO announced the state of the pandemic and the search for the vaccine which was developed and approved for use in December 2020 [
24].
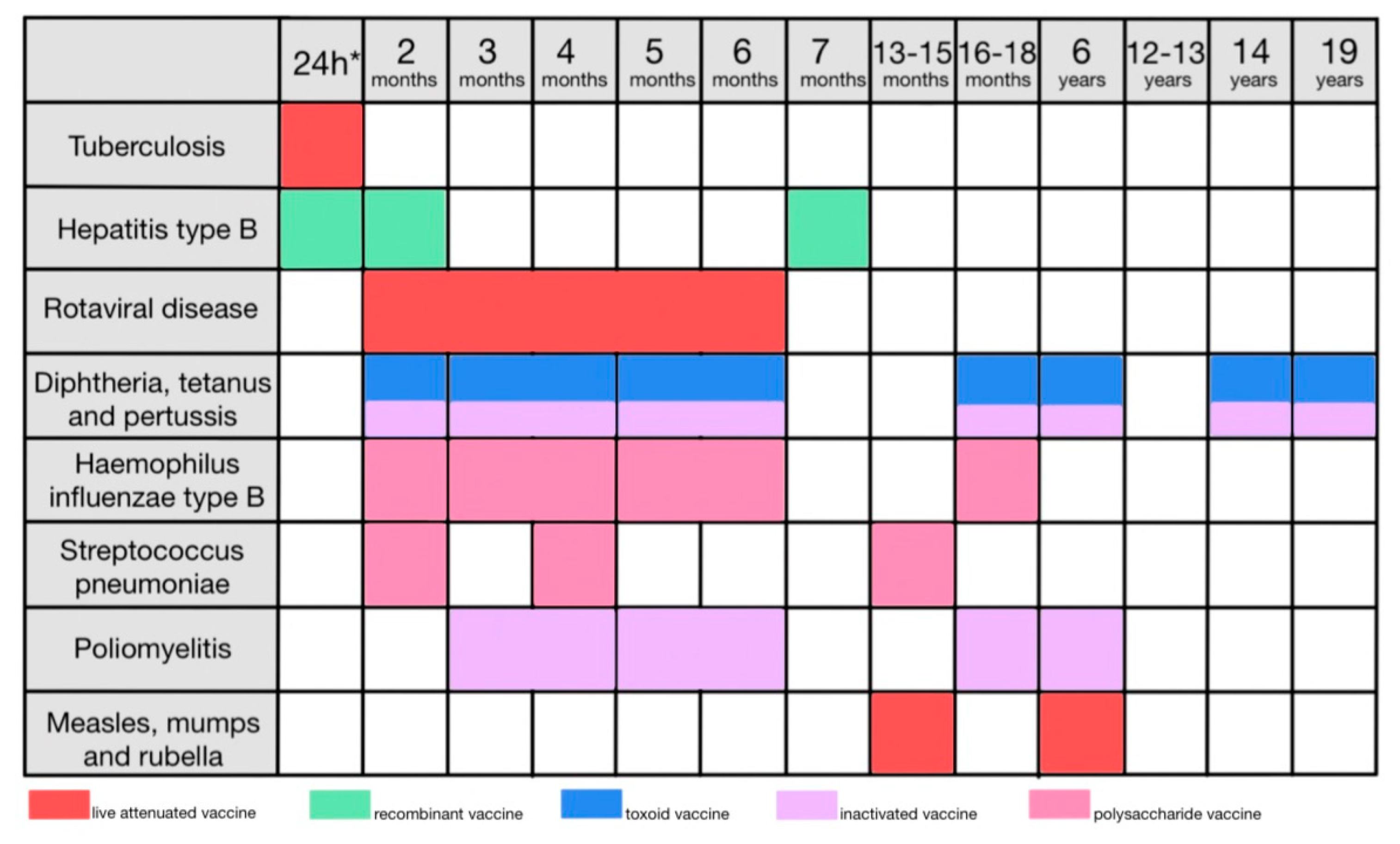
5. Types of vaccines
The most basic method of categorizing vaccines is based on whether they contain live or killed pathogens [
8,
15].
5.1. Live-attenuated vaccines
They contain live pathogens that have been modified to be harmless and not result in disease [
28]. Despite that, all the antigenic properties remain unchanged making this type of vaccine the most immunogenic of all [
8]. The immune response is strong and almost identical to one generated by an actual infection. That is why one dose is usually sufficient enough to achieve a life-long immunization [
15]. Another consequence of containing live pathogens is that they have to be stored in cold conditions which restricts their use in regions with limited access to refrigerators [
29].
Live-attenuated vaccines need additional caution in comparison to other types of vaccines due to the presence of live pathogens in the formulation. They are contraindicated during pregnancy, in severe congenital immunodeficiencies, and in states of immunosuppression [
28]. It is important to note, however, that despite an active phase of AIDS being a contraindication to live vaccines, the HIV infection itself, is not [
8].
5.2. Inactivated vaccines
They contain killed pathogens creating a lesser immune reaction and, therefore, a shorter-lasting immunity [
8]. They oftentimes require a booster shot to prolong the disease resistance [
28].
5.3. Toxoid vaccines
Created using strains of bacteria that produce strong toxins that are denatured in the vaccine manufacturing process [
15]. That modified toxin is safe while retaining its antigenic properties [
8]. They may require booster shots [
28].
5.4. Subunit, polysaccharide and conjugate vaccines
They contain fragments of pathogens that generate an immune response. Subunit vaccines contain proteins with immunizing properties, whereas polysaccharide vaccines are fragments of bacterial capsules[
8]. Polysaccharide vaccines are not effective in children under the age of 2 as until that age antibody production by B-cell is T-cell mediated [
30]. Conjugate vaccines contain a polysaccharide antigen bound to an immunogenic protein which induces a T-cell response. This provides antibody production in children under the age of 2 [
30]. They may require booster shots [
28].
5.5. Recombinant vaccines
They consist of particular particles of pathogens which are obtained through genetic engineering [
8]. They guarantee the purity and effectiveness of the vaccine [
31,
32] lowering the risk of allergic reactions. They require booster shots [
28,
32].
5.6. mRNA (messenger RNA) vaccines
They work by delivering an mRNA coding a particular antigen to the host whose muscle cells from the injection site start producing the protein antigen [
8]. This technology was used to produce a COVID-19 vaccine in which a coronavirus “spike” protein was used as an antigen [
29]. They are viewed as the future of vaccines due to their high effectiveness, low risk of adverse effects, relatively low attainment costs [
33], short development and production times, and flexibility to new variants [
34].
5.7.Viral vector vaccines
They use a modified virus to deliver the antigenic protein to the host [
8,
28]. The vectors have been altered to be replication-defective and therefore do not produce progeny virions [
35]. The most common vectors are adenoviruses [
35,
36] but influenza virus, measles virus, fowlpox virus, and herpes simplex virus have also been used [
29,
35].
The type of vaccine dictates the vaccination schedule, need for booster doses, and specific contraindications (
Table 1).
6. Contraindications to vaccines
Literature is consistent that there is one contraindication to all vaccines: a severe anaphylactic reaction to a previous dose of the vaccine or to the vaccine component [
8,
34,
37].
Acute infection, with or without fever, or exacerbation of the chronic disease is not an absolute contraindication. However, it is advised to postpone the vaccine until after the infection [
8,
37]. Even though the ongoing infection does not lower the efficacy of immunization, its natural progression may interfere with the vaccine’s adverse effects [
37] resulting in either overreporting or underreporting. Vaccines may be administered in the presence of a contraindication if the benefits outweigh the risks [
8,
38,
39].
6.1. Contraindications to live vaccines
Live vaccines are contraindicated in immunocompromised patients [
8,
37,
40] (
Table 2). The cases of infection in immunocompromised patients caused by the strain of virus used in a vaccine are not unheard of [
40]. Those patients are at a high risk of acquiring infectious diseases, however, so the decision about vaccinations should be made after considering the patient’s individual condition.
Live vaccines are contraindicated during pregnancy [
8] as pregnant women are at a higher risk of infectious diseases and poor outcomes to the fetus [
41], especially during the third trimester [
42]. The safety of the preservatives used in vaccines has also been questioned [
41]. There are, however, vaccines catered to pregnant women to bypass this contraindication. An inactivated influenza vaccine is recommended for women who are pregnant during the influenza season [
41] and it is recognized that maternal vaccinations lower the infant hospitalization rates due to laboratory-confirmed influenza [
3].
Breast-feeding is not a contraindication to any routine adult immunizations [
41].
7. A history of the Preventative Vaccinations Program in Poland
The system of common preventive vaccinations for children in Poland was first implemented in 1960 and included three vaccines. A live vaccine against tuberculosis was administered subcutaneously in the first month of the child’s life. An attenuated smallpox vaccine was administered at the age of 6-7 months and then again at 7 years of age. Lastly, a combined DTP vaccine against diphtheria, tetanus, and pertussis was given at 3, 4 and 5 months, and repeated between 18 and 24 months. At the age of 7, a child would receive a booster shot against diphtheria and tetanus. In 1965 a typhoid vaccine was added to the program for 12, 15 and 18-year-olds. In 1972 guidelines regarding the typhoid vaccine were updated and doses were scheduled for 5, 6, 14, 18 and 20-year-olds. 1972 was also a year when an oral poliomyelitis vaccine entered the system, administered between 18 and 24 months with a booster shot at the age of 7. 1975 brought an update to a poliomyelitis vaccine pattern. It was now administered in three primary doses at 6, 7 and 8 months of age and a booster shot between 18 and 24 months. From now on children would also receive a measles vaccine at 13-15 months. The typhoid vaccine pattern was updated again and would include a single dose at the age of 6. Two years later , DTP and poliomyelitis vaccine patterns were updated again. They were both administered in three primary doses at 3, 4-5 and 6 months old with a booster shot at 18-24 months and an additional DT booster at 6 years old [
43]. Since 1980 typhoid and smallpox vaccines were no longer a part of the childhood vaccination system due to the successful eradication of these diseases on Polish territories [
44]. In that same year, diphtheria and tetanus booster shot was scheduled for 14-year-olds and a tetanus booster for 18-year-olds. The program remained unchanged until 1989 when the rubella vaccine was introduced for children at the age of 13 and a poliomyelitis booster shot for 11-year-olds. Only one year later another booster for poliomyelitis was introduced for 6-year-olds and in 1991 measles booster for 10-year-olds. In 1994 a tuberculous vaccine booster shot was introduced to be administered at the age of 7 and in 1996 decision was made to administer the primary dose in the first 24 hours of the newborn’s life and that has remained unchanged until the present day. In that same year, a hepatitis B vaccine was introduced with three primary doses at 24 hours, 2 months and 3 months, and a booster shot at 12 months. In 1998 the third primary dose of hepatitis B was removed and the poliomyelitis vaccination program was completely revised [
43,
45]. The intramuscular poliomyelitis vaccine was administered at the age of 3 to 4 months and followed by several doses of oral vaccine, at the ages of 5, 6-7, 16-18 months, 6 and 11 years. The dose at 11 years old was removed from the vaccination calendar in 2000. The year 2004 brought some changes to the timing of DTP and poliomyelitis vaccines [
43]. They were scheduled almost identically which made the vaccination process more convenient to the parents. Also, the poliomyelitis vaccine had three primary doses administered intramuscularly and only the booster administered orally. That was also the year when a MMR vaccine was introduced and scheduled for 13-14 months of age with a measles booster at 7 years and a rubella booster at 13. One year later these boosters were swapped for a MMR booster between the age of 12 and 13, and finally in 2006 were set for the age of 10. In 2007 a Haemophilus influenza type B vaccine was introduced with a schedule mirroring those of poliomyelitis and DTP. In 2016 a poliomyelitis booster was changed from oral to intramuscular to match other doses. In 2017 a pneumococcal conjugate vaccine was introduced for children in second, fourth and fourteenth month of life. In 2019 a MMR booster was moved from 10 to 6 years of age therefore matching the time of DTP and poliomyelitis boosters. Since 2019 there have been no major changes in the childhood vaccinations calendar except the addition of a rotavirus vaccine in 2021 for infants between 2 and 6 months of age [
43,
46,
47].
8. Law regulations of the Preventive Vaccinations Program in Poland
The need for mass immunization of children due to outbreaks of infectious diseases has resulted in the creation of mandatory childhood vaccination programs in many countries [
48]. Although the policies vary across counties they often center around school entries [
49]. Most counties however opt for unified immunization schedules with an option to implement an individualized vaccine calendar if the child’s health prohibits them from following the usual schedule [
8,
49,
50]. With the increasing popularity of and the parents' reluctance and distrust towards vaccines, the responsibility of the government to provide immunization programs is even greater [
51,
52,
53,
54]. Non-compliance penalties are often implemented, and the educational penalty (meaning the limitation of access to school until the vaccine is received) is the most common [
55].
Italian Pediatric Society has compared 31 European Countries and 35% of them, Poland included, have mandatory vaccines against diphtheria, tetanus, pertussis, hepatitis B, Haemophilus influenzae type B, poliovirus, measles, mumps, and rubella [
56]. Mandatory vaccinations are funded by the government whereas the cost of the recommended vaccines is covered by the patient [
57].
8.1. Law in Poland
Poland has a program of mandatory childhood vaccinations [
46] that applies not only to Polish citizens but to all people who reside on the territory of Poland for longer than 3 months [
8,
57]. The country-wide program of mandatory vaccinations is regulated by the Statute from the 5th of December 2008 about prevention and combating infections and infectious diseases in humans [
57]. Regulation of the Minister of Health from the 18th of August 2011 in regard to mandatory preventative vaccinations established vaccinations against 13 diseases as mandatory [
58] with a 14th added in 2020 [
59] and currently the list consists of diphtheria, tuberculosis, invasive infection with Haemophilus influenzae type B, invasive infection with Streptococcus pneumoniae, pertussis, mumps, measles, chickenpox, polio, rubella, tetanus, hepatitis type B, rabies, and infection with rotaviruses.
The administration of a vaccination must be preceded by a doctor’s examination confirmed by a certificate which is valid for 24 hours. If the examination finds a person unable to receive a vaccination for a prolonged period of time, that patient is directed under specialist care. A person issuing a birth certificate for the newborn is obliged to create an immunization card that remains at the health care provider and a booklet of childhood vaccinations that is given to the patient’s caregivers. Both documents are filled with confirmations of the following vaccinations during the course of the child’s life [
57].
8.2. Adverse effects reporting
A doctor or feldsher suspecting or identifying an adverse effect is obliged by law [
57] to report it to the district sanitary inspector within 24 hours of raising the suspicion. The district sanitary inspector fills the report with additional information in regard to the circumstances of the case, reports it to the national sanitary inspector and maintains a record of all vaccine-related adverse effects that happened in their district [
8,
57]. If the adverse effects are severe enough to perform the conditions mentioned in the Statute [
57], the patient is entitled to compensation.
8.3. Non-compliance punishments
The Statute from the 5th of December 2008 about prevention and combating infections and infectious diseases in humans does not establish clear punishment for evading mandatory vaccinations [
57]. If parents do not fulfill the obligation they first receive a summons to appear at the vaccination visit. Following legal actions include a warning and a fine of up to 10,000 złotych (PLN) at a time [
60].
8.4. Ukrainian war crisis
Poland is also facing challenges following the war in Ukraine and the incoming migrants with 1.56 million registered Ukrainian refugees [
61]. Many of them have not been vaccinated against the diseases included in the Polish vaccination program [
62]. The vaccination schedule for Ukraine [
63] is similar to Poland, however, the vaccination rates are much lower [
62]. Ukraine is one of the countries with the most cases of measles in recent years (4667) with the vast majority having occurred in unvaccinated people [
51]. Ukrainian refugees have the same right to health care in Poland as its citizens, which includes access to vaccinations [
8,
61]. Many parents, however, cannot provide reliable information regarding the immunization status of their children. The poliomyelitis vaccine is the most problematic with 12% of parents not knowing if their child has received it [
62].
9. Mandatory vaccinations for children and adolescents in Poland
The program of mandatory vaccinations is arranged in a schedule for the parents to follow and is mandatory on all children (
Figure 2).
9.1. Tuberculosis
9.1.1. The disease
Tuberculosis is an airborne infectious disease caused by Mycobacterium tuberculosis transmissible during an active pulmonary stage [
64]. In 75% of cases it involves the lungs but generally may include any organ [
65]. Until 2020 tuberculosis was a leading cause of death due to a single agent worldwide [
66,
67]. Patients with active pulmonary tuberculosis experience fever, fatigue, weight loss, and persistent cough [
64]. A combination of isoniazid, rifampicin, ethambutol, and pyrazinamide, followed by a combination of isoniazid and rifampicin only is the standard treatment with liver damage being a common adverse effect of this treatment [
65]. A major risk factor for tuberculosis is HIV as in 2016 22% of patients who died of TB were HIV positive [
66].
In 2021 there were 3553 cases of tuberculosis reported in Poland, which is 316 more than in 2020 but 1522 less than in 2019 and 3101 less than in 2009 [
68]. The persistent downward trend in tuberculosis morbidity, however, results more from increased detectability of the infected and their effective treatment than from vaccinations [
8].
9.1.2. Vaccination
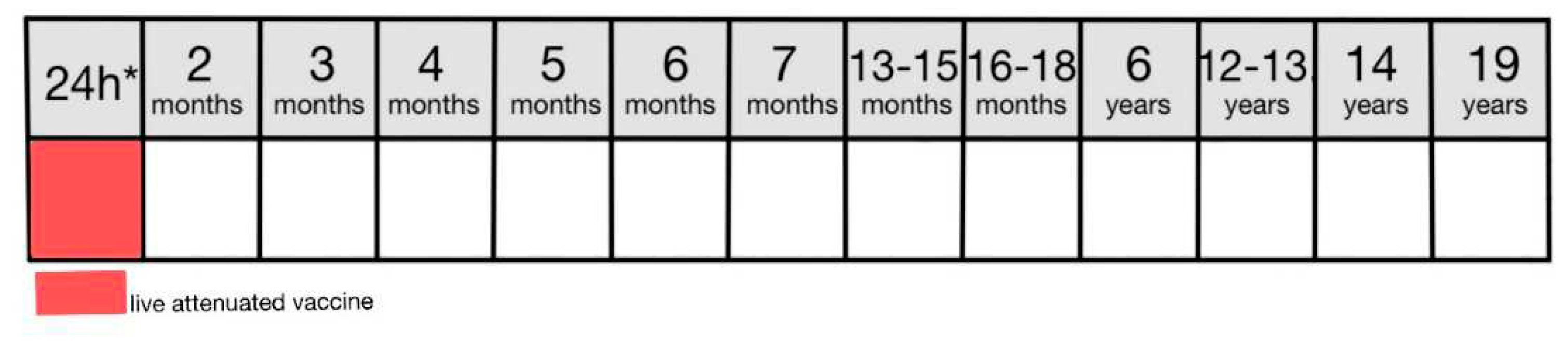
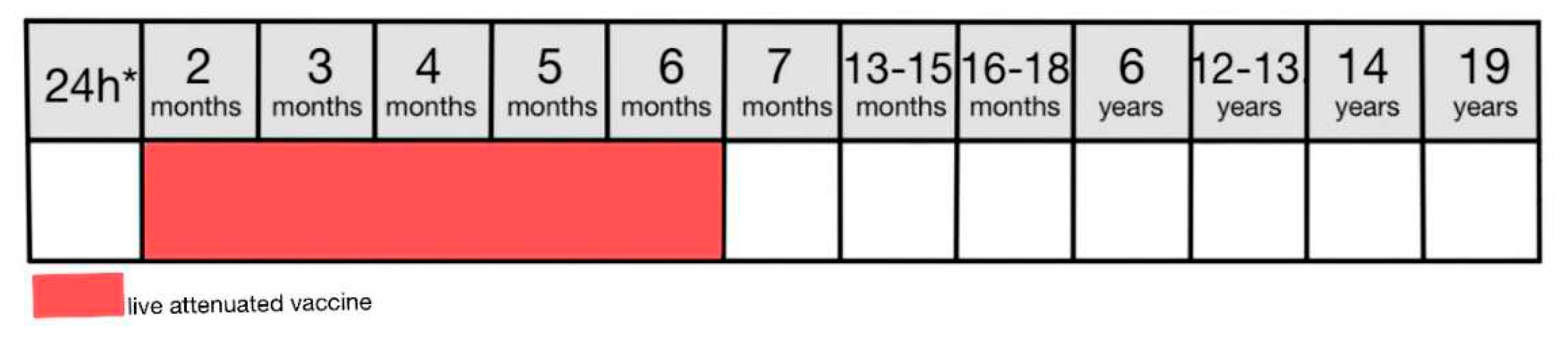
In Poland, a BCG vaccine is used which is a live attenuated vaccine produced from the strain of Mycobacterium bovis BCG Moreau [
8,
69,
70]. This vaccine is mandatory for all newborns and should be administered on the first day of life (
Figure 3) or before discharge from the neonatal ward [
8,
46]. In 2021 immunization rate for infants was 92.2% [
71,
72]. BCG vaccine is particularly effective in the prevention of tuberculous meningitis in infants and young children [
69]. The vaccine is administered subcutaneously using the Mantoux method in the upper third of the arm in a single dose regardless of the patient’s age [
8]. Literature expresses some concerns considering the efficacy of the BCG vaccine in providing lifelong immunity [
8,
69]. Despite that, WHO has not recommended repeating the dose as there is no evidence supporting the use of a booster dose in this case [
8].
After the correct administration of the vaccine, a blister should form and subside after a few minutes. After 2-3 weeks an infiltration should form with a small blister in the middle that progresses to a sore that heals spontaneously in 2-3 months forming a 3-10mm scar. A minor lymphadenopathy is also normal and not concerning [
8].
9.1.3. Contraindications
All the contraindications to live vaccines also apply to the BCG vaccine. In addition to that, a BCG vaccine is contraindicated in the following circumstances [
8]:
9.1.4. Adverse effects
The regional response rate is high at 76.6% [
73]. The most common adverse effects are not life-threatening or severe and include abscess, lymphadenopathy, excessive infiltration, ulceration, and erythema [
74]. Rarely, a BCG vaccine may result in purulent lymphadenitis or a BCG infection [
8] with almost all patients suffering from these complications being HIV positive [
74]. The vaccine is considered safe [
73] and used in Poland and worldwide [
69].
9.2. Hepatitis type B
9.2.1. The disease
Hepatitis B virus (HBV) is a DNA virus that causes acute and chronic liver infections [
75]. It is transferred through contact with infected blood or semen [
76]. Antiviral treatment is not effective in acute hepatitis B which presents with jaundice and elevated serum alanine transaminase levels and positive testing for hepatitis B core or surface antigens [
75,
77]. If the antigens are present for over 6 months we consider it a chronic disease [
75] which greatly increases the risk of liver cirrhosis and hepatocellular carcinoma [
76]. Long-term antiviral treatment is possible in chronic hepatitis B to lower the risk of complications [
75]. Patients with renal impairment, the elderly, and pregnant women, however, have an increased risk of adverse effects such as nephrotoxicity, lactic acidosis, and resistance to treatment [
77]. The age of acquiring the infection determines the course of the disease and the risk of its progression to chronic hepatitis with infants being the most susceptible to poor outcomes [
78]. 80%–90% of infants infected during their first year of life and 30%–50% of children infected before the age of 6 years develop chronic hepatitis, as opposed to 1%–5% of patients who get infected as adults [
79,
80].
9.2.2. Vaccination
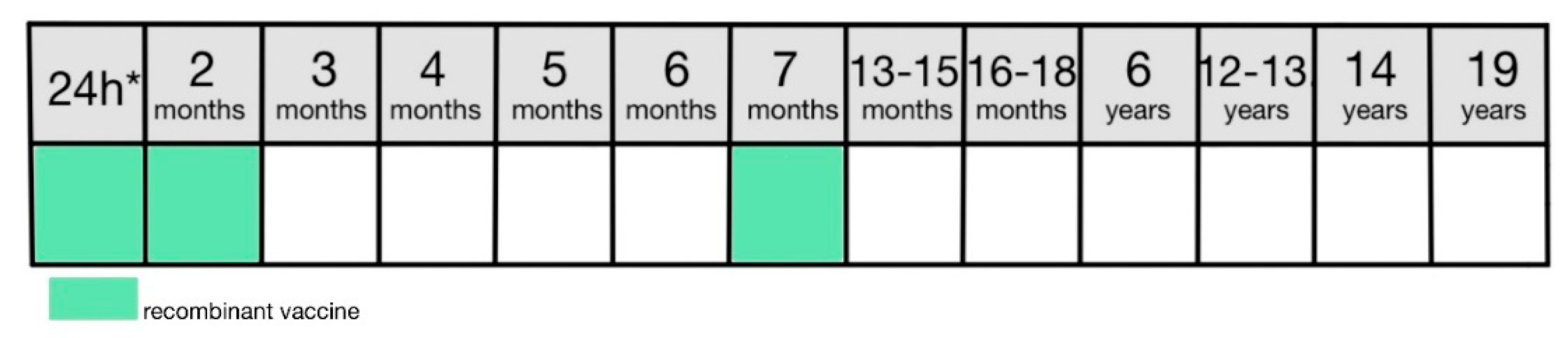
The hepatitis B vaccine is recommended for all newborns within the first 24 hours from birth [
8,
75]. In Poland, a recombinant intramuscular HBV B vaccine is used [
8] which is highly immunogenic with an efficacy rate of 95% in infants, children and adults [
78]. The main objectives are to prevent infections, prevent transmission of the virus and prevent hepatitis D as it only develops in patients infected with HBV B [
8]. In Poland, the standard vaccination consists of two doses administered respectively in the first 24 hours of life and in the second month of life. The booster dose is scheduled for the seven-month-olds (
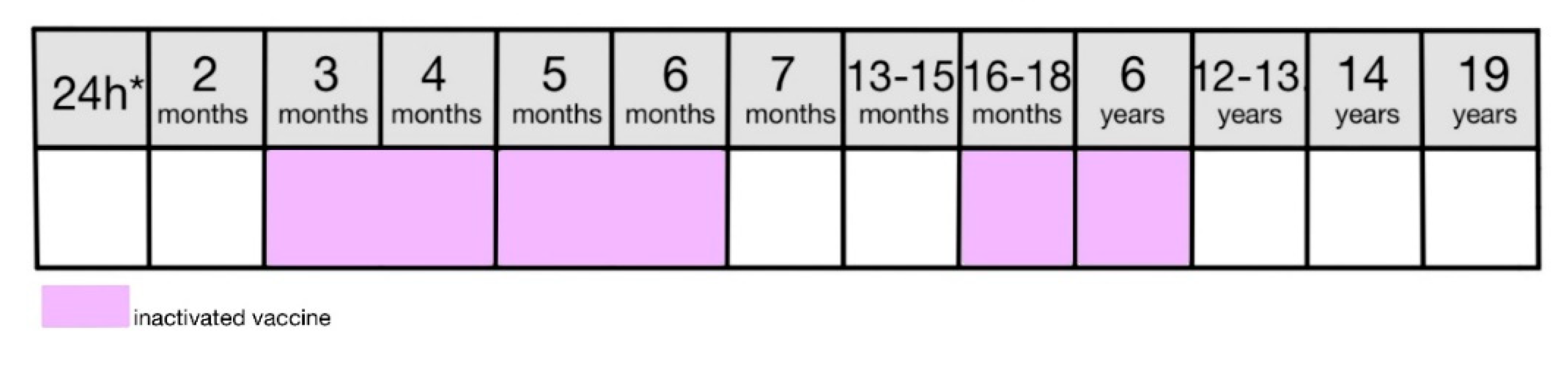
Figure 4) [
8]. In 2021 immunization rate for 2-year-olds was 97.2% [
71,
72].
9.2.3. Contraindications
There are no specific contraindications to this vaccine [
8]. It is recommended to postpone the vaccine in newborns weighing less than 2000g [
75].
9.2.4. Adverse effects
The adverse effects are minor and regional usually including erythema and tenderness [
8].
9.3. Rotaviral disease
9.3.1. The disease
Diarrhoeal diseases are one of the leading causes of death in children under the age of 5, causing over 500,000 deaths annually worldwide, the majority in low-income countries [
81]. Rotaviral infections are responsible for over 200,000 deaths globally each year despite the vaccinations [
82]. Environmental enteropathy, which is more prevalent in low-income countries, lowers the efficacy of the vaccine and is responsible for higher morbidity and mortality rates in those regions [
83]. In low-income countries, 60-80% of severe rotavirus disease cases concern children between 12 and 15 months of age whereas in industrialized countries 75% of cases happen between 3 and 35 months [
84]. The virus causes severe dehydrating gastroenteritis through the destruction of absorptive enterocytes and stimulation of intestinal secretion [
82].
Since 2015 number of cases of acute diarrhoea in children under the age of 2 has decreased by 53.1% with 33,943 cases of rotaviral infections reported [
72]. In 2021 in Poland, there were 20,384 cases reported of acute diarrhea in children up to the age of 2 including 7,417 cases caused by rotaviruses.
9.3.2. Vaccination
The rotavirus vaccine was introduced into the Polish system of mandatory childhood vaccinations in 2021 and provides protection against severe rotaviral disease lowering hospitalization and mortality rates [
8,
72]. However, it does not provide immunization against rotavirus strains responsible for mild cases. The vaccine contains live attenuated viruses and is administered orally [
83]. There are two formulations available under the brand names Rotarix and RotaTeq. Rotarix is administered at 6 weeks of age with a second dose 4 weeks after the first. RotaTeq follows the same schedule but adds a third dose 4 weeks after the second with no booster doses recommended (
Figure 5). If the vaccination course is not completed before the age of 24 weeks for Rotarix or 32 weeks for RotaTeq it should not be continued regardless of the number of administered doses [
8].
9.3.3. Contraindications
In addition to standard contraindications for live vaccines, the rotavirus vaccine is contraindicated in patients with a history of intestinal intussusception or gastrointestinal tract defects predisposing to intestinal intussusception. Vaccination should be postponed in case of acute infections presenting with vomiting or diarrhea [
8].
9.3.4. Adverse effects
Both vaccines are considered safe with no specific adverse effects [
83]. However, due to the way of administration appetite loss, constipation, diarrhea, or vomiting may occur [
8].
9.4. Diphtheria, pertussis and tetanus
9.4.1. The disease
Diphtheria is an acute respiratory infection caused predominantly by toxigenic Corynebacterium diphtheriae strains and sporadically by toxigenic C. ulcerans and C. pseudotuberculosis strains transmitted by direct contact or respiratory secretions [
85]. Most commonly it presents as a classic respiratory diphtheria with the formation of membranous inflammation of the throat and possible transfer to other organs such as the myocardium and peripheral nerves which contributes to its fatality [
86]. Treatment consists of antitoxin administration. The disease is reportable to the WHO due to its high mortality rate [
87].
In 2022, over 5,800 cases of respiratory diphtheria have been reported to the WHO worldwide with the overwhelming majority of cases from India, Indonesia, Niger, and Nigeria. In Europe, the countries with the highest number of cases reported in 2022 were The United Kingdom Of Great Britain and Northern Ireland, Switzerland, and France with respectively 88, 86, and 60 cases [
88]. In Poland, in the years 2001-2021, there have been no reported cases of diphtheria [
72]. The second most common form of the disease is cutaneous diphtheria with a much milder course and significantly lower mortality rate [
85,
87].
Individual diphtheria vaccines are available but used rarely with all routine immunizations performed using the DTP formulations [
8].
Pertussis is an acute, extremely infectious illness caused by a Gram-negative bacterium Bordetella pertussis transmitted through the respiratory route and droplets [
89,
90]. A classic pertussis clinical presentation includes paroxysmal cough, whooping, and postussive vomiting without serious fever after a few days of non-specific symptoms [
90]. Pertussis is possible in vaccinated individuals with non-specific, milder, or no symptoms [
89] as the immunity provided by the vaccine wanes 5-10 years after the last dose [
91]. Antibiotic treatment with macrolides in the early stage before the occurrence of the cough reduces the severity and duration of the illness [
89]. The highest hospitalization and mortality rate is observed in infants under the age of 3 months as they have not yet received the vaccination [
90]. The vaccine has an 89% efficacy rate for preventing serious disease or death from pertussis [
91].
In 2022 in Europe, the highest number of cases of pertussis was in Germany with 3,462 cases reported, whereas worldwide pertussis was the most prevalent in China with 38,295 cases [
92]. In 2021 in Poland, 180 cases of pertussis were reported, compared to 4,956 in 2015 [
72].
Tetanus is a disease caused by the toxin of the bacterium Clostridium tetani and presents with muscle spasms and dysfunction of the autonomic nervous system [
93]. Spores of the bacteria are present worldwide in the soil and gastrointestinal tracts of animals, including humans. Infection happens through a wound or skin break contaminated with soil or feces containing the spores [
94]. The incubation period ranges from 4 to 14 days and the longer the distance between the wound and central nervous system, the longer the incubation period. Symptoms start with localized muscle spasms near the site of the injury [
95]. Generalized tetanus makes up over 80% of cases and is characterized by spasms of the masseter muscle, resulting in trismus and lockjaw [
94]. Treatment includes post-exposition vaccination, anti-tetanus immunoglobulin, or administration of metronidazole [
95].
Neonatal tetanus happens due to poor umbilical cord hygiene with an onset of symptoms 3-7 days after the delivery with the majority of the cases reported in East Asia and sub-Saharan Africa [
94].
In 2022, 6651 cases of tetanus were reported to the WHO worldwide, including 2076 cases of neonatal tetanus [
96,
97,
98]. In Poland in 2021, there were 5 cases of tetanus reported, compared to 2 cases in 2020 and 12 cases in 2015 [
72].
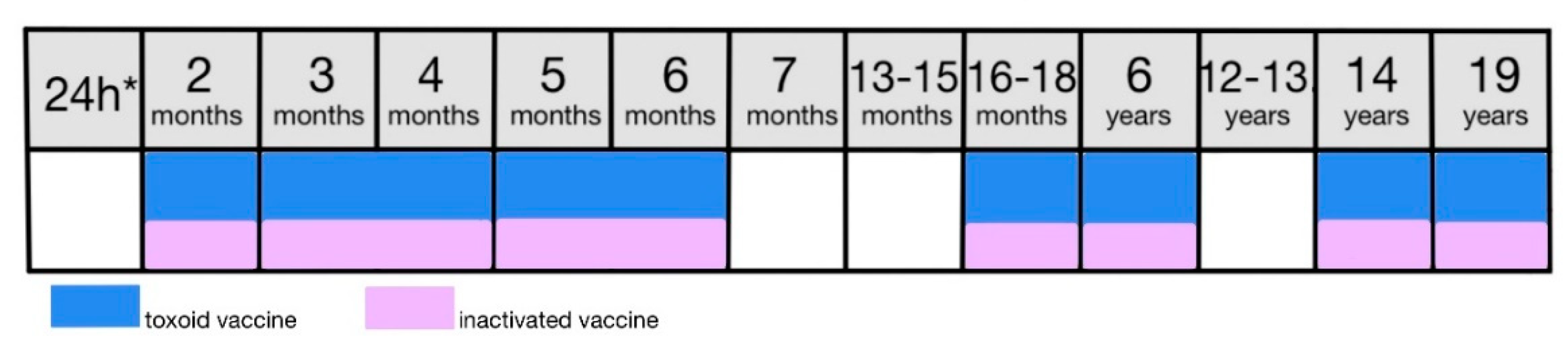
9.4.2. Vaccination
In Poland, a combined diphtheria, tetanus, and pertussis vaccine is used and two different types are available. Both versions contain a diphtheria toxoid and tetanus toxoid obtained from the diphtheria and tetanus toxins in addition to a pertussis component [
8]. DTPw (whole-cell) vaccine contains whole inactivated bacteria and the DTPa vaccine (acellular) contains pertussis antigens [
99]. The vaccine is administered in a 7-dose scheme consisting of 4 doses of basic vaccination and 3 booster doses [
46]. The primary dose at the age of 2 months, the second dose at 4 months, the third at 5-6 months, and a fourth at 16-18 months are executed using a DTPw vaccine. The first booster is administered the the age of 6 with a DTPa vaccine, second at the age of 14 with a dTaP vaccine and the course is completed with a third booster using a Td formulation (
Figure 6) [
8,
46]. DTPw according to its registration shall be used only for children younger than 3 and therefore children who have not completed their basic vaccination and are 3 or older should complete the course with a DTPa vaccine [
8].
In Poland in 2021, the immunization rate for basic doses against pertussis, diphtheria and tetanus was 83.8%, in comparison to 92.4% in 2015 [
72].
9.4.3. Contraindications
In case of serious contraindications for DTPw, immunization should be performed using the DTPa vaccine. If DTPa is also contraindicated, tetanus and diphtheria immunizations should be performed with pertussis vaccination omitted [
8].
In addition to standard contraindications true to all vaccines, both DTPw and DTPa have some specific ones. Both formulations are contraindicated in patients with progressive diseases of the nervous system. Additionally, DTPw is advised against in patients who suffered from serious adverse effects following the previous dose whereas DTPa is in those who developed an encephalopathy during the 7 days after the previous dose [
8].
9.4.4. Adverse effects
DTPw vaccine is responsible for over half of the adverse effects of vaccines reported in Poland. The adverse effects that forbid the administration of the following doses are [
8]:
Seizures, with or without fever, within 3 days from the vaccination,
Inconsolable cry lasting at least 3 hours within 24 hours from the vaccination,
Fever of 40.5 °C without other cause within 3 days from the vaccination.
The hypotonic-hyporesponsive episode is also a known adverse effect of a DTPw vaccination. Although some sources suggest withholding from the following doses after such an episode, there is no consensus or clear recommendation in that matter [
8,
100,
101].
9.5. Haemophilus influenzae type B
9.5.1. The disease
Haemophilus influenzae is a Gram-negative bacterium transferred through the respiratory route [
102]. It causes both mild infections of the upper respiratory tract as well as invasive ones including bacterial meningitis being the most serious presentation [
103]. Children under the age of two are the most susceptible to an invasive infection and its poor outcomes [
104]. Meningitis develops in approximately 66% of children with invasive infections and 15-30% of convalescents suffer from permanent neurological deficits such as deafness, epilepsy, or difficulty walking [
105,
106]. With increasing resistance of the bacteria to antibiotics, vaccines are crucial in order to reduce morbidity [
102].
In Poland in 2021, there were 52 cases of invasive infection with Haemophilus influenzae reposted, compared to 78 in 2020 and 62 in 2015 [
72].
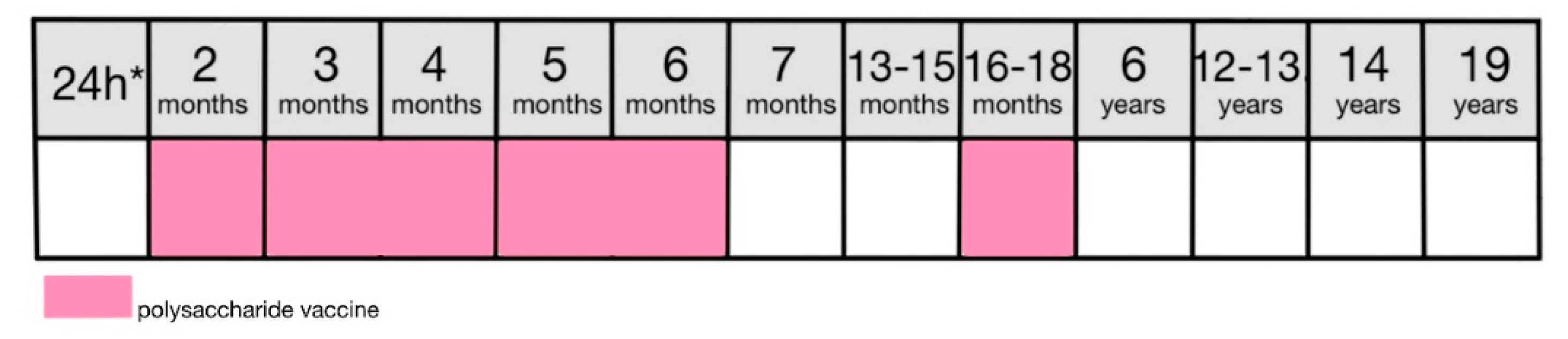
9.5.2. Vaccination
Vaccination is performed using the conjugate polysaccharide vaccine which ensures its efficacy in the targeted age group. Conjugate vaccines solved the problem of polysaccharide vaccines not being effective in children under the age of five. Binding the PS to the immunogenic proteins induces T-cells to produce antibodies as before the age of two antibody production is T-cell dependent [
104].
The scheme of vaccination is dependent on the child’s age. The Polish system of mandatory childhood vaccinations, however, suggests a 4-dose approach with 3 basic doses at 2, 3-4, and 5-6 months with a booster dose at 16-18 months (
Figure 7) [
8].
In Poland in 2021, the immunization rate was 93.8% for basic vaccination and 83.7% for a complete course, in comparison to respectively 97.8% and 92.5% in 2015 [
72].
9.5.3. Contraindications
There are no specific contraindications [
8].
9.5.4. Adverse effects
Sometimes mild regional or systemic (fever) adverse effects may occur. There are no specific adverse effects [
8].
9.6. Streptococcus pneumoniae
9.6.1. The disease
Streptococcus pneumoniae is a Gram-positive opportunistic bacteria carried in the human nasopharynx, more prevalent in children than in adults and transferred through close contact and aerosols [
107,
108].
It is the most prevalent cause of bacterial pneumonia [
108] but also commonly causes otitis media, meningitis, and sepsis [
107] with over 500,000 children worldwide annually dying due to pneumococcal infection [
109]. Pneumococcal virulence factors differ between strains and include the capsule, the toxin, and surface-associated proteins allowing biofilm formation, with the capsule being the most important [
110]. Children who are HIV-positive, have hematological cancers, or are immunodeficient have an increased risk of invasive pneumococcal disease [
111].
In 2021 in Poland, 932 cases of invasive Streptococcus pneumoniae infection were reported, compared to 629 in 2020 and 979 in 2015 [
72].
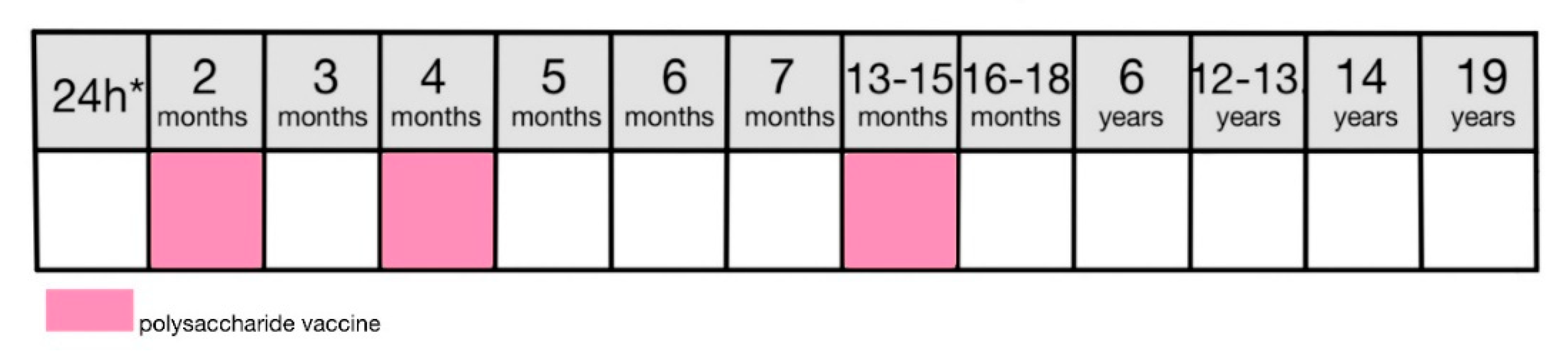
9.6.2. Vaccination
In Poland pneumococcal vaccine is administered in a three-dose scheme: at 6 weeks, 4 months and 13-15 months
Figure 8), and is mandatory for all children born after 31.12.2016. The oldest variation of the vaccine contained polysaccharides from 23 strains most commonly causing infection in young children [
109]. In Poland, basic vaccinations are performed with 10 or 13-valent formulation (PCV-10 or PCV-13) [
8]. As polysaccharide vaccines are not effective in children under the age of 2, linking it to the carrier protein ensures T-cell-dependant antibody production and lasting immunity [
30,
109].
The vaccine efficacy has been diminishing lately with the increasing prevalence of pneumonia cases caused by non-vaccine type S. pneumoniae strains [
109].
In Poland, in 2021 immunization rate was 94.0% for the primary dose and 87.9% for the full vaccination compared to respectively 93.3% and 87.8% in 2020 [
72].
9.6.3. Contraindications
There are no specific contraindications [
8].
9.6.4. Adverse effects
There are no specific adverse effects. Regional adverse effects are usually mild and systemic ones may include a fever [
8].
9.7. Poliomyelitis
9.7.1. The disease
Poliomyelitis is caused by an enterovirus transmitted through the fecal-oral route [
112]. The poliovirus incubates for 3-6 days and paralysis appears in 7-21 days [
113], with children under the age of five being the most susceptible to infection [
112]. 75% of the infections are asymptomatic. However, 0.5% of the infected will develop paralysis due to meningitis 1-3 weeks after the gastrointestinal or flu-like symptoms [
112,
114]. Muscle weakness and paralysis usually concern lower limbs but in severe cases may also involve breathing and swallowing muscles [
114]. Patients with symptoms suggestive of polio should seek medical attention and have a stool sample collected for testing for the presence of the virus. There is no specific cure or treatment for poliomyelitis, however, occupational physiotherapy is beneficial [
112,
114].
In 2003 polio was declared eradicated in Europe [
24] and currently only Pakistan, Nigeria and Afghanistan remain endemic sources of the virus [
115]. In the years 2001-2021 in Poland there have been no reported cases of poliomyelitis caused by a wild strain of poliovirus. There has been however one case per year reported in 2003, 2004, 2009, 2010, and 2014 caused by the strain used in vaccines [
72].
9.7.2. Vaccination
There are two types of polio vaccine. The oral poliomyelitis vaccine (OPV) is a live attenuated vaccine. There have been reports of OPV causing the infection and consecutive paralysis and therefore it has been slowly replaced with IPV (inactivated poliomyelitis vaccine) [
72,
115]. In April 2016 OPV was removed in Poland from routine use in mandatory childhood vaccinations and is no longer advised for recommended vaccinations [
8].
IPV contains three serotypes of inactivated polioviruses and induces close to 100% immunity [
8,
115]. It also includes a trace of antibiotics (streptomycin, neomycin, polymyxin) which may induce an anaphylactic reaction in those who are allergic [
8]. In Poland, basic vaccination consists of three doses administered respectively at 4, 5-6, and 16-18 months and the booster dose is scheduled for 6 years of age (
Figure 9) [
46]. The immunization rate for the standard vaccination (I + II dose) in 2021 was 83.9% and for the booster dose was 82.8% [
71,
72].
9.7.3. Contraindications
A specific contraindication to IPV is a history of allergy to streptomycin, neomycin, or polymyxin in addition to standard contraindications to live vaccines [
8].
9.7.4. Adverse effects
No severe adverse effects are associated with IPV [
115].
9.8. Measles, mumps and rubella (MMR)
9.8.1. The disease
Measles is an acute, febrile, highly contagious disease caused by the measles virus transmitted through a respiratory route [
116]. It remains the second most common cause of death due to a vaccine-preventable disease among children under the age of five [
117]. It should be suspected in patients presenting with a classic triad of the three “Cs”: cough, conjunctivitis, and coryza (runny nose) followed by a characteristic erythematous, maculopapular rash [
116,
118]. The rash usually starts behind the ears and on the face before spreading to the trunk and extremities [
119]. 1-2 days before the appearance of the rash, Koplik’s spots can be observed on the buccal mucosa as small white papules [
116]. In mild measles cases, patients recover in approximately a week with clinical symptoms diminishing a few days after the onset of the rash [
119]. Clinical presentation may differ in vaccine-modified measles or HIV-positive patients with less pronounced rash [
116].
Due to measles-associated immunosuppression patients are at a higher risk for secondary bacterial infections such as pneumonia or otitis media [
120]. 10-20 % of patients in industrialized countries develop nervous complications of measles including acute disseminated encephalomyelitis (ADEM) [
119] acute post-infection measles encephalitis, measles inclusion body encephalitis (MIBE), and subacute sclerosing panencephalitis (SSPE) which contribute to measles morbidity [
117].
In 2021 there were 13 cases of measles reported in Poland which is 3.7 times less than in 2015 [
72].
Mumps is caused by the mumps virus and spreads through the respiratory route [
121]. It is usually benign and characterized by pain and swelling of the parotid glands [
122]. The virus may spread to different organs and in some instances lead to viral encephalitis or sudden onset deafness due to the infection of the central nervous system [
121]. A few weeks after infection complications may occur [
122] and include meningitis, encephalitis, deafness, and pancreatitis. It may also cause orchitis and oophoritis in men and women respectively increasing the risk of infertility [
123].
In 2022 approximately 370,000 cases of mumps were reported worldwide with the vast majority in Senegal (125,256) and China (104,016) [
124]. In Poland, in 2021 484 cases were reported compared to 2208 in 2015 [
72].
Rubella is an acute disease caused by the rubella virus which is transmitted through the respiratory route and direct contact [
125]. The incubation period lasts 14-21 days and the disease usually presents with a rash in children and with prodromal symptoms preceding the rash by approximately 5 days in adolescents and adults [
126]. Although rubella is considered a mild illness, a congenital primary rubella infection may lead to congenital rubella syndrome with 100,000 cases reported each year [
125]. Congenital rubella includes typical anomalies such as hearing loss, congenital cataracts, or bone lesions [
127]. In 2022 there were over 1,500 cases of congenital rubella syndrome reported worldwide, with almost 90% of cases reported from Afghanistan, India, Indonesia, and Bangladesh [
128]. In Poland in 2021, 50 cases of rubella were reported, compared to 2007 in 2015 [
72].
9.8.2. Vaccination
Since 2004 in Poland the MMR vaccine has been used which contains live attenuated viruses [
8]. The use of a combined vaccine in comparison to single-component vaccinations does not decrease their effectiveness or the quality of immunization [
129]. It also includes gelatin and a trace neomycin which may induce an anaphylactic reaction in those who are allergic [
8]. It is administered in two doses, a primary dose at 13-15 months and a booster at the age of 6 (
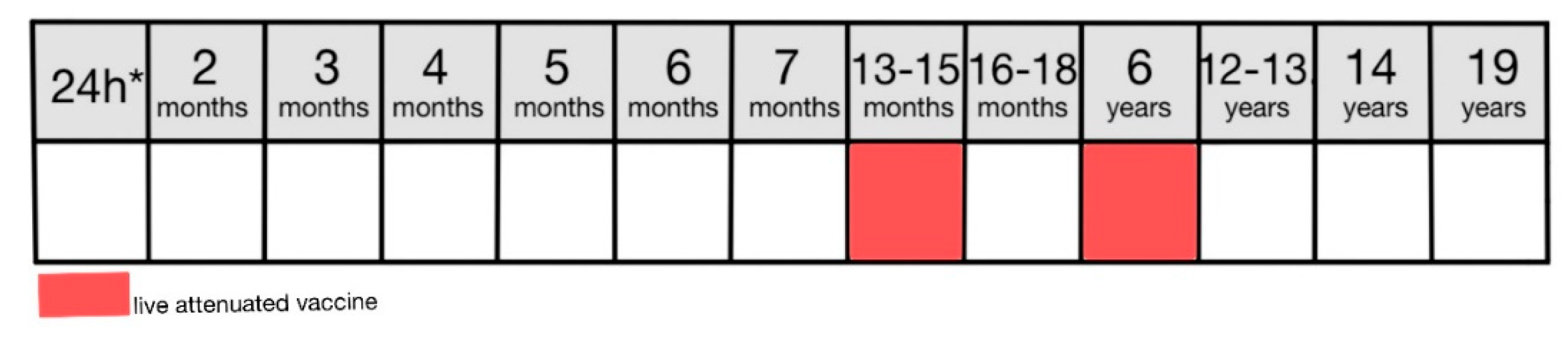
Figure 10) [
8,
46].
The immunization efficacy for each component of the vaccine is different with over 95% immunity to measles and rubella, whereas for mumps it ranges between 79 and 95% [
130]. That disproportion is speculated to result from either mumps virus mutating or waning immunity as even the wild-strain mumps virus infection does not guarantee lifelong immunity [
131].
In Poland, the vaccination rate in 2021 was 90.8% for the primary dose and 85.6% for the booster compared to respectively 98.2% and 53.5% in 2015 [
72].
9.8.3. Contraindications
A specific contraindication to an MMR vaccine is a history of allergy to neomycin or gelatin in addition to standard contraindications to live vaccines [
8].
As the viruses are propagated inside the chicken embryos, the question has emerged whether this vaccine is safe for children with egg albumen allergy. Despite this production method the vaccine does not include enough of the protein to induce an allergic reaction and is therefore safe [
8,
132].
9.8.4. Adverse effects
The most common adverse effects include fever 5-12 days after the vaccination, febrile seizures and rubella-like or measles-like rash [
8].
9.8.5. Controversies
After the publication of Andrew Wakefield’s paper in the Lancet magazine in 1998 linking cases of autism to an MMR vaccine controversies considering the safety of the vaccine emerged and set the cornerstone for the antivaccine movement [
133,
134,
135,
136]. The publication was in 2003 found fraudulent and retracted but the damage was already done [
133]. The decrease in trust in vaccinations, especially the MMR vaccine, led to outbreaks of resurgence of preventable diseases such as measles [
137]. Many later studies have proven that the MMR vaccine does not increase the risk of autism even in high-risk children with a sibling with autism [
138].
10. Mandatory vaccinations for people with a high risk of infection due to clinical or epidemiological reasons in Poland
10.1. Hepatitis type B
Hepatitis B vaccine is mandatory for people who have not been previously vaccinated and meet one of the following conditions [
8,
47]:
Students of medical schools or other schools that offer medical education.
Students of medical universities or other universities that offer medical education.
People working medical field who are at risk of hepatitis B virus infection due to a line of work.
People at risk of hepatitis B virus infection due to direct contact with an infected person.
People infected with a hepatitis C virus.
People with advanced renal insufficiency and glomerular filtration <30 ml/min.
Patients on chronic dialysis.
10.2. Chickenpox
10.2.1. The disease
Chickenpox is a childhood disease caused by the Varicella Zoster Virus (VZV) from the herpesvirus family. It is transmitted through the respiratory route and aerosols and presents with an itchy rash with vesicles filled with serous fluid that later turns into scabs. The rash is accompanied by systemic symptoms in the form of fever and malaise [
139]. In 30% of patients who experience chickenpox in childhood the latent infection may reactivate later in life in the form of shingles [
140]. Chickenpox may lead to serious complications in all age groups. Children under the age of four suffer usually cutaneous complications such as secondary bacterial infection, in children aged 5-14 nervous complications are observed, whereas, in children older than 14, pulmonary complications are the most prevalent [
141,
142].
In Poland, 150,000-220,000 cases of chickenpox are reported annually, the majority in children under the age of 10, and 1,000-1,300 patients are hospitalized due to the severe course of the illness [
143].
10.2.2. Vaccination
The Varicella Zoster Vaccine is a live-attenuated vaccine administered in two doses with 6 weeks space, targeted for children over 9 months of age [
8,
144]. The vaccination is mandatory for children under the age of 12 who meet one of the following conditions [
8,
47].
Children with severe immunodeficiency, with acute lymphoblastic leukemia in remission, HIV-positive, prior to immunosuppressive treatment or chemotherapy.
Children from the environment of people mentioned in point 1, who have not been previously vaccinated for chickenpox.
Children resident in orphanages, nursing homes, educational health centers, interventionist pre-adoption centers, and homes for mothers with minor children or pregnant women.
Children attending nursery or children’s clubs.
There is a possibility of a post-exposure vaccination within 72 hours of the exposition [
8].
10.2.3. Contraindications
All contraindications to live vaccines apply. There are no specific contraindications [
8].
For children with acute lymphoblastic leukemia, the possibility of a chickenpox infection may be a mortal danger. Therefore those children should receive a chickenpox vaccine despite the contraindication in the form of malignancy-related immunodeficiency [
8].
It is crucial to ensure that children qualified for chemotherapy receive the vaccination prior to the beginning of the treatment, as they are at a high risk of serious complications and research suggests that 20% of autologous hematopoietic stem cell graft recipients get infected with chickenpox [
141].
10.2.4. Adverse effects
Mild regional adverse effects may occur in approximately 20% of patients. 3-5% of patients may present with chickenpox-like rash 5-26 days after the immunization, either at the site of vaccination or systemically [
8].
11. Mandatory post-exposure vaccinations in Poland
11.1. Diphtheria
Diphtheria vaccination is mandatory for people at risk for infection due to direct contact with a sick patient. Immunization is conducted using either a single diphtheria vaccine (D or d) or a combined diphtheria and tetanus (Td) formulation [
8,
47].
11.2. Tetanus
Tetanus vaccination is mandatory for patients at risk for infection due to a wound exposed to soil, feces, or other defilement that may contain tetanus spores. Immunization is conducted using either a single tetanus vaccine (T), a combined diphtheria and tetanus (Td) formulation, or a combined diphtheria, tetanus acellular pertussis (Tdap) formulation [
8,
47].
11.3. Rabies
11.3.1. The disease
Rabies is a disease caused by an animal-mediated rabies virus which left untreated is virtually fatal. In 99% of cases, the virus is transmitted by dogs through contact with the saliva of an infected animal and 40% of cases concern children under the age of 15 [
145]. The disease has five stages: incubation, prodrome, acute neurological period, coma, and death. The incubation period lasts usually 30-90 days, although extremely rarely may range from 5 days to 2 years [
146]. Prodromal symptoms are usually non-specific and stretch across 2-10 days [
146,
147]. The acute neurological period takes the form of either furious or paralytic rabies. Furious rabies occurs in 67% of patients and presents with inspiratory spasms, hyperactivity, hallucinations, hydrophobia, aerophobia, and signs of autonomic dysfunction, however, those symptoms may not be present simultaneously and during a coma [
145,
147]. Paralytic rabies present in 33% of patients also involves signs of central nervous dysfunction but with ascending pure motor weakness, flaccid paralysis, and areflexia [
147].
It is estimated that annually rabies is responsible for 60,000 deaths worldwide [
148]. In Poland, since 2003 there have been no reported cases of rabies in humans [
8].
11.3.2. Vaccination
Eliminating rabies in animal carriers and immunization of people are the two ways of rabies prevention [
145]. Herd immunity for rabies does not exist due to a low immunization rate in the population [
8]. In compliance with a regulation of the Minister of Agriculture and Rural Development since 2004 in Poland there is an active program of rabies eradication [
149]. Due to a red fox (Vulpes vulpes) being the main reservoir of the virus in Poland, a program of fox vaccinations was put into place in 2013 with oral rabies vaccine distributed in regions with the highest infection rates [
150].
Annually almost 30 million people worldwide receive rabies post-exposure prophylaxis (PEP) [
145]. WHO recommends vigorous wound washing using soap and water as the first step of PEP [
151]. The vaccine is then administered in one of two schemes: 5-dose Essen scheme (on days 0,3,7,14, and 28) or 4-dose Zagrzeb scheme (2 doses on day 0, one dose on days 7 and 21) [
8].
In Poland, post-exposition rabies vaccination is mandatory for patients who had contact with an animal with rabies or are suspected of rabies [
8]:
Patients bitten by a wild animal.
Patient bitten by a rabid domestic animal or a domestic animal suspected of rabies that has not tested negatively for rabies (either vitally or post-mortem).
Patients who had contact with saliva or brain substance of a rabid animal either through mucosa or damaged skin.
11.3.3. Contraindications
No contraindications as the administration of post-exposure rabies vaccine is considered a life-saving therapy [
8].
11.3.4. Adverse effects
There are no specific adverse effects [
8].
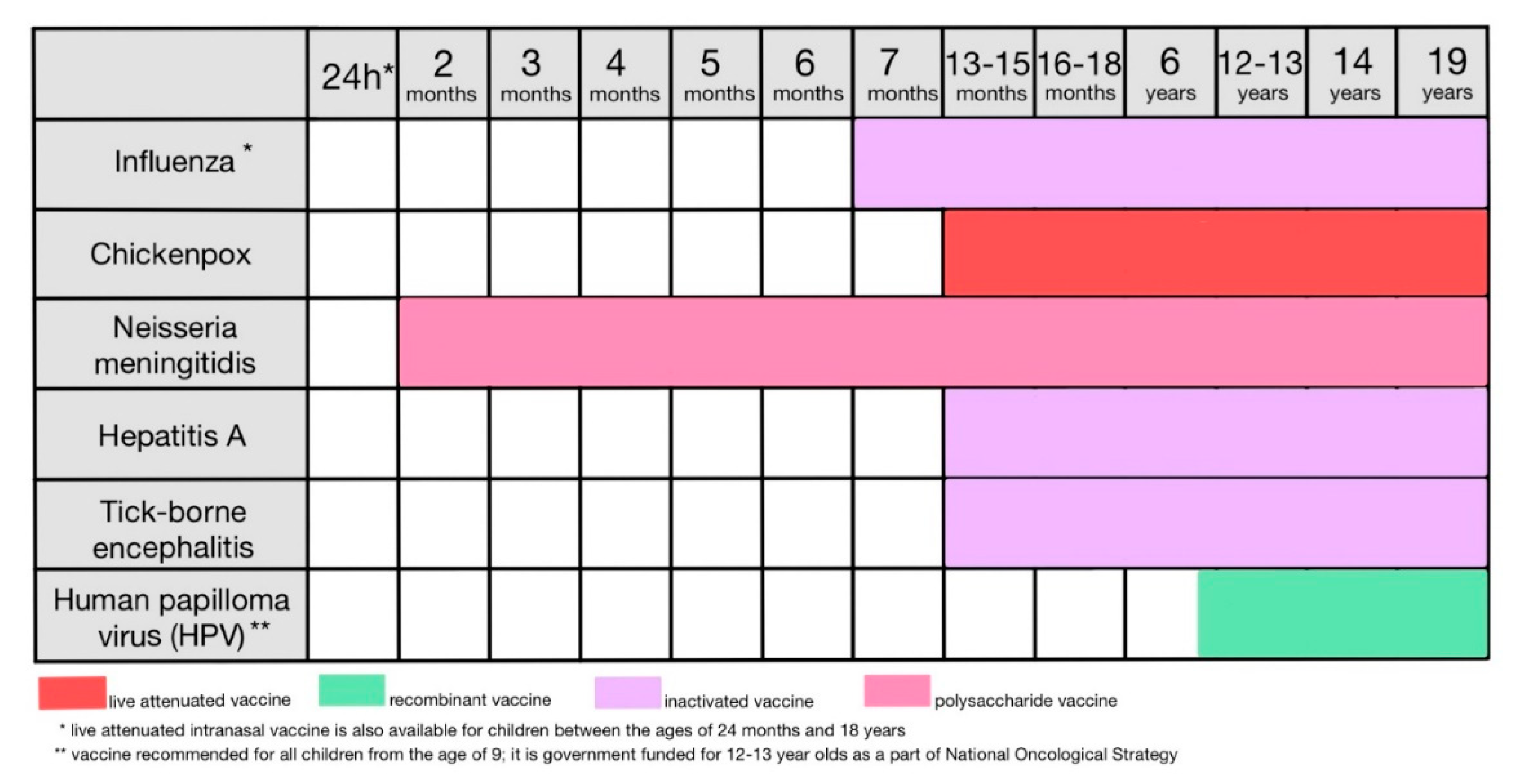
12. Childhood vaccinations recommended as an extension of the mandatory vaccinations program (Figure 11)
12.1. Influenza
12.1.1. The disease
The influenza virus is one of the most prevalent causes of respiratory infections and one with a high pandemic potential due to its mutability and easy transmission [
152,
153]. Influenza A, B, and C viruses infect humans and attack the respiratory epithelium, with the majority of cases having the influenza A virus etiology [
154,
155]. Influenza virus infections are characterized by their seasonality with a peak in the number of cases from November to February [
154]. Symptoms include fever, malaise, cough, sore throat, headache, muscle and joint aches, and rhinorrhea, usually with an abrupt onset [
156]. Infants, young children, the elderly, and people with chronic infections influenza virus infection carry a high risk of hospitalization, complications, and decease [
152]. It is estimated that annually 3-5 million patients suffer a severe case of influenza and 290,000-650,000 patients because of it [
156]. In Poland, in 2020 and 2021 there were respectively 3160711 and cases 2973793 of influenza reported [
72].
12.1.2. Vaccination
There are three types of influenza vaccine used commonly in Poland: intramuscular inactivated vaccine type “split”, intramuscular inactivated vaccine type “subunit” and live attenuated vaccine administered intranasally [
8]. Due to the virus’ mutability, the vaccine is valid for only one season as it contains strains of the virus valid for that particular year based on the predictions of the WHO [
157].
In Poland, the influenza vaccine is recommended for patients post-transplantation, children since the age of 6 months and adults with chronic diseases (with an emphasis on respiratory distress, asthma, and chronic obstructive pulmonary disease), people in states of impaired immunity, children with risk factors (such as HIV infection, congenital immunodeficiency, post-splenectomy, before or after transplantation) from the age of 6 months until 18 years of age, children with congenital heart defects, pregnant women and women trying to conceive [
8,
47]. Patient covers the price of vaccine themselves [
8].
Children up to the age of 9 who have never previously received an influenza vaccine, should be vaccinated with 2 doses with a 4-week break between doses. Otherwise, patients receive one dose of the vaccine per season [
8].
12.1.3. Contraindications
The vaccine should not be administered in children younger than 6 months [
158].
A specific contraindication to the intramuscular influenza vaccine is a history of Guillain-Barre syndrome during the 6 weeks following the previous dose. A chicken albumen allergy used to be a contraindication as the vaccine contains a small amount of it. Currently, it is advised that in such patients the vaccination is performed under the strict supervision of a doctor with access to an anaphylaxis response kit [
8].
In addition to standard contraindications to live vaccines, nasal influenza vaccine is also contraindicated in patients [
8]:
treated with oseltamivir, zanamivir or amantadine. Treatment should be withheld from 2 days before the vaccination until 14 days after.
with severe asthma.
children with severe immunodeficiencies.
children and adolescents under the age of 18 treated with salicylates.
People remaining in close contact with an immunodeficient person (due to a risk of infection with a vaccine strain virus).
12.1.4. Adverse effects
Adverse effects are usually mild and regional. A specific but extremely rare adverse effect in adults is Guillain-Barre syndrome [
8,
158].
12.2. Chickenpox
Chickenpox vaccination is recommended for those who have not been previously vaccinated and have not been ill with chickenpox, with emphasis on healthcare workers, students of medical schools or medical universities, pregnant women, and women planning conception [
8,
47].
12.3. Neisseria meningitidis
12.3.1. The disease
Neisseria meningitidis is an opportunistic bacteria found commonly in the nasopharynx of healthy individuals which may cause invasive meningococcal disease (IMD) in the form of meningitis and septicemia in immunocompromised individuals [
159]. It is transmitted through the respiratory route, aerosols, and direct contact with the nasal secretions of the infected, with infants aged 3-12 months at the highest risk for infection [
160]. Classic symptoms of meningitis include fever, headache, and meningeal signs with an abrupt onset. After the development of septicemia the symptoms progress to vomiting, severe malaise, chills, and a characteristic dark ecchymosed rash [
161]. In neonates, clinical presentation may be atypical with irritability and difficulty feeding shortly followed by a sudden clinical decline [
162].
Despite the access to antibiotics the fatality of IMD in Europe varies between 4.5% and 9.7% [
163]. In 2019 meningococcal infection was responsible for an estimated 250,000 deaths and leaving 20% of the convalescents with life-long neurological consequences [
164]. In Poland in 2021, there were 20 cases of invasive meningococcal disease reported, compared to 106 in 2020 and 220 in 2015 [
72].
12.3.2. Vaccination
In Poland three formulations of the vaccine are available: conjugated monovalent vaccine, conjugated polyvalent vaccine, and recombined vaccine combined with bacterial proteins. The exact dosing scheme depends on the formulation but usually consists of 2 basic doses and one booster dose [
8]. Patients cover the cost of the vaccination themselves.
In Poland, a vaccination against Neisseria meningitidis is recommended for all infants from the age of 6 or 8 weeks (depending on the type of vaccine), children and adults with congenital or acquired immunodeficiency, and people at risk of infection such as healthcare workers or children attending nurseries [
8,
47].
12.3.3. Contraindications
There are no specific contraindications to that vaccine [
8].
12.3.4. Adverse effects
Conjugate vaccines usually produce regional adverse effects in all age groups, with infants also occasionally experiencing a lack of apetite, irritability, and vomiting [
8].
Drowsiness and atypical crying are known adverse effects of a combined vaccine [
8].
12.4. Hepatits type A
12.4.1. The disease
Hepatitis A virus is transmitted through fecal-oral route, direct contact or consumption of contaminated food or water and presents with jaundice, hyperbilirubinemia, pain in the upper right quadrant of the stomach, and anorexia. In children, on the other hand, it oftentimes remains asymptomatic [
165]. HAV produces an acute infection that may lead to an acute liver failure. The infection does not, however, show the tendency to become chronic [
166]. Symptoms develop following the 2-week incubation period and include fever, malaise, jaundice, and dark urine, with a possibility of atypical presentation [
167]. HAV accounts for 0.5% of mortality because of hepatitis with over 7,000 people dying due to it in 2016 [
166]. In Poland, in 2021 there were 92 cases of hepatitis A infection reported, compared to 111 in 2020 and 49 in 2015 [
72].
12.4.2. Vaccination
An inactivated HAV vaccine is available in adult and pediatric formulations, with pediatric being intended for children over 12 months of age [
8]. The vaccine is administered in two doses with a 6-12 month gap between the doses, with the second one being crucial for long-term immunization [
168].
In Poland, the vaccination is recommended for all children since preschool age and adolescents who have not been previously infected with HAV, people at a high risk of infection due to their line of work (healthcare workers, people working in the production or distribution of food and people working in municipal waste disposal), and people traveling to regions of the endemic distribution of HAV [
8,
47].
12.4.3. Contraindications
There are no specific contraindications to this vaccine [
8].
12.4.4. Adverse effects
Adverse effects might be regional or systemic, usually mild [
8].
12.5. Tick-borne encephalitis
12.5.1. The disease
Tick-borne encephalitis is a disease caused by a flavivirus transmitted by a bite of a tick. The disease has recently increased its geographical range with reported incidence rates doubling between 2015 and 2020 [
169,
170,
171]. Predominantly asymptomatic, in 2-30% of patients it causes encephalitis [
170]. After the incubation period of approximately 8-10 days, the first phase of the illness begins with symptoms such as fever, malaise, fatigue, and body aches that last around 5 days. A 7-day symptom-free interval follows and then the disease progresses to the second phase with symptoms ranging from mild meningitis to severe encephalitis [
172]. As there is no specific treatment, preventative actions in the form of vaccinations are crucial [
170]. In Poland, in 2022 there were 445 cases of tick-borne encephalitis, in comparison to 210 in 2021 and 149 in 2015 [
173].
12.5.2. Vaccination
The vaccine contains inactivated viruses propagated in chicken embryos and is administered in three basic doses (day 0, 1-3 days after the first dose, 5-12 months after the second dose) and booster doses administered every 3 years starting 3 years after the end of basic vaccination course [
8,
170].
In Poland, the vaccine is recommended for people residing in areas where the disease is especially prevalent, with an emphasis on people working in forest exploitation, soldiers, firefighters, board guards, people who frequently practice outdoor activities that may put them at risk of a tick bite, and children at summer camps [
8,
47].
12.5.3. Contraindications
The vaccine is contraindicated in patients with a history of allergy to chicken albumen, neomycin or gentamycin in addition to the standard vaccine contraindications [
8].
12.5.4. Adverse effects
There are no specific adverse effects [
8].
12.6. Human papilloma virus (HPV)
12.6.1. The disease
HPV infection is estimated to be the most common sexually transmitted disease both in heterosexual and homosexual contacts [
174] with most infections happening between the ages of 18 and 30 [
175]. The virus infects squamous epithelia with low-risk subtypes responsible for genital warts and high-risk subtypes, such as HPV16 and HPV18, associated with different types of anogenital cancer with emphasis on cervical, anal and penile cancers [
174,
175]. Cervical cancer is the fourth most common cancer in women and accounted for 342,000 deaths in 2020 [
176]. The World Health Assembly in 2020 affiliated “The Global strategy towards eliminating cervical cancer as a public health problem”, which includes a three-step approach. Primary prevention establishes HPV vaccinations in girls aged 9-14, secondary prevention includes high-sensitivity testing for HPV infection and immediate treatment in case of a positive result while tertiary prevention consists of treatment of invasive cancer [
176,
177].
In Poland, despite screening for cervical cancer, the disease is frequently diagnosed at an advanced stage and entails approximately 50% mortality rate [
8]. In 2020 3,862 new cases of cervical cancer were diagnosed and 2,137 women died because of it [
178].
12.6.2. Vaccination
HPV vaccines are polyvalent, recombined formulations, containing antigens of either 2,4 or 9 strains of the virus, with children between 9 and 13 (Gardasil) or 14 (Cervarix and Gardasil 9) years of age receiving 2 doses, and children older than 13 (Gardasil) or 14 (Cervarix and Gardasil 9) receiving three doses [
8]. If the vaccine course is interrupted, there is no need to repeat the previously administered doses [
179].
In Poland, the vaccine is recommended for all children from the age of 9 [
8,
47]. As it is not a mandatory vaccination, patients cover the cost themselves. However, in 2023, a nationwide program was launched targeted at both girls and boys at the ages of 12 and 13, that funds the two-dose course of the 2-valent Cervarix vaccine or 9-valent Gardasil 9 vaccine [
180].
12.6.3. Contraindications
There are no specific contraindications [
8].
12.6.4. Adverse effects
There are no specific adverse effects. Regional redness or swelling at the site of the injection may occur together with short-lasting fever or urticaria [
8].
13. Recommended vaccinations for adults who did not receive mandatory vaccination in childhood
If an adult has not received their mandatory vaccinations in childhood despite the absence of absolute contraindications, the Program of Protective Vaccinations recommends that they get vaccinated later in life [
47]. The rotavirus vaccine, however, is exempt from that recommendation as it can be administered only in children below the age of either 24 or 32 weeks depending on the formulation [
8].
13.1. Tuberculosis
The vaccine is recommended for children under the age of 15 who have previously not been vaccinated [
8,
47].
13.2. Hepatitis B
The vaccine is recommended for people who have not previously been vaccinated and are at risk of infection or its severe course. Groups mentioned in the vaccination program include people who may be infected due to their line of work or dangerous lifestyle practices, immunocompromised patients, patients pre-surgery, the elderly, and women who are pregnant or planning to conceive [
8,
47].
13.3. Diphtheria, pertussis and tetanus
In addition to unvaccinated individuals, the vaccine is recommended for all people and to be administered every 10 years in place of a combined diphtheria and tetanus booster dose. It is also encouraged for people at risk of infection with an emphasis on healthcare workers, pregnant women between 27 and 36 weeks of pregnancy, and people from the surrounding of newborns and infants [
8,
47].
13.4. Streptococcus pneumoniae
The vaccine is recommended for individuals over 50 years old, people with chronic illnesses, people with anatomical or physiological asplenia, people with immunodeficiencies regardless of the cause, and people addicted to alcohol or cigarettes [
8,
47].
13.5. Haemophilus influenzae type B
The vaccination is recommended for children under the age of 6 who have not been vaccinated according to the mandatory vaccination program and people with immunodeficiencies if the doctor has decided so [
8,
47].
13.6. Measles, mumps, rubella (MMR)
The vaccine is recommended for healthcare workers and students of medical schools and universities. In addition, it is highly encouraged for women working around children as a preventative measure against congenital rubella [
8,
47].
13.7. Poliomyelitis
The vaccination is recommended to all people over the age of 19 who have not previously been vaccinated, with an emphasis on people traveling to areas of endemic distribution of polio [
8,
47].
14. Recommended vaccinations for people traveling to regions with endemic occurrences of the diseases
14.1. Cholera
Cholera is an acute disease presenting with severe watery diarrhea caused by Vibrio cholerae and is responsible for an estimated 100,000 deaths annually worldwide [
181]. The bacteria is transmitted through consumption of contaminated food or water with symptoms appearing after an incubation period ranging from 12 hours to 5 days [
182]. The main objective of treatment is to reduce both the severity and the duration of the illness which is achieved through rehydration therapy and employment of antibiotics such as doxycycline, azithromycin, and tetracycline [
183,
184].
In Poland, cholera vaccination is recommended to people traveling to areas of endemic distribution of cholera such as South-East Asia and Africa [
8,
47]. An inactivated oral vaccine is administered in 3 basic doses and one booster in children aged 2-6 and 2 basic doses and one booster in patients over the age of 6 [
8,
184]. The specific contraindication is an acute gastrointestinal disorder and adverse effects include stomachache, nausea, vomiting and diarrhea [
8].
14.2. Typhoid
Typhoid or typhoid fever is caused by Salmonella enterica serotype Typhi (S Typhi) with an estimated 9 million cases and 110,000 deaths annually worldwide [
185]. The disease presents with prolonged high fever, malaise, abdominal pain, nausea, and diarrhea or constipation [
186] with 10-15% of untreated patients developing serious complications [
185]. Although the disease may be treated with antibiotics, the increasing resistance to azithromycin and third-generation cephalosporins poses a new challenge in managing typhoid.
In Poland, the typhoid vaccine is recommended to people traveling to areas of endemic distribution of typhoid such as Asia, Africa, Latin America, and the Middle East, and to people who may come in contact with the bacteria due to their line of work [
8,
47,
187]. In the case of the latter, the employer covers the cost of the vaccination. There are four formulations available: live oral vaccine, live subcutaneous vaccine, intramuscular polysaccharide vaccine, and combined typhoid and tetanus vaccine. There are no specific contraindications or adverse effects [
8].
14.3. Rabies
Rabies vaccine contains an inactivated rabies virus and its main objective is to provide protection for the vaccinated individual rather than to create herd immunity. The vaccine is performed using the same formulation as for post-exposure prophylaxis [
8].
The vaccination consists of 3 basic doses on days 0, 7, and 21 (or 28) and boosters every 2-5 years [
8]. WHO allows basic vaccination consisting of two doses: on days 0 and 7 [
151]. Vaccination is recommended for people traveling to regions of endemic rabies, in which instance the patient covers the cost of the vaccine. It is also recommended for people who may come in contact with rabies due to their line of work. The cost of the vaccine in that case is reimbursed by the employer [
8].
There are no specific contraindications [
8].
14.4. Yellow fever
Yellow fever is a hemorrhagic fever caused by a flavivirus transmitted by mosquitos [
188] with 200,000 cases and 30,000 deaths reported annually worldwide [
189]. Initial symptoms include fever, shivers, muscle pains, lack of appetite, vomiting, nausea, and headaches [
188,
189]. The course of infection may vary from asymptomatic to liver failure resulting in coagulopathy and death in 20-60% of cases [
188,
190]. Due to the lack of effective treatment, preventative actions in the form of protection from mosquito bites and vaccinations are crucial [
191].
In Poland, yellow fever vaccination is recommended for people traveling to areas of endemic distribution of yellow fever such as South America and Africa [
8,
188]. A live vaccine is administered in one dose and from the 10th day after the vaccination, the immunity is believed to be life-long [
8,
192]. All contraindications to live vaccines also apply to the yellow fever vaccine. Due to containing traces of chicken albumen, it is contraindicated in those who are allergic [
8]. In addition to mild regional adverse effects, neurotropic and viscerotropic diseases are known, extremely rare adverse effects associated with yellow fever vaccine [
192,
193].
15. Conclusions
The creation of immunizations was a huge step in combating infectious diseases and still remains one of the most important tools of protection of public health. Poland is an example of a country which implemented a program of mandatory childhood vaccinations and watched the number of cases of infectious diseases plummet. Often evaluations of the program and its infection rates provide an effective, long-term, and cost-effective strategy for infectious disease prevention. Nowadays Poland is dealing with the consequences of antivaccine movements and an immigrant crisis due to a war in Ukraine and the number of cases is rising again. The creation of new vaccine formulations makes them available to a wider range of patients who were previously unfit to receive them due to particular contraindications such as chicken albumen allergy. Maintenance of high immunization rates, combating vaccine hesitancy, and searching for new formulations of vaccines should be a priority in order to preserve herd immunity against vaccine-preventable diseases and public safety.
Author Contributions
Conceptualization: Aleksandra Kamińska, Katarzyna Plata-Nazar; Data research: Aleksandra Kamińska; Writing - original draft preparation: Aleksandra Kamińska; Writing - review and editing: Aleksandra Kamińska, Katarzyna Plata-Nazar; Supervision: Katarzyna Plata-Nazar
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Child mortality (under 5 years) [Internet]. [cited 2023 Oct 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-under-5-mortality-in-2020.
- Bhutta ZA, Saeed MA. Childhood Infectious Diseases: Overview. In: International Encyclopedia of Public Health [Internet]. Elsevier; 2008 [cited 2023 Oct 8]. p. 620–40. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7148616/#mc0447title.
- Walker JL, Zhao H, Dabrera G, Andrews N, Thomas SL, Tsang C, et al. Assessment of Effectiveness of Seasonal Influenza Vaccination During Pregnancy in Preventing Influenza Infection in Infants in England, 2013-2014 and 2014-2015. J Infect Dis [Internet]. 2020 Jan 1 [cited 2023 Aug 24];(221 (1)):16–20. Available from: https://pubmed.ncbi.nlm.nih.gov/31711165/.
- Hardt K, Bonanni P, King S, Santos JI, El-Hodhod M, Zimet GD, et al. Vaccine strategies: Optimising outcomes. Vaccine [Internet]. 2016 Dec [cited 2023 Oct 8];34(52):6691–9. Available from: https://pubmed.ncbi.nlm.nih.gov/27887796/.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov [Internet]. 2018 Apr 12 [cited 2023 Oct 8];17(4):261–79. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5906799/.
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy. Hum Vaccin Immunother [Internet]. 2013 Aug 8 [cited 2023 Oct 8];9(8):1763–73. Available from: https://pubmed.ncbi.nlm.nih.gov/23584253/.
- Jacobson RM, St. Sauver JL, Finney Rutten LJ. Vaccine Hesitancy. Mayo Clin Proc [Internet]. 2015 Nov [cited 2023 Oct 8];90(11):1562–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26541249/.
- Ciechanowski P, Mrożek-Budzyn D. Wakcynologia praktyczna [Internet]. IX. α-medica press; 2023 [cited 2023 Sep 12]. Available from: https://pubmed.ncbi.nlm.nih.gov/28302312/.
- Faenza I, Blalock WL. Innate Immunity: A Balance between Disease and Adaption to Stress. Biomolecules [Internet]. 2022 May 23 [cited 2023 Jul 22];(12 (5)):737. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9138980/. 23 May.
- Parkin J, Cohen B. An overview of the immune system. Lancet [Internet]. 2001 Jun 2 [cited 2023 Jul 24];(357(9270)):1777–89. Available from: https://pubmed.ncbi.nlm.nih.gov/11403834/.
- Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H. Innate-adaptive immunity interplay and redox regulation in immune response. . Redox Biol [Internet]. 2020 Oct [cited 2023 Aug 9];37(101759). Available from: https://pubmed.ncbi.nlm.nih.gov/33086106/.
- Kennedy RB, Ovsyannikova IG, Palese P, Palese P PGA. Current Challenges in Vaccinology. Front Immunol [Internet]. 2020 Jun 25 [cited 2023 Aug 17];(11):1181. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7329983/.
- Bonilla FA, Oettgen HC. Adaptive immunity. Allergy Clin Immunol [Internet]. 2010 Feb [cited 2023 Aug 9];(125). Available from: https://pubmed.ncbi.nlm.nih.gov/20061006/.
- Marcotte H, Hammarström L. Passive Immunization: Toward Magic Bullets. Mucosal Immunol [Internet]. 2015 [cited 2023 Aug 15];1403–34. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7150278/.
- Bartlett BL, Pellicane AJ, Tyring SK. Vaccine immunology. Dermatol Ther [Internet]. 2009 [cited 2023 Aug 16];Mar-Apr(22 (2)):104–9. Available from: https://pubmed.ncbi.nlm.nih.gov/19335722/.
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. . Immunity [Internet]. 2008 May [cited 2023 Aug 14];(28 (5)):710–22. Available from: https://pubmed.ncbi.nlm.nih.gov/18468462/.
- Pulendran B, Davis MM. The science and medicine of human immunology. Science (1979) [Internet]. 2020 Sep 25 [cited 2023 Aug 14];(369(6511)). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7872131/.
- Okwo-Bele, J.M.; Cherian, T. The expanded programme on immunization: A lasting legacy of smallpox eradication. Vaccine 2011, 29, D74–D79. [Google Scholar] [CrossRef] [PubMed]
- Ashby B, Best A. Herd immunity. Curr Biol [Internet]. 2021 Feb 22 [cited 2023 Aug 15];(31 (4)):R174R177. Available from: https://pubmed.ncbi.nlm.nih.gov/33621500/.
- WHO. WHO- how vaccines work [Internet]. [cited 2023 Aug 16]. Available from: https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work?gclid=EAIaIQobChMIzafbheXcgAMV_wWiAx3A7Qq5EAAYASAAEgJKrPD_BwE.
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature [Internet]. 2007 May [cited 2023 Jul 17];17;447(7142):279–83. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7095142/.
- Casanova, J.L.; Abel, L. The Genetic Theory of Infectious Diseases: A Brief History and Selected Illustrations. Annu Rev Genomics Hum Genet. 2013, 14, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Pandemics in history. [Internet]. [cited 2023 Jul 19]. Available from: https://www.mphonline.org/worst-pandemics-in-history/.
- WHO- a brief history of vaccinations. [Internet]. [cited 2023 Jul 20]. Available from: https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination?topicsurvey=ht7j2q)&gclid=EAIaIQobChMIr9P73M6dgAMV-QWiAx0PkQ-9EAAYASAAEgJYufD_BwE.
- Bird A. James Jurin and the avoidance of bias in collecting and assessing evidence on the effects of variolation. J R Soc Med [Internet]. 2019 Mar [cited 2023 Jul 20];(112 (3)):119–23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6423525/.
- Kayser, V.; Ramzan, I. Vaccines and vaccination: history and emerging issues. Hum Vaccin Immunother. 2022, 17, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M. Parenteral, non-live rotavirus vaccine: recent history and future perspective. Clin Exp Vaccine Res. 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- US Departament of Health and Human Services. Vaccine Types [Internet]. [cited 2023 Aug 17]. Available from: https://www.hhs.gov/immunization/basics/types/index.html.
- historyofvaccines.org. History of vaccines- vaccine types [Internet]. [cited 2023 Aug 18]. Available from: https://historyofvaccines.org/vaccines-101/what-do-vaccines-do/different-types-vaccines.
- He Q, Xiao J, Liu M, Li Z, Xu J, Zhang Z. Immunogenicity of meningococcal polysaccharide conjugate vaccine as a replacement for meningococcal polysaccharide vaccine in children in Guangzhou, China. Vaccine 2022, 40, 1370–5. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, K.R. Recombinant vaccine technology in veterinary medicine. Vet Clin North Am Small Anim Pract [Internet]. 2001 May [cited 2023 Aug 17];(31 (3)):535–8. Available from: https://pubmed.ncbi.nlm.nih.gov/11446102/.
- Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). . Drugs [Internet]. 2006 [cited 2023 Aug 17];(66 (9)):1263–73. Available from: https://pubmed.ncbi.nlm.nih.gov/16827602/.
- Xu S, Yang K, Li R, Zhang L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci [Internet]. 2020 Sep 9 [cited 2023 Aug 18];(21 (18)):6582. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7554980/.
- Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther [Internet]. 2022 Mar 23 [cited 2023 Aug 18];(7 (1)):94. Available from: https://pubmed.ncbi.nlm.nih.gov/35322018/.
- Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology [Internet]. 2006 Jan 5 [cited 2023 Aug 18];(344 (1)):230–9. Available from: https://pubmed.ncbi.nlm.nih.gov/16364753/.
- Crosby CM, Matchett WE, Anguiano-Zarate SS, Parks CA, Weaver EA, Pease LR, et al. Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J Virol [Internet]. 2017 Jan 3 [cited 2023 Aug 18];(91 (2)). Available from: https://pubmed.ncbi.nlm.nih.gov/27807231/.
- Smith M. Vaccine safety: medical contraindications, myths, and risk communication. Pediatr Rev [Internet]. 2015 Jun [cited 2023 Aug 18];(36 (6)):227–38. Available from: https://pubmed.ncbi.nlm.nih.gov/26034253/.
- COMMITTEE ON INFECTIOUS DISEASES. Recommendations for Prevention and Control of Influenza in Children, 2021-2022. . Pediatrics [Internet]. 2021 Oct [cited 2023 Aug 21];e2021053745(148 (4)). Available from: https://pubmed.ncbi.nlm.nih.gov/34493538/.
- Liu S, Wang J, Liu Y, Xu Y, Che X, Gu W, et al. Survey of contraindications in children’s routine vaccination in Hangzhou, China. Hum Vaccin Immunother [Internet]. 2017 Jul 3 [cited 2023 Aug 21];(13 (7)):1539–43. Available from: https://pubmed.ncbi.nlm.nih.gov/28406739/.
- Kamei K. Live attenuated vaccines in patients receiving immunosuppressive agents. Pediatr Nephrol [Internet]. 2023 Apr 20 [cited 2023 Aug 22];1–12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10115603/.
- Raney EC, El-Ibiary SY. Immunizations and pregnancy: an update for pharmacists. J Am Pharm Assoc (2003) [Internet]. 2012 [cited 2023 Aug 24];(52 (6)). Available from: https://pubmed.ncbi.nlm.nih.gov/23229984/.
- Tanner AR, Dorey RB, Brendish NJ, Clark TW. Influenza vaccination: protecting the most vulnerable. Eur Respir Rev 2021 Jan 13;30(159) [Internet]. 2021 Jan 13 [cited 2023 Aug 24];(30 (159)). Available from: https://pubmed.ncbi.nlm.nih.gov/33650528/.
- szczepienia.info. Historyczny kalendarz szczepień [Internet]. [cited 2023 Jul 16]. Available from: https://szczepienia.pzh.gov.pl/kalendarz-szczepien/.
- Szukała Michał. Nauka w Polsce. [cited 2023 Oct 5]. Epidemie na ziemiach polskich. Available from: https://naukawpolsce.pl/aktualnosci/news%2C81227%2Cepidemie-na-ziemiach-polskich.html.
- Światowy Program Eradykacji Poliomyelitos [Internet]. [cited 2023 Oct 5]. Available from: https://www.gov.pl/web/psse-radziejow/swiatowy-program-eradykacji-poliomyelitos.
- szczepienia.info, Narodowy Instytut Zdrowia Publicznego. Kalendarz szczepień obowiązkowych na rok 2023 [Internet]. 2023 [cited 2023 Aug 29]. Available from: https://szczepienia.pzh.gov.pl/kalendarz-szczepien-2023-2/.
- KOMUNIKAT GŁÓWNEGO INSPEKTORA SANITARNEGO z dnia 28 października 2022 r. w sprawie Programu Szczepień Ochronnych na rok 2023. Dziennik Urzędowy Ministra Zdrowia Oct 28, 2022.
- Attwell K, Navin MC, Lopalco PL, Jestin C, Reiter S, Omer SB. Recent vaccine mandates in the United States, Europe and Australia: A comparative study. . Vaccine [Internet]. 2018 Nov 19 [cited 2023 Aug 10];(36 (48)):7377–84. Available from: https://www.sciencedirect.com/science/article/pii/S0264410X18313719.
- Vanderslott S, Marks T. Charting mandatory childhood vaccination policies worldwide. Vaccine [Internet]. 2021 Jul 5 [cited 2023 Aug 10];(39 (30)):4054–62. Available from: https://pubmed.ncbi.nlm.nih.gov/34119351/.
- szczepienia.gov. Na czym polega indywidualny kalendarz szczepień? [Internet]. [cited 2023 Aug 16]. Available from: https://szczepienia.pzh.gov.pl/faq/czym-polega-indywidualny-kalendarz-szczepien/.
- Coombes R. Europe steps up action against vaccine hesitancy as measles outbreaks continue. BMJ [Internet]. 2017 Oct 16 [cited 2023 Aug 12];(359:j4803). Available from: https://pubmed.ncbi.nlm.nih.gov/29038341/.
- Hussain A, Ali S, Ahmed M, Hussain S. The Anti-vaccination Movement: A Regression in Modern Medicine. Cureus [Internet]. 2018 Jul 3 [cited 2023 Oct 5]; Available from: https://pubmed.ncbi.nlm.nih.gov/30186724/.
- Dubé E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines [Internet]. 2015 Jan 2 [cited 2023 Oct 5];14(1):99–117. Available from: https://pubmed.ncbi.nlm.nih.gov/25373435/.
- Doustmohammadi S, Cherry JD. The sociology of the antivaccine movement. Emerg Top Life Sci [Internet]. 2020 Sep 8 [cited 2023 Oct 5];4(2):241–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32463081/.
- Gravagna K, Becker A, Valeris-Chacin R, Mohammed I, Tambe S, Awan FA, et al. Global assessment of national mandatory vaccination policies and consequences of non-compliance. Vaccine [Internet]. 2020 Nov 17 [cited 2023 Aug 10];(38 (49)):7865–73. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8562319/.
- Bozzola E, Spina G, Russo R, Bozzola M, Corsello G, Villani A. Mandatory vaccinations in European countries, undocumented information, false news and the impact on vaccination uptake: the position of the Italian pediatric society. . Ital J Pediatr [Internet]. 2018 Jun 14 [cited 2023 Aug 10];(44 (1)):67. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6001041/.
- Ustawa z dnia 5 grudnia 2008 r. o zapobieganiu oraz zwalczaniu zakażeń i chorób zakaźnych u ludzi. Dz. U. z 2023 r. poz. 1284, 909. Poland: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20082341570/U/D20081570Lj.pdf.
- Rozporządzenie ministra zdrowia z dnia 18 sierpnia 2011 r. w sprawie obowiązkowych szczepień ochronnych. Dz. U. 2011 nr 182 poz. 1086 Poland: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20111821086/O/D20111086.pdf.
- Rozporządzenie ministra zdrowia z dnia 9 października 2020 r. zmieniające rozporządzenie w sprawie obowiązkowych szczepień ochronnych. Dz. U. 2020 poz. 1964 Poland: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200001964/O/D20201964.
- Żaczkiewicz-Zborska Katarzyna. prawo.pl. [cited 2023 Oct 5]. Sądy administracyjne bezwzględne wobec rodziców nieszczepiących dzieci. Available from: https://www.prawo.pl/zdrowie/kary-nakladane-na-rodzicow-nieszczepiacych-dzieci,515880.
- WHO. WHO’s response to the Ukraine crisis: annual report, 2022 [Internet]. 2023 Mar [cited 2023 Aug 12]. Available from: https://www.who.int/publications/i/item/WHO-EURO-2023-5897-45662-68308.
- Statistics of Poland, WHO. Health of refugees from Ukraine in Poland 2022 [Internet]. 2022 [cited 2023 Aug 12]. Available from: https://stat.gov.pl/en/events/international-events/health-of-refugees-from-ukraine-in-poland-2022-survey-findings,6,1.
- WHO. Vaccination schedule for Ukraine [Internet]. [cited 2023 Aug 12]. Available from: https://immunizationdata.who.int/pages/schedule-by-country/ukr.html?DISEASECODE=&TARGETPOP_GENERAL=.
- Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers [Internet]. 2016 Oct 27 [cited 2023 Aug 26];(2). Available from: https://pubmed.ncbi.nlm.nih.gov/27784885/.
- Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The Diagnosis and Treatment of Tuberculosis. Dtsch Arztebl Int [Internet]. 2019 Oct 25 [cited 2023 Aug 26]; Available from: https://pubmed.ncbi.nlm.nih.gov/31755407/.
- Koch A, Mizrahi V. Mycobacterium tuberculosis. Trends Microbiol [Internet]. 2018 Jun [cited 2023 Aug 26];26(6):555–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29580884/.
- Tuberculosis in Poland in 2020. Przegl Epidemiol [Internet]. 2023 Mar 30 [cited 2023 Aug 26];528–46. Available from: https://pubmed.ncbi.nlm.nih.gov/37017237/.
- Główny Urząd Statystyczny. Zachorowania na gruźlicę 2009-2021. [Internet]. 2023 [cited 2023 Aug 26]. Available from: https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/zachorowania-na-niektore-choroby-zakazne,20,1.html.
- Fatima S, Kumari A, Das G, Dwivedi VP. Tuberculosis vaccine: A journey from BCG to present. Life Sci [Internet]. 2020 Jul [cited 2023 Aug 26];252:117594. Available from: https://pubmed.ncbi.nlm.nih.gov/32305522/.
- Schwarz MGA, Corrêa PR, Malaga W, Guilhot C, Mendonça-Lima L. Mycobacterium bovis BCG moreau is naturally deficient in homologous recombination. Tuberculosis [Internet]. 2020 Jul [cited 2023 Aug 26];123:101956. Available from: https://pubmed.ncbi.nlm.nih.gov/32741533/.
- Główny urząd statystyczny. Udział dzieci zaszczepionych w latach 2010-2021.
- Rocznik Statystyczny Rzeczpospolitej Polskiej 2022 [Internet]. Warszawa/Warsaw; 2022 [cited 2023 Aug 29]. Available from: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-rzeczypospolitej-polskiej-2022,2,22.html.
- Dommergues MA, Rocque F de La, Guy C, Lécuyer A, Jacquet A, Guérin N, et al. Local and regional adverse reactions to BCG-SSI® vaccination: A 12-month cohort follow-up study. Vaccine [Internet]. 2009 Nov [cited 2023 Aug 28];27(50):6967–73. Available from: https://pubmed.ncbi.nlm.nih.gov/19800440/.
- Montagnani C, Esposito S, Galli L, Chiappini E, Principi N, de Martino M. Recommendations for pediatric tuberculosis vaccination in Italy. Hum Vaccin Immunother [Internet]. 2016 Mar 3 [cited 2023 Aug 28];12(3):644–50. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4964763/.
- Wilkins T, Sams R, Carpenter M. Hepatitis B: Screening, Prevention, Diagnosis, and Treatment. . Am Fam Physician [Internet]. 2019 Mar 1 [cited 2023 Aug 28];99(5):314–23. Available from: https://pubmed.ncbi.nlm.nih.gov/30811163/.
- Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. The Lancet [Internet]. 2014 Dec [cited 2023 Aug 28];384(9959):2053–63. Available from: https://pubmed.ncbi.nlm.nih.gov/24954675/.
- Dekker SE, Green EW, Ahn J. Treatment and Prevention of Acute Hepatitis B Virus. Clin Liver Dis [Internet]. 2021 Nov [cited 2023 Aug 28];25(4):711–24. Available from: https://pubmed.ncbi.nlm.nih.gov/34593149/.
- Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B Vaccines. J Infect Dis [Internet]. 2021 Sep 30 [cited 2023 Aug 28];224(Supplement_4):S343–51. Available from: https://pubmed.ncbi.nlm.nih.gov/34590138/.
- McMahon, B.J. The natural history of chronic hepatitis B virus infection. Hepatology 2009, 49, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.L.; Lau, J.Y. Clinical aspects of hepatitis B virus infection. Lancet 1993, 342, 1340–4. [Google Scholar] [CrossRef] [PubMed]
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis [Internet]. 2017 Sep [cited 2023 Aug 30];17(9):909–48. Available from: https://pubmed.ncbi.nlm.nih.gov/28579426/.
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primers [Internet]. 2017 Nov 9 [cited 2023 Aug 30];3(1):17083. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5858916/.
- Carvalho MF, Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother [Internet]. 2019 Jun 3 [cited 2023 Aug 30];15(6):1237–50. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6663136/.
- Dennehy PH. Rotavirus Infection. Infect Dis Clin North Am [Internet]. 2015 Dec [cited 2023 Aug 30];29(4):617–35. Available from: https://pubmed.ncbi.nlm.nih.gov/26337738/. 2633.
- Sharma NC, Efstratiou A, Mokrousov I, Mutreja A, Das B, Ramamurthy T. Diphtheria. Nat Rev Dis Primers [Internet]. 2019 Dec 5 [cited 2023 Sep 7];5(1):81. Available from: https://pubmed.ncbi.nlm.nih.gov/31804499/.
- Muscat M, Gebrie B, Efstratiou A, Datta SS, Daniels D. Diphtheria in the WHO European Region, 2010 to 2019. Eurosurveillance [Internet]. 2022 Feb 24 [cited 2023 Sep 7];27(8). Available from: https://pubmed.ncbi.nlm.nih.gov/35209973/.
- Truelove SA, Keegan LT, Moss WJ, Chaisson LH, Macher E, Azman AS, et al. Clinical and Epidemiological Aspects of Diphtheria: A Systematic Review and Pooled Analysis. Clinical Infectious Diseases [Internet]. 2020 Jun 24 [cited 2023 Sep 7];71(1):89–97. Available from: https://pubmed.ncbi.nlm.nih.gov/31425581/.
- World Health Organisation. Diphtheria - number of reported cases [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/diphtheria---number-of-reported-cases.
- Nguyen VTN, Simon L. Pertussis. Primary Care: Clinics in Office Practice [Internet]. 2018 Sep [cited 2023 Sep 7];45(3):423–31. Available from: https://pubmed.ncbi.nlm.nih. 3011.
- Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr [Internet]. 2016 May 6 [cited 2023 Sep 7];4(3). Available from: https://pubmed.ncbi.nlm.nih.gov/27337481/.
- Kuchar E, Karlikowska-Skwarnik M, Han S, Nitsch-Osuch A. Pertussis: History of the Disease and Current Prevention Failure. In 2016 [cited 2023 Sep 7]. p. 77–82. Available from: https://pubmed.ncbi.nlm.nih.gov/27256351/.
- World Health Organization. Pertussis- number of reported cases. [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/pertussis-number-of-reported-cases.
- Yen LM, Thwaites CL. Tetanus. The Lancet [Internet]. 2019 Apr [cited 2023 Sep 7];393(10181):1657–68. Available from: https://pubmed.ncbi.nlm.nih.gov/30935736/.
- Rhinesmith E, Fu L. Tetanus Disease, Treatment, Management. Pediatr Rev [Internet]. 2018 Aug 1 [cited 2023 Sep 7];39(8):430–2. Available from: https://pubmed.ncbi.nlm.nih.gov/30068747/.
- Finkelstein P, Teisch L, Allen CJ, Ruiz G. Tetanus: A Potential Public Health Threat in Times of Disaster. Prehosp Disaster Med [Internet]. 2017 Jun 20 [cited 2023 Sep 7];32(3):339–42. Available from: https://pubmed.ncbi.nlm.nih.gov/28215195/.
- World Health Organization. Neonatal tetanus- number of reported cases [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/neonatal-tetanus---number-of-reported-cases.
- World Health Organization. Total tetanus- number of reported cases [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/total-tetanus---number-of-reported-cases.
- World Health Organization. Tetanus reported cases and incidence [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://immunizationdata.who.int/pages/incidence/ttetanus.html?CODE=Global&DISEASE=NTETANUS&YEAR=.
- Aaby P, Ravn H, Benn CS. The WHO Review of the Possible Nonspecific Effects of Diphtheria-Tetanus-Pertussis Vaccine. Pediatric Infectious Disease Journal [Internet]. 2016 Nov [cited 2023 Sep 8];35(11):1247–57. Available from: https://pubmed.ncbi.nlm.nih.gov/27753772/.
- DuVernoy TS, Braun MM. Hypotonic–Hyporesponsive Episodes Reported to the Vaccine Adverse Event Reporting System (VAERS), 1996–1998. Pediatrics [Internet]. 2000 Oct 1 [cited 2023 Sep 8];106(4):e52–e52. Available from: https://pubmed.ncbi.nlm.nih.gov/11015547/.
- szczepienia.pzh.gov.pl. Epizod hipotoniczno-reaktywny jako niepożądany odczyn poszczepienny- jak postępować przy kolejnych szczepieniach? [Internet]. 2022 [cited 2023 Sep 8]. Available from: https://szczepienia.pzh.gov.pl/faq/epizod-hipotoniczno-reaktywny-jako-niepozadany-odczyn-poszczepienny-jak-postepowac-przy-kolejnych-szczepieniach/.
- World Health Organization. Haemophilus influezae type b [Internet]. [cited 2023 Sep 8]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/hib.
- Wen S, Feng D, Chen D, Yang L, Xu Z. Molecular epidemiology and evolution of Haemophilus influenzae. Infection, Genetics and Evolution [Internet]. 2020 Jun [cited 2023 Sep 8];80:104205. Available from: https://pubmed.ncbi.nlm.nih.gov/31981610/.
- Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology [Internet]. 2004 Oct [cited 2023 Sep 8];113(2):163–74. Available from: https://pubmed.ncbi.nlm.nih.gov/15379976/.
- Briere EC, Rubin L, Moro PL, Cohn A, Clark T, Messonnier N. Prevention and control of haemophilus influenzae type b disease: recommendations of the advisory committee on immunization practices (ACIP). Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. MMWR Recomm Rep [Internet]. 2014 Feb 28 [cited 2023 Sep 8];(63):1–14. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6301a1.htm.
- szczepienia.pzh.gov.pl. Szczepionka przeciw Hib [Internet]. 2022 [cited 2023 Sep 8]. Available from: https://szczepienia.pzh.gov.pl/szczepionki/hib/.
- Marquart ME. Pathogenicity and virulence of Streptococcus pneumoniae : Cutting to the chase on proteases. Virulence [Internet]. 2021 Dec 31 [cited 2023 Sep 6];12(1):766–87. Available from: https://pubmed.ncbi.nlm.nih.gov/33660565/.
- Kim GL, Seon SH, Rhee DK. Pneumonia and Streptococcus pneumoniae vaccine. Arch Pharm Res [Internet]. 2017 Aug 22 [cited 2023 Sep 6];40(8):885–93. Available from: https://pubmed.ncbi.nlm.nih.gov/28735461/.
- Briles DE, Paton JC, Mukerji R, Swiatlo E, Crain MJ. Pneumococcal Vaccines. Microbiol Spectr [Internet]. 2019 Dec 19 [cited 2023 Sep 6];7(6). Available from: https://pubmed.ncbi.nlm.nih.gov/31858954/.
- Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clinical Microbiology and Infection [Internet]. 2010 May [cited 2023 Sep 6];16(5):411–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20132250/.
- Rose MA, Christopoulou D, Myint TTH, Schutter I. The burden of invasive pneumococcal disease in children with underlying risk factors in North America and Europe. Int J Clin Pract [Internet]. 2014 Jan 19 [cited 2023 Sep 6];68(1):8–19. Available from: https://pubmed.ncbi.nlm.nih.gov/23869711/.
- Pucchio AMR, Alabdulraheem A, Salvadori MI. Polio. Can Med Assoc J [Internet]. 2022 Nov 15 [cited 2023 Aug 29];194(44):E1509–E1509. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9828935/.
- Itasca (IL): American Academy of Pediatrics. Poliovirus infections. n: Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd ed. 2021. 601–607 p.
- Walter K, Malani PN. What Is Polio? JAMA [Internet]. 2022 Oct 25 [cited 2023 Aug 29];328(16):1652. Available from: https://pubmed.ncbi.nlm.nih.gov/36112391/.
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol [Internet]. 2015 May [cited 2023 Aug 29];10(5):791–808. Available from: https://pubmed.ncbi.nlm.nih.gov/25824845/.
- Moss WJ. Measles. The Lancet [Internet]. 2017 Dec [cited 2023 Aug 30];390(10111):2490–502. Available from: https://pubmed.ncbi.nlm.nih.gov/28673424/.
- Griffin DE. Measles virus and the nervous system. In 2014 [cited 2023 Aug 30]. p. 577–90. Available from: https://pubmed.ncbi.nlm.nih.gov/25015505/.
- Porter A, Goldfarb J. Measles: A dangerous vaccine-preventable disease returns. Cleve Clin J Med [Internet]. 2019 Jun [cited 2023 Aug 30];86(6):393–8. Available from: https://pubmed.ncbi.nlm.nih.gov/31204978/.
- Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers [Internet]. 2016 Jul 14 [cited 2023 Aug 30];2(1):16049. Available from: https://pubmed.ncbi.nlm.nih.gov/27411684/.
- de Vries RD, McQuaid S, van Amerongen G, Yüksel S, Verburgh RJ, Osterhaus ADME, et al. Measles Immune Suppression: Lessons from the Macaque Model. PLoS Pathog [Internet]. 2012 Aug 30 [cited 2023 Aug 30];8(8):e1002885. Available from: https://pubmed.ncbi.nlm.nih.gov/22952446/.
- Rubin S, Kennedy R, Poland G. Emerging Mumps Infection. Pediatric Infectious Disease Journal [Internet]. 2016 Jul [cited 2023 Sep 5];35(7):799–801. Available from: https://pubmed.ncbi.nlm.nih.gov/27097351/.
- Su SB, Chang HL, Chen KT. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. Int J Environ Res Public Health [Internet]. 2020 Mar 5 [cited 2023 Sep 5];17(5):1686. Available from: https://pubmed.ncbi.nlm.nih.gov/32150969/.
- Hviid A, Rubin S, Mühlemann K. Mumps. The Lancet [Internet]. 2008 Mar [cited 2023 Sep 5];371(9616):932–44. Available from: https://pubmed.ncbi.nlm.nih.gov/18342688/.
- World Health Organisation. Mumps- number of reported cases [Internet]. [cited 2023 Sep 5]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/mumps---number-of-reported-cases.
- Winter AK, Moss WJ. Rubella. The Lancet [Internet]. 2022 Apr [cited 2023 Sep 6];399(10332):1336–46. Available from: https://pubmed.ncbi.nlm.nih.gov/35367004/.
- Vander Straten MR, Tyring SK. Rubella. Dermatol Clin [Internet]. 2002 Apr [cited 2023 Sep 6];20(2):225–31. Available from: https://pubmed.ncbi.nlm.nih.gov/12120437/.
- Gudeloglu E, Akillioglu M, Bedir Demirdag T, Unal NA, Tapisiz AA. Congenital Rubella syndrome: A short report and literature review. Trop Doct [Internet]. 2023 Jan 1 [cited 2023 Sep 6];53(1):171–5. Available from: https://pubmed.ncbi.nlm.nih.gov/36321169/.
- World Health Organisation. Congenital rubella syndrome- number of reported cases. [Internet]. 2023 [cited 2023 Sep 7]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/congenital-rubella-syndrome---number-of-reported-cases.
- Miller E. MMR Vaccine: Review of Benefits and Risks. Journal of Infection [Internet]. 2002 Jan [cited 2023 Sep 6];44(1):1–6. Available from: https://pubmed.ncbi.nlm.nih.gov/11972410/.
- Latner DR, Hickman CJ. Remembering Mumps. PLoS Pathog [Internet]. 2015 May 7 [cited 2023 Sep 6];11(5):e1004791. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423963/.
- Plotkin SA. Commentary. Pediatric Infectious Disease Journal [Internet]. 2013 Apr [cited 2023 Sep 6];32(4):381–2. Available from: https://pubmed.ncbi.nlm.nih.gov/23552675/.
- Afzal MA, Pipkin PA, Minor PD. Absence of chicken myelin basic protein residues in commercial formulations of MMR vaccine. Vaccine [Internet]. 2000 Oct 15 [cited 2023 Sep 6];19(4–5):442–6. Available from: https://pubmed.ncbi.nlm.nih.gov/11027807/.
- Godlee F, Smith J, Marcovitch H. Wakefield’s article linking MMR vaccine and autism was fraudulent. BMJ [Internet]. 2011 Jan 5 [cited 2023 Sep 7];342(jan05 1):c7452–c7452. Available from: https://pubmed.ncbi.nlm.nih.gov/21209060/.
- Wakefield AJ. MMR vaccination and autism. The Lancet [Internet]. 1999 Sep [cited 2023 Sep 7];354(9182):949–50. Available from: https://pubmed.ncbi.nlm.nih.gov/10489978/.
- Wakefield A, Murch S, Anthony A, Linnell J, Casson D, Malik M, et al. RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. The Lancet [Internet]. 1998 Feb [cited 2023 Sep 7];351(9103):637–41. Available from: https://pubmed.ncbi.nlm.nih.gov/9500320/.
- Gerber JS, Offit PA. Vaccines and Autism: A Tale of Shifting Hypotheses. Clinical Infectious Diseases [Internet]. 2009 Feb 15 [cited 2023 Oct 8];48(4):456–61. Available from: https://pubmed.ncbi.nlm.nih.gov/19128068/.
- DeStefano F, Shimabukuro TT. The MMR Vaccine and Autism. Annu Rev Virol [Internet]. 2019 Sep 29 [cited 2023 Sep 7];6(1):585–600. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6768751/.
- Jain A, Marshall J, Buikema A, Bancroft T, Kelly JP, Newschaffer CJ. Autism Occurrence by MMR Vaccine Status Among US Children With Older Siblings With and Without Autism. JAMA [Internet]. 2015 Apr 21 [cited 2023 Sep 7];313(15):1534. Available from: https://pubmed.ncbi.nlm.nih.gov/25898051/.
- World Health Organization. Varicella [Internet]. [cited 2023 Sep 9]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/varicella.
- Bolton L. Herpes Zoster (Shingles). Wounds [Internet]. 2018 May [cited 2023 Sep 9];30(5):144–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29847305/.
- Lo Presti C, Curti C, Montana M, Bornet C, Vanelle P. Chickenpox: An update. Med Mal Infect [Internet]. 2019 Feb [cited 2023 Sep 9];49(1):1–8. Available from: https://pubmed.ncbi.nlm.nih.gov/29789159/.
- Macartney K, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. In: Macartney K, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2008 [cited 2023 Sep 9]. Available from: https://pubmed.ncbi.nlm.nih.gov/18646079/.
- szczepienia.pzh.gov.pl. Szczepienia przeciw ospie wietrznej. 2023 Aug 31 [cited 2023 Sep 9]; Available from: https://szczepienia.pzh.gov.pl/szczepionki/ospa-wietrzna/.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother [Internet]. 2019 Mar 4 [cited 2023 Sep 9];15(3):645–57. Available from: https://pubmed.ncbi.nlm.nih.gov/30427766/.
- World Health Organization. Rabies [Internet]. 2023 [cited 2023 Sep 9]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/rabies.
- Rupprecht CE. Rhabdoviruses: Rabies Virus. In: Baron S, editor. Medical Microbiology [Internet]. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996 [cited 2023 Sep 9]. Available from: https://pubmed.ncbi.nlm.nih.gov/21413354/.
- Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol [Internet]. 2013 May [cited 2023 Sep 9];12(5):498–513. Available from: https://pubmed.ncbi.nlm.nih.gov/23602163/.
- Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, et al. Rabies. Nat Rev Dis Primers [Internet]. 2017 Nov 30 [cited 2023 Sep 9];3(1):17091. Available from: https://pubmed.ncbi.nlm.nih.gov/29188797/.
- MIN ROLNICTWA I ROZWOJU WSI. ROZPORZĄDZENIE MINISTRA ROLNICTWA I ROZWOJU WSI z dnia 1 marca 2022 r. w sprawie wprowadzenia programu zwalczania wścieklizny na lata 2022 i 2023. 2022.
- MIN. ROLNICTWA I ROZWOJU WSI. Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 17 grudnia 2013 r. w sprawie przeprowadzania ochronnych szczepień lisów wolno żyjących przeciwko wściekliźnie. 2013.
- World Health Organization. Rabies Vaccines: WHO Position Paper. Weekly Epidemiological Record 2018, 16, 201–20. [Google Scholar]
- Taubenberger JK, Kash JC. Influenza Virus Evolution, Host Adaptation, and Pandemic Formation. Cell Host Microbe [Internet]. 2010 Jun [cited 2023 Sep 10];7(6):440–51. Available from: https://pubmed.ncbi.nlm.nih.gov/20542248/.
- Coelingh K, Olajide IR, MacDonald P, Yogev R. Efficacy and effectiveness of live attenuated influenza vaccine in school-age children. Expert Rev Vaccines [Internet]. 2015 Oct 3 [cited 2023 Sep 10];14(10):1331–46. Available from: https://pubmed.ncbi.nlm.nih.gov/26372891/.
- World Health Organization. INFLUENZA LABORATORY SURVEILLANCE INFORMATION- Virus detection by subtype reported to FluNet [Internet]. 2023 [cited 2023 Sep 10]. Available from: https://app.powerbi.com/view?r=eyJrIjoiZTkyODcyOTEtZjA5YS00ZmI0LWFkZGUtODIxNGI5OTE3YjM0IiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9.
- Hutchinson EC. Influenza Virus. Trends Microbiol [Internet]. 2018 Sep [cited 2023 Sep 10];26(9):809–10. Available from: https://pubmed.ncbi.nlm.nih.gov/29909041/.
- Worls Health Organozation. Influenza (seasonal) [Internet]. [cited 2023 Sep 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
- Live attenuated influenza vaccine for children. Drug Ther Bull [Internet]. 2017 Oct 11 [cited 2023 Sep 10];55(10):114–7. Available from: https://pubmed.ncbi.nlm.nih.gov/29021248/.
- Nypaver C, Dehlinger C, Carter C. Influenza and Influenza Vaccine: A Review. J Midwifery Womens Health [Internet]. 2021 Jan [cited 2023 Sep 10];66(1):45–53. Available from: https://pubmed.ncbi.nlm.nih.gov/33522695/.
- Hollingshead S, Tang CM. An Overview of Neisseria meningitidis. In 2019 [cited 2023 Sep 10]. p. 1–16. Available from: https://pubmed.ncbi.nlm.nih.gov/30877666/.
- World Health Organization. Meningococcal meningitis [Internet]. [cited 2023 Sep 10]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/meningococcal-meningitis.
- Centers For Disease Control And Prevention. Meningococcal Disease [Internet]. 2022 [cited 2023 Sep 10]. Available from: https://wwwnc.cdc.gov/travel/diseases/meningococcal-disease.
- Filippakis D, Gkentzi D, Dimitriou G, Karatza A. Neonatal meningococcal disease: an update. The Journal of Maternal-Fetal & Neonatal Medicine [Internet]. 2022 Nov 2 [cited 2023 Sep 10];35(21):4190–5. Available from: https://pubmed.ncbi.nlm.nih.gov/33233995/.
- Ceyhan M, Ozsurekci Y, Lucidarme J, Borrow R. Characterization of invasive Neisseria meningitidis isolates recovered from children in Turkey during a period of increased serogroup B disease, 2013–2017. Vaccine [Internet]. 2020 Apr [cited 2023 Sep 10];38(19):3545–52. Available from: https://pubmed.ncbi.nlm.nih.gov/32199701/.
- World Health Organization. Defeating meningitis by 2030 global road map - Highlights from WHO launch event [Internet]. 2018 [cited 2023 Sep 10]. Available from: https://www.who.int/initiatives/defeating-meningitis-by-2030.
- Abutaleb A, Kottilil S. Hepatitis A. Gastroenterol Clin North Am [Internet]. 2020 Jun [cited 2023 Sep 11];49(2):191–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32389358/.
- World Health Organization. Hepatitis A [Internet]. 2023 [cited 2023 Sep 11]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a.
- Jeong SH, Lee HS. Hepatitis A: Clinical Manifestations and Management. Intervirology [Internet]. 2010 [cited 2023 Sep 11];53(1):15–9. Available from: https://pubmed.ncbi.nlm.nih.gov/20068336/.
- Bell BP. Hepatitis A vaccine. Semin Pediatr Infect Dis [Internet]. 2002 Jul [cited 2023 Sep 11];13(3):165–73. Available from: https://pubmed.ncbi.nlm.nih.gov/12199612/.
- Johnson N, Migné C V., Gonzalez G. Tick-borne encephalitis. Curr Opin Infect Dis [Internet]. 2023 Jun [cited 2023 Sep 11];36(3):198–202. Available from: https://pubmed.ncbi.nlm.nih.gov/37093044/.
- Phipps LP, Johnson N. JMM Profile: Tick-borne encephalitis virus. J Med Microbiol [Internet]. 2022 May 31 [cited 2023 Sep 11];71(5). Available from: https://pubmed.ncbi.nlm.nih.gov/35604825/.
- Pustijanac E, Buršić M, Talapko J, Škrlec I, Meštrović T, Lišnjić D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms [Internet]. 2023 Jun 22 [cited 2023 Sep 11];11(7):1634. Available from: https://www.mdpi.com/2076-2607/11/7/1634.
- Lindquist L, Vapalahti O. Tick-borne encephalitis. The Lancet [Internet]. 2008 May [cited 2023 Sep 11];371(9627):1861–71. Available from: https://pubmed.ncbi.nlm.nih.gov/18514730/.
- Wojewódzka Stacja Sanitarno- Epidemiologiczna w Poznaniu. Kleszczowe zapalenie mózgu [Internet]. [cited 2023 Sep 11]. Available from: https://www.gov.pl/web/wsse-poznan/kleszczowe-zapalenie-mozgu.
- Ntanasis-Stathopoulos I, Kyriazoglou A, Liontos M, A Dimopoulos M, Gavriatopoulou M. Current trends in the management and prevention of human papillomavirus (HPV) infection. J BUON [Internet]. 2020 [cited 2023 Sep 11];25(3):1281–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32862567/.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol [Internet]. 2010 May [cited 2023 Sep 11];117(2):S5–10. Available from: https://pubmed.ncbi.nlm.nih.gov/20304221/.
- World Health Organization. Cervical cancer [Internet]. 2022 [cited 2023 Sep 11]. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- World Health Organization, Cervical cancer elimination initiative. Global strategy to accelerate the elimination of cervical cancer as a public health problem [Internet]. 2020 Nov [cited 2023 Sep 11]. Available from: https://www.who.int/publications/i/item/9789240014107.
- szczepienia.pzh.gov.pl. Europejski Tydzień Profilaktyki Raka Szyjki Macicy 2023 [Internet]. 2023 [cited 2023 Sep 11]. Available from: https://szczepienia.pzh.gov.pl/europejski-tydzien-profilaktyki-raka-szyjki-macicy-2023/.
- Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep [Internet]. 2015 Mar 27 [cited 2023 Sep 11];64(11):300–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25811679/.
- Ministerstwo Zdrowia. Szczepienia przeciw HPV [Internet]. [cited 2023 Sep 11]. Available from: https://www.gov.pl/web/zdrowie/hpv.
- Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. The Lancet. 2017 Sep;390(10101):1539–49.
- World Health Organization. Cholera [Internet]. [cited 2023 Sep 12]. Available from: https://www.who.int/health-topics/cholera#tab=tab_1.
- Kanungo S, Azman AS, Ramamurthy T, Deen J, Dutta S. Cholera. The Lancet [Internet]. 2022 Apr [cited 2023 Sep 12];399(10333):1429–40. Available from: https://pubmed.ncbi.nlm.nih.gov/35397865/.
- Hsueh BY, Waters CM. Combating Cholera. F1000Res [Internet]. 2019 Apr 30 [cited 2023 Sep 12];8:589. Available from: https://pubmed.ncbi.nlm.nih.gov/31069064/.
- Khanam F, Ross AG, McMillan NAJ, Qadri F. Toward Typhoid Fever Elimination. International Journal of Infectious Diseases [Internet]. 2022 Jun [cited 2023 Sep 12];119:41–3. Available from: https://pubmed.ncbi.nlm.nih.gov/35338009/.
- World Health Organization. Typhoid [Internet]. 2023 [cited 2023 Sep 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/typhoid.
- Ajibola O, Mshelia M, Gulumbe B, Eze A. Typhoid Fever Diagnosis in Endemic Countries: A Clog in the Wheel of Progress? Medicina (B Aires) [Internet]. 2018 Apr 25 [cited 2023 Sep 12];54(2):23. Available from: https://pubmed.ncbi.nlm.nih.gov/30344254/.
- Monath TP, Vasconcelos PFC. Yellow fever. Journal of Clinical Virology [Internet]. 2015 Mar [cited 2023 Sep 12];64:160–73. Available from: https://pubmed.ncbi.nlm.nih.gov/25453327/.
- World Health Organization. Yellow Fever [Internet]. 2015 [cited 2023 Sep 12]. Available from: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=9476:yellow-fever&Itemid=0&lang=en#gsc.tab=0.
- Tuells J, Henao-Martínez AF, Franco-Paredes C. Yellow Fever: A Perennial Threat. Arch Med Res [Internet]. 2022 Nov [cited 2023 Sep 12];53(7):649–57. Available from: https://pubmed.ncbi.nlm.nih.gov/36404585/.
- Reno E, Quan NG, Franco-Paredes C, Chastain DB, Chauhan L, Rodriguez-Morales AJ, et al. Prevention of yellow fever in travellers: an update. Lancet Infect Dis [Internet]. 2020 Jun [cited 2023 Sep 12];20(6):e129–37. Available from: https://pubmed.ncbi.nlm.nih.gov/32386609/.
- Thomas R. Yellow fever vaccine-associated viscerotropic disease: current perspectives. Drug Des Devel Ther [Internet]. 2016 Oct [cited 2023 Sep 12];Volume 10:3345–53. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5066857/.
- Cohen M, Nguyen M, Nix CD, Case B, Nickerson JP, Bernard J, et al. Case Report: Yellow Fever Vaccine-Associated Neurotropic Disease and Associated MRI, EEG, and CSF Findings. Front Neurol [Internet]. 2022 Feb 18 [cited 2023 Sep 12];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8931735/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).