Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
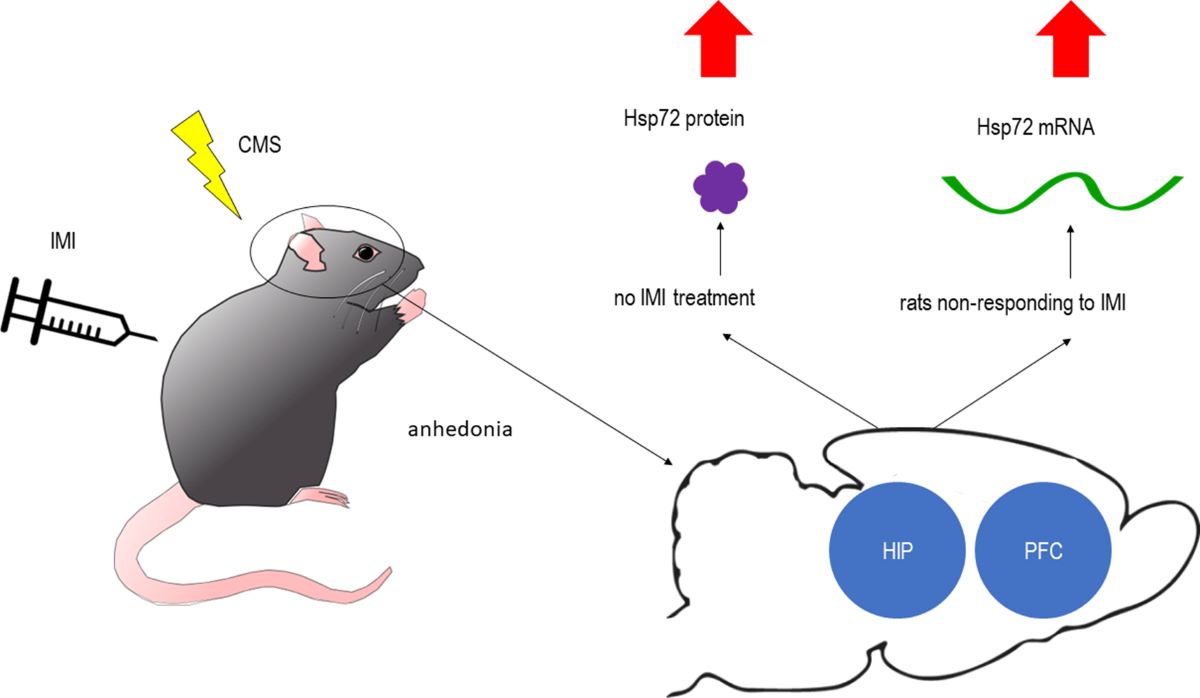
Abstract
Keywords:
1. Introduction
2. Results
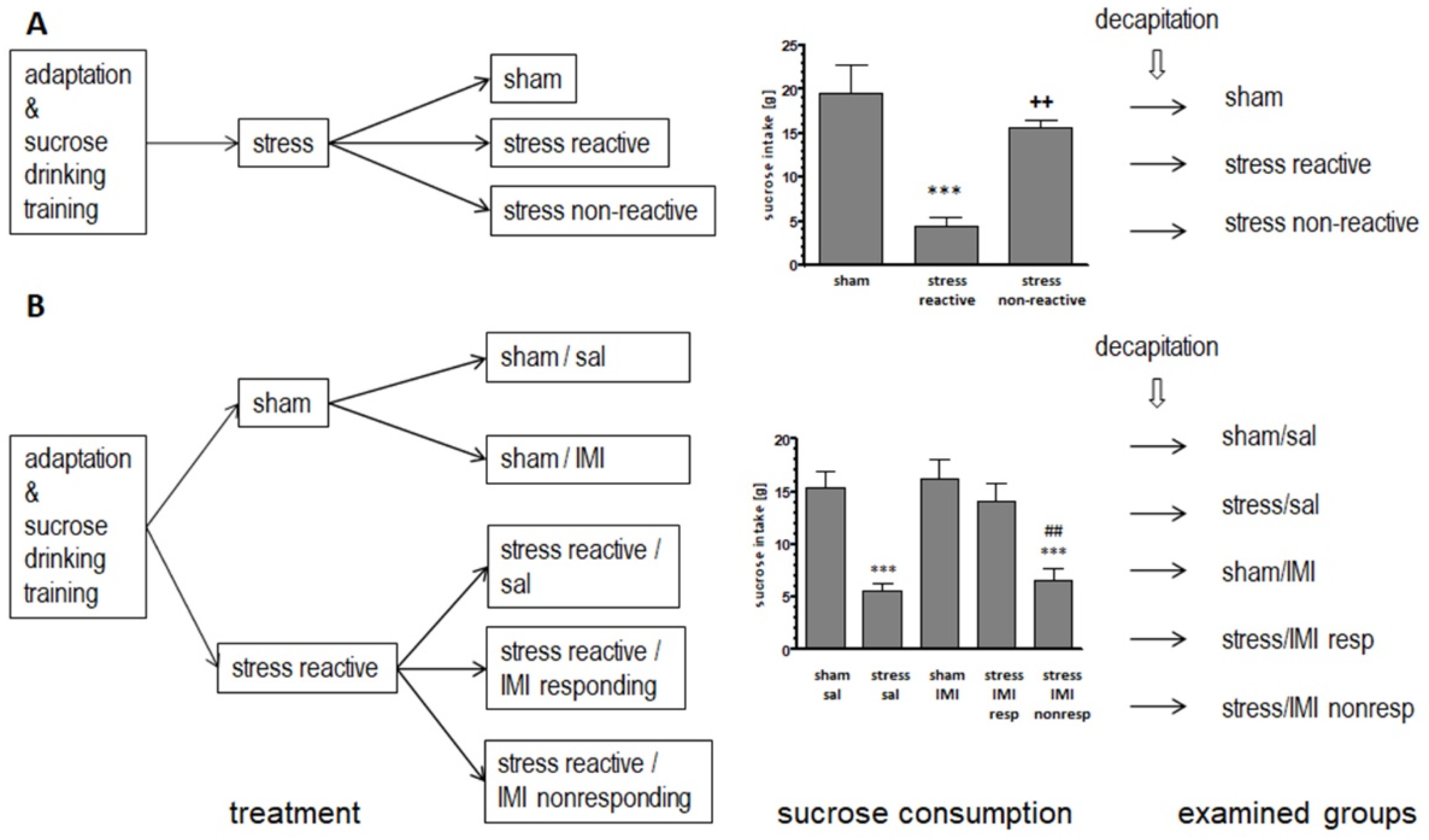
2.1. Evaluation of sucrose consumption in stress reactive vs. stress nonreactive animals and in imipramine responding vs. imipramine nonresponding animals: generating of experimental groups for biochemical studies
2.2. Effects of three weeks of CMS on the mRNA expression of HSP70 and HSP90 family members
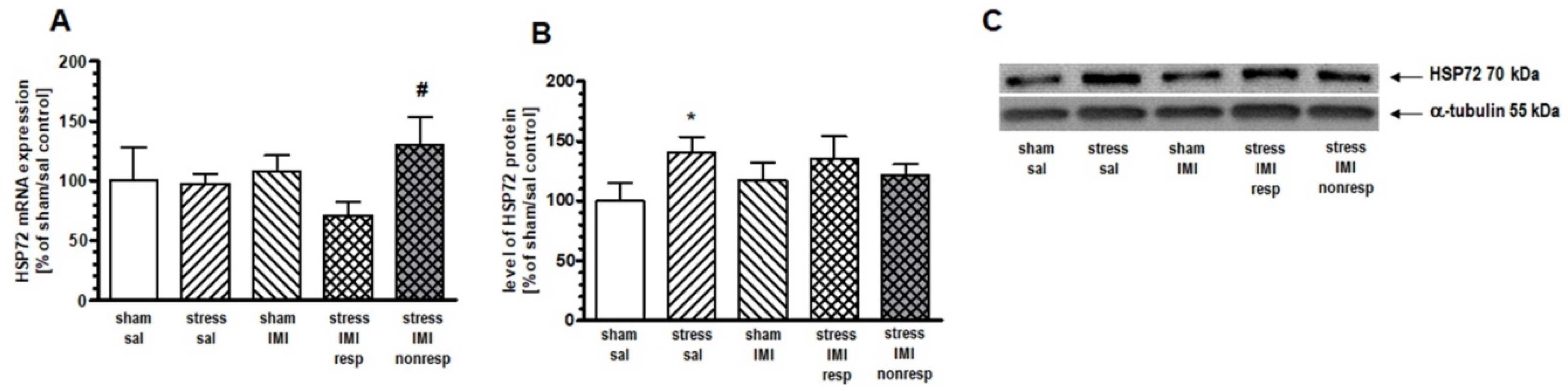
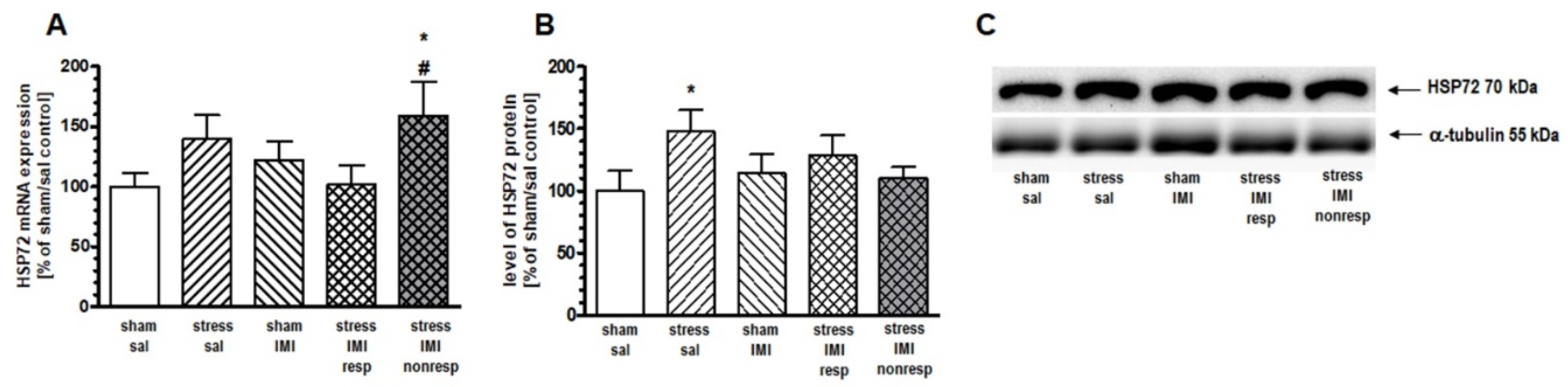
2.3. Effects of eight weeks of CMS on the mRNA expression of HSP70 family members
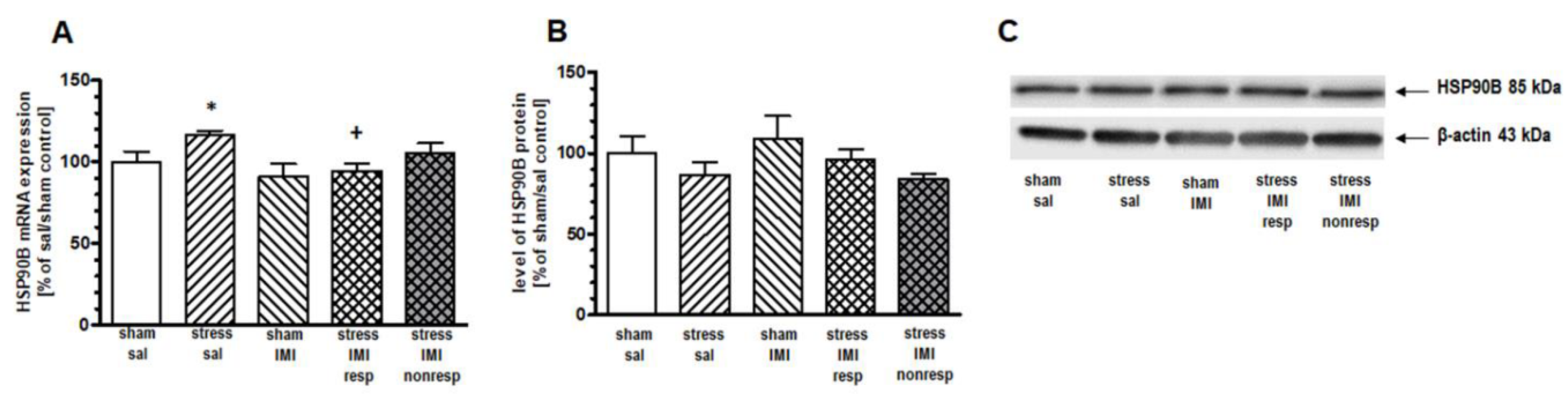
2.4. Effects of eight weeks of CMS on the mRNA expression of HSP90 family members
| Treatment | HSC70 mRNA level[% of sal/sham control ± SEM] 1 | |
| PFC | HIP | |
| sham/sal | 100,00 ± 21,14 | 100,00 ± 7,69 |
| stress/sal | 88,95 ± 22,11 | 107,79 ± 3,66 |
| sham/IMI | 83,96 ± 14,87 | 91,40 ± 6,75 |
| stress/IMI resp | 96,63 ± 17,67 | 97,95 ± 4,17 |
| stress/IMI nonresp | 79,81 ± 5,98 | 107,40 ± 7,57 |
| 1 way ANOVA | F(4,25) = 0.88, p > 0.05 | F(4,25) = 1.23, p >0.05 |
| Treatment | mRNAs level [% of sal/sham control ± SEM] 1 |
||
| PFC | HIP | ||
| HSP90A | HSP90B | HSP90A | |
| sham/sal | 100,00± 7,39 | 100,00 ± 5,20 | 100,00 ± 1,80 |
| stress/sal | 100,80 ± 8,78 | 100,85 ± 5,86 | 104,98 ± 3,90 |
| sham/IMI | 109,89 ± 7,37 | 101,33 ± 4,24 | 98,07 ± 3,59 |
| stress/IMI resp | 101,45 ± 3,75 | 111,74 ± 6,95 | 102,90 ± 3,44 |
| stress/IMI nonresp | 97,86 ± 5,12 | 104,32 ± 6,17 | 100,62 ± 4,34 |
| 1 way ANOVA |
F(4,25) = 0.47, p > 0.05 |
F(4,25) = 1.29, p > 0.05 |
F(4,25) = 0.58, p > 0.05 |
| Treatment | mRNAs level in Thal [% of sal/sham control ± SEM]1 |
|||
| HSP72 | HSC70 | HSP90A | HSP90B | |
| sham/sal | 100,00 ± 7,50 | 100,00 ± 3,20 | 100,00 ± 11,30 | 100,00 ± 2,89 |
| stress/sal | 95,60 ± 8,43 | 109,00 ± 4,50 | 105,99 ± 5,38 | 99,01 ± 3,87 |
| sham/IMI | 99,80 ± 4,93 | 101,90 ± 4,70 | 108,00 ± 4,86 | 101,84 ± 4,12 |
| stress/IMI resp | 97,07 ± 9,94 | 112,00 ± 9,10 | 101,25 ± 7,51 | 97,88 ± 8,39 |
| stress/IMI nonresp | 115,74 ± 8,34 | 99,00 ± 5,00 | 100,72 ± 5,32 | 99,01 ± 7,58 |
| 1 way ANOVA |
F(4,25) = 1.05, p > 0.05 |
F(4,25) = 1.04, p > 0.05 |
F(4,25) = 0.23, p > 0.05 |
F(4,25) = 0.07, p > 0.05 |
2.5. Effects of eight weeks of CMS on the cytosolic protein expression of HSPs
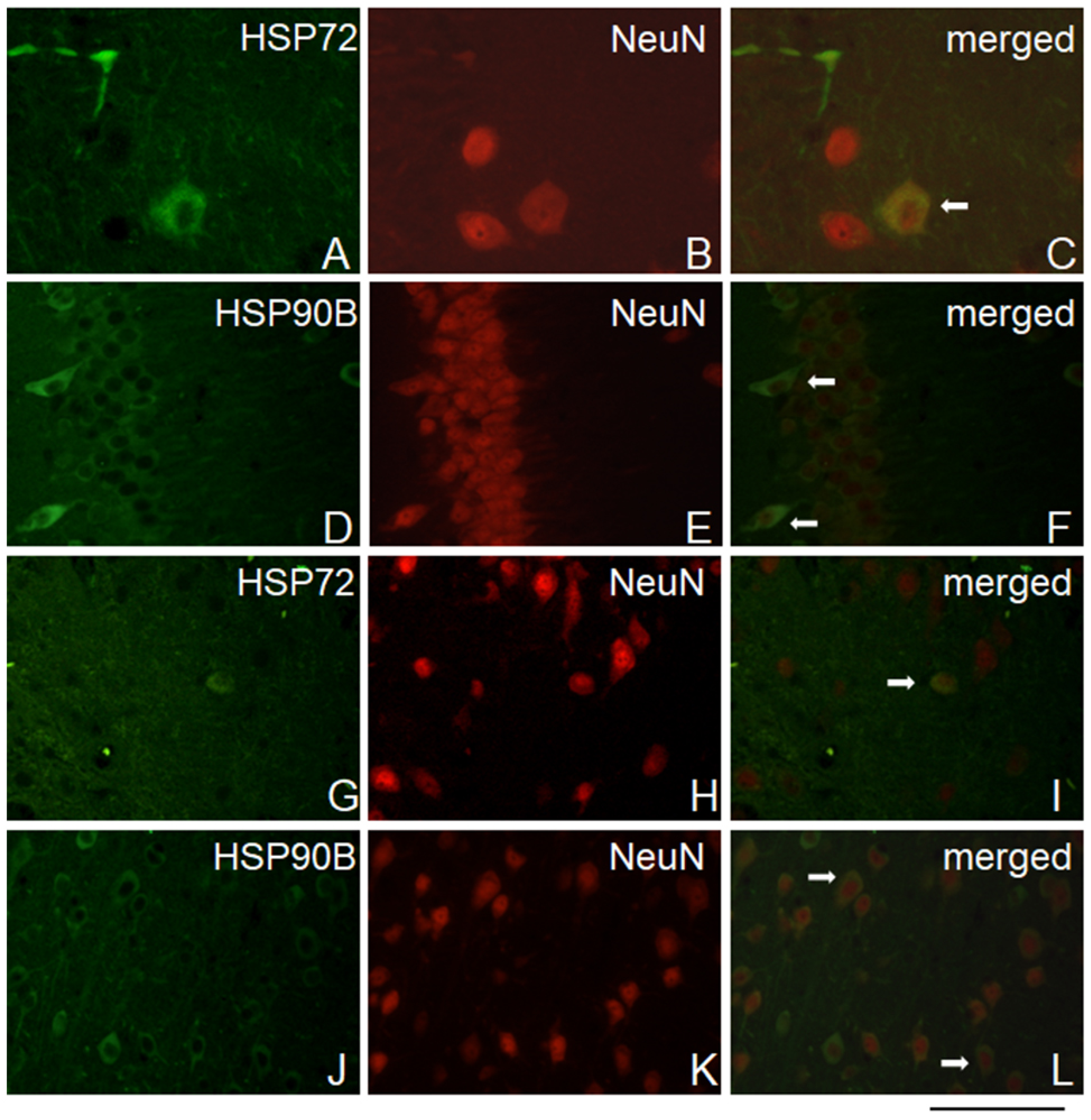
2.6. HSP72 and HSP90B colocalize with neuronal cells
3. Discussion
3.1. Applicability of experimental groups generated in the CMS model
3.2. Evaluation of the effects of CMS and IMI therapy on the cerebral expression of HSP
3.2.1. HSPA family
3.2.2. HSPC family
3.3. Localization and dynamics of CMS-induced changes in HSP expression
4. Materials and Methods
4.1. Animals
4.2. Sucrose consumption test
4.3. Chronic mild stress protocol
4.3.1. Stress-reactive and nonreactive animals
4.3.2. Imipramine responding and nonresponding animals
4.4. Drug administration
4.5. Tissue preparation
4.6. Real-time analysis of HSP mRNA levels
4.7. Immunoblot analysis of HSP72 and HSP90B protein levels
4.8. Immunofluorescence analysis
4.9. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritossa, F. Discovery of the Heat Shock Response. Cell Stress. Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the Nomenclature of the Human Heat Shock Proteins. Cell Stress. Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat Shock Proteins: Cellular and Molecular Mechanisms in the Central Nervous System. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Ran, R.; Parmentier-Batteur, S.; Nee, A.; Sharp, F.R. Geldanamycin Induces Heat Shock Proteins in Brain and Protects against Focal Cerebral Ischemia. J. Neurochem. 2002, 81, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 Chaperone Machinery*. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef]
- Chaudhuri, T.K.; Paul, S. Protein-Misfolding Diseases and Chaperone-Based Therapeutic Approaches. FEBS J. 2006, 273, 1331–1349. [Google Scholar] [CrossRef]
- Bei, E.S.; Salpeas, V.; Alevizos, B.; Anagnostara, C.; Pappa, D.; Moutsatsou, P. Pattern of Heat Shock Factor and Heat Shock Protein Expression in Lymphocytes of Bipolar Patients: Increased HSP70-Glucocorticoid Receptor Heterocomplex. J. Psychiatr. Res. 2013, 47, 1725–1736. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the Human Heat Shock Protein Hsp70 in Protection against Stress-Induced Apoptosis. Mol. Cell. Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef]
- Tsuchiya, D.; Hong, S.; Matsumori, Y.; Kayama, T.; Swanson, R.A.; Dillman, W.H.; Liu, J.; Panter, S.S.; Weinstein, P.R. Overexpression of Rat Heat Shock Protein 70 Reduces Neuronal Injury after Transient Focal Ischemia, Transient Global Ischemia, or Kainic Acid-Induced Seizures. Neurosurgery 2003, 53, 1179. [Google Scholar] [CrossRef]
- Matsumori, Y.; Northington, F.J.; Hong, S.M.; Kayama, T.; Sheldon, R.A.; Vexler, Z.S.; Ferriero, D.M.; Weinstein, P.R.; Liu, J. Reduction of Caspase-8 and -9 Cleavage Is Associated With Increased c-FLIP and Increased Binding of Apaf-1 and Hsp70 After Neonatal Hypoxic/Ischemic Injury in Mice Overexpressing Hsp70. Stroke 2006, 37, 507–512. [Google Scholar] [CrossRef]
- Klucken, J.; Shin, Y.; Masliah, E.; Hyman, B.T.; McLean, P.J. Hsp70 Reduces α-Synuclein Aggregation and Toxicity*. J. Biol. Chem. 2004, 279, 25497–25502. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Coccurello, R.; Bielawski, A.; Zelek-Molik, A.; Vetulani, J.; Kowalska, M.; D’Amato, F.R.; Nalepa, I. Brief Maternal Separation Affects Brain A1-Adrenoceptors and Apoptotic Signaling in Adult Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, M.; Russo, S.J. Anhedonia and the Brain Reward Circuitry in Depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.M.; Morais, M.; Marques, F.; Pinto, L.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. Stress-Induced Anhedonia Is Associated with Hypertrophy of Medium Spiny Neurons of the Nucleus Accumbens. Transl. Psychiatry 2013, 3, e266–e266. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Validity, Reliability and Utility of the Chronic Mild Stress Model of Depression: A 10-Year Review and Evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Nalepa, I.; Antkiewicz-Michaluk, L.; Sanchez, C. Behavioural and Biochemical Studies of Citalopram and WAY 100635 in Rat Chronic Mild Stress Model. Pharmacol. Biochem. Behav. 2002, 72, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Lason, M.; Tota-Glowczyk, K.; Niemczyk, M.; Litwa, E.; Willner, P. Rapid Antidepressant Effects of Deep Brain Stimulation of the Pre-Frontal Cortex in an Animal Model of Treatment-Resistant Depression. J. Psychopharmacol. 2018, 32, 1133–1140. [Google Scholar] [CrossRef]
- Escribá, P.V.; Ozaita, A.; García-Sevilla, J.A. Increased MRNA Expression of A2A-Adrenoceptors, Serotonin Receptors and μ-Opioid Receptors in the Brains of Suicide Victims. Neuropsychopharmacol. 2004, 29, 1512–1521. [Google Scholar] [CrossRef]
- Zelek-Molik, A.; Taracha, E.; Nawrat, D.; Bielawski, A.; Lehner, M.; Płaźnik, A.; Nalepa, I. Effects of Morphine and Methadone Treatment on MRNA Expression of Gα(i) Subunits in Rat Brains. Pharmacol. Rep. 2010, 62, 1197–1203. [Google Scholar] [CrossRef]
- Dowell, J.; Elser, B.A.; Schroeder, R.E.; Stevens, H.E. Cellular Stress Mechanisms of Prenatal Maternal Stress: Heat Shock Factors and Oxidative Stress. Neurosci. Lett. 2019, 709, 134368. [Google Scholar] [CrossRef]
- Tripathy, K.; Sodhi, M.; Kataria, R.S.; Chopra, M.; Mukesh, M. In Silico Analysis of HSP70 Gene Family in Bovine Genome. Biochem. Genet. 2021, 59, 134–158. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Galigniana, M.D.; Harrell, J.M.; DeFranco, D.B. Role of Hsp90 and the Hsp90-Binding Immunophilins in Signalling Protein Movement. Cell. Signal. 2004, 16, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The Heat Shock Protein 70 Family: Highly Homologous Proteins with Overlapping and Distinct Functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.C.; Morimoto, R.I. The Human Cytosolic Molecular Chaperones Hsp90, Hsp70 (Hsc70) and Hdj-1 Have Distinct Roles in Recognition of a Non-Native Protein and Protein Refolding. EMBO J. 1996, 15, 2969–2979. [Google Scholar] [CrossRef]
- Nollen, E.A.A.; Brunsting, J.F.; Roelofsen, H.; Weber, L.A.; Kampinga, H.H. In Vivo Chaperone Activity of Heat Shock Protein 70 and Thermotolerance. Mol. Cell Biol. 1999, 19, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-H.; Zhou, X.-M.; Cui, J.-Z.; Wang, K.-J.; Feng, Y.; Zhang, H.-A. Neuroprotective Effects of Dexmedetomidine on Traumatic Brain Injury: Involvement of Neuronal Apoptosis and HSP70 Expression. Mol. Med. Rep. 2018, 17, 8079. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.I.; Chu, K.; Lee, S.T.; Song, E.C.; Jung, K.H.; Kim, E.H.; Park, D.K.; Kang, K.M.; Kim, M.; Roh, J.K. Pharmacological Induction of Heat Shock Protein Exerts Neuroprotective Effects in Experimental Intracerebral Hemorrhage. Brain Res. 2007, 1135, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Matsuki, N. A 72 KDa Heat Shock Protein Is Protective against the Selective Vulnerability of CA1 Neurons and Is Essential for the Tolerance Exhibited by CA3 Neurons in the Hippocampus. Neuroscience 2002, 109, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, I.; Peraile, I.; Orio, L.; Colado, M.I.; O’Shea, E. Evidence for a Role of Hsp70 in the Neuroprotection Induced by Heat Shock Pre-Treatment against 3,4-Methylenedioxymethamphetamine Toxicity in Rat Brain. J. Neurochem. 2007, 101, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.A.; Harlow, B.L.; Soares, C.N.; Otto, M.W.; Cohen, L.S.; Minuzzi, L.; Gelain, D.P.; Moreira, J.C.F.; Frey, B.N. A Longitudinal Study of Neurotrophic, Oxidative, and Inflammatory Markers in First-Onset Depression in Midlife Women. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Elaković, I.; Brkljačić, J.; Matić, G. Long-Term Imipramine Treatment Affects Rat Brain and Pituitary Corticosteroid Receptors and Heat Shock Proteins Levels in a Gender-Specific Manner. J. Neural Transm. 2007, 114, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Boyer, P.-A.; Mocaër, E. Effect of Agomelatine in the Chronic Mild Stress Model of Depression in the Rat. Neuropsychopharmacol. 2003, 28, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, D.; Gavrilovic, L.; Dronjak, S.; Radojcic, M.B. Brain Glucocorticoid Receptor and Heat Shock Protein 70 Levels in Rats Exposed to Acute, Chronic or Combined Stress. Neuropsychobiology 2005, 51, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Samson, J.; Sheeladevi, R.; Ravindran, R.; Senthilvelan, M. Stress Response in Rat Brain after Different Durations of Noise Exposure. Neurosci. Res. 2007, 57, 143–147. [Google Scholar] [CrossRef]
- Hamed, R.; Elmalt, H.; Salama, A.; Younes, S.; Ahmed, A. Biomarkers of Oxidative Stress in Major Depressive Disorder. Open Access Maced. J. Med. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Yoshino, Y.; Dwivedi, Y. Elevated Expression of Unfolded Protein Response Genes in the Prefrontal Cortex of Depressed Subjects: Effect of Suicide. J. Affect. Disord. 2020, 262, 229–236. [Google Scholar] [CrossRef]
- Bachis, A.; Cruz, M.I.; Nosheny, R.L.; Mocchetti, I. Chronic Unpredictable Stress Promotes Neuronal Apoptosis in the Cerebral Cortex. Neurosci. Lett. 2008, 442, 104–108. [Google Scholar] [CrossRef]
- Yao, S.; Peng, M.; Zhu, X.; Cheng, M.; Qi, X. Heat Shock Protein72 Protects Hippocampal Neurons from Apoptosis Induced by Chronic Psychological Stress. Int. J. Neurosci. 2007, 117, 1551–1564. [Google Scholar] [CrossRef]
- Politi, P.; Brondino, N.; Emanuele, E. Increased Proapoptotic Serum Activity in Patients with Chronic Mood Disorders. Arch. Med. Res. 2008, 39, 242–245. [Google Scholar] [CrossRef]
- Eilat, E.; Mendlovic, S.; Doron, A.; Zakuth, V.; Spirer, Z. Increased Apoptosis in Patients with Major Depression: A Preliminary Study. J. Immunol. 1999, 163, 533–534. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Slotwinska, M.; Stachura, A.; Marmurowska-Michalowska, H.; Dubas-Slemp, H.; Bojarska-Junak, A.; Kandefer-Szerszen, M. Accelerated Apoptosis of Blood Leukocytes and Oxidative Stress in Blood of Patients with Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 686–694. [Google Scholar] [CrossRef]
- Peng, C.-H.; Chiou, S.-H.; Chen, S.-J.; Chou, Y.-C.; Ku, H.-H.; Cheng, C.-K.; Yen, C.-J.; Tsai, T.-H.; Chang, Y.-L.; Kao, C.-L. Neuroprotection by Imipramine against Lipopolysaccharide-Induced Apoptosis in Hippocampus-Derived Neural Stem Cells Mediated by Activation of BDNF and the MAPK Pathway. Eur. Neuropsychopharmacol. 2008, 18, 128–140. [Google Scholar] [CrossRef]
- Larsen, M.H.; Hay-Schmidt, A.; Ronn, L.C.; Mikkelsen, J.D. Temporal Expression of Brain-Derived Neurotrophic Factor (BDNF) MRNA in the Rat Hippocampus after Treatment with Selective and Mixed Monoaminergic Antidepressants. Eur. J. Pharmacol. 2008, 578, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.A.; Galloway, M.P.; Duman, R.S.; Russell, D.S.; D’Sa, C. Repeated Unpredictable Stress and Antidepressants Differentially Regulate Expression of the Bcl-2 Family of Apoptotic Genes in Rat Cortical, Hippocampal, and Limbic Brain Structures. Neuropsychopharmacol. 2008, 33, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Peng, C.-H.; Yang, Y.-P.; Wu, C.-C.; Hsu, W.-M.; Wang, H.-J.; Chan, K.-H.; Chou, Y.-P.; Chen, S.-J.; Chang, Y.-L. Desipramine Activated Bcl-2 Expression and Inhibited Lipopolysaccharide-Induced Apoptosis in Hippocampus-Derived Adult Neural Stem Cells. J. Pharmacol. Sci. 2007, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-H.; Ku, H.-H.; Tsai, T.-H.; Lin, H.-L.; Chen, L.-H.; Chien, C.-S.; Ho, L.L.-T.; Lee, C.-H.; Chang, Y.-L. Moclobemide Upregulated Bcl-2 Expression and Induced Neural Stem Cell Differentiation into Serotoninergic Neuron via Extracellular-Regulated Kinase Pathway. Br. J. Pharmacol. 2006, 148, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Fleshner, M. Adrenergic Receptors Mediate Stress-Induced Elevations in Extracellular Hsp72. J. Appl. Physiol. 2005, 99, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.H.; Okazaki, M.; Hu, Z.-W.; Miller, J.W.; Hoffman, B.B. Activation of Heat Shock Protein (Hsp)70 and Proto-Oncogene Expression by A1 Adrenergic Agonists in Rat Aorta with Age. J. Clin. Investig. 1996, 97, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Brown, J.M.; Ao, L.; Banerjee, A.; Harken, A.H. Norepinephrine Induces Cardiac Heat Shock Protein 70 and Delayed Cardioprotection in the Rat through A1 Adrenoceptors. Cardiovasc. Res. 1996, 32, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, A.; De Cian, M.-C.; Cueff, A.; Poulet, S.A. Noradrenaline and A-Adrenergic Signaling Induce the Hsp70 Gene Promoter in Mollusc Immune Cells. J. Cell Sci. 2001, 114, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Gavrilyuk, V.; Landreth, G.E.; O’Banion, M.K.; Weinberg, G.; Feinstein, D.L. Noradrenergic Depletion Increases Inflammatory Responses in Brain: Effects on IκB and HSP70 Expression. J. Neurochem. 2003, 85, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Liu, X.; Liu, D.X.; Jiang, H.; Mao, X.Q.; Wang, C.; Pan, F. Effects of Different Adrenergic Blockades on the Stress Resistance of Wistar Rats. Neurosci. Lett. 2012, 511, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Martinez-Vicente, M.; Arias, E.; Kaushik, S.; Sulzer, D.; Cuervo, A.M. Constitutive Upregulation of Chaperone-Mediated Autophagy in Huntington’s Disease. J. Neurosci. 2011, 31, 18492–18505. [Google Scholar] [CrossRef] [PubMed]
- Furay, A.R.; Murphy, E.K.; Mattson, M.P.; Guo, Z.; Herman, J.P. Region-Specific Regulation of Glucocorticoid Receptor/HSP90 Expression and Interaction in Brain. J. Neurochem. 2006, 98, 1176–1184. [Google Scholar] [CrossRef]
- Galigniana, N.M.; Ballmer, L.T.; Toneatto, J.; Erlejman, A.G.; Lagadari, M.; Galigniana, M.D. Regulation of the Glucocorticoid Response to Stress-Related Disorders by the Hsp90-Binding Immunophilin FKBP51. J. Neurochem. 2012, 122, 4–18. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid Receptor Function Regulated by Coordinated Action of the Hsp90 and Hsp70 Chaperone Cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef]
- Baker, J.D.; Ozsan, I.; Rodriguez Ospina, S.; Gulick, D.; Blair, L.J. Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease. Int. J. Mol. Sci. 2018, 20. [Google Scholar] [CrossRef]
- Guo, Y.; Guettouche, T.; Fenna, M.; Boellmann, F.; Pratt, W.B.; Toft, D.O.; Smith, D.F.; Voellmy, R. Evidence for a Mechanism of Repression of Heat Shock Factor 1 Transcriptional Activity by a Multichaperone Complex*. J. Biol. Chem. 2001, 276, 45791–45799. [Google Scholar] [CrossRef] [PubMed]
- Vamvakopoulos, N.O. Tissue-Specific Expression of Heat Shock Proteins 70 and 90: Potential Implication for Differential Sensitivity of Tissues to Glucocorticoids. Mol. Cell. Endocrinol. 1993, 98, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Saleh, A.; Nakazawa, A.; Kumar, S.; Srinivasula, S.M.; Kumar, V.; Weichselbaum, R.; Nalin, C.; Alnemri, E.S.; Kufe, D.; et al. Negative Regulation of Cytochrome C-Mediated Oligomerization of Apaf-1 and Activation of Procaspase-9 by Heat Shock Protein 90. EMBO J. 2000, 19, 4310–4322. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Tran, I.C.; Backos, D.S.; Harrell, J.M.; Chinkers, M.; Pratt, W.B.; Esteban, J.A. Independent Functions of Hsp90 in Neurotransmitter Release and in the Continuous Synaptic Cycling of AMPA Receptors. J. Neurosci. : Off. J. Soc. Neurosci. 2004, 24, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Shors, T.J. Stress, Anxiety, and Dendritic Spines: What Are the Connections? Neuroscience 2013, 251, 108–119. [Google Scholar] [CrossRef]
- Licznerski, P.; Duman, R.S. Remodeling of Axo-Spinous Synapses in the Pathophysiology and Treatment of Depression. Neuroscience 2013, 251, 33–50. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, Depression, and Mood Disorders: Structural Remodeling in the Brain. Metab. : Clin. Exp. 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Gourley, S.L.; Swanson, A.M.; Koleske, A.J. Corticosteroid-Induced Neural Remodeling Predicts Behavioral Vulnerability and Resilience. J. Neurosci. 2013, 33, 3107–3112. [Google Scholar] [CrossRef]
- Patel, D.; Anilkumar, S.; Chattarji, S.; Buwalda, B. Repeated Social Stress Leads to Contrasting Patterns of Structural Plasticity in the Amygdala and Hippocampus. Behav. Brain Res. 2018, 347, 314–324. [Google Scholar] [CrossRef]
- Godsil, B.P.; Kiss, J.P.; Spedding, M.; Jay, T.M. The Hippocampal–Prefrontal Pathway: The Weak Link in Psychiatric Disorders? Eur. Neuropsychopharmacol. 2013, 23, 1165–1181. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Wang, P.W.; Gado, M.H.; Csernansky, J.G.; Vannier, M.W. Hippocampal Atrophy in Recurrent Major Depression. Proc. Natl. Acad. Sci. USA 1996, 93, 3908–3913. [Google Scholar] [CrossRef]
- Lacerda, A.L.; Keshavan, M.S.; Hardan, A.Y.; Yorbik, O.; Brambilla, P.; Sassi, R.B.; Nicoletti, M.; Mallinger, A.G.; Frank, E.; Kupfer, D.J.; et al. Anatomic Evaluation of the Orbitofrontal Cortex in Major Depressive Disorder. Biol. Psychiatry 2004, 55, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Botteron, K.N.; Raichle, M.E.; Drevets, W.C.; Heath, A.C.; Todd, R.D. Volumetric Reduction in Left Subgenual Prefrontal Cortex in Early Onset Depression. Biol. Psychiatry 2002, 51, 342–344. [Google Scholar] [CrossRef]
- Viena, T.D.; Rasch, G.E.; Allen, T.A. Dual Medial Prefrontal Cortex and Hippocampus Projecting Neurons in the Paraventricular Nucleus of the Thalamus. Brain Struct. Funct. 2022, 227, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Curtis, G.R.; Oakes, K.; Barson, J.R. Expression and Distribution of Neuropeptide-Expressing Cells Throughout the Rodent Paraventricular Nucleus of the Thalamus. Front. Behav. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Musazzi, L.; Treccani, G.; Popoli, M. Functional and Structural Remodeling of Glutamate Synapses in Prefrontal and Frontal Cortex Induced by Behavioral Stress. Front. Psychiatry 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zelek-Molik, A.; Bobula, B.; Gądek-Michalska, A.; Chorązka, K.; Bielawski, A.; Kuśmierczyk, J.; Siwiec, M.; Wilczkowski, M.; Hess, G.; Nalepa, I. Psychosocial Crowding Stress-Induced Changes in Synaptic Transmission and Glutamate Receptor Expression in the Rat Frontal Cortex. Biomolecules 2021, 11, 294. [Google Scholar] [CrossRef]
- Papp, M.; Willner, P. Models of Affective Illness: Chronic Mild Stress in the Rat. Current Protocols 2023, 3, e712. [Google Scholar] [CrossRef]
- Zelek-Molik, A.; Costanzi, M.; Rafa-Zabłocka, K.; Kreiner, G.; Roman, A.; Vetulani, J.; Rossi-Arnaud, C.; Cestari, V.; Nalepa, I. Fear Memory-Induced Alterations in the MRNA Expression of G Proteins in the Mouse Brain and the Impact of Immediate Posttraining Treatment with Morphine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 221–231. [Google Scholar] [CrossRef]
| Brain structure/ Treatment |
Level of mRNAs [% of sham control ± SEM] 1 |
|||
| HSP72 | HSC70 | HSP90A | HSP90B | |
| A. PFC | ||||
| sham | 100,00 ± 10,98 | 100,00 ± 7,91 | 100,00 ± 5,86 | 100,00 ± 7,70 |
| stress reactive | 93,63 ± 10,57 | 96,64 ± 6,89 | 96,84 ± 5,97 | 94,30 ± 5,92 |
| stress non-reactive | 92,76 ± 8,94 | 96,33 ± 3,96 | 100,43 ± 2,96 | 85,88 ± 3,80 |
| 1 way ANOVA |
F(2,15) = 0.15, p > 0,05 |
F(2,15) = 0.10, p > 0.05 |
F(2,15) = 0.15, p > 0.05 |
F(2,15) = 1.39, p > 0.05 |
| B. HIP | ||||
| sham | 100,00 ± 10,58 | 100,00 ± 6,83 | 100,00 ± 3,00 | 100,00 ± 8,26 |
| stress reactive | 89,10 ± 8,30 | 99,04 ± 3,88 | 88,06 ± 2,82 * | 100,47 ± 5,64 |
| stress non-reactive | 81,66 ± 16,30 | 78,67 ± 12,15 | 91,93 ± 2,04 | 85,89 ± 13,92 |
| 1 way ANOVA |
F(2,15) = 0.57, p > 0.05 |
F(2,15) = 2.08, p > 0.05 |
F(2,15) = 5.28, p < 0.05 |
F(2,15)=0.51, p > 0.05 |
| C. Thal | ||||
| sham | 100,00 ± 14,45 | 100,00 ± 7,50 | 100,00 ± 9,72 | 100,00 ± 14,09 |
| stress reactive | 119,54 ± 18,15 | 116,78 ± 3,39 | 127,81 ± 3,76 | 136,17 ± 7,84 |
| stress non-reactive | 112,58 ± 15,72 | 108,21 ± 11,09 | 99,51 ± 20,64 | 121,24 ± 19,32 |
| 1 way ANOVA |
F(2,15) = 0.31, p > 0.05 |
F(2,15) = 0.98, p > 0.05 |
F(2,15)=1.37, p>0.05 |
F(2,15) = 1.37, p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





