1. Introduction
Myiasis, derived from the Greek word “myia” meaning fly, is defined as the infestation of Diptera larvae in vertebrate humans and animals for at least one period of their lives and spending their lives feeding on their living and dead tissues, liquid substances of their bodies and digested food [
1]. Myiasis is divided into groups as cutaneous, nasopharyngeal, ocular, intestinal, or urogenital myiasis according to the affected body parts and tissues and as obligatory, facultative, and accidental myiasis according to the degree of parasitism displayed by the fly [
1,
2]. Traumatic myiasis, a type of cutaneous myiasis, is defined as a parasitic infestation that occurs as a result of the feeding of fly larvae in traumatic lesions in the cutaneous tissues of vertebrate hosts [
3,
4,
5]. It is seen in domestic and wild animals, especially in tropical and subtropical climatic zones. This infestation results in significant economic losses and health issues in farm animals and is also prevalent among humans with low economic and educational development [
6]. Species that cause traumatic myiasis are found in the families
Calliphoridae and
Sarcophagidae [
4,
5,
7]. In
Calliphoridae, blowflies consist of moderately large flies that lay eggs into fresh or cooked meat or dung. They are also able to lay eggs on living animals. Wohlfahrtia magnifica, belonging to the
Sarcophagidae family, is an obligate parasite found in wounds and natural orifices of mammals, including humans. It is common in Europe, Asia, North Africa, the Middle East, and the Mediterranean region [
1,
8,
9].
Although its cases caused by various species have been reported in many animals around the world [
4,
5,
10,
11,
12],
Wohlfahrtia magnifica and
Lucilia sericata have been reported as the most common species in Türkiye [
10,
13,
14,
15,
16]. In addition, traumatic myiasis cases caused by
Lucilia sericata and
Calliphora vicina in wild birds [
10,
13,
14,
17,
18,
19,
20] and
Chrysomya megacephala,
Lucilia spp, and
Calliphora vicina in turtles have been reported [
21,
22,
23]. To our knowledge, there have been no reported cases of myiasis in Euphrates soft-shelled turtles and wild goats. To determine the fly species of the larvae, certain larval features must be analyzed, which may not always be feasible. In recent years, in these cases, molecular methods have been used to determine the species of larvae [
24,
25]. MtDNA is a widely used molecular tool in metazoan taxa for taxonomic, population, and evolutionary studies. It has high copy numbers, is easier to isolate, and has a high phylogenetic signal and mutation rates. In recent years, DNA barcoding using CO1 sequences has emerged as an effective tool for molecular identification and phylogenetic characterization of various insect species [
26,
27,
28,
29,
30].
This study was conducted to evaluate the traumatic myiasis cases detected during the examination of animals brought to Dicle University Wildlife Rescue, Rehabilitation, Training, Practice, and Research Center with wound complaints between 2020-2022. For this purpose, microscopic and molecular identification and characterization of larvae collected from wounds were performed.
2. Material and methods
2.1. Study period and study area
The study was conducted in the Dicle University Wildlife Rescue, Rehabilitation, Training, Practice, and Research Center in Diyarbakır province (37°55´ N, 40°14´ E, 670 m) located in southeastern Anatolia, Turkey between 2020-2022.
2.2. Animals and larvae collection
During the examination of eight wild animals brought with complaints of injury, traumatic myiasis was detected. Before beginning the treatment process, the larvae present in the wound were collected using forceps. These larvae were then stored in 70% ethyl alcohol until species identification and molecular analysis could be carried out. The data concerning the infested animals are illustrated in
Table 1. Species identification was carried out as described in the literature [
1].
2.3. Molecular analysis
Total genomic DNA (gDNA) isolation from 11 third instar larvae samples with a commercial tissue DNA isolation kit (Thermo Fisher Scientific, Waltham, MA). gDNA samples were stored at -20 °C until use. The region of the cytochrome oxidase subunit I gene (CO1) gene was amplified by PCR using conserved specific primers previously described by Otranto et al. (UEA7 and UEA10) [
31]. 9 µl of each PCR product was mixed with 1 µl of TriTrack loading dye (ThermoScientific, R1161) and loaded on 1.4% agarose gel.
COI sequences obtained from larvae were sequenced in both directions on a 3100 ABI PRISM genetic analyzer (Applied Biosystems, Foster City, CA.). Sequences were edited with CodonCode Aligner software version 11.0 (CodonCode Corporation, Dedham, MA, USA). BLAST algorithms and databases from the National Center for Biotechnology (
http://www.ncbi.nlm.nih.gov) undertook nucleotide sequence analysis. Pairwise calculations and phylogenetic trees were performed with MEGA 7 software [
32].
3. Results
The 3rd stage larvae collected from the wounds on the wings of four buzzards (
Buteo buteo) were identified as
L. sericata, C. vomitria, and
C. vicina, and the larvae collected from white stork (
Ciconia ciconia) were identified as
L. sericata. It was determined that the larvae obtained from the wild goat (
Capra aegagrus) were
W. magnifica larvae, and
L. sericata larvae were responsible for the traumatic myiasis occurring in the Euphrates soft-shelled turtles (
Rafetus euphraticus) and turtle (
Testudinidae) (
Table 1 and
Figure 1,
Figure 2 and
Figure 3).
The eleven sequences obtained have been deposited in GenBank under accession numbers OR642793-OR642793. The average intraspecies nucleotide divergence between
L. sericata larvae was 0.0018 (0.0000–0.0046) (
Table 2).
C. vomitoria sequences of COI were 100% identical between them. The interspecific divergence between
C. vomitoria and
C. vicina was 0.042 (Table 3).
4. Discussion
Many injured wild animals, damaged for various reasons, are brought for treatment and recovery to the Wildlife Rescue, Rehabilitation, Training, Application, and Research Centers established in many parts of Turkey, which is the wealthiest country in Europe in terms of wild animal diversity. Traumatic myiasis was detected in 8 animals brought to the Dicle University Wildlife Rescue, Rehabilitation, Training, Practice, and Research Center for various reasons such as injury wire entrapment.
Myiasis agents detected in poultry in studies conducted around the world are Calliphora vicina, Lucilia sericata, Lucilia cuprina, Wohlfahrtia vigil, Wohlfahrtia magnifica, Wohlfahrtia opaca, Cuterebra buccata and Dermatobia hominis [
18,
19,
20,
33]. In our country, the species detected in wild birds are L. sericata and C. vicina [
10,
13,
14,
17]. In this study, myiasis was detected in two species of wild birds, hawk, and stork, and the species responsible for myiasis were determined to be C. vicina, L. sericata, and C. vomitoria. Cases of traumatic myiasis caused by Calliphoridae reported in hawks worldwide are limited. A case of traumatic myiasis caused by L. cuprina and L. sericata was reported in two Harris’ hawks in Peru [
34], L. sericata and C. vicina have been reported in hawks in Turkey [
10,
13,
14]. In our study, only L. sericata larvae were found in two of the four hawks, while C. vicina, C. vomitoria, and L. sericata larvae were found in one, and C. vomitoria and L. sericata larvae were found in one. C. vomitoria larvae, which are the causative agent of tertiary myiasis, are rarely encountered in traumatic myiasis cases [
5]. Myiasis cases caused by these two Calliphora species in December and April can be explained by the fact that these flies are densely seen in the region during these months [
35]. Traumatic myiasis cases caused by L. sericata in white storks have been reported in Austria and Turkey [
14,
17,
36]. In our study, the larvae we collected from the wing wound were determined to be L. sericata.
Euphrates Softshell Turtle
(Rafetus euphraticus) and Wild Goat
(Capra aegagrus) have most recently been assessed for
The IUCN Red List of Threatened Species in 2016 and 2020, respectively.
Rafetus euphraticus is listed as Endangered under criteria A4c, and
Capra aegagrus is listed as Near Threatened under criteria A2cd [
37,
38]
Diagnosis and treatment of any disease in these endangered animals is vital. In this study, Euphrates Softshell Turtle and wild goat, which was examined with complaints of injury, was diagnosed with traumatic myiasis. It was determined that the larvae collected from the Euphrates turtle were L. sericata, and the case of traumatic myiasis in the Euphrates turtle was reported for the first time in the world in our study. It was determined that the larvae collected from the wound in the gluteal region of Capra aegagrus belonged to W. magnifica. To our knowledge, no traumatic myiasis is reported in Capra aegagrus, and in this study, traumatic myiasis occurring in W. magnifica is presented for the first time.
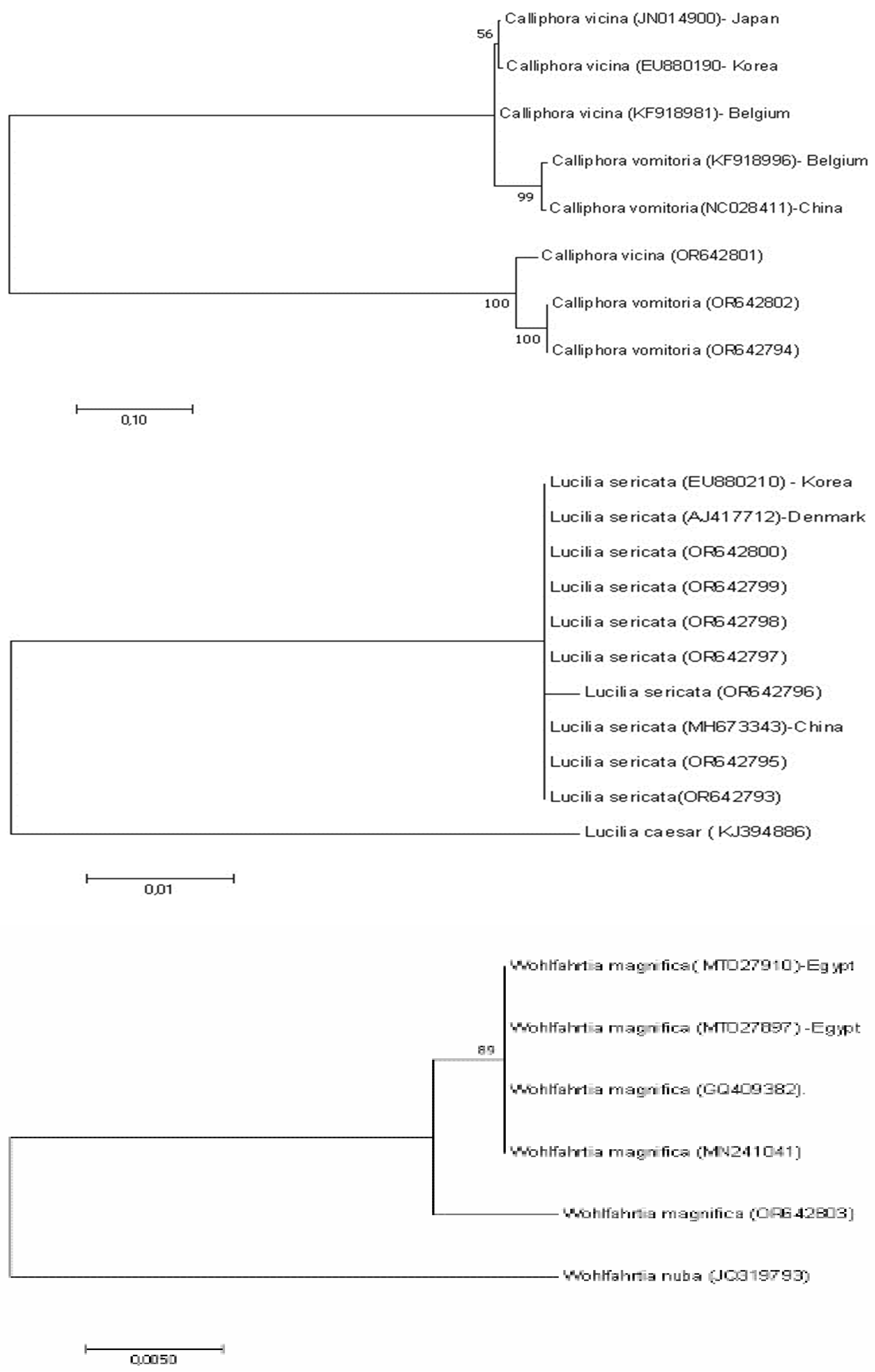
Molecular analysis of DNA obtained from the larvae identified in this study confirms the morphological diagnosis. As a result of comparing the 644 base pairs obtained from the COI gene of
L. sericata with the reference sequences, 99.7 to 100% identity was shown with China, Austria, Denmark, UK, and Korea (
Figure 4). Intraspecies nucleotide divergence between
Lucilia sericata larvae in this study was 0.0018 (0.0000–0.0046) and similar to our study, Gao, Fu [
27] found the intraspecific divergence of traumatic myiasis caused by
Lucilia caesar larvae to be 0.0022.
The interspecific divergence between
C. vomitoria and
C. vicina was 0.042. Two sequences of
C. vomitoria were 100% identical between them. The nucleotide sequences were compared with reference sequences from the NIH, and they had an identity of 99.8 to 100%, to
C. vomitoria from Belgium, China, and the United States and 99.5 to 99.8%, to
C. vicina from Japan, Belgium, and Korea (
Figure 4). It was determined that the
W. magnifica larva collected from a single animal showed an identity of 99.52 to 99.44% in Egypt (
Figure 4)
5. Conclusion
This study presents myiasis cases caused by different fly species in different wild animals. However, this study reported traumatic myiasis for the first time in a Euphrates turtle. Traumatic myiasis causes serious health problems in many animals, but this infestation, which occurs due to various injuries and can be fatal, especially in endangered wild animals, is significant. In the habitats of wild animals, the number of flies should be reduced by setting traps against flies that cause myiasis, along with precautions to be taken against injuries to these animals.
Author Contributions
Conceptualization, D.N.S.İ. and A.E.; methodology, D.N.S.İ.; software, A.E.; validation, D.N.S.İ. and A.E.; formal analysis, D.N.S.İ.; investigation, A.E.; resources, A.E.; data curation, D.N.S.İ.; writing—original draft preparation, D.N.S.İ.; writing-review and editing, D.N.S.İ.; visualization, A.E.; supervision, D.N.S.İ. and A.E.; project administration, D.N.S.İ. and A.E.; funding acquisition, D.N.S.İ. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zumpt, F. Myiasis in man and animals in the Old World. A textbook for physicians, veterinarians and zoologists. Myiasis in Man and Animals in the Old World. A Textbook for Physicians, Veterinarians and Zoologists, 1965. [Google Scholar]
- Wall, R.; Shearer, D. Myiasis. Chapter 5. In Veterinary ectoparasites: Biology, pathology and control, 2nd ed.; Blackwell Science Ltd: United Kingdom, 2001; pp. 114–142. [Google Scholar]
- Hall, M.; Wall, R. Myiasis of humans and domestic animals. Advances in Parasitology 1995, 35, 257–334. [Google Scholar] [CrossRef]
- Hall, M. Traumatic myiasis of sheep in Europe: a review. Parassitologia 1997, 39, 409–413. [Google Scholar]
- Francesconi, F.; Lupi, O. Myiasis. Clinical microbiology reviews 2012, 25, 79–105. [Google Scholar] [CrossRef]
- Hall, M.J.; Wall, R.L.; Stevens, J.R. Traumatic myiasis: a neglected disease in a changing world. Annual Review of Entomology 2016, 61, 159–176. [Google Scholar] [CrossRef]
- Scholl, P.J.; Colwell, D.D.; Cepeda-Palacios, R. Myiasis (Muscoidea, Oestroidea). In Medical and Veterinary Entomology; Elsevier, 2019; pp. 383–419. [Google Scholar]
- Pezzi, M.; Bonacci, T.; Leis, M.; Mamolini, E.; Marchetti, M.G.; Krčmar, S.; Chicca, M.; Del Zingaro, C.N.F.; Faucheux, M.J.; Scapoli, C. Myiasis in domestic cats: a global review. Parasites & Vectors 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Wall, R.L.; Shearer, D. Myiasis. Chapter 5. In Veterinary ectoparasites: Biology, pathology and control, 2nd ed.; Blackwell Science Ltd, John Wiley & Sons: United Kingdom, 2008. [Google Scholar]
- Dik, B.; Uslu, U.; Isik, N. Myiasis in animals and humanbeings in Turkey. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2012, 18. [Google Scholar] [CrossRef]
- İpek, D.N.S.; Şaki, C.E. External myiasis on cows, sheep and goats in Diyarbakır province. Journal of the Faculty of Veterinary Medicine, Dicle University, 1–7.
- Bonacci, T.; Curia, G.; Scapoli, C.; Pezzi, M. Wohlfahrtiosis in Italy: a case in a puppy and overview of geographical distribution. Acta Veterinaria Brno 2020, 89, 171–177. [Google Scholar] [CrossRef]
- Dik, B.; Kandir, E.H. Ectoparasites in some wild birds (Aves) in Turkey. Progress in Nutrition 2021, 23, e2021261. [Google Scholar] [CrossRef]
- Ütük, A.E.; Şaki, C.E. Wound myiasis caused by Lucilia sericata in two white storks (Ciconia ciconia) and in a common buzzard (Buteo buteo). Etlik Veteriner Mikrobiyoloji Dergisi 2017, 28, 73–75. [Google Scholar] [CrossRef]
- Ipek, D.N.S.; İpek, P. A case of traumatic myiasis in a domestic rabbit (Oryctolagus cuniculus) caused by Lucilia sericata. Türkiye Parazitolojii Dergisi 2012, 36, 54. [Google Scholar] [CrossRef]
- Gökçen, A.; Sevgili, M. A case of cutaneous myiasis in a gazella (Gazella subgutturosa) in Turkey. Atatürk Üniversitesi Veteriner Bilimleri Dergisi 2007, 2. [Google Scholar]
- Yaman, M.; Zerek, A.; Akküçük, Ş. A case of traumatic myiasis in a white stork (Ciconia ciconia) caused by Lucilia sericata (Diptera: Calliphoridae). Eurasian Journal of Veterinary Sciences 2018, 34. [Google Scholar] [CrossRef]
- Araghi, M.; Eskandari, F.; Gilasian, E. Avian wound myiasis caused by Calliphora vicina Robineau-Desvoidy (Diptera: Calliphoridae) in an immature migrating eastern imperial eagle (Aquila heliacal Savigny)(Aves: Accipitridae) in south-western Iran. Journal of Veterinary Science & Technology 2015, 6, 212. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. Parasitic diseases of wild birds; John Wiley & Sons, Ltd., Publication, 2009. [Google Scholar]
- Pezzi, M.; Krčmar, S.; Mendicino, F.; Carlomagno, F.; Bonelli, D.; Scapoli, C.; Chicca, M.; Leis, M.; Bonacci, T. Lucilia sericata (Diptera: Calliphoridae) as agent of myiasis in a goose in Italy and a review of myiasis by this species in birds. Insects 2022, 13, 542. [Google Scholar] [CrossRef]
- Knotek, Z.; Fischer, O.; Jekl, V.; Knotková, Z. Fatal myiasis caused by Calliphora vicina in Hermann´ s Tortoise (Testudo hermanni). Acta Veterinaria Brno 2005, 74, 123–128. [Google Scholar] [CrossRef]
- McMullen, D.B. Cutaneous myiasis in a box turtle. Proceedings of the Oklahoma Academy of Science 1940, 23–25. [Google Scholar]
- Gould, W.; Georgi, M. Myiasis in two box turtles. Journal of the American Veterinary Medical Association 1991, 199, 1067–1068. [Google Scholar] [CrossRef]
- Traversa, D.; Otranto, D. A new approach for the diagnosis of myiasis of animals: the example of horse nasal myiasis. Veterinary Parasitology 2006, 141, 186–190. [Google Scholar] [CrossRef]
- de Azeredo-Espin, A.M.L.; Lessinger, A.C. Genetic approaches for studying myiasis-causing flies: molecular markers and mitochondrial genomics. Genetica 2006, 126, 111–131. [Google Scholar] [CrossRef]
- Hebert, P.D.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Systematic Biology 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, Y.; Yan, L.; Hu, D.; Jiang, B.; Zhang, D. First record of traumatic myiasis obtained from forest musk deer (Moschus berezovskii). International Journal for Parasitology: Parasites and Wildlife 2021, 16, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of birds through DNA barcodes. PLoS biology 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Li, X.-y.; Pape, T.; Zhang, D. Gasterophilus flavipes (Oestridae: Gasterophilinae): A horse stomach bot fly brought back from oblivion with morphological and molecular evidence. PLoS One 2019, 14, e0220820. [Google Scholar] [CrossRef]
- Otranto, D.; Traversa, D.; Guida, B.; Tarsitano, E.; Fiorente, P.; Stevens, J. Molecular characterization of the mitochondrial cytochrome oxidase I gene of Oestridae species causing obligate myiasis. Medical and Veterinary Entomology 2003, 17, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Cooper, J.; Cooper, M.; Krone, O.; Newton, I.; Peakall, D.; Zucca, P. Foot conditions. In Birds of Prey: Health and Disease, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 121–131. [Google Scholar]
- Gomez-Puerta, L.A.; Cribillero, N.G.; Silva, W.; Ayala, P. Cloacal myiasis by Lucilia spp.(Diptera: Calliphoridae) in a rooster (Gallus gallus domesticus) and two Harris’s hawks (Parabuteo unicinctus). Parasitology International 2021, 83, 102363. [Google Scholar] [CrossRef]
- Sayin İpek, D.N.; Sakı, C.E.; Özer, E. Seasonal distributions of external myiasis flies determined in Diyabakır province. Kafkas Universitesi Veteriner Fakultesi Dergisi 2011, 17. [Google Scholar] [CrossRef]
- Hinaidy, H.; Frey, H. Weitere Fakultativmyiasis-Fälle bei Wirbeltieren in Österreich. Wien Tierärztl Monat 1984, 71, 237–238. [Google Scholar]
- Ghaffari, H.; Taskavak, E.; Turkozan, O.; Mobaraki, A. Rafetus euphraticus. The IUCN Red list of threatened species 2017: e. T19070A1956551, 2017.
- Amininasab, S.M.; Zamani, N.; Taleshi, H.; Xu, C.C. Ensemble modelling the distribution and habitat suitability of wild goat Capra aegagrus in southwestern Iran. Biodiversity 2023, 24, 124–136. [Google Scholar] [CrossRef]
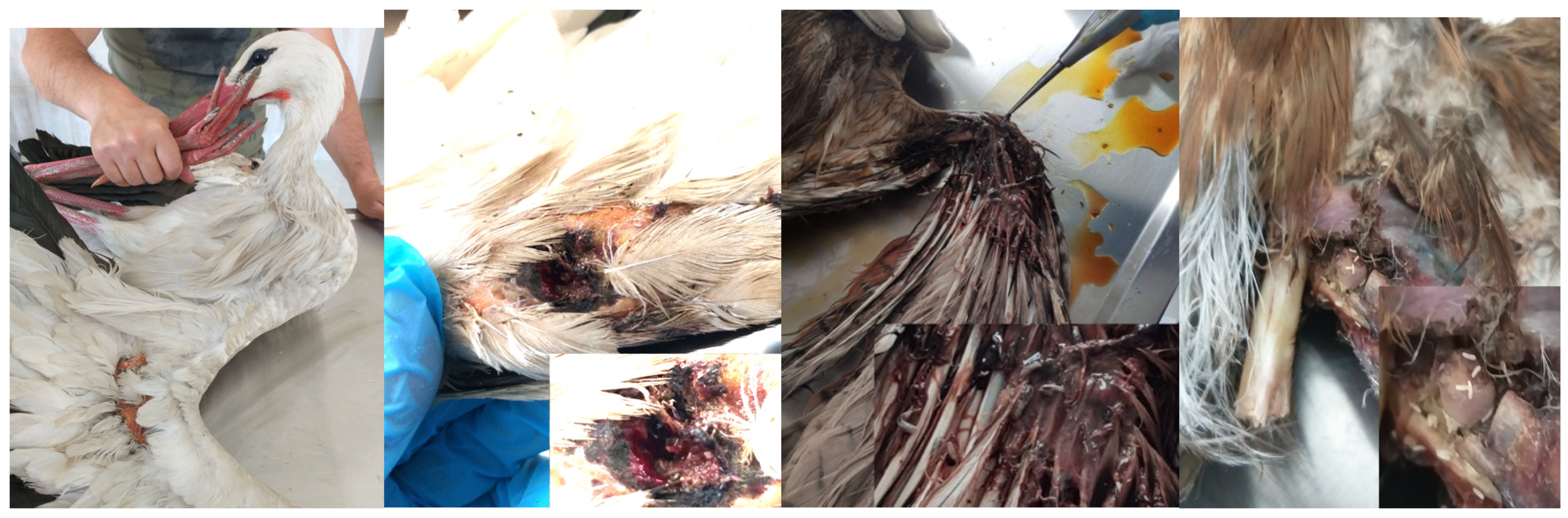
Figure 1.
a- White stork (Ciconia ciconia) with wing wound, b- The stork’s wing infested with numerous L. sericata larvae, c- Ventral view of buzzard wing wound and different species larvae d- The buzzard’s wing with fractured and numerous L. sericata larvae in wound.
Figure 1.
a- White stork (Ciconia ciconia) with wing wound, b- The stork’s wing infested with numerous L. sericata larvae, c- Ventral view of buzzard wing wound and different species larvae d- The buzzard’s wing with fractured and numerous L. sericata larvae in wound.
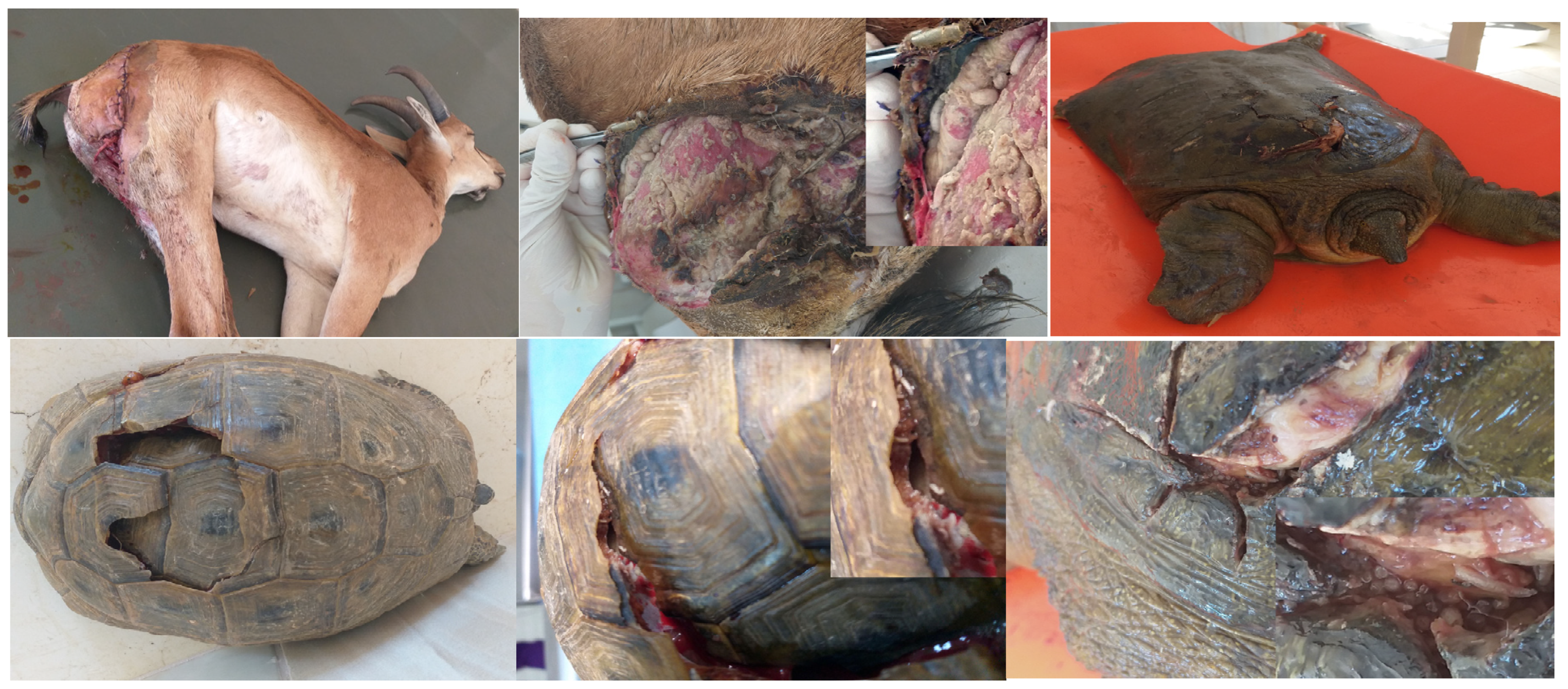
Figure 2.
a- Wild goat (Capra aegagrus) with gluteal region wound, b- The wild goats’ wound infested with numerous W. magnifica larvae, c- Euphrates soft-shelled turtles (Rafetus euphraticus) with under carapace wound, e-The Euphrates soft-shelled turtles myiasis caused by L. sericata, f- Turtle (Testudinidae) with under carapace wound, g- numerous L. sericata larvae in wound.
Figure 2.
a- Wild goat (Capra aegagrus) with gluteal region wound, b- The wild goats’ wound infested with numerous W. magnifica larvae, c- Euphrates soft-shelled turtles (Rafetus euphraticus) with under carapace wound, e-The Euphrates soft-shelled turtles myiasis caused by L. sericata, f- Turtle (Testudinidae) with under carapace wound, g- numerous L. sericata larvae in wound.
Figure 3.
The third stage larvae of Calliphora vomitoria, Calliphora vicina, Lucilia sericata, Wohlfahrtia magnifica a- C. vomitoria, cephalon skeleton, lateral view b- C. vomitoria, posterior spiracles c- C. vicina, cephalon skeleton, lateral view d- C. vicina, posterior spiracles e- L. sericata, cephalon skeleton, lateral view f- L. sericata, posterior spiracles g- W magnifica, cephalon skeleton, lateral view h- W. magnifica, posterior spiracles.
Figure 3.
The third stage larvae of Calliphora vomitoria, Calliphora vicina, Lucilia sericata, Wohlfahrtia magnifica a- C. vomitoria, cephalon skeleton, lateral view b- C. vomitoria, posterior spiracles c- C. vicina, cephalon skeleton, lateral view d- C. vicina, posterior spiracles e- L. sericata, cephalon skeleton, lateral view f- L. sericata, posterior spiracles g- W magnifica, cephalon skeleton, lateral view h- W. magnifica, posterior spiracles.
Figure 4.
Phylogenetic relationships of Lucilia, Calliphora, and Wohlfahrtia collected from wild animals from Türkiye, inferred by the Maximum Likelihood method of the partial COI gene sequences, based on genetic distances calculated by the Tamura 3-parameter model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option).
Figure 4.
Phylogenetic relationships of Lucilia, Calliphora, and Wohlfahrtia collected from wild animals from Türkiye, inferred by the Maximum Likelihood method of the partial COI gene sequences, based on genetic distances calculated by the Tamura 3-parameter model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option).
Table 1.
Animals’ species, date of infections, body location, larvae species in animals suffering from traumatic myiasis.
Table 1.
Animals’ species, date of infections, body location, larvae species in animals suffering from traumatic myiasis.
| Animal (no.) |
Animal Species |
Date |
Body Location |
Identified Larvae |
| 1 |
Buteo buteo |
December 2020 |
Left Wing |
C. vomitoria, L. sericata |
| 2 |
Buteo buteo |
May 2022 |
Right Wing |
C. vomitoria, L. sericata, C. vicina |
| 3 |
Ciconia ciconia |
April 2021 |
Left Wing |
L. sericata |
| 4 |
Buteo buteo |
May 2022 |
Left Wing |
L. sericata |
| 5 |
Buteo buteo |
April 2022 |
Left Wing |
L. sericata |
| 6 |
Testudinidae |
June 2022 |
Under carapace |
L. sericata |
| 7 |
Capra aegagrus |
July 2022 |
Gluteal |
W. magnifica |
| 8 |
Rafetus euphraticus |
September2022 |
Under carapace |
L. sericata |
Table 2.
Pairwise comparison of nucleotide sequence differences in the COI among Lucilia sericata isolates and Calliphora spp. isolates in this study.
Table 2.
Pairwise comparison of nucleotide sequence differences in the COI among Lucilia sericata isolates and Calliphora spp. isolates in this study.
| Species |
|
|
|
|
|
|
|
|
Lucilia sericata (OR642795) |
|
0.0024 |
0.0025 |
0.0020 |
0.000 |
0.0020 |
0.0020 |
|
Lucilia sericata (OR642796) |
0.0046 |
|
0.0022 |
0.0015 |
0.0015 |
0.0015 |
0.0015 |
|
Lucilia sericata (OR642799) |
0.0046 |
0.0031 |
|
0.0016 |
0.0015 |
0.0015 |
0.0016 |
|
Lucilia sericata (OR642793) |
0.0031 |
0.0015 |
0.0016 |
|
0.0000 |
0.0000 |
0.0000 |
|
Lucilia sericata (OR642797) |
0.0031 |
0.0015 |
0.0015 |
0.0000 |
|
0.0000 |
0.0000 |
|
Lucilia sericata (OR642798) |
0.0031 |
0.0015 |
0.0015 |
0.0000 |
0.0000 |
|
0.0000 |
|
Lucilia sericata (OR642800) |
0.0031 |
0.0015 |
0.0015 |
0.0000 |
0.0000 |
0.0000 |
|
| Species |
|
|
|
|
Calliphora vicina (OR642801) |
|
0.0072 |
0.0073 |
|
Calliphora vomitoria (OR642794) |
0.0427 |
|
0.0000 |
|
Calliphora vomitoria (OR642802) |
0.0431 |
0.0000 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).