1. INTRODUCTION
Regenerative medicinal drugs deal with the transformation or reconstruction of cells, organs, and tissues. This technological knowledge of regenerative medicine is of interest in repairing damaged tissues and renewing them by affecting the body's repair mechanisms. Regenerative medication could also be a medication approach that involves cell use. [
1] The tissue engineering sector is concerned with the integration of body scaffolds, cells, and biomolecules together with tissues and the development of biomaterials. [
1,
2]
Regenerative substances might be a field of tissue engineering; however, research on self-recovery with biological substances to regenerate cellular tissues and organs is lacking. [
3,
4]
1.2. Advantages of Regenerative Medicine
An alternative to tissue engineering
Apply the technique of organ and tissue transplantation
Applicable to prevent tissue and organ deficiency
Enables production of a new biomaterial [
5]
2. REGENERATIVE MEDICINE AND TISSUE ENGINEERING
‘‘Cells are the constructing blocks of tissues and simple devices of the body.’’ The cell shapes the grid structures, which is called a matrix. [
6]
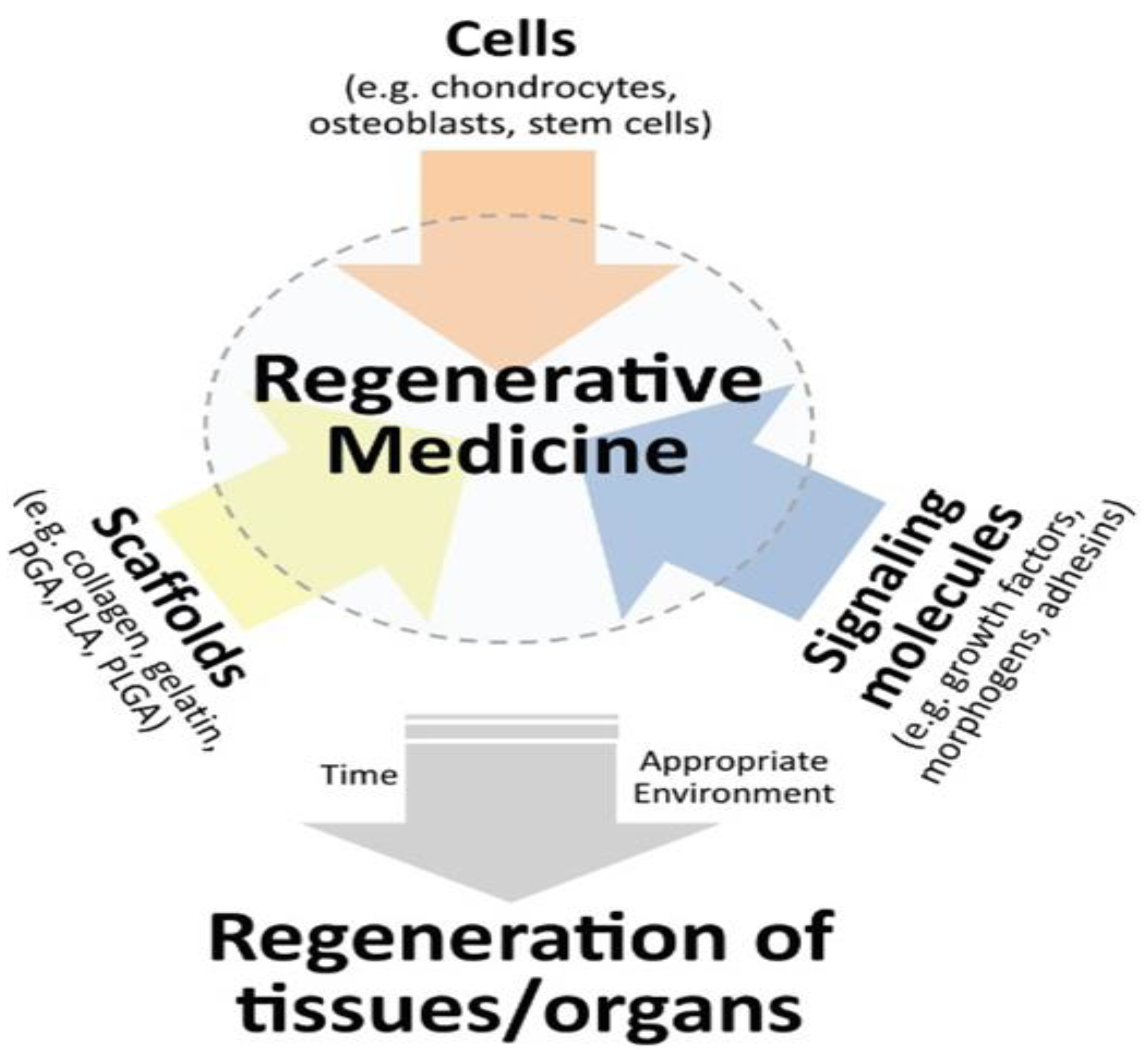
Figure 1.
Regenerative medicine triad. Combining three key elements-cells, biomaterials and signalling molecules to get regenerated tissue-engineered neo-organs.[
7].
Figure 1.
Regenerative medicine triad. Combining three key elements-cells, biomaterials and signalling molecules to get regenerated tissue-engineered neo-organs.[
7].
2.1. Create a New Tissue
The stem cell-giving organ was peeled, and the remaining collagen scaffold was used for new tissue production. This process is used in bioengineering science in the body tissues.[
8]
After scaffolding is formed, many materials, from protein to plastic, can be introduced into cells with or without a cocktail of growth factors. Tissue growth occurs when ambient conditions are appropriate. [
8,
9,
10]
3. ADHESION BARRIERS
Adhesion occurs when blood flow, inflammation, wound wear, and surgical wounds heal for several reasons while adhering to neighboring tissues or organs. There is much stronger adhesion with the spread of cells into tissues.
Adhesion barriers separate organs from tissues within the body. It is used during recovery to reduce the incidence of abnormal internal injuries after surgery. This is a medical implant. Examples of commonly used materials include animal membranes, gold foil, mineral oil, rubber, Teflon plates, and pharmaceuticals and materials.[
11]
3.1. Biggest Disadvantages of Adhesions
Intestinal obstruction
Postoperative morbidity
Chronic pelvic pain
Chronic abdominal pain
Adhesions have disadvantages that have led to the development of new surgical approaches. They performed some work to minimize tissue damage and adhesion formation. [
11,
12]
3.2. Adhesions Barriers
Postoperative adhesion pain will result in serious issues, such as inflammation. It has a negative effect on health, and dangerous conditions could result in organ failure and, consequently, life may finish. It is best-known that 60-95% of adhesion occur, particularly when open surgery is performed. To address such issues, the adhesion hindrance barrier square measure is often utilized in operations. Anti-adhesion barriers function as physical barriers between broken organs and tissue surfaces. The barrier typically dissolves at weekly intervals during recovery and is absorbed by the body. Adhesion interference barriers square measure often employed in operations.[
13]
Generally, it may end up within the termination of life because of the loss of organs. It is known that 60-95% of adhesion occur, especially after open surgery. Adhesion-prevention barriers are frequently used in operations to prevent such problems. [
12,
13]
Anti-adhesion barriers serve as physical barriers to separate damaged tissues or organ surfaces. Thus, tissues do not adhere to each other as they heal. The barrier dissolves within a few days of recovery and is then absorbed by the body. Examples of these barriers may be solutions, gels, and films. [
13,
14,
15]
Therefore, anti-adhesion materials must have certain properties. These properties act as barriers during wound healing and subsequent degradation. It should not be toxic. Toxic substances should not be produced through degradation or metabolism. [
16]
3.3. Examples of Antiadhesive Materials
Polysaccharides
Proteins
Natural polymers
Water-soluble synthetic polymers
Water-insoluble synthetic polymers
Dye-based natural polymers
PEG
Oxygenated regenerated cellulose orc
Sodium carboxymethylcellulose CMC
Dextran sulphate
Sodium hyaluronate
PLA
PGA
PLGA
Collagen
These materials are often used either alone or in combination.
Some materials are less effective as anti-adhesion barriers than others. They are gels, liquids, foams, etc. This makes it less feasible to repair wounds because the material cannot be embedded in the wound and falls owing to gravity. In this case, the impact of the anti-adhesion barrier does not continue because the low-relative-molecular-mass materials square measure chop-chop is absorbed. Subsequently, they are less successful in treating wounds and decreasing adherence. However, the implant of the anti-adhesion obstruction does not last long as a barrier does not last long as low-molecular-weight particles are competent for fast assimilation. Gel-type anti-adherent materials are difficult to accurately fix to wound areas, such as continuously moving abdominal organs and tissues. Another substance is hyaluronic corrosive. It is found in creatures and human tissues. It has superior biocompatibility. It corrupts in 1-3 days and is utilized as an anti-adhesion material because it retains a parcel of water and diminishes its quality because it gets water. For these reasons, multilayer anti-adhesion materials have been created using multiple materials with high biocompatibility and superior properties. Multilayer barriers provide user comfort. This effectively prevents blood and cell infiltration. It minimizes foreign-body reactions in surgical applications. The multilayered anti-adhesion barriers consist essentially of three layers, which are hydrophobic biodegradable and include a biocompatible polymer base, a hydrophilic bio-welded polymer layer, and an anti-adhesion barrier. [
11,
18,
19]
In summary, their square measures some properties that have received anti-adhesion materials.
Therefore, the pore size should be precisely controlled.
The anti-adhesion material ought to be ready to hook up with space for an explicit amount.
Minimizing foreign-body reactions.
The anti-adhesion obstruction should be flexible.
It ought to have prevalent mechanical properties for ease of use amid surgery.
No deformation occurred during the desired period.
The wound should be covered so that there is no open space.
Should not tear or ruin when folded or rolled.
Should be transported using small surgical instruments. [
11,
19]
4. CELL ADHESION
‘‘Cell adhesion is the binding of cells to neighbouring cells by molecules.” Adhesives that cause cell association in biological structures are referred to as CAM. Cell adhesion molecules (CAMs) are called proteins those membrane-bound proteins. [
19]
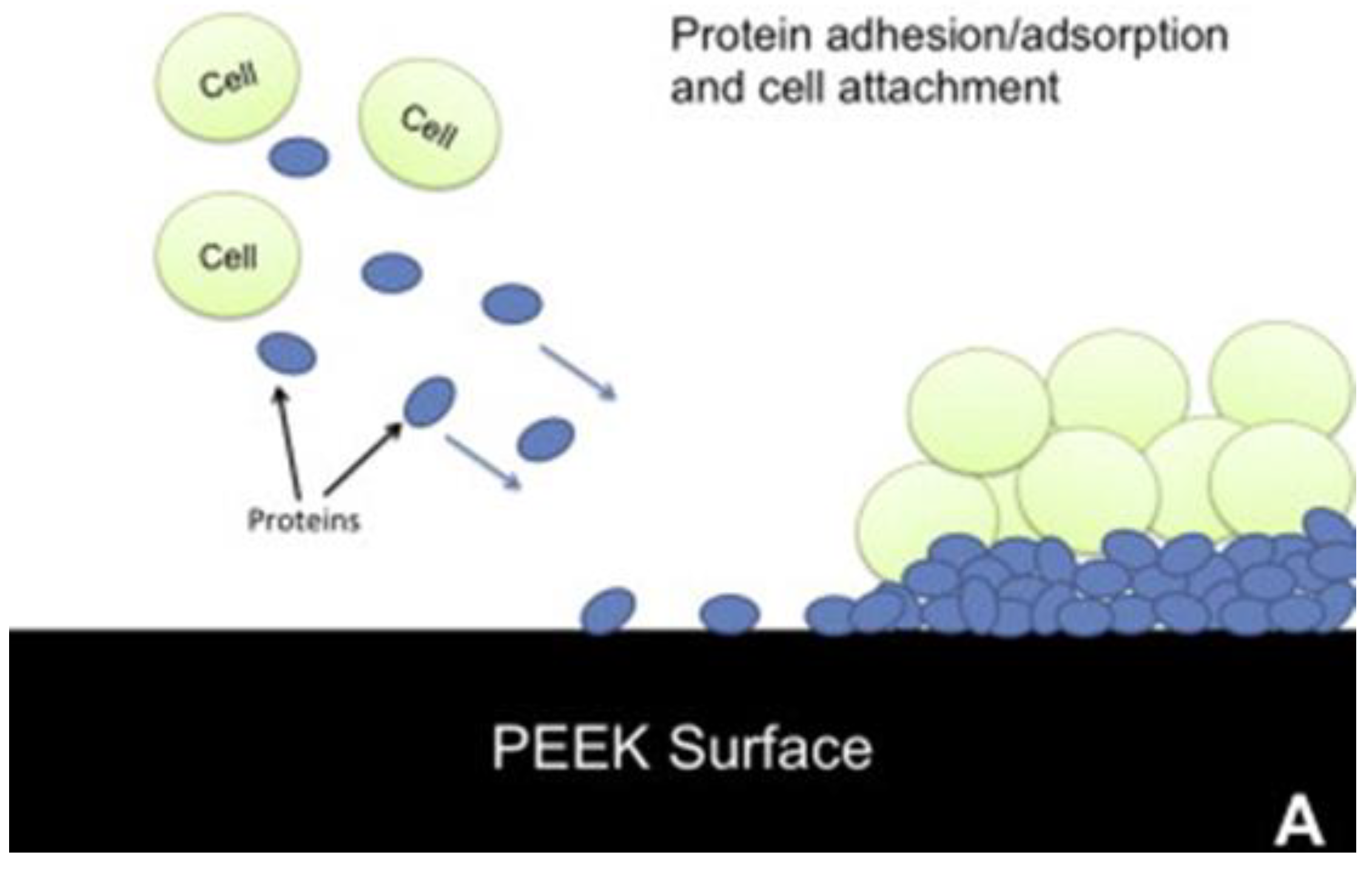
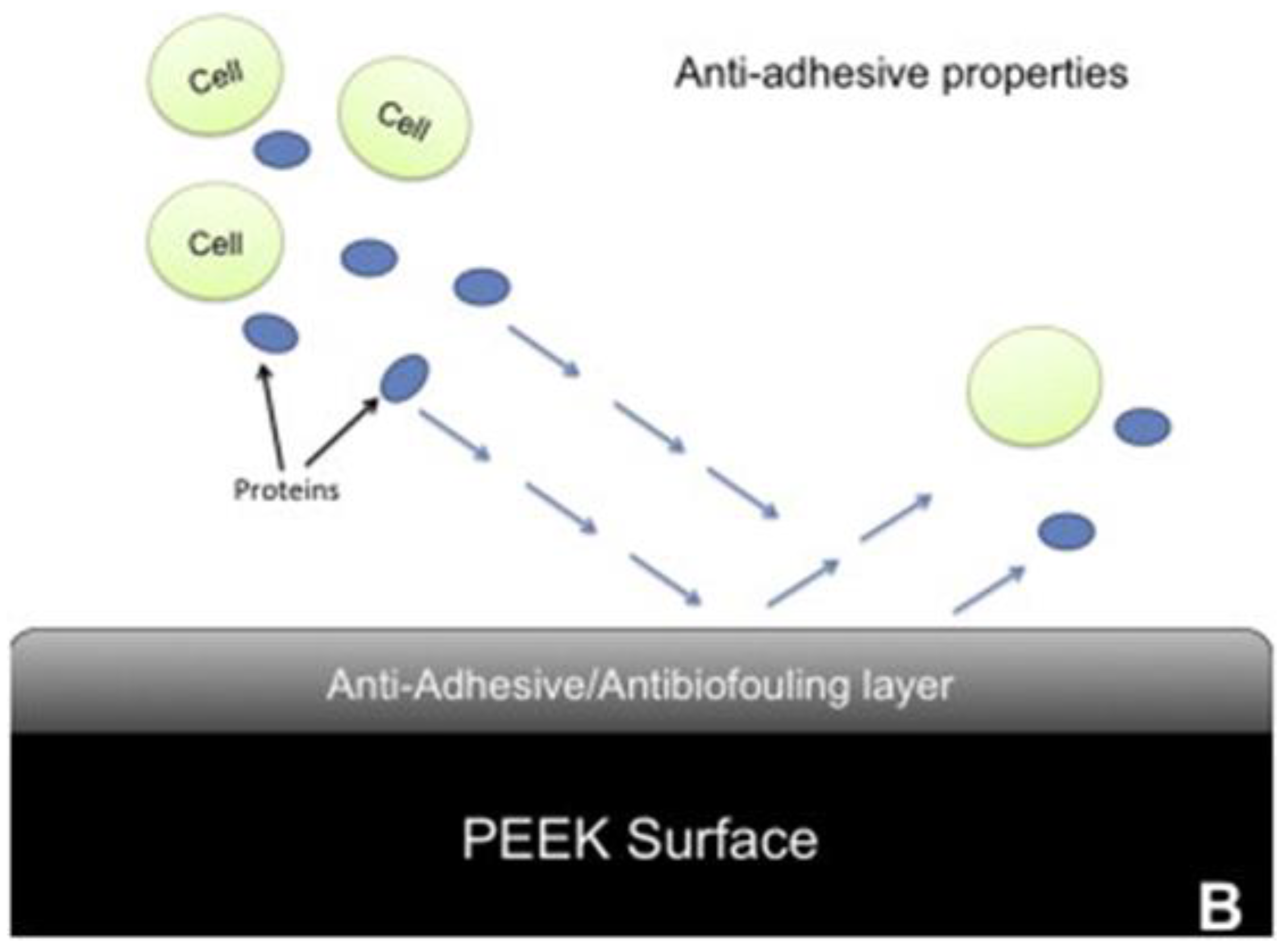
Figure 2.
On an untreated PEEK surface (A), proteins and cells accumulate, but with the low-friction PMPC layer attached (B), the cells and proteins attach less.[
19].
Figure 2.
On an untreated PEEK surface (A), proteins and cells accumulate, but with the low-friction PMPC layer attached (B), the cells and proteins attach less.[
19].
4.1. The Most Important Functions of Cell Adhesion Molecules
Cell adhesion can occur directly or indirectly. Gel-like structures containing molecules launched into the cavities via the cells are bound to the tissue matrix, and cell adhesion occurs. Adhesion molecules are shaped by several interactions between proteins on the surface. [
20,
21,
22]
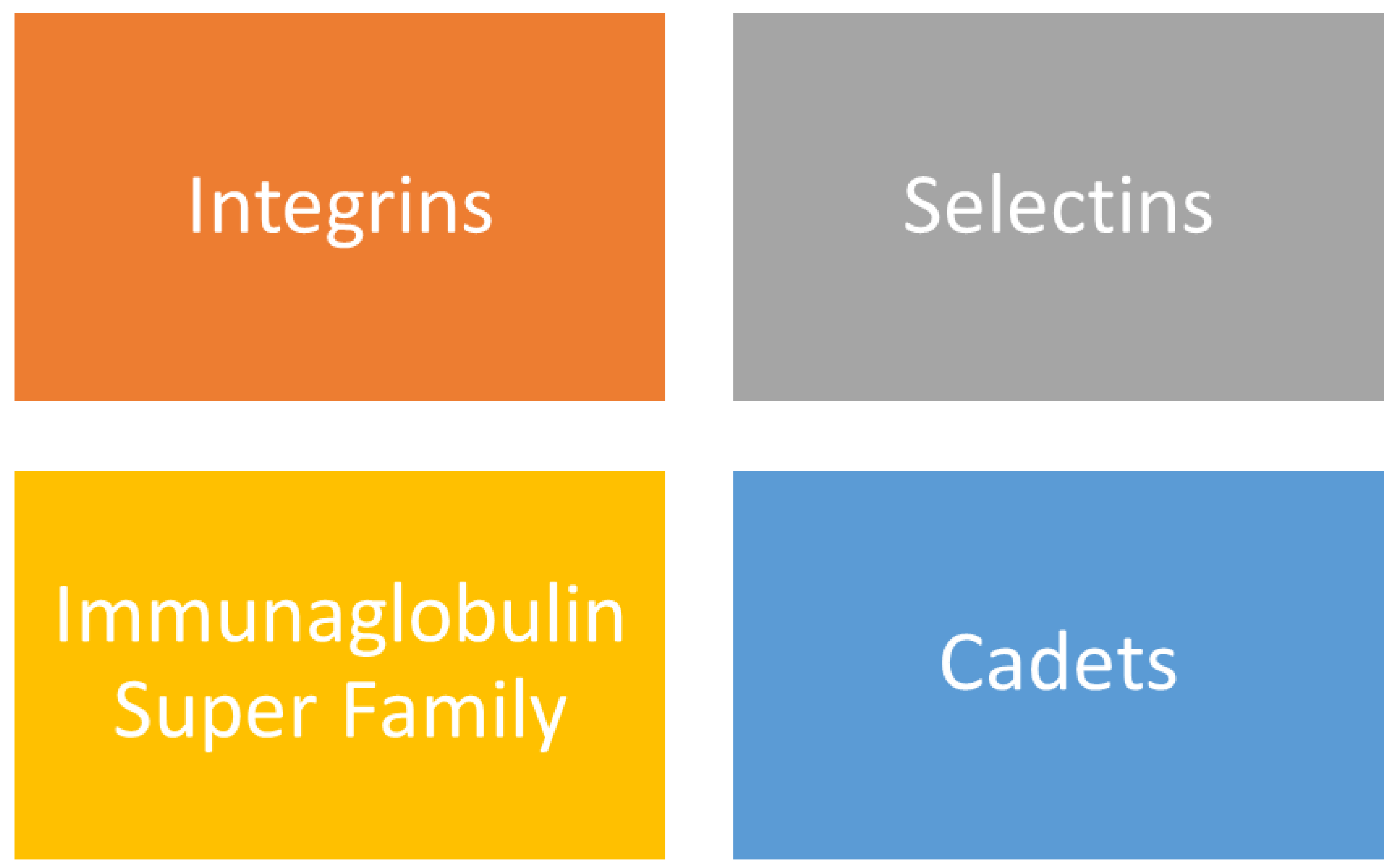
Figure 3.
Classification of Cell Adhesion Molecules [
23,
24].
Figure 3.
Classification of Cell Adhesion Molecules [
23,
24].
4.2. Cell Adhesion Types
4.2.1. Abdominal adhesions: The general World Health Organization has performed abdominal or girdle surgery. [24,25]
4.2.2. Pelvic adhesions: Uterine ovaries are a type of adhesions that involve any organ, such as the fallopian tubes or bladder. It is usually caused by an infection that causes adhesion to the fallopian tubes. [24,25]
4.2.3. Heart adhesions: This is a type of adhesion formed by limiting the effect of cardiac function due to the wound on the tissues above the heart. [24,25]
5. SIGNS OF ADHESION
It is difficult to diagnose as it does not have a definite medical indication.
Most Common Symptoms:
Adhesions involving the genital area and uterus can cause pain.
Pericardial adhesions can cause chest pain.
Adhesion evokes minimal internal organ obstruction, which is of pressing importance. It triggers pain within the abdomen and cramps.
Regurgitation due to nausea.
In the progressive method, high vital signs become noticeable.
It is not every bitter adhesion that matters. [
11,
26,
27]
5.1. When Should Adhesions Be Treated?
Adhesions generally manifest a few days after surgical treatment, but occasionally, they no longer produce signs for a long time. If excessive physique temperature causes unreasonable and extreme belly pain, pelvic pain, chest pain, continuous vomiting, abdominal swelling, back pain, and fainting dizziness, gastrointestinal bleeding should be consulted immediately by the doctor. [
26,
27,
28]
5.2. Adhesion Diagnosis
Laparoscopy
Blood tests
X-ray
BT scans
Nevertheless, an authoritative conclusion can be drawn during surgery. [
27,
28]
6. FOR WHICH APPLICATION DO WE REQUIRE LOW CELL ADHESION
Biomaterials that should not interact with blood should not adhere to cells, as they may pose a danger as a result of interaction with blood. Artificial heart valves and blood vessels are some examples of this. To prevent thrombosis, the artery is blocked by a substance carried in the bloodstream. As a result, high adhesions trigger wound formation, which can be shown to reduce intestinal movements by restricting bowel movements through pressure on the intestines, disrupting the digestive tract, and causing intestinal obstruction. In addition, low adhesion is expected in human tissue materials used in the intestine. In cases of high adhesion, the resulting wounds can restrict bowel movements, disrupt the digestive tract, and cause intestinal obstruction. It can even cause death in advanced dimensions. [
28,
29,
30]
7. TISSUE SCAFFOLD PRODUCTION METHODS
The designed construction scaffolders should have the following properties.
Large surface area and pore structure in the 3D structure
Contribute to the development of cell and tissue scaffold
Enough porosity
Allow cell suspension
Interconnected pore structure
Production methods determine the scaffolding structures. Scaffold training is designed to support tissue regeneration, cell differentiation, and other natural functions. The most suitable scaffolds should be biocompatible, should not elicit unwanted host responses, be degradable during the regeneration of cellular components, and should not show toxic effects. [
31,
32,
33]
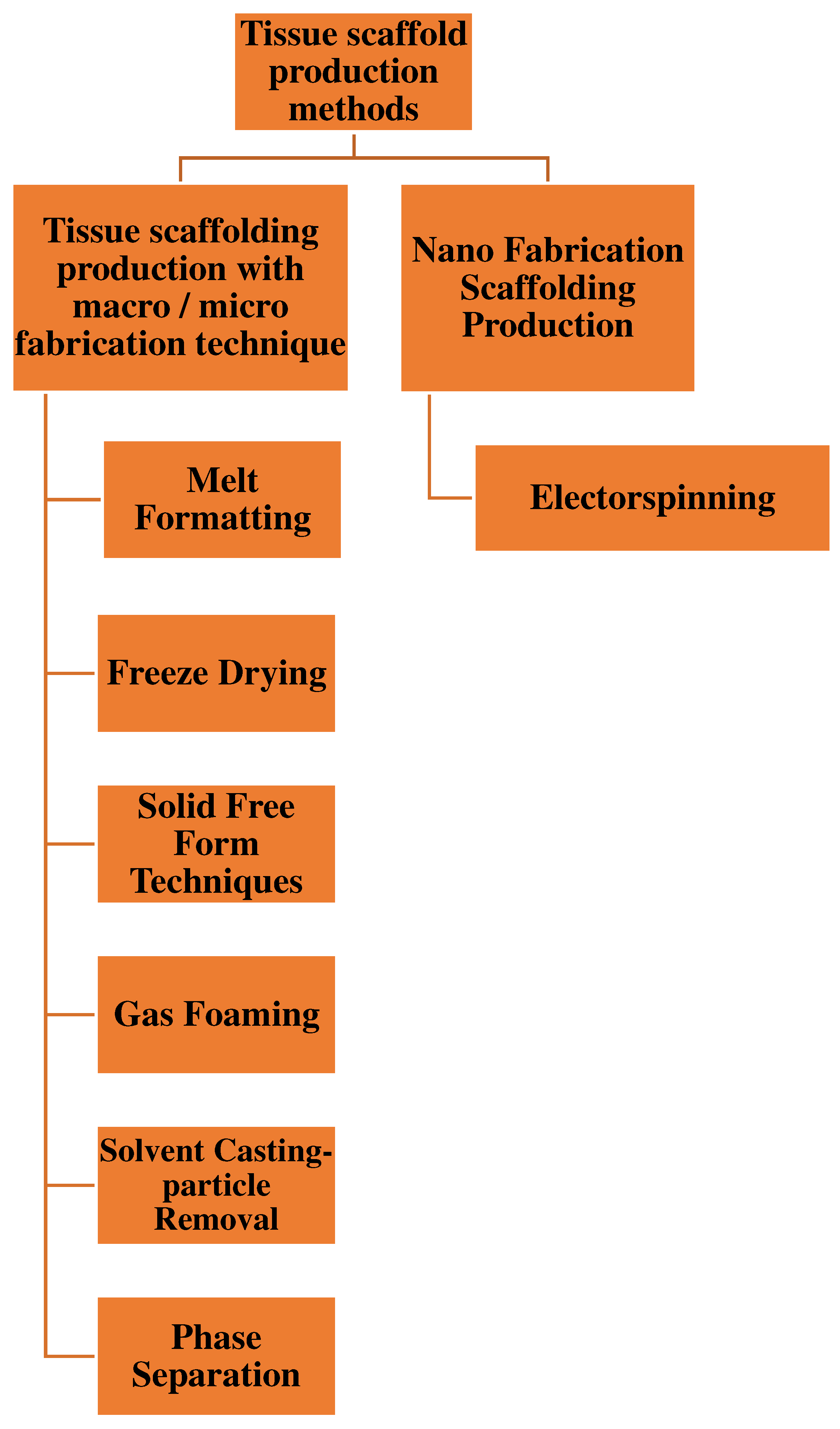
Figure 4.
Tissue scaffold production methods[
33].
Figure 4.
Tissue scaffold production methods[
33].
7.1. Melt Formatting
PLGA tissue scaffolds were obtained by filtering the composites formed using a melding technique. It takes place at low temperature and has the capacity for managed delivery and incorporation of bioactive molecules that is due to the fact no natural solvent is used. [
32,
33]
7.2. Freeze Drying
It is frozen to have a porous matrix structure, and then drying together with freezing takes place simultaneously, and the solution freezes to form ice crystals. By freeze-drying, ice crystals are destroyed to form small pores. [
32,
33]
7.3. Solid Free Form Techniques
The physical material was created using computer-aided design. The technological structure of the tissue used to produce a 3D structure can be designed using programs such as CAD/CAM programs. [
32,
33]
7.4. Gas Foaming
Porous matrices are preferred for obtaining their structures. The PLGA tablets were converted into solids and compressed. The process is carried out by applying CO
2 at high pressure, after which the pressure is reduced to the ambient level. As a result, pores were formed owing to CO
2 gas. [
32,
33]
7.5. Solvent Casting-Particle Removal
For thermal applications, specific pore and volume ratios, and pore sizes that control the placement of the material in the cell, biodegradable polymers are used for different applications. [
32,
33]
7.6. Phase Separation
This is a frequently used method for producing porous polymer membranes. However, this does not require special equipment. [
32,
33]
7.7. Electrospinning Method
It is an old method that consists of a combination of small-diameter fibers and polymers. Electrospinning is based on the transfer of liquid solutions to different points using a controlled electric field force with a change in structure and size. In this method, a syringe was used and the polymer solution was placed in the syringe. The positive end of the power delivery to offer a high voltage is connected to the metal stop of the syringe, even as it is grounded from the collector plate. The floor is received by generating an electric-powered discipline between the syringe and the collector. Within the collector, fibers with sub-micron diameters are produced, which occur at an invisible velocity. Cellular behavior can be managed by nanotopographic patterning on the biomaterial surface. Therefore, tissue engineering has great potential. [
32,
33,
34]
8. Conclusions
Regenerative medicine focuses on repairing or replacing damaged cells, tissues, and organs using biological substances. It encompasses the use of cells, biomaterials, and signaling molecules to generate regenerated tissue-engineered neoorgans. Tissue engineering, on the other hand, involves the integration of scaffolds, cells, and biomolecules to produce tissues. Although tissue engineering has its advantages, regenerative medicine presents itself as an alternative to organ and tissue transplantation.
Tissue damage can result in adhesion, a process by which tissues stick together. Adhesion barriers are used to separate the tissues and organs within the body during recovery. These barriers are suitable physical films, fabrics, gels, etc., that prevent tissue adhesion. On the other hand, anti-adhesion barriers are used to prevent adhesions during surgery. Examples of anti-adhesive materials include polysaccharides, proteins, natural polymers, water-soluble, insoluble synthetic polymers, and dye-based natural polymers.
Regenerative medicine involves the utilization of cells, biomaterials, and signaling molecules to regenerate tissues and engineered organs. Tissue engineering involves integration of scaffolds, cells, and biomolecules to produce tissues and biomaterials. Stem cells, scaffolds, and growth factors have been used in regenerative medicine and tissue engineering to create new tissues.
Adhesion barriers play a critical role in preventing abnormal postoperative internal injuries. Examples of adhesion barriers include physical films, fabrics, gels, and anti-adhesion materials such as polysaccharides, proteins, and synthetic polymers. In the fields of Regenerative Medicine and Tissue Engineering, it is crucial to focus on developing therapies that can effectively and safely target specific cell types, tissues, and diseases. Bioprinting technology can be used to create complex tissue structures, such as the liver and kidneys, using patient-specific cells. Additionally, new methods for expanding and differentiating stem cells, such as induced pluripotent stem cells (iPSCs), should be developed to increase their therapeutic potential. Research on scaffold materials and biomaterials that can support cell growth and tissue regeneration is warranted. Collaboration with experts in fields, such as biomaterials, engineering, and computer science, is crucial for the development of new technologies and therapies. The ethical and regulatory challenges associated with cell-based therapies must be considered and addressed. Advocating increased funding and support for regenerative medicine research is essential for accelerating the development of new therapies. Collaborative and interdisciplinary approaches to regenerative medicine research should be fostered to bring together experts from various fields. It is important to keep up with current advancements in regenerative medicine and tissue engineering, including new developments in gene editing, biofabrication, and regenerative immunology. Communicating findings and advancements to the public is necessary to raise awareness and generate support for the field.
References
- Chistiakov, D. (2012). Liver Regenerative Medicine: Advances and Challenges. Cells Tissues Organs, 196(4), pp.291-312. [CrossRef]
- Iversen, S. (1956). THE NUCLEO-CYTOPLASMIC RATIO IN REGENERATING RAT LIVER CELLS. Cells Tissues Organs, 27(4), pp.303-308. [CrossRef]
- Atala, A. (2007). Engineering tissues, organs and cells. Journal of Tissue Engineering and Regenerative Medicine, 1(2), pp.83-96.
- Johns, D. and Athanasiou, K. (2007). Improving Culture Conditions for Temporomandibular Joint Disc Tissue Engineering. Cells Tissues Organs, 185(4), pp.246-257. [CrossRef]
- Chhabra, P., Mirmira, R. and Brayman, K. (2009). Regenerative medicine and tissue engineering: contribution of stem cells in organ transplantation. Current Opinion in Organ Transplantation, 14(1), pp.46-50. [CrossRef]
- Sakiyama-Elbert (2008). Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemBook.
- Mooneylab.seas.harvard.edu. (2019). Tissue Engineering and Regenerative Medicine. [online] Available at: https://mooneylab.seas.harvard.edu/musculoskeletal-tissue-engineering [Accessed 27 Dec. 2019].
- X. Wang, M. Ali and C. Lacerda, "A Three-Dimensional Collagen-Elastin Scaffold for Heart Valve Tissue Engineering", Bioengineering, vol. 5, no. 3, p. 69, 2018. [CrossRef]
- "Can stem cells from one tissue be grown into many other types of cells?", Nature Reports Stem Cells, 2007. [CrossRef]
- "Can stem cells from one tissue be grown into many other types of cells?", Nature Reports Stem Cells, 2007. [CrossRef]
- B. polymers et al., "EP1937323A1 - Multi-layered antiadhesion barrier - Google Patents", Patents.google.com, 2019. [Online]. Available: https://patents.google.com/patent/EP1937323A1/en. [Accessed: 27- Dec- 2019].
- D. Hanein, A. Yarden, H. Sabanay, L. Addadi and B. Geiger, "Cell-Adhesion to Crystal Surfaces: Adhesion-Induced Physiological Cell Death", Cell Adhesion and Communication, vol. 4, no. 4-5, pp. 341-353, 1996. [CrossRef]
- M. Persson and J. van der Linden, "Intraoperative field flooding with warm humidified CO2 may help to prevent adhesion formation after open surgery", Medical Hypotheses, vol. 73, no. 4, pp. 521-523, 2009. [CrossRef]
- M. Reed, M. Damodarasamy and W. Banks, "The extracellular matrix of the blood–brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease", Tissue Barriers, vol. 7, no. 4, p. 1651157, 2019. [CrossRef]
- M. Mizee and H. de Vries, "Blood-brain barrier regulation", Tissue Barriers, vol. 1, no. 5, p. e26882, 2013. [CrossRef]
- Palatinsky, E. A. (2001). Antiadhesion Barrier Gels in Peripheral Nerve Surgery. Seminars in Neurosurgery, 12(01), 093–099. [CrossRef]
- L. Fu, “Delivery Systems in Wound Healing and Nanomedicine,” Wound Healing - New insights into Ancient Challenges, Dec. 2016.
- L. Tayebi and K. Moharamzadeh, “Introduction to oral and dental tissue engineering,” Biomaterials for Oral and Dental Tissue Engineering, pp. 3–6, 2017.
- S. M. Kurtz, PEEK biomaterials handbook. London: William Andrew, an imprint of Elsevier, 2019.
- R. G. Lebaron and K. A. Athanasiou, “Extracellular Matrix Cell Adhesion Peptides: Functional Applications in Orthopedic Materials,” Tissue Engineering, vol. 6, no. 2, pp. 85–103, 2000. [CrossRef]
- R. G. Lebaron and K. A. Athanasiou, “Extracellular Matrix Cell Adhesion Peptides: Functional Applications in Orthopedic Materials,” Tissue Engineering, vol. 6, no. 2, pp. 85–103, 2000. [CrossRef]
- Goldstein, “Cell Adhesion,” Electrical Engineering Handbook Tissue Engineering and Artificial Organs, 2006.
- M. Lotfi, M. Nejib, and M. Naceur, “Cell Adhesion to Biomaterials: Concept of Biocompatibility,” Advances in Biomaterials Science and Biomedical Applications, 2013.
- Warocquier-Cleront M.R, Legris C, Degrange M, Sigot-Luizard M.F. Correlation between substratum roughness and wettability, cell adhesion and cell migration. J. Biomed. Mater. Res., 36 (1), 1997, 99-108.
- J. H. Wilson and T. Hunt, Molecular biology of the cell, 4th edition: a problems approach. New York: Garland Science, 2002.
- “Hardin Eugene[au] - PubMed Result - National Center for ...” [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed?term=Hardin Eugene[au]. [Accessed: 27-Dec-2019].
- “Adhesion Definition, Causes, Treatment & Scar Tissue Pain ...” [Online]. Available: https://www.emedicinehealth.com/adhesions_general_and_after_surgery/article_em.htm. [Accessed: 27-Dec-2019].
- “Adhesions.” [Online]. Available: https://gutscharity.org.uk/advice-and-information/conditions/adhesions/.
- “Exam 6 Study Guide - Pathophysiology Nu545 with Miller at University of South Alabama,” STUDYBLUE. [Online]. Available: https://www.studyblue.com/notes/note/n/exam-6-study-guide/deck/21047358. [Accessed: 27-Dec-2019].
- “Laparoscopic Colon Surgery: Minimally Invasive Procedure ...” [Online]. Available: https://laparoscopic.md/surgery/colon. [Accessed: 27-Dec-2019].
- “TEKSTİL VE MÜHENDİS (Journal of Textiles and Engineer ...” [Online]. Available: https://dergipark.org.tr/tr/download/article-file/137907. [Accessed: 27-Dec-2019].
- “NANOLİF YAPILI POLİMERİK DOKU İSKELELERİ.” [Online]. Available: http://dergi.tekstilvemuhendis.org.tr/article/download/5000076781/5000070865. [Accessed: 27-Dec-2019].
- Ravichandran R., Sundarrajan S., Venugopal J. R., Mukherjee S., Ramakrishna S., (2012), Advances in Polymeric Systems for Tissue Engineering and Biomedical Applications, Macromolecular Bioscience, 12, 3, 286–311. [CrossRef]
- B. Subia, J. Kundu, and S. C., “Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications,” Tissue Engineering, Jan. 2010.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).