Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
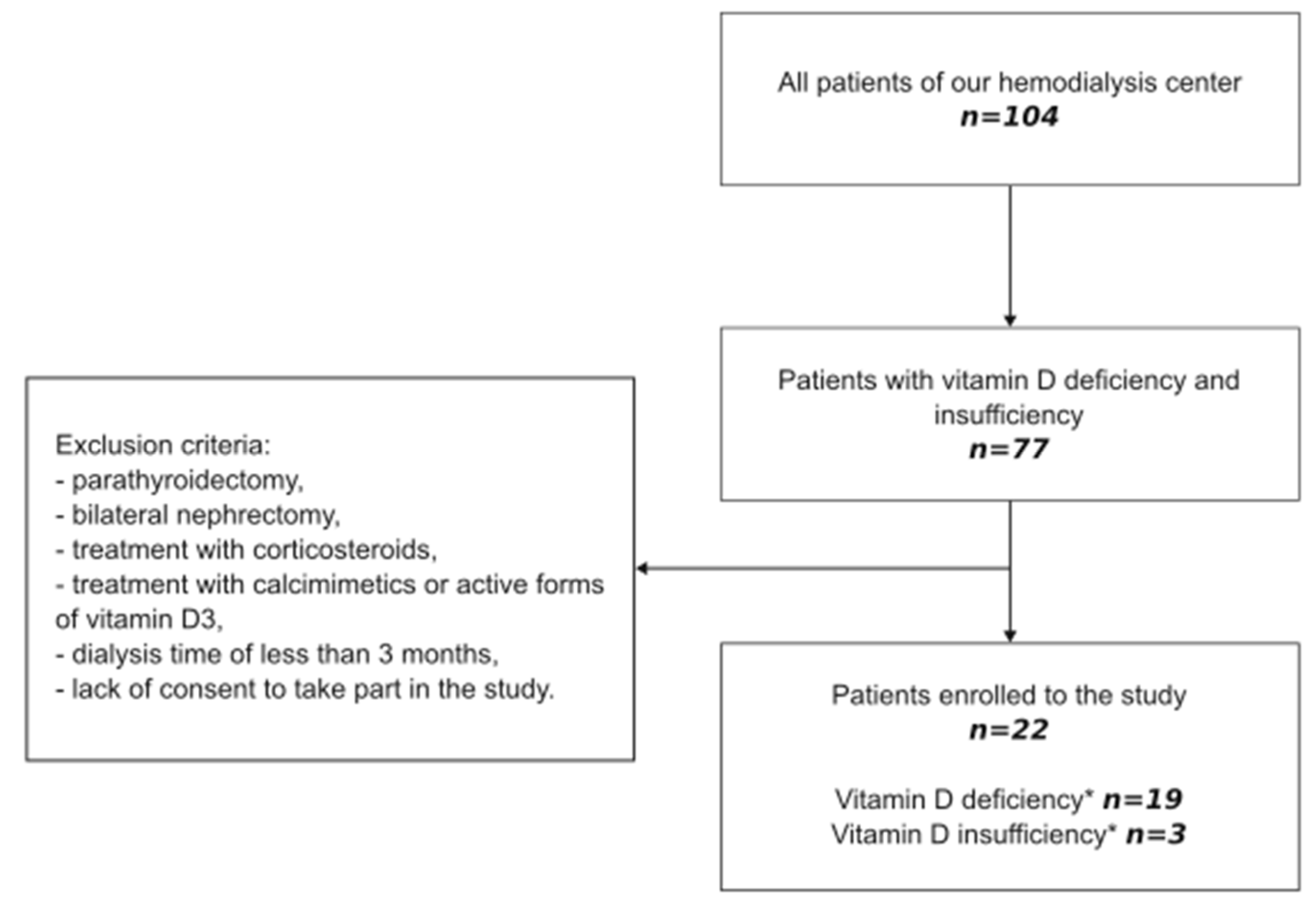
2.1. Study Design
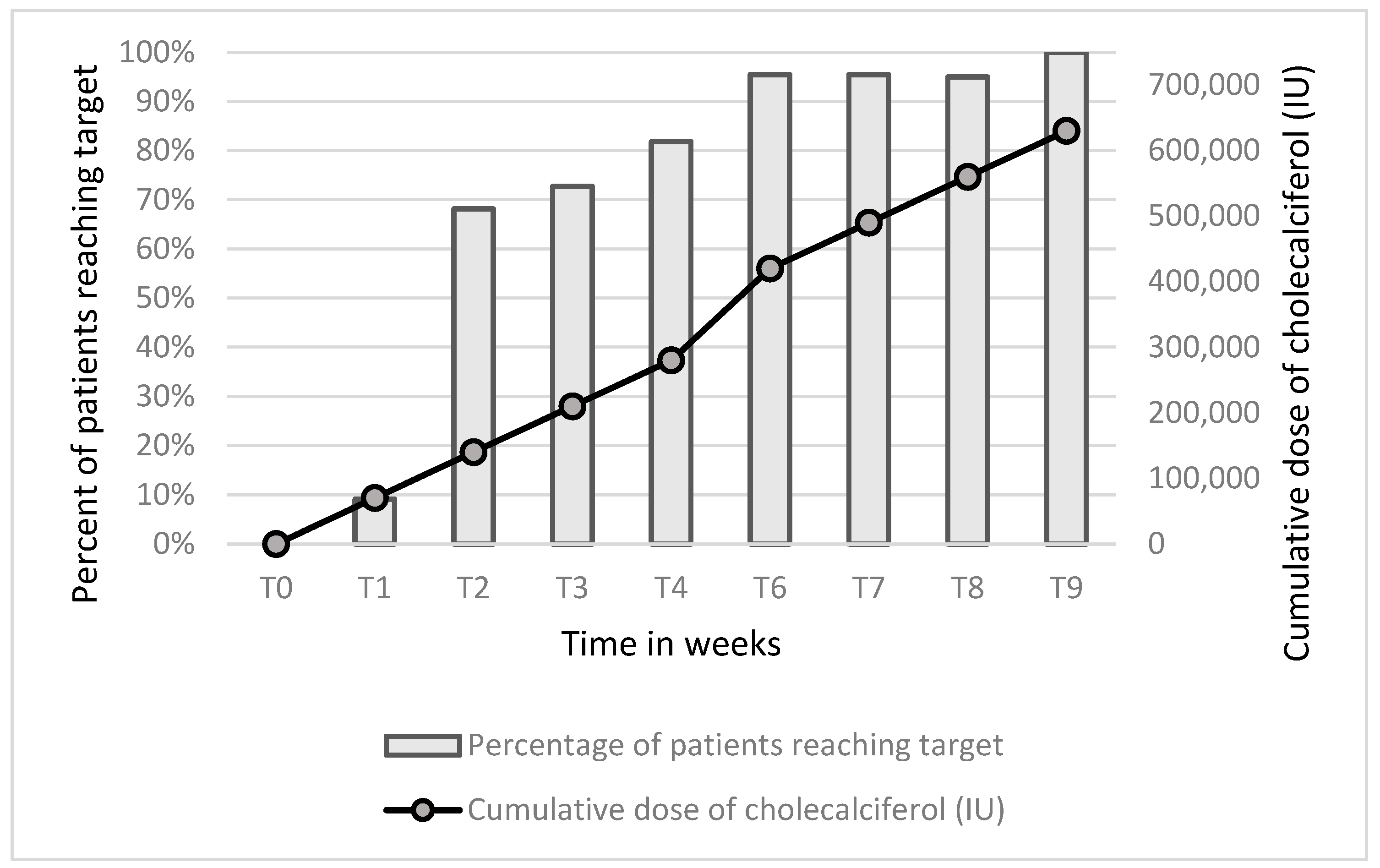
2.2. Treatment with Cholecalciferol
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
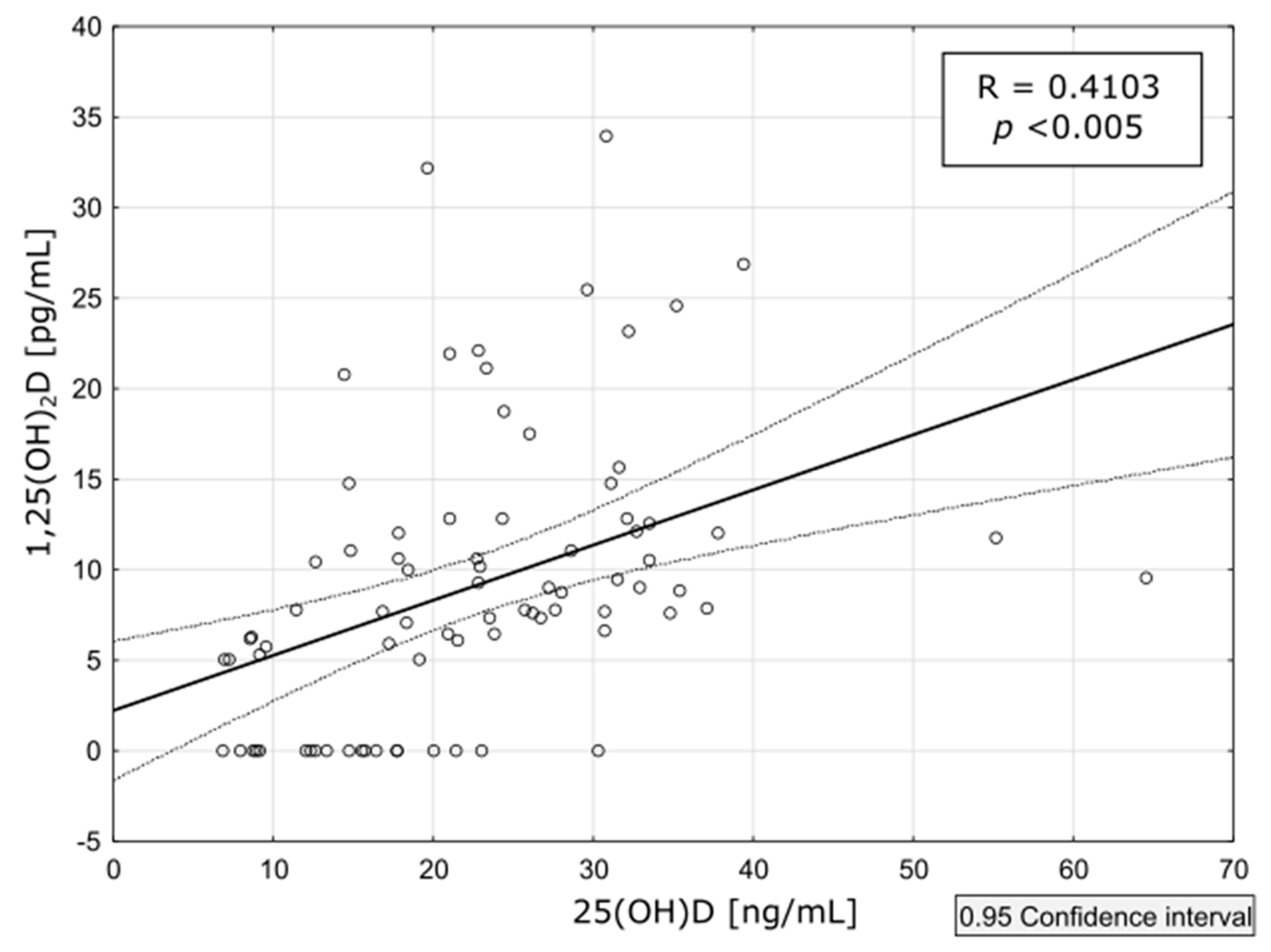
3.4. PTH Levels
3.5. Safety Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; He, C.; Gong, M.; Wu, S.; He, J. The Effects of Vitamin D on All-Cause Mortality in Different Diseases: An Evidence-Map and Umbrella Review of 116 Randomized Controlled Trials. Front. Nutr. 2023, 10, 1132528. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Baena, C. P.; Prabhakaran, D.; Hoshen, M. B.; Feldman, B. S.; Pan, A.; Johnson, L. Vitamin D and Risk of Cause Specific Death : Systematic Review and Meta-Analysis of Observational Cohort and Randomised Intervention Studies. BMJ (Clinical research ed.), 2014, 348, g1903. [CrossRef]
- Pilz, S.; Iodice, S.; Zittermann, A.; Grant, W. B.; Gandini, S. Vitamin D Status and Mortality Risk in CKD: A Meta-Analysis of Prospective Studies. Am. J. Kidney Dis. 2011, 58, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Souberbielle, J. C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients. 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, P.; Polewska, K.; Och, A.; Susmarska, A.; Puchalska-Reglí nska, E.; Parczewska, A.; Biedunkiewicz, B.; Szabat, K.; Renke, M.; Tylicki, L.; et al. Angiotensin Converting Enzyme Inhibitors May Increase While Active Vitamin D May Decrease the Risk of Severe Pneumonia in SARS-CoV-2 Infected Patients with Chronic Kidney Disease on Maintenance Hemodialysis. Viruses. 2022, 14(3), 451. [Google Scholar] [CrossRef]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C. C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients. 2022, 14, 1483. [Google Scholar] [CrossRef]
- Kim SM, Choi HJ, Lee JP, Kim DK, Oh YK, Kim YS, L. C. Prevalence of Vitamin D Deficiency and Effects of Supplementation with Cholecalciferol in Patients with Chronic Kidney Disease. J Ren Nutr. 2014, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Szupryczyńska, N.; Dębska-Ślizień, A.; Borek, P.; Kaczkan, M.; Rutkowski, B.; Małgorzewicz, S. Dietary Intake of Vitamins in Different Options of Treatment in Chronic Kidney Disease: Is There a Deficiency? Transplant. Proc. 2016, 48, 1427–1430. [Google Scholar] [CrossRef]
- Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Vitamins and Microelement Bioavailability in Different Stages of Chronic Kidney Disease. Nutrients. 2017, 9, 282. [Google Scholar] [CrossRef]
- Jean, G.; Terrat, J. C.; Vanel, T.; Hurot, J. M.; Lorriaux, C.; Mayor, B.; Chazot, C. Evidence for Persistent Vitamin D 1-Alpha-Hydroxylation in Hemodialysis Patients: Evolution of Serum 1,25-Dihydroxycholecalciferol after 6 Months of 25-Hydroxycholecalciferol Treatment. Nephron. Clin. Pract. 2008, 110, c58–65. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef]
- Płudowski, P.; Kos-kudła, B.; Walczak, M.; Fal, A.; Jackowska, T.; Helwich, E.; Mazur, A. Guidelines for Preventing and Treating Vitamin D Deficiency : A 2023 Update in Poland. Nutrients. 2023, 15(3), 695. [Google Scholar] [CrossRef]
- Matuszkiewicz-Rowińska, J.; Kulicki, P.; Zebrowski, P.; Klatko, W.; Sokalski, A.; Niemczyk, S.; Wypych-Birecka, M.; Małyszko, J. Cholecalciferol vs. Small Doses of Alfacalcidol vs. Placebo in Chronic Kidney Disease Patients on Hemodialysis: A Randomized Parallel Group Study. Front. Med. 7811. [Google Scholar] [CrossRef]
- Wasse, H.; Huang, R.; Long, Q.; Singapuri, S.; Raggi, P.; Tangpricha, V. Efficacy and Safety of a Short Course of Very-High-Dose Cholecalciferol in Hemodialysis. Am. J. Clin. Nutr. 2012, 95, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Wasse, H. Vitamin D Therapy in Kidney Disease: More Vitamin D Is Necessary. Am. J. Kidney Dis. 2014, 64, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Guella, A.; Abduelkarem, A. R.; Hassanein, M. M. The Effects and Safety of High Dose Vitamin D3 in Hemodialysis Patients. Pharm. Pract. (Granada). 2023, 21, 2773. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D Supplementation: Cholecalciferol, Calcifediol, and Calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Cicarma, E.; Porojnicu, A. C.; Lagunova, Z.; Dahlback, A.; Juzeniene, A.; Moan, J. Sun and Sun Beds : Inducers of Vitamin D and Skin Cancer. Anticancer Res. 2009, 29, 3495–3500. [Google Scholar] [PubMed]
- Mieczkowski, M.; Żebrowski, P.; Wojtaszek, E.; Stompór, T.; Przedlacki, J.; Bartoszewicz, Z.; Sierdziński, J.; Wańkowicz, Z.; Niemczyk, S.; Matuszkiewicz-Rowińska, J. Long-Term Cholecalciferol Administration in Hemodialysis Patients: A Single-Center Randomized Pilot Study. Med. Sci. Monit. 2014, 20, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, F.; Quack, I.; Schieren, G.; Sellin, L.; Rattensperger, D.; Holland-letz, T.; Weiner, S. M.; Rump, L. C. High-Dose Cholecalciferol to Correct Vitamin D Deficiency in Haemodialysis Patients. Nephrol Dial Transpl. 2008, 23 (July), 4016–4020. [Google Scholar] [CrossRef]
- Massart, A.; Debelle, F. D.; Racape, J.; Gervy, C.; Husson, C.; Dhaene, M.; Wissing, K. M.; Nortier, L. Original Investigation Biochemical Parameters After Cholecalciferol Repletion in Hemodialysis: Results From the VitaDial Randomized Trial. Am J Kidney Dis. 2014, 64, 696–705. [Google Scholar] [CrossRef]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Monthly Cholecalciferol Administration in Haemodialysis Patients : A Simple and Efficient Strategy for Vitamin D Supplementation. Nephrol Dial Transpl. 2009, 24, 3799–3805. [Google Scholar] [CrossRef]
- Descombes, E.; Fellay, B.; Hemett, O. M.; Magnin, J. L.; Fellay, G. Oral Postdialysis Cholecalciferol Supplementation in Patients on Maintenance Hemodialysis: A Dose-Response Approach. Int. J. Nephrol. 2014, 30–39. [Google Scholar] [CrossRef]
- Huish, S. A.; Jenkinson, C.; Dunn, J. A.; Meredith, D. J.; Bland, R.; Hewison, M. Low Serum 1,25(OH)2D3 in End-Stage Renal Disease : Is Reduced 1α-Hydroxylase the Only Problem ? Endocr. Connect. 2021, 10, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Matias, P. J.; Laranjinha, I.; Ávila, G.; Azevedo, A.; Jorge, C.; Ferreira, C.; Aires, I.; Amaral, T.; Gil, C.; Ferreira, A. Long-Term Cholecalciferol Supplementation in Hemodialysis Patients: Effects on Mineral Metabolism, Inflammation, and Cardiac Parameters. Semin. Dial. 2023, 36, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Armas, L. A. G.; Andukuri, R.; Barger-Lux, J.; Heaney, R. P.; Lund, R. 25-Hydroxyvitamin D Response to Cholecalciferol Supplementation in Hemodialysis. Clin. J. Am. Soc. Nephrol. 2012, 7, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Sprenger-Mähr, H.; Mündle, M.; Lhotta, K. Efficacy and Safety of Body Weight-Adapted Oral Cholecalciferol Substitution in Dialysis Patients with Vitamin D Deficiency. BMC Nephrol. 2015, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, J. G. Vitamin D Toxicity – A Clinical Perspective. Front Endocrinol (Lausanne). 2018, 20, 550. [CrossRef]
- Brandenburg, V.; Ketteler, M. Vitamin D and Secondary Hyperparathyroidism in Chronic and the Future. Nutrients, 2022, 14, 3009. [CrossRef]
- Marckmann, P.; Agerskov, H.; Thineshkumar, S.; Bladbjerg, E. M.; Sidelmann, J. J.; Jespersen, J.; Nybo, M.; Rasmussen, L. M.; Hansen, D.; Scholze, A. Randomized Controlled Trial of Cholecalciferol Supplementation in Chronic Kidney Disease Patients with Hypovitaminosis D. Nephrol. Dial. Transplant. 2012, 27, 3523–3531. [Google Scholar] [CrossRef]
- Friedl, C.; Zitt, E. Vitamin D Prohormone in the Treatment of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease. Int J Nephrol Renov. Dis. 2017, 10, 109–122. [Google Scholar] [CrossRef]
| Gender (Men/Women) | 16/6 |
|---|---|
| Causes of ESRD (n/%) | |
| Ischemic nephropathy/ no histology | 5 / 22.7 |
| Autosomal dominant polycystic kidney disease | 4 / 18.2 |
| Diabetic nephropathy in type 2 diabetes/no histology | 6 / 27.7 |
| Glomerulonephritis/no histology | 2 / 9.1 |
| Chronic hypertensive nephropathy/no histology | 1 / 4.5 |
| Congenital renal malformation | 1 / 4.5 |
| Unknown | 2 / 9.1 |
| Age (years) | 72.5 (67–81) |
| Charlson Comorbidity Index (points) | 8 (2–14) |
| Dialysis vintage (months) | 25.0 (12–56) |
| Body Mass Index (kg/m2) | 25.27 (22.50–28.76) |
| Parameters | T0 | Tmax | p |
|---|---|---|---|
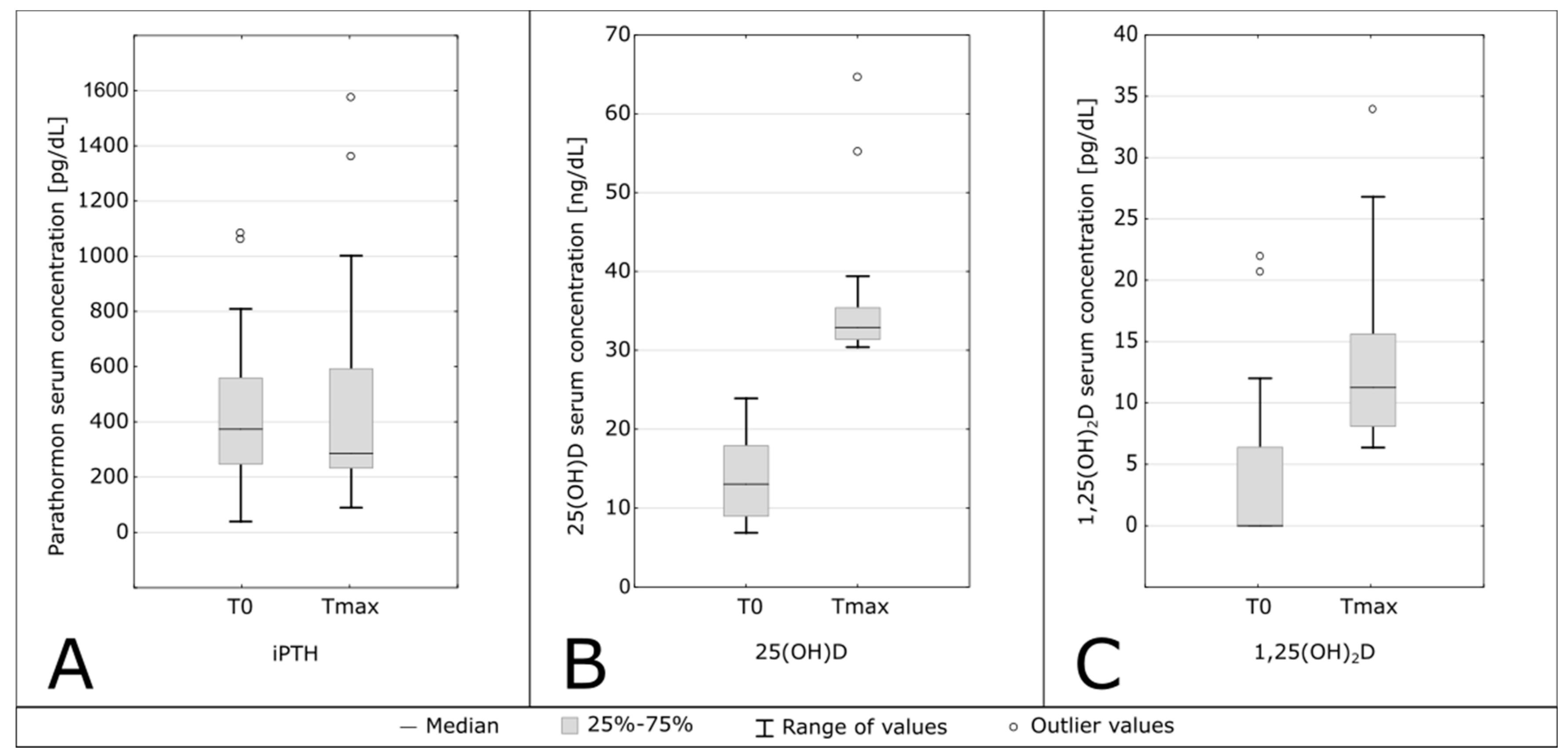
| 25(OH)D [ng/mL] | 13.05 (9.00–17.90) | 32.9 (31.4–35.4) | <0.001 |
| 1,25(OH)2 D [pg/mL] | 0.00 (0.00–6.38) | 6.37 (8.11–15.60) | <0.001 |
| iPTH [pg/mL] | 373.50 (247–558) | 236 (146–537.5) | 0.70 |
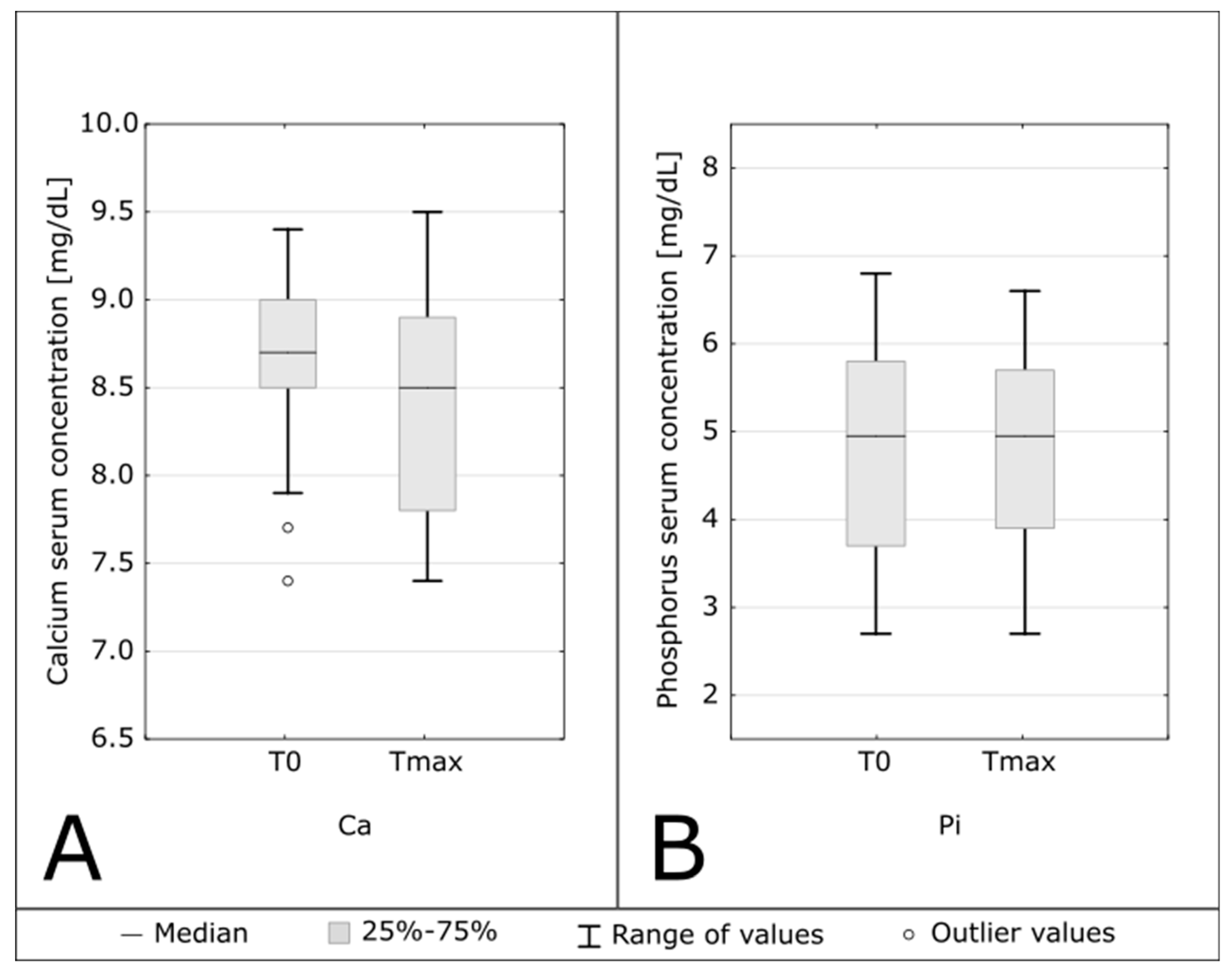
| Calcium [mg/dL] | 8.7 (8.5–9.0) | 8.5 (7.8–8.9) | 0.048 |
| Phosphorus [mg/dL] | 4.95 (3.7–5.8) | 4.95 (3.9–5.7) | 0.68 |
| Serum CaxP [mg2/dL2] | 48.778 (42.416–52.7) | 43.7 (36.16–48.36) | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





