Submitted:
06 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General methods
2.2. Synthesis
2.3. Prediction of Activity Spectra for Substances (PASS)
2.4. Evaluation of in vitro antimicrobial activities
2.5. Computational method for molecular docking
2.6. DFT calculations
2.7. Hydrophile-lipophile balance (HLB) calculation
2.8. ADMET analysis
3. Results and discussion
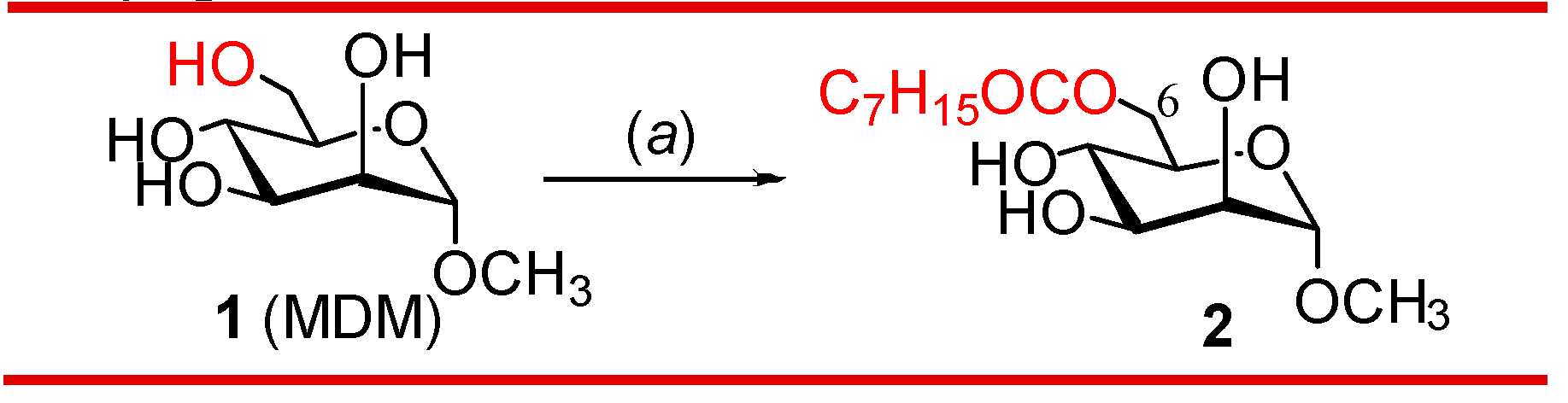
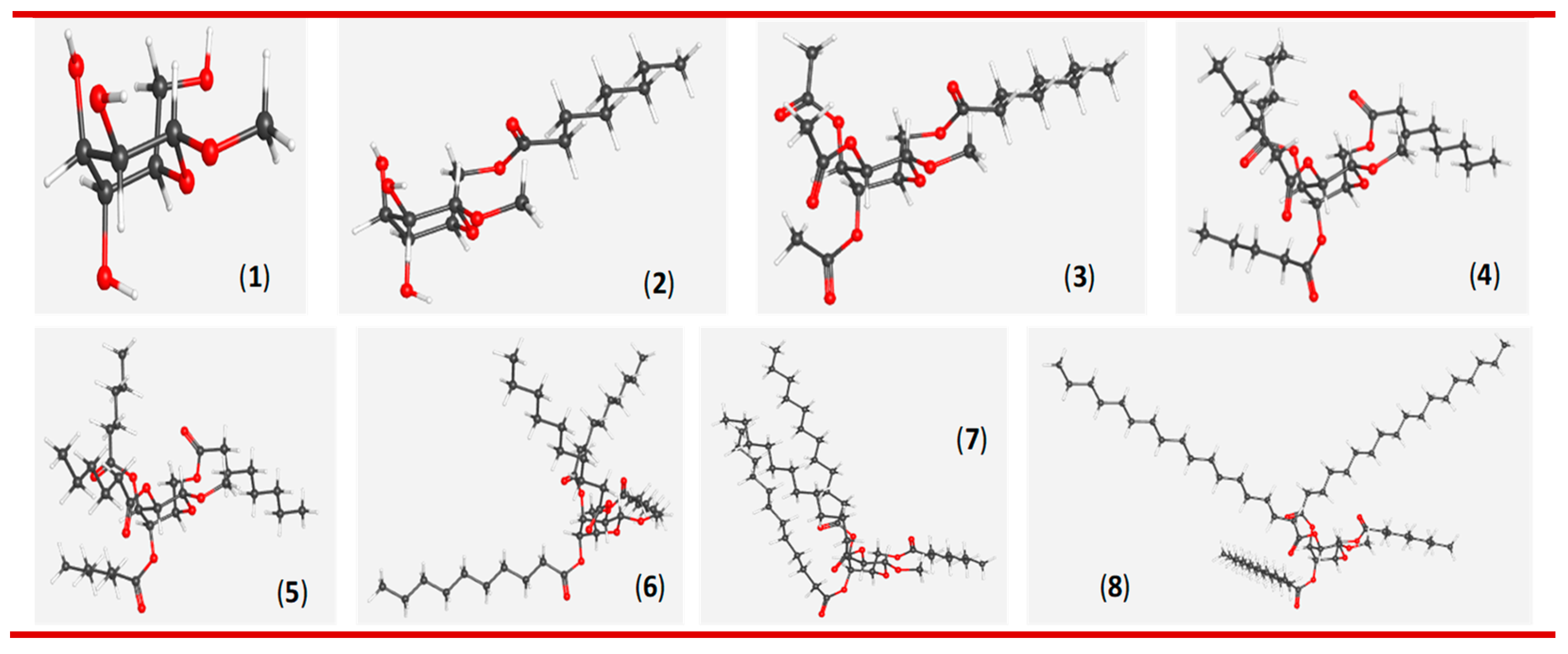
3.1. One-step regioselective octanoylation of mannopyranoside 1
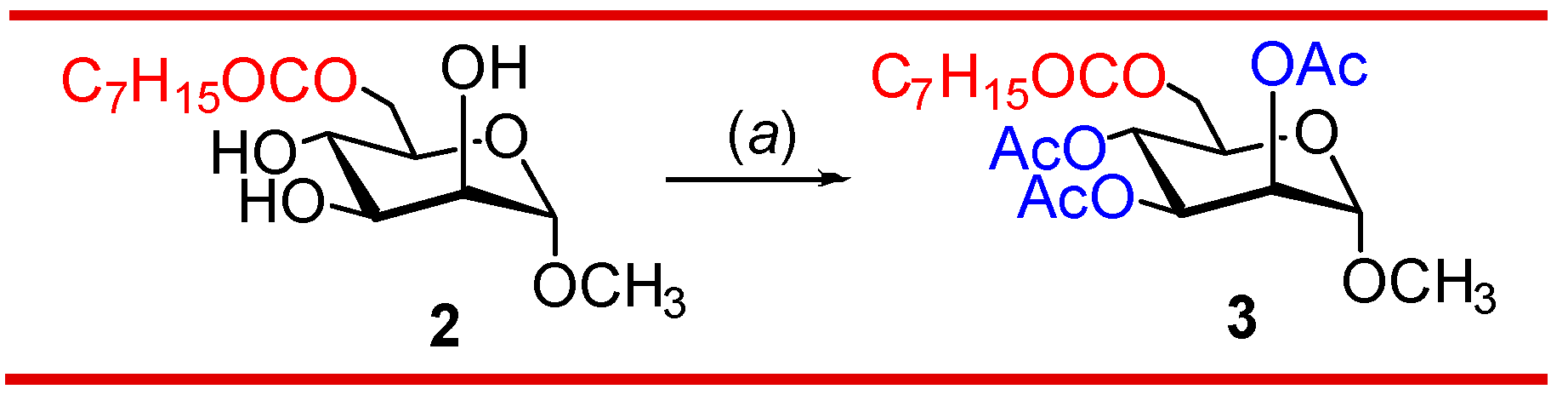
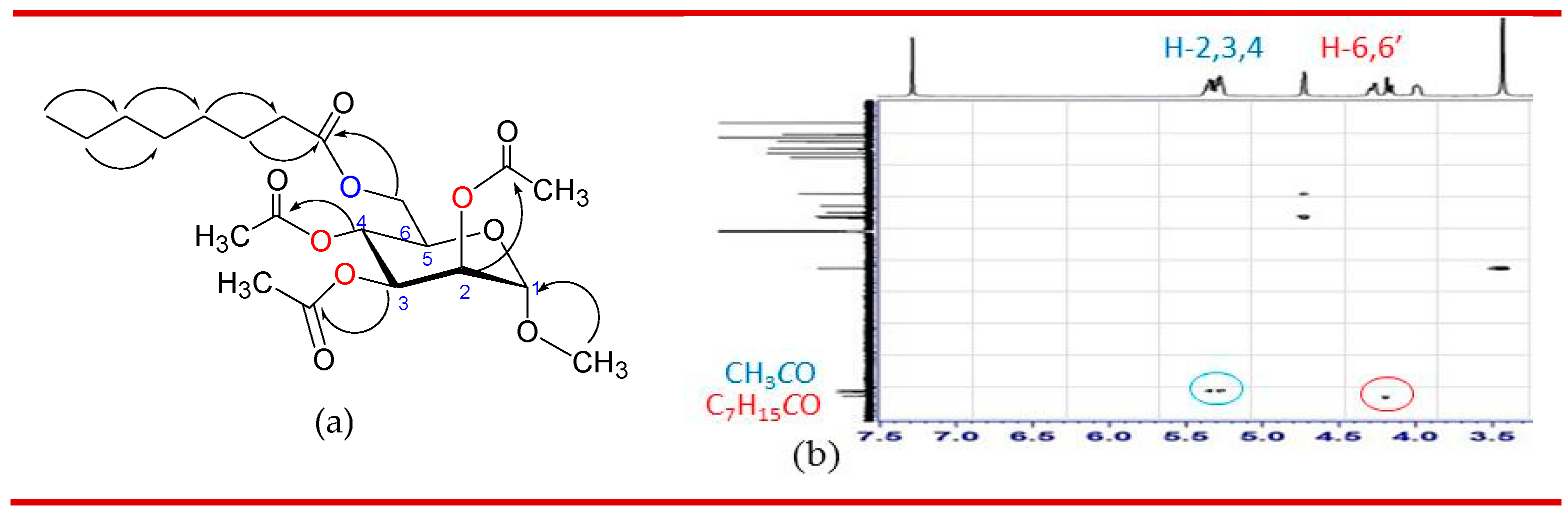
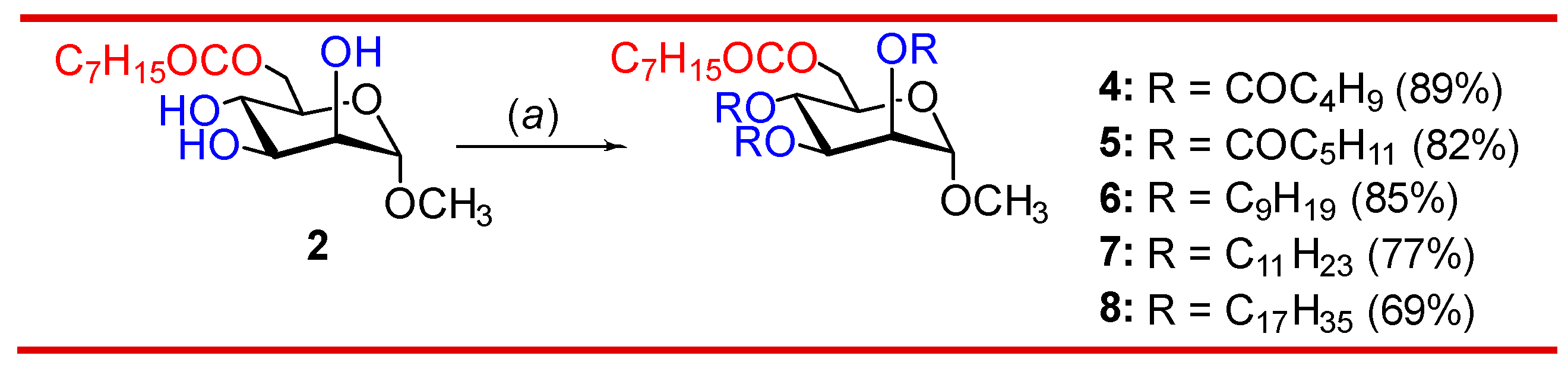
3.2. Synthesis of 2,3,4-tri-O-acylates of 6-O-octanoate 2
3.3. Computational-based biological activity evaluation
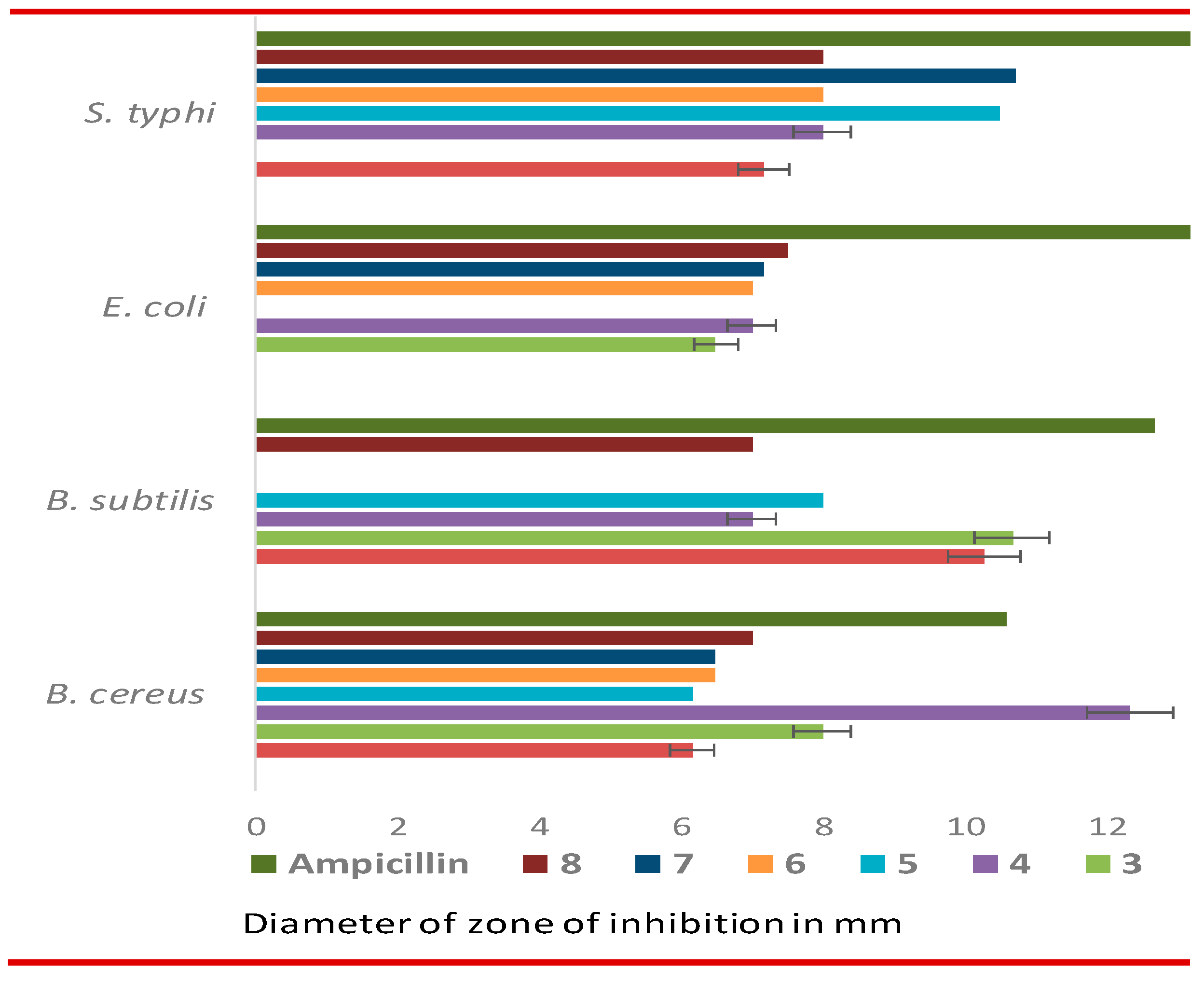
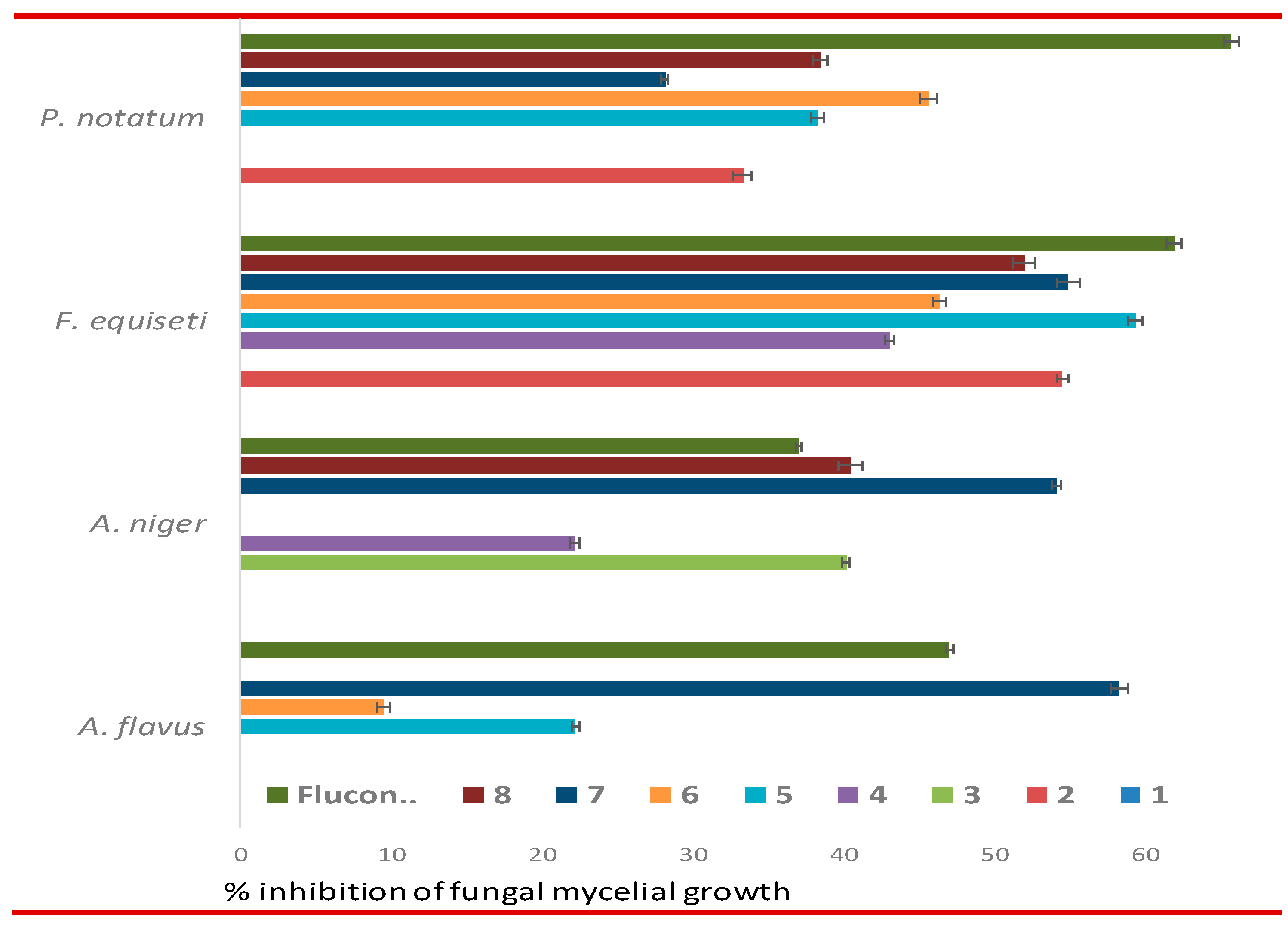
3.4. In vitro antimicrobial evaluation of MDM esters
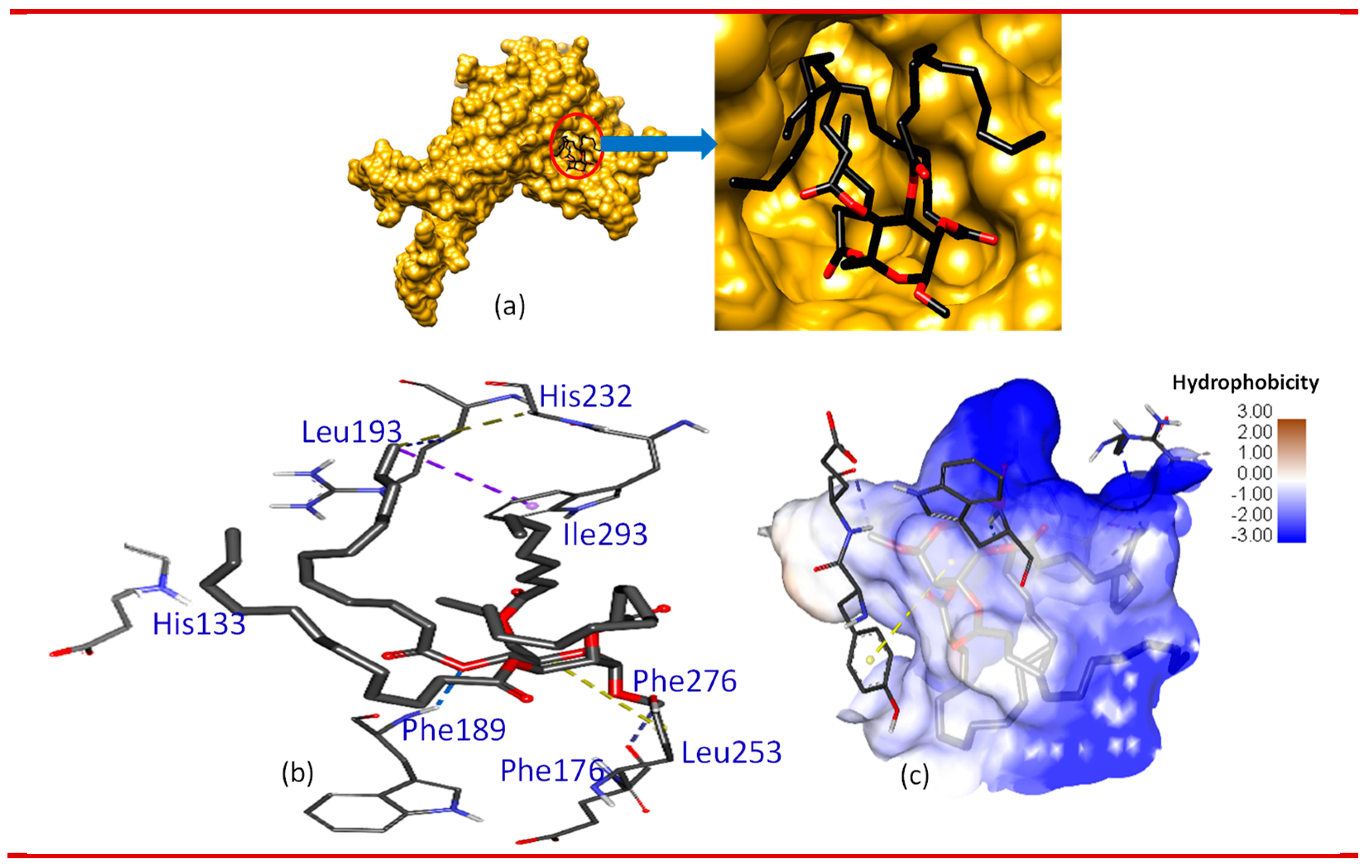
3.5. Molecular docking and nonbonding interactions
3.6. DFT-based thermodynamic properties
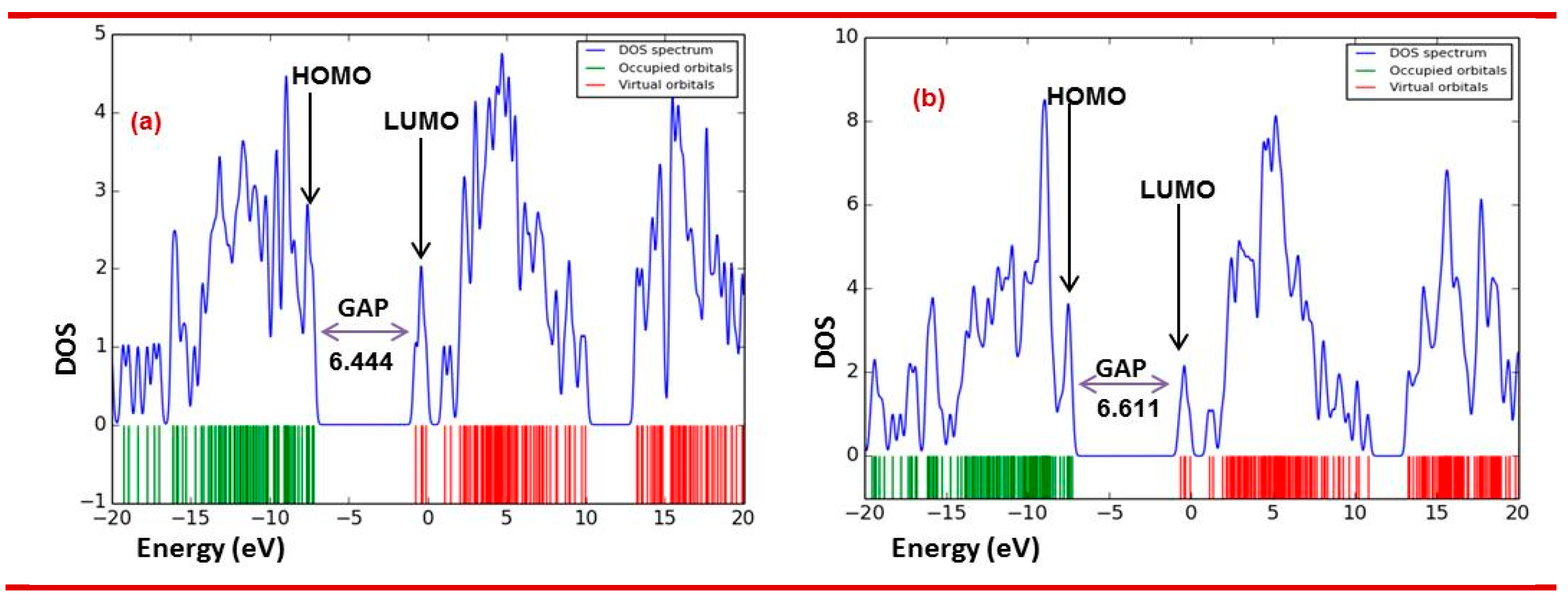
3.6.1. Molecular orbitals (MO) analysis
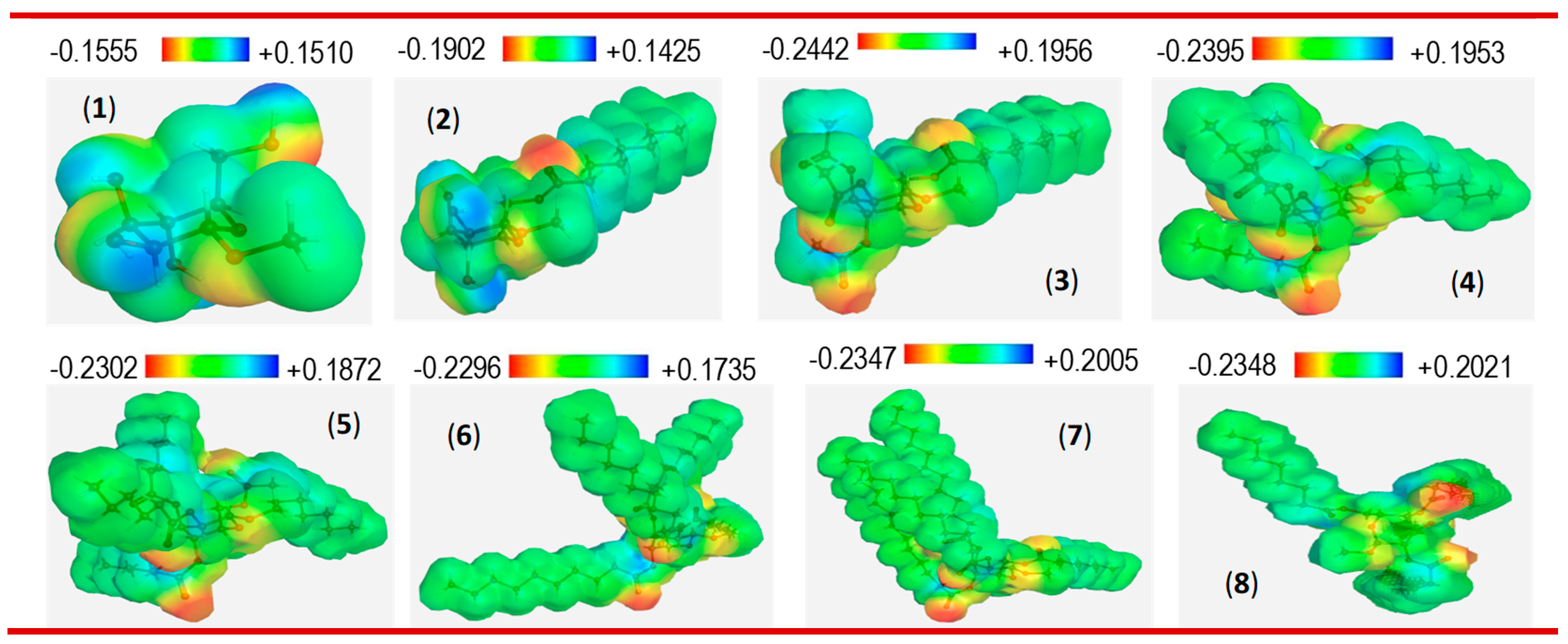
3.6.2. Molecular electrostatic potential (MEP) analysis
3.7. Drug-likeness and ADMET analysis of 2-8
3.8. Structure-activity relationship (SAR)
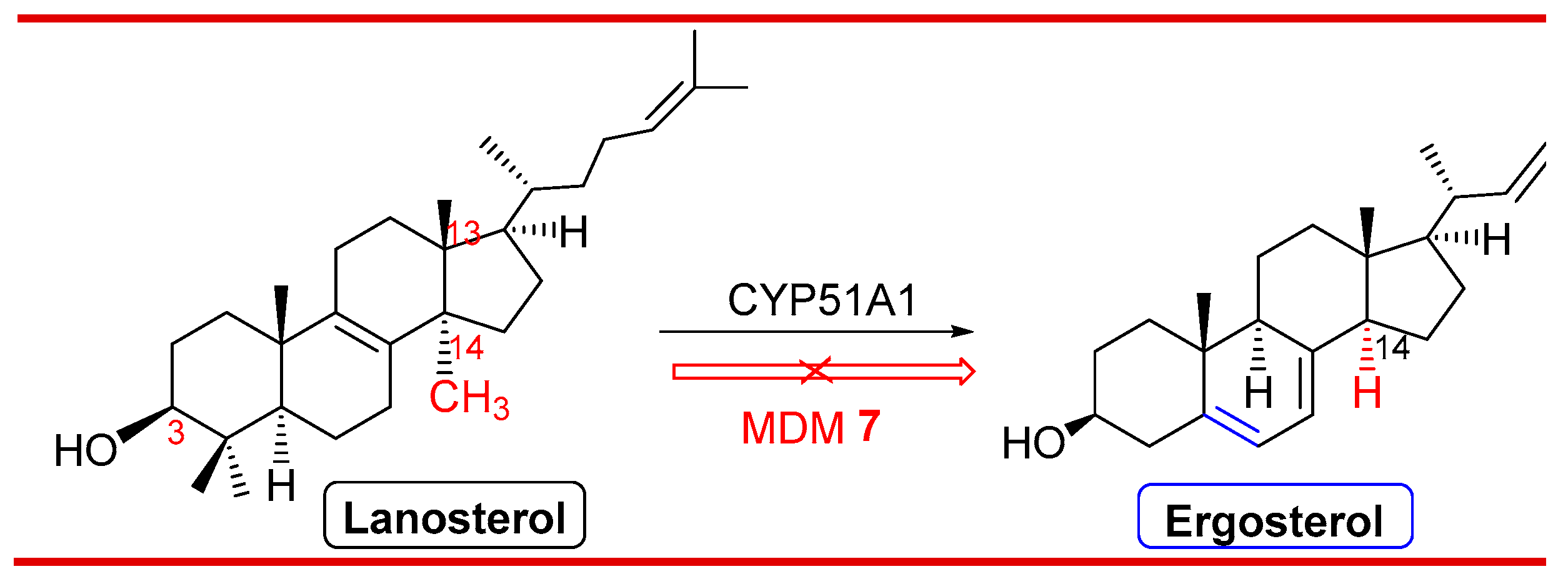
3.9. Probable antifungal mechanism of action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Romao-Dumaresq, A.S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase mediated synthesis of sugar fatty acid esters. Process. Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfact. Deter. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Nobmann, P.; Bourke, P.; Dunne, J.; Henehan, G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against Staphylococcus aureus and MRSA. J. Appl. Microbiol. 2010, 108, 2152–2161. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.Y.; Hao, T.Y.; Li, S.R. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; et al. Unsaturated fatty acids lactose esters: cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.-Y.; Shi, Y.-G.; Wu, Y.; Bian, L.-Q.; Zhu, Y.-J.; Huang, X.-Y.; et al. Lipase-catalyzed synthesis of sucrose monolaurate and its antibacterial property and mode of action against four pathogenic bacteria. Molecules 2018, 23, e1118. [Google Scholar] [CrossRef] [PubMed]
- Staron, J.; Dabrowski, J.M.; Cichon, E.; Guzik. M. Lactose esters: synthesis and biotechnological applications. Critic. Rev. Biotech. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Zhang, B.C.; Xu, H. Synthesis of some monosaccharide-related ester derivatives as insecticidal and acaricidal agents. Bioorg. Med. Chem. Lett. 2017, 27, 4336–4340. [Google Scholar] [CrossRef]
- Okamoto, H.; Sakai, T.; Danjo, K. Effect of sucrose fatty acid esters on transdermal permeation of lidocaine and ketoprofen. Biol. Pharmaceutic. Bull. 2005, 28, 1689–1694. [Google Scholar] [CrossRef]
- Ei-Laithy, H.M.; Shoukry, O.; Mahran. L.G. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: Preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2011, 77, 43–55. [Google Scholar] [CrossRef]
- Okamoto, H.; Sakai, T.; Tokuyama, C.; Danjo, K. Sugar ester J-1216 enhances percutaneous permeation of ionized lidocaine. J. Pharm. Sci. 2011, 100, 4482–4490. [Google Scholar] [CrossRef]
- Alama, T.; Katayama, H.; Hirai, S.; Ono, S.; Kajiyama, A.; Kusamori, K. et al. Enhanced oral delivery of alendronate by sucrose fatty acids esters in rats and their absorption-enhancing mechanisms. Int. J. Pharm. 2016, 515, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A. et al. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.L.; Stewart, S.G.; Hai, Y.W.; Li, X.; Banwell, M.G.; Lan, P. Sucrose fatty acid esters: Synthesis, emulsifying capacities, biological activities and structure-property profiles. Critic. Rev. Food Sci. Nutr. 2021, 61, 3297–3317. [Google Scholar] [CrossRef]
- Sanaullah, A.F.M.; Bhuiyan, M.M.H.; Matin, M.M. Stearoyl glucopyranosides: Selective synthesis, PASS analysis, in vitro antimicrobial, and SAR study. Egyp. J. Chem. 2022, 65, 329–338. [Google Scholar] [CrossRef]
- AlFindee, M.N.; Zhang, Q.; Subedi, Y.P.; Shrestha, J.P.; Kawasaki, Y.; Grilley, M. et al. One-step synthesis of carbohydrate esters as antibacterial and antifungal agents. Bioorg. Med. Chem. 2018, 26, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Katayama, S.; Matsubara, M.; Honda, Y.; Kuwahara, M. Antibacterial carbohydrate monoesters suppressing cell growth of Streptococcus mutans in the presence of sucrose. Curr. Microbiol. 2000, 41, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Snoch, W.; Stepien, K.; Prajsnar, J.; Staron, J.; Szaleniec, M.; Guzik, M. Influence of chemical modifications of polyhydroxyalkanoate-derived fatty acids on their antimicrobial properties. Catalysts 2019, 9, e510. [Google Scholar] [CrossRef]
- Petkova, N.; Vassilev, D.; Grudeva, R.; Tumbarski, Y.; Vasileva, I. et al. "Green" Synthesis of sucrose octaacetate and characterization of its physicochemical properties and antimicrobial activity. Chem. Biochem. Engin. Quart. 2017, 31, 395–402. [Google Scholar] [CrossRef]
- Matin, M.M.; Nath, A.R.; Saad, O.; Bhuiyan, M.M.H.; Kadir, F.A.; Hamid, S.B.A. et al. Synthesis, PASS-predication and in vitro antimicrobial activity of benzyl 4-O-benzoyl-α-L-rhamnopyranoside derivatives. Int. J. Mol. Sci. 2016, 17, e1412. [Google Scholar] [CrossRef]
- Matin, M.M.; Bhattacharjee, S.C.; Chakraborty, P.; Alam, M.S. Synthesis, PASS predication, in vitro antimicrobial evaluation and pharmacokinetic study of novel n-octyl glucopyranoside esters. Carbohydr. Res. 2019, 485, e107812. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Leveiller, F.; Franchi, D.; de Jong, H.; Linden, H. When poor solubility becomes an issue: From early stage to proof of concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef]
- Aronson, M.; Medalia, O.; Schori, L.; Mirelman, D.; Sharon, N.; Ofek. I. (1979). Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J. Infect. Dis. 1979, 139, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Imai, K.; Yoshikawa, S.; Kawada, K.; Uchibor, T. Surface activities, biodegradability and antimicrobial properties of n-alkyl glucosides, mannosides and galactosides. J. Am. Oil Chem. Soc. 1990, 67, 996–1001. [Google Scholar] [CrossRef]
- Okamoto, H.; Sakai, T.; Tokuyama, C.; Danjo, K. Sugar ester J-1216 enhances percutaneous permeation of ionized lidocaine. J. Pharm. Sci. 2011, 100, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Chen, L.Q.; Wu, X.B. Thermotropic liquid crystalline and surface-active properties of n-alkyl alpha-D-mannopyranosides. J. Mol. Liq. 2018, 269, 947–955. [Google Scholar] [CrossRef]
- Hanee, U.; Rahman, M.R.; Matin, M.M. Synthesis, PASS, in silico ADMET, and thermodynamic studies of some galactopyranoside esters. Phys. Chem. Res. 2021, 9, 591–603. [Google Scholar] [CrossRef]
- Matin, M.M.; Bhuiyan, M.M.H.; Kabir, E.; Sanaullah, A.F.M.; Rahman, M.A.; Hossain, M.E. et al. Synthesis, characterization, ADMET, PASS predication, and antimicrobial study of 6-O-lauroyl mannopyranosides. J. Mol. Struct. 2019, 1195, 189–197. [Google Scholar] [CrossRef]
- Matin, M.M.; Uzzaman, M.; Chowdhury, S.A.; Bhuiyan, M.M.H. In vitro antimicrobial, physicochemical, pharmacokinetics and molecular docking studies of benzoyl uridine esters against SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2022, 40, 3668–3680. [Google Scholar] [CrossRef]
- Matin, M.M.; Chakraborty, P.; Alam, M.S.; Islam, M.M.; Hanee. U. Novel mannopyranoside esters as sterol 14 alpha-demethylase inhibitors: Synthesis, PASS predication, molecular docking, and pharmacokinetic studies. Carbohydr. Res. 2020, 496, e108130. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.J.; Yu, H. et al. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: significant factors affecting antibacterial activity of lauric acid esters. Food Sci. Biotechnol. 2018, 27, 401–409. [Google Scholar] [CrossRef]
- Wagh, A.; Shen, S.; Shen, F.A.; Miller, C.D.; Walsh, M.K. Effect of lactose monolaurate on pathogenic and nonpathogenic bacteria. Appl. Environ. Microbiol. 2012, 78, 3465–3468. [Google Scholar] [CrossRef]
- Nakayama, M.; Tomiyama, D.; Ikeda, K.; Katsuki, M.; Nonaka, A.; Miyamoto, T. Antibacterial effects of monoglycerol fatty acid esters and sucrose fatty acid esters on bacillus spp. Food Sci. Technol. Res. 2015, 21, 431–437. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative study of surface-active properties and antimicrobial activities of disaccharide monoesters. Plos One 2014, 9, e114845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, W.; Cao, X.; Feng, F. Characterization of enzymatically prepared sugar medium-chain fatty acid monoesters. J. Sci. Food Agric. 2015, 9, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Hasan, M.S.; Uzzaman, M.; Bhuiyan, M.M.H.; Kibria, S.M.; Hossain, M.E.; Roshid, M.H.O. Synthesis, spectroscopic characterization, molecular docking, and ADMET studies of mannopyranoside esters as antimicrobial agents. J. Mol. Struct. 2020, 1222, 128821. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing of anaerobic bacteria: approved standard-8th ed. CLSI document, Approved Standard M11-A8. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Grover, R.K.; Moore, J.D. Toximetric studies of fungicides against the brown rot organisms Sclerotinia flucticola and S. laxa. Phytopathol. 1962, 52, 876–880. [Google Scholar]
- Akash, S.; Kumer, A.; Chandro, A.; Chakma, U.; Matin, M.M. Quantum calculation, docking, ADMET and molecular dynamics of ketal and non-ketal forms of D-glucofuranose against bacteria, black & white fungus, and triple-negative breast cancer. Bioint. Res. Appl. Chem. 2023, 13, 374. [Google Scholar] [CrossRef]
- Kumer, A.; Chakma, U.; Chandro, A.; Howlader, D.; Akash, S.; Kobir, M.E.; Hossain, T.; Matin, M.M. Modified D-glucofuranose computationally screening for inhibitor of breast cancer and triple breast cancer: Chemical descriptor, molecular docking, molecular dynamics and QSAR. J. Chilean Chem. Soc. 2022, 67, 5623–5635. [Google Scholar] [CrossRef]
- Kumer, A.; Chakma, U.; Matin, M.M. Bilastine based drugs as SARS-CoV-2 protease inhibitors: Molecular docking, dynamics, and ADMET related studies. Orbital: Electron. J. Chem. 2022, 14, 15–23. [Google Scholar] [CrossRef]
- Matin, M.M.; Ibrahim, M.; Anisa, T.R.; Rahman, M.R. Synthesis, characterization, in silico optimization, and conformational studies of methyl 4-O-palmitoyl-α-L-rhamnopyranosides. Malaysian J. Sci. 2022, 41, 91–105. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cos. Chem. 1954, 5, 249–256. [Google Scholar]
- Syed, S.B.; Arya, H.; Fu, I.-H.; Yeh, T.-K.; Periyasamy, L.; Hsieh, H.-P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Bhuiyan, M.M.H.; Kibria, S.M.; Hasan, M.S. Synthesis, PASS predication of antimicrobial activity and pharmacokinetic properties of hexanoyl galactopyranosides and experimental evaluation of their action against four human pathogenic bacteria and four fungal strains. Pharm. Chem. J. 2022, 56, 627–637. [Google Scholar] [CrossRef]
- Matin, P.; Hanee, U.; Alam, M.S.; Jeong, J.E.; Matin, M.M.; Rahman, M.R.; Mahmud, S.; Alshahrani, M.M.; Kim, B. Novel galactopyranoside esters: Synthesis, mechanism, in vitro antimicrobial evaluation and molecular docking studies. Molecules 2022, 27, 4125. [Google Scholar] [CrossRef] [PubMed]
- AFM Sanaullah, MM Matin, MR Rahman, SMA Nayeem, Acyl glucopyranosides: Synthesis, PASS predication, antifungal activities, and molecular docking. Org. Communications 2022, 15, 32–43. [CrossRef]
- Ahuja, R.; Sidhu, A.; Bala, A.; Arora, D.; Sharma, P. Structure based approach for twin-enzyme targeted benzimidazolyl-1,2,4-triazole molecular hybrids as antifungal agents. Arabian J. Chem. 2020, 13, 5832–5848. [Google Scholar] [CrossRef]
- Chugunova, E.; Shaekhov, T.; Khamatgalimov, A.; Gorshkov, V.; Burilov, A. DFT Quantum-Chemical Calculation of Thermodynamic Parameters and DSC Measurement of Thermostability of Novel Benzofuroxan Derivatives Containing Triazidoisobutyl Fragments. Int. J. Mol. Sci. 2022, 23, 1471. [Google Scholar] [CrossRef]
- Rahman, M.A.; Matin, M.M.; Kumer, A.; Chakma, U.; Rahman, M.R. Modified D-glucofuranoses as new black fungus protease inhibitors: Computational screening, docking, dynamics, and QSAR study. Phy. Chem. Res. 2022, 10, 195–209. [Google Scholar] [CrossRef]
- Munawar, A.; Zaman, F.; Ishaq, M.W.; Hassan, K.A.; Masood, S.; Ali, Z.; et al. Comparative study to characterise the pharmaceutical potential of synthesised snake venom Bradykinin-Potentiating peptides in vivo. Curr. Med. Chem. 2022, 29, 6422–6432. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.; Matin, M.M.; Miah, S.M.R.; Devi, P. Synthesis, antimicrobial, and DFT studies of some benzyl 4-O-acyl-α-L-rhamnopyranosides. Orbital: Electron. J. Chem. 2021, 13, 250–258. [Google Scholar] [CrossRef]
- Sanaullah, A.F.M.; Devi, P.; Hossain, T.; Sultan, S.B.; Badhon, M.M.U.; Hossain, M.E.; Uddin, J.; Patwary, M.A.M.; Kazi, M.; Matin, M.M. Rhamnopyranoside-based fatty acid esters as antimicrobials: Synthesis, spectral characterization, PASS, antimicrobial, and molecular docking studies. Molecules 2023, 28, 986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.X.; Li, W.H.; Zhou, Y.D.; Shen, J.; Wu, Z.R.; Liu, G.X. et al. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inform. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.P. et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Nobmann, P.; Smith, A.; Dunne, J.; Henehan, G.; Bourke, P. The antimicrobial efficacy and structure activity relationship of novel carbohydrate fatty acid derivatives against Listeria spp. and food spoilage microorganisms. Int. J. Food Microb. 2009, 128, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta. 2007, 1770, 467–477. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
| Type of SFAE | SFAEs* | Active against | MIC | Ref. |
|---|---|---|---|---|
| monoester | 6-O-lauroylsucrose | Listeria monocytogenes, Bacillus subtilis | 2.5 mM | [7] |
| Listeria monocytogenes, Staphylococcus aureus | 0.4 mM | [32] | ||
| Streptococcus suis | 0.02 mg/mL | [33] | ||
| 6-O-myristoylsucrose | Bacillus megaterium | 32 ng/mL | [34] | |
| 6-O-caproylsucrose | Bacillus cereus, Bacillus subtilis, Staphylococcus aureus | 2.5 mM | [5] | |
| 6-O-nonanoylsucrose | Candida parapsilosis | 0.313 mg/mL | [19] | |
| 6-O-methacryloylsucrose | Listeria monocytogenes, Micrococcus flavus, Enterobacter cloacae, E. coli | 0.24 μM | [20] | |
| 6-O-nervonoyllactose | Listeria monocytogenes, Escherichia coli, Staphylococcus enteritidis, Enterococcus faecalis, Yersinia enterocolitica, Candida albicans | 0.064 mg/mL | [6] | |
| 6-O-lauroylmaltose | Staphylococcus aureus | 0.25 mg/mL | [35] | |
| Methyl 6-O-lauroyl- α-D-glucopyranoside | Staphylococcus aureus, Escherichia coli, Candida albicans | 0.188 mg/mL | [36] | |
| Methyl 6-O-lauroyl- β-D-glucopyranoside | Staphylococcus aureus | 0.04 mM | [4] | |
| Methyl 6-O-lauroyl-α-D-mannopyranoside | Staphylococcus aureus | 0.04 mM | [24] | |
| oligoester | 6-O-methacryloylsucrose heptaacetate | Aspergillus versicolor, Penicillium funiculosum, Penicillium ochrochloron | 0.28 μM | [20] |
| Octyl 2,4-di-O-decanoyl-3,6- di-O-valeroyl-β-D-glucopyranoside | Escherichia coli, Aspergillus flavus, Aspergillus niger, Candida albicans | 68.4% | [22] | |
| Benzyl 4-O-benzoyl- α-L-rhamnopyranoside | Candida albicans | 65.0% | [21] | |
| Methyl 2,3,4-tri-O-lauroyl-6-O- octanoyl-α-D-mannopyranoside | Aspergillus flavus, Aspergillus niger | 58.2% | This work |
| Entry | Reagent (C7H15COCl) (equivalent) | Solvent | Catalyst | Temperature (°C) | Product | Yield (2)% |
|---|---|---|---|---|---|---|
| 1. | 1.2 | pyridine | - | 0 - 25 | 4 & mixturea | 37 |
| 2. | 0.98 | pyridine | - | 0 - 22 | 4 & mixture | 61 |
| 3. | 0.98 | pyridine | DMAP | 0 - 22 | 4 & mixture | 49 |
| 4. | 1.1 | Pyridine & CHCl3 | - | 0 - 22 | 4 & mixture | 43 |
| 5. | 1.1 | Pyridine & CHCl3 | DMAP | 0 - 22 | 4 & mixtureb | 33 |
| 6 | 1.1 | CHCl3 | DMAP | 0 - 22 | 4 & mixtureb | 39 |
| Drugs | Biological activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Antibacterial | Antifungal | Anticarcinogenic | Antioxidant | |||||
| Pa* | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| 3 | 0.528 | 0.014 | 0.669 | 0.012 | 0.731 | 0.008 | 0.667 | 0.004 |
| 4 | 0.558 | 0.012 | 0.675 | 0.011 | 0.769 | 0.010 | 0.530 | 0.008 |
| 5 | 0.551 | 0.012 | 0.673 | 0.011 | 0.675 | 0.010 | 0.461 | 0.008 |
| 6 | 0.551 | 0.012 | 0.673 | 0.011 | 0.614 | 0.012 | 0.461 | 0.008 |
| 7 | 0.551 | 0.012 | 0.673 | 0.011 | 0.614 | 0.012 | 0.461 | 0.008 |
| 8 | 0.551 | 0.012 | 0.673 | 0.011 | 0.614 | 0.012 | 0.461 | 0.008 |
| 9 | 0.551 | 0.012 | 0.673 | 0.011 | 0.614 | 0.012 | 0.463 | 0.008 |
| 10 | 0.551 | 0.012 | 0.673 | 0.011 | 0.614 | 0.012 | 0.463 | 0.008 |
| Drug | Diameter of zone of inhibition in mm (100 µg dw / disc) | |||
|---|---|---|---|---|
| B. cereus | B. subtilis | E. coli | S. typhi | |
| 1 | NI | NI | NI | NI |
| 2 | 6.17±0.29 | *10.27±0.46 | NI | 7.17±0.29 |
| 3 | 8.00±0.50 | *10.67±0.58 | 6.50±0.40 | NI |
| 4 | *12.33±0.58 | 7.00±0.40 | 7.00±00 | 8.00±0.30 |
| 5 | 6.17±0.29 | 8.00±1.00 | NI | *10.50±0.50 |
| 6 | 6.50±0.50 | NI | 7.00±1 | 8.00±0.35 |
| 7 | 6.50±0.50 | NI | 7.17±0.29 | *10.73±0.46 |
| 8 | 7.00±1.00 | 7.00±0.20 | 7.50±0.5 | 8.00±0.00 |
| Ampicillin** | *10.60±0.53 | *12.67±0.58 | *15.54±0.5 | *13.23±0.41 |
| Drug | % Inhibition of fungal mycelial growth (100 µg dw / mL PDA) | |||
|---|---|---|---|---|
| A. flavus | A. niger | F.equiseti | P. notatum | |
| 1 | NI | NI | NI | NI |
| 2 | NI | NI | *54.57±0.41 | 33.33±0.58 |
| 3 | NI | 40.23±0.25 | NI | NI |
| 4 | NI | 22.22±0.27 | 43.13±0.32 | NI |
| 5 | 22.22±0.26 | NI | *59.37±0.55 | 38.27±0.40 |
| 6 | 9.53±0.45 | NI | 46.42±0.47 | 45.67±0.58 |
| 7 | *58.33±0.58 | *54.17±0.29 | *54.93±0.76 | 28.20±0.26 |
| 8 | NI | 40.53±0.76 | *52.00±0.72 | 38.50±0.50 |
| **Fluconazole | 47.03±0.25 | 37.10±0.17 | *62.00±0.50 | *65.73±0.46 |
| Drugs/Compounds | Lanosterol 14α-demethylase(3LD6) | Fungi (kcal/mol) | ||
|---|---|---|---|---|
| A. flavus(1R51) | A. niger(1KUL) | A. aculeatus(1IA5) | ||
| 1 | -5.3 | -5.4 | -4.6 | -4.3 |
| 2 | -6.7 | -6.3 | -4.7 | -5.2 |
| 3 | -6.4 | -6.3 | -4.8 | -6.7 |
| 4 | -5.8 | -6.1 | -4.4 | -6.2 |
| 5 | -5.7 | -5.6 | -4.3 | -5.8 |
| 6 | -5.3 | -5.7 | -4.3 | -5.4 |
| 7 | -7.7 | -7.1 | -3.8 | -4.1 |
| 8 | -5.0 | -5.1 | -3.1 | -4.9 |
| FCZ* | -6.5 | -7.0 | -6.5 | -5.4 |
| NST | -5.6 | -6.1 | -4.6 | -4.4 |
| PCD | -8.9 | -7.8 | -6.4 | -5.9 |
| CA | -4.9 | -4.7 | -4.2 | -3.9 |
| LA | -5.0 | -4.8 | -4.3 | -4.5 |
| SA | -4.8 | -5.3 | -3.8 | -5.0 |
| Comp. No. | MF* | MW(g/mol) | RB3LYP, EE (Hartree) | ΔH (Hartree) | G(Hartree) | μ (Debye) | WebMO Mechanics | |
|---|---|---|---|---|---|---|---|---|
| TE | VWE | |||||||
| 1 | C7H14O6 | 194.18 | -726.22423 | -725.9849 | -726.0398 | 3.3323 | 62.3786 | 44.0835 |
| 2 | C15H28O7 | 320.38 | -1114.6733 | -1114.2110 | -1114.2941 | 2.9772 | 67.2912 | 43.0505 |
| 3 | C21H34O10 | 446.49 | -1572.4892 | -1571.9035 | -1572.0140 | 8.4446 | 78.3025 | 60.8014 |
| 4 | C30H52O10 | 572.73 | -1926.2212 | -1925.3637 | -1925.5067 | 7.1562 | 91.5670 | 59.2060 |
| 5 | C33H58O10 | 614.81 | -2044.1349 | -2043.1875 | -2043.3400 | 6.5944 | 92.7009 | 64.1183 |
| 6 | C45H82O10 | 783.13 | -2502.8194 | -2501.5084 | -2501.6961 | 6.1728 | 109.2732 | 83.0093 |
| 7 | C51H94O10 | 867.29 | -2737.4306 | -2735.9377 | -2736.1367 | 7.5728 | 94.3915 | 56.8761 |
| 8 | C69H130O10 | 1119.76 | -3441.2472 | -3432.3005 | -3432.5009 | 6.8291 | 127.6629 | 86.6837 |
| Drug | 𝜀HOMO | 𝜀LUMO | Gap | Hardness (η) | Softness (S) |
|---|---|---|---|---|---|
| 1 | -7.014 | -1.288 | 5.726 | 2.863 | 0.349 |
| 2 | -7.048 | -0.307 | 6.641 | 3.371 | 0.297 |
| 3 | -7.215 | -0.771 | 6.444 | 3.222 | 0.310 |
| 4 | -7.299 | -0.752 | 6.547 | 3.274 | 0.305 |
| 5 | -7.292 | -0.681 | 6.611 | 3.306 | 0.302 |
| 6 | -6.819 | -0.313 | 6.506 | 3.253 | 0.307 |
| 7 | -6.731 | -0.577 | 6.154 | 3.077 | 0.325 |
| 8 | -7.296 | -0.663 | 6.633 | 3.317 | 0.301 |
| Compound | MW | HLB | TPSA | MLog P | Aqueous solubility | Lipinski rule | Human intestinal absorption | P-glycoprotein inhibitor | Carcinogen | Acute oral toxicity | Rat acute toxicity LD50 | BBB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| follow | violation | ||||||||||||
| 1 | 194.18 | 10.29 | 99.38 | -2.40 | Soluble | Yes | 0 | -0.8373 | NI 0.9393 | NC 0.9654 | III | 1.1350 | N |
| 2 | 320.38 | 8.98 | 105.45 | -0.21 | Soluble | Yes | 0 | -0.6797 | NI 0.7283 | NC 0.9671 | III | 2.0091 | N |
| 3 | 346.49 | 9.31 | 123.66 | 1.00 | Soluble | Yes | 0 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
| 4 | 572.74 | 7.26 | 123.66 | 2.85 | Soluble | Yes | 1 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
| 5 | 614.82 | 6.76 | 123.66 | 3.41 | Poor | Yes | 1 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
| 6 | 783.14 | 5.31 | 123.66 | 5.49 | Poor | No | 2 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
| 7 | 867.30 | 4.79 | 123.66 | 6.44 | Insoluble | No | 2 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
| 8 | 1119.79 | 3.71 | 123.66 | 9.06 | Insoluble | No | 2 | 0.8974 | I 0.8144 | NC 0.9027 | III | 1.9138 | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





