1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease of the 21s century, with a prevalence rates range between 23% and 32% depending on the geographical region [
1,
2]. NAFLD is seen in 47·3–63·7% of people with type 2 diabetes and up to 80% of people with obesity, because of its close association with the metabolic syndrome (MS) [
3,
4], and with lipid accumulation, inflammation, excessive calorie intake, genetic susceptibility, and insulin resistance [
5]. Despite not being included as one of the criteria for diagnosing MS, NAFLD, the pro-inflammatory state and endothelial dysfunction are known to be associated with the metabolic, physiological and biochemical changes of this syndrome [
6,
7].
The intestinal microbiota establishes a symbiotic relationship with its host, contributing nutrients and energy by metabolizing dietary components in the large intestine, including cholesterol [
8]. Several studies suggest that the intestinal microbiome represents an environmental factor that contributes to the development of NAFLD [
9]. The intestinal microbiota may vary according to the stage of NAFLD, and more studies are needed to confirm the influence of specific bacteria on liver diseases [
10].
Fructooligosaccharides (FOS) are widely used prebiotics, being a source of energy and an essential nutrient for intestinal bacteria, which carry out their fermentation and promote the colonization and activities of beneficial bacteria, improve the metabolism of the intestinal microbiota, improve host immunity and reduce inflammation [
11]. Many probiotics and prebiotics have been linked to maintaining intestinal microbiota homeostasis and reducing
NAFLD-associated dysregulation of hepatic carbohydrate and lipid metabolism. Therefore, microbiota-based treatments are beneficial for the prevention and treatment of NAFLD, however, more studies are needed to understand the mechanisms used by FOSs [
10]
.
To elucidate the potential benefits of FOS in improving gastrointestinal health, systemic inflammation and metabolic parameters, it is necessary to establish an animal experimental protocol that evaluates a dietary intervention with this prebiotic in the long term. It is expected that the provision of high doses of FOS will contribute to the consolidation of benefits, as well as to the investigation of possible harms, in experimental animal models. Although the use of 25% of FOS was verified in the study by Mao et al (2018), this amount is too high if we consider the tolerance limit in humans. The present work invested in methodological differences by analyzing high doses of FOS supplemented in diets with two types of lipid composition and offered in the long term, which is little described in similar studies in the literature. Thus, the effects of 15% FOS supplementation for four months on metabolic parameters and the production of short-chain fatty acids in the feces of C57BL mice fed both a normolipid and hyperlipid diet rich in fiber were evaluated.
2. Materials and Methods
2.1. Animals and Diet
Sixty male C57BL mice weighing 20 g at the beginning of the experiment were obtained from the Central Animal House of the Faculty of Medicine of Ribeirão Preto (FMRP), University of São Paulo (USP), and maintained under controlled conditions of temperature (24 ± 2 °C) and of humidity and on a light (7:00 am–7:00 pm)/dark (7:00 pm–7:00 am) cycle. Water and food were supplied ad libitum. Animals were handled according to Brazilian College of Animal Experimentation recommendations and all procedures were approved by the Ethics Committee of FMRP (protocol no. 10/2016).
The animals underwent a period of adaptation to the environment and diet for 7 days. During this period, experimental diets were gradually introduced. Animals were randomly assigned to six experimental groups of 6 mices each: control (C), normolipid rich in fiber (F), normolipid supplemented with FOS (FOS), high fat (HL), high fat with high fiber (HLF) and high fat with FOS (HLFOS). For FOS supplementation in the diets, the product Orafti® SIPX (2016) from Beneo Animal Nutrition was used, which consists of powdered chicory inulin containing a mixture of oligosaccharides and polysaccharides composed of fructose units. The soybean oil (®Liza) was purchased at the local market. Vitamin, mineral mix, choline, and L-cystine were purchased from Rhoster (Araçoiaba da Serra, Brazil). All diets were based on the AIN-93 and are described in
Table 1 [
12].
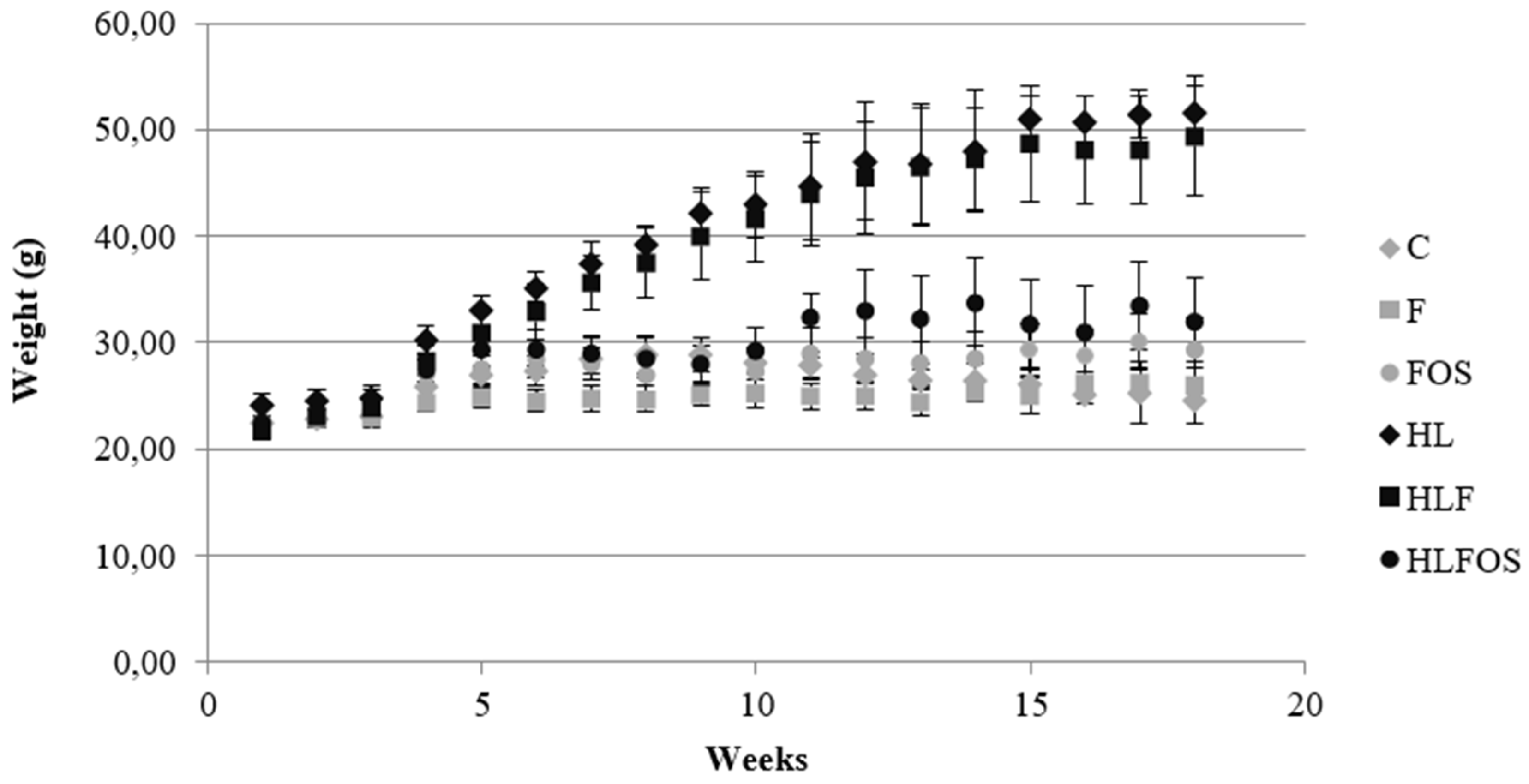
Food intake and weight were determined per cage (4 animals/cage) over a period of 18 weeks and are reported as mean food intake and weight in g/day. Weight was measured weekly in the morning, between 8:00 and 9:00. The food intake was measured twice a week, always leaving sufficient quantity available.
At the end of experiment, animals were starved for 12 hours and then anesthetized with ketamine and xylazine diluted in saline at the proportion of 1 : 1:2 ml. It was administrated dose applications of 10 µl/g weight each. Blood was immediately collected by cardiac puncture, left to rest at room temperature for 30 minutes, and centrifuged at 3500 rpm at 4°C for serum separation afterward. Serum was stored at −80°C for later analysis. Liver and colon were weighed and frozen in aluminum parts for further analysis. The animals’ feces were extracted from the final portion of the intestine after euthanasia and stored in eppendorf tubes and frozen at -20°C.
2.2. Histopathological Analysis of the Intestine
Colon fragments were sectioned in an annular cross-section (proximal, middle and distal thirds), and the material was immersed in buffered formalin. The fragments were analyzed together, choosing well-oriented villi, with apparent and continuous basal, medial and apical portions [
13]. For this analysis, a conventional light microscope with 20x magnification was used. Subsequently, the images were analyzed using the Image J software to quantify the intestinal muscle thickness and the total diameter of the intestinal lumen.
2.3. Histopathological Analysis of the Liver
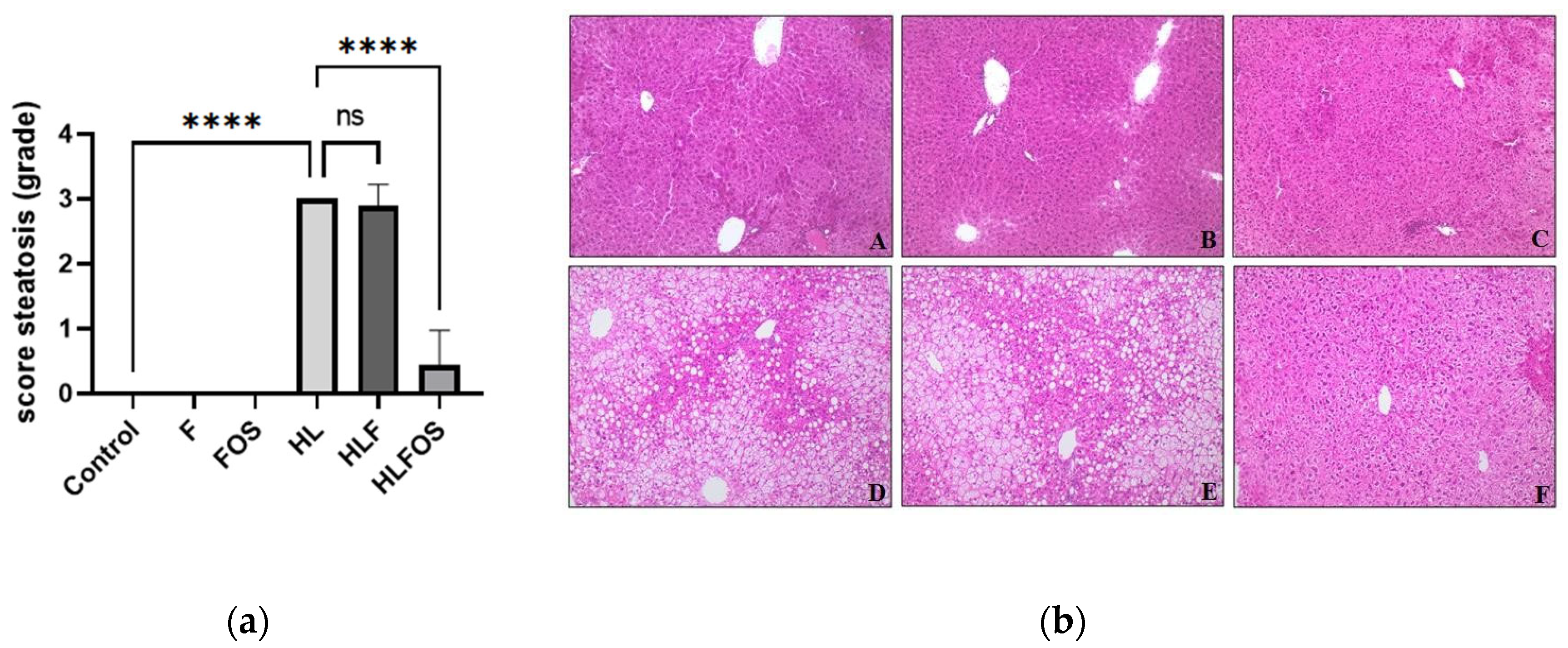
Liver fragments were fixed in 10% buffered formalin for 24 hours and embedded in paraffin. Histological preparations containing 4 µm thick sections were stained with hematoxylin and eosin (H&E). Hepatic steatosis was evaluated semi-quantitatively and classified in crosses, according to Oh et al. (1998) [
14] with some modifications. The steatosis score is associated with the morphological location of the liver (zone 1, 2 and 3), with crosses being assigned according to the degree of steatosis: without steatosis (0Y); 1-25% in zone 3 only (1Y); 25-50% only in zone 3 (2Y); 50-75% encompassing zones 2 and 3 (3Y) and 75-100% involving zones 1, 2 and 3 (4Y). In addition, the presence of inflammatory infiltrate (L: mild; M: moderate and I: intense) and Mallory's bodies (A: absent; FC: few corpuscles and MC: many corpuscles) were also evaluated. For these analyses, a conventional light microscope with magnifications of 20 and 40 times was used.
2.4. Biochemical and Hepatic Analyses
Protein determination in liver and serum was performed using a commercial kit using the Biuret method (Labtest Diagnóstica S.A., Brazil). Total cholesterol (TC) and serum triacylglycerides (TAG), as well as in the liver, were determined using commercial kits from Labtest (Labtest Diagnóstica S.A., Brazil). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) transaminases were quantitatively determined using a commercial Labtest kit (Labtest Diagnóstica S.A., Brazil) in a serum sample with continuous UV kinetic reaction. For the determination and quantification of total fat in the liver, the method proposed by Bligh and Dyer (1959) [
15] was used.
2.5. Analysis of Glycemia
At the end of experiment, animals glycemia was determined using obtained samples from animal's tail and the freestyle lite Abbot®A glucometer.
2.6. Analysis of Lipid Peroxidation and Antioxidant Parameters
The analysis of the Total Antioxidant Capacity (TAC) was performed based on the methodology described by Erel (2004) [
16]. The Gerard-Monnier et al. method [
17] with some modifications, as it is thoroughly described in S1 text section, was used to determine the hepatic malondialdehyde (MDA). Vitamin A (α-tocopherol) was determined by adapted Arnaud et al. method [
18]. Complete methodology is detailed and described in S1 text section.
2.7. Determination of Short Chain Fatty Acids in Feces
The determination of short-chain fatty acids in feces was performed based on the methodology of Zhao, Nyman and Jonsson (2006) [
19] with adaptations. Complete methodology is detailed and described in S1 text section.
2.8. Statistical Analysis
One-way analysis of variance (ANOVA) was applied to the data of the various groups, with the Tukey post-test, using the GraphPad Prism software, version 5.00 for Windows (GraphPad Software, San Diego, CA, USA), with the level of significance set up at p < 0.05. Data are reported as mean ± standard deviation.
4. Discussion
Although NAFLD, which has no specific medical treatment, is not part of the diagnostic criteria for MS, its presence increases the risk of a cardiovascular event in these individuals [
20,
21]. Many studies have suggested that NAFLD progression can be inhibited with the use of prebiotics, probiotics and symbiotics [
22,
23,
24], but the mechanisms of the effects of FOS on body composition, metabolic parameters, production of short-chain fatty acids in feces and NAFLD need further understanding. The present study evaluated the long-term effects of 15% FOS supplementation compared to 15% microcrystalline cellulose in experimental animal models with obesity and metabolic syndrome induced by a high-fat diet, as well as in animals receiving a normolipid diet. The findings indicate that FOSs promoted a reduction in body weight, retroperitoneal adipose tissue and serum cholesterol and prevented NAFLD in C57BL mice with obesity and metabolic syndrome induced by a high-fat diet.
Despite the fact that supplementation with FOS did not interfere with the monthly caloric intake regardless of the lipid content of the diet, from the fifth experimental week onwards, a lower weight of the animals that received a high-fat diet with FOS (HLFOS) was verified, and at the end of the eighteen weeks the weight of the HLFOS group was equal to that of the groups that received a normolipid diet, which was not observed with the diet enriched with microcrystalline cellulose. In addition, there was a reduction in the weight of the retroperitoneal adipose tissue in the HLFOS group compared to the other groups that received a high-fat diet. Such results are similar to the study by Mao et al.[
25], who verified a reduction in body weight in mice receiving a normolipid diet rich in FOS (25%), but the same was not observed in animals that received only 5% of this prebiotic.
The benefits of FOS in reducing weight gain may be due to the production of SCFA from intestinal bacteria, as Lu et al (2016)[
26] found that the long-term offer of a high-fat diet supplemented with 5% of the main SCFA, alone or mixed, stimulated beige adipogenesis, increasing fat oxidation and energy expenditure mediated by GPR43 and GPR41 activation. In addition, SCFA are also related to increased plasma concentration of PYY (Peptide YY), which is an intestinal peptide hormone secreted in the postprandial period and which decreases intestinal motility and exerts anorexigenic effects in eutrophic and with obesity individuals. [
27,
28]. The divergence of findings regarding the effects of FOS on food intake and body composition can be explained by the different methodologies found, whether in relation to the duration of the experiment, the type of diet or the amount of FOS.
Animals that received a high-fat diet had higher liver weight, presence of hepatic steatosis and increased ALT, so that long-term FOS supply prevented the development of NAFLD and reduced liver weight and ALT levels, while the same was not observed in animals that received a high-fat diet with high doses of cellulose. These results were consistent with those found by Matsumoto et al. (2017) [
29] in C57BL mice with NAFLD induced by a choline-deficient diet, in which the supply of 5% FOS for 3 weeks improved liver parameters with a reduction in ALT levels, as well as in the degree of steatosis, in the presence inflammation and hepatic ballooning. Prevention of NAFLD was associated with decreased intestinal permeability and SCFA production by intestinal bacteria.
In our study, it was expected that the offer of FOS would improve fasting glycemia and serum triglycerides in experimental animal models of obesity and MS, but there was no statistically significant difference in these analyses, with only a reduction in serum cholesterol being observed in the HLFOS group compared to to the HL and the HLF. These findings were consistent with Mao et al. (2018) [
25], whose supply of FOS in a normolipid diet did not interfere with the analyzed serum biochemical parameters, even with the use of high amounts of this prebiotic.
Considering that the oxidative stress promoted by the increase of fatty acids in the liver is an important factor in the pathogenesis of non-alcoholic steatohepatitis resulting from NASH, it is necessary to develop therapeutic targets that can act in this way [
30]. The present study did not find alteration in the total antioxidant capacity between the groups, and the supply of FOS did not interfere in the dosages of hepatic MDA and retinol, regardless of the lipid content of the diet. Based on these findings, it cannot be concluded that the supply of 15% FOS, in the long term, improved the antioxidant system in experimental animal models of obesity and MS induced by a high-fat diet.
Histological analysis of the colon showed that the high-fat diet increased the diameter of the intestinal lumen and reduced the enteric muscle thickness, whereas the supplementation with fructooligosaccharides was able to reverse these changes. Although the supply of FOS in the normolipid diet did not change the diameter of the intestinal lumen compared to group C, a reduction in the enteric musculature was observed. Wedel et al (2006)[
31] demonstrated that severe colorectal motility disorders, such as idiopathic megacolon and slow-transit constipation, are associated with deficient expression of proteins linked to intestinal smooth muscle contraction. In a review study, it was found that both an increase and a decrease in smooth muscle contractility was present in intestinal inflammation, and the functional deficiency of smooth muscle cells can occur due to changes in the activities of muscarinic receptors and ion channels [
32]. Thus, the evidence that fermentable dietary fibers influence enteric muscle thickness and intestinal lumen diameter suggests that changes in gastrointestinal health do not only involve the modulation of intestinal microbiota and mucosal permeability.
The prebiotic effect of FOS is responsible for the modulation of the intestinal microbiota and the modifications in SCFA, and currently the modulation of the intestinal microbiota appears to be a promising direction for the treatment of NASH[
33,
34,
35]. The production of SCFA by intestinal bacteria plays an important role in liver health, as on the one hand it contributes to increased intestinal absorption and, consequently, caloric intake, and on the other hand it suppresses colon inflammation via activation of GPR43, protecting the liver against toxic components coming from the portal vein [
36,
37]. Furthermore, the benefits of FOS in non-alcoholic hepatic steatosis would be related to its prebiotic properties, which can be confirmed with the results of the present study, since NASH induced by the high-fat diet was only prevented by offering FOS, while that supplementation with microcrystalline cellulose, which does not present prebiotic characteristics in monogastric animals, did not have the same effects.
In the present study, the supply of high doses of FOS showed significant benefits in experimental animal models of obesity and MS induced by a high-fat diet, but the same was not observed in the groups that received a normolipid diet with FOS. Furthermore, no signs of liver toxicity or worsening of metabolic parameters resulting from the use of high doses of fructooligosaccharides were found, indicating that the amount used in the long term does not seem to trigger possible harm to the health of the animal models in this experiment. Thus, the present study contributes to the development of new research with FOS, either to explore the mechanisms by which this prebiotic exerts different effects according to the lipid content of the diet, or to invest in experimental protocols that define therapeutic amounts of fructooligosaccharides in prevention and treatment of obesity, MS and NAFLD.