Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
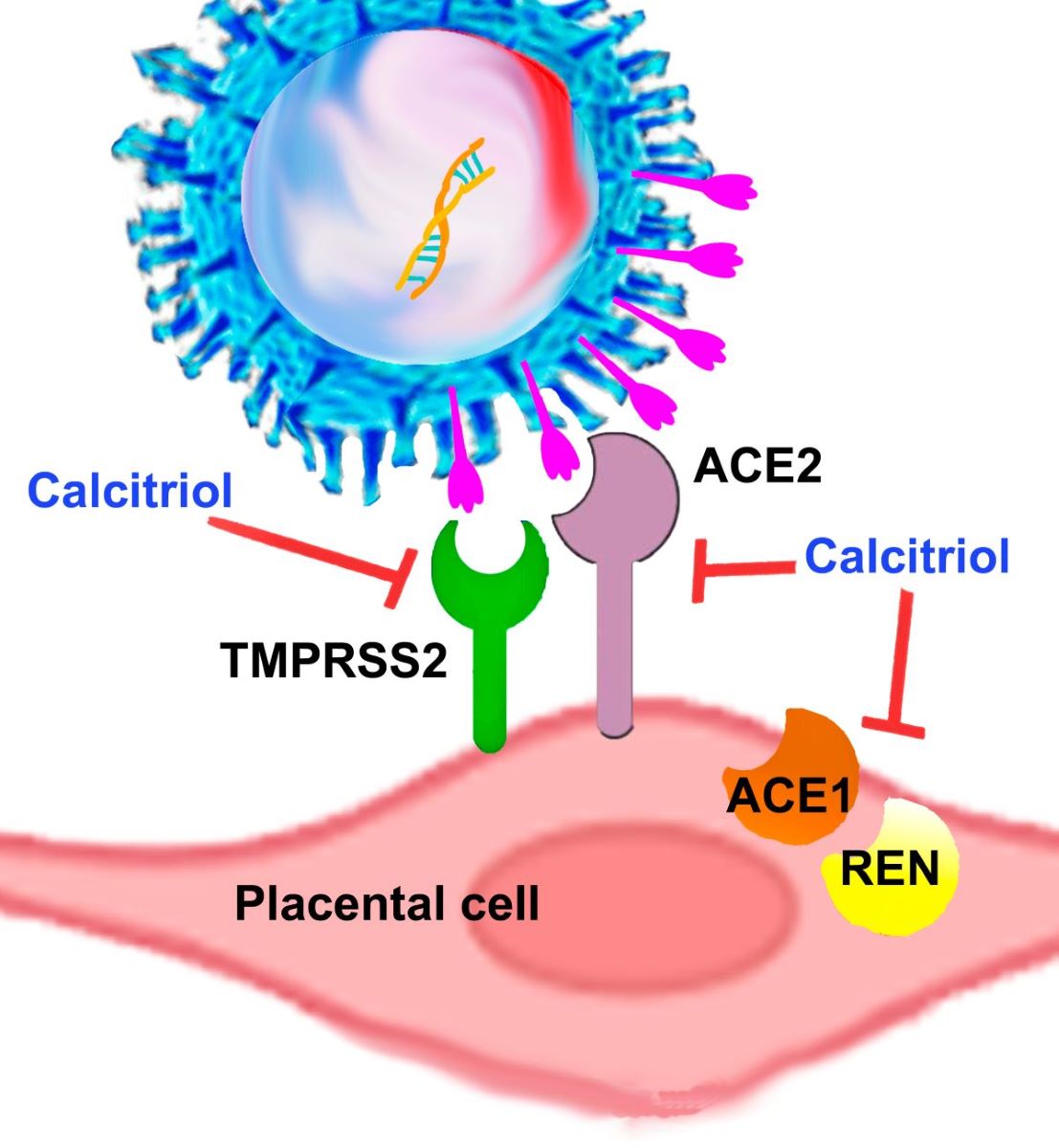
Keywords:
1. Introduction
2. Results
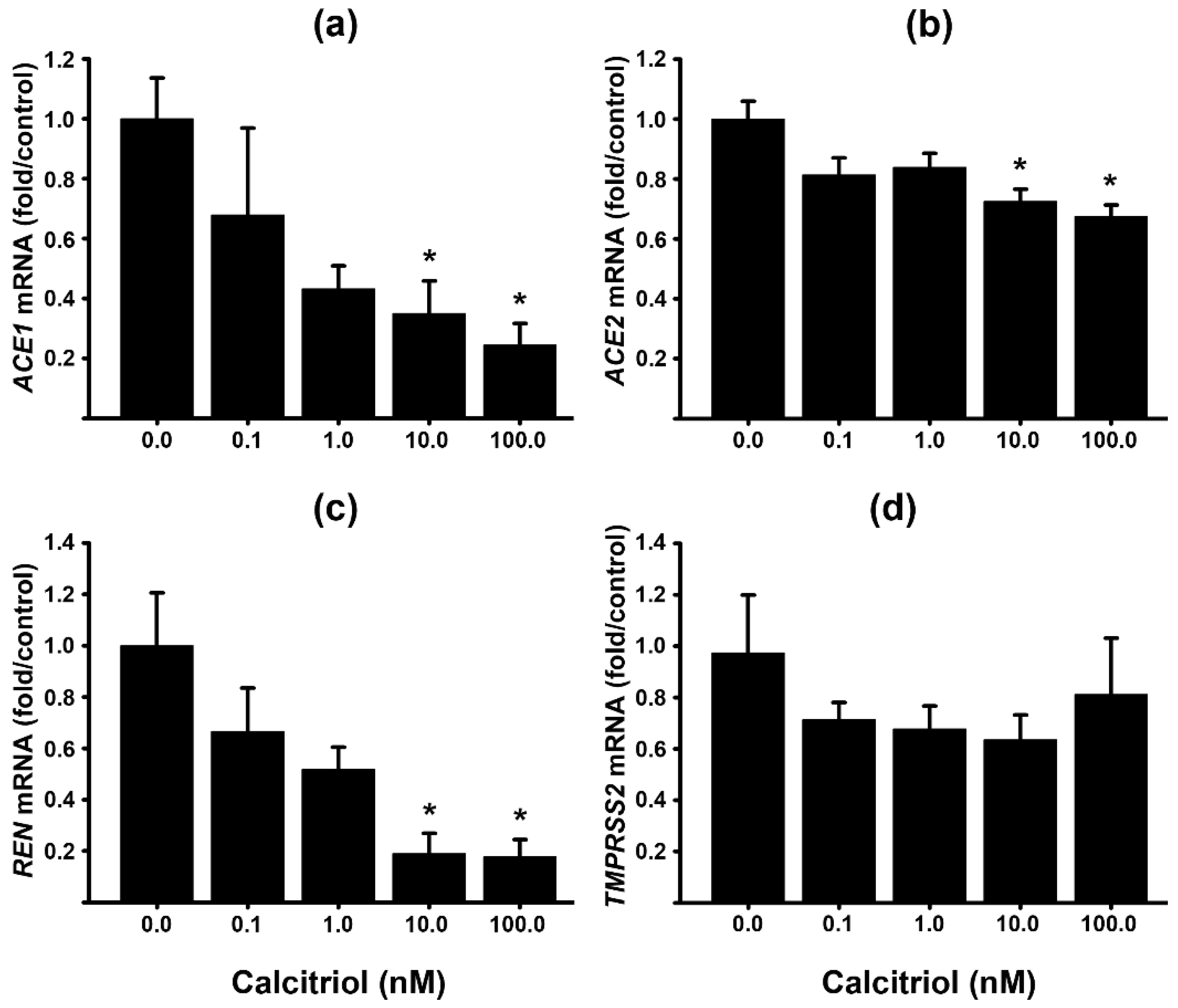
2.1. Calcitriol and Calcidiol Downregulate ACE1, ACE2, TMPRSS2 and Renin Gene Expression in Cultured Syncytiotrophoblast from Human Placentas
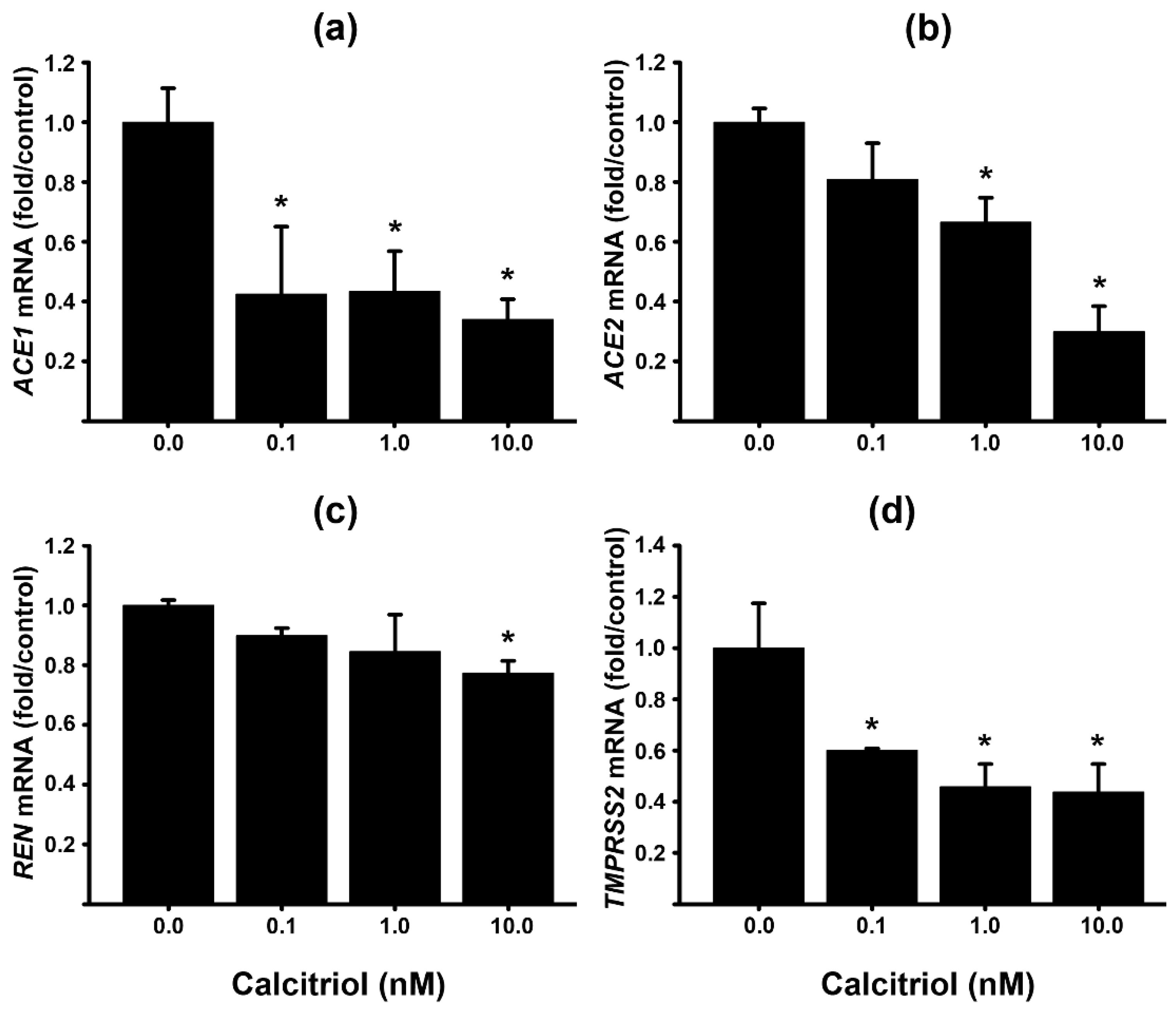
2.2. Calcitriol Inhibits RAS Components in the Placental Cell Line HTR-8/SVneo
2.3. Syncytiotrophoblast Cells Exibit Greater Expression of RAS Components than HTR8 EVT Cells, While ACE1/ACE2 Ratio is Higher in the Latter
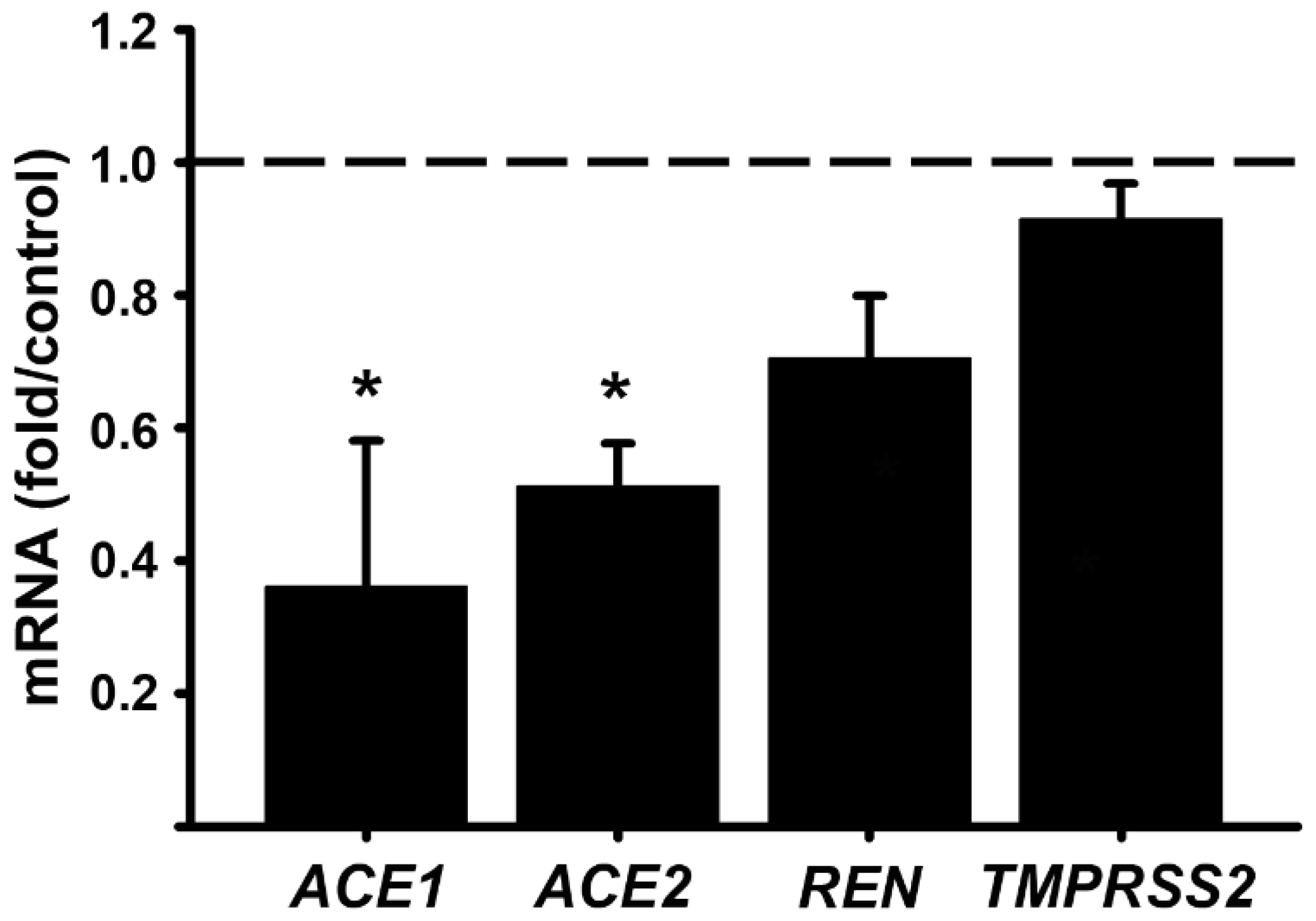
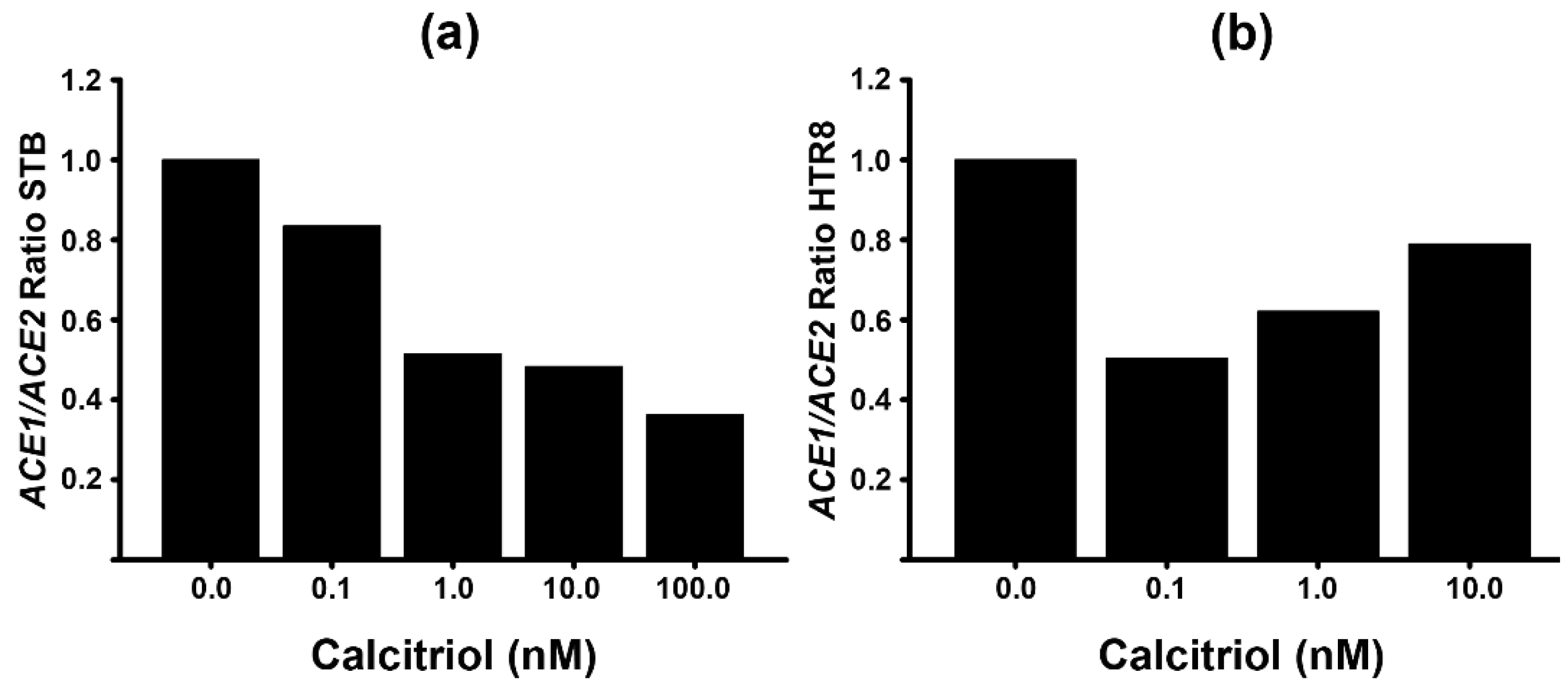
2.4. Calcitriol Reduces ACE1/ACE2 Ratio
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures
4.3. Reverse Transcription and Real-Time PCR Amplifications (qPCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Noyola-Martinez, N.; Barrera, D.; Hernandez, G.; Avila, E.; Halhali, A.; Larrea, F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol 2009, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Kaplan, A.T.; Lagishetty, V.; Ouyang, Y.B.; Ouyang, Y.; Simmons, C.F.; Equils, O.; Hewison, M. Vitamin D and the regulation of placental inflammation. J Immunol 2011, 186, 5968–5974. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J Investig Med 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Kaplan, A.T.; Low, J.; Nguyen, L.; Liu, G.Y.; Equils, O.; Hewison, M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 2009, 80, 398–406. [Google Scholar] [CrossRef]
- Barrera, D.; Avila, E.; Hernandez, G.; Halhali, A.; Biruete, B.; Larrea, F.; Diaz, L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol 2007, 103, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.; Avila, E.; Hernandez, G.; Mendez, I.; Gonzalez, L.; Halhali, A.; Larrea, F.; Morales, A.; Diaz, L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol 2008, 6, 3. [Google Scholar] [CrossRef]
- Stephanou, A.; Ross, R.; Handwerger, S. Regulation of human placental lactogen expression by 1,25-dihydroxyvitamin D3. Endocrinology 1994, 135, 2651–2656. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Li, Y.C. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 2003, 88, 327–331. [Google Scholar] [CrossRef]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 2004, 89-90, 387–392. [Google Scholar] [CrossRef]
- Forman, J.P.; Scott, J.B.; Ng, K.; Drake, B.F.; Suarez, E.G.; Hayden, D.L.; Bennett, G.G.; Chandler, P.D.; Hollis, B.W.; Emmons, K.M.; et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 2013, 61, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kristal-Boneh, E.; Froom, P.; Harari, G.; Ribak, J. Association of calcitriol and blood pressure in normotensive men. Hypertension 1997, 30, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Hanni, A.; Lithell, H.; Hvarfner, A.; Sorensen, O.H.; Ljunghall, S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens 1995, 8, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Lithell, H.; Skarfors, E.; Wide, L.; Ljunghall, S. Reduction of blood pressure by treatment with alphacalcidol. A double-blind, placebo-controlled study in subjects with impaired glucose tolerance. Acta Med Scand 1988, 223, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wengle, B.; Wide, L.; Ljunghall, S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am J Hypertens 1989, 2, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 2001, 86, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Olivares-Huerta, A.; Garcia-Quiroz, J.; Avila, E.; Halhali, A.; Quesada-Reyna, B.; Larrea, F.; Zaga-Clavellina, V.; Diaz, L. Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Grover, S.; Brandt, J.S.; Reddy, U.M.; Ananth, C.V. Chronic hypertension, perinatal mortality and the impact of preterm delivery: a population-based study. BJOG 2022, 129, 572–579. [Google Scholar] [CrossRef]
- Tamanna, S.; Lumbers, E.R.; Morosin, S.K.; Delforce, S.J.; Pringle, K.G. ACE2: a key modulator of the renin-angiotensin system and pregnancy. Am J Physiol Regul Integr Comp Physiol 2021, 321, R833–R843. [Google Scholar] [CrossRef]
- Marques, F.Z.; Pringle, K.G.; Conquest, A.; Hirst, J.J.; Markus, M.A.; Sarris, M.; Zakar, T.; Morris, B.J.; Lumbers, E.R. Molecular characterization of renin-angiotensin system components in human intrauterine tissues and fetal membranes from vaginal delivery and cesarean section. Placenta 2011, 32, 214–221. [Google Scholar] [CrossRef]
- Pringle, K.G.; Tadros, M.A.; Callister, R.J.; Lumbers, E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J Clin Invest 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Molina, R.L.; Tsai, T.C.; Dai, D.; Soto, M.; Rosenthal, N.; Orav, E.J.; Figueroa, J.F. Comparison of Pregnancy and Birth Outcomes Before vs During the COVID-19 Pandemic. JAMA Netw Open 2022, 5, e2226531. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef]
- Moukayed, M. A Narrative Review on the Potential Role of Vitamin D(3) in the Prevention, Protection, and Disease Mitigation of Acute and Long COVID-19. Curr Nutr Rep 2023, 12, 215–223. [Google Scholar] [CrossRef]
- Ashique, S.; Gupta, K.; Gupta, G.; Mishra, N.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Dureja, H.; Zacconi, F.; Oliver, B.G.; et al. Vitamin D-A prominent immunomodulator to prevent COVID-19 infection. Int J Rheum Dis 2023, 26, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Bin-Jumah, M.N.; Nadeem, M.S.; Kazmi, I. Vitamin D attenuates COVID-19 complications via modulation of proinflammatory cytokines, antiviral proteins, and autophagy. Expert Rev Anti Infect Ther 2022, 20, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Vanegas-Cedillo, P.E.; Bello-Chavolla, O.Y.; Ramirez-Pedraza, N.; Rodriguez Encinas, B.; Perez Carrion, C.I.; Jasso-Avila, M.I.; Valladares-Garcia, J.C.; Hernandez-Juarez, D.; Vargas-Vazquez, A.; Antonio-Villa, N.E.; et al. Serum Vitamin D Levels Are Associated With Increased COVID-19 Severity and Mortality Independent of Whole-Body and Visceral Adiposity. Front Nutr 2022, 9, 813485. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, J.F.; Urcuqui-Inchima, S. Vitamin D Supplementation: A Potential Approach for Coronavirus/COVID-19 Therapeutics? Front Immunol 2020, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Kliman, H.J.; Nestler, J.E.; Sermasi, E.; Sanger, J.M.; Strauss, J.F., 3rd. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 1986, 118, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2012, 303, E928–935. [Google Scholar] [CrossRef]
- Diaz, L.; Sanchez, I.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J Clin Endocrinol Metab 2000, 85, 2543–2549. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Garcia-Quiroz, J.; Avila, E.; Caldino-Soto, F.; Halhali, A.; Larrea, F.; Diaz, L. Lipopolysaccharide and cAMP modify placental calcitriol biosynthesis reducing antimicrobial peptides gene expression. Am J Reprod Immunol 2018, 79, e12841. [Google Scholar] [CrossRef]
- Lye, P.; Dunk, C.E.; Zhang, J.; Wei, Y.; Nakpu, J.; Hamada, H.; Imperio, G.E.; Bloise, E.; Matthews, S.G.; Lye, S.J. ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth. Cells 2021, 10. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. ACE/ACE2 Ratio: A Key Also in 2019 Coronavirus Disease (COVID-19)? Front Med (Lausanne) 2020, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.; Lonati, E.; Russo, S.; Cazzaniga, E.; Bulbarelli, A.; Palestini, P. Effects of PM2.5 Exposure on the ACE/ACE2 Pathway: Possible Implication in COVID-19 Pandemic. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Zhang, J.; Nakpu, J.; Hamada, H.; Dunk, C.E.; Li, S.; Imperio, G.E.; Nadeem, L.; Kibschull, M.; Lye, P.; et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol 2021, 224, 298 e291–298 e298. [Google Scholar] [CrossRef]
- Valdespino-Vazquez, M.Y.; Helguera-Repetto, C.A.; Leon-Juarez, M.; Villavicencio-Carrisoza, O.; Flores-Pliego, A.; Moreno-Verduzco, E.R.; Diaz-Perez, D.L.; Villegas-Mota, I.; Carrasco-Ramirez, E.; Lopez-Martinez, I.E.; et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J Med Virol 2021, 93, 4480–4487. [Google Scholar] [CrossRef] [PubMed]
- Patane, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM 2020, 2, 100145. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Ye, Z.W.; Yuan, S.; Li, Z.; Zhang, W.; Ong, C.P.; Tang, K.; Ka Ki Tam, T.T.; Guo, J.; Xuan, Y.; et al. Human early syncytiotrophoblasts are highly susceptible to SARS-CoV-2 infection. Cell Rep Med 2022, 3, 100849. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Neil, J.A.; Tan, J.P.; Rudraraju, R.; Mohenska, M.; Sun, Y.B.Y.; Walters, E.; Bediaga, N.G.; Sun, G.; Zhou, Y.; et al. A placental model of SARS-CoV-2 infection reveals ACE2-dependent susceptibility and differentiation impairment in syncytiotrophoblasts. Nat Cell Biol 2023, 25, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, G.V. Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics-Guided Tracing of SARS-CoV-2 Targets in Human Cells. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol 2019, 93. [Google Scholar] [CrossRef]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 1999, 59, 4180–4184. [Google Scholar]
- Shen, L.W.; Mao, H.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef]
- Abate, B.B.; Kassie, A.M.; Kassaw, M.W.; Aragie, T.G.; Masresha, S.A. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open 2020, 10, e040129. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin Rev Allergy Immunol 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Shook, L.L.; Bordt, E.A.; Meinsohn, M.C.; Pepin, D.; De Guzman, R.M.; Brigida, S.; Yockey, L.J.; James, K.E.; Sullivan, M.W.; Bebell, L.M.; et al. Placental Expression of ACE2 and TMPRSS2 in Maternal Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Are Placental Defenses Mediated by Fetal Sex? J Infect Dis 2021, 224, S647–S659. [Google Scholar] [CrossRef]
- Song, Y.; Qayyum, S.; Greer, R.A.; Slominski, R.M.; Raman, C.; Slominski, A.T.; Song, Y. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study. J Biomol Struct Dyn 2022, 40, 11594–11610. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Slominski, R.M.; Raman, C.; Slominski, A.T. Novel CYP11A1-Derived Vitamin D and Lumisterol Biometabolites for the Management of COVID-19. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, D.K.; Colenso, C.K.; Toelzer, C.; Gupta, K.; Sessions, R.B.; Davidson, A.D.; Berger, I.; Schaffitzel, C.; Spencer, J.; Mulholland, A.J. Molecular Simulations suggest Vitamins, Retinoids and Steroids as Ligands of the Free Fatty Acid Pocket of the SARS-CoV-2 Spike Protein*. Angew Chem Int Ed Engl 2021, 60, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Mohammad, T.; Slominski, R.M.; Hassan, M.I.; Tuckey, R.C.; Raman, C.; Slominski, A.T. Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes. Am J Physiol Endocrinol Metab 2021, 321, E246–E251. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Ishida, J.; Sugiyama, F.; Horiguchi, H.; Murakami, K.; Fukamizu, A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 1996, 274, 995–998. [Google Scholar] [CrossRef]
- Tamara Siblini, M.B., Sanjana Kulkarni, Muhammad Aslam. Coronavirus Disease 2019 (COVID-19) and Hypertensive Disorders of Pregnancy: Is the Placenta the Problem? 2023. [CrossRef]
- Gomez, J.; Albaiceta, G.M.; Garcia-Clemente, M.; Lopez-Larrea, C.; Amado-Rodriguez, L.; Lopez-Alonso, I.; Hermida, T.; Enriquez, A.I.; Herrero, P.; Melon, S.; et al. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene 2020, 762, 145102. [Google Scholar] [CrossRef]
- Granger, J.P.; Alexander, B.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens 2001, 14, 178S–185S. [Google Scholar] [CrossRef]
- Alexander, B.T.; Cockrell, K.L.; Massey, M.B.; Bennett, W.A.; Granger, J.P. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 2002, 15, 170–175. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.J.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic Histiocytic Intervillositis With Trophoblast Necrosis Is a Risk Factor Associated With Placental Infection From Coronavirus Disease 2019 (COVID-19) and Intrauterine Maternal-Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Live-Born and Stillborn Infants. Arch Pathol Lab Med 2021, 145, 517–528. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zheng, L.B.; Wang, Y.L.; Li, X.; Zhang, X.Q.; Cao, B. [Regulatory Effect of Vitamin D on Renin Expression at Maternal-Fetal Interface]. Sichuan Da Xue Xue Bao Yi Xue Ban 2022, 53, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Rysz, S.; Al-Saadi, J.; Sjostrom, A.; Farm, M.; Campoccia Jalde, F.; Platten, M.; Eriksson, H.; Klein, M.; Vargas-Paris, R.; Nyren, S.; et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat Commun 2021, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br J Pharmacol 2020, 177, 4825–4844. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Chang, H.S.; Yang, Y.P.; Lin, T.W.; Lai, W.Y.; Lin, Y.Y.; Chang, C.C. The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J Chin Med Assoc 2021, 84, 821–826. [Google Scholar] [CrossRef]
| Gene | STB | HTR8 | P |
|---|---|---|---|
| ACE1 | 2.7 x 10−6 ± 7.2 x 10−7 | 1.7 x 10−6 ± 3.4 x 10−7 | 0.58 |
| ACE2 | 2.9 x 10−2 ± 4.6 x 10−3 | 2.3 x 10−5 ± 3.8 x 10−6 | <0.001 |
| TMPRSS2 | 1.8 x 10−2 ± 8.8 x 10−3 | 1.8 x 10−4 ± 6.5 x 10−5 | <0.001 |
| REN | 3.2 x 10−2 ± 5.3 x 10−3 | 8.7 x 10−5 ± 3.3 x 10−5 | <0.001 |
| Gene | Upper Primer | Lower Primer | Probe Number |
|---|---|---|---|
| ACE1 | ctgctcatctgctgggagac | ttgtctgggaaaggcaccac | 33 |
| ACE2 | ttctgtcacccgattttcaa | tcccaacaatcgtgagtgc | 4 |
| TMPRSS2 | acctgatcacaccagccatg | tcaccctggcaagaatcgac | 4 |
| GAPDH | agccacatcgctgagacac | gcccaatacgaccaaatcc | 60 |
| REN | tacctttggtctcccgacag | ttgagggcattctcttgagg | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





