Submitted:
07 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study group
Definitions
Laboratory markers
Echocardiographic findings
Statistical Analysis
Approval of the Bioethics Committee
Results
Discussion
Limitations
Conclusion
Author Contributions
Funding
Data availability
Conflicts of interest
References
- Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, Olson E, Perry JD, Prendergast BD, Spry MJ, Steeds RP, Tayebjee MH, Watkin R British Society for Antimicrobial Chemotherapy; British Heart Rhythm Society; British Cardiovascular Society; British Heart Valve Society; British Society for Echocardiography. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Rep\ort of a joint Working Party project on behalf of the BritishSociety for Antimicrobial Chemotherapy (BSAC, host organization), BritishHeart Rhythm Society (BHRS), British Cardiovascular Society (BCS), BritishHeart Valve Society (BHVS) and British Society for Echocardiography (BSE).J AntimicrobChemother. 2015; 70: 325-359.
- Polewczyk A, Jacheć W, Polewczyk AM, Tomasik A, Janion M, Kutarski A. Infectious complications in patients with cardiac implantable electronic devices: risk factors, prevention, and prognosis.Pol Arch Intern Med. 2017; 29;127:597-607.
- Uslan DZ, Sohail MR, St Sauver JL, Friedman PA, Hayes DL, Stoner SM, Wilson WR, Steckelberg JM, Baddour LM. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
- Polewczyk A, Janion M, Podlaski R, Kutarski A. Clinical manifestations of lead-dependent infective endocarditis: analysis of 414 cases Eur J Clin Microbiol Infect Dis 2014; 33:1601–1608.
- Wyllie D. H., Bowler I. C., Peto T. E. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. Journal of Clinical Pathology. 2004;57:950–955.
- Meshaal MS, Nagi A, Eldamaty A, Elnaggar W, Gaber M, Rizk H. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as independent predictors of outcome in infective endocarditis (IE). Egypt Heart J. 2019; 18;71:13.
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo JC, Epstein LM, Sághy L, Snygg-Martin U, Starck C, Tascini C, Strathmore N. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) European Heart Journal (2020) 41, 2012–2032.
- Nowosielecka D, Jacheć W, Polewczyk A, Tułecki Ł, Kleinrok A, Kutarski A. The role of transesophageal echocardiography in predicting technical problems and complications of transvenous lead extractions procedures. Clin Cardiol. 2021; 44:1233-1242.
- Neul-Bom Y, Choonhee S, Soo-Jung U. Role of the Neutrophil-Lymphocyte Count Ratio in the Differential Diagnosis between Pulmonary Tuberculosis and Bacterial Community-Acquired Pneumonia. Ann Lab Med 2013;33:105-110.
- Ishizuka M, Shimizu T, Kubota K. Neutrophil-to-Lymphocyte Ratio Has a Close Association With Gangrenous Appendicitis in Patients Undergoing Appendectomy. Int Surg 2012;97:299–304.
- Kahramanca S, Ozgehan G, Seker D, Gökce EI, Seker G, Tunç G, Küçükpınar T, Kargıcı H. Neutrophil-to-lymphocyte ratio as a predictor of acute appendicitis. Ulus Travma Acil CerrahiDerg 2014;20:19-22.
- Kekilli M, Tanoglu A, Sakin YS, Kurt M, Ocal S, Bagci S. Is the neutrophil to lymphocyte ratio associated with liver fibrosis in patients with chronic hepatitis B? World J Gastroenterol 2015; 14; 21: 5575-558.
- de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC.Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192.
- Gharebaghi N, ValizadeHasanloei MA, MedizadehKhalifani A, Pakzad S, Lahooti D. Neutrophil-to-lymphocyte ratio in patients with gram-negative sepsis admitted to intensive care unit. AnaesthesiolIntensiveTher. 2019;51:11-16.
- Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017 ;45:299-307.
- Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. ExpertRevCardiovascTher. 2016;14:573-7.
- Hong D, Choi KH, Song YB, Lee JM, Park TK, Yang JH, Hahn JY, Choi JH, Choi SH, Kim SM, Choe Y, Kim EK, Chang SA, Lee SC, Oh JK, Gwon HC. Prognostic implications of post-percutaneous coronary intervention neutrophil-to-lymphocyte ratio on infarct size and clinical outcomes in patients with acute myocardial infarction. Sci Rep. 2019; 4;9:9646.
- Lo R, D’AncaM, Cohen T, Kerwin T. Incidence and prognosis of pacemaker lead-associated masses: a study of 1,569 transesophageal echocardiograms. J Invasive Cardiol 2006; 18:599–601.
- Downey BC, Juselius WE, Pandian NG, et al. Incidence and significance of pacemaker and implantable cardioverter-defibrillator lead masses discovered during transesophageal echocardiography. Pacing Clin Electrophysiol 2011; 34:679–683.
- Dundar C, Tigen K, Tanalp C, et al. The prevalence of echocardiographic accretions on the leads of patients with permanent pacemakers. J Am Soc Echocardiogr 2011; 24:803–807.
- Golzio PG, Errigo D, Peyracchia M, Gallo E, Frea S, Castagno D, Budano C, Giustetto C, Rinaldi M. Prevalence and prognosis of lead masses in patients with cardiac implantable electronic devices without infection. J Cardiovasc Med (Hagerstown). 2019;20:372-378.
- Kołodzińska A, Kutarski A, Koperski Ł, Grabowski M, Małecka B, Opolski G. Differences in encapsulating lead tissue in patients who underwent transvenous lead removal. Europace 2012;14 :994-1001.
- Kolodzińska K, Kutarski A, Grabowski M, Jarzyna I, Małecka B, Opolski G. Abrasions of the outer silicone insulation of endocardial leads in their intracardiac part: a new mechanism of lead-dependent endocarditis. Europace.2012;14:903-10.
- Novak M, Dvorak P, Kamaryt P, Slana B, Lipoldova J.Autopsy and clinical context in deceased patients with implanted pacemakers and defibrillators: intracardiac findings near their leads and electrodes. Europace. 2009;11:1510-6.
- Rodríguez-Alfonso B, Mitjavila Casanovas M, Castro Urda V, Cobo Marcos M, Sánchez Romero I, Ramos-Martínez A. PET/CT with 18 F-FDG in suspected intracardiac device-related infections: analysis of performance and diagnostic usefulness. Rev Esp Cardiol 2021;74:238-246.
- Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM, Zucchelli G, Doria R, Menichetti F, Bongiorni MG, Lazzeri E, Mariani G. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging 2013; 6:1075–1086.
- Gürol G, Çiftci İH, Terizi HA, Atasoy AR, Ozbek A, Köroğlu M. Are There Standardized Cutoff Values for Neutrophil-Lymphocyte Ratios in Bacteremia or Sepsis? J. Microbiol. Biotechnol. 2015, 25, 521–525.
- Chen Y, Ye LJ, Wu Y, Shen BZ, Zhang F, Qu Q, Qu J. Neutrophil-Lymphocyte Ratio in Predicting Infective Endocarditis: A Case-Control Retrospective Study Mediators Inflamm 2020; 27;2020:8586418.
- Bozbay M, Ugur M, Uyarel H, Cicek G, Koroglu B, Tusun E, Sunbul M, Murat A, Sari I, Eren M. Neutrophil-to-lymphocyte ratio as a prognostic marker in infective endocarditis: in-hospital and long-term clinical results. J Heart Valve Dis 2014 ;23:617-623.
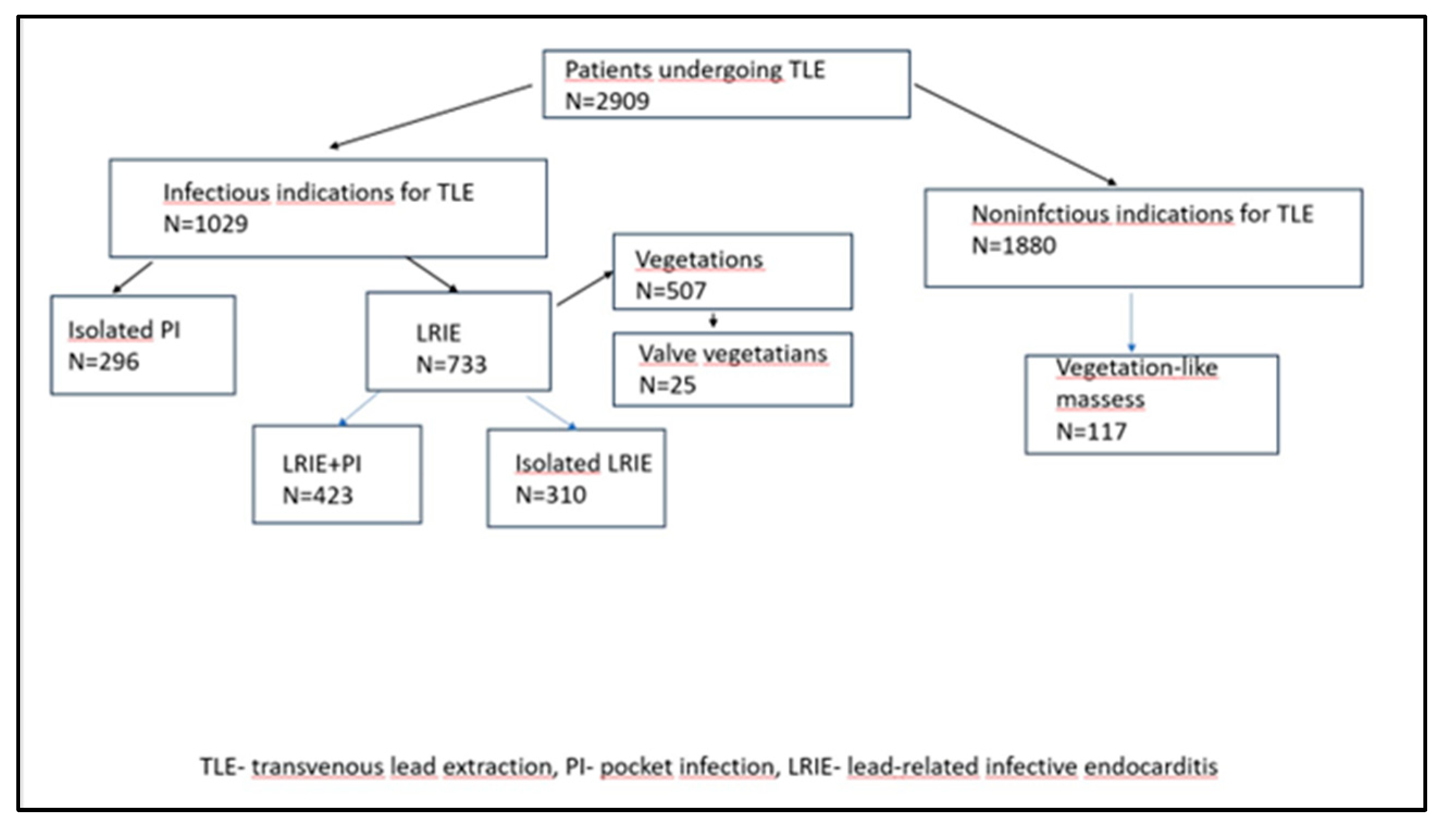
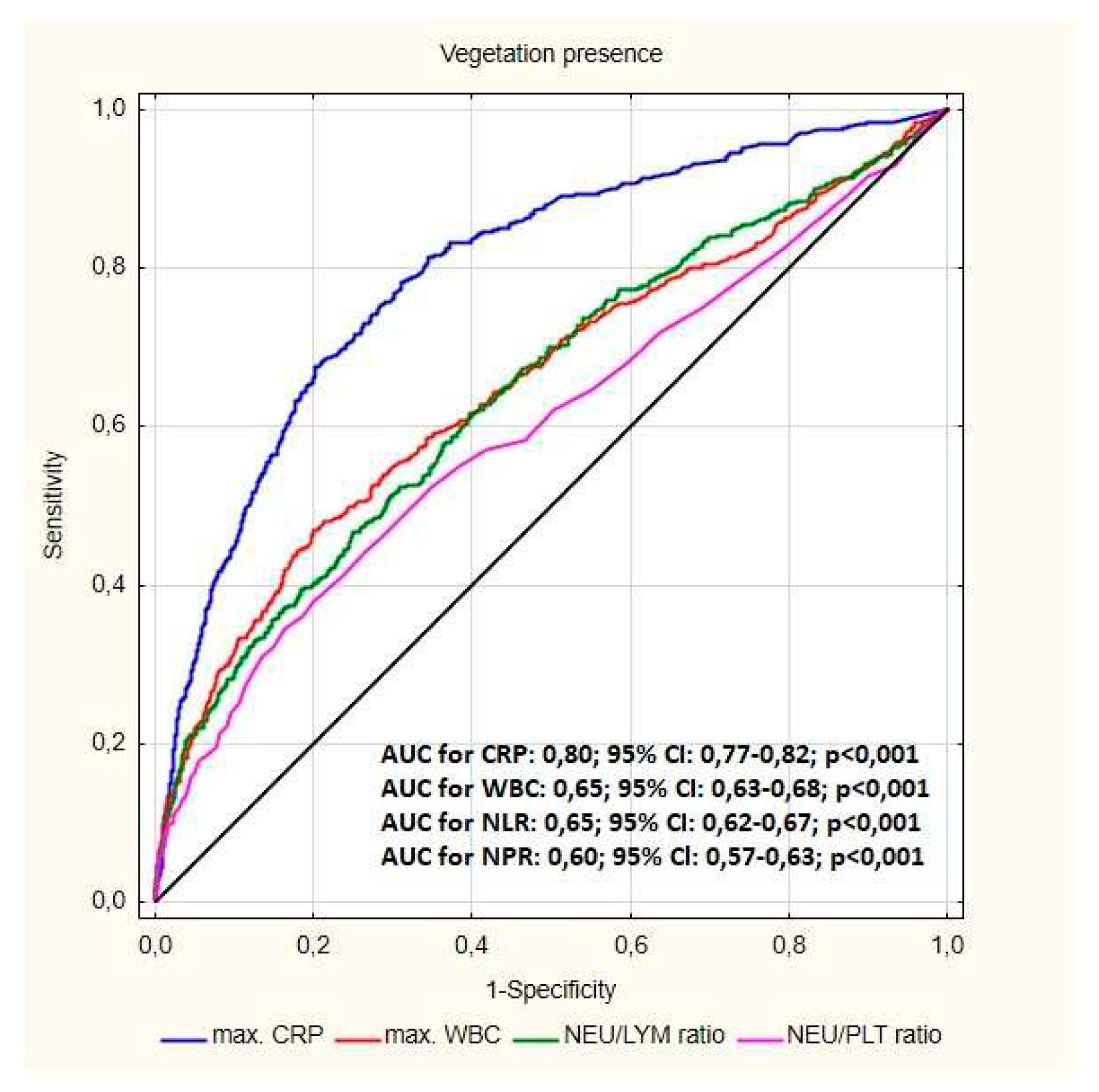
| All patients N=2909 |
|
|---|---|
| Patient’s age during TLE [years] median (Q1-Q3) | 69 (59-77) |
| Patient's age during first system implantation [years] median (Q1-Q3) | 61 (51-69) |
| Sex (% of female patients) (n, %) | 1340 (46.06) |
| LVEF [%] median (Q1-Q3) | 54 (36.00-60.00) |
| Renal failure (any) (n, %) | 885 (28.70) |
| Diabetes t.2 (n, %) | 775 (26.64) |
| Carlson's comorbidity index [number of points] median, (Q1 - Q3) | 4.00 (2.00-6.00) |
| Infectious indications for TLE (n, %) | 1029 (36.37) |
| LRIE (with and without pocket infection) (n, %) | 733(25.20) |
| Isolated pocket infections (n, %) | 296 (10.18) |
| Vegetations (n, %) | 507 (17.40) |
| Non-infectious indications for TLE (n, %) | 1880 (64.63) |
| Vegetations-like masses (n, %) | 117 (4.02) |
| Infectious indications for TLE N=1029 |
Non-infectious indications for TLE N=1880 |
p | |
|---|---|---|---|
| Patient’s age during TLE [years] median (Q1-Q3) | 70(61-78) | 68(58-76) | <0.001 |
| Patient's age during first system implantation [years] median (Q1-Q3) | 63(54-71) | 60(48-68) | <0.001 |
| Sex (% of female patients) (n, %) | 306 (29.74) | 828(44.04) | 0.001 |
| LVEF [%] median (Q1-Q3) | 50 (36.00.00-60) | 55(35.00-60.00) | <0.001 |
| Renal failure (any) (n, %) | 271(26.34) | 352 (18.72) | <0.001 |
| Diabetes t.2 (n, %) | 232(22.55) | 332 (17.66) | <0.001 |
| Carlson's comorbidity index [number of points] median, (Q1 - Q3) | 4.00(3.00-7.00) | 4.00(2.00-5.50) | <0.001 |
| Hemoglobin (g/dl) (lowest) (mean, SD) | 12.5(11.0-13.30) | 13.30(12.10-14.40) | <0.001 |
| Hematocrit (%) (lowest) median (Q1-Q3) | 37.20(33.00-40.90) | 39.90(36.20-42.90) | <0.001 |
| Platelets/ul (lowest) median Q1-Q3) |
210.0(164.0-272.0) | 197.0(160.0-241.0) | 0.420 |
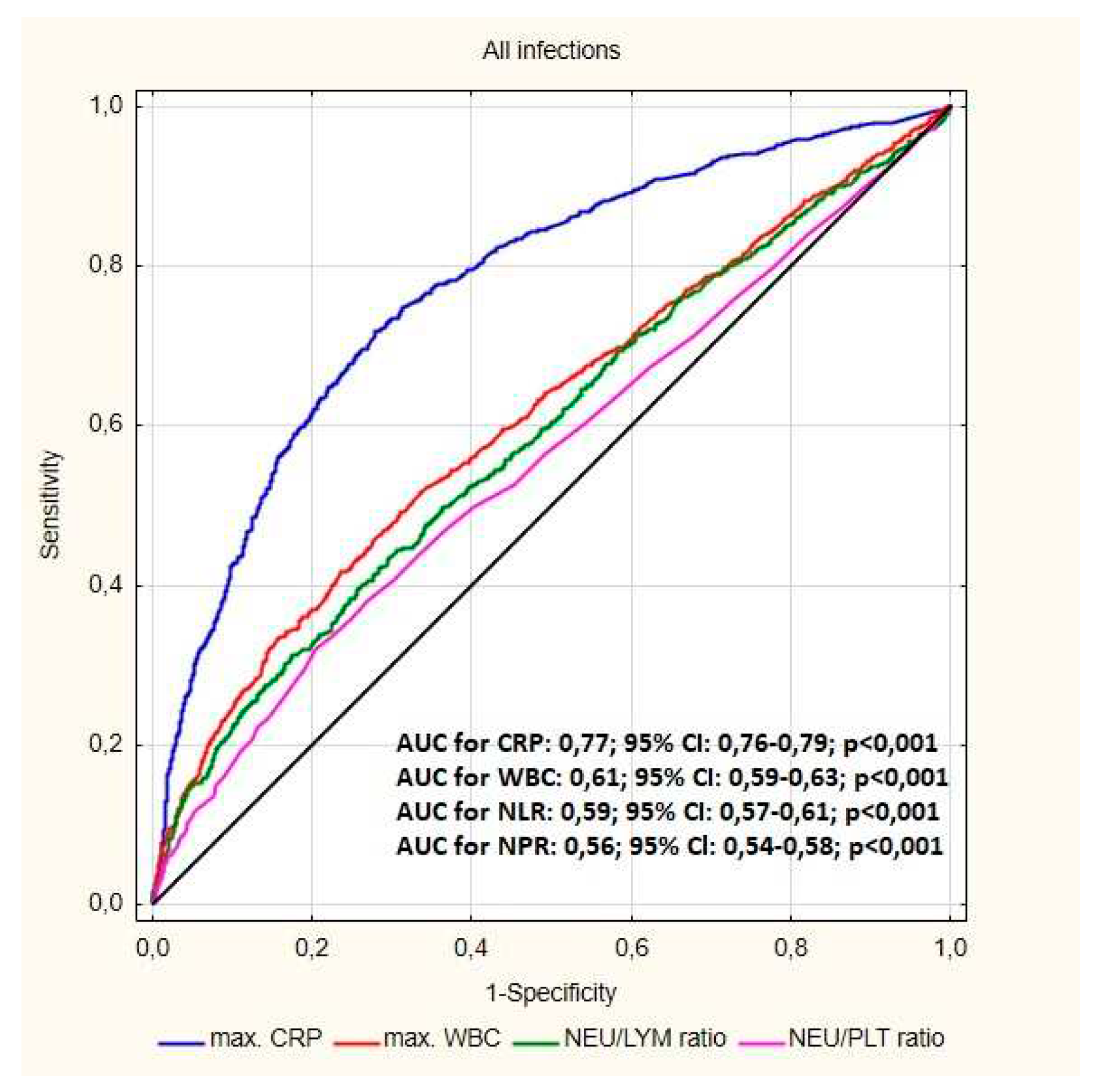
| Max WBC/ul (mean.SD) | 8185(6600-10360) | 7210(6070-8630) | 0.032 |
| Neutrophil count/ul (max) median (Q1-Q3) | 5.29(3.90-7.40) | 4.30(3.50-5.50) | 0.018 |
| Neutrophil % median (Q1-Q3) |
66.15(58.60-74.00) | 62.90(56.60-69.10) | 0.002 |
| Lymphocyte count/ul (max) median (Q1-Q3) | 1.60(1.30-2.30) | 1.70(1.30-2.19) | <0.001 |
| Lymphocyte% median (Q1-Q3) |
22.30(16.20-29.10) | 24.60(19.20-30.50) | <0.001 |
| Max ESR (mm/h) median (Q1-Q3) |
25.00(1.00-50.00) | 11.00(6.00-20.00) | <0.001 |
| Max CRP (mg/dl) median (Q1-Q3) |
17.57(5.07-60.40) | 2.00(0.60-7.17) | <0.001 |
| Max Procalcitonin (ug/L) median (Q1-Q3) |
0.10(0.06-0.30) | 0.07(0.04-0.125) | 0.154 |
| NLR median (Q1-Q3) | 3.07(2.12-4.91) | 2.59(1.86-3.57) | <0.001 |
| NLR % median (Q1-Q3) | 3.07(2.12-4.84) | 2.57(1.86-3.58) | <0.001 |
| NPR median (Q1-Q3) | 0.02(0.02-0.04) | 0.01(0.01-0.03) | 0.008 |
| LPR % median (Q1-Q3) | 0.10(0.07-0.15) | 0.13(0.10-0.17) | 0.001 |
| LPR (median IQR) | 0.01(0.01-0.01) | 0.01(0.01-0.01) | 0.003 |
| Parameters | Presence of vegetation-like masses | Presence of vegetations | p |
|---|---|---|---|
| Hemoglobin (mg/dl) (lowest) median (Q1-Q3) | 13.70 (11.90-14.70) | 11.90 (10.30-13.20) | <0.001 |
| Hematocrit (%) (lowest) median (Q1-Q3) | 40.20 (36.00-43.90) | 35.90 (31.20-39.70) | <0.001 |
| Platelets/ul (lowest) median (Q1-Q3) | 200.0 (163.0-252.0) | 215.0 (157.0-278.0) | 0.063 |
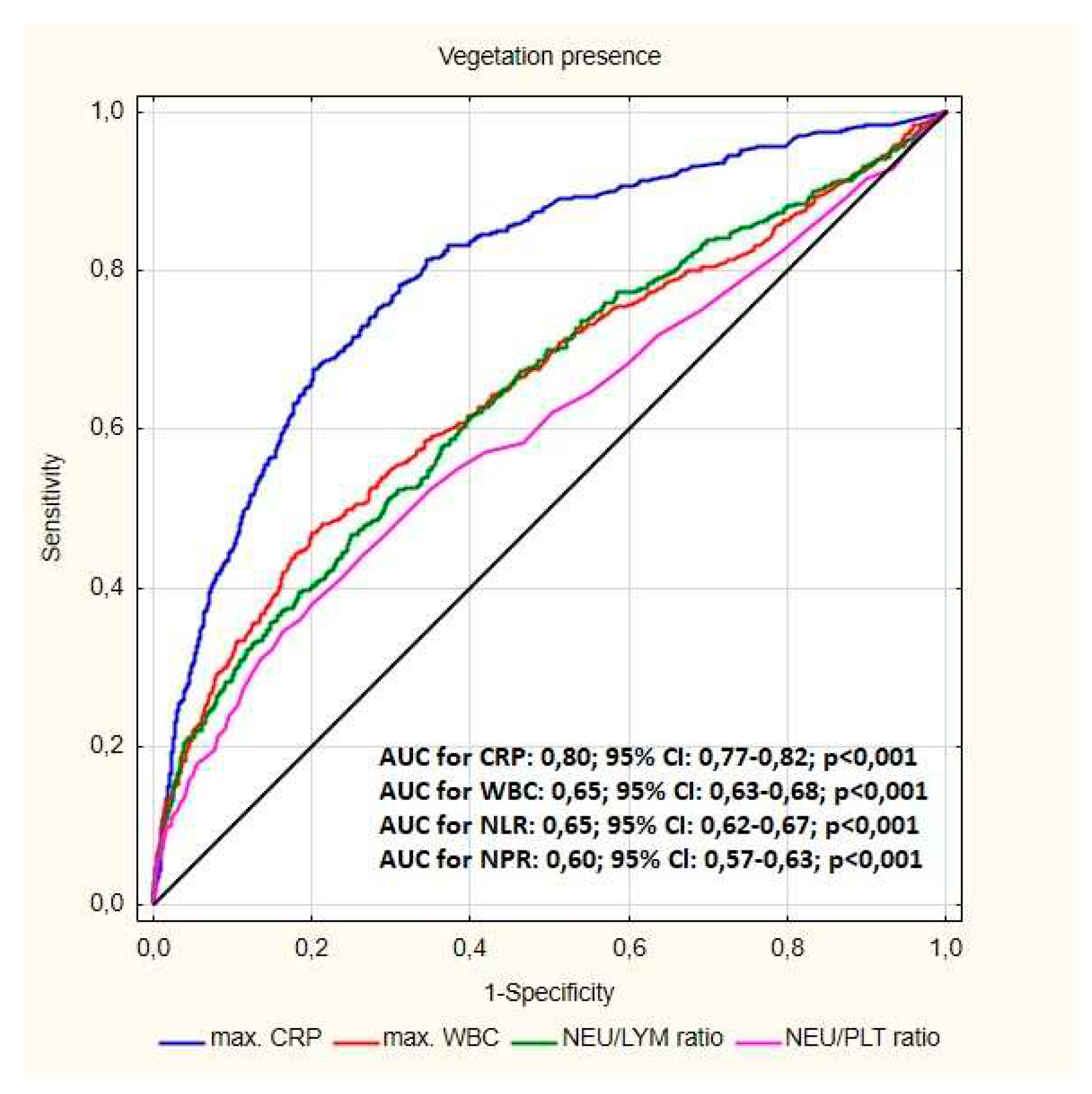
| MaxWBC/ul median (Q1-Q3) | 7440 (6060-8640) | 8880 (7000-11400) | <0.001 |
| Neutrophil count /ul(max) median (Q1-Q3) | 4.50 (3.56-5.54) | 5.70 (4.10-8,30) | <0.001 |
| Neutrophil % median (Q1-Q3) | 63.40 (54.80-69.80) | 67.70 (60.40-76.20) | <0.001 |
| Lymphocyte count/ul(max) median (Q1-Q3) | 1.65 (1.38-2.10) | 1.60 (1.20-2.20) | 0.927 |
| Lymphocyte% median (Q1-Q3) | 24.30 (18.70-31.90) | 19.80 (13.60-26.10) | <0.001 |
| Max ESR (mm/h)median (Q1-Q3) | 10.50 (5.00-19.50) | 30.00 (13.00-54.00) | <0.001 |
| MaxCRP (mg/dl) median (Q1-Q3) | 3.00 (0.67-10.75) | 32.58 (9.30-90.00) | <0.001 |
| Max Procalcitonin (ug/L)median (Q1-Q3) | 0.05 (0.04-1,52) | 0.12(0,07-0.50) | 0.581 |
| NLR median (Q1-Q3) | 2.61 (1.72-3.67) | 3.37 (2.35-5.55) | <0.001 |
| NLR % median (Q1-Q3) | 2.61 (1.70-3,81) | 3.39 (2,37-5.56) | <0.001 |
| NPR median (Q1-Q3) | 0.02 (0.02-0.03) | 0.03 (0.02-0,04) | 0..008 |
| LPR % median (Q1-Q3) | 0.13 (0.09-0.18) | 0.09 (0.06-0,14) | 0.025 |
| LPR median (Q1-Q3) | 0.01(0.01-0.01) | 0.01 (0.01-0.01) | 0.937 |
| Parameters | LRIE | Isolated pocket infection | p |
|---|---|---|---|
| Hemoglobin (g/dl) (lowest) median (Q1-Q3) | 11.90 (10.50-13.30) | 13.20 (12.00-14.30) | <0.001 |
| Hematocrit (%) (lowest) median (Q1-Q3) | 33.95 (29.15-37.80) | 39.30 (35.90-42.10) | <0.001 |
| Platelets /ul(lowest) median (Q1-Q3) | 222.0 (159.0-293.0) | 202.00 (166.0 -250.5) | 0.001 |
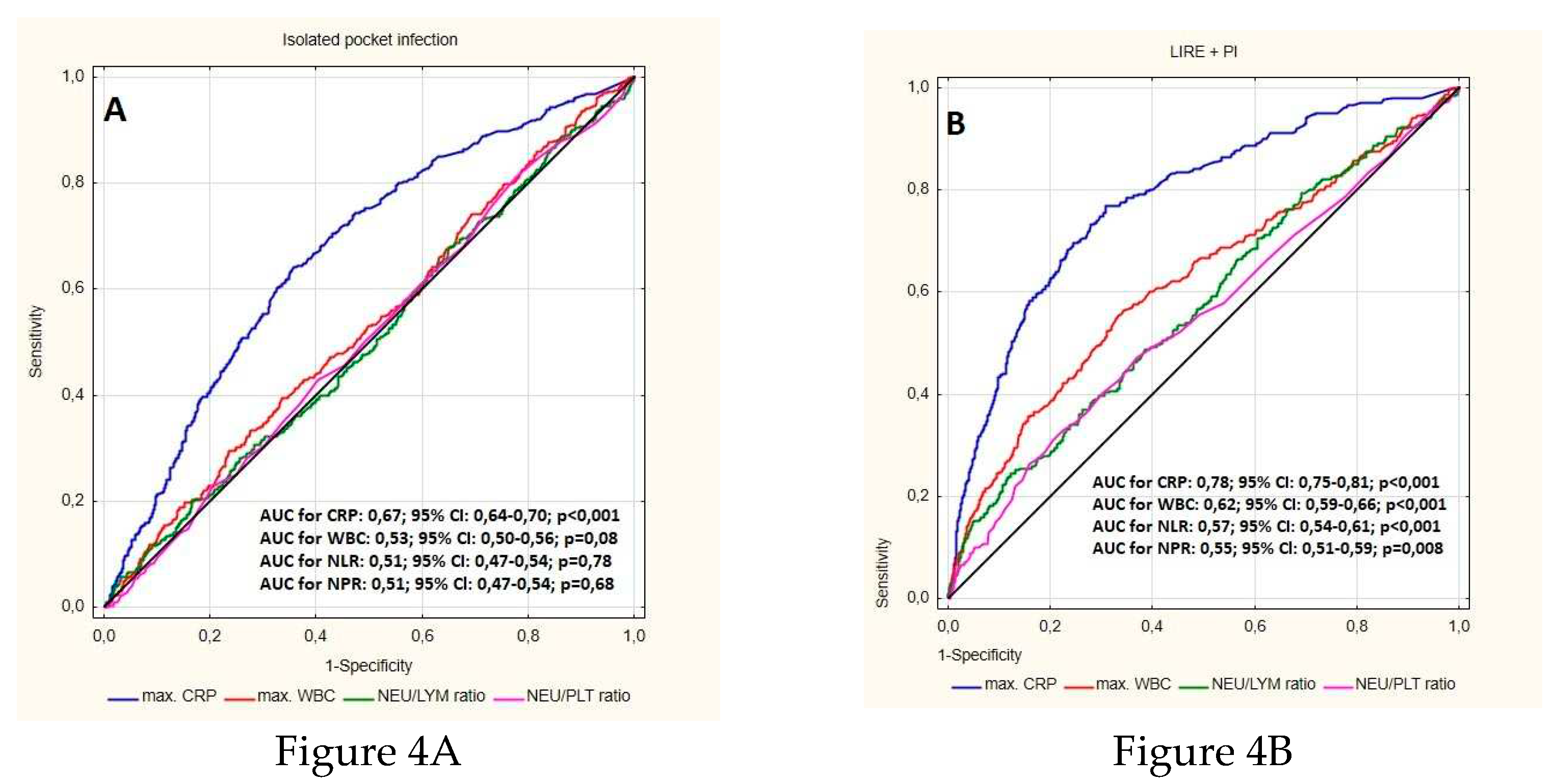
| MaxWBC/ul (mean.SD) | 10100 (7550-13300) | 7370(6280-8900) | <0.001 |
| Neutrophil count/ul (max) median (Q1-Q3) | 6.80 (4.65-9.50) | 4.40 (3.60-5.80) | <0.001 |
| Neutrophil % median (Q1-Q3) | 71.10 (63.75-78.45) | 62.75 (56.25-70.50) | <0.001 |
| Lymphocyte count/ul (max) median (Q1-Q3) | 1.60 (1.02-2.2.0) | 1.70 (1.30-2.29) | 0.93 |
| Lymphocyte% median (Q1-Q3) | 17.00(11.45-23.30) | 24.70 (18.80-30.30) | <0.001 |
|
Max ESR (mm/h) median (Q1-Q3) |
44.50 (22.00-68.00) | 15.00 (8.00-30.00) | <0.001 |
| MaxCRP (mg/dl ) median (Q1-Q3) | 65.00 (24,70-120.7) | 7.30 (2.20-19.20) | <0.001 |
| Max Procalcitonin (ug/L)median (Q1-Q3) | 0.23 (0.10-1.53) | 0.08 (0.05-0.10) | 0.03 |
| NLR median (Q1-Q3) | 4.11 (2.72-6.98) | 2.55 (1.85-3.70) | <0.001 |
| NLR % median (Q1-Q3) | 4.24 (2.75-6.93) | 2.56 (1.87-3.73) | <0.001 |
| NPR median (Q1-Q3) | 0.03 (0.02-0.05) | 0.02 (0.02-0.03) | <0.001 |
| LPR % median (Q1-Q3) | 0.08 (0.05-0.11) | 0.12 (0.08-0.16) | 0.001 |
| LPR median (Q1-Q3) | 0.01 (0.00-0.01) | 0.01 (0.01-0.01) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





