1. Introduction
For a long time, butchers use the dry ageing process to enhance the beef quality and preserve it. Nowadays the consumer interest in dry aged beef is increasing all over the world creating a high niche in the food service market [
1,
2]. The process consists in a selection of unpackaged beef primal cuts that are placed in a room with controlled environment for several weeks. The main dry aged meat goals are related to flavor intensification resulting beefier and nuttier and succulence and tenderness improvement [
3]. In addition, dry aging also modifies the microbiological, physical, and chemical characteristics of beef [
4]. The juices are absorbed into the meat, there is chemical breakdown of protein and fat constituents [
1,
2], there is a water diffusion from the interior to surface and then it is evaporated to the environment leading to flavor compound concentration [
5]. Dry ageing costs are higher than conventional processing methods, in one hand due to the high ageing shrinkage, trim loss, contamination risk and requirements of ageing conditions and other hand because only the highest grade of beef marbling are selected for dry ageing [
1]. The process guidelines include the days of ageing, storage temperature, RH and air flow [
1,
5]. The average length of dry ageing can be from 14 to 40 days. However, the rate is dependent of the temperature, lower temperature required more time to producing the desired results of this process [
1]. The optimum is between 0 °C and 4 °C and it is critical to dry ageing. High temperatures improve the enzymatic process but can promote rapid bacterial growth [
1]. To control the process, it is crucial control not only the temperature but the RH. If the RH is too high, the spoilage bacteria risk is higher too, creating an unpleasant viscous surface. On the other hand, low RH can limit bacterial growth and promote the surface dehydration and the weight loss [
1,
5]. The recommended value range is 61% to 85% [
1].
After slaughter during the conversion of muscle to meat, the pH drops as the hydrogen accumulate until reach the isoelectric point. The meat pH can be important during the dry ageing process as it affects the muscle’s ability to bind water [
5]. Regarding dry aged meat some studies reported a similar pH for fresh meat and others, a positive correlation between the pH, from 5.8 to 6.9 and the growth of lactic acid bacteria (LAB) on dark cutting samples [
6]. Throughout the dry process, it creates a dynamic microbiome on the meat surface (crust), in particular, various
Lactobacillus spp. including
L. sakei and
L. plantarum [
7,
8]. There is a limit growth bacteria and emergence of beneficial molds [
1]. The continuously changing of bacteria, yeasts and molds that metabolizes and produces metabolites on the meat affects its quality and safety [
2,
7]. Bacteria can contribute to meat spoilage, such lactic acid group can cause greening of the meat,
Pseudomonas spp. metabolizes glucose, lactate and amino acid resulting on off-odor [
2,
9]. Nevertheless, there are some prevalent bacteria during the dry ageing like, lactic acid, yeasts and molds like
Mucor spp. and
Penicillium spp. [
2,
6]. On other hand, pathogenic bacteria like
Listeria monocytogenes, entero-hemorrhagic
Escherichia coli and
Salmonella sp. can be present during the dry ageing but the studies indicate a tendency to reduce along the time [
6,
9]. Despite the unknown correlation of the microbial flora and the dry aged meat quality, the recent studies show positive effects of some specific molds like
Pilaira anomala,
Thamnidium spp. and
Debaryomyces hansenii by releasing proteases and collagenolytic enzymes that break down myofibrils and improve the flavor [
2,
6,
9]. As the current consumer interest grow, the need of food safety supportive studies grows too. In Europe, there is no specific regulation for dry aged meat in particular microbiological criteria. The Regulation (EC) nº 2073/2005 establish the microbiological limits for general foodstuffs and it is the only one so far. Therefore, the European Food Safety Authority (EFSA) recently published in its journal a scientific opinion about microbiological safety of dry aged meat [
6].
Tenderness, flavor, and juiciness are important factors for consumers to determine the acceptability and palatability of beef [
10]. On the appearance of dry beef, the color showed to be more darker and color stable comparing with wet aged beef [
11,
12]. The studies comparing sensory quality of dry aged beef are inconsistent, some reported no effects of dry aged beef on sensorial traits [
12,
13], while others, mostly in Europe, Japan and New Zealand have shown beneficial effects of dry ageing on consumer sensory assessments of quality [
11,
12,
14].
Dry aged beef is a niche market and expensive once requires not only premium beef carcass, un exclusive and controlled process but also, high trimming and weight losses. However, the consumer perception of these products is controversial, some unquestionably liked, others are afraid and because of the lack of regulation it is crucial the process standardization since persist the doubt of the dry aged beef safety. Therefore, this study was designed to characterize the beef microbiological and physicochemical changes during dry aging inside a Portuguese company as well as their consumer perception and acceptance.
2. Materials and Methods
2.1. Study planification
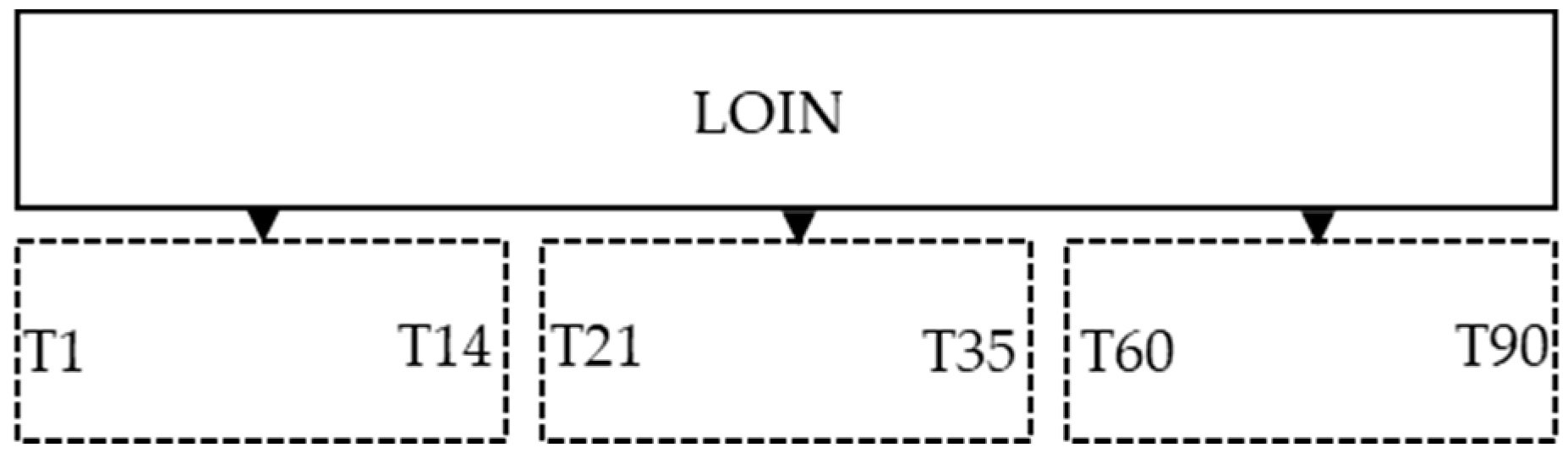
This study was outlined in a certificated company for the dry-ageing meat process in Portugal, in order to evaluate the safety and consumer acceptance of the dry ageing process. Twelve loins (
longissimus lumborum) from 6 animals with similar characteristics (gender, age and weight) where selected for the study. In order to have cutting surface with the specific aging time (
Figure 1) the loins where divided in three pieces (about 5.0 kg each) and dry aging time maximum was 90 days. At the end of the study, the leftover meat was discharged.
2.2. Dry aged process guidelines
On the facilities, were followed the dry aged company practices like, controlled refrigerator room temperature, RH and air flow, UV light continuously and the day 1 was consider as the beginning of the aging and it correspond five days after slaughter. Before each sample collection, the intramuscular pH of each piece was measured with a commercial equipment (HANNA®, Romania) and registered the environment temperature and RH.
2.3. Laboratory analysis
2.3.1. Sample collection
On day 1, 14, 21, 35, 60 and 90 of dry aging, from a cutting surface of the piece of each loin, were hygienically taken two samples (total of 24 samples/time) and transported to the laboratory at 4°C within 1h30minutes. The samples were taken from two different sites of the piece surface, similar to a cut beef, with approximately 400g (with superficial and lean meat).
2.3.2. Microbiological analysis
On the laboratory, the packed samples were placed in a refrigerated room until the preparation for the different steps. From the 12 samples were aseptically taken 10 g and 25 g x 2 of superficial meat (crust) and the same procedure for 12 samples of lean meat, to microbiological analysis. To quantify total aerobic bacterial populations and pathogens,10 g of the samples (superficial and lean meat) were added to 90 ml of 0.1% buffered peptone water and the samples were then homogenized for 60 s using a stomacher. The homogenates were then serially diluted and 1 or 0.1 ml portions of the diluted suspensions were surface-plated were poured or spread on non-selective and selective agar plates. To quantify different groups of bacteria, the following media and conditions were used: Plate Count Agar at 30°C for 72 hours for the total mesophilic aerobic (ISO 4833-1) and at 7°C for 10 days for the total psycrothrophic aerobic bacteria (ISO 174410); Baird-Parker agar at 37 °C for 48 h for coagulase-positive staphylococci (ISO 6888–2); Tryptone Bile Glucuronic agar at 44 ºC for 24 h for E. coli (ISO 16649–2); Violet Red Bile Glucose at 37 °C for 24 h for Enterobacteriaceae (ISO 21528–2); Cephalothin-Sodium-Fusidate-Cetrimide agar at 37 °C for 24 h for Pseudomonas spp. (ISO 13720); Chloramphenicol Glucose agar at 25 °C for 5 days and Saboraud Dextrose agar at 30 °C for 7 days, for yeasts and molds (ISO 21527–1); Man-Rogosa-Sharpe at 30 °C for 3 days for mesophilic lactic acid bacteria (ISO 15214); Chromogenic medium agar for detection, isolation and enumeration of L. monocytogenes at 37 °C for 24–48 h for Listeria spp. and L. monocytogenes (ISO 11290–2/A1). Hektoen Enteric agar at 37°C for 24h for Salmonella spp. (ISO 6579-1). The search of L. monocytogenes was performed using 25g, in 225 ml of Fraser I and Fraser broth and then spread on Chromogenic medium agar (ISO 11290:1998-1 / AFNOR Validation CHR-21/1-12/01). The search of Salmonella sp. was performed using 25g in 225 ml of Buffered Peptone Water medium and then with selective enrichments – Rappaport Vassiliadis broth and Muller Kauffman Tetrathionate-novobiocin spreads on Chromogenic medium agar and Hektoen Enteric agar (ISO 6579:2002 / NP 870:1988). After incubation, plates with colonies were counted using a spiral grid and the number of CFU/g was calculated and log10 transformed.
2.3.3. Physicochemical parameters
The color of the meat was measured on the crust and on lean meat, on the total of the 24 samples each time. The samples were measured at room temperature with a chroma meter (CR410, Konica Minolta Co., Osaka, Japan) that had been calibrated using a standard white tile. CIE L* (lightness), a* (redness), b* (yellowness) values were measured at three random locations on each sample. The aw was quantified in lean meat, at day 1 and 60 (total 12/time) using a aw-kryometer (Rotronic HigroLab®, China).
The intramuscular pH was performed has described on subsection 2.2.
2.4. Sensorial analysis
The sensorial traits evaluation was made each time by an untrained consumer panel (total of 6) which they classified 2 pieces from each sample, one whole (crust+lean meat) and one trimmed (total of 24/time). The sensory analysis scope was to evaluate the perception of the raw meat, as color, smell, intensity and overall acceptability with a classification score of 0-7 (0-less; 7-lot).
2.5. Statistical analysis
Microbiological enumeration data were log10 transformed for analysis. Descriptive statistics, including means and correlation values, were calculated for microflora, pH, color and sensory evaluation. To assess differences among distinct times, non- parametric analysis Kruskal-Wallis tests were performed, followed by Bonferroni correction for multiple pairwise comparisons. A significant level of 5% was used. Microbiological counts were visualized using violin plots generated with ggplot2 package in R version 4.3.0. Principal components analysis (PCA) was employed to separate meat and crust samples and to explore the interrelations among variables. Correlation matrices, for crust and meat samples, were also examined to assess the importance of variables in group separation. Partial least squares-discriminant analysis (PLS-DA) was conducted to further differentiate between group types, using two components for prediction. Variable importance in projection (VIP) scores were generated and plotted, using with mixOmics package, to identify influential variables in separation of groups. The quality of the PLS-DA is given by the classification error rate (based on 5-fold cross-validation strategy).
3. Results and Discussion
3.1. Dry aged process and meat microbiological and physicochemical status
During the entire study the variation of temperature and RH on dry aged refrigerated room, was 4.3 - 2.1 °C and 69.0 - 55.4 %, respectively. In order to evaluate the surface microbiome dinamics and the trimming effect, data were analysed separated for the superficial beef (crust) and for lean meat (meat) with significant differences between them for most variables (P<0.05).
3.1.1. Microbiological counts
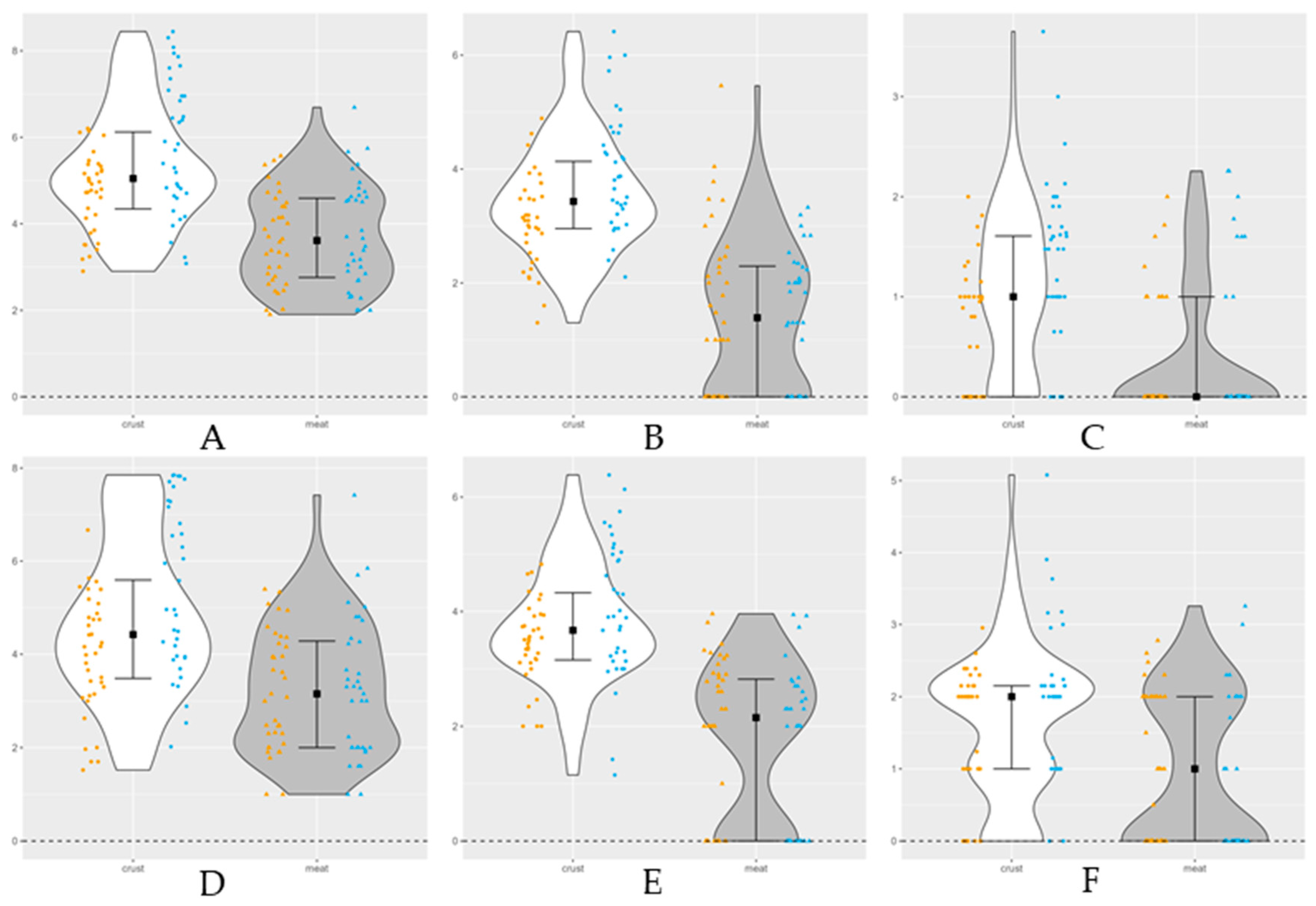
At beginning of dry aging, the microbial enumerations are higher on crust than in meat, significantly for Enterobacteriaceae (
Table 1). During time the crust counts increase, significantly, on mesophilic bacteria, LAB, Enterobacteriaceae,
Pseudomonas spp. and molds, with higher numbers at day 90. No significant differences were observed for yeasts (P>0.05). In turn, the lean meat counts decrease with aging time, specially the mesophilic and psycrotrophic bacteria, LAB,
Pseudomonas spp. and yeasts (P<0.05). Molds increased counts with time on both samples, besides no significant differences were observed in lean meat
(P>0.05). The less counts comparing to the initial values and over drying on lean meat were at day 21 on mesophilic and psycrotrophic bacteria, LAB,
Pseudomonas spp. and yeasts corresponding to molds higher peak. The lower counts on lean meat on overall traits suggest beef dry aged optimal time of 21 days with an opposite relation between the bacteria and yeasts counts and the molds counts. At day 14 when most bacteria were low, the molds counts were higher, suggesting the dynamic microbiome of the dry aged beef [
15]. The differences on counts between the group types can be related to trimming practices. Mesophilic bacteria, LAB, yeasts and
Pseudomonas spp. had higher median values on crust and meat group (
Figure 2). The pathogens count like
Listeria spp.,
Salmonella sp.
Staphylococcus spp.,
E. coli were all negative on enumeration for each time and for the crust and lean meat. These results are consistent to the references [
6,
9] The dry aged process parameters, like refrigerator temperature, HR and forced air, meat a
w and pH could be a positive influence for development of food borne pathogens [
9].
The significant bacteria and yeast increase counts during dry aged was demonstrated in other studies [
3,
6]. Recently, Gowda et al., reported great variation on microbiological counts between loins, once they were coming from different companies with different process and aging times [
9]. The study showed, at the end of ripening process, high numbers of psycrotrophic bacteria,
Pseudomonas spp., LAB and
B. thermosphacta on both tissue types [
9,
16]. However, other study reported that with a long ripening period there was a decrease on LAB counts and increase the yeasts counts and observed similar numbers in both adipose tissue and lean meat [
17].
Molds were detected at 19 days of aging [
9], and on the present study were detected at day 14, in contrast with other studies that referred the beginning of molds growth 3 weeks after the initial aging time [
1].
3.1.2. Color, pH and water activity
The a
w on meat had an average at day 1 of 0.985 ± 0.009 and at day 60 of 0.976 ± 0.013. The a
w can be related to the meat microbiological profile and process parameters. Some studies reported that low a
w of the beef surface may reduce the ability to bacteria spoilage and growth of pathogens [
13]. Nevertheless, in this study the lower microbiological counts were at day 21 suggesting the need of further studies in order to correlate the variables. Although the lack of results during time, the present showed a
w tendency to decrease with time. Although some studies refer inhibition of growth of food borne pathogens at a
w under 0.93 with mold toleration [
9].
The presented dry aged process revealed effects on pH and color
(P<0.05). Despite the fluctuation of values during the different times, the pH increased and differs significantly from day 1 at day 21 and until day 90 (
Table 2), always with values < 6.1. Previous studies reported no pH influence with beef drying [
3,
17] and significant differences with the aging method [
12]. It is known that meat pH is related to Maillard reactions during cooking and therefore the meat flavor. With this assumption, higher pH on dry aged beef can promote flavor improvement [
12,
14,
17].
Significant differences on surface color (CIE L*a*b*) were found on both types of samples for a* and b*
(P<0.05) during aging (
Table 2), suggesting the time effect on instrumental color. The crust and lean meat became less luminous (L*) but no significant differences were observed over the aged time. For redness (a*) and yellowness (b*) moderate and high significant differences were observed, respectively, in both crust and lean meat. Previous studies revealed significant color (L*, a* and b*) changes on dry aged beef, with lower values for L* and b* and higher for a* [
11,
12]. Nevertheless, the instrumental color measure the reflectance (wavelengths 630/580 nm) which is closely what the eye can see and this is correlated by differences on muscle components, moisture and pigment concentration [
12].
3.2. Consumer perception of dry aged meat
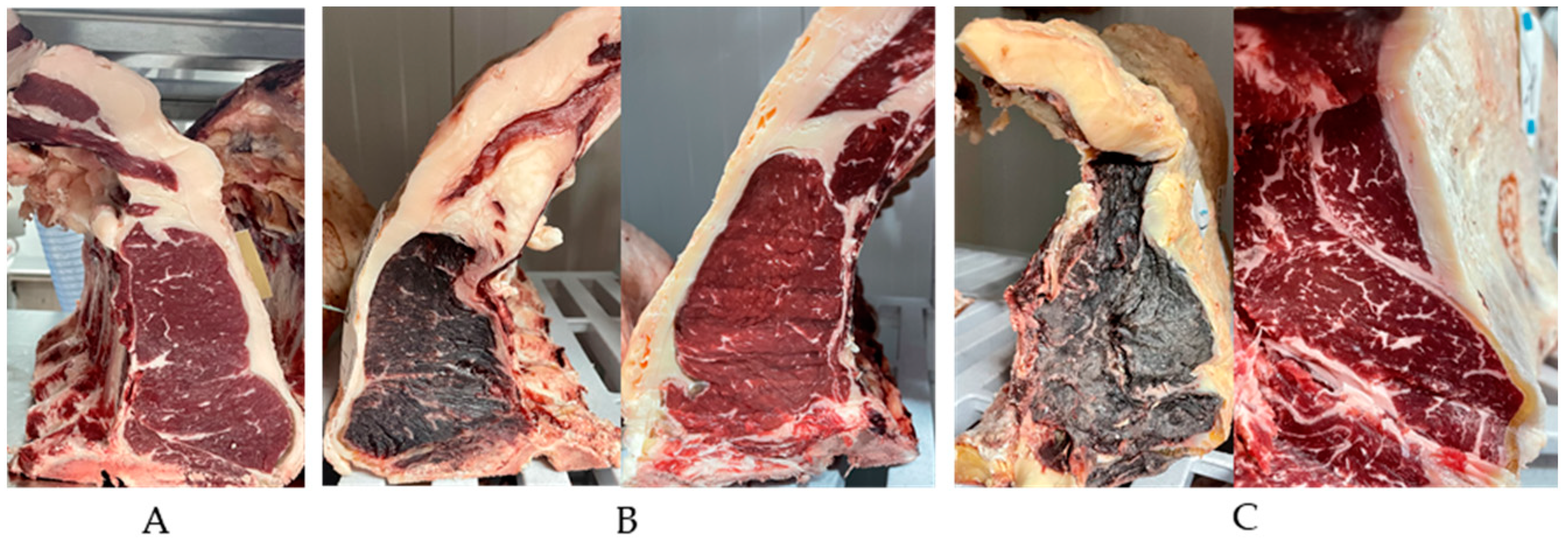
The consumer panel was constituted by untrained people who mimic the Portuguese consumer profile. The sensorial analysis was mainly on beef color, odor and overall acceptability, that was intended by freshness, where the panel visualized the whole sample, without trimming (crust + lean meat) and the trimmed sample (meat). The loins were refrigerator stored and on each colleting day, the samples were collected from each cutting surface of the piece (explained on material and methods). The panel observations where made according with the beef appearance, with a score of 0 (less) until 7 (lot). The present results were calculated through the average of sensory panel on each sample and time. The
Figure 3 represent examples of the loin pieces used in the study, and showed the overall color differences of the dry aged beef before and after trimming and with time effect. Regarding the sensorial evaluation of the color, the panel considered the whole and trimmed samples less redness and browner (P<0.05) (
Table 3). The apparent viscosity increases with aging and the odors, on both sample types, the intensity increased with time (P<0.05) and therefore the sweet, buttery and rancidity odors with predominance of the sweet (P<0.05). Nevertheless, the values were lower on lean meat samples (
Table 3). The overall meat approval was evaluated by freshness score and decrease with time (P<0.05) and it is higher on lean meat samples. The results are in lined with the expectations, once the dry aged process leads to evaporative losses (dehydration) and muscle oxidation (browner and less red). The sweet odor can be related to the nutty flavors of the dry aged beef and maybe a signal of the drying quality [
1]. Regarding to freshness trait, on day 90 the score was 3.48 for lean meat, showing the consumer acceptation of the dry aged beef until 90 days. The majority of beef dry aged sensorial analysis based on eating quality attributes showed that dry aged beef had higher tenderness, flavor and overall liking when comparing to wet aged beef [
3,
11]. Berger et al. reported significant palatability improvement of dry aged beef comparing with wet aged and the positive quality impact of aging is reported by other studies [
3,
11,
16,
18].
3.3. Multivariate analysis and correlations
The overall variables behavior was evaluated by partial least squares – discriminant analysis (PLS-DA) (
Figure 4) and principal component analysis (PCA) (
Figure 5) in order to separate and distinguished differences based on each group and variables were measured by scores known by variable importance in projection (VIP) (
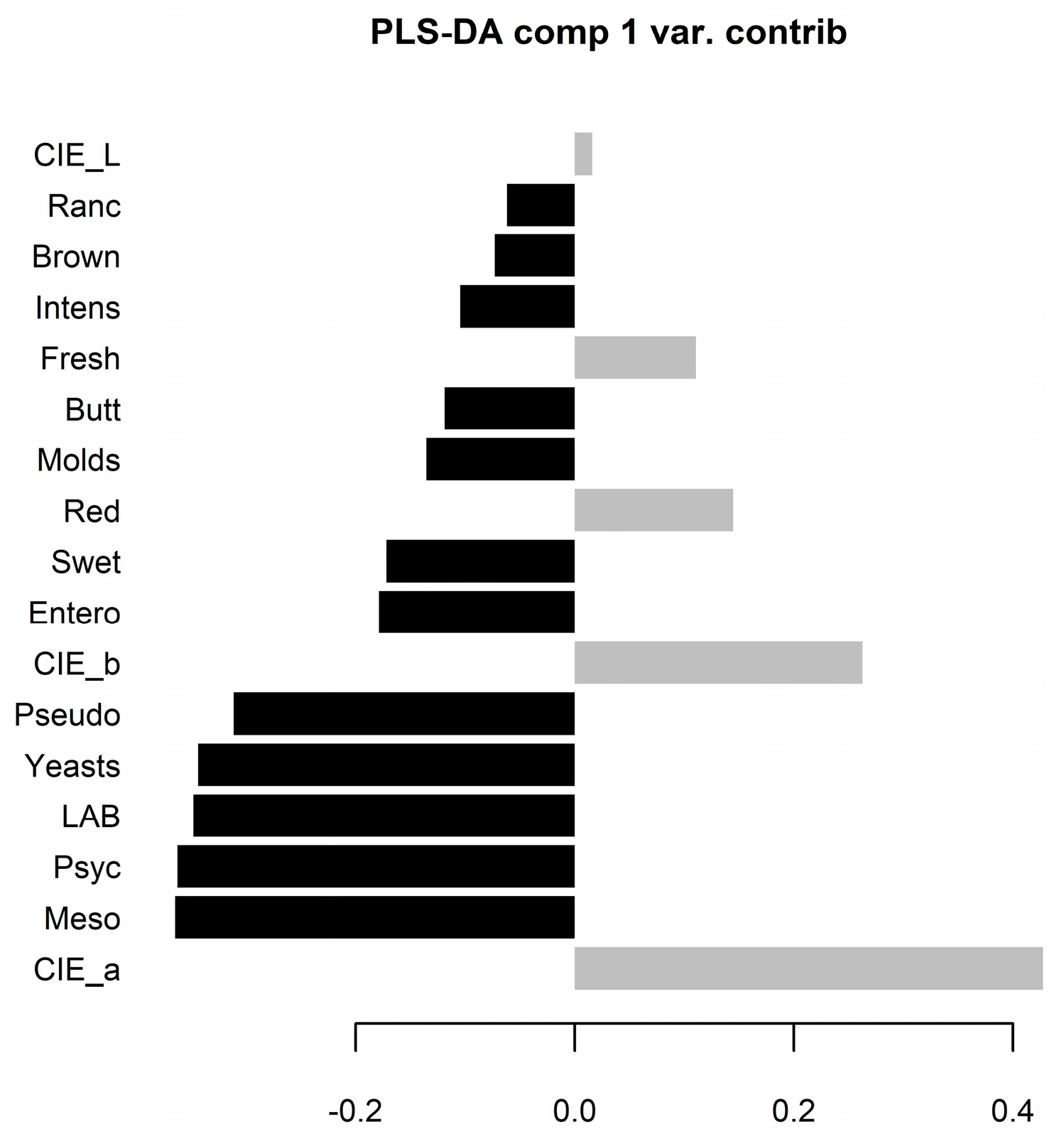
Figure 6).
The PLS-DA showed the lean meat samples on the positive side of comp 1, more associated to positive color atributtes (
Figure 5), namely red color and fresh meat; and the crust samples (points) more related to comp 2 on its positive side, associated to the microtiota of spoilage, specially mesophilic and psycrotrophic bacteria and Pseudomonas spp. with significantly importance (
Figure 5).
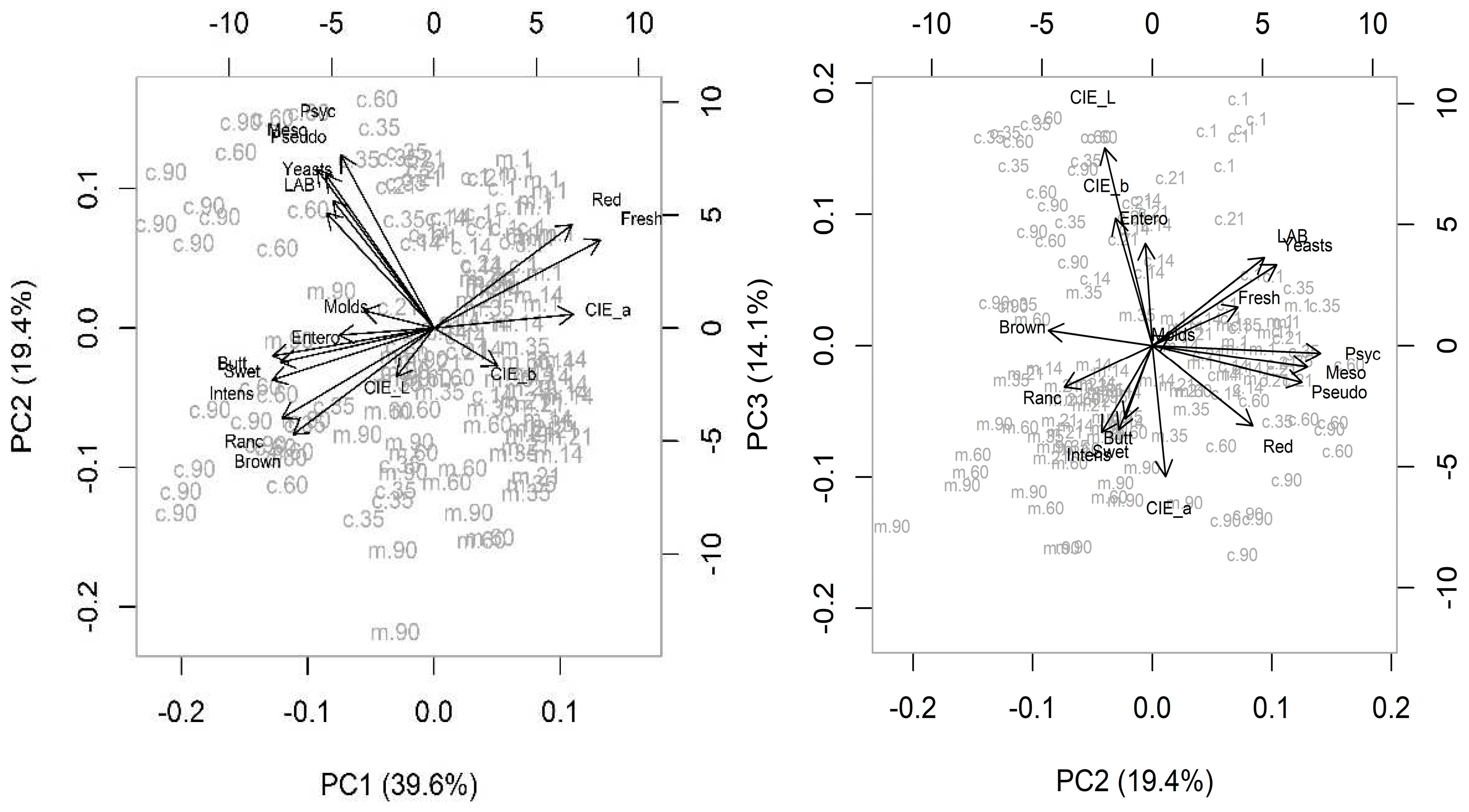
The
Figure 5 illustrate the biplot for principal components the samples were separated by type (crust or meat) along PC1 and aging deteriorative process (PC2). The relations between the variables are showed on PC1:PC2 and PC2:PC3, and together represented 63.1% of explained variance. On crust samples, the microbiological traits, except molds and Enterobacteriaceae, are strongly related and located in the positive side of PC2, increased with drying. For other hand, the color L* contribute strongly to PC3 and are associated to crust samples. On meat samples the mainly variables related are sensory positive attributes redness and freshness in the positive side of PC1, associated to the fresh samples (days 1, 14 and 21) and are correlated with the CIE a*, and negatively associated to the undesirable attributes (brown color, off odors intensity) on the opposite side. The color a* had a high relation with group meat and the values decrease with aging.
Figure 5 show variables with significantly importance: LAB, Pseudomonas spp. yeasts and sensory attributes namely rancid odor and brown color on crust and CIE a* and red attribute on lean meat. These results could mean that the color of the meat (CIE a* - redness) is highly related with aging, decreasing with time (
Table 2). On crust, the LAB and yeasts could be a microbiological indicator to aging, increasing with time showing significant differences, except for yeasts on crust samples as observed by Bischof et al. [
19].
In addition to PCA, partial least squares-discriminant analysis (PLS-DA) was performed to separate between groups of observations and it can discriminate differences based on each group. In this study, PLS-DA showed low classification error rate of 2.7%, when we used all data, only four sample were not well classified, one from the crust and three from lean meat. With test validation sample the result was similar to the analysis performed with all data with only one meat sample (3.3%) not well classified. These results show a good predictive ability thus in other studies [
20]. The aging period were clearly distinguished based on the color variables, specially a* and red color more related with freshness in the lean meat; and the microbiological variables and off-odors in the crust.
5. Conclusions
The current process shows that aging loins at <4.0 °C with RH <65.0% can be safety on microbiological perspective. This study reveals high crust counts with aging on mesophils, psycotrophics, LAB, Pseudomonas spp. with average values of 4.7 and 5.8 Log10CFU respectively. On lean meat the counts were, in general 2-3 Log lower than in crust. Molds only present growth and increase on crust samples during time, similar to the references.
The meat color (CIE a* - redness) is highly related with aging, decreasing with time with crust becoming browner. However, the meat freshness score, on day 90 was 3.48 for lean meat, showing the consumer acceptation of the dry aged beef until 90 days.
Nevertheless, the wide range of parameters that is used in practice and the higher loads that were occasionally found, suggests that future studies should also assess microbiome community and their influence on the growth/survival of pathogens. Therefore, we cannot completely rule out the potential growth of psycotrophic pathogens during storage, though this still needs to be assessed. Hygiene during retail practices and proper storage of the end product remains of critical importance and need further studies to assess que quality and safety of the end product.
The present data reveal that drying did not adversely impact the surface meat color and overall acceptability immediately after the trimming. Further research in characterizing more descriptive sensory attributes of the dry aged beef loin by a trained/certified sensory panel, conducting lipid/protein oxidation measurements and/or identifying volatile compounds governing the unique flavor of dry-aged beef loins would be warranted.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, A.R., P.T., and C.S.; methodology, A.R., K.S., P.T. and C.S..; software, I.O. and F.S.; validation, P.T. and C.S..; formal analysis, A.R., I.O. and C.S.; investigation, A.R., C.S..; resources, F.S. and C.S..; data curation, I.O.; writing—original draft preparation, A.R. and C.S.; writing—review and editing, A.R., P.T. and C.S.; visualization, I.O., K.S., F.S. and C.S.; supervision, P.T. and C.S.; project administration, F.S. and C.S.; funding acquisition, I.O., F.S and C.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the projects UIDB/CVT/00772/2020 and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT).
Acknowledgments
The participation of IO is supported by the project UID/MULTI/04621/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dashdorj D., V. K. Tripathi, S. Cho, Y. Kim, and I. Hwang, “Dry aging of beef; Review,” Journal of Animal Science and Technology, vol. 58. Korean Society of Animal Sciences and Technology, 2016. [CrossRef]
- Kim S., J. C. Kim, S. Park, J. Kim, Y. Yoon, and H. Lee, “Identification of microbial flora in dry aged beef to evaluate the rancidity during dry aging,” Processes, vol. 9, no. 11, Nov. 2021. [CrossRef]
- Berger J. et al., “Dry-aging improves meat quality attributes of grass-fed beef loins,” Meat Sci, vol. 145, pp. 285–291, Nov. 2018. [CrossRef]
- Smaldone G. et al., “Microbiological, rheological and physical-chemical characteristics of bovine meat subjected to a prolonged ageing period,” Ital J Food Saf, vol. 8, no. 3, pp. 131–136, 2019. [CrossRef]
- Ribeiro F. A. et al., “Ultimate pH effects on dry-aged beef quality,” Meat Sci, vol. 172, Feb. 2021. [CrossRef]
- Koutsoumanis K. et al., “Microbiological safety of aged meat,” EFSA Journal, vol. 21, no. 1, Jan. 2023. [CrossRef]
- Ryu S. et al., “Diversity and characteristics of the meat microbiological community on dry aged beef,” J Microbiol Biotechnol, vol. 28, no. 1, pp. 105–108, Jan. 2018. [CrossRef]
- Kim H. et al., “Evaluation of probiotic characteristics of newly isolated lactic acid bacteria from dry-aged hanwoo beef,” Food Sci Anim Resour, vol. 41, no. 3, pp. 468–480, May 2021. [CrossRef]
- Gowda T. K. G. M., L. De Zutter, G. Van Royen, and I. Van Damme, “Exploring the microbiological quality and safety of dry-aged beef: A cross-sectional study of loin surfaces during ripening and dry-aged beef steaks from commercial meat companies in Belgium,” Food Microbiol, vol. 102, Apr. 2022. [CrossRef]
- Kim M., J. Choe, H. J. Lee, Y. Yoon, S. Yoon, and C. Jo, “Effects of aging and aging method on physicochemical and sensory traits of different beef cuts,” Food Sci Anim Resour, vol. 39, no. 1, pp. 54–64, 2019. [CrossRef]
- Kim Y. H. B., R. Kemp, and L. M. Samuelsson, “Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins,” Meat Sci, vol. 111, pp. 168–176, Jan. 2016. [CrossRef]
- Ha M. et al., “Effects of different ageing methods on colour, yield, oxidation and sensory qualities of Australian beef loins consumed in Australia and Japan,” Food Research International, vol. 125, Nov. 2019. [CrossRef]
- Smith R. D. et al., “Dry versus wet aging of beef: Retail cutting yields and consumer palatability evaluations of steaks from US Choice and US Select short loins,” Meat Sci, vol. 79, no. 4, pp. 631–639, Aug. 2008. [CrossRef]
- Li X., J. Babol, A. Wallby, and K. Lundström, “Meat quality, microbiological status and consumer preference of beef gluteus medius aged in a dry ageing bag or vacuum,” Meat Sci, vol. 95, no. 2, pp. 229–234, Oct. 2013. [CrossRef]
- Capouya R., T. Mitchell, D. I. Clark, D. L. Clark, and P. Bass, “A Survey of Microbial Communities on Dry-Aged Beef in Commercial Meat Processing Facilities,” Meat and Muscle Biology, vol. 4, no. 1, Mar. 2020. [CrossRef]
- Li X., J. Babol, W. L. P. Bredie, B. Nielsen, J. Tománková, and K. Lundström, “A comparative study of beef quality after ageing longissimus muscle using a dry ageing bag, traditional dry ageing or vacuum package ageing,” Meat Sci, vol. 97, no. 4, pp. 433–442, 2014. [CrossRef]
- Ahnström M. L., M. Seyfert, M. C. Hunt, and D. E. Johnson, “Dry aging of beef in a bag highly permeable to water vapour,” Meat Sci, vol. 73, no. 4, pp. 674–679, Aug. 2006. [CrossRef]
- Oh H., H. J. Lee, J. Lee, C. Jo, and Y. Yoon, “Identification of Microorganisms Associated with the Quality Improvement of Dry-Aged Beef Through Microbiome Analysis and DNA Sequencing, and Evaluation of Their Effects on Beef Quality,” J Food Sci, vol. 84, no. 10, pp. 2944–2954, Oct. 2019. [CrossRef]
- Bischof G. et al., “Metabolic and microbial analyses of the surface and inner part of wet-aged and dry-aged beef.,” J Food Sci, Oct. 2023. [CrossRef]
- Kim H. C., K. H. Baek, Y. J. Ko, H. J. Lee, D. G. Yim, and C. Jo, “Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy,” Molecules, vol. 25, no. 13, Jul. 2020. [CrossRef]
Figure 1.
Scheme of the study design of the cutting surface during the aging plan.
Figure 1.
Scheme of the study design of the cutting surface during the aging plan.
Figure 2.
Violin plot of dry aged beef microbiological counts on crust and lean meat with a limit detection of 1 log10 CFU/g (lower dashed line). The separated samples, crust (n=72) and meat (n=72) are presented by mirrored density plots including the median and first and third quartile. Points (crust) and triangles (meat) represented individual observations at dry aging beginning (yellow) and ending (blue). A: total mesophilic bacteria; B: lactic acid bacteria; C: Enterobacteriaceae; D: Molds; E: yeasts; F: Pseudomonas spp.
Figure 2.
Violin plot of dry aged beef microbiological counts on crust and lean meat with a limit detection of 1 log10 CFU/g (lower dashed line). The separated samples, crust (n=72) and meat (n=72) are presented by mirrored density plots including the median and first and third quartile. Points (crust) and triangles (meat) represented individual observations at dry aging beginning (yellow) and ending (blue). A: total mesophilic bacteria; B: lactic acid bacteria; C: Enterobacteriaceae; D: Molds; E: yeasts; F: Pseudomonas spp.
Figure 3.
Beef loin used for the study. A: Cutting surface at day 1; B: Cutting surface at day 35 (left image represents the crust and right image represents the meat after trimming); c: Cutting surface at day 60 (left image represents the crust and right image represents the meat after trimming).
Figure 3.
Beef loin used for the study. A: Cutting surface at day 1; B: Cutting surface at day 35 (left image represents the crust and right image represents the meat after trimming); c: Cutting surface at day 60 (left image represents the crust and right image represents the meat after trimming).
Figure 4.
Partial least squares-discriminant analysis (PLS-DA) from the crust samples (points) and lean meat samples (triangles).
Figure 4.
Partial least squares-discriminant analysis (PLS-DA) from the crust samples (points) and lean meat samples (triangles).
Figure 5.
Principal components analysis (PCA) biplots illustrating the sample type variation, crust (c) and meat (m) (PC1) and the aging time (PC2; PC3) on variable patterns: mesophilic, psycotrophic and LAB, Enterobacteriaceae, Pseudomonas spp., yeasts, molds, CIE L*, a*, b*, sensorial analysis: redness, brown, intensity, sweetness, buttery, rancidity and freshness.
Figure 5.
Principal components analysis (PCA) biplots illustrating the sample type variation, crust (c) and meat (m) (PC1) and the aging time (PC2; PC3) on variable patterns: mesophilic, psycotrophic and LAB, Enterobacteriaceae, Pseudomonas spp., yeasts, molds, CIE L*, a*, b*, sensorial analysis: redness, brown, intensity, sweetness, buttery, rancidity and freshness.
Figure 6.
Partial least squares – discriminant analysis (PLS-DA) demonstrating variable importance projection (VIP) scores from the crust (black columns) and meat (grey columns) from the train data 5-fold cross-validation.
Figure 6.
Partial least squares – discriminant analysis (PLS-DA) demonstrating variable importance projection (VIP) scores from the crust (black columns) and meat (grey columns) from the train data 5-fold cross-validation.
Table 1.
Microbiological counts (means), expressed in Log10 CFU/g, on crust and lean meat, accordingly aging time and sampling type (crust and lean meat).
Table 1.
Microbiological counts (means), expressed in Log10 CFU/g, on crust and lean meat, accordingly aging time and sampling type (crust and lean meat).
| Trait |
Type |
1 d |
|
14 d |
|
21 d |
|
35 d |
|
60 d |
|
90 d |
|
P |
| Mesophiles |
C |
4.940 |
ab |
4.310 |
b |
4.870 |
ab |
5.100 |
ab |
6.150 |
ab |
6.240 |
a |
0.0161 |
| M |
4.600 |
a |
3.330 |
bc |
2.980 |
c |
3.400 |
abc |
3.550 |
abc |
4.340 |
ab |
0.0027 |
| P |
0.3262 |
|
0.0387 |
|
0.0003 |
|
0.0068 |
|
<0.0001 |
|
0.0121 |
|
|
| Psycrothrophics |
C |
4.740 |
a |
4.540 |
a |
5.300 |
a |
4.780 |
a |
5.880 |
a |
5.720 |
a |
0.1561 |
| M |
4.500 |
a |
3.310 |
a |
3.250 |
a |
3.410 |
a |
3.330 |
a |
3.550 |
a |
0.0485 |
| P |
0.4776 |
|
0.0100 |
|
0.0004 |
|
0.0885 |
|
0.0020 |
|
0.0004 |
|
|
| LAB* |
C |
3.600 |
ab |
2.460 |
c |
3.190 |
bc |
3.500 |
ab |
3.690 |
ab |
4.740 |
a |
0.0001 |
| M |
3.150 |
a |
0.440 |
c |
1.050 |
bc |
1.260 |
bc |
1.680 |
b |
1.240 |
bc |
<0.0001 |
| P |
0.1978 |
|
0.0002 |
|
<0.0001 |
|
0.0001 |
|
0.0002 |
|
<0.0001 |
|
|
| Enterobacteriaceae |
C |
0.840 |
abc |
0.670 |
bc |
0.490 |
c |
1.320 |
ab |
1.360 |
ab |
1.710 |
a |
0.0008 |
| M |
0.280 |
a |
0.390 |
a |
0.380 |
a |
0.730 |
a |
0.380 |
a |
0.150 |
a |
0.5482 |
| P |
0.0343 |
|
0.2835 |
|
0.6299 |
|
0.1228 |
|
0.0048 |
|
0.0003 |
|
|
|
Pseudomonas spp. |
C |
3.800 |
ab |
3.410 |
b |
4.670 |
ab |
4.710 |
ab |
5.600 |
a |
5.820 |
a |
0.0083 |
| M |
4.290 |
a |
2.750 |
ab |
2.640 |
b |
2.940 |
ab |
3.190 |
ab |
3.640 |
ab |
0.0244 |
| P |
0.2189 |
|
0.3121 |
|
0.0014 |
|
0.0056 |
|
0.0024 |
|
0.0224 |
|
|
| Yeasts |
C |
3.500 |
a |
3.270 |
a |
3.620 |
a |
3.670 |
a |
4.320 |
a |
4.160 |
a |
0.1333 |
| M |
3.120 |
a |
1.720 |
b |
1.260 |
b |
1.610 |
b |
1.330 |
b |
2.020 |
ab |
0.0013 |
| P |
0.1600 |
|
0.0019 |
|
0.0001 |
|
0.0006 |
|
0.0001 |
|
0.0029 |
|
|
| Molds |
C |
0.830 |
b |
1.920 |
a |
1.350 |
ab |
1.520 |
ab |
2.040 |
a |
2.320 |
a |
0.0086 |
| M |
0.690 |
a |
1.170 |
a |
1.510 |
a |
0.690 |
a |
0.940 |
a |
1.020 |
a |
0.3473 |
| P |
0.6615 |
|
0.0185 |
|
0.6594 |
|
0.0509 |
|
0.0204 |
|
0.0344 |
|
|
Table 2.
Means of instrumental surface color and pH on crust and lean meat, accordingly aging time and sampling type (crust and lean meat).
Table 2.
Means of instrumental surface color and pH on crust and lean meat, accordingly aging time and sampling type (crust and lean meat).
| Variable |
Type |
1 d |
|
14 d |
|
21 d |
|
35 d |
|
60 d |
|
90 d |
|
P |
| pH |
C |
------ |
|
------ |
|
------ |
|
------ |
|
------ |
|
------ |
|
|
| M |
5.610 |
c |
5.770 |
bc |
5.800 |
ab |
5.850 |
ab |
5.740 |
ab |
6.010 |
a |
<0.0001 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CIE L* |
C |
57.520 |
a |
46.590 |
a |
51.660 |
a |
48.660 |
a |
52.400 |
a |
45.950 |
a |
0.3131 |
| M |
35.110 |
a |
36.060 |
a |
36.370 |
a |
35.410 |
a |
34.340 |
a |
34.110 |
a |
0.3119 |
| P |
0.0000 |
|
0.0043 |
|
0.0226 |
|
0.0205 |
|
0.3401 |
|
1.0000 |
|
|
| CIE a* |
C |
13.350 |
a |
10.720 |
ab |
11.520 |
a |
8.250 |
ab |
6.460 |
ab |
3.770 |
c |
0.0019 |
| M |
24.460 |
ab |
24.440 |
a |
24.380 |
ab |
22.740 |
ab |
21.820 |
b |
16.560 |
c |
<0.0001 |
| P |
<0.0001 |
|
0.0000 |
|
0.0000 |
|
0.0000 |
|
0.0000 |
|
0.0000 |
|
|
|
CIE b*
|
C |
14.740 |
a |
10.460 |
bc |
12.650 |
ab |
15.540 |
ab |
12.920 |
ab |
7.480 |
c |
<0.0001 |
| M |
14.170 |
a |
13.800 |
ab |
12.920 |
abc |
12.580 |
bc |
12.080 |
cd |
10.550 |
d |
<0.0001 |
| P |
1.0000 |
|
0.0071 |
|
0.9538 |
|
0.2598 |
|
0.3118 |
|
0.0004 |
|
|
Table 3.
Sensory attributes of the whole beef (C+M) and meat (M) by the untrained panel.
Table 3.
Sensory attributes of the whole beef (C+M) and meat (M) by the untrained panel.
| Sensory attributes |
Type |
1 d |
|
14 d |
|
21 d |
|
35 d |
|
60 d |
|
90 d |
|
P |
| Color |
Redness |
C+M |
5.810 |
a |
5.100 |
ab |
4.860 |
b |
3.770 |
bc |
2.920 |
c |
2.690 |
c |
<0.0001 |
| M |
5.990 |
a |
5.620 |
ab |
5.420 |
bc |
4.690 |
cd |
4.480 |
d |
4.660 |
d |
<0.0001 |
| P |
0.6882 |
|
0.0081 |
|
0.0023 |
|
0.0564 |
|
0.0558 |
|
0.0085 |
|
|
| Brown |
C+M |
0.080 |
c |
0.910 |
bc |
1.640 |
b |
3.710 |
a |
4.450 |
a |
4.400 |
a |
<0.0001 |
| M |
0.230 |
d |
1.050 |
cd |
1.760 |
bc |
2.330 |
ab |
3.060 |
a |
3.300 |
a |
<0.0001 |
| P |
0.1055 |
|
0.9763 |
|
1.0000 |
|
0.0429 |
|
0.0109 |
|
0.2020 |
|
|
| Viscosity |
C+M |
0.200 |
c |
0.530 |
abc |
0.340 |
abc |
0.280 |
bc |
0.940 |
ab |
1.670 |
a |
0.0017 |
| M |
0.250 |
b |
0.670 |
b |
0.610 |
b |
0.650 |
b |
1.260 |
a |
1.460 |
a |
<0.0001 |
| P |
0.5186 |
|
0.6166 |
|
0.1068 |
|
0.0076 |
|
0.4839 |
|
0.9768 |
|
|
| Odor |
Intensity |
C+M |
0.450 |
d |
1.180 |
c |
1.550 |
c |
2.300 |
b |
3.340 |
a |
4.380 |
a |
<0.0001 |
| M |
0.220 |
d |
0.910 |
c |
1.340 |
bc |
0.600 |
b |
2.940 |
a |
3.250 |
a |
<0.0001 |
| P |
0.3918 |
|
0.4010 |
|
0.1553 |
|
0.0493 |
|
0.3684 |
|
0.0223 |
|
|
| Sweet |
C+M |
0.800 |
c |
1.180 |
c |
1.260 |
c |
1.760 |
b |
2.080 |
b |
3.580 |
a |
<0.0001 |
| M |
0.840 |
c |
0.820 |
c |
1.160 |
bc |
0.550 |
bc |
1.660 |
b |
3.120 |
a |
<0.0001 |
| P |
0.9076 |
|
0.0077 |
|
0.5804 |
|
0.0128 |
|
0.0820 |
|
0.3254 |
|
|
| Buttery |
C+M |
0.610 |
c |
1.140 |
bc |
1.300 |
b |
1.250 |
b |
2.870 |
a |
2.990 |
a |
<0.0001 |
| M |
0.680 |
c |
0.970 |
bc |
1.180 |
b |
0.390 |
b |
1.840 |
a |
2.200 |
a |
<0.0001 |
| P |
0.8610 |
|
0.1806 |
|
0.4332 |
|
0.4862 |
|
0.0012 |
|
0.0125 |
|
|
| Rancidity |
C+M |
0.110 |
c |
0.570 |
b |
0.680 |
b |
0.950 |
b |
1.360 |
b |
3.110 |
a |
<0.0001 |
| M |
0.150 |
d |
0.480 |
c |
0.620 |
bc |
0.410 |
c |
1.200 |
ab |
2.340 |
a |
<0.0001 |
| P |
0.5242 |
|
0.3836 |
|
0.8849 |
|
0.4506 |
|
0.7710 |
|
0.0335 |
|
|
| Freshness |
C+M |
6.320 |
a |
5.240 |
b |
5.270 |
b |
4.300 |
c |
3.700 |
c |
2.580 |
d |
<0.0001 |
| M |
6.380 |
a |
5.640 |
b |
5.540 |
b |
0.360 |
c |
4.280 |
d |
3.480 |
d |
<0.0001 |
| P |
0.7056 |
|
0.0090 |
|
0.1517 |
|
0.0026 |
|
0.0865 |
|
0.0137 |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).