Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
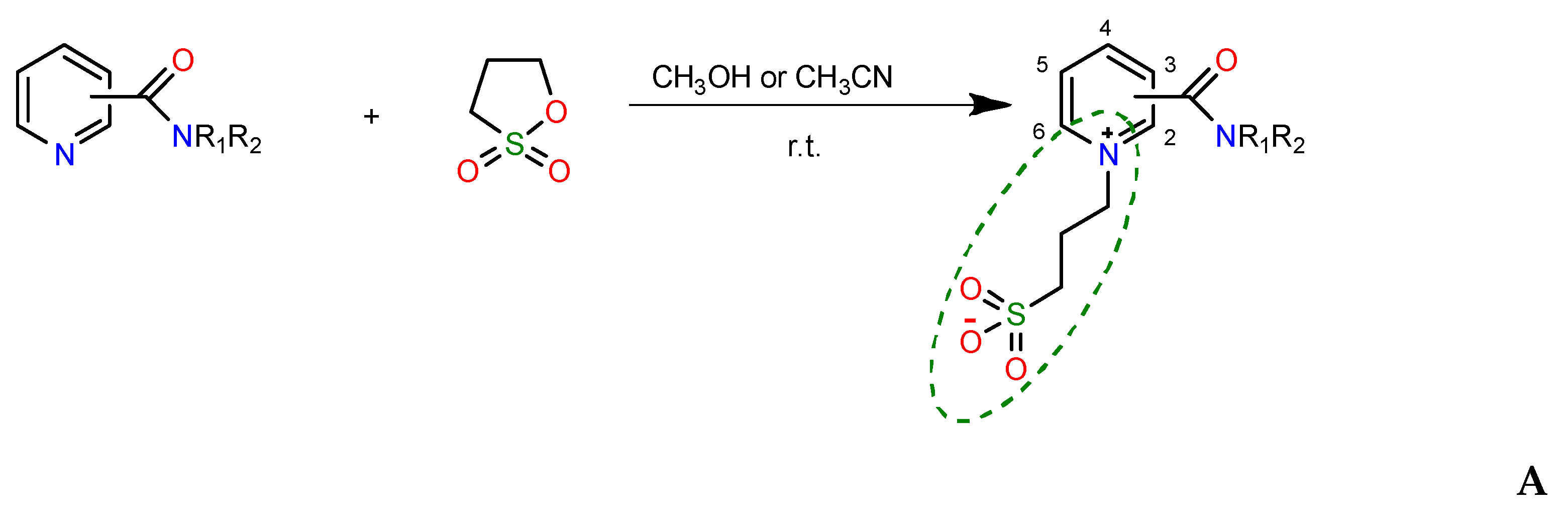
1. Introduction
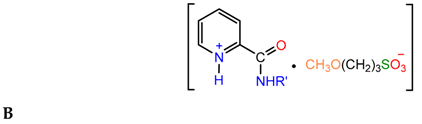
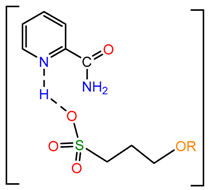
2. Results
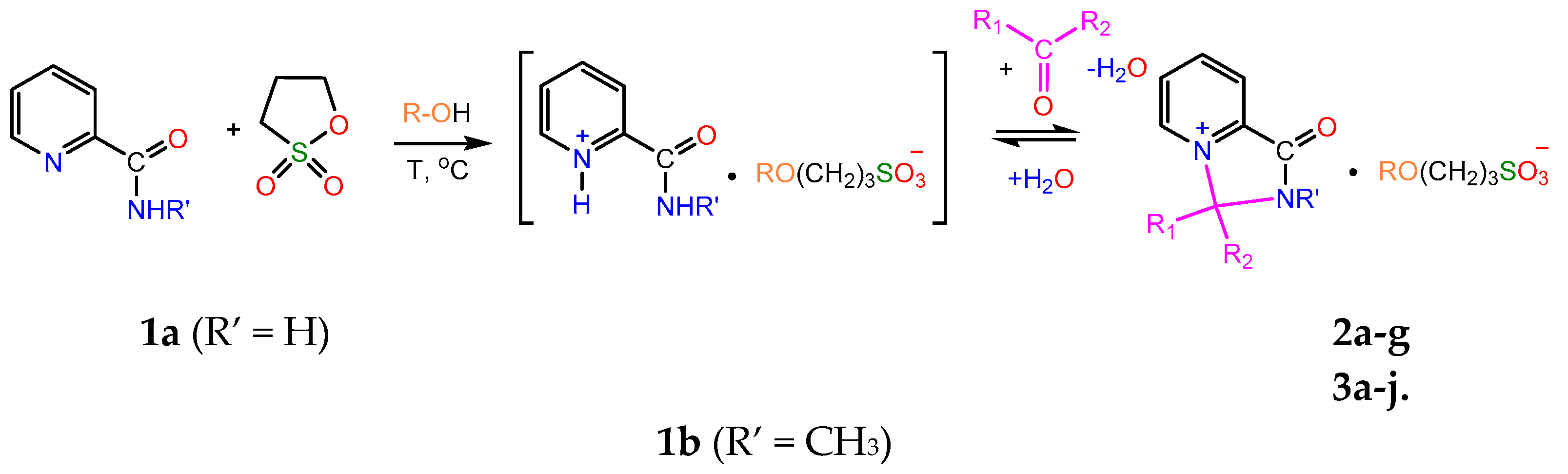
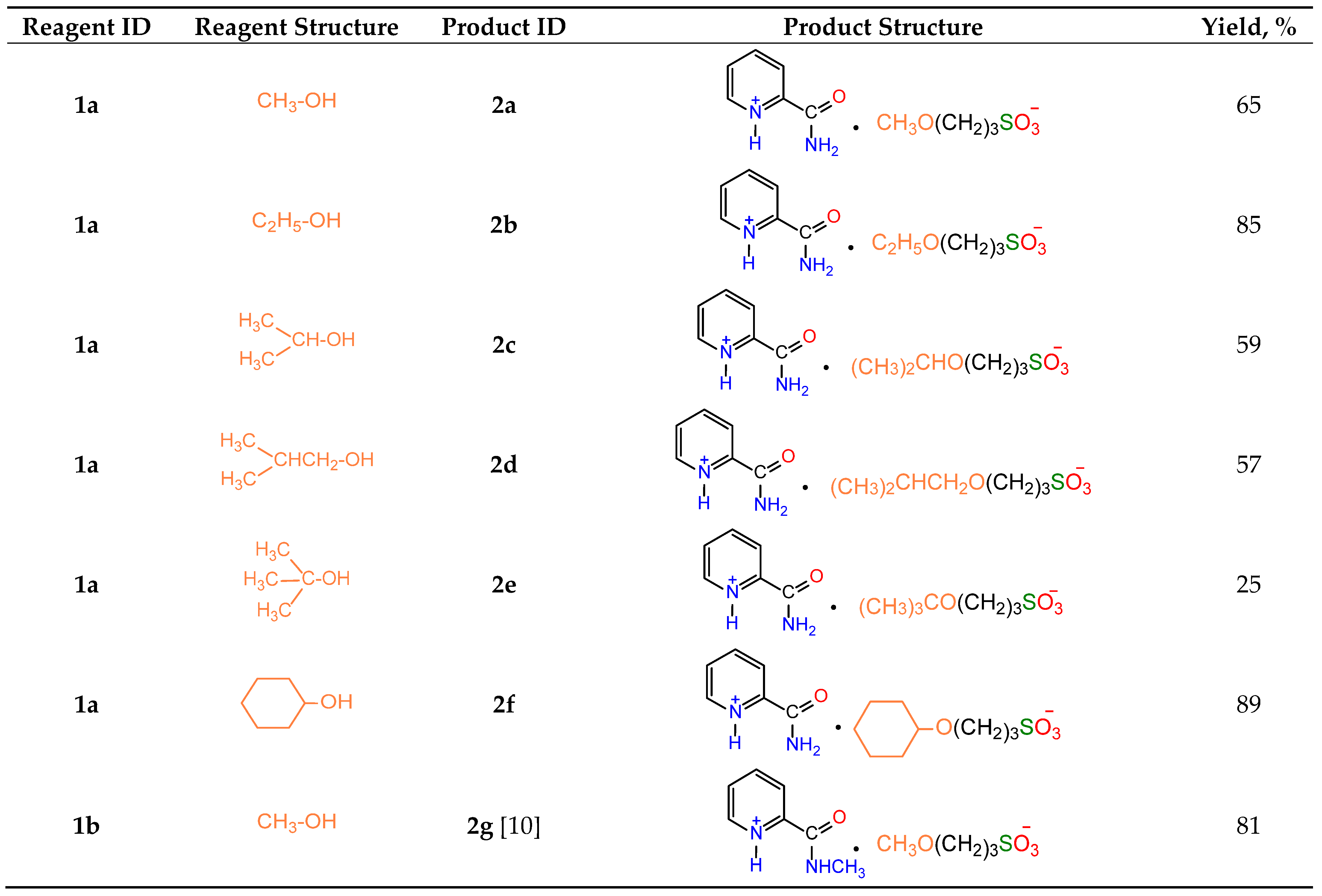
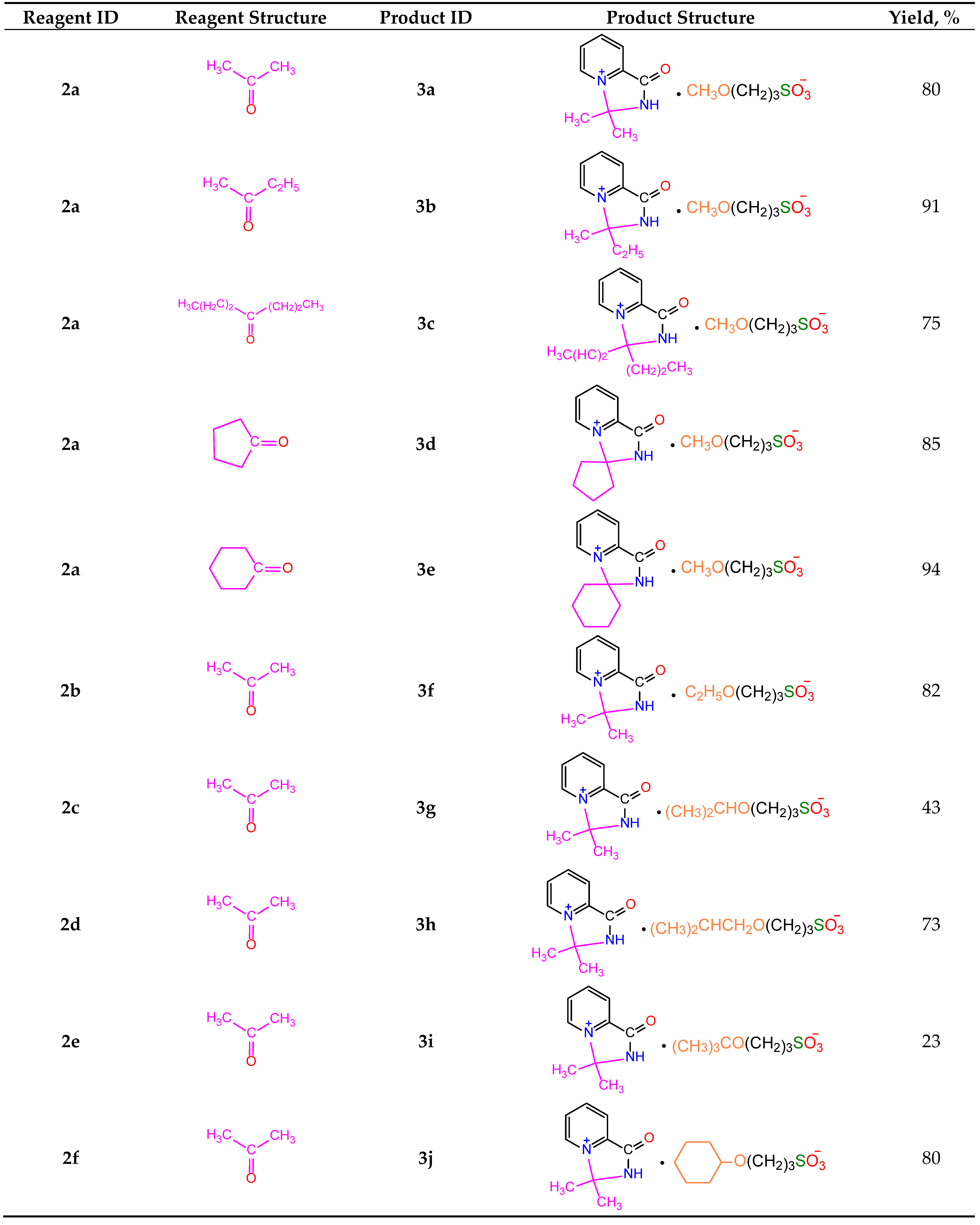
2.1. Synthesis
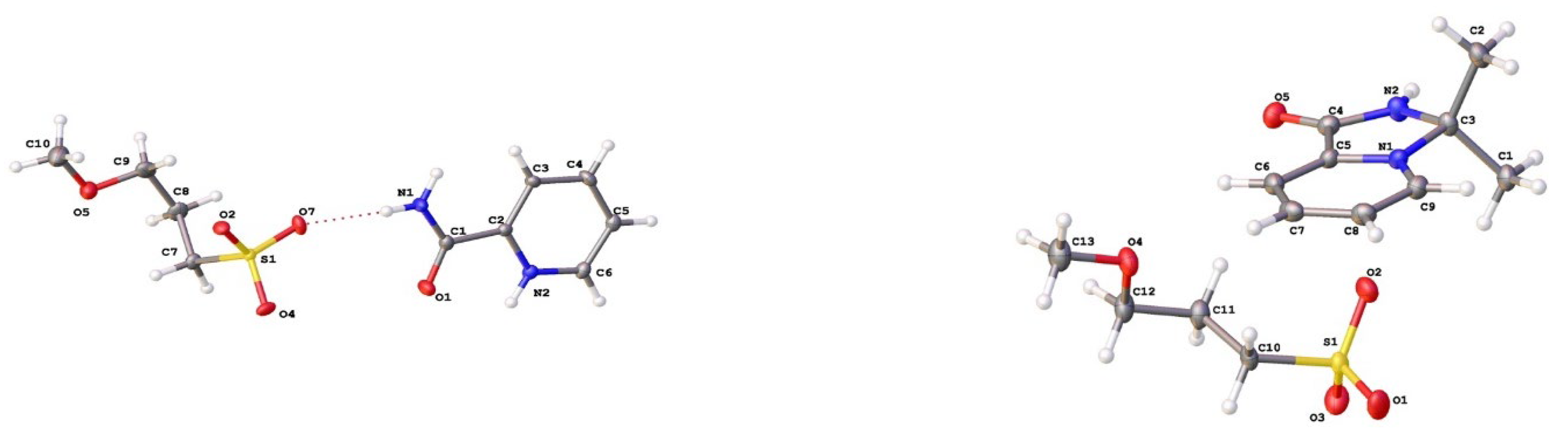
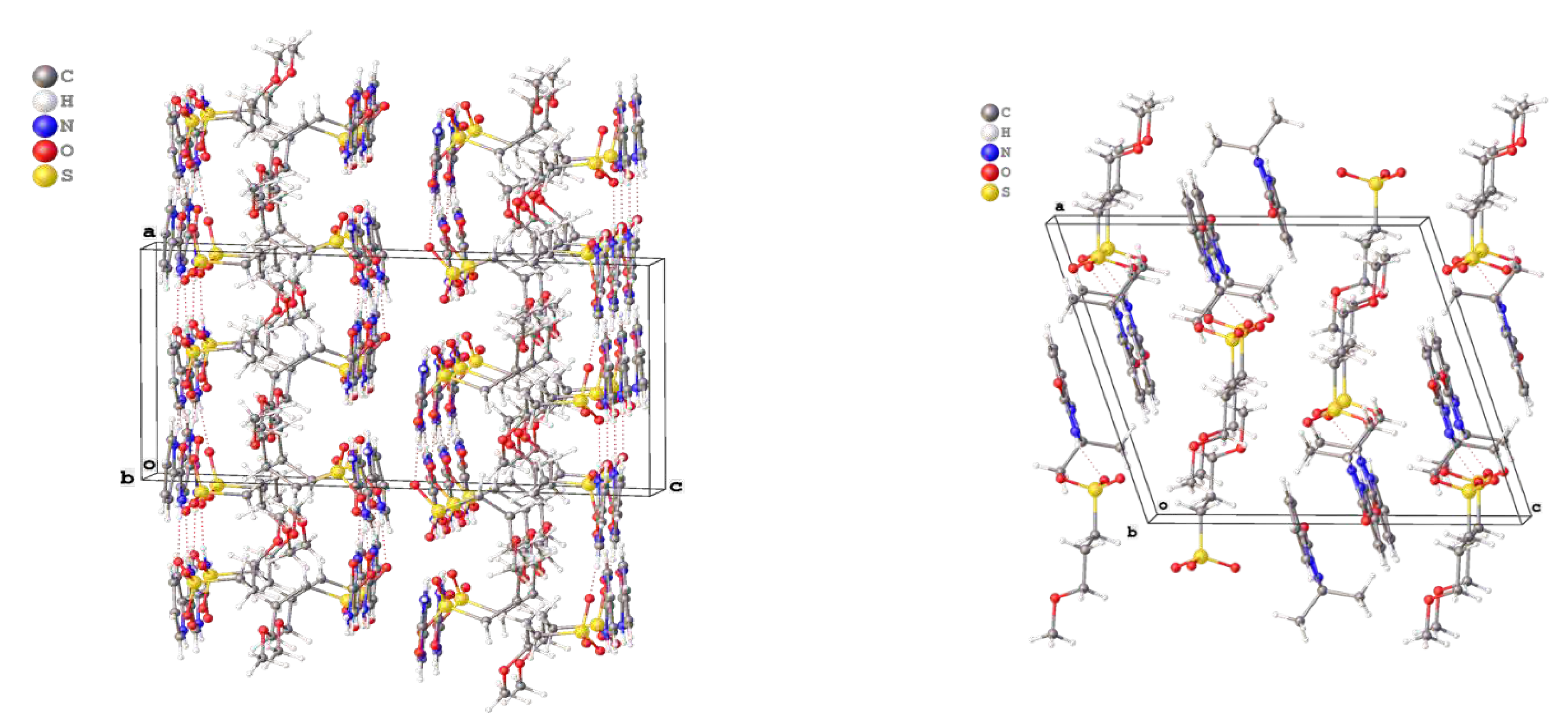
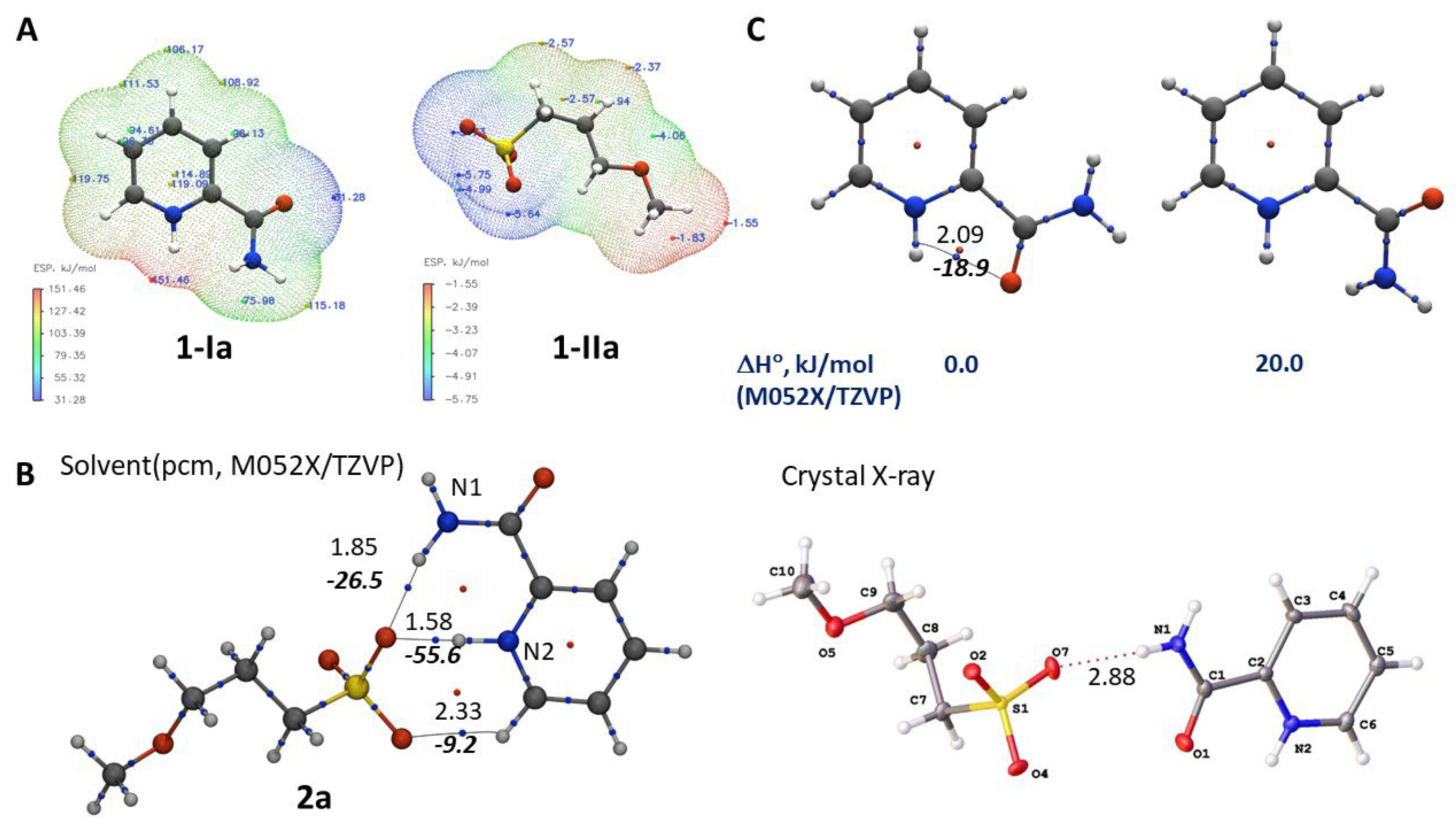
2.2. X-ray Study
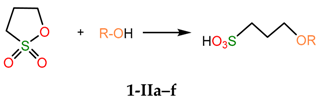
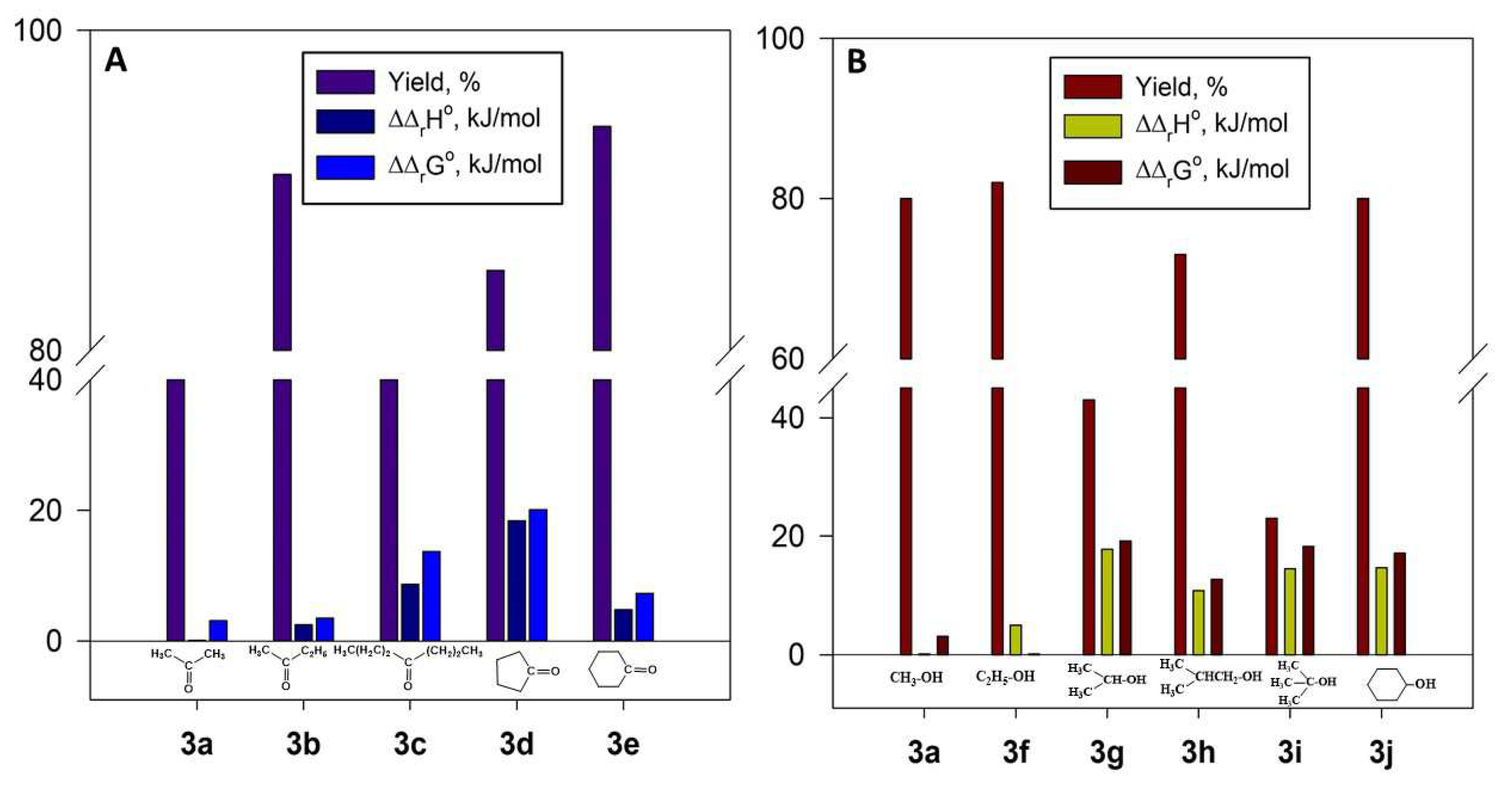
2.3. Reactions of Hydrolysis and Alcoholysis of Compounds 3a, 3d and 3e
2.4. Theoretical Study
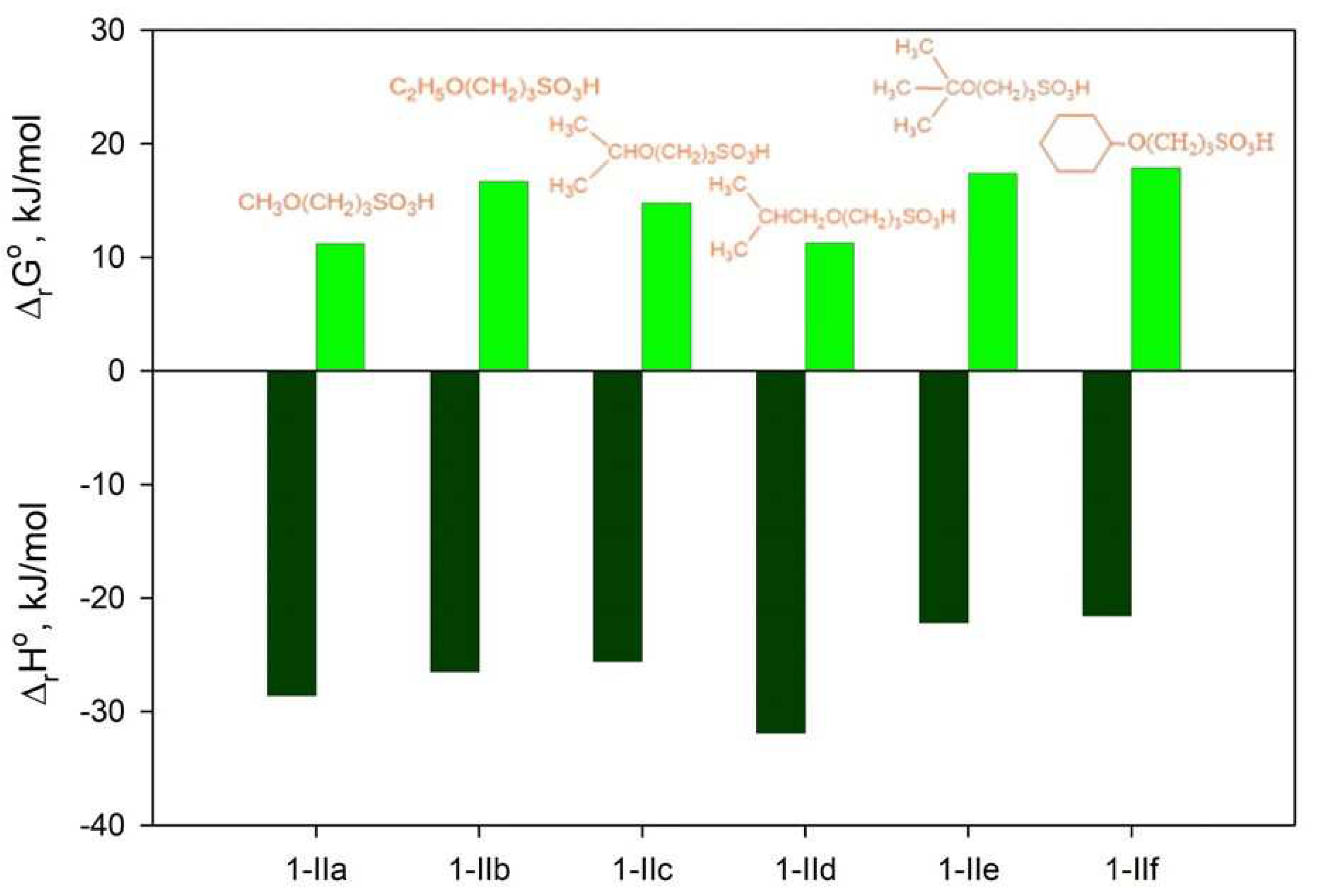
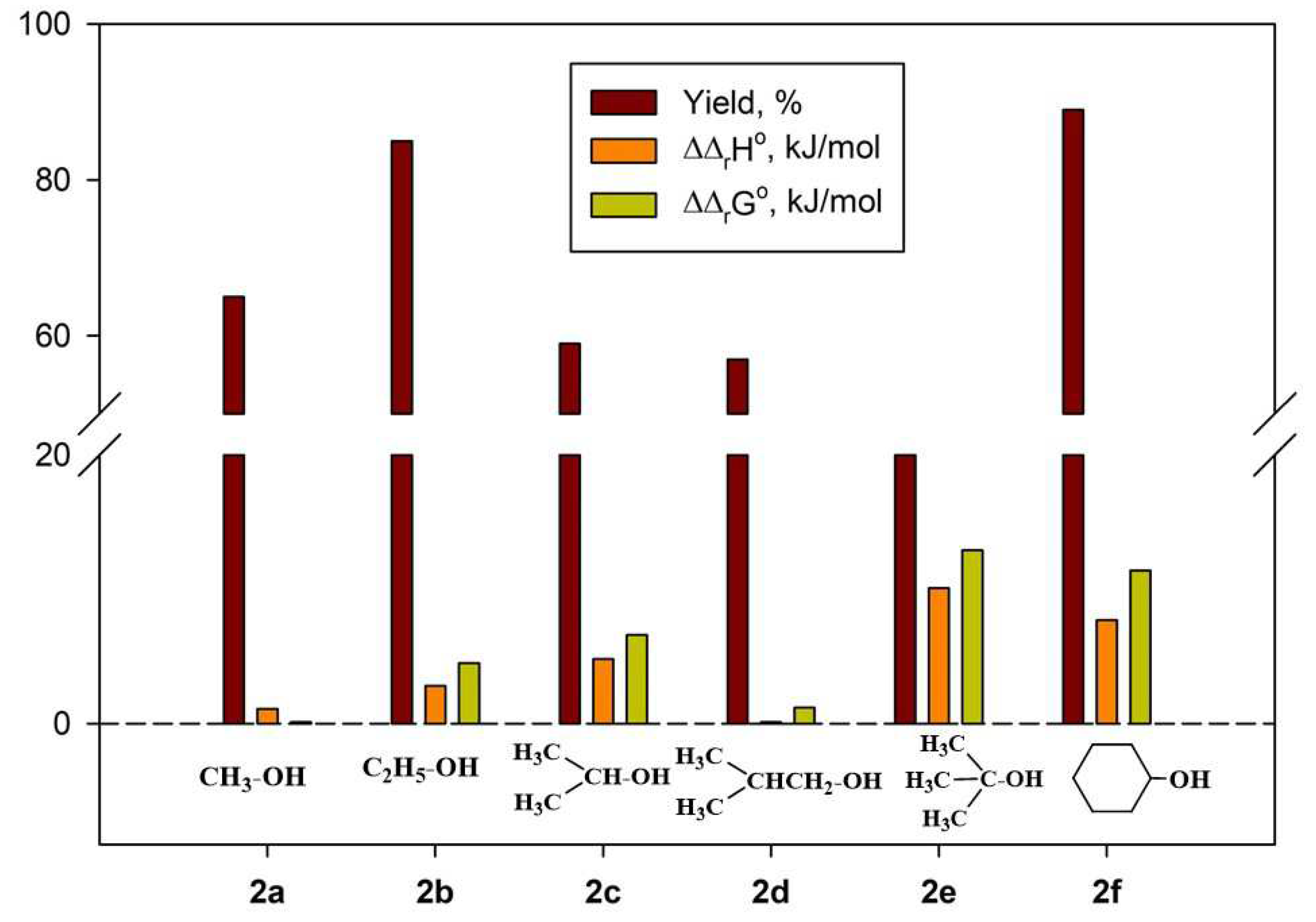
2.4.1. Thermodynamic Parameters of Formation for Compounds 2a-f
2.4.2. Structures of the Cation–Anion Complexes
2.4.3. Thermodynamic Parameters of 2a–g Formation
2.4.4. Thermodynamic Parameters of 3a-j Formation
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis
- a)
- 0.11 g (0.3 mmol) of 3a in 3 mL of water was stirred for 7 days at room temperature. After evaporation, 0.10 g (91%) of 3a was obtained.
- b)
- 0.36 g (1.1 mmol) of 3a in 4 mL of water was refluxed for 4 h. After evaporation and recrystallisation of the residue from CH3CN, 0.10 g (32%) of 2a was obtained.
- c)
- 0.31 g (0.97 mmol) of 3a in 4 mL of water was stirred at 70–80 °C for 5 h. After evaporation, 0.17 g (63%) of 2a was obtained.
- d)
- 0.11 g (0.3 mmol) of 3d in 4 mL of water was stirred at 70–80 °C for 5 h. After evaporation, 0.08 g of a mixture of 3a and 3d was obtained.
- e)
- 0.16 g (0.4 mmol) of 3e in 4 mL of methanol was refluxed for 16 h. After evaporation, 0.10 g of a mixture of 2a and 3e was obtained.
- f)
- 0.11 g (0.3 mmol) of 3e in 4 mL of water was stirred at 70–80 °C for 5 h. After evaporation, 0.08 g (97%) of 3a was obtained.
3.2. Calculation Details
3.3. X-ray Crystallographic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeBruler, C.; Hu, B.; Moss, J.; Liu, X.; Luo, J.; Sun, Y.; and Liu, T.L. Designer Two-Electron Storage Viologen Anolyte Materials for Neutral Aqueous Organic Redox Flow Batteries. Chem. 2017, 3, 961–978. [Google Scholar] [CrossRef]
- Ichikawa, T.; Kato, T.; and Ohno, H. (2012) 3D Continuous Water Nanosheet as a Gyroid Minimal Surface Formed by Bicontinuous Cubic Liquid-Crystalline Zwitterions. J. of the Amer. Chem. Soc. 2012, 134, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; El-Mekawy, R.E.-D.; and AbdelAal, M.T. Synthesis and antimicrobial evaluation of some new N-pyridinium, quinolinium, and isoquinolinium sulfonate derivatives. Phosphorus, Sulfur, and Silicon and the Related Elements 2016, 191, 1148–1154. [Google Scholar] [CrossRef]
- Frederickson, M.; Vuillard, L.; and Abell, C. Novel selenium containing non-detergent sulphobetaines. Tetrahedron Lett. 2003, 44, 7925–7928. [Google Scholar] [CrossRef]
- Bondock, S.; Khalifa, W.; Fadda, A.A. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur. J.l of Med. Chem. 2007, 42, 948–954. [Google Scholar] [CrossRef]
- Mostafa S. Amany, Bialy A. El Serry, Bayoumi A. Waleed, Ueda Youki, Ikeda Masanori, Nobuyuki, K.; Ali, A.M. Synthesis and In Vitro Activity of Pyrrolo [3,4-d]pyrimidine-2,5-diones as Potential Non-nucleoside HCV Inhibitors. Curr. Enz. Inhib. 2016, 12, 170–176. [CrossRef]
- Tian, X.; Liu, T.; Fang, B.; Wang, A.; Zhang, M.; Hussain, S.; Luo, L.; Zhang, R.; Zhang, Q.; Wu, J.; Battaglia, G.; Li, L.; Zhang, Z.; and Tian, Y. NeuN-Specific Fluorescent Probe Revealing Neuronal Nuclei Protein and Nuclear Acids Association in Living Neurons under STED Nanoscopy. ACS Appl. Mater. & Interf. 2018, 10, 31959–31964. [Google Scholar]
- Yan, P.; Acker, C.D.; and Loew, L.M. Tethered Bichromophoric Fluorophore Quencher Voltage Sensitive Dyes. ACS Sens. 2018, 3, 2621–2628. [Google Scholar] [CrossRef]
- Willner, I.; Ford, W.E. Preparation of zwitterionic electron acceptors and donors. J. of Heteroc. Chem. 1983, 20, 1113–1114. [Google Scholar] [CrossRef]
- Kramarova, E.P.; Borisevich, S.S.; Khamitov, E.M.; Korlyukov, A.A.; Dorovatovskii, P.V.; Shagina, A.D.; Mineev, K.S.; Tarasenko, D.V.; Novikov, R.A.; Lagunin, A.A.; Boldyrev, I.; Ezdoglian, A.A.; Karpechenko, N.Y.; Shmigol, T.A.; Baukov, Y.I.; and Negrebetsky, V.V. Pyridine Carboxamides Based on Sulfobetaines: Design, Reactivity, and Biological Activity. Molecules 2022, 27, 7542. [Google Scholar] [CrossRef]
- Koizumi, Y.; Arai, M.; Tomoda, H.; Omura, S. Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization. Biochim Biophys Acta 2004, 1693, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, W.J.; Kim, C.U.; Shih, K.M.; McGregor, D.N. Hetacillin (R)- and (S)-sulfoxides. Synthesis and structure-activity relationships. J. Med. Chem. 1978, 21, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Burns, H.D.; Dannals, R.F.; Langström, B.; Ravert, H.T.; Zemyan, S.E.; Duelfer, T.; Wong, D.F.; Frost, J.J.; Kuhar, M.J.; and Wagner, H.N. (3-N-[11C]methyl)spiperone, a ligand binding to dopamine receptors: radiochemical synthesis and biodistribution studies in mice. J. Nuc.l Med. 1984, 25, 1222–1227. [Google Scholar]
- Barrow, J.C.; Rittle, K.E.; Ngo, P.L.; Selnick, H.G.; Graham, S.L.; Pitzenberger, S.M.; McGaughey, G.B.; Colussi, D.; Lai, M.T.; Huang, Q.; Tugusheva, K.; Espeseth, A.S.; Simon, A.J.; Munshi, S.K.; Vacca, J.P. Design and synthesis of 2,3,5-substituted imidazolidin-4-one inhibitors of BACE-1. Chem. Med. Chem. 2007, 2, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Augelli-Szafran, C.E.; Roth, B.D.; Essenburg, A.; Hamelehle, K.L.; Krause, B.R.; Stanfield, R.L. Imidazolidinones and pyrazolones as novel ACAT inhibitors: Chemistry and biological activity. Bioorg. Med. Chem. Lett. 1994, 4, 1095–1100. [Google Scholar] [CrossRef]
- Rinnová, M.; Nefzi, A.; Houghten, R.A. Opioid activity of 4-imidazolidinone positional analogues of Leu-Enkephalin. Bioorg. Med. Chem. Lett. 2002, 12, 3175–3178. [Google Scholar]
- Liu, J.; Cui, G.; Zhao, M.; Cui, C.; Ju, J.; and Peng, S. Dual-acting agents that possess reversing resistance and anticancer activities: Design, synthesis, MES-SA/Dx5 cell assay, and SAR of Benzyl 1,2,3,5,11,11a-hexahydro-3,3-dimethyl-1-oxo-6H-imidazo [3’,4’:1,2]pyridin [3,4-b]indol-2-substitutedacetates. Bioorg. Med. Chem. 2007, 15, 7773–7788. [Google Scholar] [CrossRef]
- Vale, N.; Matos, J.; Gut, J.; Nogueira, F.; do Rosário, V.; Rosenthal, P.J.; Moreira, R.; Gomes, P. Imidazolidin-4-one peptidomimetic derivatives of primaquine: synthesis and antimalarial activity. Bioorg. Med. Chem. Lett. 2008, 18, 4150–4153. [Google Scholar]
- Vale, N.; Prudêncio, M.; Marques, C.A.; Collins, M.S.; Gut, J.; Nogueira, F.; Matos, J.; Rosenthal, P.J.; Cushion, M.T.; do Rosário, V.E.; Mota, M.M.; Moreira, R.; Gomes, P. Imidazoquines as antimalarial and antipneumocystis agents. J. Med. Chem. 2009, 52, 7800–7807. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Qian, K.; Zhang, X.; Lee, K.H.; Wu, J.; Liu, Y.N.; Peng, S. Benzyl 1,2,3,5,11,11a-hexahydro-3,3-dimethyl-1-oxo-6H-imidazo [3’,4’:1,2]pyridin [3,4-b]indole-2-substituted acetates: One-pot-preparation, anti-tumor activity, docking toward DNA and 3D QSAR analysis. Bioorg. Med. Chem. 2010, 18, 1910–1917. [Google Scholar] [CrossRef]
- Mostardeiro, M.A.; Ilha, V.; Dahmer, J.; Caro, M.S.; Dalcol, II, da Silva, U.F.; Morel, A.F. Cyclopeptide alkaloids: stereochemistry and synthesis of the precursors of discarines C and D and myrianthine A. J. Nat. Prod. 2013, 76, 1343–1350. [CrossRef]
- Ross, T.M.; Battista, K.; Bignan, G.C.; Brenneman, D.E.; Connolly, P.J.; Liu, J.; Middleton, S.A.; Orsini, M.; Reitz, A.B.; Rosenthal, D.I.; Scott, M.K.; Vaidya, A.H. A selective small molecule NOP (ORL-1 receptor) partial agonist for the treatment of anxiety. Bioorg. Med. Chem. Lett. 2015, 25, 602–606. [Google Scholar] [CrossRef]
- Morgan, B.J.; Kozlowski, M.C.; Song, A.; Wang, W. (2016) (5S)-2,2,3-Trimethyl-5-(phenylmethyl)-4-imidazolidinone. In Encyclopedia of Reagents for Organic Synthesis (EROS), 2016, 1-5.
- Bedos, P.; Amblard, M.; Subra, G.; Dodey, P.; Luccarini, J.M.; Paquet, J.L.; Pruneau, D.; Aumelas, A.; Martinez, J. A rational approach to the design and synthesis of a new bradykinin B(1) receptor antagonist. J. Med. Chem. 2000, 43, 2387–2394. [Google Scholar] [CrossRef]
- Vale, N.; Nogueira, F.; do Rosário, V.E.; Gomes, P.; Moreira, R. Primaquine dipeptide derivatives bearing an imidazolidin-4-one moiety at the N-terminus as potential antimalarial prodrugs. Eur. J. Med. Chem. 2009, 44, 2506–2516. [Google Scholar] [CrossRef]
- Blackmore, T.R.; and Thompson, P.E. (2012) ChemInform Abstract: Imidazolidin-4-ones: Their Syntheses and Applications. Heterocycl. 2011, 83, 1953–1975. [Google Scholar] [CrossRef]
- Curtius, T. Hydrazide und Azide organischer Säuren I. Abhandlung. Journal für Praktische Chem. 1894, 50, 275–294. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Eickhoff, J.A.; Minakova, A.S.; Gu, Z.; Lu, P.; Zakarian, A. Cutting-Edge and Time-Honored Strategies for Stereoselective Construction of C–N Bonds in Total Synthesis. Chem. Rev. 2016, 116, 4441–4557. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M.; Sarkar, A. The Curtius Rearrangement: Applications in Modern Drug Discovery and Medicinal Chemistry. Chem. Med. Chem. 2018, 13, 2351–2373. [Google Scholar] [CrossRef]
- Bariwal, J.; Van der Eycken, E. C–N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Gomez, S.; Peters, J.A.; Maschmeyer, T. (2002) The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv. Synt. & Cat. 2002, 344, 1037–1057. [Google Scholar]
- Swamy, K.C.; Kumar, N.N.; Balaraman, E.; Kumar, K.V. Mitsunobu and related reactions: advances and applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar]
- Magni, L.; Örtengren, B.; Sjöberg, B.; Wahlqvist, S. (1967) Stability, Absorption and Excretion Studies with Hetacillin. Scand. J. Clin. and Lab. Invest. 1967, 20, 195–201. [Google Scholar] [CrossRef]
- Brown, D.M.; Hannan, D.P.; Langley, P.F. Biotransformation of hetacillin to ampicillin in man. Tox. Appl. Pharm. 1969, 15, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, .; J.L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Jr. Montgomery, J.A.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.; Heyd J. J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; J.; Millam, M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Fox, D.J. (2016) Gaussian 09, Revision C. Wallingford CT.
- Zhurko, G.A. ChemCraft. Version 1.6 (build 332).
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theor. Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 15410. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A.; Bader, R.F.W. Calculation of magnetic response properties using atoms in molecules. Chem. Phys. Lett. 1992, 194, 1–8. [Google Scholar] [CrossRef]
- Keith, T.A.; Bader, R.F.W. Calculation of magnetic response properties using a continuous set of gauge transformations. Chemical Physics Lett. 1993, 210, 223–231. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; Abel, R.; Friesner, R.A.; Harder, E.D. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Bruker. (2016) APEX3, RLATT, CELL_NOW, TWINABS, SAINT-Plus and SADABS.
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. 2015, A 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sec. 2015, C 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; and Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
 |
 |
| Datablock | 2a | 3a |
|---|---|---|
| Formula moiety | C4H9O4S, C6H7N2O | 2(C4H9O4S), 2(C9H11N2O) |
| Brutto formula | C10H16N2O5S | C26H40N4O10S2 |
| Formula weight | 276.31 | 632.74 |
| Diffractometer | Bruker QUEST | Bruker QUEST |
| Scan mode | ω and ϕ scans | ω and ϕ scans |
| Anode [Wavelength, Å] | MoKα [0.71073] microfocus sealed X-ray tube | MoKα [0.71073] microfocus sealed X-ray tube |
| Crystal Dimensions, mm | 0.04 × 0.07 × 0.14 | 0.03 × 0.05 × 0.1 |
| Crystal colour | colourless | colourless |
| Crystal system | monoclinic | monoclinic |
| a, Å | 9.9079(5) | 12.422(2) |
| b, Å | 12.6799(6) | 8.7237(15) |
| c, Å | 19.8194(10) | 14.699(2) |
| α, ° | 90 | 90 |
| β, ° | 92.227(3) | 109.106(6) |
| γ, ° | 90 | 90 |
| Volume, Å3 | 2488.1(2) | 1505.1(4) |
| Density, g cm–3 | 1.475 | 1.396 |
| Temperature, K | 100 | 100 |
| Tmin/Tmax | 0.497553/0.746069 | 0.5954/0.7461 |
| μ, mm⁻¹ | 0.276 | 0.238 |
| Space group | P1211 | P121/n1 |
| Z | 8 | 2 |
| F(000) | 1168 | 672 |
| Reflections collected | 29400 | 13353 |
| Independent reflections | 29400 | 3544 |
| Reflections (I > 2σ(I)) | 27603 | 3030 |
| Parameters | 654 | 193 |
| Rint | 0.00 | 0.0598 |
| 2θmin – 2θmax, ° | 3.814 – 55.750 | 5.226 – 55.752 |
| wR2 (all reflections) | 0.1531 | 0.2011 |
| R1(I > σ(I)) | 0.0572 | 0.0730 |
| GOF | 1.055 | 0.972 |
| ρmin/ρmax, e Å–3 | –0.748/1.719 | –0.445/0.676 |
| Restraints | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





