Submitted:
07 November 2023
Posted:
08 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Gene | Chromosome location |
Disorder name | Penetrance and lifetime risk of HM |
Malignancy Types | Other manifestations |
| DDX41 | 5q35.3 | Familial MDS/AML with mutated DDX41 |
penetrance is incomplete | MDS, AML, t-MN, solid tumors, especially colon and prostate cancer and melanoma, but not yet definitively linked |
cytopenia, macrocytosis, autoimmune diseases |
| TP53 | 17p13.1 | Li-Fraumeni syndrome | lifetime risk of HM is about 6% | MDS, AML, lymphoma, ALL, t-MN, MM, osteosarcoma, breast cancer, brain tumors, soft tissue sarcoma, adrenocortica carcinoma and other solid tumors |
None |
| CEBPA | 19q13.1 | Familial AML with mutated CEBPA |
>80% lifetime risk of AML | AML | None |
| RUNX1 | 21q22.12 | Familial platelet disorder with propensity to myeloid malignancy |
unknown | MDS, AML, ALL, other lymphoid malignancies |
thrombocytopenia, platelet dysfunction, atopic and autoimmune disorders |
| ANKRD26 | 10p12.1 | Thrombocytopenia 2 | penetrance for thrombocytopenia is near complete, lifetime risk of HM is about 8% |
MDS, AML, CML, MPN, ALL, CLL, MM |
thrombocytopenia, leucocytosis, erythrocytosis, mild bleeding tendency |
| ETV6 | 12p13.2 | Thrombocytopenia 5 | Penetrance for thrombo- cytopenia is near complete |
ALL, MDS, AML, CMML, MM, GI cancers |
thrombocytopenia, macrocytosis, platelet dysfunction |
| SAMD9 | 7q21.2 | MIRAGE Syndrome | unknown | MDS, AML, CMML | bone marrow failure, cytopenia, infections, growth restriction, adrenal hypoplasia, enteropathy, genital abnormalities |
| SAMD9L | 7q21.2 | Ataxia Pancytopenia Syndrome |
unknown | MDS, AML, CMML | Systemic autoinflammatory disease, bone marrow failure, Ataxia |
| GATA2 | 3q21.3 | GATA2 deficiency syndrome |
penetrance is incomplete | MDS, AML, CMML, ALL | immunodeficiency, bone marrow failure, monocytopenia, lymphopenia, neutropenia, other cytopenia, infections, lymphedema, congenital deafness, pulmonary alveolar proteinosis, venous and arterial thrombosis |
| HM, Hematological malignancies; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; t-MN, therapy-related myeloid neoplasms; MM, multiple myeloma; MPN, myeloproliferative neoplasm; | |||||
2. Genes of Syndromes without Pre-Existing Disease or Organ Dysfunction
2.1. DDX41
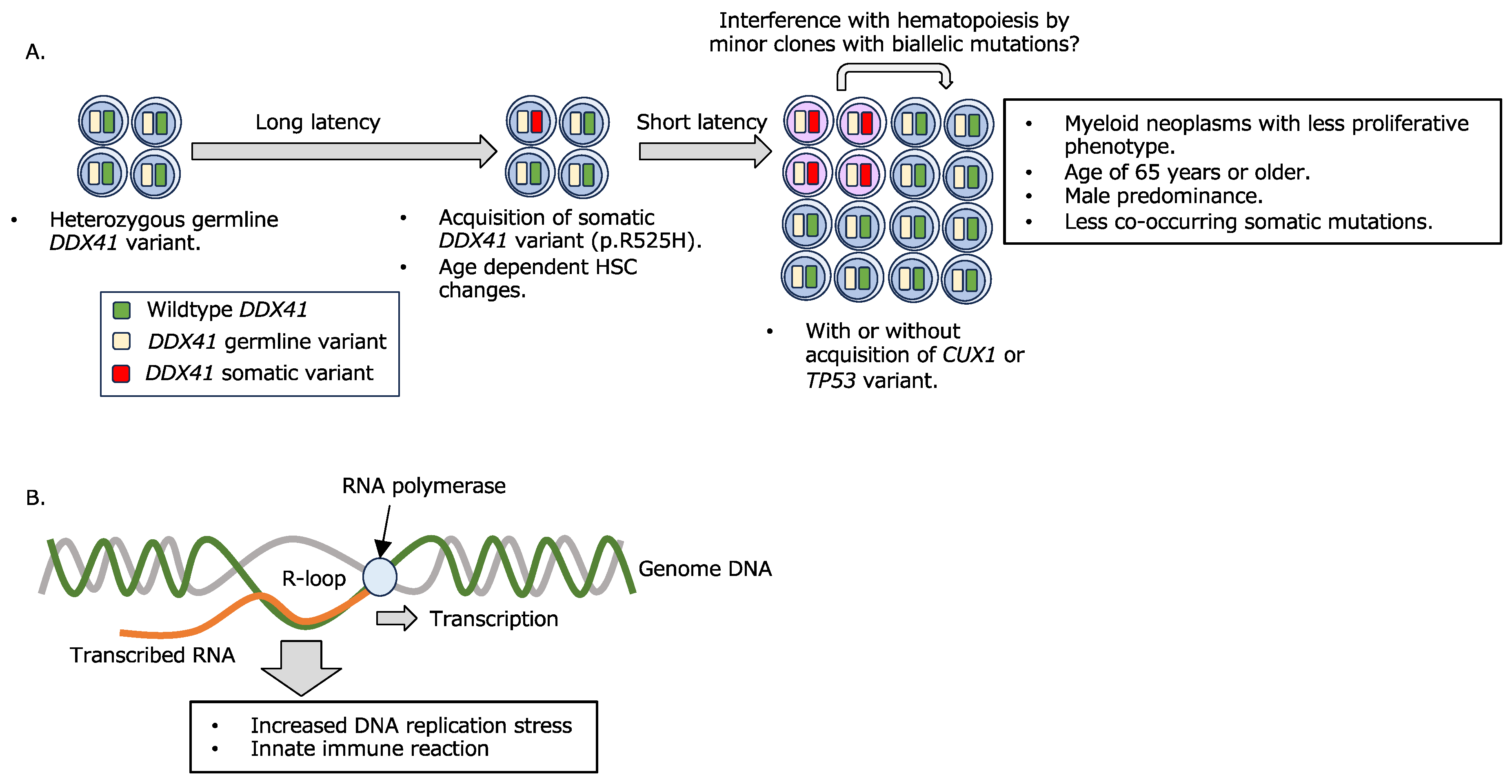
- A.
- A combination of germline and somatic DDX41 variants confers myeloid disease development.
- B.
- R-loop formation and its consequence.
2.2. TP53
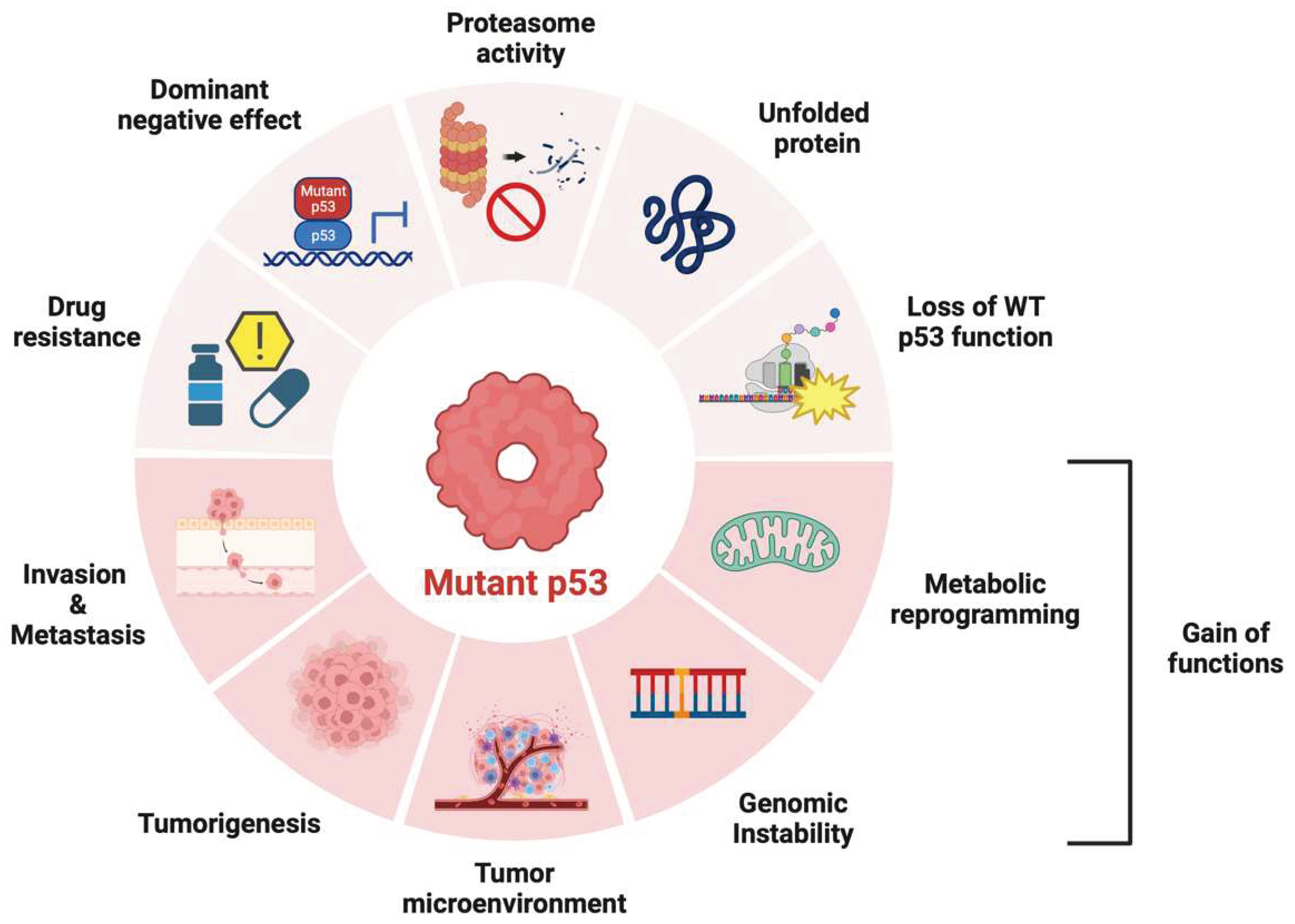
2.3. CEBPA
3. Genes of Syndromes Associated with Preexisting Platelet Disorders
3.1. RUNX1
3.2. ANKRD26
3.3. ETV6
4. Genes of Syndromes Associated with Other Organ Dysfunction
4.1. SAMD9/SAMD9L
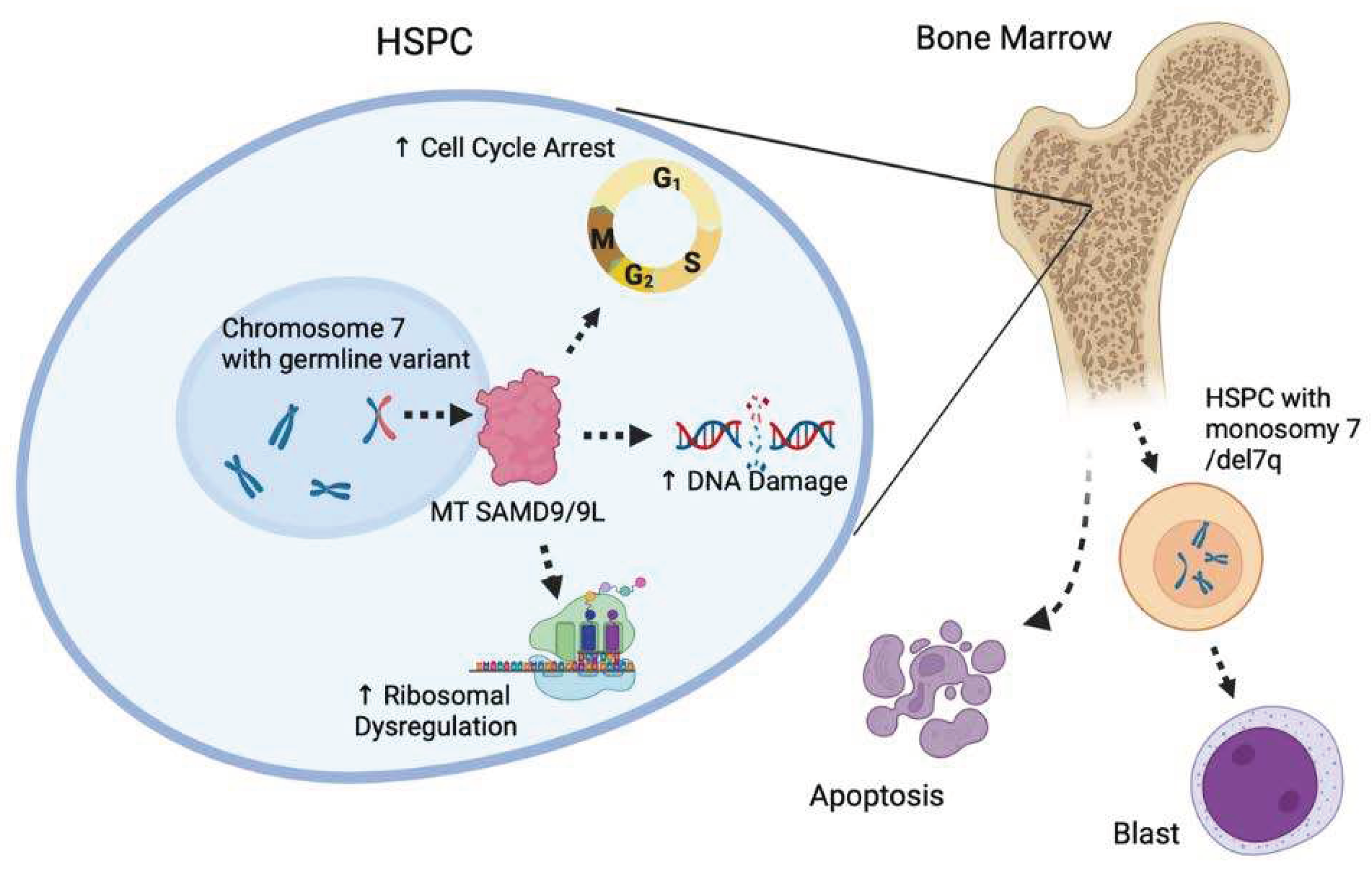
4.2. GATA2
4.3. IBMFS
5. Conclusions & Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corces-Zimmerman, M.R. and R. Majeti, Pre-leukemic evolution of hematopoietic stem cells: the importance of early mutations in leukemogenesis. Leukemia, 2014. 28(12): p. 2276-82. [CrossRef]
- Godley, L.A., Germline mutations in MDS/AML predisposition disorders. Curr Opin Hematol, 2021. 28(2): p. 86-93. [CrossRef]
- Fenwarth, L., et al., Hereditary Predisposition to Acute Myeloid Leukemia in Older Adults. Hemasphere, 2021. 5(4): p. e552. [CrossRef]
- Guijarro, F., et al., Germ line variants in patients with acute myeloid leukemia without a suspicion of hereditary hematologic malignancy syndrome. Blood Adv, 2023. [CrossRef]
- Churpek, J.E., Familial myelodysplastic syndrome/acute myeloid leukemia. Best Pract Res Clin Haematol, 2017. 30(4): p. 287-289. [CrossRef]
- Hamidi, A., et al., Clinical guideline variability in the diagnosis of hereditary hematopoietic malignancy syndromes. Leuk Lymphoma, 2023. 64(9): p. 1562-1565. [CrossRef]
- Stieglitz, E. and M.L. Loh, Genetic predispositions to childhood leukemia. Ther Adv Hematol, 2013. 4(4): p. 270-90. [CrossRef]
- Babushok, D.V. and M. Bessler, Genetic predisposition syndromes: when should they be considered in the work-up of MDS? Best Pract Res Clin Haematol, 2015. 28(1): p. 55-68. [CrossRef]
- Kotmayer, L., K. Kallay, and C. Bodor, [Hereditary haematological malignancies]. Magy Onkol, 2020. 64(1): p. 43-55.
- Furutani, E. and A. Shimamura, Germline Genetic Predisposition to Hematologic Malignancy. J Clin Oncol, 2017. 35(9): p. 1018-1028. [CrossRef]
- Zahid, M.F., et al., Cytogenetic Abnormalities in Myelodysplastic Syndromes: An Overview. Int J Hematol Oncol Stem Cell Res, 2017. 11(3): p. 231-239. [CrossRef]
- Yoshida, M., et al., Prevalence of germline GATA2 and SAMD9/9L variants in paediatric haematological disorders with monosomy 7. Br J Haematol, 2020. 191(5): p. 835-843. [CrossRef]
- Sahoo, S.S., E.J. Kozyra, and M.W. Wlodarski, Germline predisposition in myeloid neoplasms: Unique genetic and clinical features of GATA2 deficiency and SAMD9/SAMD9L syndromes. Best Pract Res Clin Haematol, 2020. 33(3): p. 101197. [CrossRef]
- Rafei, H. and C.D. DiNardo, Hereditary myeloid malignancies. Best Pract Res Clin Haematol, 2019. 32(2): p. 163-176. [CrossRef]
- Zhang, M.Y., et al., Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet, 2015. 47(2): p. 180-5. [CrossRef]
- Tawana, K., A.L. Brown, and J.E. Churpek, Integrating germline variant assessment into routine clinical practice for myelodysplastic syndrome and acute myeloid leukaemia: current strategies and challenges. Br J Haematol, 2022. 196(6): p. 1293-1310. [CrossRef]
- Arber, D.A., et al., The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 2016. 127(20): p. 2391-405. [CrossRef]
- Bohnsack, K.E., et al., Cellular functions of eukaryotic RNA helicases and their links to human diseases. Nat Rev Mol Cell Biol, 2023. [CrossRef]
- Fairman-Williams, M.E., U.P. Guenther, and E. Jankowsky, SF1 and SF2 helicases: family matters. Curr Opin Struct Biol, 2010. 20(3): p. 313-24. [CrossRef]
- Makishima, H., et al., Germ line DDX41 mutations define a unique subtype of myeloid neoplasms. Blood, 2023. 141(5): p. 534-549. [CrossRef]
- Yang, F., et al., Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult patients with AML. Blood, 2022. 139(8): p. 1208-1221. [CrossRef]
- Li, P., et al., The genetic landscape of germline DDX41 variants predisposing to myeloid neoplasms. Blood, 2022. 140(7): p. 716-755. [CrossRef]
- Cheloor Kovilakam, S., et al., Prevalence and significance of DDX41 gene variants in the general population. Blood, 2023. [CrossRef]
- Makishima, H., T.V. Bowman, and L.A. Godley, DDX41-associated susceptibility to myeloid neoplasms. Blood, 2023. 141(13): p. 1544-1552. [CrossRef]
- Sébert, M., et al., Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood, 2019. 134(17): p. 1441-1444. [CrossRef]
- Molteni, E., et al., Prevalence and clinical expression of germline predisposition to myeloid neoplasms in adults with marrow hypocellularity. Blood, 2023.
- Kadono, M., et al., Biological implications of somatic DDX41 p.R525H mutation in acute myeloid leukemia. Exp Hematol, 2016. 44(8): p. 745-754.e4. [CrossRef]
- Tierens, A., et al., Biallelic disruption of DDX41 activity is associated with distinct genomic and immunophenotypic hallmarks in acute leukemia. Front Oncol, 2023. 13: p. 1153082. [CrossRef]
- Badar, T., et al., Clinical and molecular correlates of somatic and germline DDX41 variants in patients and families with myeloid neoplasms. Haematologica, 2023. [CrossRef]
- Kobayashi, S., et al., Donor cell leukemia arising from preleukemic clones with a novel germline DDX41 mutation after allogenic hematopoietic stem cell transplantation. Leukemia, 2017. 31(4): p. 1020-1022. [CrossRef]
- Rolles, B., et al., DDX41 germline variants causing donor cell leukemia indicate a need for further genetic workup in the context of hematopoietic stem cell transplantation. Blood Cancer J, 2023. 13(1): p. 73. [CrossRef]
- Berger, G., et al., Re-emergence of acute myeloid leukemia in donor cells following allogeneic transplantation in a family with a germline DDX41 mutation. Leukemia, 2017. 31(2): p. 520-522. [CrossRef]
- Hirsch, P., et al., Successive relapses from donor and host cells in a patient with DEAD-box helicase 41 (DDX41)-associated myelodysplastic syndrome: The lessons to be learned. Br J Haematol, 2022. 199(4): p. 623-626. [CrossRef]
- Huo, L., et al., Causative germline variant p.Y259C of DDX41 recurrently identified in acute lymphoblastic leukaemia. Br J Haematol, 2023. 202(1): p. 199-203.
- Jelloul, F.Z., et al., DDX41 mutations in patients with non-myeloid hematologic neoplasms. Am J Hematol, 2023. 98(8): p. E193-e196.
- Ma, J., et al., DDX41 is needed for pre- and postnatal hematopoietic stem cell differentiation in mice. Stem Cell Reports, 2022. 17(4): p. 879-893. [CrossRef]
- Chlon, T.M., et al., Germline DDX41 mutations cause ineffective hematopoiesis and myelodysplasia. Cell Stem Cell, 2021. 28(11): p. 1966-1981.e6. [CrossRef]
- Chen, L., et al., The Augmented R-Loop Is a Unifying Mechanism for Myelodysplastic Syndromes Induced by High-Risk Splicing Factor Mutations. Mol Cell, 2018. 69(3): p. 412-425.e6. [CrossRef]
- Nguyen, H.D., et al., Spliceosome Mutations Induce R Loop-Associated Sensitivity to ATR Inhibition in Myelodysplastic Syndromes. Cancer Res, 2018. 78(18): p. 5363-5374. [CrossRef]
- Singh, S., et al., SF3B1 mutations induce R-loop accumulation and DNA damage in MDS and leukemia cells with therapeutic implications. Leukemia, 2020. 34(9): p. 2525-2530. [CrossRef]
- Cusan, M., et al., SF3B1 mutation and ATM deletion co-drive leukemogenesis via centromeric R-loop dysregulation. J Clin Invest, 2023.
- Weinreb, J.T., et al., Excessive R-loops trigger an inflammatory cascade leading to increased HSPC production. Dev Cell, 2021. 56(5): p. 627-640.e5. [CrossRef]
- Mosler, T., et al., R-loop proximity proteomics identifies a role of DDX41 in transcription-associated genomic instability. Nat Commun, 2021. 12(1): p. 7314. [CrossRef]
- Shinriki, S., et al., DDX41 coordinates RNA splicing and transcriptional elongation to prevent DNA replication stress in hematopoietic cells. Leukemia, 2022. 36(11): p. 2605-2620. [CrossRef]
- Polprasert, C., et al., Inherited and Somatic Defects in DDX41 in Myeloid Neoplasms. Cancer Cell, 2015. 27(5): p. 658-70. [CrossRef]
- Yoshida, K., et al., Frequent pathway mutations of splicing machinery in myelodysplasia. Nature, 2011. 478(7367): p. 64-9. [CrossRef]
- Cvitkovic, I. and M.S. Jurica, Spliceosome database: a tool for tracking components of the spliceosome. Nucleic Acids Res, 2013. 41(Database issue): p. D132-41. [CrossRef]
- Singh, R.S., et al., DDX41 is required for cGAS-STING activation against DNA virus infection. Cell Rep, 2022. 39(8): p. 110856.
- Crossley, M.P., et al., R-loop-derived cytoplasmic RNA-DNA hybrids activate an immune response. Nature, 2023. 613(7942): p. 187-194. [CrossRef]
- Challakkara, M.F. and R. Chhabra, snoRNAs in hematopoiesis and blood malignancies: A comprehensive review. J Cell Physiol, 2023. 238(6): p. 1207-1225. [CrossRef]
- Dong, J., et al., Small but strong: Pivotal roles and potential applications of snoRNAs in hematopoietic malignancies. Front Oncol, 2022. 12: p. 939465. [CrossRef]
- Tungalag, S., et al., Ribosome profiling analysis reveals the roles of DDX41 in translational regulation. Int J Hematol, 2023. 117(6): p. 876-888. [CrossRef]
- Ramdzan, Z.M. and A. Nepveu, CUX1, a haploinsufficient tumour suppressor gene overexpressed in advanced cancers. Nat Rev Cancer, 2014. 14(10): p. 673-82. [CrossRef]
- Imgruet, M.K., et al., Loss of a 7q gene, CUX1, disrupts epigenetically driven DNA repair and drives therapy-related myeloid neoplasms. Blood, 2021. 138(9): p. 790-805. [CrossRef]
- Leroy, B., M. Anderson, and T. Soussi, TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat, 2014. 35(6): p. 672-88. [CrossRef]
- Hernandez Borrero, L.J. and W.S. El-Deiry, Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer, 2021. 1876(1): p. 188556.
- Eisenstein, M., p53: an anticancer protein's chequered past and promising future. Nature, 2022. 603(7899): p. S1.
- Usman, R.M., et al., Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia Pac J Clin Oncol, 2021. 17(3): p. 193-208. [CrossRef]
- Mantovani, F., L. Collavin, and G. Del Sal, Mutant p53 as a guardian of the cancer cell. Cell Death Differ, 2019. 26(2): p. 199-212. [CrossRef]
- Lapke, N., et al., Missense mutations in the TP53 DNA-binding domain predict outcomes in patients with advanced oral cavity squamous cell carcinoma. Oncotarget, 2016. 7(28): p. 44194-44210. [CrossRef]
- Hansen, S., T.R. Hupp, and D.P. Lane, Allosteric regulation of the thermostability and DNA binding activity of human p53 by specific interacting proteins. CRC Cell Transformation Group. J Biol Chem, 1996. 271(7): p. 3917-24. [CrossRef]
- Alvarado-Ortiz, E., et al., Mutant p53 Gain-of-Function: Role in Cancer Development, Progression, and Therapeutic Approaches. Front Cell Dev Biol, 2020. 8: p. 607670. [CrossRef]
- Gencel-Augusto, J. and G. Lozano, p53 tetramerization: at the center of the dominant-negative effect of mutant p53. Genes Dev, 2020. 34(17-18): p. 1128-1146. [CrossRef]
- Zhu, G., et al., Mutant p53 in Cancer Progression and Targeted Therapies. Front Oncol, 2020. 10: p. 595187. [CrossRef]
- Keymling, M., et al., [Li-Fraumeni syndrome]. Radiologie (Heidelb), 2022. 62(12): p. 1026-1032.
- Sejben, A., et al., [Li-Fraumeni syndrome]. Orv Hetil, 2019. 160(6): p. 228-234.
- Qian, M., et al., TP53 Germline Variations Influence the Predisposition and Prognosis of B-Cell Acute Lymphoblastic Leukemia in Children. J Clin Oncol, 2018. 36(6): p. 591-599. [CrossRef]
- Comeaux, E.Q. and C.G. Mullighan, TP53 Mutations in Hypodiploid Acute Lymphoblastic Leukemia. Cold Spring Harb Perspect Med, 2017. 7(3). [CrossRef]
- Swaminathan, M., et al., Hematologic malignancies and Li-Fraumeni syndrome. Cold Spring Harb Mol Case Stud, 2019. 5(1). [CrossRef]
- Preudhomme, C., et al., Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA). Blood, 2002. 100(8): p. 2717-23. [CrossRef]
- Pathak, A., et al., Whole exome sequencing reveals a C-terminal germline variant in CEBPA-associated acute myeloid leukemia: 45-year follow up of a large family. Haematologica, 2016. 101(7): p. 846-52. [CrossRef]
- Tawana, K. and J. Fitzgibbon, CEBPA-Associated Familial Acute Myeloid Leukemia (AML), in GeneReviews((R)), M.P. Adam, et al., Editors. 1993: Seattle (WA).
- West, A.H., L.A. Godley, and J.E. Churpek, Familial myelodysplastic syndrome/acute leukemia syndromes: a review and utility for translational investigations. Ann N Y Acad Sci, 2014. 1310(1): p. 111-8. [CrossRef]
- Godley, L.A., Inherited predisposition to acute myeloid leukemia. Semin Hematol, 2014. 51(4): p. 306-21. [CrossRef]
- Harrigan, A.M. and A.M. Trottier, Hereditary acute myeloid leukemia associated with C-terminal CEBPA germline variants. Fam Cancer, 2023. 22(3): p. 331-339. [CrossRef]
- Pabst, T., et al., Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet, 2001. 27(3): p. 263-70.
- Frohling, S., et al., CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol, 2004. 22(4): p. 624-33. [CrossRef]
- Brown, A.L., C.N. Hahn, and H.S. Scott, Secondary leukemia in patients with germline transcription factor mutations (RUNX1, GATA2, CEBPA). Blood, 2020. 136(1): p. 24-35. [CrossRef]
- Xiao, H., et al., First report of multiple CEBPA mutations contributing to donor origin of leukemia relapse after allogeneic hematopoietic stem cell transplantation. Blood, 2011. 117(19): p. 5257-60. [CrossRef]
- Homan, C.C., H.S. Scott, and A.L. Brown, Hereditary platelet disorders associated with germ line variants in RUNX1, ETV6, and ANKRD26. Blood, 2023. 141(13): p. 1533-1543.
- Galera, P., A. Dulau-Florea, and K.R. Calvo, Inherited thrombocytopenia and platelet disorders with germline predisposition to myeloid neoplasia. Int J Lab Hematol, 2019. 41 Suppl 1: p. 131-141. [CrossRef]
- Asou, N., The role of a Runt domain transcription factor AML1/RUNX1 in leukemogenesis and its clinical implications. Crit Rev Oncol Hematol, 2003. 45(2): p. 129-50.
- Forster, A., et al., Beyond Pathogenic RUNX1 Germline Variants: The Spectrum of Somatic Alterations in RUNX1-Familial Platelet Disorder with Predisposition to Hematologic Malignancies. Cancers (Basel), 2022. 14(14). [CrossRef]
- Okumura, A.J., et al., t(8;21)(q22;q22) Fusion proteins preferentially bind to duplicated AML1/RUNX1 DNA-binding sequences to differentially regulate gene expression. Blood, 2008. 112(4): p. 1392-401.
- Homan, C.C., et al., The RUNX1 database (RUNX1db): establishment of an expert curated RUNX1 registry and genomics database as a public resource for familial platelet disorder with myeloid malignancy. Haematologica, 2021. 106(11): p. 3004-3007. [CrossRef]
- Kamath-Loeb, A.S., et al., Accurate detection of subclonal variants in paired diagnosis-relapse acute myeloid leukemia samples by next generation Duplex Sequencing. Leuk Res, 2022. 115: p. 106822. [CrossRef]
- Zharlyganova, D., et al., High frequency of AML1/RUNX1 point mutations in radiation-associated myelodysplastic syndrome around Semipalatinsk nuclear test site. J Radiat Res, 2008. 49(5): p. 549-55.
- Sendker, S., et al., RUNX1 mutation has no prognostic significance in paediatric AML: a retrospective study of the AML-BFM study group. Leukemia, 2023. 37(7): p. 1435-1443. [CrossRef]
- Sood, R., Y. Kamikubo, and P. Liu, Role of RUNX1 in hematological malignancies. Blood, 2017. 129(15): p. 2070-2082. [CrossRef]
- Hayashi, Y., et al., Myeloid neoplasms with germ line RUNX1 mutation. Int J Hematol, 2017. 106(2): p. 183-188. [CrossRef]
- Bellissimo, D.C. and N.A. Speck, RUNX1 Mutations in Inherited and Sporadic Leukemia. Front Cell Dev Biol, 2017. 5: p. 111. [CrossRef]
- Ng, I.K., et al., Preleukemic and second-hit mutational events in an acute myeloid leukemia patient with a novel germline RUNX1 mutation. Biomark Res, 2018. 6: p. 16. [CrossRef]
- Brown, A.L., et al., RUNX1-mutated families show phenotype heterogeneity and a somatic mutation profile unique to germline predisposed AML. Blood Adv, 2020. 4(6): p. 1131-1144. [CrossRef]
- Hong, D., et al., RUNX1-dependent mechanisms in biological control and dysregulation in cancer. J Cell Physiol, 2019. 234(6): p. 8597-8609. [CrossRef]
- Deuitch, N., et al., RUNX1 Familial Platelet Disorder with Associated Myeloid Malignancies, in GeneReviews((R)), M.P. Adam, et al., Editors. 1993: Seattle (WA).
- Vyas, H., et al., Prevalence and natural history of variants in the ANKRD26 gene: a short review and update of reported cases. Platelets, 2022. 33(8): p. 1107-1112. [CrossRef]
- Ferrari, S., et al., A novel RUNX1 mutation with ANKRD26 dysregulation is related to thrombocytopenia in a sporadic form of myelodysplastic syndrome. Aging Clin Exp Res, 2021. 33(7): p. 1987-1992. [CrossRef]
- Sullivan, M.J., E.L. Palmer, and J.P. Botero, ANKRD26-Related Thrombocytopenia and Predisposition to Myeloid Neoplasms. Curr Hematol Malig Rep, 2022. 17(5): p. 105-112. [CrossRef]
- Kennedy, A.L. and A. Shimamura, Genetic predisposition to MDS: clinical features and clonal evolution. Blood, 2019. 133(10): p. 1071-1085. [CrossRef]
- Melazzini, F., et al., Clinical and pathogenic features of ETV6-related thrombocytopenia with predisposition to acute lymphoblastic leukemia. Haematologica, 2016. 101(11): p. 1333-1342. [CrossRef]
- Noetzli, L., et al., Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet, 2015. 47(5): p. 535-538. [CrossRef]
- Wang, Q., et al., ETV6 mutation in a cohort of 970 patients with hematologic malignancies. Haematologica, 2014. 99(10): p. e176-8. [CrossRef]
- Di Paola, J. and C.C. Porter, ETV6-related thrombocytopenia and leukemia predisposition. Blood, 2019. 134(8): p. 663-667.
- Rodriguez-Hernandez, G., et al., The Second Oncogenic Hit Determines the Cell Fate of ETV6-RUNX1 Positive Leukemia. Front Cell Dev Biol, 2021. 9: p. 704591. [CrossRef]
- Filipiuk, A., et al., Genetic Disorders with Predisposition to Paediatric Haematopoietic Malignancies-A Review. Cancers (Basel), 2022. 14(15). [CrossRef]
- Schafer, D., et al., Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood, 2018. 131(7): p. 821-826. [CrossRef]
- Wong, J.C., et al., Germline SAMD9 and SAMD9L mutations are associated with extensive genetic evolution and diverse hematologic outcomes. JCI Insight, 2018. 3(14). [CrossRef]
- Tesi, B., et al., Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood, 2017. 129(16): p. 2266-2279. [CrossRef]
- Pastor, V.B., et al., Constitutional SAMD9L mutations cause familial myelodysplastic syndrome and transient monosomy 7. Haematologica, 2018. 103(3): p. 427-437. [CrossRef]
- Mekhedov, S.L., K.S. Makarova, and E.V. Koonin, The complex domain architecture of SAMD9 family proteins, predicted STAND-like NTPases, suggests new links to inflammation and apoptosis. Biol Direct, 2017. 12(1): p. 13. [CrossRef]
- Nagamachi, A., et al., Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell, 2013. 24(3): p. 305-17. [CrossRef]
- Nagamachi, A., et al., Multiorgan failure with abnormal receptor metabolism in mice mimicking Samd9/9L syndromes. J Clin Invest, 2021. 131(4).
- Meng, X. and Y. Xiang, RNA granules associated with SAMD9-mediated poxvirus restriction are similar to antiviral granules in composition but do not require TIA1 for poxvirus restriction. Virology, 2019. 529: p. 16-22. [CrossRef]
- Zhang, F., et al., Human SAMD9 is a poxvirus-activatable anticodon nuclease inhibiting codon-specific protein synthesis. Sci Adv, 2023. 9(23): p. eadh8502. [CrossRef]
- Davidsson, J., et al., SAMD9 and SAMD9L in inherited predisposition to ataxia, pancytopenia, and myeloid malignancies. Leukemia, 2018. 32(5): p. 1106-1115. [CrossRef]
- Nagata, Y., et al., Germline loss-of-function SAMD9 and SAMD9L alterations in adult myelodysplastic syndromes. Blood, 2018. 132(21): p. 2309-2313. [CrossRef]
- Yahata, T., et al., Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood, 2011. 118(11): p. 2941-50. [CrossRef]
- Zhou, T., et al., Myelodysplastic syndrome: an inability to appropriately respond to damaged DNA? Exp Hematol, 2013. 41(8): p. 665-74.
- Thomas, M.E., 3rd, et al., Pediatric MDS and bone marrow failure-associated germline mutations in SAMD9 and SAMD9L impair multiple pathways in primary hematopoietic cells. Leukemia, 2021. 35(11): p. 3232-3244.
- Milyavsky, M., et al., A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell, 2010. 7(2): p. 186-97. [CrossRef]
- Parker, J.E., et al., The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood, 2000. 96(12): p. 3932-8.
- Tanase-Nakao, K., T.S. Olson, and S. Narumi, MIRAGE Syndrome, in GeneReviews((R)), M.P. Adam, et al., Editors. 1993: Seattle (WA).
- Yoshizaki, K., et al., MIRAGE syndrome with recurrent pneumonia probably associated with gastroesophageal reflux and achalasia: A case report. Clin Pediatr Endocrinol, 2019. 28(4): p. 147-153. [CrossRef]
- Viaene, A.N. and B.N. Harding, The Neuropathology of MIRAGE Syndrome. J Neuropathol Exp Neurol, 2020. 79(4): p. 458-462. [CrossRef]
- Basilious, A., et al., Lacrimal Gland Hypoplasia and Corneal Anesthesia in MIRAGE Syndrome: A Case Report and Literature Review. Cornea, 2022. 41(8): p. 1041-1044.
- Janjua, D., et al., MIRAGE Syndrome Enteropathy Responding to Pancrelipase Despite Normal Pancreatic Fecal Elastase: A Case Report. Am J Case Rep, 2022. 23: p. e937057. [CrossRef]
- Gorcenco, S., et al., Ataxia-pancytopenia syndrome with SAMD9L mutations. Neurol Genet, 2017. 3(5): p. e183. [CrossRef]
- Vaughan, D., et al., Ataxia pancytopenia syndrome due to SAMD9L mutation presenting as demyelinating neuropathy. J Peripher Nerv Syst, 2020. 25(4): p. 433-437. [CrossRef]
- King-Robson, J., et al., Ataxia-Pancytopenia Syndrome due to a de Novo SAMD9L Mutation. Neurol Genet, 2021. 7(3): p. e580. [CrossRef]
- Raskind, W.H., D.H. Chen, and T. Bird, SAMD9L Ataxia-Pancytopenia Syndrome, in GeneReviews((R)), M.P. Adam, et al., Editors. 1993: Seattle (WA).
- de Pater, E., et al., Gata2 is required for HSC generation and survival. J Exp Med, 2013. 210(13): p. 2843-50.
- Santiago, M., et al., The Clinical Spectrum, Diagnosis, and Management of GATA2 Deficiency. Cancers (Basel), 2023. 15(5).
- Wehr, C., et al., A novel disease-causing synonymous exonic mutation in GATA2 affecting RNA splicing. Blood, 2018. 132(11): p. 1211-1215. [CrossRef]
- Oleaga-Quintas, C., et al., Inherited GATA2 Deficiency Is Dominant by Haploinsufficiency and Displays Incomplete Clinical Penetrance. J Clin Immunol, 2021. 41(3): p. 639-657. [CrossRef]
- Calvo, K.R. and D.D. Hickstein, The spectrum of GATA2 deficiency syndrome. Blood, 2023. 141(13): p. 1524-1532. [CrossRef]
- McReynolds, L.J., K.R. Calvo, and S.M. Holland, Germline GATA2 Mutation and Bone Marrow Failure. Hematol Oncol Clin North Am, 2018. 32(4): p. 713-728. [CrossRef]
- Mir, M.A., et al., Spectrum of myeloid neoplasms and immune deficiency associated with germline GATA2 mutations. Cancer Med, 2015. 4(4): p. 490-9. [CrossRef]
- Wlodarski, M.W., M. Collin, and M.S. Horwitz, GATA2 deficiency and related myeloid neoplasms. Semin Hematol, 2017. 54(2): p. 81-86. [CrossRef]
- Spinner, M.A., et al., GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood, 2014. 123(6): p. 809-21. [CrossRef]
- Hsu, A.P., L.J. McReynolds, and S.M. Holland, GATA2 deficiency. Curr Opin Allergy Clin Immunol, 2015. 15(1): p. 104-9.
- Shimamura, A., Aplastic anemia and clonal evolution: germ line and somatic genetics. Hematology Am Soc Hematol Educ Program, 2016. 2016(1): p. 74-82. [CrossRef]
- Wlodarski, M.W., et al., Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood, 2016. 127(11): p. 1387-97; quiz 1518. [CrossRef]
- Park, M., Overview of inherited bone marrow failure syndromes. Blood Res, 2022. 57(S1): p. 49-54. [CrossRef]
- Li, J. and J.R. Bledsoe, Inherited bone marrow failure syndromes and germline predisposition to myeloid neoplasia: A practical approach for the pathologist. Semin Diagn Pathol, 2023. [CrossRef]
- Deng, J. and L.J. McReynolds, Inherited bone marrow failure syndromes: a review of current practices and potential future research directions. Curr Opin Pediatr, 2023. 35(1): p. 75-83. [CrossRef]
- Dokal, I. and T. Vulliamy, Inherited bone marrow failure syndromes. Haematologica, 2010. 95(8): p. 1236-40.
- Bhandari, J., P.K. Thada, and Y. Puckett, Fanconi Anemia, in StatPearls. 2023: Treasure Island (FL) ineligible companies. Disclosure: Pawan Thada declares no relevant financial relationships with ineligible companies. Disclosure: Yana Puckett declares no relevant financial relationships with ineligible companies.
- Dufour, C. and F. Pierri, Modern management of Fanconi anemia. Hematology Am Soc Hematol Educ Program, 2022. 2022(1): p. 649-657.
- Thakur, B. and K.M. Hiwale, Fanconi Anemia: A Rare Genetic Disorder. Cureus, 2023. 15(5): p. e38899. [CrossRef]
- Da Costa, L., T. Leblanc, and N. Mohandas, Diamond-Blackfan anemia. Blood, 2020. 136(11): p. 1262-1273.
- Da Costa, L.M., I. Marie, and T.M. Leblanc, Diamond-Blackfan anemia. Hematology Am Soc Hematol Educ Program, 2021. 2021(1): p. 353-360.
- Gadhiya, K. and C. Wills, Diamond Blackfan Anemia, in StatPearls. 2023: Treasure Island (FL) ineligible companies. Disclosure: Christina Wills declares no relevant financial relationships with ineligible companies.
- AlSabbagh, M.M., Dyskeratosis congenita: a literature review. J Dtsch Dermatol Ges, 2020. 18(9): p. 943-967. [CrossRef]
- Gitto, L., et al., Dyskeratosis congenita. Autops Case Rep, 2020. 10(3): p. e2020203.
- Garofola, C., A. Nassereddin, and G.P. Gross, Dyskeratosis Congenita, in StatPearls. 2023: Treasure Island (FL) ineligible companies. Disclosure: Ali Nassereddin declares no relevant financial relationships with ineligible companies. Disclosure: Gary Gross declares no relevant financial relationships with ineligible companies.
- Nelson, N., et al., Functional genomics for curation of variants in telomere biology disorder associated genes: A systematic review. Genet Med, 2023. 25(3): p. 100354. [CrossRef]
- Cordell, V. and L. Osoba, Pregnancy in a patient with Schwachman-Diamond syndrome. BMJ Case Rep, 2015. 2015. [CrossRef]
- Woodward, E.R. and S. Meyer, Fanconi Anaemia, Childhood Cancer and the BRCA Genes. Genes (Basel), 2021. 12(10).
- Alter, B.P., et al., Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol, 2010. 150(2): p. 179-88. [CrossRef]
- van Dooijeweert, B., et al., GATA-1 Defects in Diamond-Blackfan Anemia: Phenotypic Characterization Points to a Specific Subset of Disease. Genes (Basel), 2022. 13(3).
- Mello, F.V., et al., Maturation-associated gene expression profiles during normal human bone marrow erythropoiesis. Cell Death Discov, 2019. 5: p. 69. [CrossRef]
- Savage, S.A. and C. Dufour, Classical inherited bone marrow failure syndromes with high risk for myelodysplastic syndrome and acute myelogenous leukemia. Semin Hematol, 2017. 54(2): p. 105-114. [CrossRef]
- Feurstein, S., et al., Telomere biology disorder prevalence and phenotypes in adults with familial hematologic and/or pulmonary presentations. Blood Adv, 2020. 4(19): p. 4873-4886. [CrossRef]
- Victorelli, S. and J.F. Passos, Telomeres and Cell Senescence - Size Matters Not. EBioMedicine, 2017. 21: p. 14-20. [CrossRef]
- Myers, K.C., S.M. Davies, and A. Shimamura, Clinical and molecular pathophysiology of Shwachman-Diamond syndrome: an update. Hematol Oncol Clin North Am, 2013. 27(1): p. 117-28, ix. [CrossRef]
- Tan, S., et al., EFL1 mutations impair eIF6 release to cause Shwachman-Diamond syndrome. Blood, 2019. 134(3): p. 277-290. [CrossRef]
- Godley, L.A., DNAJC21: the new kid on the SDS block. Blood, 2017. 129(11): p. 1413-1414. [CrossRef]
- Tawana, K., M.W. Drazer, and J.E. Churpek, Universal genetic testing for inherited susceptibility in children and adults with myelodysplastic syndrome and acute myeloid leukemia: are we there yet? Leukemia, 2018. 32(7): p. 1482-1492. [CrossRef]
- Roloff, G.W. and E.A. Griffiths, When to obtain genomic data in acute myeloid leukemia (AML) and which mutations matter. Blood Adv, 2018. 2(21): p. 3070-3080. [CrossRef]
- Padella, A., et al., Targeting PARP proteins in acute leukemia: DNA damage response inhibition and therapeutic strategies. J Hematol Oncol, 2022. 15(1): p. 10. [CrossRef]
- Godley, L.A. and A. Shimamura, Genetic predisposition to hematologic malignancies: management and surveillance. Blood, 2017. 130(4): p. 424-432. [CrossRef]
- Saygin, C., et al., Allogeneic hematopoietic stem cell transplant outcomes in adults with inherited myeloid malignancies. Blood Adv, 2023. 7(4): p. 549-554. [CrossRef]
- Williams, L., et al., Genetics of donor cell leukemia in acute myelogenous leukemia and myelodysplastic syndrome. Bone Marrow Transplant, 2021. 56(7): p. 1535-1549. [CrossRef]
- Gibson, C.J., et al., Donor Clonal Hematopoiesis and Recipient Outcomes After Transplantation. J Clin Oncol, 2022. 40(2): p. 189-201. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





