1. Introduction
The focus of the United Nations' sustainable development goal number 13 is to address climate change, as its severe impact is affecting every country worldwide. Greenhouse gas emissions have increased by over 50% since 1990, and 2019 marked the second warmest year on record and the end of the warmest decade ever recorded. Carbon dioxide (CO
2) and other greenhouse gas levels in the atmosphere reached new records in 2019. Although there was a temporary reduction in emissions due to the pandemic, emissions are expected to rise as the global economy recovers. Urgent and coordinated action is necessary to combat both the pandemic and climate change for the sake of human survival. Additionally, Goal 7 of the United Nations Sustainable Development Goals emphasizes the importance of affordable and sustainable energy for all, ensuring access to reliable and inexpensive energy sources. The use of fossil fuels without measures to reduce greenhouse gas emissions contributes to global climate change. It is evident that enhancing energy efficiency and promoting renewable energy sources is crucial for mitigating climate change and reducing risks to ecosystems. The Intergovernmental Panel on Climate Change (IPCC) highlighted the need to halve fossil fuel emissions within 11 years to limit global warming to 1.5 degrees Celsius above pre-industrial levels (
IPCC 2018). The concentration of atmospheric carbon dioxide has increased by 49% from 1750 to 2020 (
Global Carbon Project 2021). Non-renewable energy sources alone are insufficient to meet the current and growing energy demand (
Pinaki et al. 2015). Motor vehicles are a significant source of pollution, responsible for 70% of carbon monoxide and 19% of carbon dioxide emissions globally (
Deenanath et al. 2012). The International Monetary Fund (IMF) estimates that there are currently over 1 billion cars worldwide, with projections indicating a rise to approximately 2.9 billion cars by 2050, mostly in developing countries (World Energy Outlook 2022). These statistics underscore the need to take action to reduce the impact of greenhouse gas emissions from motor vehicles.
Global ethanol production has been increasing overall, but it experienced a decline in 2020 due to the COVID-19 pandemic. The United States is the largest producer of ethanol, followed by Brazil, the European Union, China, and Canada (
Edeh 2021). The rest of the world generated a significant amount of ethanol as well (
Edeh 2021). In Africa, where many countries are net oil importers, there is a growing need to develop alternative fuels for energy self-sufficiency and socio-economic benefits (
Sekoai & Yoro 2016). Biofuels are seen as a potential solution, and governments worldwide have implemented policies to promote their use and reduce CO
2. In Ghana, there have been attempts to establish biofuel policies, such as using biodiesel blends for government vehicles and increasing the production of jatropha and cassava/sugarcane for bioethanol (
Sielhorst 2008). However, the initiatives faced challenges and failed to reach their targets due to factors like the discovery of crude oil, changes in political power, and a lack of political commitment (
Abubakari et al. 2016). Various crops, including cassava, sugarcane, maize, and jatropha oil seeds, have been identified as potential feedstocks for bioethanol and biodiesel production in Ghana (
Sekoai & Yoro 2016).
Bioethanol is a leading biofuel that has experienced significant market growth. It is produced by fermenting the starch and sugar components of plant and biomass materials (
Edjekouane et al. 2020). Bioethanol serves as a high-octane fuel and a replacement for lead in gasoline, enhancing its octane rating (
Kumar & Kataria 2019). When bioethanol is blended with gasoline, it oxygenates the fuel mixture, resulting in cleaner combustion and reduced pollutant emissions (
Adetunji 2015). Compared to other generations of bioethanol production, the focus of this study is on second-generation bioethanol, which utilizes lignocellulosic biomass. Lignocellulose found abundantly in both woody and non-woody plants, is the most common renewable energy source globally and a by-product of various plant processing materials (
Visser et al 2013). Its primary components are cellulose, hemicellulose, and lignin. The use of second-generation bioethanol offers numerous advantages over other generations due to the widespread availability and sustainability of lignocellulosic biomass (
Kumar & Kataria 2019). Cassava, scientifically known as
Manihot esculenta, is a starchy root crop commonly used as a food source in developing nations (
Kehinde et al. 2021). It is extensively cultivated in tropical and subtropical regions of Asia, South America, and Africa. Nigeria, the Democratic Republic of Congo, Thailand, Indonesia, Brazil, and Ghana are among the top cassava producers, with global production reaching 291 million tonnes in 2019 according to United Nations FAOSTAT. In Ghana alone, approximately 3.8 million metric tons of cassava peels are generated annually (Aboagye et al. 2021).
Cassava peels are an ideal feedstock for bioethanol production due to their low lignin content, facilitating the separation of fermentable sugars for bioethanol processing. Moreover, their abundant availability and affordability make cassava peels a viable option for bioethanol production (Aboagye et al. 2021).
Fungi are widely recognized as effective microorganisms for breaking down lignocellulose into its main components. Cellulolytic enzymes, such as cellulases and amylases, are crucial for efficient cellulose hydrolysis, and fungi, particularly species like Aspergillus, Rhizopus, and Penicillium, are known to produce these enzymes abundantly.
Aspergillus niger, in particular, is highly regarded for its enzyme production capabilities and is considered safe for use in various biotechnological applications by the US FDA (
Schuster 2002). Enzymes play a significant role in the cost of bioethanol production and producing them on-site can help reduce expenses. Among the enzyme-catalyzed approaches, Simultaneous Saccharification and Fermentation (SSF) is preferred due to its ability to achieve higher yields and concentrations of ethanol. This method combines enzymatic cellulose hydrolysis with the simultaneous fermentation of glucose to ethanol, resulting in improved efficiency and cost-effectiveness. The SSF technique also avoids the need for expensive equipment and minimizes the risk of contamination by organisms sensitive to ethanol. The use of yeast in the cellulolytic enzyme complex helps enhance yields and saccharification rates by reducing the accumulation of inhibitory sugars in the reactor. The objective of this research is to analyze the composition of cassava peels, isolate and characterize degrading fungi from fresh and decaying peels, produce a crude enzyme blend using cassava peels, optimize enzyme production, and utilize the enzyme cocktail for the production of bioethanol through simultaneous saccharification and fermentation using cassava peels as the substrate.
2. MATERIALS AND METHODS
CHAPTER 1
2.1. Location of study
The present study was conducted at the laboratories of the Department of Biochemistry and Biotechnology and the Department of Chemical Engineering all in Kwame Nkrumah University of Science and Technology (KNUST), Kumasi in the Ashanti region of Ghana.
2.2. Sources of experimental materials
The feedstock of cassava peels that served as substrate was procured from the Gari processing facility at Asuboi in the Ayesuano district close to Suhum on the Accra-Kumasi Highway in the Eastern Region of Ghana. From the same cassava processing factory's disposal site, two more samples of Fresh Cassava Peels (FCP) and Decayed Cassava Peels (DCP) for fungal isolation were collected.
2.3. Sample preparation
The cassava peels used as feedstock were taken through a series of steps. Firstly, they were washed with water to remove any unwanted particles and dust. Then, the peels were dried in the sun for a period of 7 days to eliminate moisture. Once dried, the cassava peels were manually crushed using a mortar and pestle, resulting in fine particles. These fine particles were then placed in a sealed poly bag and stored under room conditions for future processing. Additionally, fresh and partially decayed cassava peels were washed, crushed separately, and stored for further processing as well.
2.4. Chemical analysis of cassava peels
This was carried out in the Nutrition Laboratory of the Animal Science Department, KNUST. The insoluble fibre in cassava peel which is, cellulose, hemicellulose, and lignin content was determined as Acid Detergent Fibre (ADF), Neutral Detergent Fibre (NDF), and Acid Detergent Lignin (ADL) according to the method of analysis used by Van Soest (van Soest 1995). The analysis of starch from the cassava peels was also conducted using the wet method as described by Noorfarahzilah et al., (2014).
2.5. Isolation of fungi
The isolation of the fungi was undertaken at the Soil Science Laboratory of Kwame Nkrumah University of Science and Technology The investigation employed the simple dilution plate method. Each sample, weighing 1 g, was diluted three-fold in 9 ml of sterile distilled water. The final dilution (10-3) was then flooded onto three separate sterile Potato Dextrose Agar (PDA) medium plates that were supplemented with chloramphenicol antibiotic to inhibit bacterial growth. The plates were incubated at room temperature (25oC-28oC) for a duration of 7 days. The resulting fungal growth was counted using a colony counter and identified using the Illustrated Genera of Imperfect Fungi Manual by (Barnett and Hunter 1972). The frequencies of occurrence for each identified fungus were determined. Subsequently, the cultures were sub-cultured until pure isolates were obtained.
2.6. Macromorphological and micromorphological characteristic identification
The fungal isolates were identified based on their macromorphological and micromorphological characteristics. For macromorphological identification, colony traits such as colour, texture, and spore structure were observed, following the guidelines provided in the handbook for the identification of fungi (
Alexopoulos 1996; Barnett and Hunter 1972). Micromorphological characteristics were examined using the Conventional Lactophenol Cotton Blue Technique (LPCB). Slides for microscopic analysis were prepared using five-day-old pure cultures. A small number of mycelia was placed on a slide, and a drop of lactophenol blue was added. A cover slip was then applied, and the sample was examined under a light microscope at a magnification of x400. Identification was conducted by comparing the observed features with relevant micrographs.
2.7. Quantitative analysis of the three fungal isolates for enzyme activity
The three fungal isolates were cultivated on Potato Dextrose Agar (PDA) medium. Subsequently, the isolates were tested for enzyme activity by incorporating 3g of cassava peels into a PDA medium. Three separate media were prepared, each inoculated with one of the three fungi and then incubated at 30°C. An iodine solution containing iodine (0.2 ml), potassium iodide (0.4 ml), and distilled water (100 ml) was flooded onto each medium. The presence of a clear zone around the fungal growth indicated the production of amylase. To identify cellulase enzymes, the plates were flooded with a 1% Congo red solution and left at 28°C for 20 minutes. Subsequently, the plates were thoroughly rinsed with a 1M sodium chloride solution. The formation of a clear zone surrounding the cellulase-producing colonies against a dark red background indicated cellulase activity.
2.8. Substrate for the enzyme production
Starch powder and Carboxymethylcellulose (CMC) powder were used for the assay of amylase, and cellulase enzymes respectively. The purpose of the assay above was to check for the presence and quantity of these enzymes which were the enzymes of interest.
2.9. Preparation of Aspergillus niger spore suspension
After the mould had been incubated for five days, its spores were harvested by saturating the culture with 10 ml of sterilized distilled water and then scraping the spores out. The spore suspensions were then decanted and combined to the appropriate volume in a 100 ml Erlenmeyer flask.
2.10. Determination of spore concentration
Using sterile distilled water, 1 ml of spore suspension was transferred from the stock into a measuring cylinder with a capacity of 100 ml. After shaking the diluted sample, a portion was pipetted into the grooves of haemacytometer and placed under a light microscope to count the spores. The average of the spore counts from the haemacytometer five zones was calculated. The average value was then multiplied by a dilution factor of 10 and 104 spores per millilitre. 2.1 x 105 spores/ml were determined to be the spore concentration.
2.11. Preparation of Growth Medium
Basal Salt Medium (BSM) which is the main medium used in this experiment was prepared according to the combinations used by (Efeovbokhan et al. 2019) with slight modifications. The following concentrations of components; 1.4 g of (NH4)2SO4, 0.3 g of MgSO4.7H2O, 2.0 g of KH2PO4, 0.5 g of FeSO4, 0.3 g of CaCl2, 0.3 g of Urea, 1 ml of Tween 20, 0.16g of MnSO4, 0.14 g of ZnSO4, 0.2 g of CoCl2, and 10 g of Starch in 1 L of deionized water for the production of enzymes. No additional carbon or nitrogen source was added. The pH of the medium was adjusted to 5.5.
2.12. Enzyme production (α-amylase and cellulase) from Aspergillus niger
Temperature and pH were the fixed conditions maintained during the enzyme production. The optimum temperature and pH were used in this research. For the enzyme production by Aspergillus niger, an optimum temperature of 30°C and a pH of 5.5 respectively were used. These fixed conditions are within the range for optimum production of amylase and cellulase enzymes as reported in the literature for Aspergillus niger.
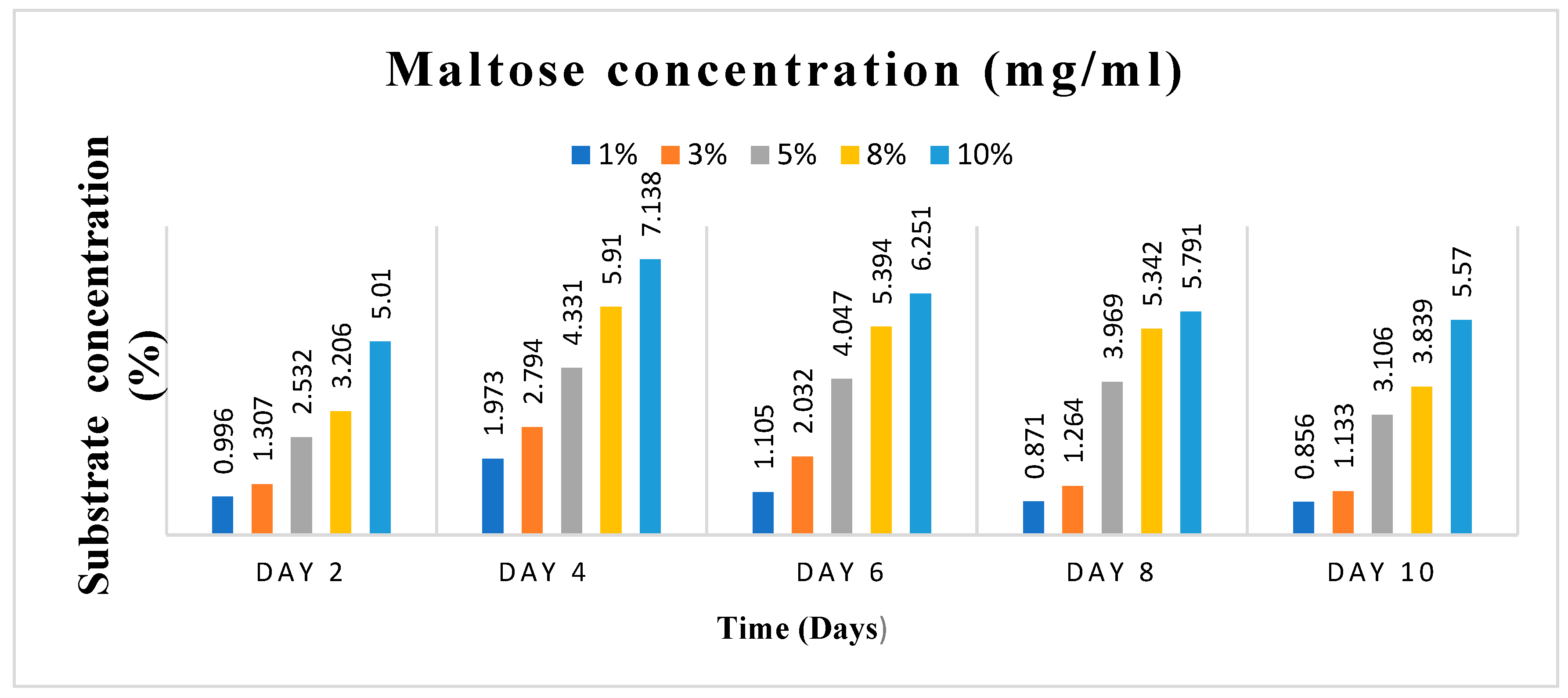
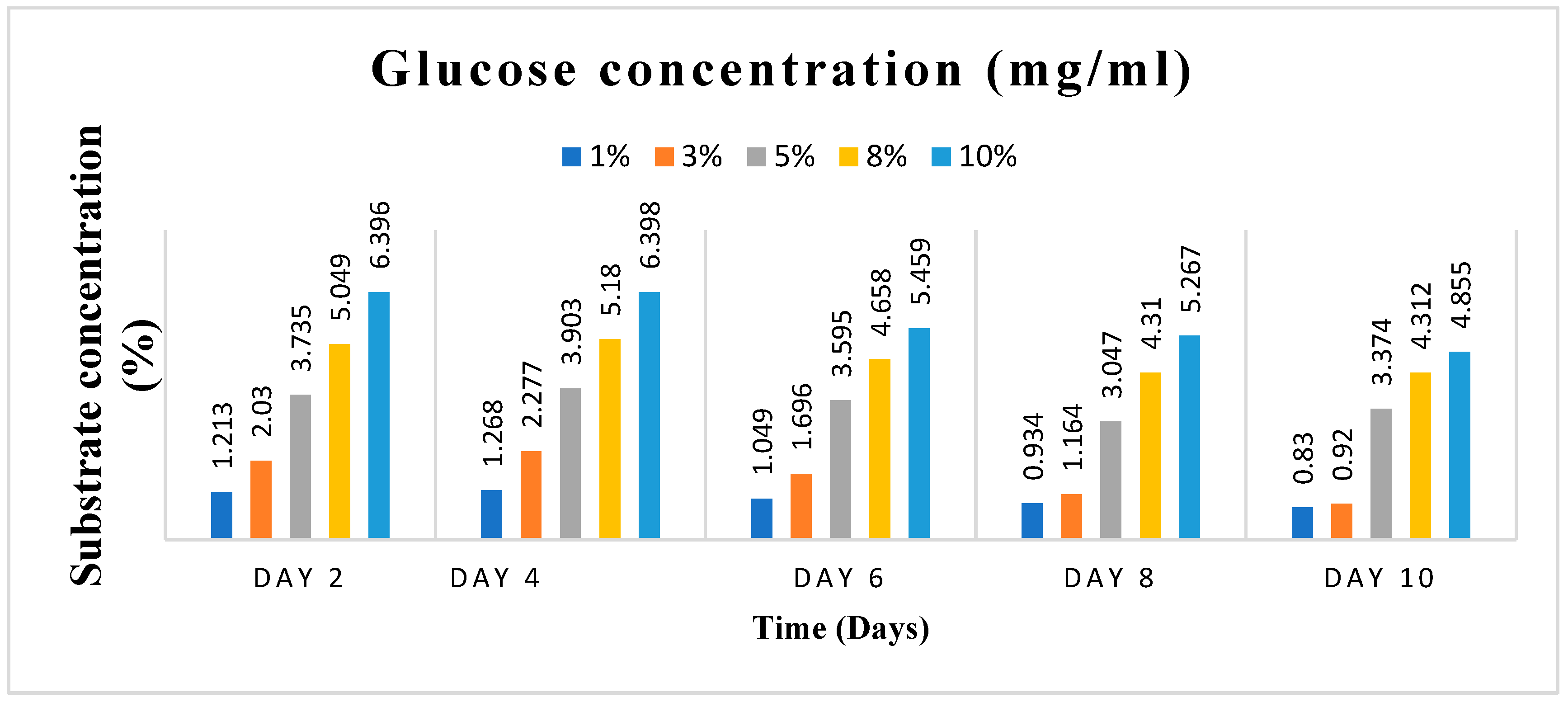
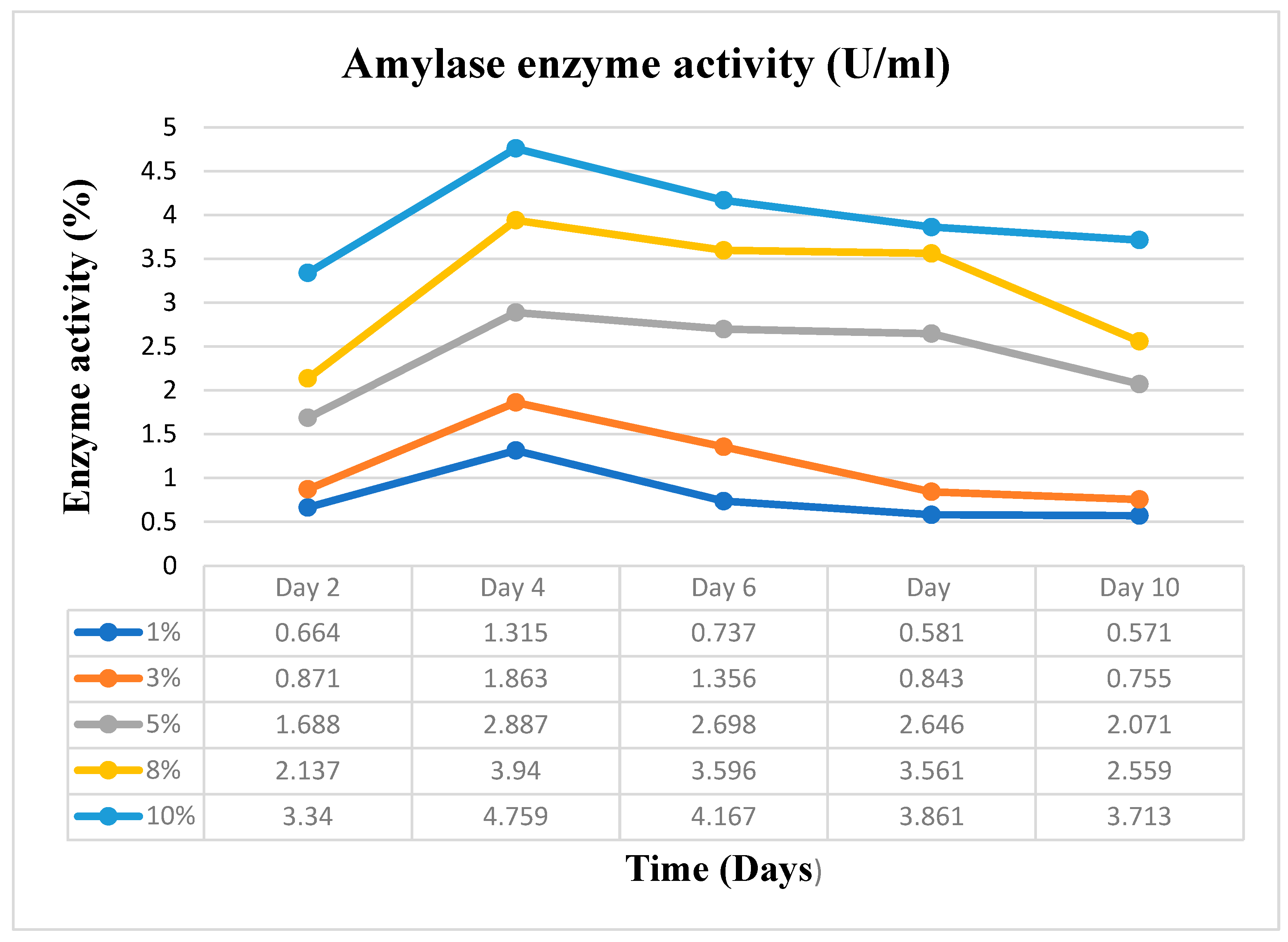
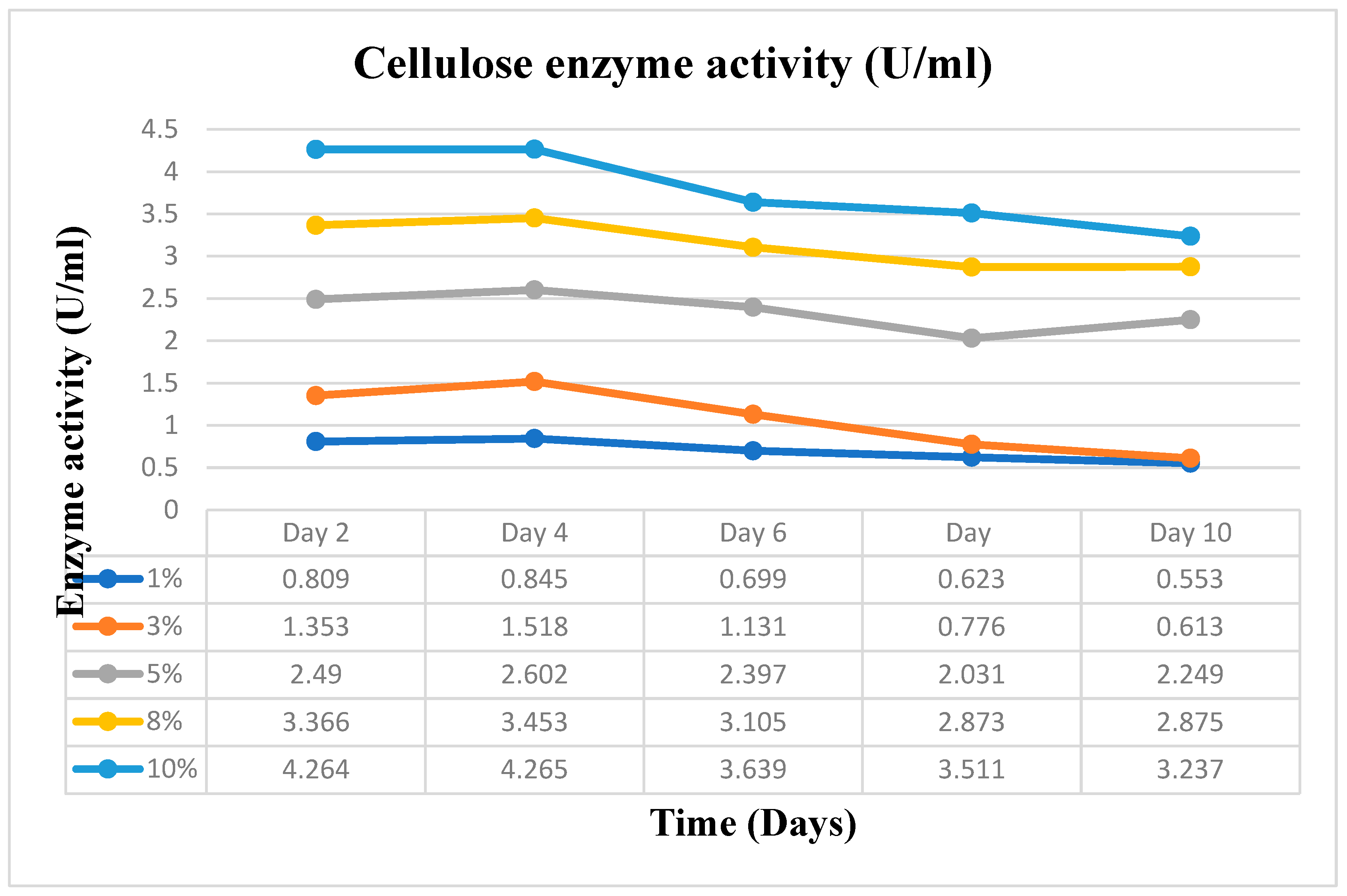
Substrate concentration and incubation time, two essential variables, were varied in this study. Varying amounts of 1, 3, 5, 8 and 10% (w/v) of the dry-weight biomass of the cassava peels were used as the substrate concentrations. On the other hand, the incubation time was varied as; 0, 2, 4, 6, 8, and 10 days. It was essential to do this to obtain the ideal substrate concentration and incubation time for the scale-up manufacture of Crude Enzyme Extract (CEE), the enzyme pool that was used to produce the bioethanol.
The five distinct substrate concentrations, 1, 3, 5, 8, and 10% w/v of the cassava peels, were added in triplicate to five different 50 ml flasks that contained 25 ml of culture media. The flasks and their contents were autoclaved for 15 minutes at 121°C and were then cooled to room temperature. After that, the flasks were inoculated with 5 ml of spore suspension of A. niger containing 2.1 x 105 spores/ml, and they were incubated in an orbital shaker at 150 rpm and 30 °C for the indicated varied number of days: 0, 2, 4, 6, 8, and 10 days. Amylase and cellulase activity tests were performed after samples were collected at the specified time intervals.
2.13. Recovery of crude enzyme extract (onsite enzymes)
At various periodic intervals indicated, samples were taken from the flasks and filtered. The filtrate which contains the crude enzymes was recovered through centrifugation at 7,000 rpm for 30 mins at 30°C. The supernatant was then decanted into a test tube for further analysis.
2.14. Measurement of enzyme activity (U/ml)
The amylase and cellulase assays were performed using the Dinitrosalicylic Acid (DNSA) technique. For the amylase activity measurement, 0.5 ml of soluble starch solution and 1 ml of potassium phosphate buffer (pH 6.8) were mixed in five separate test tubes. Similarly, for cellulase activity measurement, 0.5 ml of CMC solution and 1 ml of sodium acetate buffer (pH 5.5) were mixed in five independent test tubes. To initiate the reaction, 0.5 ml of the raw enzyme extract was added to each mixture. The tubes were then incubated at 50°C for 30 minutes. All five samples, which were collected at regular intervals for examination over the course of ten days, followed this procedure. A control test tube was also prepared for both assays. After the incubation period, 1 ml of DNS reagent was added, and the reaction was stopped by placing all the tubes in a boiling water bath at 95℃ for 5 minutes. The amount of reducing sugar released (glucose) was determined by measuring the absorbance at 540 nm using a spectrophotometer with the DNS technique. The concentration of reducing sugars was calculated by comparing the results with a standard glucose curve. In this context, one unit of amylase and cellulase activity is defined as the amount of enzyme that can release 1 mole of reducing sugars per minute under the specified assay conditions. (Monga et al. 2011).
2.15. Optimization of onsite enzymes
The optimum conditions obtained in the outcome of the initial enzyme extraction process as outlined earlier were used to scale up the enzyme extraction in a 500 ml flask. The initial experiment results proved that 10% substrate concentration and a 4-day period were the optimum variable conditions for enzyme extraction in this experiment as a temperature of 30°C and pH of 5.5 remains constant with the same already prepared Basal Salt Medium (BSM). The dinitrosalicylic acid (DNSA) method was used for the amylase and cellulase enzyme assays. The absorbance at 540nm was measured against a standard curve as was done in the initial process. The CEE was stored for the hydrolysis process.
2.16. Bioethanol production
Simultaneous Saccharification and Fermentation (SSF)
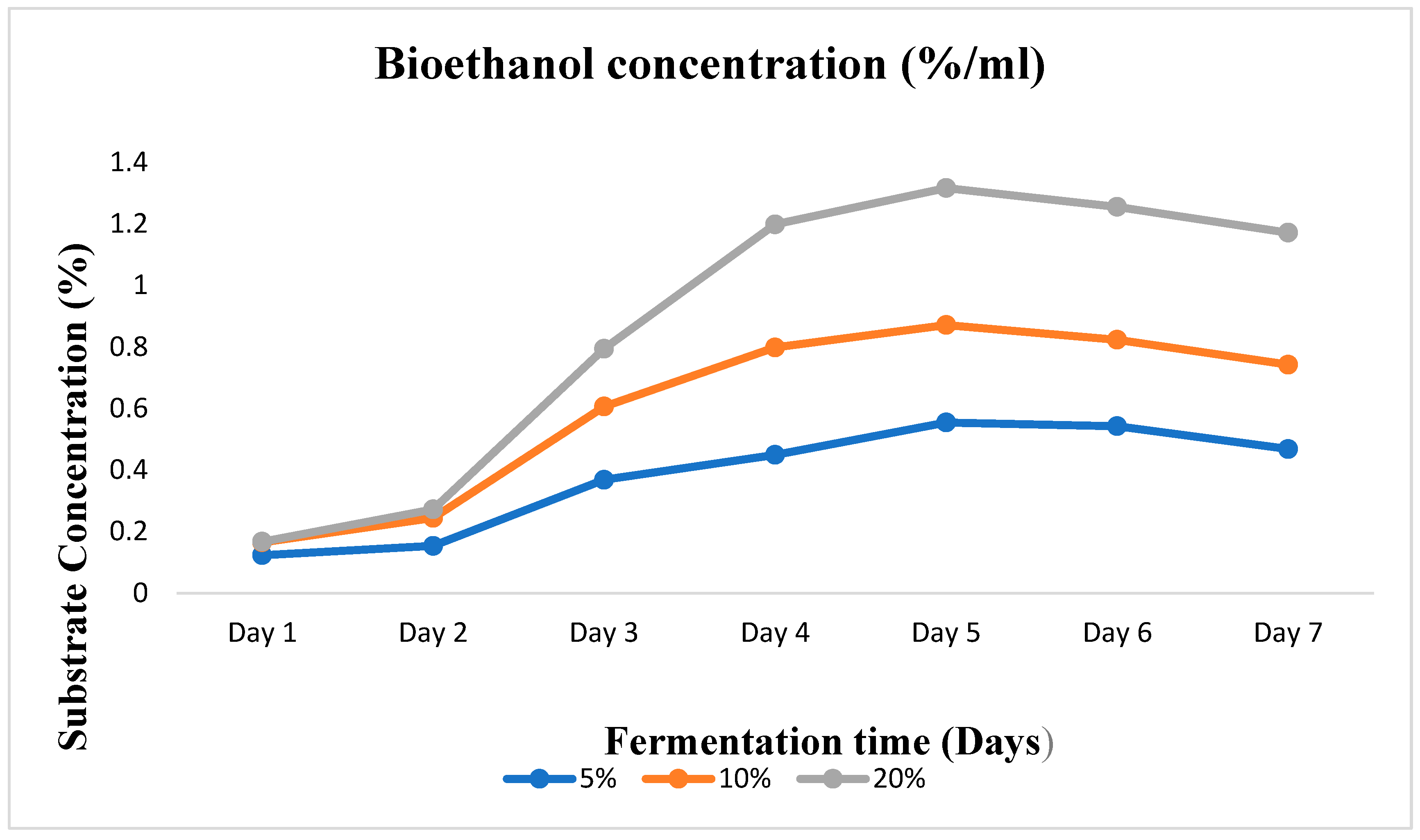
The crude enzymes produced (amylase and cellulase) were used to test for ethanol production efficiency by flask cultivation in other to get the optimum conditions for the scale-up of the final bioethanol production in a 10 L bioreactor. This initial experimental process was done in shake flasks (250 mL). An enzyme dose of 20 mL, a temperature of 50oC, and a pH of 5.0 were maintained as fixed conditions throughout the experimental period. Substrate concentrations of 5%, 10%, and 20% w/v were varied in the saccharification process. The best condition among the three that produced the highest amount of bioethanol against the fixed conditions was chosen for the scale-up in the bioreactor.
Three different conical flasks were utilized for the given substrate concentrations of 5%, 10%, and 20% w/v. In each of the three conical flasks, the already prepared cassava peel flour was dissolved in an initial volume of distilled water. To reach the target, more water was poured into the flask. The flasks were sealed with cotton wool, vigorously shaken, and then autoclaved at 121°C for 15 minutes. After that, 20 ml of the enzyme mixture which contains the cellulase and amylase was added to each flask as an inoculant, and a brewer's yeast (obtained from Anchor Yeast, South Africa) with a concentration of 1.5 g was added to the fermentation broth. Each conical flask's mixture was sealed with aluminium foil and kept under anaerobic conditions for 7 days. After 7 days, samples were taken daily, and filtered, and Gas Chromatography (GC) was used to determine the ethanol concentration. In triplicate, each experiment was carried out, and a control experiment was also prepared.
2.17. Statistical analysis
All analyses were done in triplicates and results were presented as mean standard values. One-Way Analysis of variance (ANOVA) was used for the comparison of means using Microsoft Excel 365 ProPlus. The significance of p <0.05 was accepted.