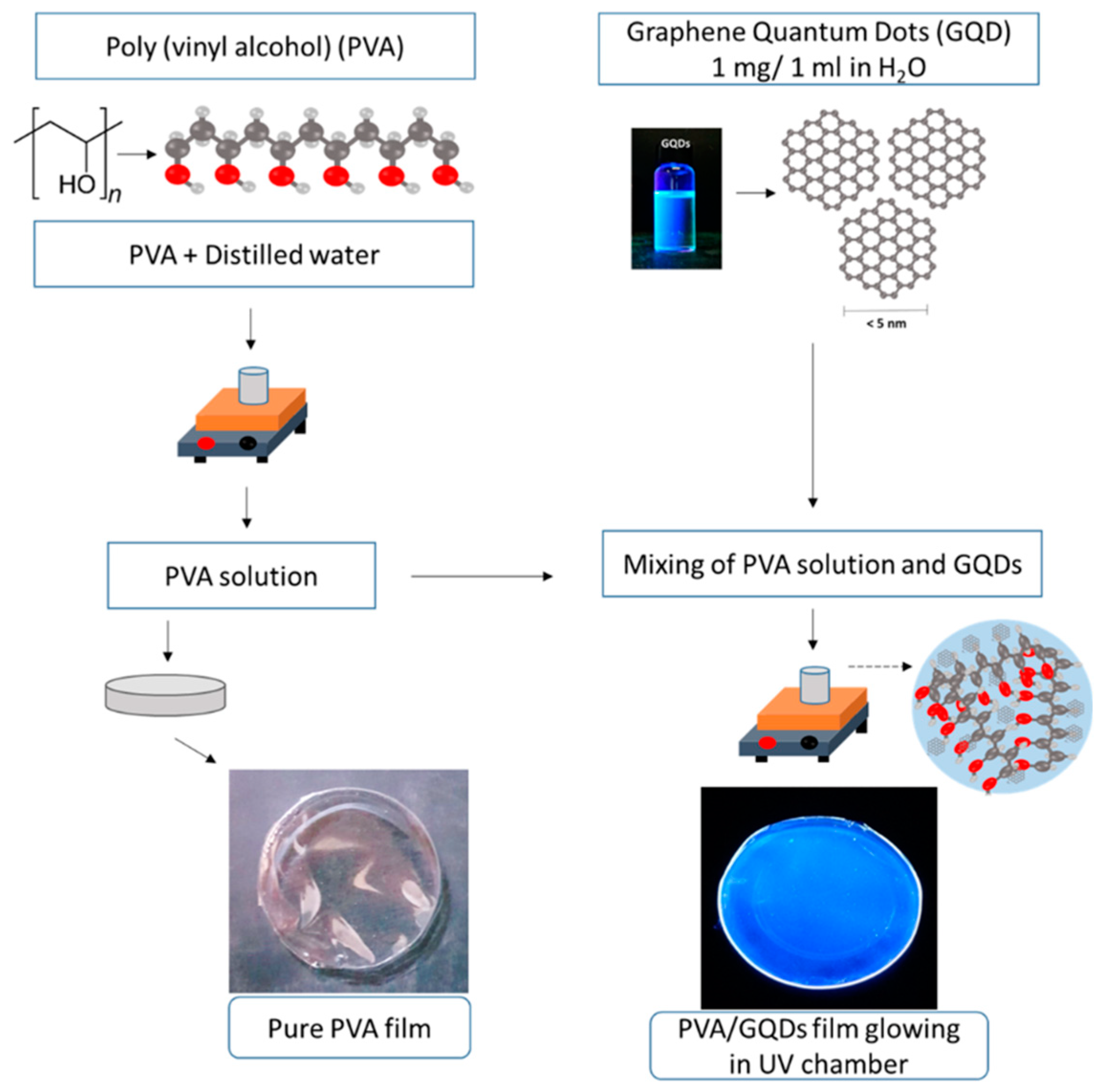
3. Results and discussion
PVA exhibits semicrystalline properties due to inter- and intra-molecular hydrogen bonding (O‒H) that provides the structural order of PVA chains [
24,
25].
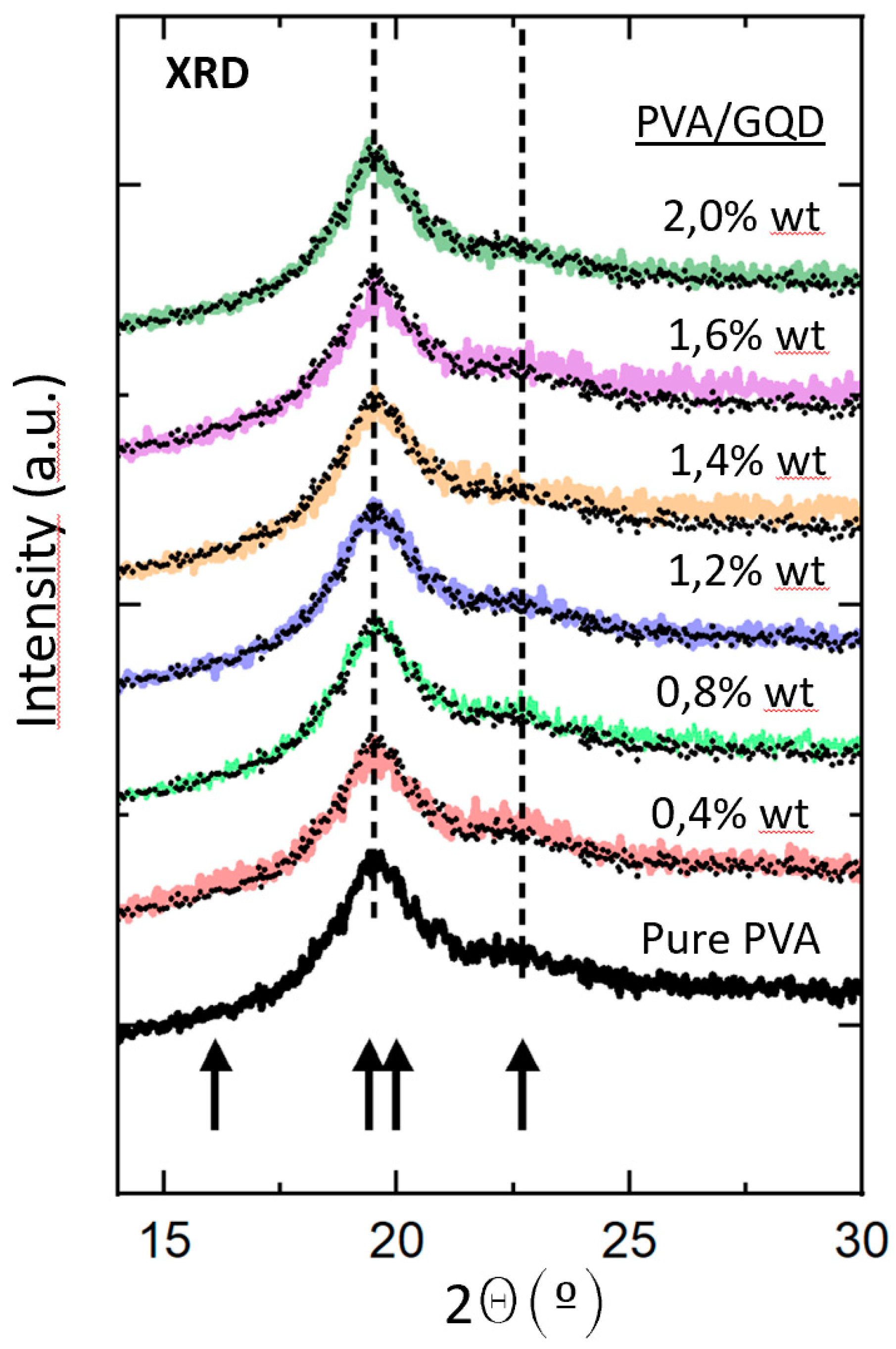
Figure 2 shows the normalized XRD patterns of the pure PVA and PVA/GQDs composite films. The lowest black curve in
Figure 2 corresponds to diffractogram of the pure PVA film. Within the considered angular range, four crystalline maxima at
indexed as (001),
,
, should be distinguished. The positions are indicated by arrows in
Figure 2. Nevertheless, in our case, peaks at 19.4º and 20.0º were not resolved.
Considering a peak at
º of FWHM of 2.1º, and applying the Debye-Scherrer equation,
where
D is the crystalline size,
K the Scherrer constant (0,98), and
the wavelength (0,154 nm), a size of
4.2 nm is estimated for the PVA crystallites, approximately the same as that of the GQD (nominal size < 5 nm).
The interplanar spacing may be estimated as
taking into account Bragg’s equation,
where
n is the order of reflection.
The % of crystallinity was calculated as by considering the ratio between the area of crystalline peaks and the total area in the XRD diffractogram.
Figure 2.
Normalized XRD patterns of the pure PVA and PVA/GQDs composite films. The dotted curve reproduces the diffractogram of the pure PVA film that has been superimposed on each of the diffractograms of the PVA/GQD nanocomposites with different GQD content, depicted in different colors for ease of comparison. The arrows indicate the position of expected maxima in crystalline PVA [
24].
Figure 2.
Normalized XRD patterns of the pure PVA and PVA/GQDs composite films. The dotted curve reproduces the diffractogram of the pure PVA film that has been superimposed on each of the diffractograms of the PVA/GQD nanocomposites with different GQD content, depicted in different colors for ease of comparison. The arrows indicate the position of expected maxima in crystalline PVA [
24].
The normalized diffractograms of the PVA/GQD films with different GQD loadings correspond to the curves in different colors in
Figure 2. In each case, the diffractogram of the pure PVA film has been superimposed (black dotted curve) on the corresponding curve for ease of comparison. As can be seen in
Figure 2, the shape of the diffractogram does not undergo any significant change for the PVA/GQD films with the different %wt loadings of GQD. This fact indicates that neither the crystallinity percentage of the films nor the average size of the PVA crystalline domains should be significantly affected by the incorporation of GQD in our PVA/GQD nanocomposite films. Moreover, it is evident that no additional peak related to the presence of GQD is distinguished [
26,
27], even for the highest GQD load considered (2%wt).
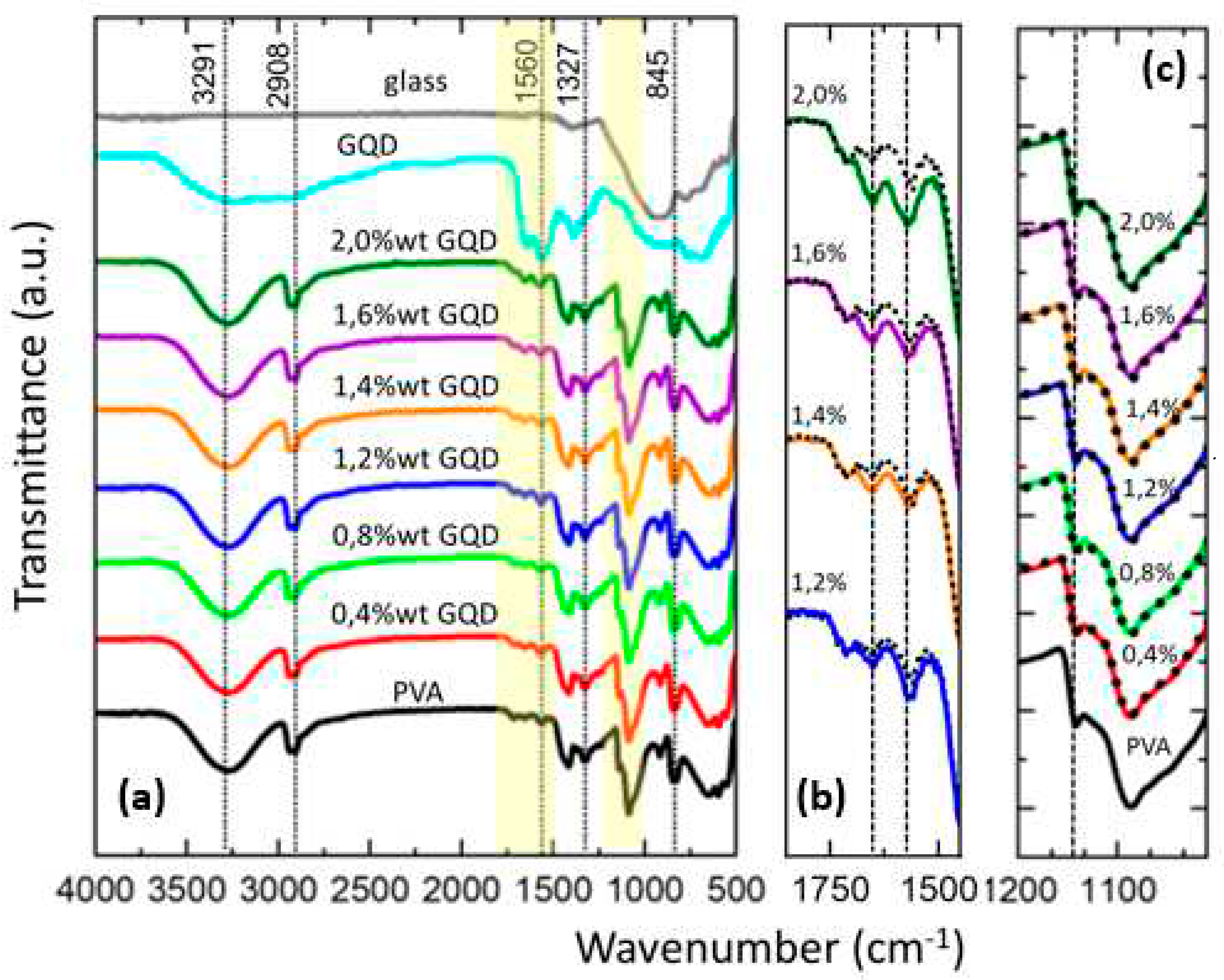
The chemical structure of PVA/GQDs composite films has been analyzed with reference to the pure PVA film using FT-IR spectroscopy.
Figure 3 shows the normalized FT-IR spectra for the pure PVA and PVA/GQD composite films. To characterize the FT-IR response of the GQD, a special sample was prepared by depositing a droplet of the GQD solution on a glass slide and waiting for 24 hours for the solvent to evaporate. The FT-IR spectra of the GQDs (on the glass slide) and of the clean glass slide without deposited GQDs are also shown in
Figure 3.
In
Figure 3a, the broad band in the range of 3600 cm
-1 to 3100 cm
-1 is attributed to –OH stretching as a result of inter- and intra-molecular hydrogen bonding of PVA. An ‒OH band around the same range does also appears in the GQD spectrum. However, in the spectra of the PVA/GQD films no significant modification of the -OH peak with respect to the spectrum of the pure PVA is apparent, neither in shape nor in position of the maximum. For the highest GQD loads, a slight shift of the OH band maximum to higher wavenumbers can be allocated (by ~10 cm
-1 for the %2wt GQD load, almost inappreciable in
Figure 3a) possibly due to the incorporation to the GQD to the PVA matrix.
The peaks at 2935 cm-1 and 2908 cm-1 are assigned to the symmetric and asymmetric CH2 stretching modes, and their shape and position remain the same for the pure PVA and the different PVA/GQD composites.
In the spectrum of GQD (cyan curve in
Figure 3a), bands appear at 1650 cm
-1 and 1560 cm
-1; however, these bands, although weak, are also found in the pure PVA and the PVA/GQD films. In pure PVA, the band at ~1654 cm
-1 has been attributed to absorbed water [
28,
29]. Given the fact that the GQD sample was prepared from an aqueous solution, it is plausible that some water molecules remain attached to the GQDs after the evaporation of the solvent and give rise to this band. In the case of GQDs, such a band has been previously assigned to in-plane stretching vibration of the sp
2 hybridized C=C bond [
30]. According to our data, it is clear that in our case, this peak can be associated with the presence of GQDs. The peak at 1560 cm
-1 is attributed to C=O stretching [
31]. Although our PVA was completely hydrolyzed (98 to 99%), some residual acetate groups in the PVA molecular chains contain carbonyl bonds that may explain the weak peak observed at ~1560 cm
-1 in pure PVA and PVA/GQD films. In addition, GQDs are expected to have attached surface carboxyl groups (‒COOH), with characteristic absorption band around 1566 cm
-1 [
30] and 1570 cm
-1 [
32].
The modification of the PVA/GQD FT-IR spectra in the spectral region from 1850 to 1450 cm
-1 is carefully investigated in
Figure 3b. There, the FT-IR spectrum of pure PVA (black dotted curve in
Figure 2b) has been superimposed on the spectra of PVA/GQD with different GQD loadings. For less than 1,2% wt GQD, no appreciable difference can be distinguished between the pure PVA and PVA/GQD spectral curves. However, for 1,2%wt GQD a slight increase in the bands coincident with those of the GQDs (cyan curve in
Figure 3a) is seen, and the increase becomes larger as the amount of GQD incorporated into PVA matrix increases.
In
Figure 3), the peaks around 1417 cm
-1 and 1327 cm
-1 are assigned to ‒OH bending (in-plane) and C‒H wagging modes of PVA [
33]. These peaks also remain identical in shape and position for the different PVA/GQD composites.
Particularly interesting is the peak at 1141 cm
-1, attributed to C-O/C-C stretching modes, which is typically used to evaluate the crystallinity of PVA using FT-IR analysis [
29,
34,
35]. The FT-IR spectra of the pure PVA and PVA/GQD composites in the spectral region from 1200 to 1019 cm-1 are carefully investigated in
Figure 3c. There, the FT-IR spectrum for pure PVA (black curve) has been vertically shifted and superimposed (black dotted curves) on the spectra of PVA/GQD with different GQD loadings. As can be seen in
Figure 3c, the spectral curves do not change in shape or position as the amount of GQD incorporated into the PVA matrix increases. These results confirm the XRD observations that the percentage crystallinity of pure PVA is not affected by the GQD loading.
Eventually, in
Figure 3a, the peaks at 1089 cm
-1, 916 cm
-1 and 845 cm
-1 are attributable to the C‒O stretching, CH
2 rocking mode and C-C stretching vibrational modes respectively [
36,
37], which do not undergo any modification in shape or position in the pure PVA films and PVA/GQD composites.
Table 1 lists the peak assignments discussed in the FT-IR spectra of
Figure 3.
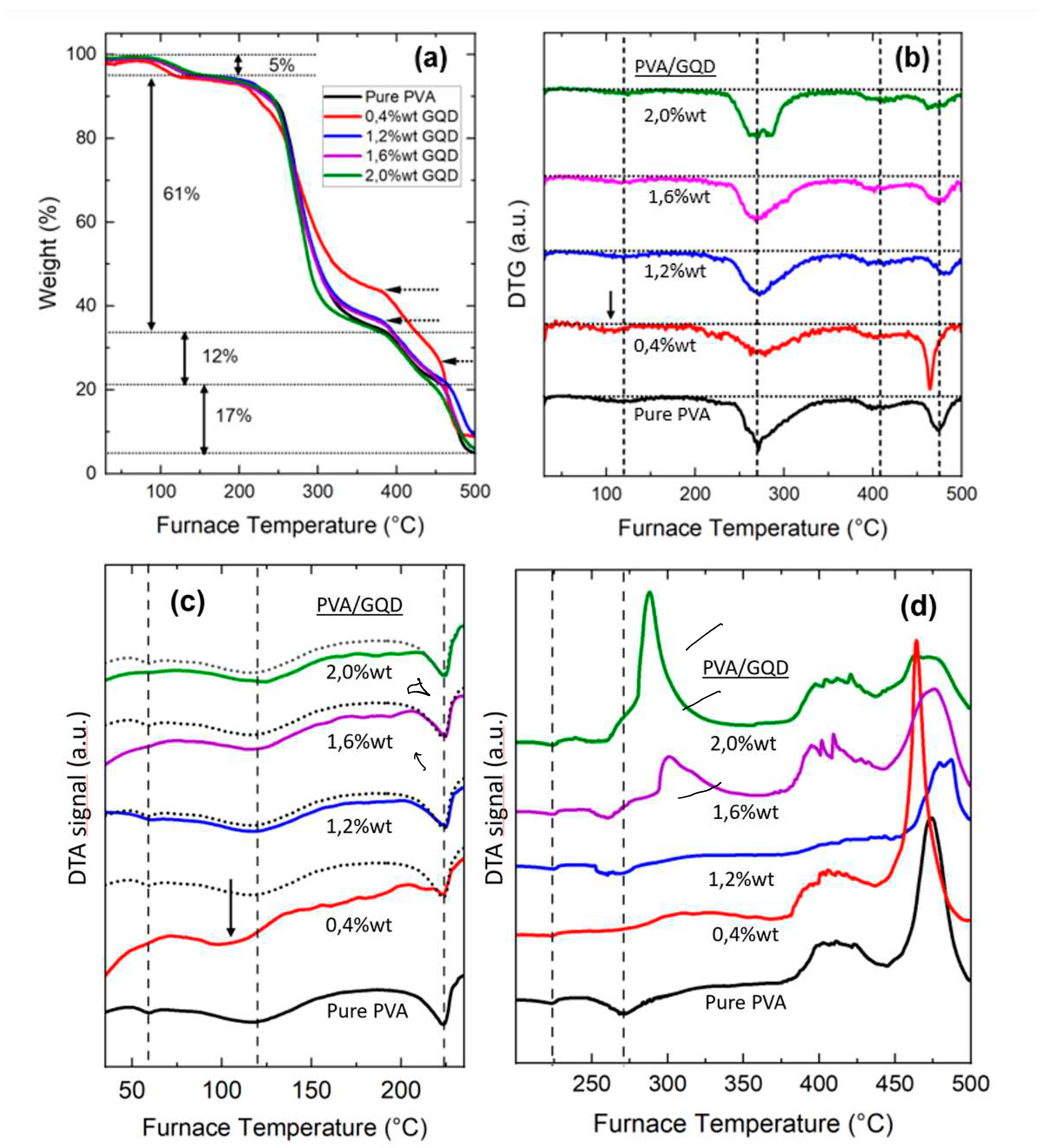
The thermal stability and thermal transition properties of the PVA/GQDs samples were studied over a varying temperature range by TG/DTA analysis.
Figure 4 shows (a) TGA thermographs, (b) Differential-Thermogravimetry (DTG) curves and (c) (d) Differential Thermal analysis (DTA) data of pure PVA and PVA/GQD composite films.
From
Figure 3a,b, four degradation phases can be distinguished. The percentage weight loss of each thermal degradation phase for PVA is indicated in
Figure 4a. As can be seen in
Figure 4, the inclusion of GQD in the PVA matrix influences the thermal behavior of the resulting nanocomposite film.
Table 2 indicates the weight loss percentage for each degradation stage (measured from
Figure 4a) and the % remaining at 500ºC.
At the first degradation stage, a ~ 5% loss of weight is observed at temperatures between 70° to 160 °C in all films, which can be attributed to the evaporation of residual water within the samples [
10,
38]. The maximum weight loss temperature occurs at ~120ºC in all cases, except for the film with 0,4%wt GQD content, where it occurs at a temperature ~ 15ºC lower (see arrow in
Figure 4b).
Figure 4c,d show the Differential Thermal Analysis data. In
Figure 4c, the dashed lines indicate the endothermic peaks corresponding to the glass transition temperature, at ~59ºC, the evaporation of the residual water, at ~120ºC , and the crystalline melting point, at ~224º C, of the pure PVA film [
39]. The curve corresponding to pure PVA has been superimposed (black dotted curves) on the curves measured for the other PVA/GQD films. No significant modification of those points is seen for the different films, except in the case of 0,4%GQD, for which the evaporation of the residual water occurs at ~15ºC less than for the pure PVA film (see arrow in
Figure 4c), in agreement with the observations in
Figure 4a,b. Moreover, the enthalpy of fusion of the different composites -area under the endothermic peak corresponding to the melting transition- is apparently similar to that of the pure PVA film for the different PVA/GQD nanocomposites, in agreement with the XRD results indicating that the percentage of crystallinity remains the same, except perhaps for the case of 0,4% GQD, whose DTA curve exhibits a positive slope.
The second degradation stage takes place at temperatures between 200 and 350 °C (
Figure 4a,b), and is attributed to the disruption of the intermolecular hydrogen bonding in PVA, with partial chain-stripping elimination reactions (removal of water, with the elimination of hydroxyl side-groups) and chain-scission reactions (formation of free radicals by PVA chain breakage), leading to the formation of polyenes as a result of the thermal degradation [
40,
41]. From
Figure 4b, for pure PVA the maximum weight loss temperature at which the degradation occurs in this second stage is at ~270ºC, at which an endothermic peak appears in the DTA measurements for the pure PVA film (
Figure 3d). Interestingly, the DTG curves (
Figure 4b) also reveal a small transition at the temperature region corresponding to the melting point, at the onset of this second degradation stage both for the pure PVA and the PVA/GQD composites. Nevertheless, when analyzing the DTA curves at temperatures close to 270ºC (see the corresponding dashed line in
Figure 4d), the response of the different composites is apparently rather different. For the cases of 1,2% and 1,6% GQD, the endothermic peak appears shifted to lower temperatures, although the maximum loss peak in
Figure 4b remains at the same position. And for the higher GQD contents, i.e. 1,6% and 2,0% GQD, DTA reveals the occurrence of an exothermic transition at this temperature, and an even more significant exothermic peaks are measured within this temperature range (see
Figure 4d). The results in
Figure 4d evidence that the presence of GQD alters the reactions taking place during this second stage of PVA degradation.
Finally, regarding the third and fourth degradation steps, above 350 °C, reactions giving rise to exothermic peaks in TDA take place (see
Figure 4d). At these stages, the occurrence of further degradation and carbonization of PVA backbone structure is expected [
42].
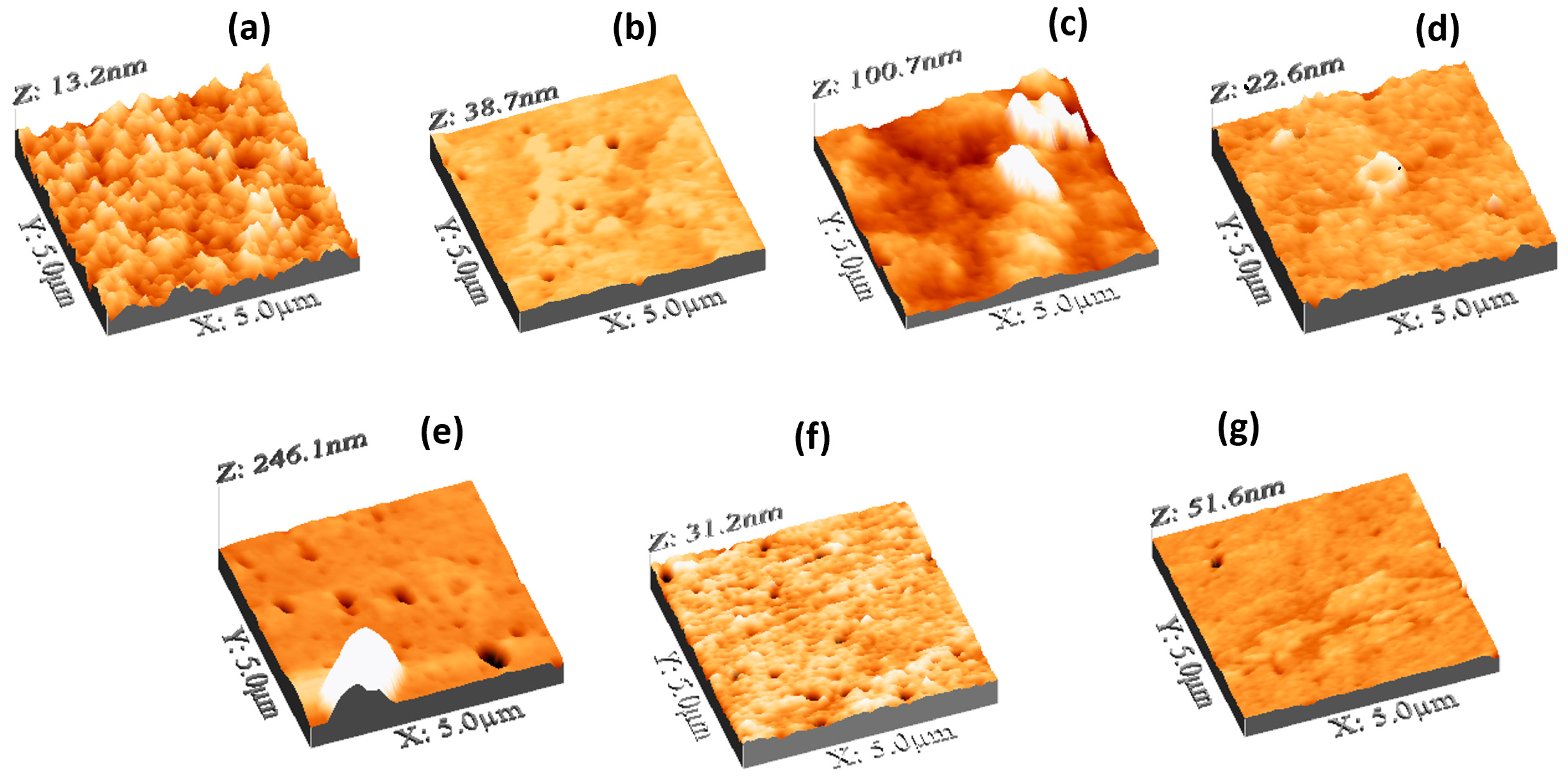
Let us study the surface features of the PVA/GQD nanocomposite films.
Figure 5 shows 3D representations of the topography measured by AFM of the pure PVA and PVA/GQD nanocomposite films prepared with different GQD concentrations. As it is apparent from
Figure 5, the incorporation of GQD within the PVA matrix has a strong impact on the film topographic features. The surface of the pure PVA film (
Figure 5a) is characterized by the presence of rounded, homogeneously distributed clusters, ~80 nm in diameter. The Root Mean Square (RMS) roughness in
Figure 5a is 1,6 nm, the surface skewness is 0,1, and the kurtosis is 3,0. However, on the sample with 0,4%wt GQD (
Figure 5b), the surface structural homogeneity has been severely disrupted; the RMS roughness is now 2,1 nm, the skewness -2,0 and the kurtosis 21,1. Surface pores are now more clearly visible on the surface, and the former cluster structures cannot be now resolved, having been replaced by a kind of extended stratified islands.
In the case of the sample with 0,8%wt GQD (
Figure 5c), the changes are even more dramatic. Although XRD, FT-IR and DTA allows us to conclude that there is no variation on the percentage crystallinity of PVA for the films with different GQD concentrations, the AFM topographic image in this film clearly reveals the presence of 3D clusters with stepped walls and facets characteristic of a crystalline morphology. We attribute these features to the formation of crystalline PVA islands, possibly with GQD’s acting as a nucleating agent for 3D crystalline PVA growth on the sample surface, as will be discussed in more detail below (see discussion related to
Figure 7) In
Figure 5c, the RMS roughness is 9,7 nm, the skewness 1,8 and the kurtosis 12.4.
On the film with 1,2%wt GQD (
Figure 5d), the surface regains a flat appearance, with a surface roughness of 1,05 nm, a skewness of 0,1 and a kurtosis of 6,7. Pores and, in some cases, characteristic PVA annular structures at the rim of the pores may be observed.
On the film with 1,4%wt GQD (
Figure 5e), 3D islands like those in
Figure 5c were again found, together with pores similar to those in
Figure 5d. Due to the presence of the 3D features and pores, the RMS roughness amounts to 12,5 nm, the skewness to 3,8 and the kurtosis to 18,4.
For the film corresponding to 1,6%wt GQD, in addition to (smaller) pores, small surface clusters aligned along a specific direction can be distinguished in
Figure 5f. In this case, the RMS roughness is 1,4 nm, the skewness -1,3 and the kurtosis 12,17.
Eventually, for the case of 2,0%wt GQD, aligned surface clusters can be observed in
Figure 5g, similar to those in
Figure 5f, but gathered now to form larger aggregates. Here, the RMS roughness is 1,7 nm, the skewness -2,3 and the kurtosis 30,2.
In the following, characteristic features of the different PVA/GQD films will be discussed in more detail taking advantage of the application of different AFM modes, where relevant.
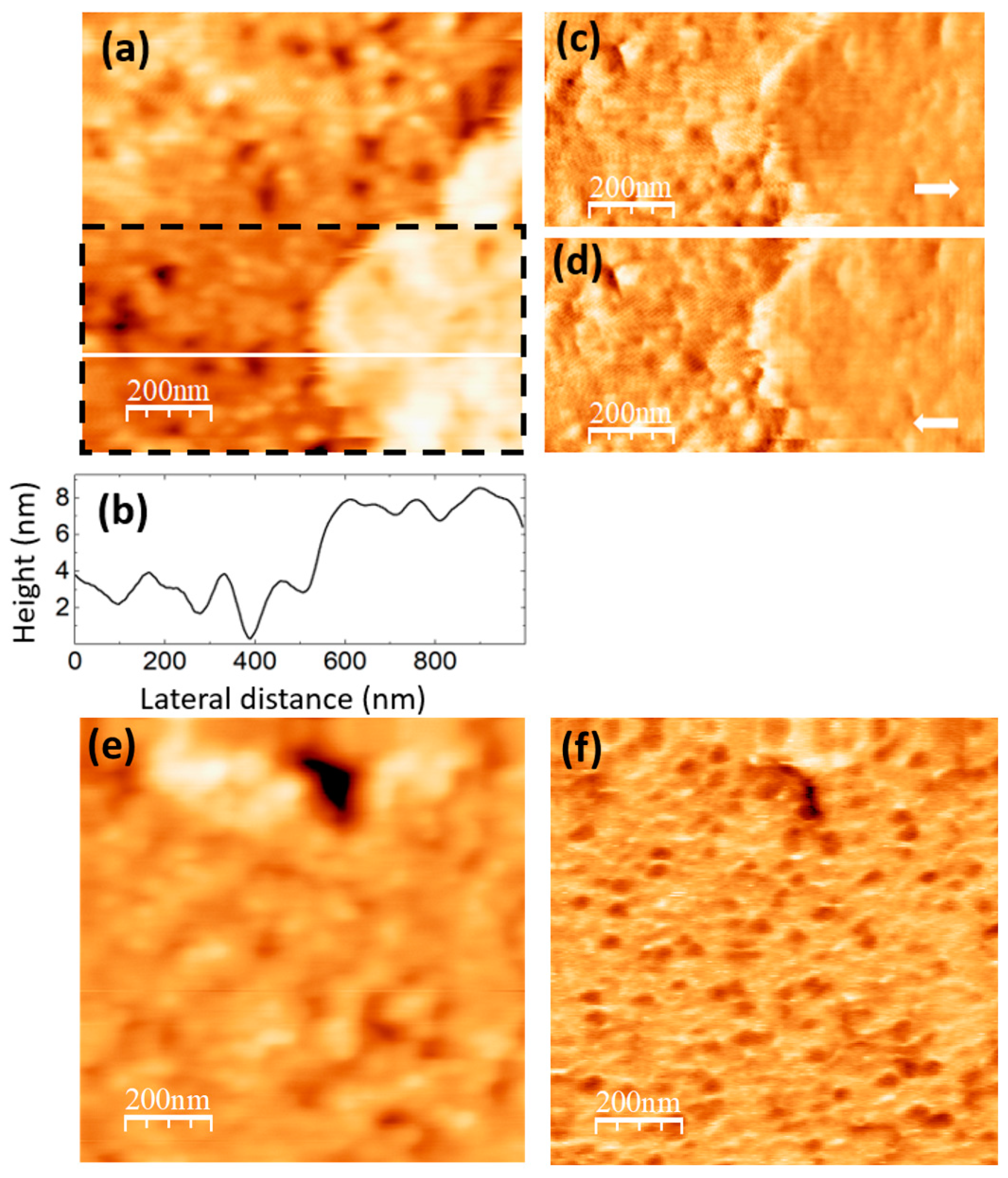
The images in
Figure 6 were recorded on the PVA/GQD film with 0,4%wt GQD.
Figure 6a shows the surface topography, recorded with contact-mode AFM.
Figure 6b corresponds to a height-contour profile along the white line in
Figure 6a. In the lower right-hand side of
Figure 6a, the presence of a terrace ~ 4 nm higher is apparent. On the lower terrace, rounded clusters ~ 80 nm in diameter similar to those on the pure PVA sample surface can be distinguished; the area is characterized by frequent “void” defects possibly consisting in displaced clusters. There are also clusters on the upper terrace, although they do not have such a well-defined rounded shape.
Figure 6c,d correspond to LFM images recorded over the area delimited by a dashed black rectangle in
Figure 6a. As it is apparent from
Figure 6c,d only a slight frictional contrast (darker in (c) and brighter in (d) over the same area) is noticeable at some areas over the higher terrace region, indicating a chemical homogeneity of the surface.
Figure 6.
PVA/GQD film with 0,4%wt GQD. (a) Contact-mode AFM topography. Color-scale range: 12 nm (b) Height-contour profile along the white line in (a). (c) (d) LFM images recorded over the surface area enclosed by the dashed black rectangle in (a), scanning from left to right (c) and from right to left (d). (e) Contact-mode AFM topography over a different surface area than (a). Color-scale range: 12 nm. (f) UFM image simultaneously recorded with (e) over the same surface area.
Figure 6.
PVA/GQD film with 0,4%wt GQD. (a) Contact-mode AFM topography. Color-scale range: 12 nm (b) Height-contour profile along the white line in (a). (c) (d) LFM images recorded over the surface area enclosed by the dashed black rectangle in (a), scanning from left to right (c) and from right to left (d). (e) Contact-mode AFM topography over a different surface area than (a). Color-scale range: 12 nm. (f) UFM image simultaneously recorded with (e) over the same surface area.
Figure 6e,f correspond to contact-mode AFM topography (e) and UFM (f) images recorded over another surface area of the same sample. The UFM image reveals that some of the clusters topographically similar in (e) exhibit, however, a lower UFM contrast, indicative of a lower stiffness. Such result may arise from a different conformation and packing of the macromolecular PVA chains within such clusters, resulting in a lower density. Also, it is observed that the higher topographic area at the top in (e) does not lead to a notably different UFM contrast in (f), in agreement with conclusions obtained when analyzing
Figure 6c,d.
The obtained results indicate that GQD interactions with PVA influence the conformation of the PVA chains with respect to those of pure PVA. GQDs can easily bind to a PVA chain via H-bonds through the chemical groups at their edges. According to the FT-IR data (see
Figure 3a,b) our GQDs must contain groups with C=O bonds, as well as OH groups at their edges, which confers them hydrophilic nature. It is certainly plausible that they alter the conformation of the PVA chains when incorporated into the PVA matrix, promoting the formation of the void defects observed in
Figure 6 (e) (i), and the re-arrangement of the PVA molecules into less dense clusters and/or new terraces. On the other hand, it is likely that these morphological changes facilitate water removal as observed by thermogravimetry for this film (see
Figure 4a–c).
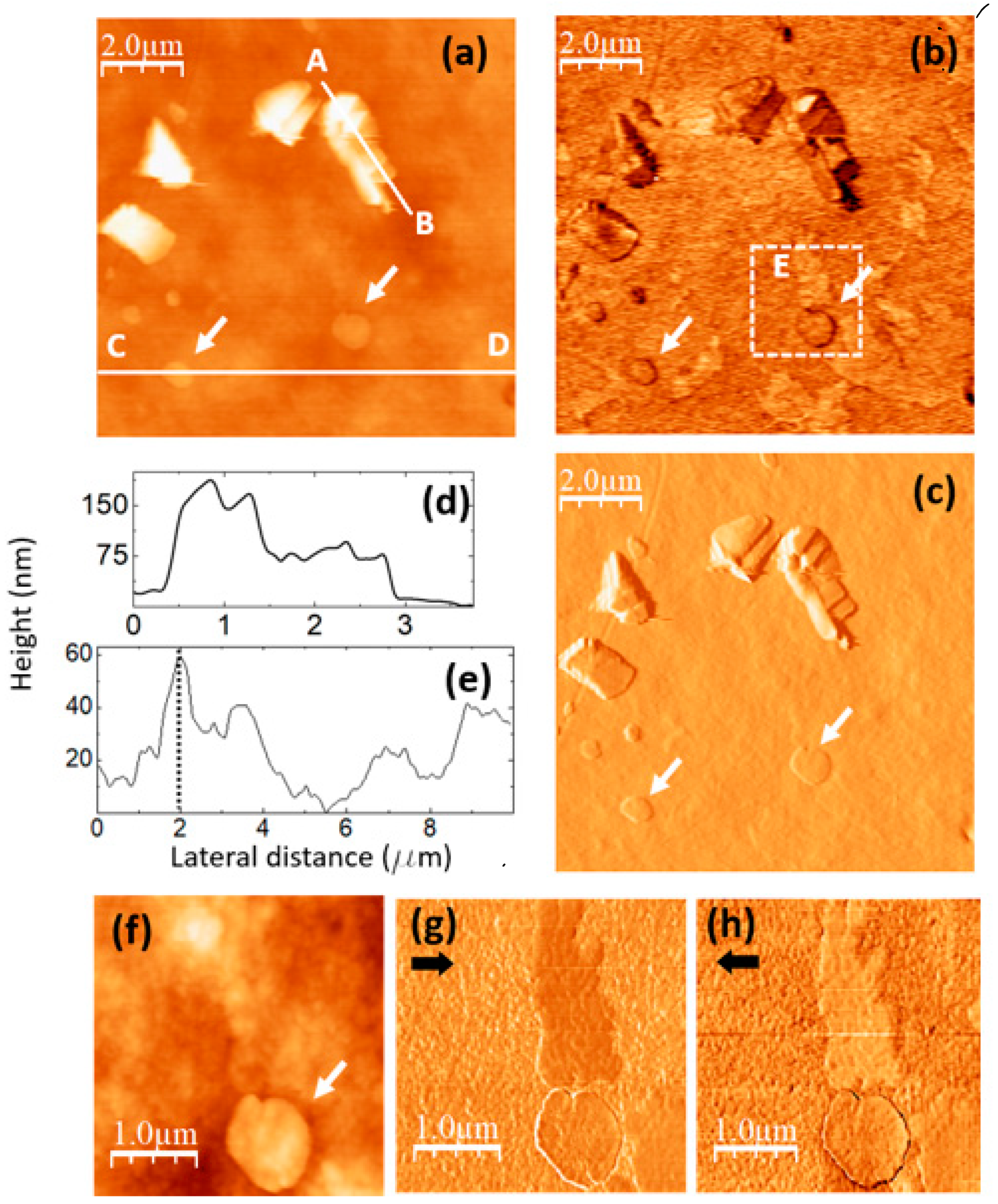
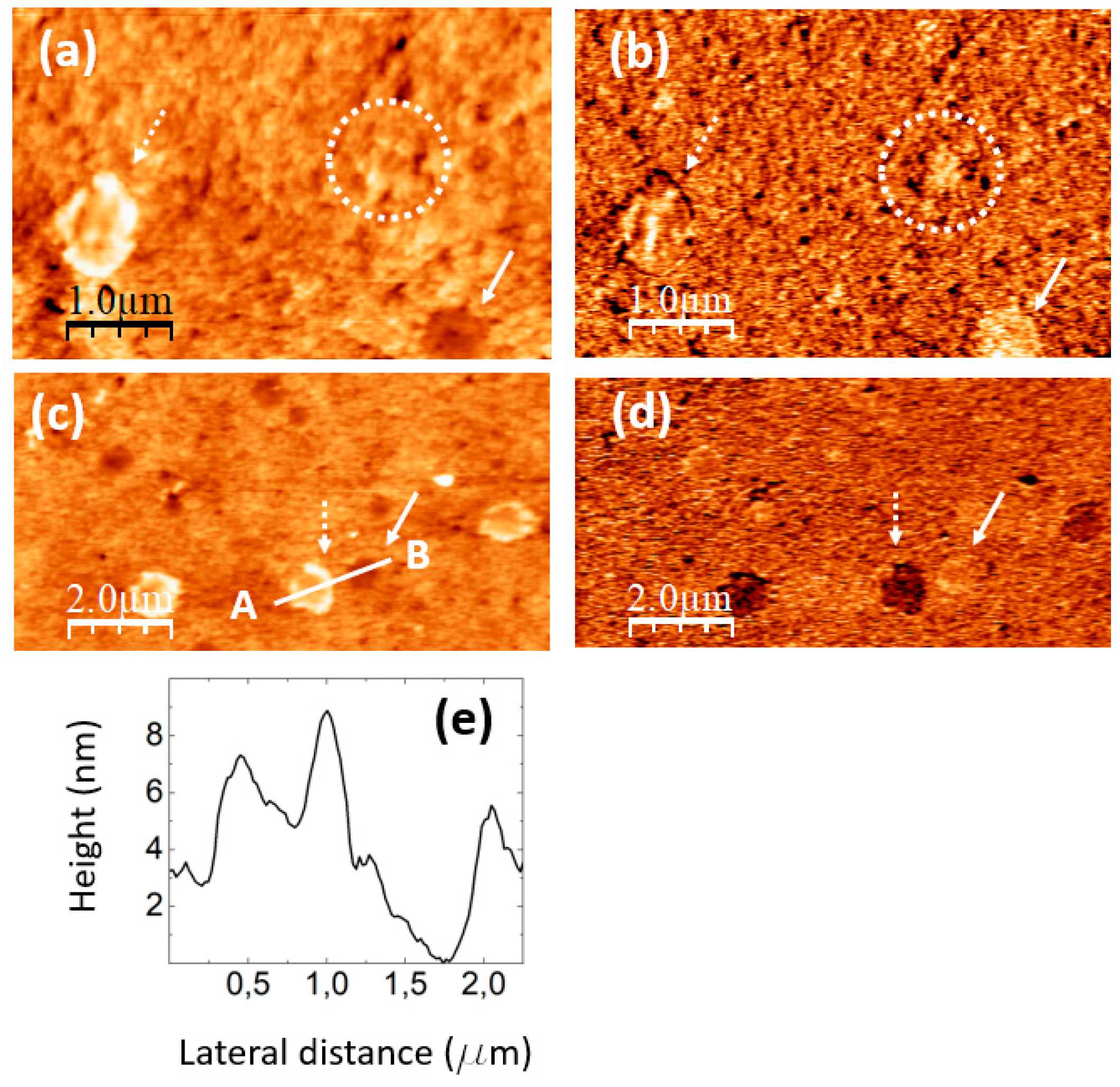
The images in
Figure 7 were recorded on a PVA/GQD film with 0,8%wt GQD.
Figure 7a,b correspond to contact-mode AFM (a) and UFM (b) images simultaneously recorded over a same surface area. As in
Figure 5c, the topography reveals the formation of 3D islands of crystalline appearance. The fact that thin inorganic layered fillers may induce the crystallization of polymer nanocomposites is already well known [
43]. It should be noted that those islands are much larger than the ~4,2 nm size estimated from the XRD data for the PVA crystalline domains, according to the Debye-Scherrer equation (see discussion related to
Figure 2). The fact that XRD on this film does not provide any indication of PVA crystal growth suggest that most probably their formation most likely occurs only on the film surface, whereas the XRD information comes not only from the surface, but from the whole PVA film. Furthermore, it could be the case that despite of the crystalline morphology of the islands, the specific domains in which the polymer atomic species are sufficiently well ordered to contribute to the XRD signal are much smaller than the island size.
Figure 7.
PVA/GQD film with 0,8%wt GQD. (a) contact-mode AFM image. Color-scale range: 208 nm (b) UFM image recorded simultaneously with (a), over the same surface area. (c) Derivative image of (a). (d) Height-contour profile recorded along the white line labelled A-B in (a). (e) Height-contour profile recorded along the lower white line labelled C-D in (a). (f) Contact-mode AFM image recorded over the area enclosed by the dashed white rectangle in (b). Color-scale range: 35 nm. (g) (h) LFM images recorded over the same surface area than (f) scanning from left to right (g) and from right to left (h).
Figure 7.
PVA/GQD film with 0,8%wt GQD. (a) contact-mode AFM image. Color-scale range: 208 nm (b) UFM image recorded simultaneously with (a), over the same surface area. (c) Derivative image of (a). (d) Height-contour profile recorded along the white line labelled A-B in (a). (e) Height-contour profile recorded along the lower white line labelled C-D in (a). (f) Contact-mode AFM image recorded over the area enclosed by the dashed white rectangle in (b). Color-scale range: 35 nm. (g) (h) LFM images recorded over the same surface area than (f) scanning from left to right (g) and from right to left (h).
The apparently crystalline 3D islands in
Figure 7a are characterized by stepped facets with characteristic orientations and angles.
Figure 7c corresponds to the derivative of the topography (
Figure 7a) and has been included to facilitate the observation of topographic slope variations.
The islands contrast in UFM (
Figure 7b) is facet-dependent, and it is probably strongly influenced by the orientation of the facet with respect to the tip. The chemical termination of the facet surface may also play an important role in the tip-sample adhesion, and thus in the resulting UFM signal.
Figure 7d shows the height-contour profile along the line A-B in
Figure 7a, according to which the island height reaches ~ 180 nm; different island facets can be appreciated.
Next to those apparently crystalline islands, flatter rounded terraces can also be distinguished in
Figure 7a, such as those marked with arrows.
Figure 7e is a height-contour profile along the line C-D in
Figure 7a that crosses one of these terraces. As can be seen in
Figure 7c, the height of this terrace (indicated by a dashed line at the contour-profile curve) is ~ 58 nm, much higher than the terraces found on the PVA/GQD film with 0,4%wt GQD (see
Figure 6). Those terraces are also distinguishable in the UFM image (
Figure 7b), but they provide no distinct UFM contrast, apart from the originated from the slope changes at their edges.
Interestingly, in
Figure 7b areas with a higher (brighter) UFM contrast are noticeable in the images at regions with no straightforwardly correlated topographic features, such as this labelled as “E”. There is no correlation between the brightest UFM zones in
Figure 7b and specific features in
Figure 7a or c.
Figure 7f–h correspond to topographic and LFM images recorded over the area within the dashed white rectangle in
Figure 7b, scanning from right to left (g) and from left to right (h). A comparison of
Figure 7b,g,h indicates that the stiffer areas in UFM (brighter contrast) exhibit lower friction (darker in (e) and brighter in (a)). Still, no clear correlation between the LFM images and the surface topography (
Figure 7f) is noticeable for this area. This type of contrast may arise from the existence of buried PVA crystallite domains in the near subsurface region, positioned very close to the surface, thereby exerting an influence on the tip-sample frictional response. PVA crystals with stiffer contrast formed in the presence of an inorganic filler surface (sodium montmorillonite) have been previously observed using AFM modes [
44].
Finally, it should be noticed that the aforementioned circular terraces (marked with arrows in
Figure 7), do not show a significant frictional contrast in
Figure 7g,h with respect to the substrate.
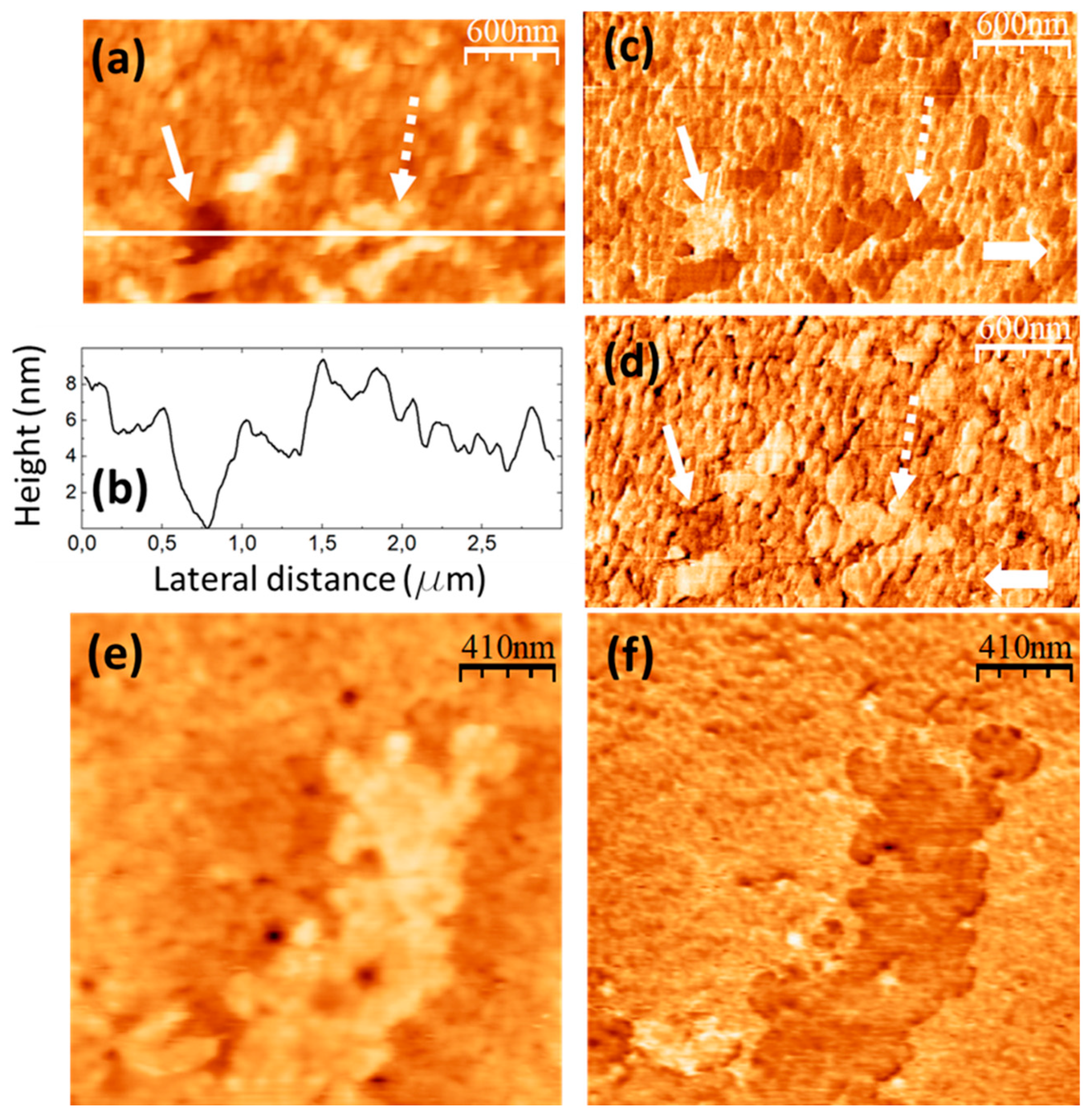
The images in
Figure 8 were recorded on a PVA/GQD film with 1,2%wt GQD.
Figure 8a,b correspond to simultaneously recorded contact-mode AFM topographic (a) and UFM (b) images;
Figure 8c,d are also simultaneously recorded contact-mode AFM topographic (c) and UFM (d) images from another surface area of the same sample.
As can be seen in
Figure 8a,c and
Figure 5d, the incorporation of a higher amount of GQD (1,2%wt) leads to the formation of circular “pores” (e.g. those marked by continuous white arrows in
Figure 8a,c) with various diameters, up to ~500 nm, and ~4 nm deep, some of them with a protruding ring at their edge (see
Figure 5d also). In addition to the pores, circular structures with a surrounding annular rim are apparent (e.g. those marked by the dashed white arrows in
Figure 8a,c). The internal diameter of such structures is ~ the same size as that of the pores, but their central area is larger than that of the substrate, their structure and origin being presumably common to those of the pores, but the latter being filled by additional molecules. In the circular structure marked by the dashed white arrow in
Figure 8a, the rim is formed by clusters ~ 125 nm in diameter and ~ 5 nm in height; some cluster similar to those at the rim is also located over the central region.
Figure 8e shows a height-contour profile along the white line in
Figure 8c from A to B that crosses one of the circular structures and a pore located nearby. Notice that the depressed central region of the circular structure is ~ 2 nm higher than the surrounding substrate.
In UFM (
Figure 8b,d) the pores usually appear more rigid (brighter contrast). At some areas, such as the one enclosed by a dashed white circle in
Figure 8a,b, the fact that the UFM image yields a stiffer contrast distinctly suggest that the topography corresponds to a covered pore area. Regarding the circular structure marked by the dashed white arrow in
Figure 8c, the UFM (darker) contrast is clearly indicative of a softer zone. We understand this contrast has its origin in modified PVA clusters located both at the edge and filling the central part.
When analyzing
Figure 5 (0,4%wt GQD loading), we observed defects which we termed “void” defects, that apparently consisted in displaced PVA surface clusters; we attributed the origin of those defects to the incorporation of a slight amount of hydrophilic GQDs into the PVA matrix, which incorporated to the PVA molecular chains via H bonding and influenced their conformation. For 1,2%wt GQD loading in
Figure 8, we observe “pores”, much larger in diameter than the “voids”, which however could have a similar origin, but this time requiring a higher amount of GQDs to induce the modifications of the PVA molecular chains conformation, in which interactions of the GQDs with each other could also play a role.
The images in
Figure 9 were recorded on a PVA/GQD film with 2,0%wt GQD.
Figure 9a corresponds to the contact-mode AFM topography,
Figure 9b is a height-contour profile along the white line in
Figure 9a,c,d are LFM images recorded over the same surface area than
Figure 9a scanning from right to left (c) and from left to right (d). Also, in this film, we find pores similar to those in
Figure 8, such as this one marked by the continuous white arrow in
Figure 9a, with a diameter of ~ 500 nm and depth of ~4 nm (see
Figure 9b). At the pore zone, LFM reveals a higher frictional contrast (brighter in (c) and darker in (d)). According to
Figure 9, for the 2,0wt% GQD load, the surface is characterized by the presence of cluster aggregates (e.g. this one marked by the dashed white arrow in
Figure 9a), that yield a clear lower frictional contrast (
Figure 9c,d).
Figure 9e,f correspond to contact-mode AFM topography and UFM image simultaneously recorded over the same surface area, different than this of
Figure 9a, on the same sample. From the figures, it is noticeable that the aggregates in
Figure 9a gather to form extended terraces, that yield a distinct softer (darker) UFM contrast, confirming that distinct phases characterized by different elastic and frictional contrast form on the film surface. It should be remarked that for GQD loading higher than 1,2%wt GQD, a GQD-related band emerges in the FT-IR spectrum (see
Figure 3). We understand that the new PVA-GQD phase observed in
Figure 9 develops as a result of the incorporation of GQD to the PVA molecular chains via H bonds, and the arrangement of the modified PVA molecules in a distinct conformation, which possibly also involves GQD-GQD interactions.
Figure 9.
PVA/GQD film with 2,0%wt GQD. (a) Contact-mode AFM topography. Color-scale range: 12 nm. (b) Height-contour profile recorded along the lower white line in (a). (c) (d) LFM images recorded over the same area than (a) scanning from left to right (c) and from right to left (d). (e) Contact-mode AFM topography on a different surface area than (a). Color-scale range: 17 nm. (f) UFM image recorded simultaneously with (e), over the same surface area.
Figure 9.
PVA/GQD film with 2,0%wt GQD. (a) Contact-mode AFM topography. Color-scale range: 12 nm. (b) Height-contour profile recorded along the lower white line in (a). (c) (d) LFM images recorded over the same area than (a) scanning from left to right (c) and from right to left (d). (e) Contact-mode AFM topography on a different surface area than (a). Color-scale range: 17 nm. (f) UFM image recorded simultaneously with (e), over the same surface area.
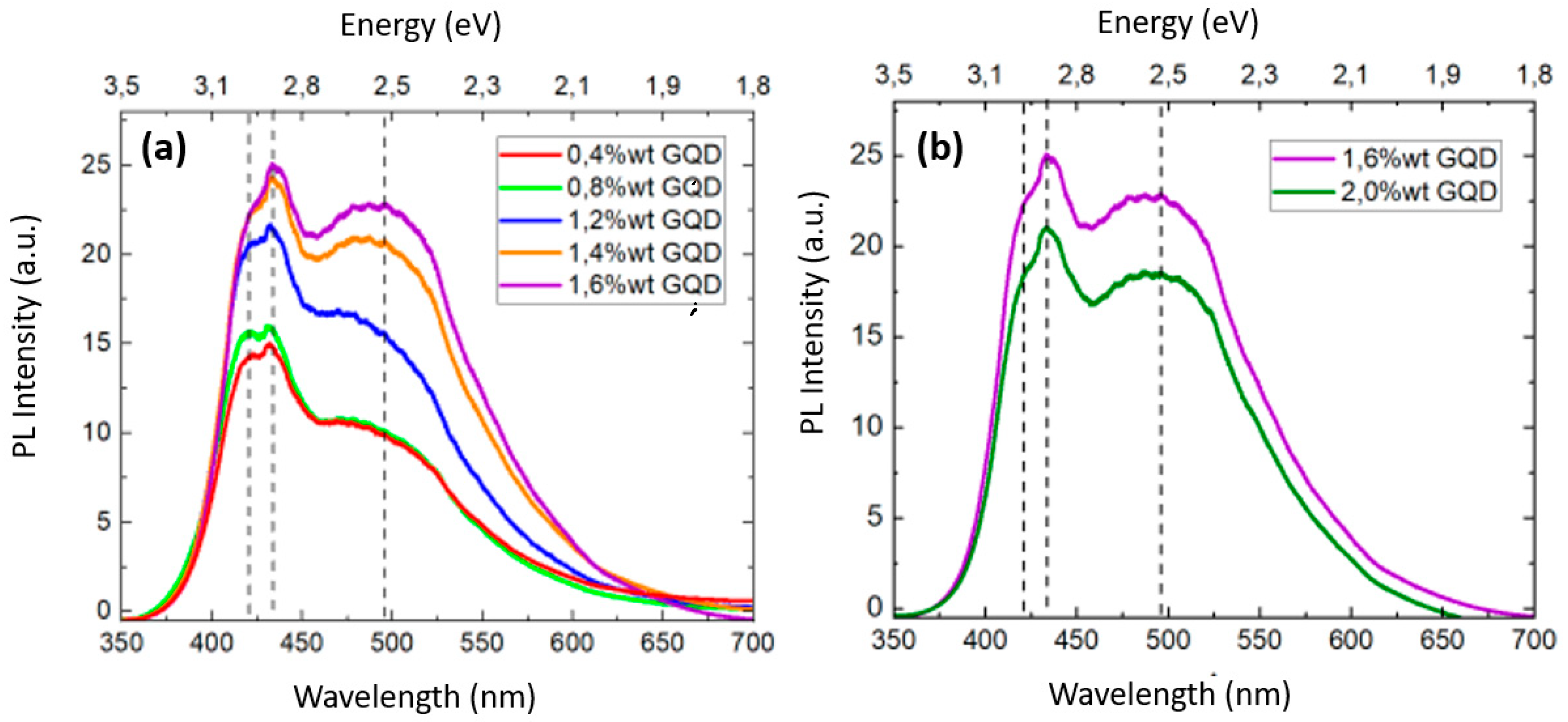
Figure 10 shows the PL spectra of the PVA/GQDs composite films. In PVA/GQD films, the PL response is due to the incorporation of the GQDs, and is excitation-dependent [
10,
12,
14]. Our PL measurements were conducted at room temperature, utilizing a 325 nm laser source. As can be seen in
Figure 10, the PL spectra of the PVA/GQD films exhibits maxima at ~420 nm, ~434 nm and ~495 nm (marked with dashed lines in
Figure 10a,b). The PL emission intensity increases as the concentration of GQDs is increased, although in a nonlinear manner. In addition, the PL curves experience significant variations in shape that reflect that the relative contribution of each spectral component varies with the amount of GQD incorporated into the PVA matrix.
To date, there is still a need for a comprehensive understanding of the mechanisms behind the PL emission of GQDs [
45,
46]. There are three primary contributing factors: size, surface structure and edge effects. The quantum confinement effect of conjugated p-domains is determined by the carbon core. Surface states are determined by the hybridization of the carbon backbone and the connected chemical groups. Various functional groups (C–OH, C=O, O–C=O etc.) introduced during the growth of GQDs can give rise to surface states with energy levels located between the p and p* states of C=C, leading to the absorption/emission bands due to electron transitions within one or more of these groups. Both the edge structure and the presence of defects/surface states can significantly alter the electronic properties of GQDs. PL emission of GQDs primarily arises from the interplay between intrinsic state emission and defect state emission. Intrinsic state emission results from the quantum size effect, zigzag edge sites or the recombination of localized electron-hole pairs whereas defect state emission originates from energy traps.
Our PVA/GQD films, transparent under natural light, exhibited a bright blue color when placed inside a UV chamber (see
Figure 1) even at the lowest considered GQD loads (0,4%wt GQD). An increase in GQD loading from 0,4 to 0,8%wt resulted in the growth of 3D islands on the PVA/GQD film surface (see
Figure 5 and
Figure 7) but the PL response did not experience significant variations (see
Figure 10a). This result suggests that when GQDs acting as nucleating agents for the growth of PVA crystallites, their PL response is quenched. However, for the 1,2%wt GQD load, a steady increase of the PL spectral response is observed. As the amount of incorporated GQD is further increased, the intensity of the PL band at lower energy (495 nm) increases, along with the emergence of the GQD-related band ~ 1650 cm
-1 in FT-IR, while the higher energy PL band (maxima at ~420 and ~434 nm) reaches a saturation value. For ~2%wt GQD (
Figure 10b), the overall PL spectral response decreases linearly. This decrease occurs when a new extended phase with clear and distinct elastic and frictional contrast is observed on the PVA/GQD film surface (see
Figure 9), and hence could both events could be correlated.
Our results emphasize the impact of the surface molecular rearrangements and morphology on the PL response of PVA/GQD films. Further experiments are planned to explore the PL behavior in higher detail.