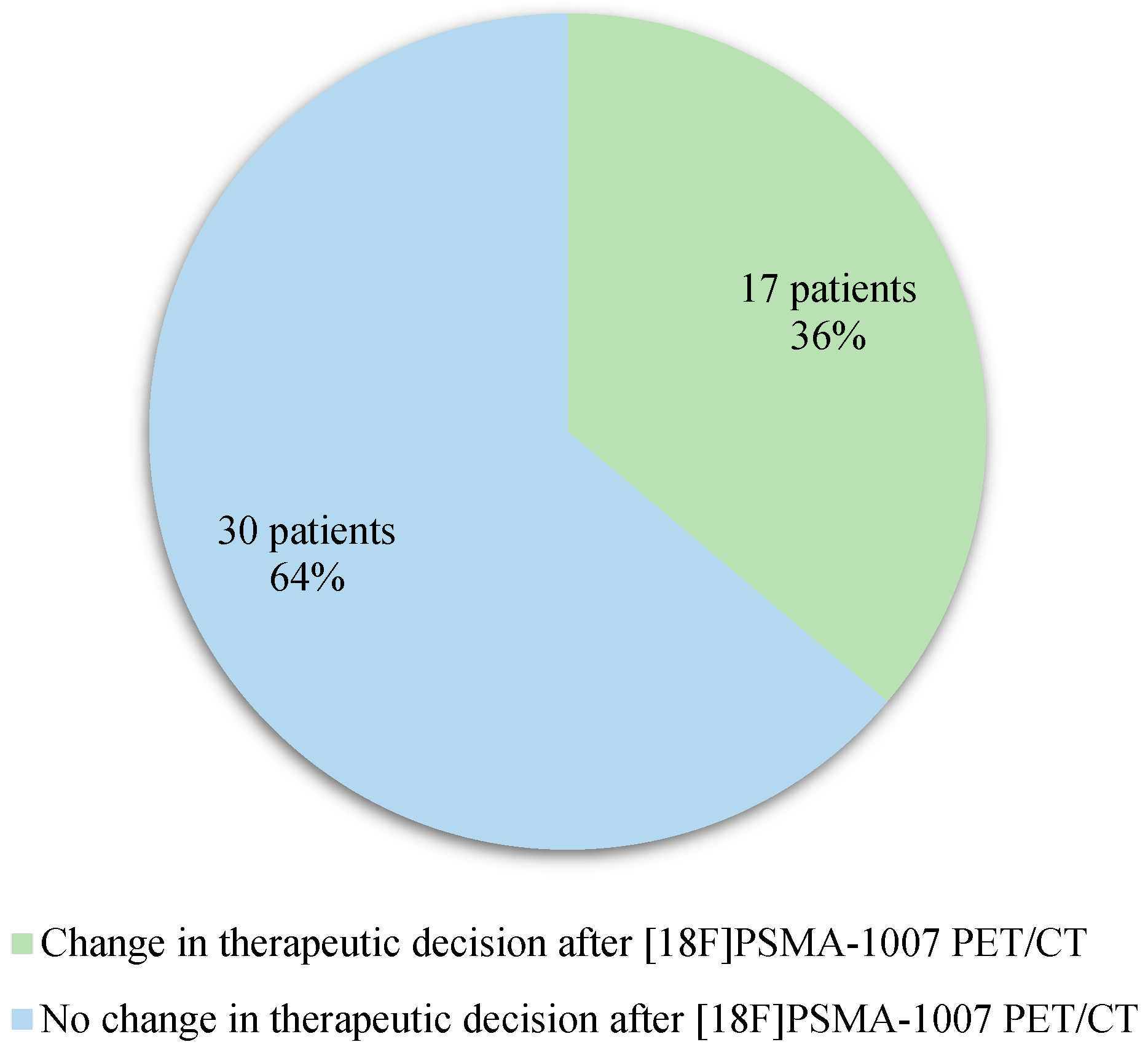INTRODUCTION
Prostate cancer is the most common male prevalent solid malignancy (1,2) in Europe, with high morbidity (3) and it is the third most common with regards of mortality (4,5).
Despite highly successful treatments like radiotherapy and radical prostatectomy, prostate cancer recurrence occurs in 20-40% of patients within 10 years following treatment (6). The biological recurrence of the disease is considered after two consecutive prostate specific antigen (PSA) values of 0.2 ng/ml after radical prostatectomy or with a PSA level of at least 2.0 ng/ml with 2 consecutive rises above the nadir after radiotherapy (7).
The major challenge is to be able to localize the recurrence (local recurrence, loco-regional or metastatic recurrence) in order to optimize the therapeutic management (loco-regional or systemic treatment: hormone therapy, chemotherapy, radiotherapy…). Imaging modalities play an important role in the restaging of prostate cancer (2,8).
Currently, Fluor-18 ([18F]) Fluorocholine (F-Choline or FCH) positron emission tomography computed tomography (PET/CT) is one of the most widely used diagnostic technique for detecting recurrent disease in prostate cancer (5,6,8). It was demonstrated that [18F]F-Choline PET/CT can better detect sites of prostate cancer recurrent in the earlier phase of PSA recurrence than bone scans and CT scans, which are the standard imaging modalities in this indication. However [18F]F-Choline PET/CT is rarely positive for patients with restaging PSA levels under 1 ng/ml (9,10).
Prostate-specific membrane antigen (PSMA) is a glutamate carboxypeptidase type II membrane protein. It is overexpressed in prostatic carcinoma cells, both in density (100-1000 times) and activity (8-10 times) compared to benign prostatic tissue. The role and function of this protein are not exactly know but the main hypothesis is that it plays a role in folate transportation and metabolism (11).
Indeed, PSMA can be labelled with two different radioactive molecules: [18Fluor] and [68Gallium]. In terms of Tumor Node Metastasis (TNM) staging, both tracers appeared widely exchangeable (12) and [18F]PSMA-1007 PET/CT performs at least equally to [68Ga]PSMA-11 (13–15). Physical half-life of the [18F] radioisotope is the longer (109 minutes versus 68 minutes for the [68Ga])(13–16) and production methods differ: a limited quantity can be obtained from the generator-produced [68Ga]. Initially, [18F]PSMA-1007 had a temporary use authorization when [18F]F-Choline PET/CT was negative or doubtful, which still remains the case when using a gallium-labelled PSMA. Since the 28th April 2022, the French Health Authority has granted early access authorization for [18F]PSMA-1007 PET/CT in patients with biological recurrent prostate cancer, after radical treatment, with a re-increase in the serum concentration of PSA.
In several studies, PSMA-ligand imaging had better detection rate compared to [18F]F-Choline (10,17). In a meta-analysis, PSMA PET/CT had a detection rate of 50% and 68%, respectively for prostate-specific antigen levels < 0.5 ng/ml and 0.5–2 ng/ml, whereas [18F]F-Choline PET/CT was rarely positive for patients with restaging PSA levels under 1 ng/ml (9,10). [18F]F-Choline PET/CT sensitivity was 77.5%, 80.7%, 85.2%, and 92.8% for the trigger PSA levels of more than 0.5, 1, 2, and 4 ng/ml, respectively (2). The performance of PSMA PET/CT is superior to [18F]F-Choline PET/CT, with an estimated detection rate of 40–60% at early BR for PSMA PET/CT (PSA < 1 ng/ml) (18–20). Compared to other imaging modalities (like magnetic resonance imagining and pelvic scan), PSMA PET/CT has shown superiority in patients with suspected biochemical recurrence (4,21,22), with high sensitivity (77-92%) and specificity (99%)(23).
The early detection of lesions, allowed by PSMA PET/CT when [18F]F-Choline PET/CT is not contributive because of too low PSA levels (< 1 ng/ml), has a major interest on therapeutic decision-making permitting to choose the best therapeutic strategy (1,6,24–26). A recent systematic review of the literature shows that PSMA PET/CT seems to be effective in identifying recurrence localization also for very low levels of PSA (< 0.5 ng/ml) and this technique provided significant changes in the therapeutic management of patients (24). Indeed, several studies have demonstrated a better detection rate of the recurrence site thanks to PSMA tracers which had a significant clinical influence, notably in the choice of treatment (27–33), but the majority of these studies focuses on [68Ga]PSMA-11 PET/CT, more difficult to perform routinely.
The aim of our study was to explore the impact on therapeutic decision-making of [18F]PSMA-1007 PET/CT in the biochemical recurrence of prostate cancer compared to [18F]F-Choline PET/CT.
METHODS
Study design and population
This study was a multicentric, prospective and observational study based on patients from two nuclear medicine centers in Angers: the university hospital center (CHU) and the cancer center “Institut de Cancérologie de l’Ouest (ICO), Paul Papin” between the first October 2021 and the first October 2022.
We included patients with biochemical recurrence for prostate cancer who underwent a [18F]PSMA-1007 PET/CT after [18F]F-Choline PET/CT during this period. According to the literature, the diagnostic of recurrence was defined as two consecutive increases in serum PSA concentration and/or PSA concentration > 2.0 ng/ml + nadir after radiation or cryotherapy and/or serum PSA concentration > 0.2 ng/ml after prostatectomy (7). We excluded minor patients, patients with contraindications to any ingredients of ABX-PSMA-1007 and all patients included in a clinical trial involving Lutetium-PSMA.
These data were collected by consulting the patients' medical records. When data were not available or not clear, a questionnaire was sent in a secure way to the prescriber (Error! Reference source not found.), after the [18F]PSMA-1007 PET/CT, to know if this exam modified the therapeutic decision-making. This questionnaire included 2 questions with different options to check: has the therapeutic management been modified by the imaging examination? What treatment has been decided for the patient (chemotherapy, hormone therapy, radiotherapy, therapeutic abstention)? This questionnaire was validated by physicians specialized in oncology and with an experience in prostate pathology.
We collected the following clinical data at enrolment: population characteristics, initial prognostic parameters (International Society of Urological Pathology (ISUP) classification, initial level of PSA, TNM classification), type of curative treatment, existence of previous recurrence before the biological recurrence for which [18F]PSMA-1007 PET/CT was realized, time to biological recurrence for which [18F]PSMA-1007 was realized and PSA levels at recurrence. We also collected technical data for dosimetry, the delay between injection and acquisition for [18F]F-Choline PET/CT and [18F]PSMA-1007 PET/CT.
A follow-up was carried out during six months to recover data concerning the therapeutic management of the patients.
PET/CT acquisition
Acquisition of images was realized on 2 different PET/CT:
A digital PET Philips Vereos with an acquisition of one minute per step with an OSEM (ordered subset expectation maximization) reconstruction, 2 iterations with 5 subsets, Field of View (FOV) of 567mm, Gaussian filter with FWHM (Full Width at Half Maximum) of 2 mm;
An analogic PET General Electric Discovery IQ5 with an acquisition of 2 minutes per step, 2 iterations with 12 subsets, FOV of 700mm, Gaussian filter with FWHM of 2mm.
The scanners were standardized in an EARL (resEARch 4 Life ©)-like protocol (34). The delivered dose was measured according to the Dose-Length-product (DLP) model and was expressed in mGy.cm (milliGray.centimeter). The acquisition was performed from the vertex to the midthigh level, arm raised when possible. After the injection of the radiotracer, a diuretic (20 mg of furosemide) was administered to the patients, to avoid urinary stasis of the tracer. The patients who performed their examination at the ICO (n=31) may also have an injection of iodinated contrast product IOMERON 350®, the dose administered depended on the patient's weight. The scanner acquisition started 55 to 60 seconds after the intravenous injection.
Interpretation
Interpretation of the images was performed on Syngo.via® post-acquisition software (Siemens healthineers) by an experienced nuclear physician. PSMA interpretation was not performed blind to [18F]F-Choline PET/CT. In case of doubt, a second advice was requested from another nuclear physician.
The physicians had access to the patient's previous examinations, clinical and biological information.
An examination was considered as positive after interpretation if a non-physiological uptake was found in the prostatectomy territory, in lymph nodes, in bone or in visceral organs. This abnormal uptake should be higher than the background (35). Uptake foci known to be false positives in literature were excluded.
PET/CT analysis was performed by using: a visual analysis and a semi-quantitative analysis with the use of the parameter “maximum standardized uptake value” (SUVmax). SUV is the ratio of the activity concentration in the lesion to the activity concentration in the whole body. It is the highest value of a single voxel in the volume of interest (VOI). SUV = (tissue radioactivity [Bq] / tissue weight [g]) / (injected activity [Bq] / body weight [g]).
The primary endpoint was to determine the therapeutic impact of the [18F]PSMA-1007 PET/CT comparative to [18F]F-Choline PET/CT. The secondary endpoints were to compare the diagnostic performance of these 2 tracers. The diagnostic performance was defined as the ability to detect uptake in an area of recurrence. An evaluation of the correlation between [18F]PSMA-1007 PET/CT parameters and known prognostic factors of prostatic neoplasia (PSA level at recurrence and ISUP grade at diagnosis) was realized.
Statistical analysis
Continuous data were described by the mean and standard deviation, interval confidence (IC), median, minimum and maximum can be specified if it was relevant. Categorical variables were described by the number and percentage of each modality of the variable. The difference between [18F]F-Choline PET/CT and [18F]PSMA-1007 PET/CT diagnostic performances was calculated with a McNemar test. A calculation of the kappa concordance coefficient was carried out and a Landis & Koch classification was use for the interpretation after calculating the observed concordance coefficient corrected by the chance concordance coefficient. Comparisons between groups were made by the Pearson’s test for continuous variables whom following a normal distribution. The analyses evaluating the associations between PSA levels at recurrence and SUVmax were performed with univariable logistic regression models. Analyses were performed using Microsoft Excel, AnaStats and R® for macOS version 4.2.2. In order to determine the correlation between the ISUP score and the SUVmax and between subgroups of PSA levels and SUVmax, a non-parametric test was carried out (Kruskal-Wallis test), as the numbers compared were small. All tests used were two-sided with an alpha threshold at 5%. Differences were considered as statistically significant when p-value was below 0.05.
RESULTS
Population description
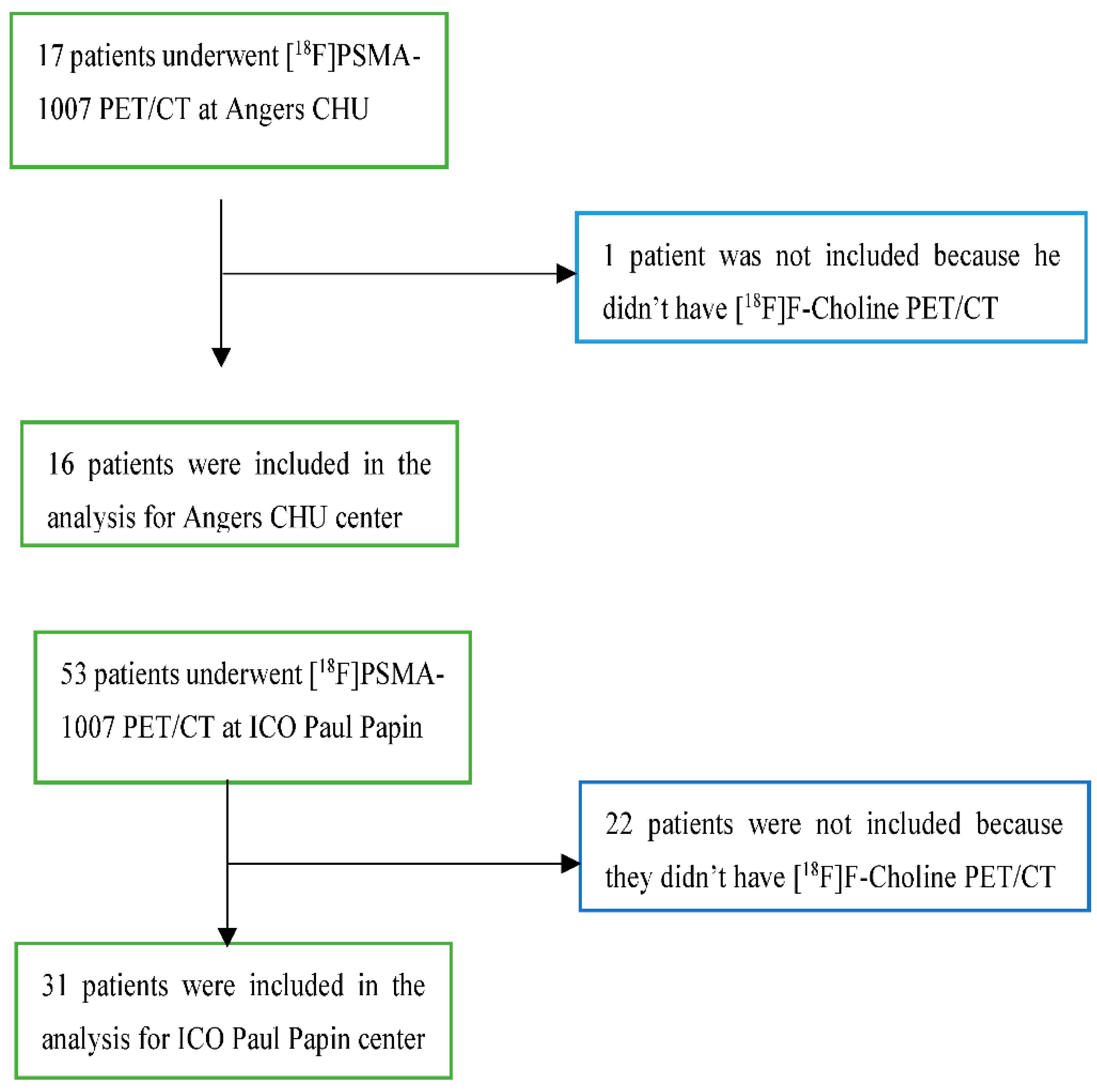
47 patients were included between the 1
st October 2021 and the 1
st October 2022. 16 patients underwent their exam in Angers CHU and 31 patients underwent their [
18F]PSMA-1007 PET/CT in ICO Paul Papin (
Error! Reference source not found.).
Figure 1.
Flow chart for patient’s inclusion.
Figure 1.
Flow chart for patient’s inclusion.
The characteristics of the patients have been described in Table I. Patients included had a PSA level at diagnosis mainly below 10 ng/ml (24 patients/47), they were mostly classified as ISUP 3 (17/47), T3N0M0. Biochemical recurrence occurred mostly in the first 5 years (16/47) of their initial management, with a mean PSA at 2.4 ng/ml.
Table 1.
Population characteristic
Table 1.
Population characteristic
| |
Total of patients n = 47 |
| Age at initial diagnostic – Mean [years] |
62 |
| Center |
University hospital of Angers
ICO Paul Papin
|
16 (34%)
31 (66%) |
| Histological type |
Adenocarcinoma |
47 (100%) |
| PSA at diagnostic [ng/ml] |
Mean
< 10 ng/ml
10-20 ng/ml
> 20 ng/ml
Missing data
|
13.2
24 (51%)
10 (21%)
7 (15%)
6 (13%) |
| ISUP at diagnostic |
1
2
3
4
5
|
5 (11%)
11 (23%)
17 (36%)
10 (21%)
4 (9%) |
| TNM: T |
T1
T2
T3
Doubt between T2 and T3
|
0 (0%)
19 (40%)
24 (51%)
4 (9%) |
| TNM: N |
Nx
N0
N1
|
1 (2%)
41 (87%)
5 (11%) |
| TNM: M |
M0
M1
|
46 (98%)
1 (2%) |
| Prostatectomy |
45 (96%) |
| Scraping |
Total
Bilateral ilio-obturator
Unilateral ilio-obturator
|
31 (66%)
30 (97%)
1 (3%) |
| Initial pelvis radiotherapy |
2 (4%) |
| Other recurrence before this study |
24 (51%) |
| Time [years] between initial diagnosis and the recurrence for which [18F]F-Choline was realized |
Mean
< 1 year
[1-5] years
]5-10] years
> 10 years
|
7.1
7 (15%)
16 (34%)
14 (30%)
10 (21%) |
| PSA at biological recurrence [ng/ml] |
Mean
< 1 ng/ml
1-2 ng/ml
> 2 ng/ml
|
2.4
14 (30%)
17 (36%)
16 (34%) |
Among the 47 patients included, considering the therapeutic decision expected after completion of the [18F]F-Choline PET/CT and what was finally decided after completion of the [18F]PSMA-1007 PET/CT (
Error! Reference source not found.), a modification of therapeutic management was observed in 36% of case (17 patients)
Figure 2.
Therapeutic impact of [18F]PSMA-1007 PET/CT.
Figure 2.
Therapeutic impact of [18F]PSMA-1007 PET/CT.
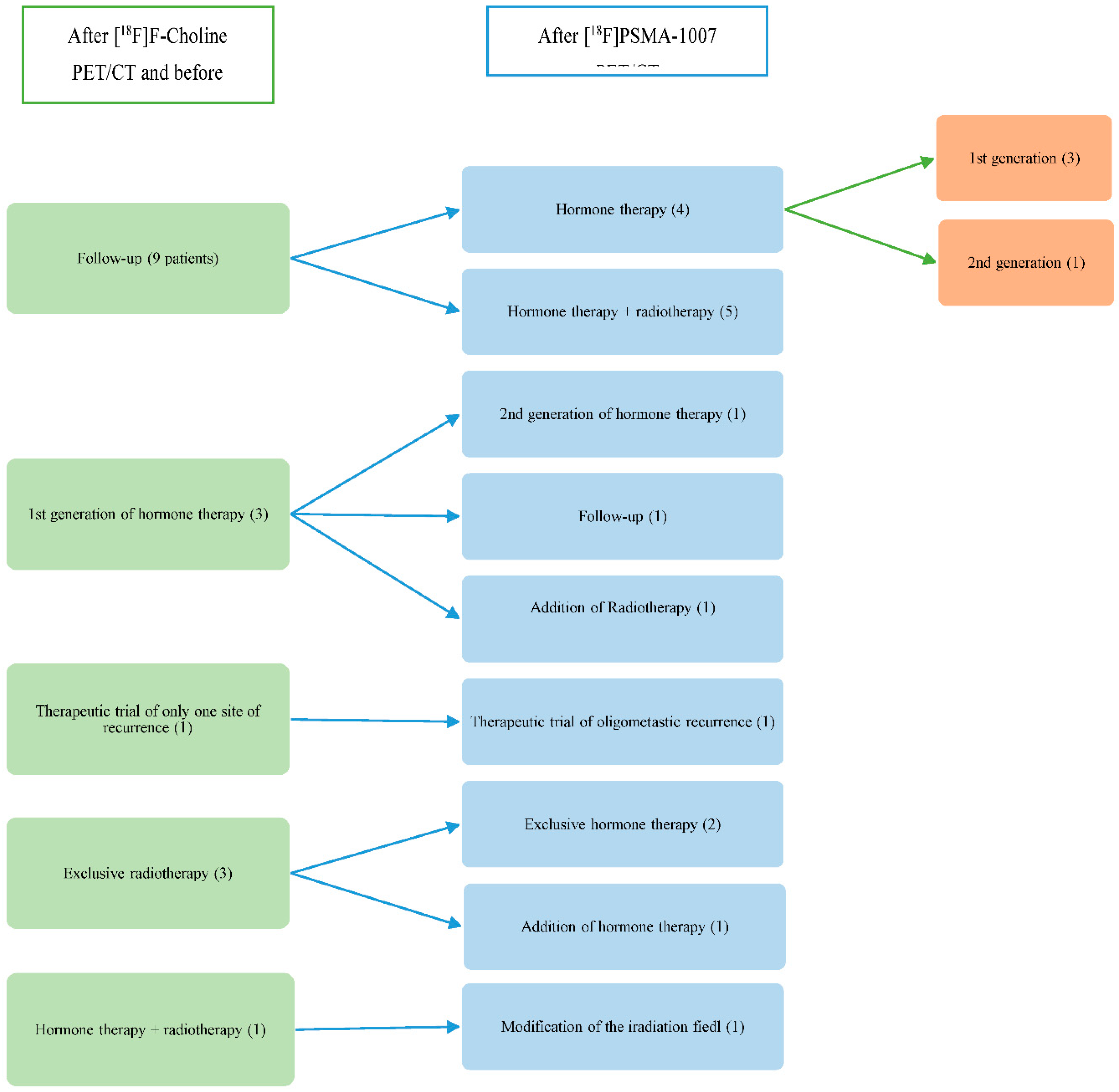
Among the 17 patients who had a modification of their therapeutic management following [
18F]PSMA-1007 PET/CT (
Error! Reference source not found.), it was decided to perform hormone therapy and radiotherapy at the site of recurrence instead of follow-up for 5 patients (29.4%). For these 5 patients, the [
18F]F-Choline PET/CT didn’t reveal any suspicious or positive localization, unlike the [
18F]PSMA-1007 PET/CT did. For 4 patients (23.5%), surveillance was abandoned for hormone therapy. [
18F]F-Choline PET/CT was negative for these 4 patients whereas [
18F]PSMA-1007 PET/CT showed subdiaphragmatic lymph node involvement for 3 patients and prostatectomy area involvement for one patient. One patient (5.9%) changed of therapeutic trial (CARLHA 2 trial to oligo-pelvis 2 trial) because the [18F]PSMA-1007 PET/CT was positive for several iliac nodes versus only one on [18F]F-Choline PET/CT. The therapeutic plan of radiotherapy was abandoned for exclusive hormone therapy in 2 patients (11.8%) because the [18F]PSMA-1007 PET/CT was negative for a single suspicious lymph node involvement and has shown a more diffuse recurrence of the disease. In one patient (5.9%), hormone therapy was added to the radiotherapy plan because the [18F]PSMA-1007 PET/CT has highlighted a recurrence in the prostatectomy area that [18F]F-Choline PET/CT had no showed and didn’t reveal any localized supra-diaphragmatic lymph node involvement as demonstrated by the initial scan. In one patient (5.9%), the treatment by hormone therapy with radiotherapy was modified after the [18F]PSMA-1007 PET/CT to expand the field of radiotherapy. For another patient (5.9%), radiation therapy was added to the hormone therapy plan after [18F]PSMA-1007 PET/CT brought to light an external iliac lymph node involvement accessible to local treatment. One patient (5.9%) who was scheduled to receive hormone therapy prior to [18F]PSMA-1007 PET/CT was finally able to have an exclusive surveillance because the lymph node suspects in [18F]F-Choline PET/CT were negative in [18F]PSMA-1007 PET/CT. For one patient (5.9%), the hormone therapy was changed to a second generation instead of a first generation because the [18F]PSMA-1007 PET/CT has shown a multiple lymph node involvement whereas [18F]F-Choline PET/CT was normal. It should be noted that the [18F]F-Choline PET/CT allowed the diagnosis of lung cancer for a patient.
Figure 3.
Therapeutic modification after [18F]PSMA-1007 PET/CT (number of patients).
Figure 3.
Therapeutic modification after [18F]PSMA-1007 PET/CT (number of patients).
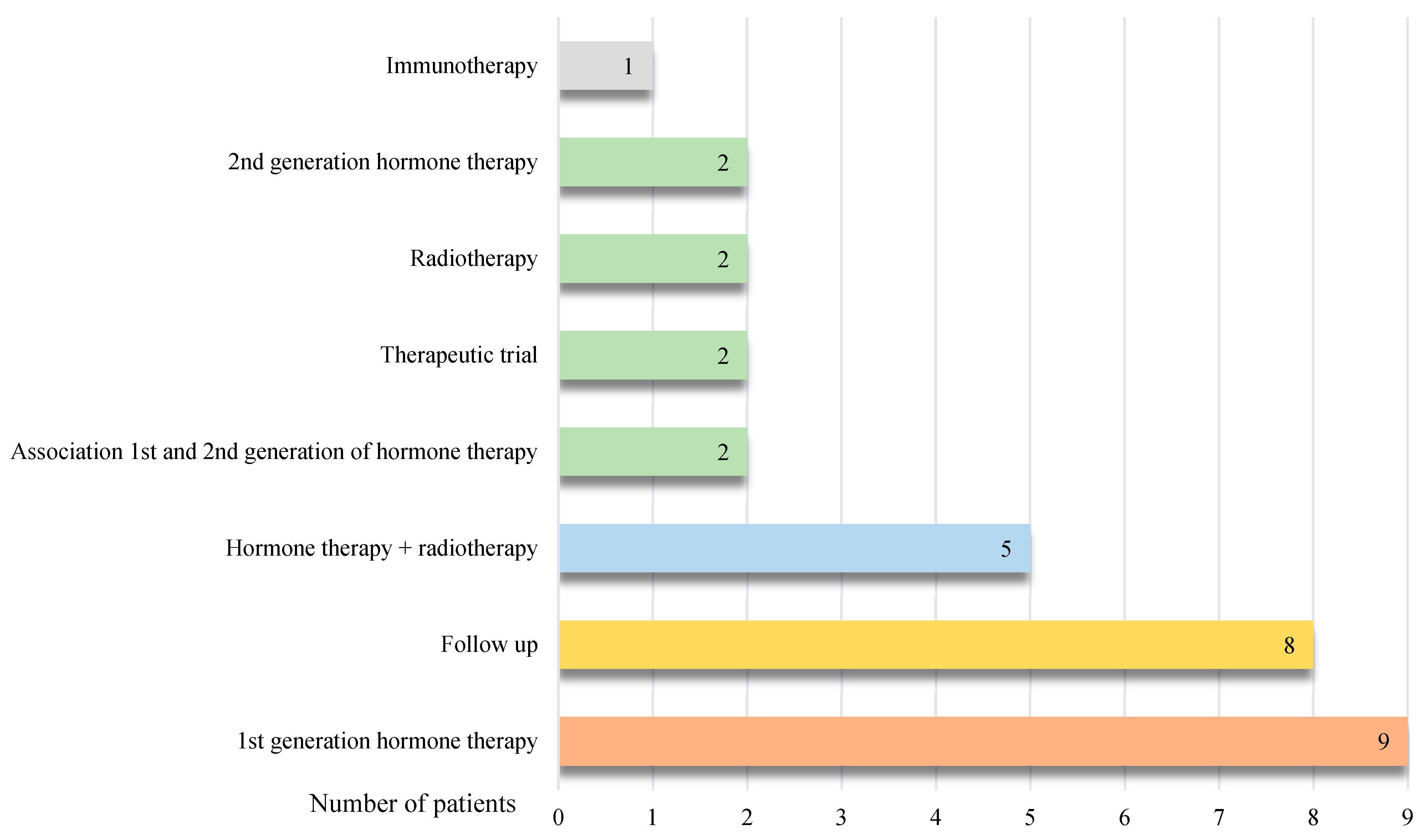
On the other side, 30 patients out of 47 did not have any change in their therapeutic management following [18F]PSMA-1007 PET/CT.
Among these 30 patients, it was decided to start a first-generation hormone therapy for 9 patients (30.0%), to continue the surveillance for 8 patients (26.7%), to make a combination of hormone therapy and radiotherapy for 5 patients (16.7%), to start a second-generation hormone therapy in 2 patients (6.7%), to maintain an inclusion in a trial for 2 patients (6.7%), to make an exclusive radiotherapy at the site of recurrence for 2 patients (6.7%), to combined 2 hormone therapies for 1 patient (3.3%) and to start an immunotherapy for 1 patient (3.3%) (
Error! Reference source not found.).
Figure 4.
Description of therapeutic management not modified after [18F]PSMA-1007 PET/CT.
Figure 4.
Description of therapeutic management not modified after [18F]PSMA-1007 PET/CT.
Diagnostic performances of [18F]Fluorocholine PET/CT and [18F]PSMA-1007 PET/CT
47 imaging examinations were studied for each of the two tracers.
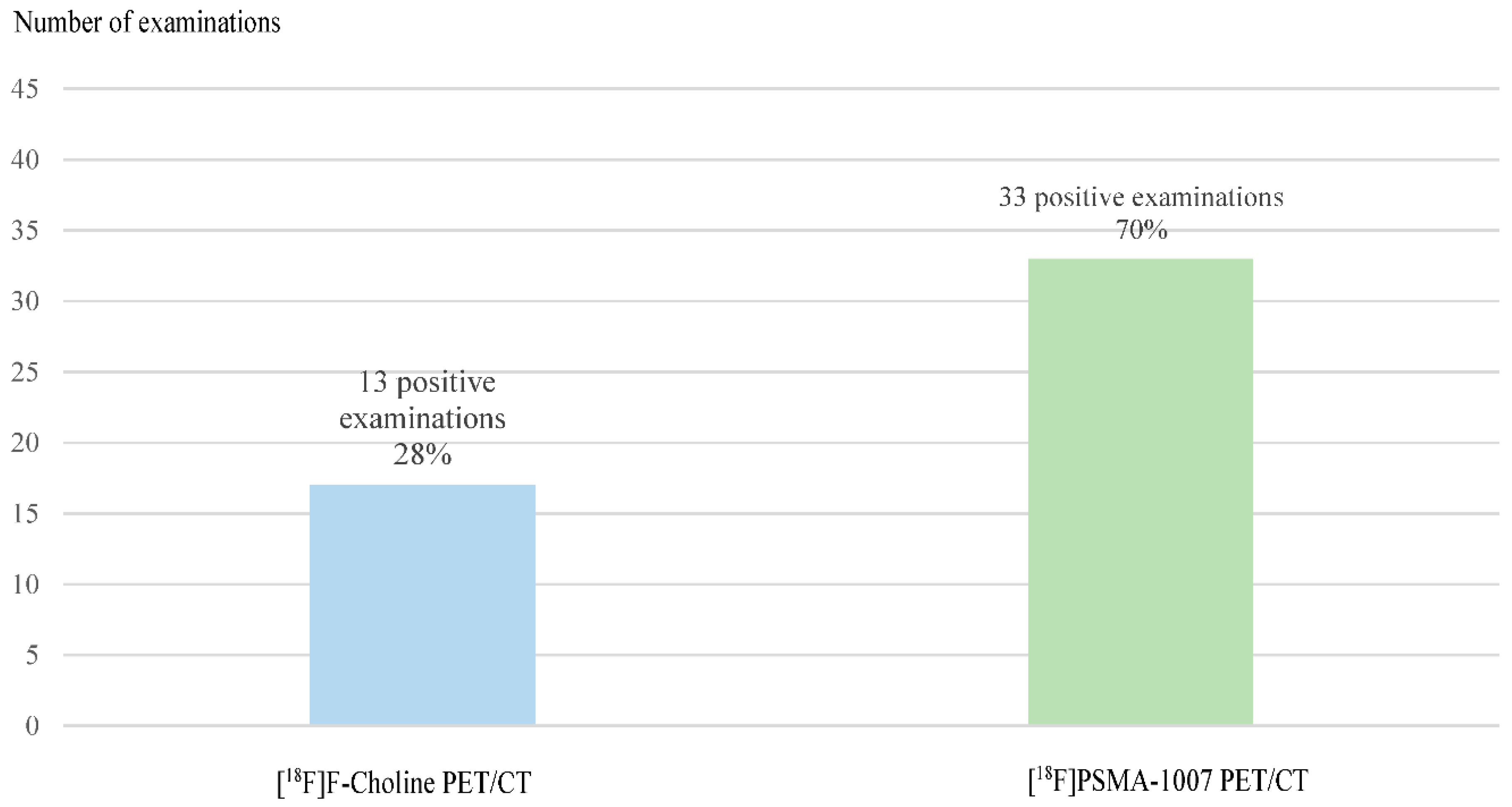
Regarding the diagnostic performance of these 2 modalities, 13 exams (28%) of [18F]F-Choline PET/CT were positive, 10 (21%) were dubious and 24 (51%) were negative. For the 13 positive [18F]F-Choline PET/CT, which didn’t fall under the conditions of the Temporary Authorization of Use, an additional [18F]PSMA-1007 PET/CT was requested because it was considered important to improve recidivism assessment (especially for distant metastases) to optimize management.
[18F]PSMA-1007 PET/CT were positive for 33 patients (70%), negative for 13 patients (28%) and doubtful in one case (2%).
Differences in detection were found between the 2 modalities for 30 patients (64%). Some differences concerned several locations (for example: lymph nodes and bone locations) in the same examination.
For 16 exams (60% of discordant exams), the difference concerned lymph nodes:
For 8 exams, [18F]F-Choline PET/CT didn’t show lymph node significant uptake whereas PSMA did. The majority of lymph node uptake foci were sub-centimetric.
For 4 exams, the number of nodes was significantly higher on the [18F]PSMA-1007 PET/CT compared to [18F]F-Choline PET/CT. They all concerned the pelvic region.
For 4 exams, [18F]PSMA-1007 PET/CT refuted the lymph node involvement described on the [18F]F-Choline PET/CT. These lymph nodes were located for 50% of the cases in the supra-diaphragmatic region.
For 8 exams (30% of discordant exams), the difference concerned bone locations:
For 7 exams, bone involvement was not visible on [18F]F-Choline PET/CT, unlike [18F]PSMA-1007 PET/CT. The majority of the lesions were localized on the axial skeleton: rachis for 4 cases and pelvis for 4 cases (Error! Reference source not found.)
For 1 exam, the [18F]F-Choline PET/CT showed a suspicious spinal injury, which was not visible on the other imaging modality.
For 5 exams (19% of discordant exams), [18F]PSMA-1007 PET/CT brought to light a recurrence in the prostatectomy area, which was not visible on [18F]F-Choline PET/CT (Error! Reference source not found.).
Finally, for one [18F]F-Choline exam, the prostatectomy area recurrence was refuted by [18F]PSMA-1007 PET/CT.
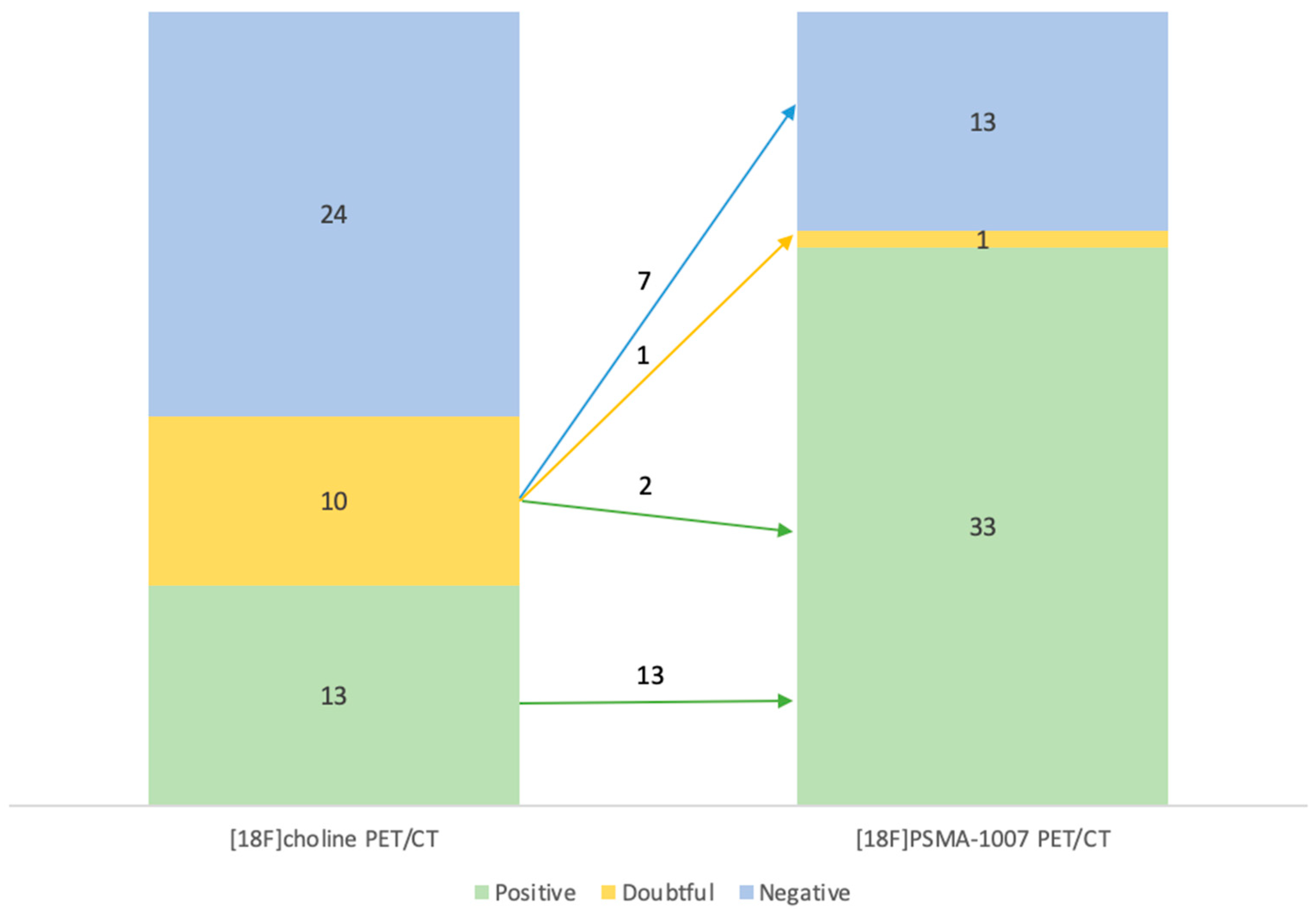
The differences between the 2 examination modalities and their results are summarized in the
Error! Reference source not found.. For example, of the 10 examinations that were doubtful for [
18F]F-Choline PET/CT, 7 were positive with [
18F]PSMA-1007 PET/CT, 2 were negative and only one was still doubtful.
Table 2.
Contingency table with the [18F]F-Choline and [18F]PSMA-1007 PET/CT results.
Table 2.
Contingency table with the [18F]F-Choline and [18F]PSMA-1007 PET/CT results.
| |
|
[18F]F-Choline PET/CT |
| |
|
Negative |
Positive |
Doubtful |
| [18F]PSMA-1007 PET/CT |
Negative |
11 |
0 |
2 |
| Positive |
13 |
13 |
7 |
| Doubtful |
0 |
0 |
1 |
The diagnostic performances of the 2 tracers for the 47 imaging examinations are represented in the
Error! Reference source not found.. The diagnostic performance is defined as the ability to detect uptake in an area of recurrence. A significant difference was found between the two diagnostic performances (p=6.523x10
-5). The higher detection rate of [
18F]PSMA-1007 PET/CT was significantly higher than the one of [
18F]F-Choline PET/CT.
Figure 5.
Diagnostic performances of [18F]F-Choline PET/CT and [18F]PSMA-1007 PET/CT for positive examinations.
Figure 5.
Diagnostic performances of [18F]F-Choline PET/CT and [18F]PSMA-1007 PET/CT for positive examinations.
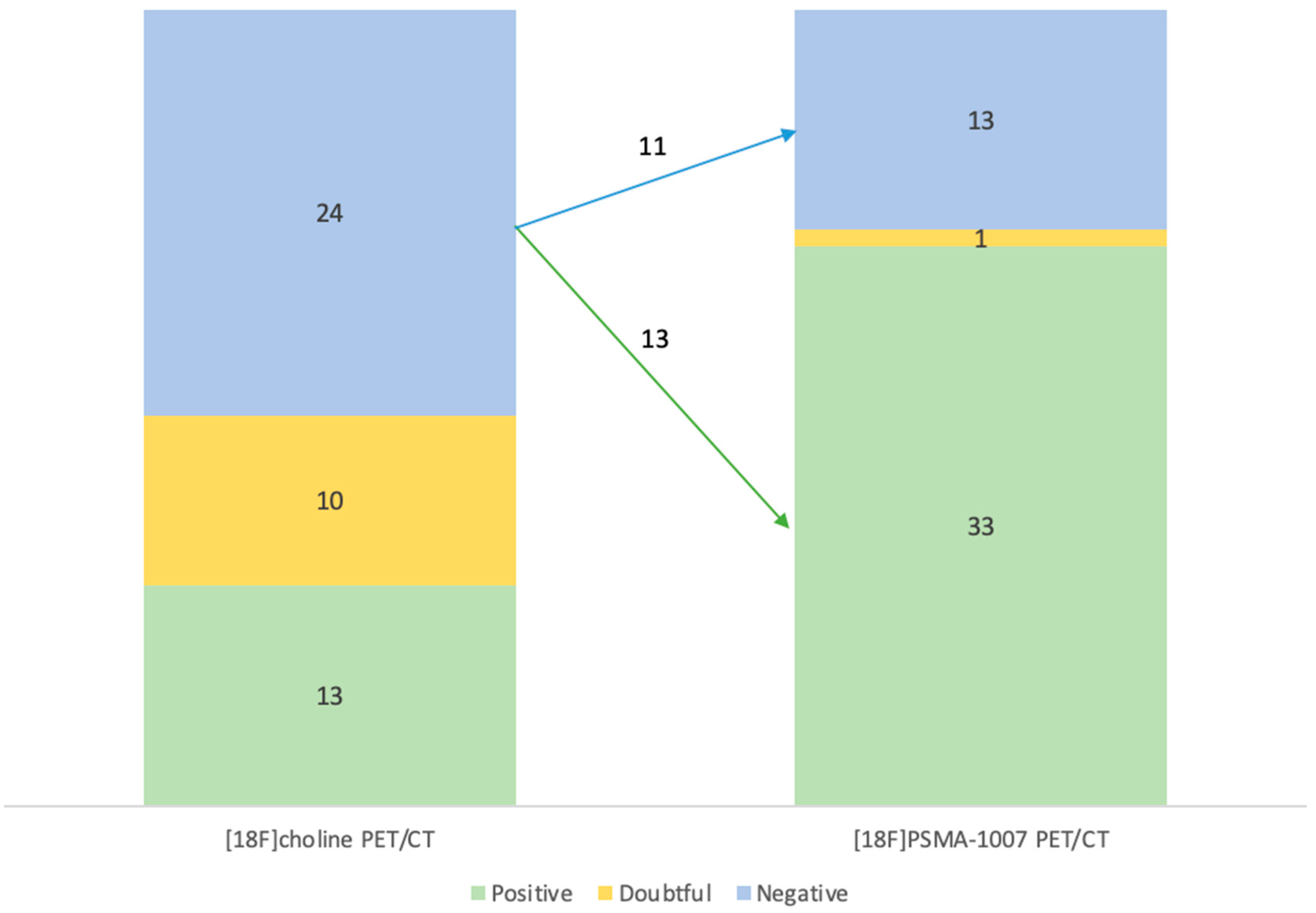
Distribution of the results of examinations of each radiotracer is represented on the Error! Reference source not found. and on the Error! Reference source not found..
Out of the 10 suspicious examinations on [18F]F-Choline PET/CT, 7 were positive, 2 were negative and 1 was still doubtful after [18F]PSMA-1007 PET/CT (Error! Reference source not found.).
Among the 24 negative examinations on [
18F]F-Choline PET/CT, 13 were finally positive and 11 were still negative in [
18F]PSMA-1007 PET/CT (
Error! Reference source not found.)
Figure 6.
Distribution of the results of the [18F]F-Choline and [18F]PSMA-1007 PET/CT and the modification of doubtful and positive [18F]F-Choline PET/CT after [18F]PSMA-1007 PET/CT (number of examinations).
Figure 6.
Distribution of the results of the [18F]F-Choline and [18F]PSMA-1007 PET/CT and the modification of doubtful and positive [18F]F-Choline PET/CT after [18F]PSMA-1007 PET/CT (number of examinations).
Figure 7.
Distribution of the results of the [18F]F-Choline and [18F]PSMA-1007 PET/CT and the modification of negative [18F]F-Choline PET/CT after [18F]PSMA-1007 PET/CT (number of examinations).
Figure 7.
Distribution of the results of the [18F]F-Choline and [18F]PSMA-1007 PET/CT and the modification of negative [18F]F-Choline PET/CT after [18F]PSMA-1007 PET/CT (number of examinations).
The kappa concordance coefficient, measuring the proportion of identical results between [18F]F-Choline PET/CT and [18F]PSMA-1007, was k=0.29, meaning a poor concordance according to the classification of Landis & Koch, which is consistent with the results found previously.
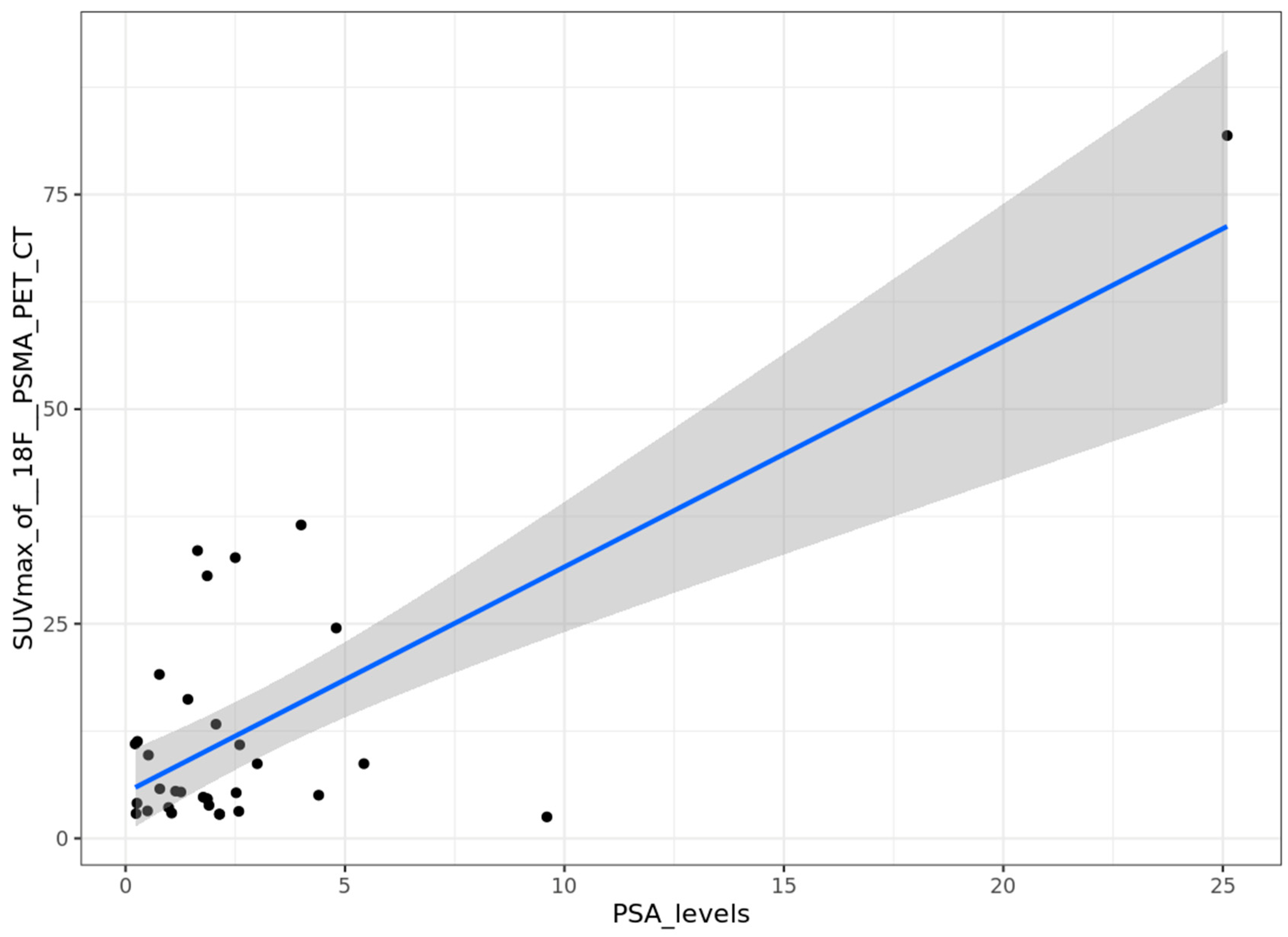
Correlation between PSA levels and SUVmax
We studied the correlation between the PSA serum level during the biological recurrence of prostatic neoplasia and the SUVmax of the most fixative lesion when [
18F]PSMA-1007 PET/CT was positive. The distribution of PSA levels and SUVmax was described in
Error! Reference source not found.. A significant correlation was found after univariable analysis (p=9.4x10
-7), with a correlation coefficient of 0.73 (
Error! Reference source not found.).
Figure 9.
Correlation between PSA serum levels (ng/ml) and SUVmax on [18F]PSMA-1007 PET/CT.
Figure 9.
Correlation between PSA serum levels (ng/ml) and SUVmax on [18F]PSMA-1007 PET/CT.
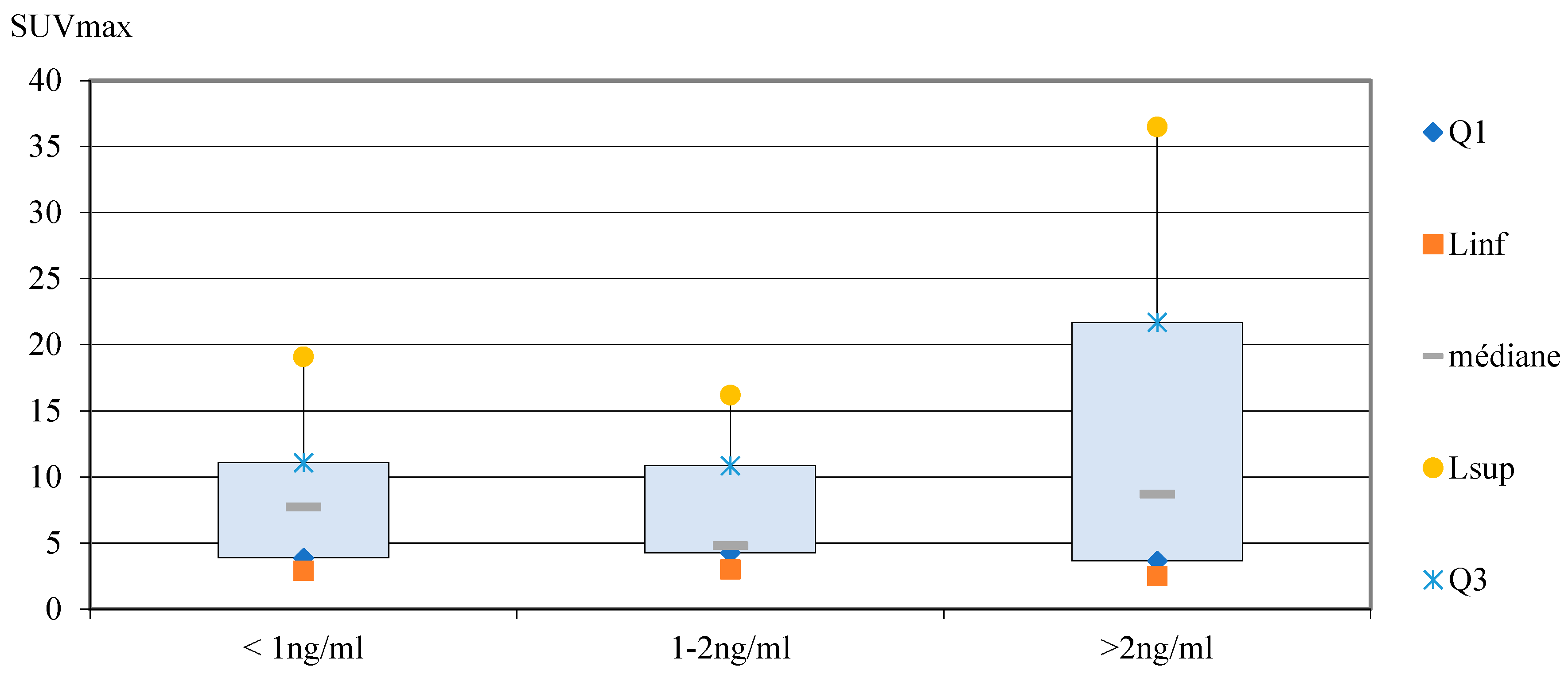
In the PSA subgroups analysis, the mean values of SUVmax [
18F]PSMA-1007 PET/CT increased with the PSA levels (8.4, 10.5 and 17.1 for < 1 ng/ml, 1-2 ng/ml and > 2 ng/ml respectively). The median values of SUVmax were 7.7, 4.8 and 8.7 for subgroups <1 ng/ml, 1-2 ng/ml and > 2 ng/ml, respectively (
Error! Reference source not found.). However, there are ranges of overlap between the different SUVmax values for each group (
Error! Reference source not found.). As the number of data compared was small, a non-parametric test was performed (Kruskal-Wallis test). The exact interpretation is that the mean rank of SUVmax is not significantly different according to PSA levels subgroups (p=0.9).
Figure 10.
Box plots showed the distribution of SUVmax in subgroups for PSA serum levels (ng/ml).
Figure 10.
Box plots showed the distribution of SUVmax in subgroups for PSA serum levels (ng/ml).
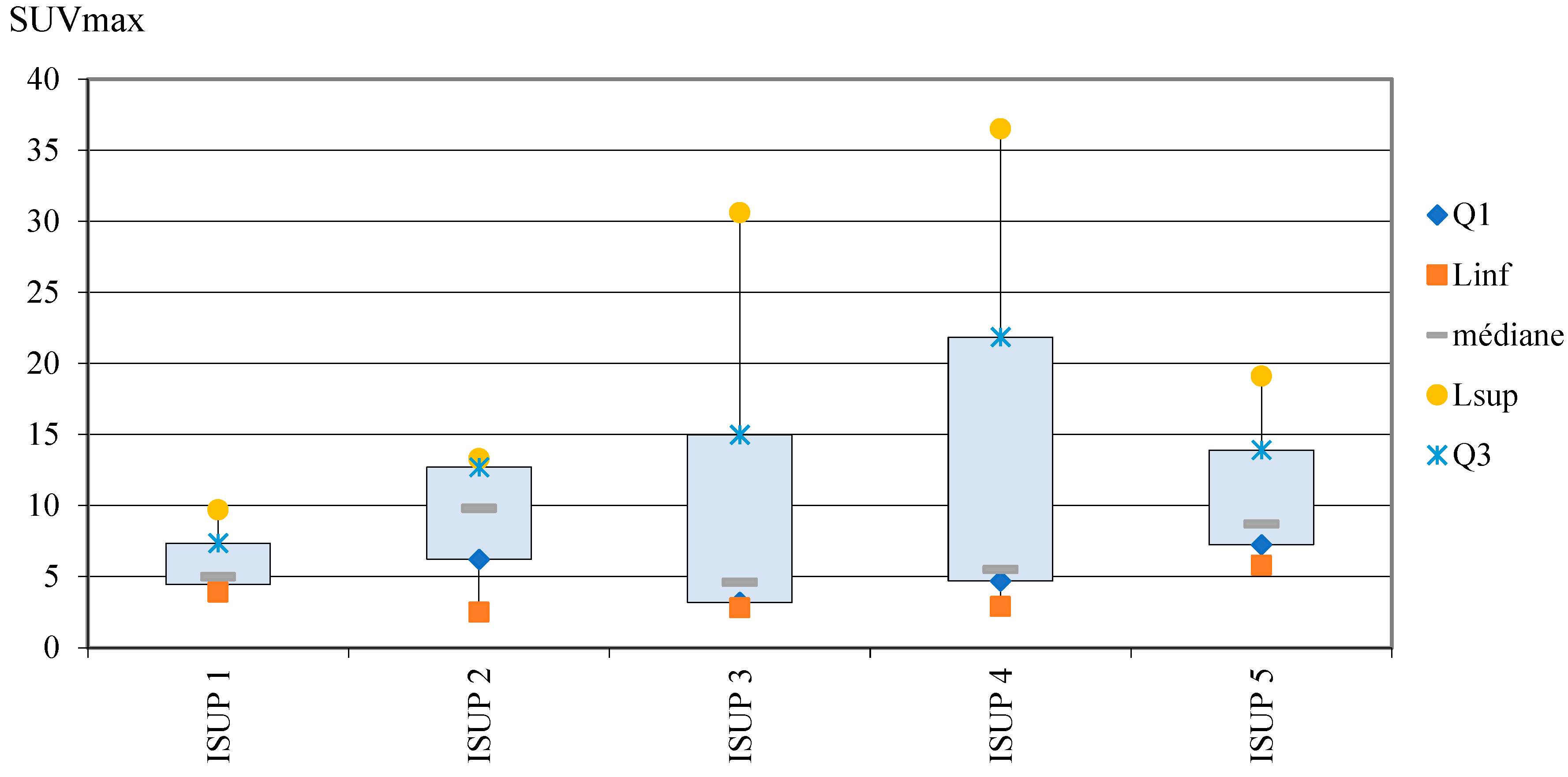
Correlation between ISUP score and SUVmax
A correlation between ISUP score and SUVmax of the selected lesion in [
18F]PSMA-1007 PET/CT was bot found in this study: the mean rank of SUVmax lesion is not significantly different according to ISUP (p=0.69). SUVmax distribution in the different ISUP groups was described in
Error! Reference source not found. and in
Error! Reference source not found..
Figure 11.
Distribution of SUVmax in ISUP groups (box plots).
Figure 11.
Distribution of SUVmax in ISUP groups (box plots).
DISCUSSION
The main objective was to evaluate the therapeutic impact of [18F]PSMA-1007 PET/CT in biological recurrence of prostate cancer. We showed a change in patient management in one more than third of cases (36% of cases). A recent prospective multicenter study (29) has shown a modification of treatment based on [68Ga]Ga-PSMA-11 PET/CT results in 56.8% among 1004 patients with biochemical recurrence in prostate cancer. Significant changes in the therapeutic management of patients after [68Ga]Ga-PSMA-11 PET/CT was also described in a systematic review of the literature (24) published in 2018 which analyzed 37 articles. In this review, the change in the treatment plans ranged from 28.6% up to 87.1%. But only four studies were prospective. Sangwon Han et al. has realized a meta-analysis to study the impact of [68Ga]Ga-PSMA-11 PET/CT on the management of patients with prostate cancer (primary staging, biochemical persistence or recurrence). Fifteen studies (1163 patients) were included. The proportion of management changes was 54% (95% IC 47–60%), highlighting the impact of [68Ga]Ga-PSMA-11 PET/CT on the management of patients with prostate cancer (36), thanks in particular to earlier management of the recurrence, improving the patient prognosis. In the literature, most of the studies are considering the impact of [68Ga]Ga-PSMA-11 instead of [18F]PSMA-1007 PET/CT imaging. The main advantage of using a [68Ga]Ga-PSMA-11 tracer is for the theranostic approach. The term theranostic is a contraction of the words diagnostic and therapeutic, which refers to the combination of a same biomarker that is used for the diagnostic ([68Ga]Ga-PSMA-11) with a different isotope ([177Lutetium], a β- emitter) leading to a therapeutic agent [177Lu]Lu-PSMA-617, for the prostate cancers.
This multicentric study have shown a significant difference between diagnostic performance of [18F]Fluorocholine PET/CT (28% of positive examinations) and [18F]PSMA-1007 PET/CT (70% of positive examinations). [18F]PSMA-1007 PET/CT improves the sensibility of detection and makes it possible to classify the quasi-majority of patients doubtful on [18F]F-Choline PET/CT. Only one of the 10 doubtful [18F]F-Choline PET/CT could not be conclusive in [18F]PSMA-1007 PET/CT. Many studies have also shown a higher detection rate for PSMA PET/CT than for [18F]F-Choline PET/CT, such as this French study of 33 patients where 76% of PSMA-PET/CT were positive versus 33% for [18F]F-Choline PET/CT, with a modification in therapeutic management for 2 of 3 patients (37). Another study performed an analysis by lesion and a total of 78 specific lesions of prostate cancer were detected in 32 patients using [68Ga]Ga-PSMA-11 PET/CT and 56 lesions were detected in 26 patients using [18F]F-Choline PET/CT, with a significant higher detection rate in [68Ga]Ga-PSMA-11 PET/CT (p=0.04) (38).
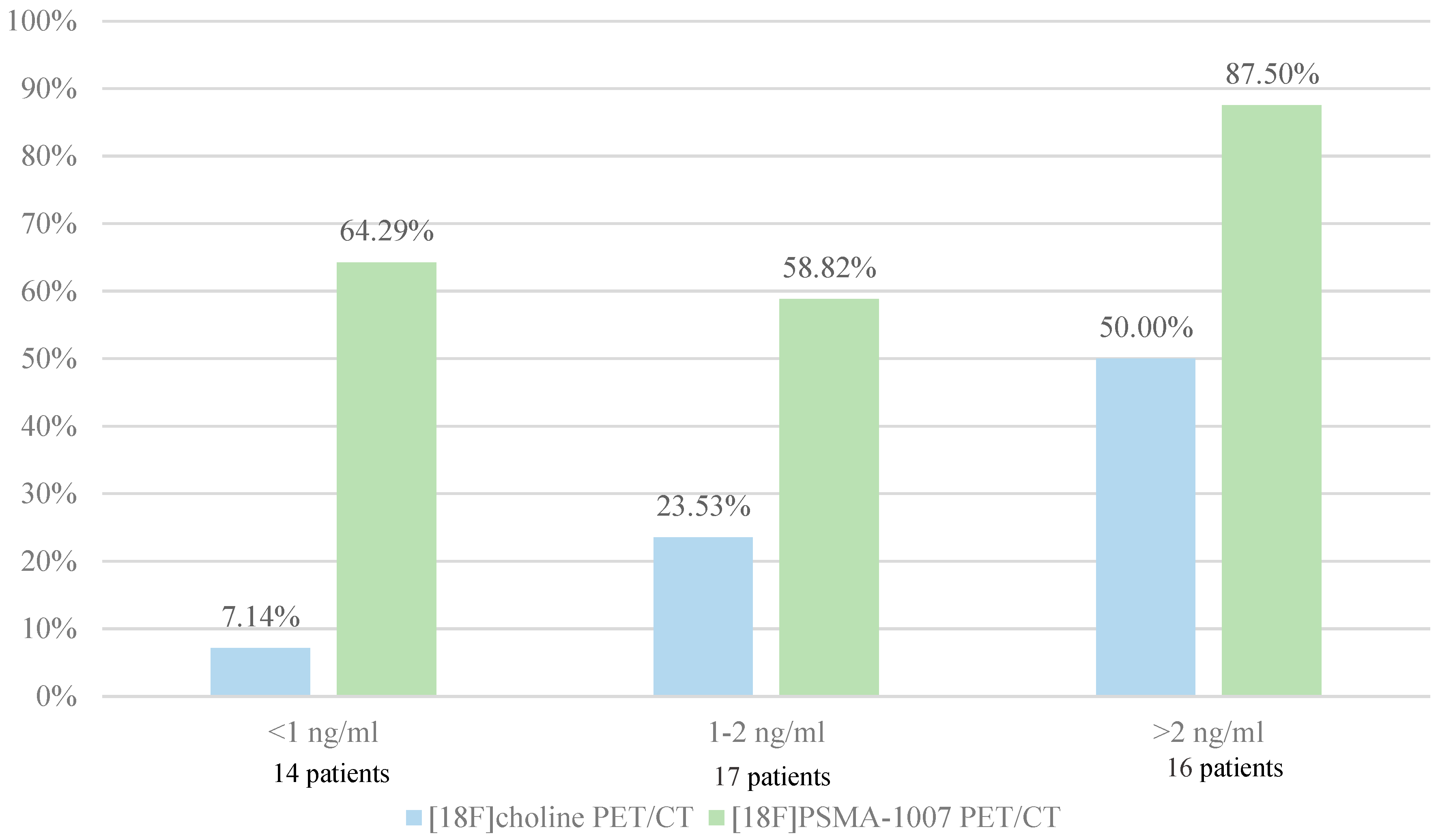
In our study, diagnostic performance by PSA subgroups was 7.14% and 64.29% for PSA serum levels lower than 1 ng/ml, 23.53% and 58.82% for PSA serum levels between 1 and 2 ng/ml and 50.00% and 87.50% for PSA serum levels higher than 2 ng/ml for [18F]F-Choline PET/CT and [18F]PSMA-1007 PET/CT respectively. Low positivity rate of [18F]F-Choline is explained by the fact that [18F]PSMA-1007 PET/CT was initially not recommended when [18F]F-Choline PET/CT was positive. In a German study of 248 patients with biological recurrence after prostatectomy, 222 (90%) patients showed pathologic uptake in [68Ga]Ga-PSMA-11 PET/CT. The detection rates were 57.9%, 72.7%, 93.0% and 96.8% for PSA serum levels of < 0.5 ng/ml, 0.2 to < 0.5 ng/ml, 0.5 to 1 ng/ml, 1 to < 2 ng/ml and ≥ 2, respectively. This trial reveals a high number of positive findings in the clinically range of low PSA values (<0.5 ng/ml), which can influence the further clinical management (39).
Our prospective multicenter study was able to show a significant correlation between the PSA serum level at recurrence and the maximum uptake of [18F]PSMA-1007 but not between the ISUP score and the intensity of fixation of [18F]PSMA-1007. Our subgroup analysis also did not reveal a significant difference between subgroups of PSA levels and uptake intensity (SUVmax). We decided to study these two parameters because they have been described in many studies as prognostic markers of positivity of PSMA-PET/CT and also as prognostic markers of the prostate neoplasia. SUVmax is the most commonly used semi-quantitative parameter in nuclear medicine. It has a considerable importance because it is not dependent on the size of the selected VOI, allowing a high reproducibility between physicians. This parameter is mainly used in [18F]Fluorodeoxyglucose (FDG) examinations to characterize lesions and assess therapeutic responses in oncology for example. SUV measurements in PSMA PET/CT or in [18F]F-Choline PET/CT are not done routinely, physicians using only visual analysis of images. Several studies, mostly performed with Gallium, have shown a correlation between PSMA uptake and PSA levels (40–43). Previous studies have investigated prognostic factors and their possible correlation with uptake intensity at PSMA PET/CT positivity and PSA levels (44).
A positive correlation between tumor grade of primary tumor and fixation intensity has been described in a study with 141 patients: the mean SUVmax values of high-risk patients were significantly higher than those of low-risk patients (18.9 ± 12.1 versus. 7.16 ± 6.2, respectively) (p < 0.001). A SUVmax cut-off of 9.1 showed a high sensitivity (78%) and specificity (81%) for detection of high risk disease (41) suggesting that the intraprostatic uptake of PSMA intensity can provide prognostic information (35).
Many other predictors of PSMA PET/CT positivity were described in literature such as PSA doubling time, PSA velocity (24) and lesion size (45). Eiber et al. has assessed [68Ga]Ga-PSMA-11 PET/CT for the detection and the localization of recurrent disease in a large cohort of patients (248 men) after radical prostatectomy. This study showed that detection rates of [68Ga]Ga-PSMA-11 PET/CT increased with a higher PSA velocity (81.8%, 82.4%, 92.1%, and 100% in <1, 1 to <2, 2 to <5, and ≥5 ng/ml/y, respectively) and in higher Gleason score (≤7 versus ≥8) (p=0.0190) (39). But the majority of these studies were retrospective with a lack of power. It is also known that, as [18F]F-Choline PET/CT, the diagnostic accuracy of [18F]PSMA-1007 PET/CT increases with the PSA serum levels (46).
This study has some limitations. Our study is a multicentric trial but the number of patients is limited to only 47 patients. The low number of patients is explained by the fact that the temporary authorization of use of PSMA-imaging was replaced on April 28th, 2022 by an early access authorization in France. The [18F]F-Choline PET/CT was therefore no longer mandatory to request a [18F]PSMA-1007 PET/CT. Few patients were included after this date. The low power of the study may also be explained by the fact that we chose to select only [18F]PSMA-1007 labelled examinations. In fact [68Ga]Ga-PSMA-11 PET/CT were mainly used in the assessment of multi-metastatic patients before vectorized internal therapy by [177Lu]Lutetium-PSMA-617. Some patients were injected with iodinated contrast product, which can increase the specificity and the sensibility of the examination. All of these differences decreased the reproducibility between examinations. Finally, acquisitions were made on 2 different machines (one analogic and one digital PET/CT) with non-overlapping parameters. The worse sensibility of detection of analogic PET/CT may have missed secondary lesion.
As we have seen previously, [18F]PSMA-1007 or [68Ga]Ga-PSMA-11 PET/CT is a major advance in the management of recurrent prostatic cancer. It allows earlier detection of recurrence sites and leads to a change in therapeutic management in a significant number of patients. However, PSMA PET/CT has also showed interesting applications for tumor primary detection, primary staging, assessment of therapeutic responses and treatment planning (47,48). We already know the main role of MRI in the positive diagnosis and staging of prostate adenocarcinoma (49,50). Multipara-metric MRI (mpMRI) is widely used by physicians to assess the risk of aggressive prostate cancer in patients with elevated PSA serum concentration and to improves tumor detection at biopsies. MRI is also the gold standard for tumor staging and helps the clinician to make a therapeutic choice especially between radical prostatectomy, radiotherapy or active surveillance (51). However, the limitation of mpMRI is its poor specificity for the differentiation of “significant” from “indolent” prostatic cancer. Furthermore, the sensitivity and specificity of mpMRI can range between 22%-85% and 50%-99% respectively, depending on technical variables and the study population (44). New technological advances have made it possible for several years to combine functional imaging by PET with the precision and the high sensibility of multimodal MRI. A recent meta-analysis and systematic review of 23 articles and 50 articles respectively, showed in primary tumors, a pooled sensitivity of PSMA-MRI of 94.9% on the patient-based analysis. At restaging, after recurrence, the pooled detection rate was 80.9% and was higher for radiolabeled PSMA (81.8%) than for [18F]F-Choline (77.3%). PSMA PET/MRI has highly sensitive in detecting primary prostate adenocarcinoma (94.9%) and a high detection rate for recurrent disease (80.9%), and the pooled detection rate was higher for radio-labelled PSMA than for [18F]F-Choline (81.8% and 77.3%, respectively)(52). [68Ga]Ga-PSMA-11 PET/CT and PET/MRI are likely to become the standard imaging modalities in the staging of intermediate-to-high-risk primary prostatic adenocarcinoma (53,54). PSMA PET might also increase the accuracy of mpMRI for biopsies guidance, mostly in patients with a high clinical suspicion when mpMRI results are negative or inconclusive (1). In the near future, the use of multi-modal imaging with the combination of MRI and PET could also find a place in the planning of external pelvic radiotherapy (55–57). Zamboglou et al. has shown in small cohort that intensity modulated radiation therapy (IMRT) dose escalation on Gross tumor volumes (GTV) based on combination of mpMRI and PSMA PET/CT was feasible. Boosting GTV-union resulted in significantly higher tumor control probability with no or minimal increase of normal tissue complication probabilities (56). However, MRI-PET remains a rare commodity, only a few centers in France are equipped.
With this increasing utilization of PSMA PET/CT imaging, the application of standardized methods to interpret images is become necessary, in particular to harmonize diagnostic interpretation criteria and unified language (56). In 2018, standardization criteria for PSMA PET/CT and PET/MRI have been proposed: The PROMISE criteria (Prostate Cancer Molecular Imaging Standardized Evaluation criteria). This score defines categories for staging the disease: a miTNM score (version 1.0). miPSMA categories were defined in relation to mean PSMA uptake in the blood pool, the liver and the parotid gland. Results are reported as 0, 1, 2, or 3 for respectively: no, low, intermediate or high PSMA expression. Scores 2 and 3 are considered as cancer lesions (58). Specific applications, such as the impact on prognosis or on management, should be evaluated in future prospective trials. More recently, in 2021, the European Association of Nuclear Medicine (EANM) has published the E-PSMA standardized reporting guidelines. This guidelines aims at the implementation of consensus statements, with a panel of experts in PSMA-PET imaging, to harmonize diagnostic interpretation criteria for PSMA-PET in prostate cancer in staging, recurrent setting, advanced setting and response to therapy (11).
CONCLUSION
This prospective multicentric observational study found a change in therapeutic management in biological recurrence of prostate cancer after [18F]PSMA-1007 PET/CT in more than one third of cases (36%).
A significant difference between diagnostic performance of [18F]F-Choline PET/CT (28%) and [18F]PSMA-1007 PET/CT (70%) was demonstrated and this study also showed a correlation between PSA serum levels at recurrence and the highest lesion uptake (SUVmax) on [18F]PSMA-1007 PET/CT.
This change in the therapeutic treatment of patients results in earlier and better management with detection of small lesions at a lower PSA serum level than is possible with [18F]F-Choline PET/CT, with a better specificity of uptake.
This study focuses on the changes in patients’ management caused by [18F]PSMA-1007 PET/CT. In a future prospective and multicentric study, it would be interesting to investigate the impact of these therapeutic modifications permitted by an earlier detection of the recurrence sites on overall survival, progression-free survival and quality of life.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, et al. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J Nucl Med. 2021 May 10;62(5):596–604.
- Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-Choline PET/CT in Prostate Cancer Patients with Biochemical Recurrence: Influence of Androgen Deprivation Therapy and Correlation with PSA Kinetics. J Nucl Med. 2013 Jun 1;54(6):833–40.
- Merriel SWD, Funston G, Hamilton W. Prostate Cancer in Primary Care. Adv Ther. 2018;35(9):1285–94.
- Tsechelidis I, Vrachimis A. PSMA PET in Imaging Prostate Cancer. Front Oncol. 2022 Jan 28;12:831429.
- Wang R, Shen G, Huang M, Tian R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer With Biochemical Recurrence: A Meta-Analysis. Front Oncol. 2021 Jun 17;11:684629.
- Rousseau C, Le Thiec M, Ferrer L, Rusu D, Rauscher A, Maucherat B, et al. Preliminary results of a 68Ga-PSMA PET/CT prospective study in prostate cancer patients with occult recurrence: Diagnostic performance and impact on therapeutic decision-making. The Prostate. 2019;79(13):1514–22.
- Lesourd M, Roumiguié M, Beauval JB. Récidive biologique après prostatectomie totale dans le cancer de la prostate : quel bilan et quel traitement en 2019 ? Prog En Urol - FMC. 2019 Mar;29(1):F13–7.
- Ferrari M, Treglia G. 18F-PSMA-1007 PET in Biochemical Recurrent Prostate Cancer: An Updated Meta-Analysis. Contrast Media Mol Imaging. 2021 Dec 18;2021:3502389.
- von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2018 Sep 1;4(5):686–93.
- Fraum TJ, Ludwig DR, Kim EH, Schroeder P, Hope TA, Ippolito JE. Prostate cancer PET tracers: essentials for the urologist. Can J Urol. 2018;13.
- Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48(5):1626–38.
- Crocerossa F, Marchioni M, Novara G, Carbonara U, Ferro M, Russo GI, et al. Detection Rate of Prostate Specific Membrane Antigen Tracers for Positron Emission Tomography/Computerized Tomography in Prostate Cancer Biochemical Recurrence: A Systematic Review and Network Meta-Analysis. J Urol. 2021 Feb;205(2):356–69.
- Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017 Apr 1;44(4):678–88.
- Witkowska-Patena E, Giżewska A, Dziuk M, Miśko J, Budzyńska A, Walęcka-Mazur A. Head-to-Head Comparison of 18F-Prostate-Specific Membrane Antigen-1007 and 18F-Fluorocholine PET/CT in Biochemically Relapsed Prostate Cancer. Clin Nucl Med. 2019 Dec;44(12):e629–33.
- Hoberück S, Löck S, Borkowetz A, Sommer U, Winzer R, Zöphel K, et al. Intraindividual comparison of [68 Ga]-Ga-PSMA-11 and [18F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res. 2021 Oct 19;11(1):109.
- Kesch C, Kratochwil C, Mier W, Kopka K, Giesel FL. 68Ga or 18F for Prostate Cancer Imaging? J Nucl Med. 2017 May 1;58(5):687–8.
- Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, et al. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology. 2021 May 1;299(2):248–60.
- Lengana T, Lawal I, Rensburg CJV, Mokoala K, Moshokoa E, Mazibuko S, et al. The Diagnostic Performance of 18F-PSMA-1007 PET/CT in Prostate Cancer Patients with Early Recurrence after Definitive Therapy with a PSA <10 ng/ml. Nukl - Nucl. 2022 Apr;61(02):120–9.
- Virgolini I, Decristoforo C, Haug A, Fanti S, Uprimny C. Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(3):471–95.
- Advantage of 18F-PSMA-1007 over 68Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion - ProQuest [Internet]. [cited 2021 Nov 24]. Available from: https://www.proquest.com/openview/fc76f63b2f6634fb9a16f3ff8b9fdcb6/1?pq-origsite=gscholar&cbl=42802.
- Krausewitz P, Ritter M. [Clinical aspects in the diagnosis and treatment of prostate cancer]. Radiol. 2021 Sep;61(9):795–801.
- Mena E, Lindenberg L, Choyke P. The Impact of PSMA PET/CT Imaging in Prostate Cancer Radiation Treatment. Semin Nucl Med. 2022 Mar 1;52(2):255–62.
- Udovicich C, Perera M, Hofman MS, Siva S, Del Rio A, Murphy DG, et al. 68Ga-prostate-specific membrane antigen-positron emission tomography/computed tomography in advanced prostate cancer: Current state and future trends. Prostate Int. 2017 Dec;5(4):125–9.
- Eissa A, Elsherbiny A, Coelho RF, Rassweiler J, Davis JW, Porpiglia F, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: a systematic review of the literature. Minerva Urol Nefrol [Internet]. 2018 Sep [cited 2021 Nov 25];70(5). Available from: https://www.minervamedica.it/index2.php?show=R19Y2018N05A0462.
- Calais J, Fendler WP, Eiber M, Gartmann J, Chu FI, Nickols NG, et al. Impact of 68Ga-PSMA-11 PET/CT on the Management of Prostate Cancer Patients with Biochemical Recurrence. J Nucl Med. 2018 Mar 1;59(3):434–41.
- Barbaud M, Frindel M, Ferrer L, Thiec ML, Rusu D, Rauscher A, et al. 68Ga-PSMA-11 PET-CT study in prostate cancer patients with biochemical recurrence and non-contributive 18F-Choline PET-CT: Impact on therapeutic decision-making and biomarker changes. The Prostate. 2019 Apr 1;79(5):454–61.
- Young S, Liu W, Zukotynski K, Bauman G. Prostate-specific membrane antigen targeted PET/CT for recurrent prostate cancer: a clinician’s guide. Expert Rev Anticancer Ther. 2021 Jun 3;21(6):641–55.
- Francolini G, Detti B, Bottero M, Zilli T, Lancia A, Bruni A, et al. Detection rate, pattern of relapse and influence on therapeutic decision of PSMA PET/CT in patients affected by biochemical recurrence after radical prostatectomy, a retrospective case series. Clin Transl Oncol. 2021 Feb 1;23(2):364–71.
- Cerci JJ, Fanti S, Lobato EE, Kunikowska J, Alonso O, Medina S, et al. Diagnostic Performance and Clinical Impact of 68Ga-PSMA-11 PET/CT Imaging in Early Relapsed Prostate Cancer After Radical Therapy: A Prospective Multicenter Study (IAEA-PSMA Study). J Nucl Med. 2022 Feb 1;63(2):240–7.
- Lasserre M, Sargos P, Barret E, Beauval JB, Brureau L, Créhange G, et al. Narrative review of PET/CT performances at biochemical recurrence in prostate cancer after radical prostatectomy and impact on patient disease management: Revue narrative à propos des performances de la TEP/TDM en cas de récidive biochimique après prostatectomie radicale dans le cancer de la prostate et impact sur la prise en charge des patients. Prog En Urol. 2022 Jun 1;32(6, Supplement 1):6S33–42.
- Murthy V, Aggarwal R, Koo PJ. The Emerging Role of Next-Generation Imaging in Prostate Cancer. Curr Oncol Rep. 2022 Jan 1;24(1):33–42.
- Karagiannis V, Wichmann V, Saarinen J, Eigeliene N, Andersen H, Jekunen A. Radiotherapy treatment modification for prostate cancer patients based on PSMA-PET/CT. Radiat Oncol Lond Engl. 2022 Jan 29;17:19.
- Artigas C, Diamand R, Shagera QA, Plouznikoff N, Fokoue F, Otte FX, et al. Oligometastatic Disease Detection with 68Ga-PSMA-11 PET/CT in Hormone-Sensitive Prostate Cancer Patients (HSPC) with Biochemical Recurrence after Radical Prostatectomy: Predictive Factors and Clinical Impact. Cancers. 2021 Oct 4;13(19):4982.
- Kaalep A, Sera T, Oyen W, Krause BJ, Chiti A, Liu Y, et al. EANM/EARL FDG-PET/CT accreditation - summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. 2018 Mar 1;45(3):412–22.
- Roberts MJ, Morton A, Donato P, Kyle S, Pattison DA, Thomas P, et al. 68Ga-PSMA PET/CT tumour intensity pre-operatively predicts adverse pathological outcomes and progression-free survival in localised prostate cancer. Eur J Nucl Med Mol Imaging. 2021 Feb 1;48(2):477–82.
- Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2018 Aug 1;74(2):179–90.
- Gauthé M, Belissant O, Girard A, Zhang Yin J, Ohnona J, Cottereau AS, et al. [PET/CT and biochemical recurrence of prostate adenocarcinoma: Added value of 68Ga-PSMA-11 when 18F-fluorocholine is non-contributive]. Progres En Urol J Assoc Francaise Urol Soc Francaise Urol. 2017 Jul;27(8–9):474–81.
- Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014 Jan 1;41(1):11–20.
- Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68 Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015 May;56(5):668–74.
- Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017 Jun 1;44(6):941–9.
- Demirci E, Kabasakal L, Şahin OE, Akgün E, Gültekin MH, Doğanca T, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019 Jan;40(1):86–91.
- Jiao J, Kang F, Zhang J, Quan Z, Wen W, Zhao X, et al. Establishment and prospective validation of an SUVmax cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by 68Ga-PSMA PET/CT: a real-world study. Theranostics. 2021 Jul 25;11(17):8396–411.
- Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of Intraprostatic Tumor Extent with 68Ga-PSMA Distribution in Patients with Prostate Cancer. J Nucl Med. 2016 Apr 1;57(4):563–7.
- Hoffmann MA, Wieler HJ, Baues C, Kuntz NJ, Richardsen I, Schreckenberger M. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology. 2019 Aug 1;130:1–12.
- Verburg FA, Pfister D, Drude NI, Mottaghy FM, Behrendt FF. PSA levels, PSA doubling time, Gleason score and prior therapy cannot predict measured uptake of [68Ga]PSMA-HBED-CC lesion uptake in recurrent/metastatic prostate cancer. Nukl - Nucl. 2017 Nov;56(06):225–32.
- Liu X, Wang Q, Zhang B, Jiang T, Zeng W. Diagnostic accuracy of 18F-PSMA-1007 PET/CT for prostate cancer in primary staging and biochemical recurrence with different serum PSA levels: A systematic review and meta-analysis. Hell J Nucl Med. 2022;25(1):88–102.
- Barbosa F de G, Queiroz MA, Nunes RF, Marin JFG, Buchpiguel CA, Cerri GG. Clinical perspectives of PSMA PET/MRI for prostate cancer. Clinics. 2018;73(Suppl 1):e586s.
- Emmett L, Papa N, Buteau J, Ho B, Liu V, Roberts M, et al. The PRIMARY Score: Using Intraprostatic 68Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J Nucl Med. 2022 Nov 1;63(11):1644–50.
- Schlemmer HP, Joachim Krause B, Schütz V, Bonekamp D, Marie Schwarzenböck S, Hohenfellner M. Imaging of Prostate Cancer. Dtsch Ärztebl Int. 2021 Oct;118(42):713–9.
- MRI in the Management of Prostate Cancer - ClinicalKey [Internet]. [cited 2023 Feb 25]. Available from: https://www.clinicalkey.fr/#!/content/playContent/1-s2.0-S0887217120300305?scrollTo=%23hl0000264.
- Abecassis JP, Ghazzar N, Peyromaure M, Giraud P. Prostate imaging: Contribution of PET PSMA and MRI. Cancer/Radiothérapie. 2020 Aug 1;24(5):423–8.
- Evangelista L, Zattoni F, Cassarino G, Artioli P, Cecchin D, dal Moro F, et al. PET/MRI in prostate cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021 Mar 1;48(3):859–73.
- Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol. 2016 Nov 1;70(5):829–36.
- Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014 May 1;41(5):887–97.
- Zamboglou C, Wieser G, Hennies S, Rempel I, Kirste S, Soschynski M, et al. MRI versus 68Ga-PSMA PET/CT for gross tumour volume delineation in radiation treatment planning of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016 May 1;43(5):889–97.
- Zamboglou C, Thomann B, Koubar K, Bronsert P, Krauss T, Rischke HC, et al. Focal dose escalation for prostate cancer using 68Ga-HBED-CC PSMA PET/CT and MRI: a planning study based on histology reference. Radiat Oncol. 2018 May 2;13(1):81.
- Evaluation of intensity modulated radiation therapy dose painting for localized prostate cancer using 68 Ga-HBED-CC PSMA-PET/CT: A planning study based on histopathology reference - ClinicalKey [Internet]. [cited 2023 Feb 25]. Available from: https://www.clinicalkey.fr/#!/content/playContent/1-s2.0-S0167814017303481?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0167814017303481%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F.
- Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J Nucl Med. 2018 Mar 1;59(3):469–78.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).