Submitted:
08 November 2023
Posted:
09 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Instruments
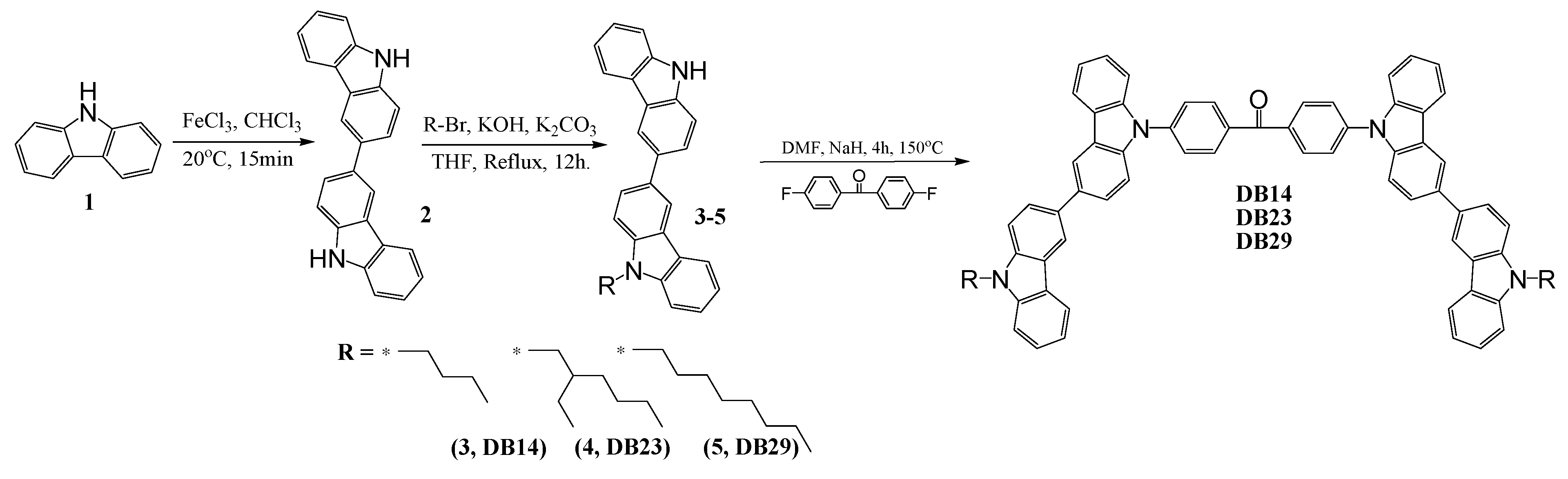
2.2. Synthesis and structure characterization of the materials
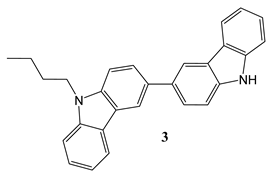
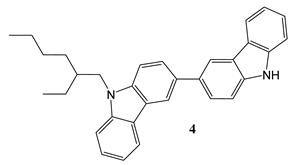
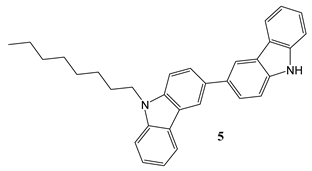
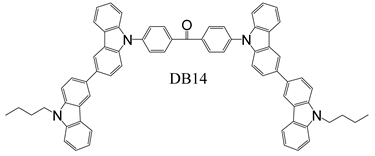
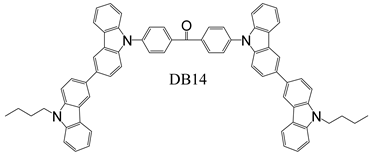
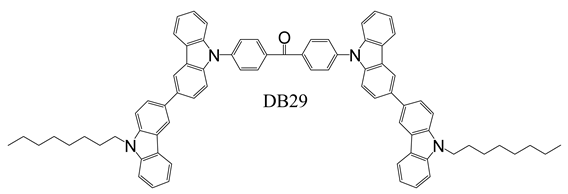
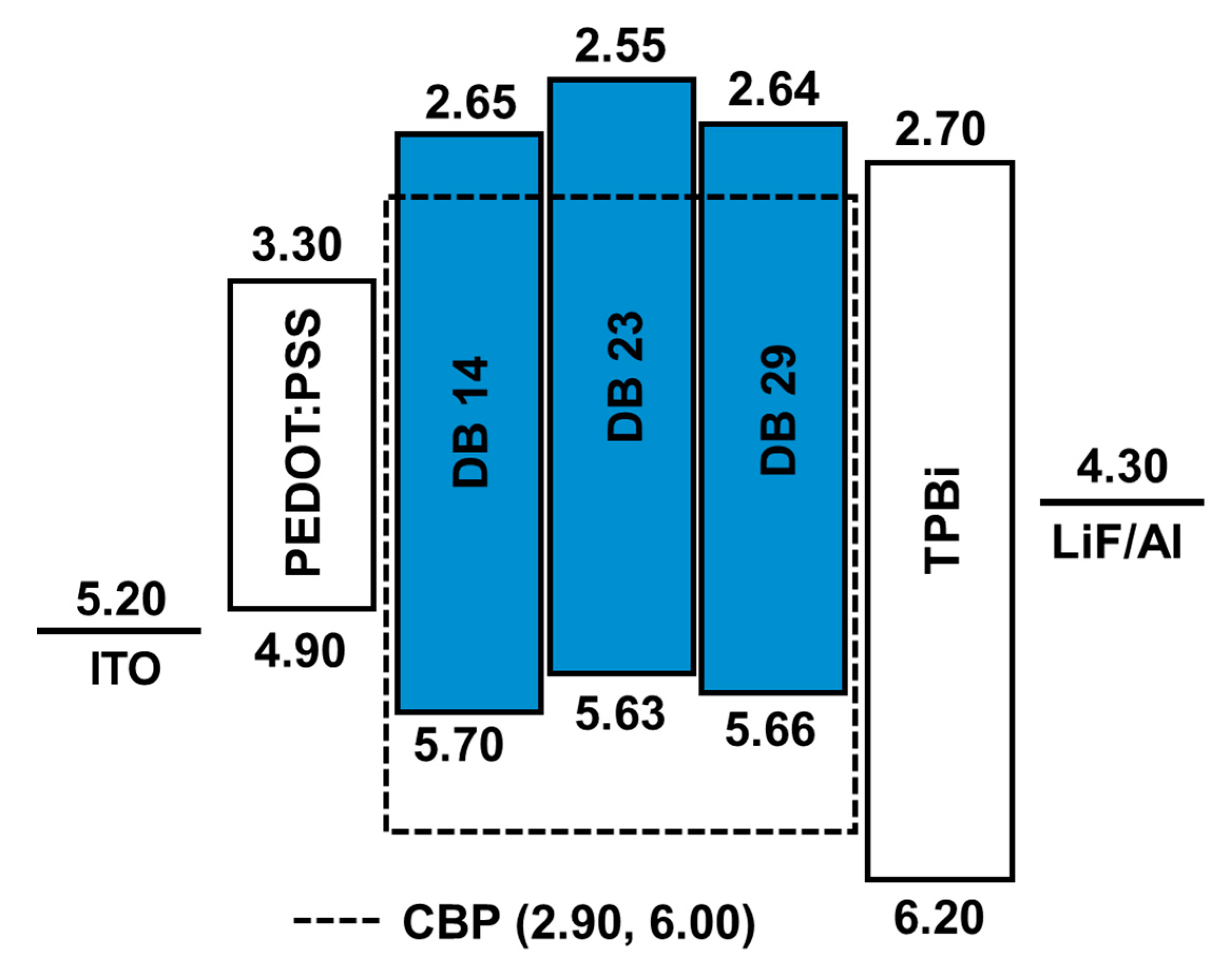
2.3. Device Fabrication
3. Result and Discussion
3.1. Material Characteristics
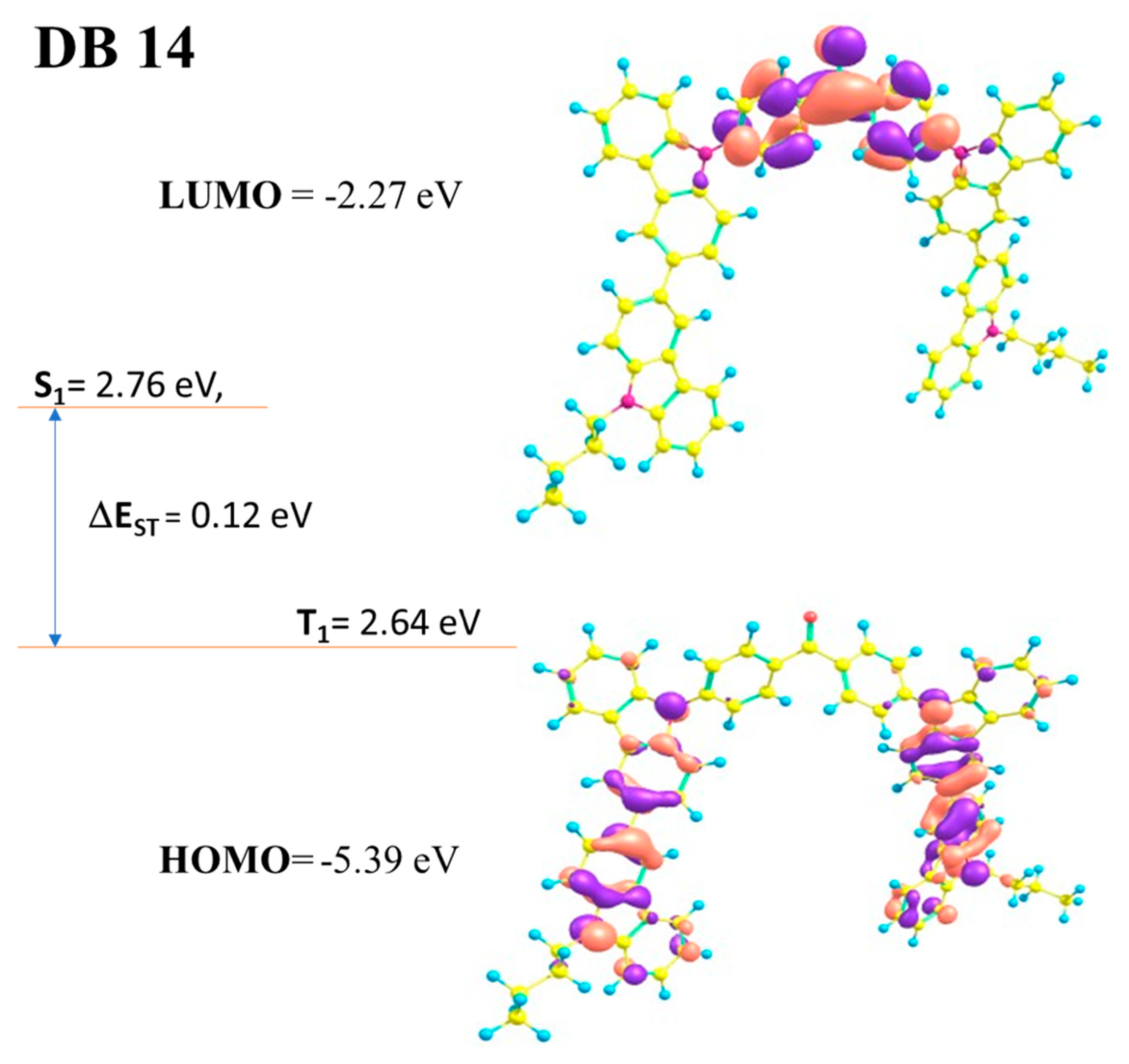
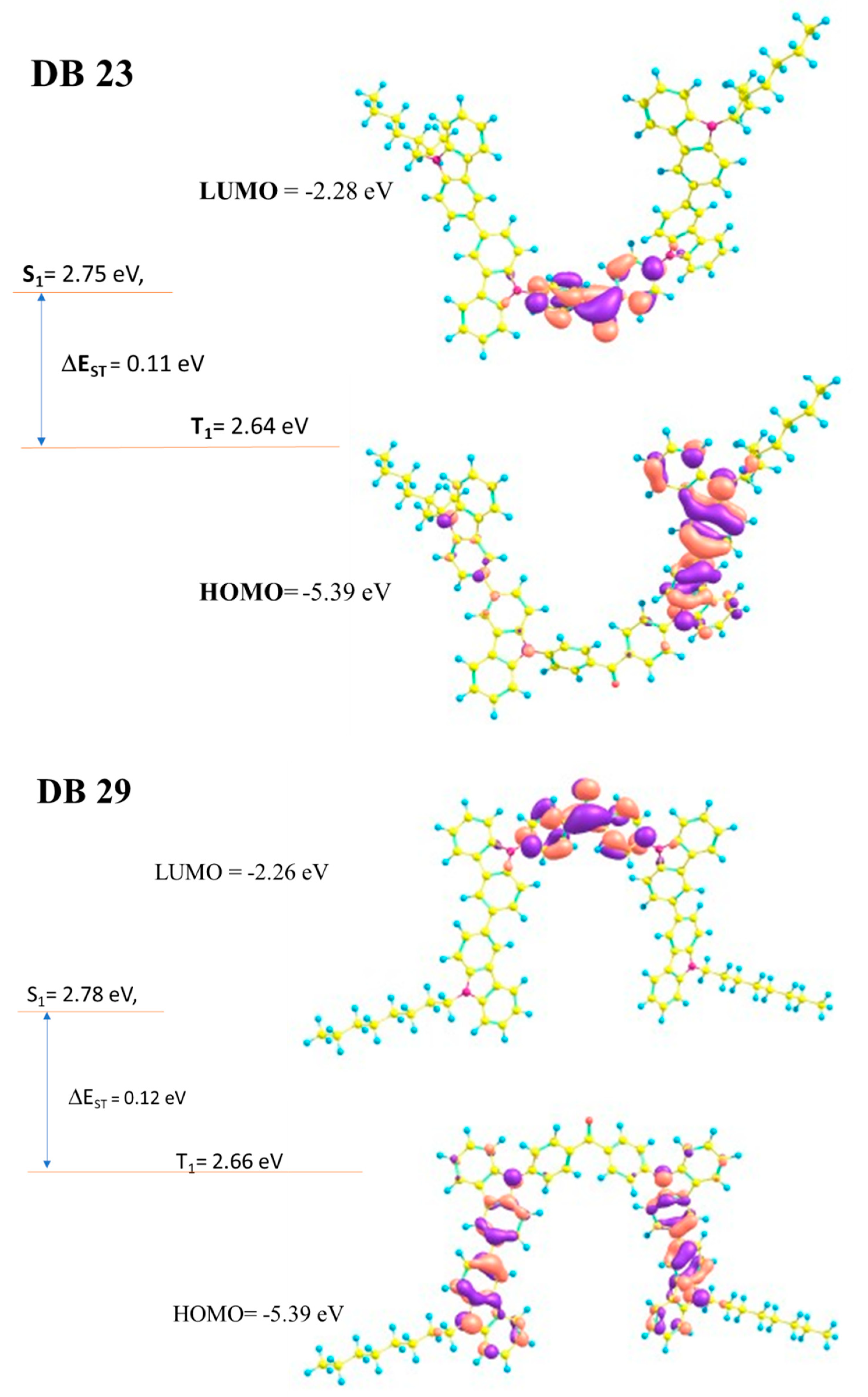
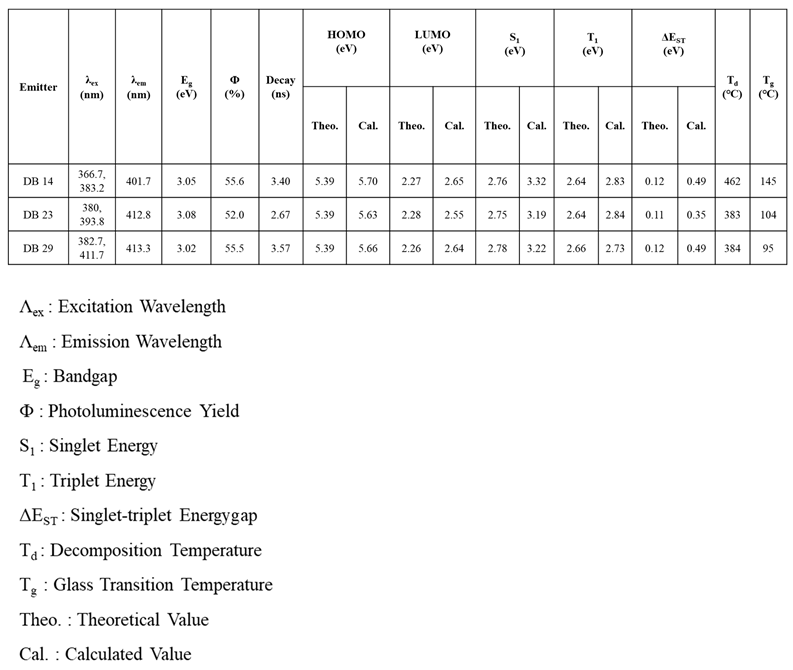
3.1.1. DFT Calculations
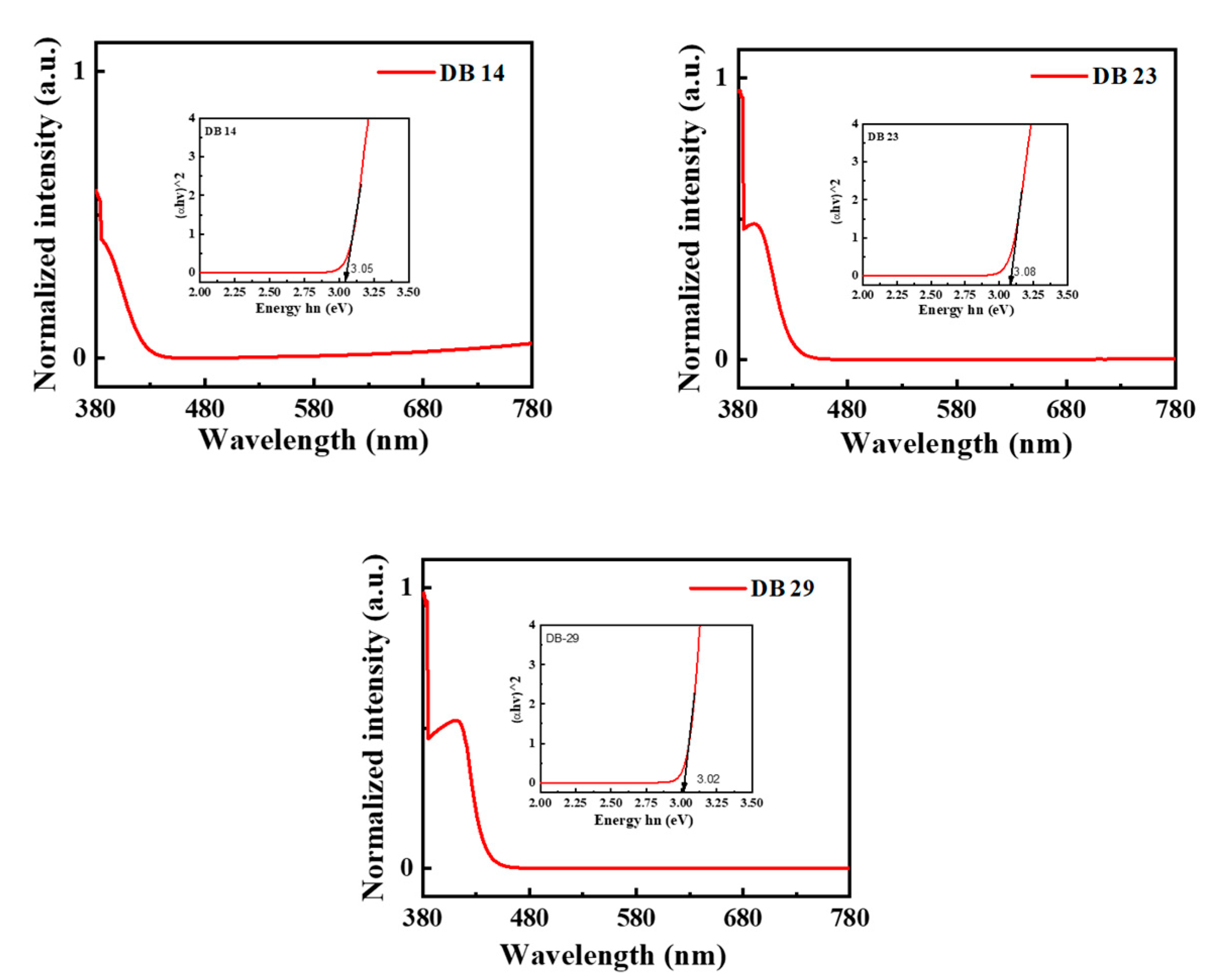
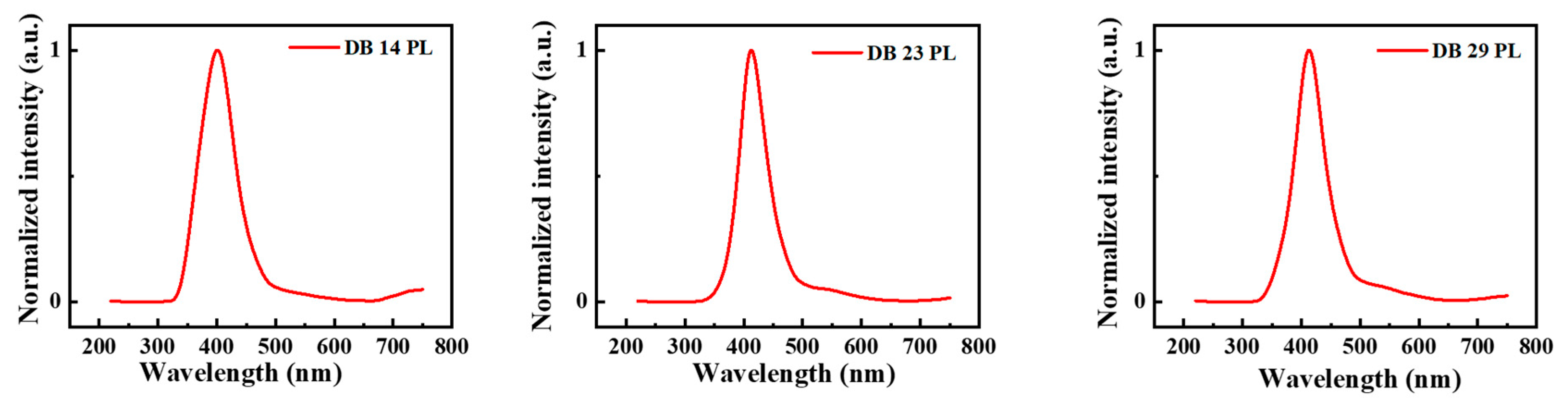
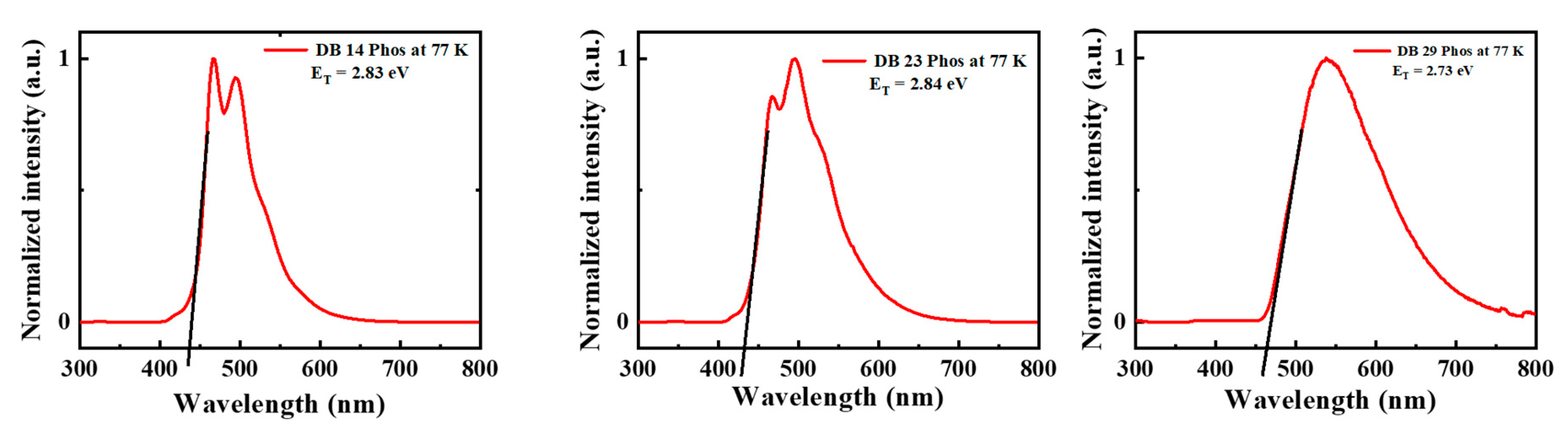
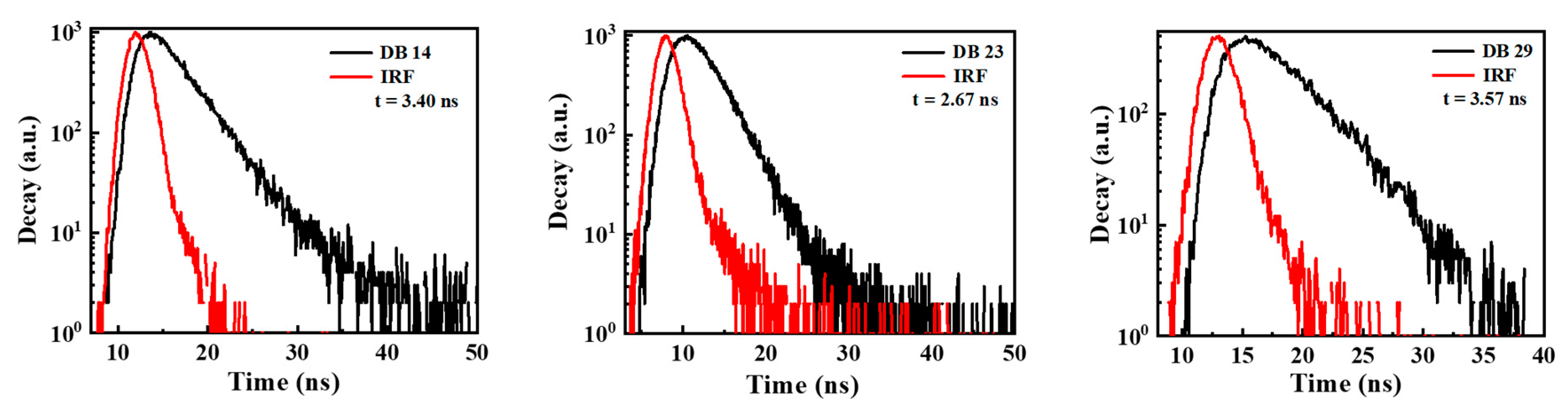
3.1.2. Photophysical Properties
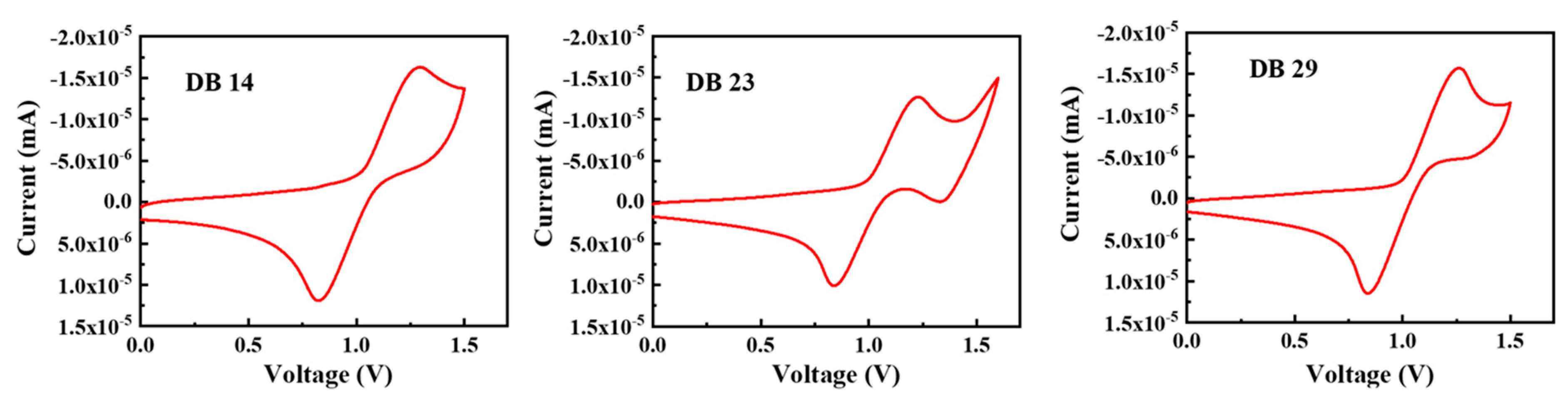
3.1.3. Electrochemical Properties
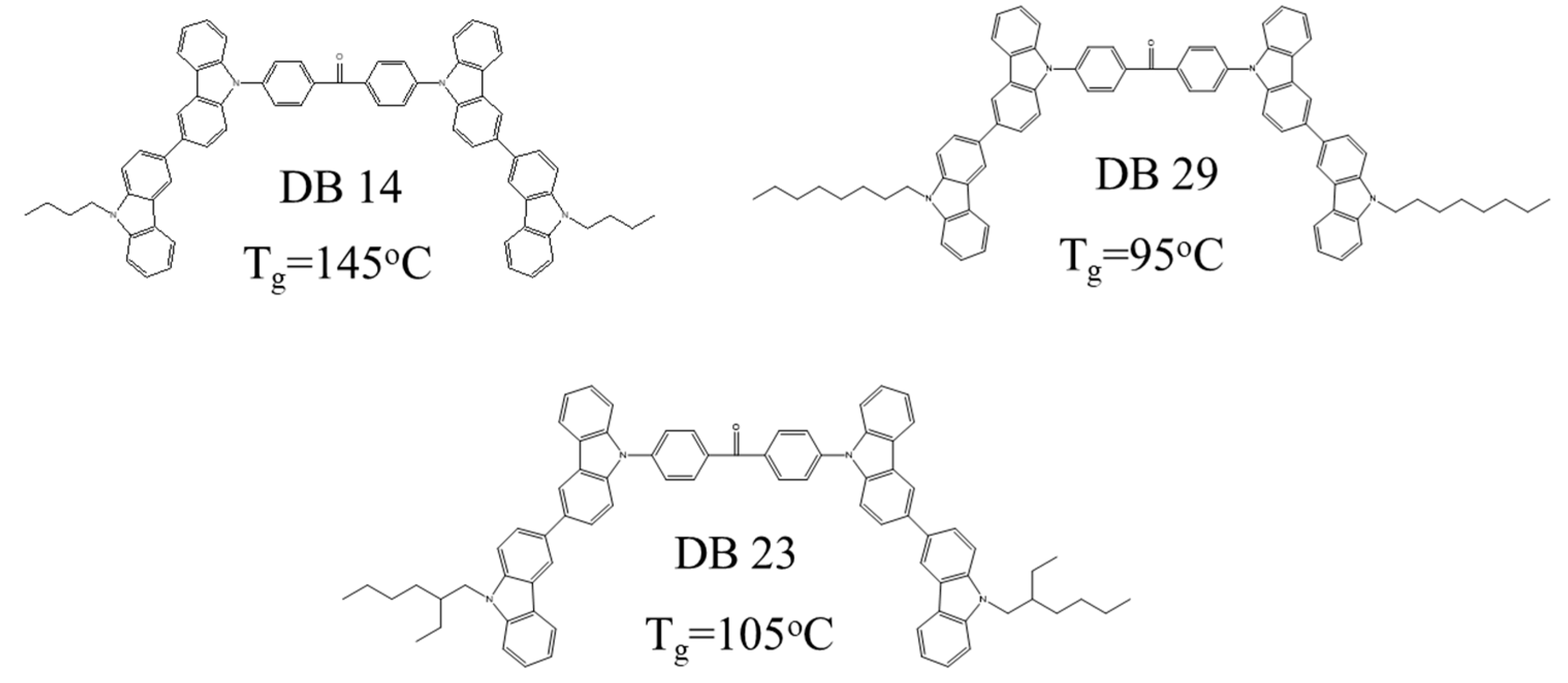
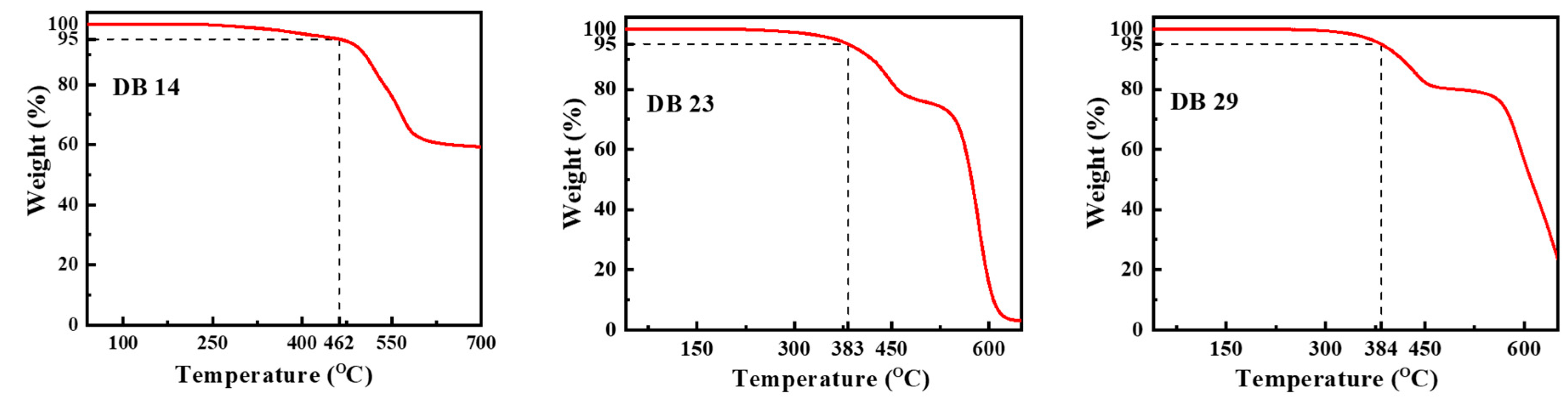
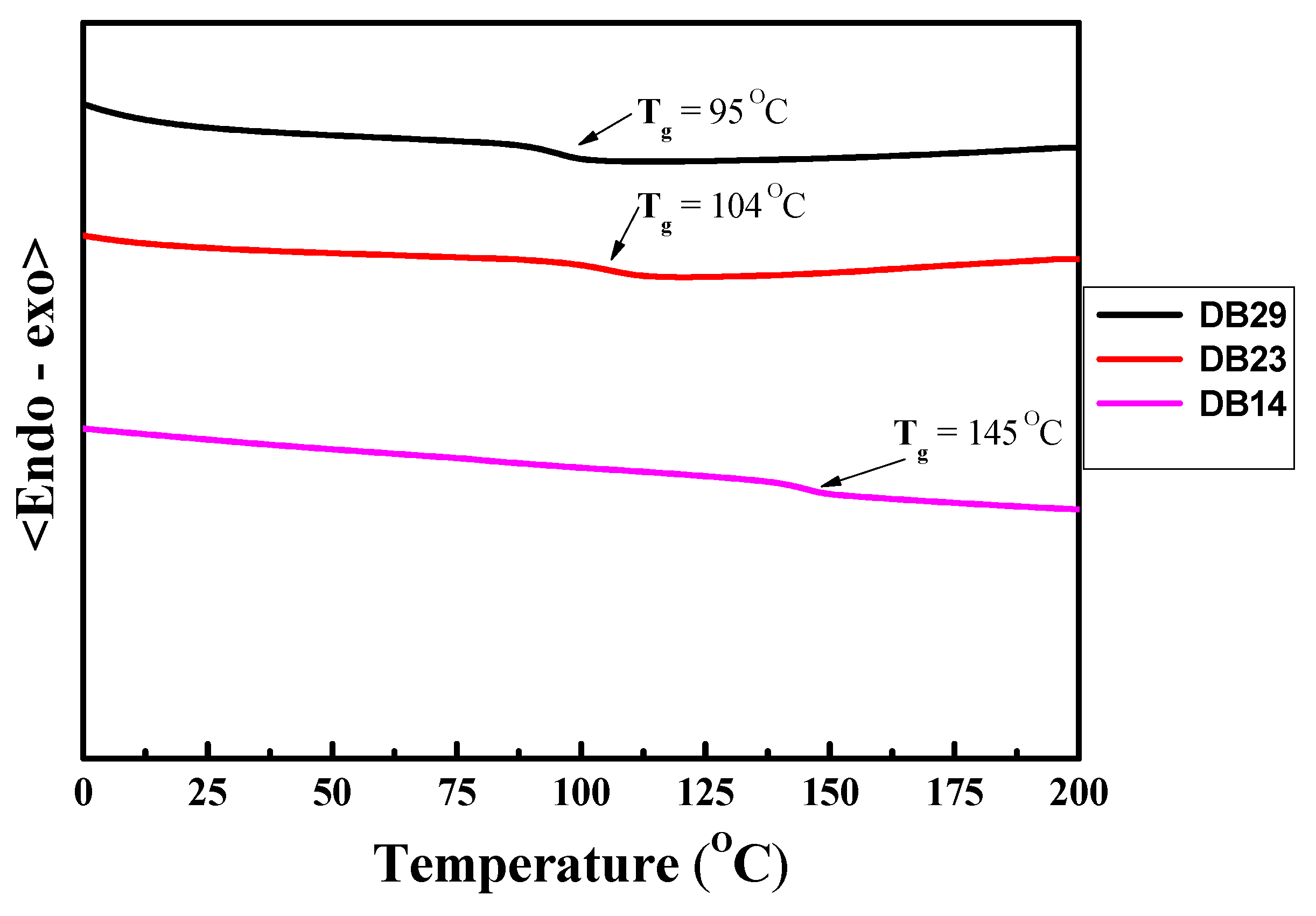
2.1.4. Thermal Properties
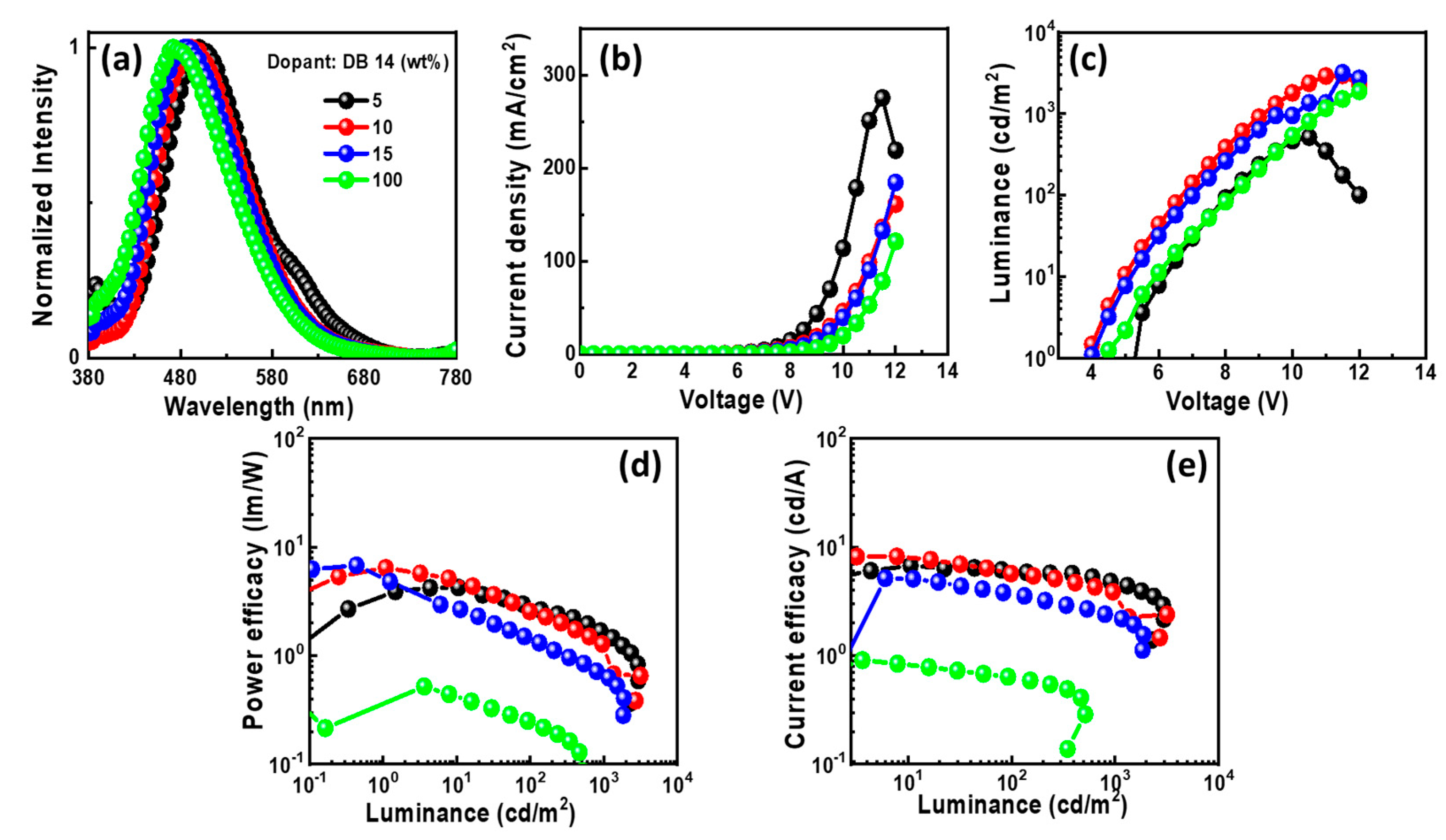
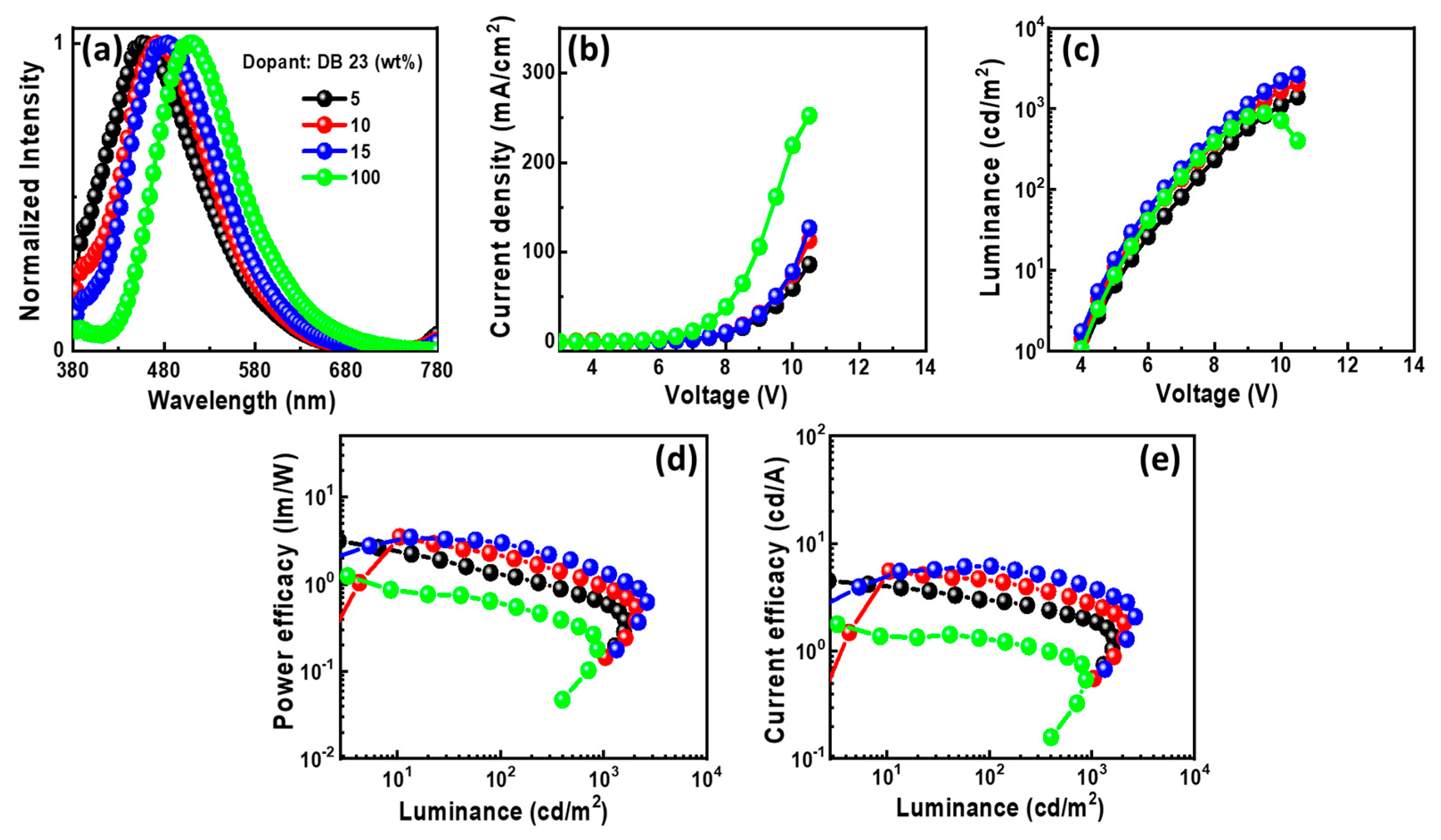
2.1.5. Electroluminescent Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Nakamura, S. Shuji Nakamura - Nobel Lecture: Background Story of the Invention of Efficient Blue InGaN Light Emitting Diodes. 2014.
- Blue LEDs-Filling the World with New Light.
- Nobel Prize ® and the Nobel Prize ® Medal Design Mark Are Registrated Trademarks of the Nobel Foundation. 2014.
- Nayak, S.R.; Siddiqui, I.; Shahnawaz; Jou, J.-H.; Vaidyanathan, S. Diphenylimidazole Based Fluorophores for Explosive Chemosensors and as Efficient Host Materials for Green Phosphorescent Organic Light Emitting Diodes. ACS Applied Optical Materials 2022. [CrossRef]
- Nayak, S.R.; Shahnawaz; Siddiqui, I.; Jou, J.H.; Patel, S.; Vaidyanathan, S. Multifunctional 4,5-Diphenyl-1 H-Imidazole-Based Luminogens as Near UV/Deep Blue Emitters/Hosts for Organic Light-Emitting Diodes and Selective Picric Acid Detection. Journal of Physical Chemistry C 2022. [CrossRef]
- Gupta, N.; Nagar, M.R.; Anamika; Gautam, P.; Maiti, B.; Jou, J.H.; Kuila, B.K. Triazine and Thiophene-Containing Conjugated Polymer Network Emitter-Based Solution-Processable Stable Blue Organic LEDs. ACS Appl Polym Mater 2022. [CrossRef]
- Solanki, J.D.; Siddiqui, I.; Gautam, P.; Gupta, V.K.; Jou, J.H.; Surati, K.R. Blue Fluorescent Zinc(II) Complexes Bearing Schiff Base Ligand for Solution-Processed Organic Light-Emitting Diodes with CIEy ≤ 0.09. Opt Mater (Amst) 2022, 134. [Google Scholar] [CrossRef]
- OLED Displays and Their Applications | Learning Corner for Beginners Available online:. Available online: https://www.electronicsforu.com/resources/oled-displays-applications (accessed on 2 May 2022).
- Siddiqui, I.; Kumar, S.; Tsai, Y.-F.; Gautam, P.; Shahnawaz; Kesavan, K.; Lin, J.-T.; Khai, L.; Chou, K.-H.; Choudhury, A.; et al. Status and Challenges of Blue OLEDs: A Review. Nanomaterials 2023, 13, 2521. [CrossRef]
- Bommireddy, P.R.; Musalikunta, C.S.; Lee, Y.-W.; Suh, Y.; Godumala, M.; Park, S.-H. Recent Endeavors and Perspectives in Developing Solution-Processable Host Materials for Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes. J Mater Chem C Mater 2023, 11, 13603–13624. [Google Scholar] [CrossRef]
- Gu, J.; Shi, W.; Zheng, H.; Chen, G.; Wei, B.; Wong, W.Y. The Novel Organic Emitters for High-Performance Narrow-Band Deep Blue OLEDs. Topics in Current Chemistry 2023 381:5 2023, 381, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cheon, H.J.; Lee, D.; Lee, W.; Kim, J.; Kim, Y.H.; Yoo, S. Toward Highly Efficient Deep-Blue OLEDs: Tailoring the Multiresonance-Induced TADF Molecules for Suppressed Excimer Formation and near-Unity Horizontal Dipole Ratio. Sci Adv 2023, 9. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, J.; Kumar Gupta, A.; Samuel, I.D.W.; Zysman-Colman, E. Merging Boron and Carbonyl Based MR-TADF Emitter Designs to Achieve High Performance Pure Blue OLEDs**. Angewandte Chemie International Edition 2023, 62, e202305182. [Google Scholar] [CrossRef] [PubMed]
- Mubarok, H.; Amin, A.; Lee, T.; Jung, J.; Lee, J.-H.; Lee, M.H. Triptycene-Fused Sterically Shielded Multi-Resonance TADF Emitter Enables High-Efficiency Deep Blue OLEDs with Reduced Dexter Energy Transfer. Angewandte Chemie 2023, 135, e202306879. [Google Scholar] [CrossRef]
- Gautam, P.; Shahnawaz; Siddiqui, I.; Blazevicius, D.; Krucaite, G.; Tavgeniene, D.; Jou, J.H.; Grigalevicius, S. Bifunctional Bicarbazole-Benzophenone-Based Twisted Donor–Acceptor–Donor Derivatives for Deep-Blue and Green OLEDs. Nanomaterials 2023, 13, 1408. Nanomaterials, 2023; 13, 1408. [CrossRef]
- Arnaboldi, S.; Benincori, T.; Mussini, P.R.; Beresneviciute, R.; Gautam, P.; Ram Nagar, M.; Krucaite, G.; Tavgeniene, D.; Jou, J.-H.; Grigalevicius, S. Naphtalimide-Based Bipolar Derivatives Enabling High-Efficiency OLEDs. Molecules 2023, Vol. 28, Page 6027 2023, 28, 6027. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Synthesis of Side-Chain Polystyrenes for All Organic Solution Processed OLEDs Available online:. Available online: https://www.researchgate.net/publication/318529009_Synthesis_of_side-chain_polystyrenes_for_all_organic_solution_processed_OLEDs (accessed on 30 June 2022).
- Peng, F.; Zhong, W.; He, J.; Zhong, Z.; Guo, T.; Ying, L. Effect of Alkyl Side Chain Length on the Electroluminescent Performance of Blue Light-Emitting Poly(Fluorene-Co-Dibenzothiophene-S,S-Dioxide). Dyes and Pigments 2021, 187, 109139. [Google Scholar] [CrossRef]
- Lee, T.; Song, C.E.; Lee, S.K.; Shin, W.S.; Lim, E. Alkyl-Side-Chain Engineering of Nonfused Nonfullerene Acceptors with Simultaneously Improved Material Solubility and Device Performance for Organic Solar Cells. ACS Omega 2021, 6, 4562–4573. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Pereira, S.; Data, P.; Monkman, A.; Pereira, S.; Monkman, A.P. Methods of Analysis of Organic Light Emitting Diodes. researchgate.net 2, 323–337.
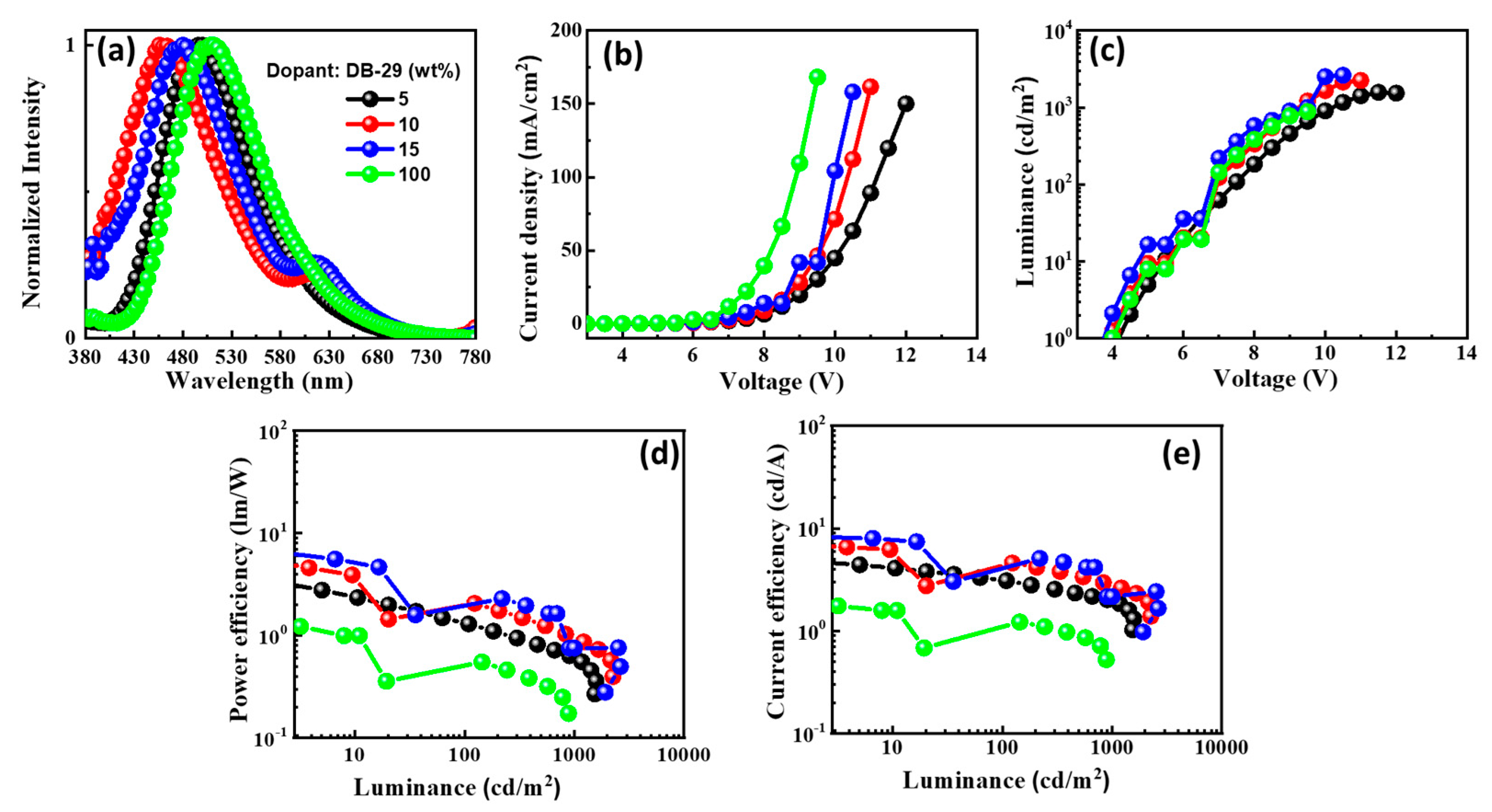
 |
| Emitter | Concentration of emitter in host material (wt%) |
Turn-on Voltage (Von)a | Power efficacy (lm/W) | Current efficacy (cd/A) | EQE (%) | CIE | Max Luminance (cd/m2) |
|---|---|---|---|---|---|---|---|
| @100/1,000 cd/m2 and max | |||||||
| DB-14 | 5.0 | 5.0 | 2.9/ 1.6/ 3.9 | 6.1/ 4.7/ 6.5 | 2.6/ 2.2/ 2.7 | (0.22, 0.36)/ (0.21, 0.32)/ - | 2951 |
| 10 | 5.1 | 2.6/ 1.3/ 4.4 | 5.7/ 3.9/ 7.6 | 2.6/ 2.0/ 3.3 | (0.21, 0.33)/ (0.20, 0.29)/ - | 3175 | |
| 15 | 5.9 | 1.4/ 1.3/ 2.4 | 3.7/ 2.3/ 4.9 | 1.9/ 1.4/ 2.4 | (0.20, 0.28)/ (0.19, 0.24)/ - | 1884 | |
| 100 | 6.1 | 0.2/ -/ 0.4 | 0.6/ -/ 0.8 | 0.3/ -/ 0.4 | (0.26, 0.40)/ - | 515 | |
| DB-29 | 5.0 | 5.4 | 0.3/ 0.6/ 2.1 | 3.1/ 2.0/ 3.9 | 2.0/ 1.4/ 2.3 | (0.19, 0.22)/ (0.22, 0.22)/ - | 1578 |
| 10 | 5.7 | 1.7/ 1.0/ 4.4 | 3.7/ 2.8/ 6.5 | 2.0/ 1.7/ 3.1 | (0.20, 0.27)/ (0.23, 0.25)/ - | 2251 | |
| 15 | 4.7 | 1.0/ 0.3/ 7.9 | 2.3/ 1.0/ 9.1 | 1.1/ 0.5/ 4.0 | (0.21, 0.31)/ (0.22, 0.27)/ - | 2631 | |
| 100 | 5.8 | 0.4/ -/ 1.4 | 0.8/ -/ 1.8 | 0.3/ -/ 0.7 | (0.26, 0.44)/ - | 884 | |
| DB-23 | 5.0 | 5.7 | 1.3/ 0.6/ 2.1 | 3.0/ 1.9/ 3.8 | 1.9/ 1.4/ 2.3 | (0.17, 0.07) | 883 |
| 10 | 5.2 | 1.4/ 0.8/ 1.7 | 2.4/ 1.7/ 2.6 | 5.1/ 3.5/ 5.3 | (0.19, 0.22)/ (0.22, 0.22)/ - | 1,620 | |
| 15 | 5.2 | 2.2/ 1.0/ 3.2 | 4.6/ 2.8/ 5.3 | 2.5/ 1.7/ 2.7 | (0.20, 0.27)/ (0.23, 0.25)/ - | 2,076 | |
| 100 | 5.1 | 0.6/ -/ 1.0 | 1.3/ -/ 1.8 | 0.5/ -/ 0.7 | (0.26, 0.44)/ - | 875 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





