1. Introduction
Post-stroke cognitive impairment (PCI) and post-stroke depressive disorder (PSD) are among most common causes of disability and ineffective rehabilitation of patients. [
1]. Meta-analysis of available studies indicates that in the first year post-stroke, 4 of 10 patients display a level of cognitive impairment that does not meet the criteria for dementia [
2]. Importantly, the development of PCI and PCD does not necessarily follow a severe ischemic stroke (IS). The reasons for the development of cognitive and affective disorders in patients after mild or moderate IS without serious post-stroke neurological consequences remain obscure. Meanwhile, the study of the pathogenesis of such disorders in patients along with neurologic recovery seems vital for understanding the causes of their development and imperative for the elaboration of strategies for their prevention and treatment.
It has been shown that the hypothalamic-pituitary-adrenal axis (HPA axis), sympathoadrenal medullary system (SAMS), and inflammatory processes are involved in the pathogenesis of both post-stroke cognitive and affective disturbances [
3]. It is assumed that IS, as a strong stressor, triggers changes in the functioning of these systems [
4]. Furthermore, the actual post-IS stress load consists of several components. The principal ones include negative experience ('accumulated stress') preceding the onset of stroke; stroke per se as a focal brain damage; the early period after the onset of damage, when the patient experiences physical and psychological suffering. The effects of each of these factors as well as their cumulative effect depends on the level of individual stress-resilience [
5].
Since PCI and PSD do not necessarily develop in all patients after IS, deep preexisting changes in the functioning of stress-response systems may be crucial for their occurrence. In this case, focal brain damage may act as an additional factor potentiating the previously existing changes in the functioning of HPA axis and/or SAMS and increasing the risk of PCI or PSD development. In clinical practice, it is fairly difficult to assess the states of stress-releasing systems preceding stroke in patients. An available approach to such assessment is retrospective measurement of cortisol level in hair [
6,
7]. Thus, it seems particularly important to search for additional sources of information associated with the development of PCI and PSD allowing to indirectly assess the state of brain structures and systems preceding IS.
Damage to the hippocampus, a unique brain region essentially associated with both memory and emotion formation, is a key link in the development of PCI and PSD. The activation of HPA axis is well known to be accompanied by the release of excessive amounts of glucocorticoids (cortisol in humans) and results in damage to structures of the limbic system of the brain, in particular the hippocampus, which has a very high density of glucocorticoid receptors (GRs) [
8,
9]. A comparative morphometric study of the hippocampus, as well as other cortical and limbic brain structures associated with memory and emotion formation, performed in the first few days after an IS, in patients with and without cognitive and/or affective impairments, could provide additional valuable information about the state of these structures prior to IS.
Chronic diseases like Alzheimer's, Parkinson's, multiple sclerosis [
10] as well as mental illnesses [
11] are believed to result from a combination of genetic and environmental factors, a concept commonly referred to as the "multiple hit" hypothesis. Leveraging two-hit or multiple-hit approaches to PCI and PSD development, we suggest that may IS represent the final hit triggering the development of delayed post-stroke consequences and may hope to reveal objective signs of previous hit(s) soon after the SI onset.
The aim of the present study was to search for associations of the volumes of cortical and limbic brain structures investigated in the acute period with the development of PCI and PSD after mild or moderate IS. Besides the hippocampus, the morphometric study included: entorhinal cortex, the main input and output of the hippocampus responsible for short-term memory formation [
12,
13]; amygdala, predominantly involved in emotion formation [
14]; supramarginal gyrus, located in the parietal lobe, part of the somatosensory association cortex mainly responsible for cognitive functions (speech and orientation) [
15]; temporal pole, associated with cognitive functions and closely interacting with the hippocampus [
16,
17]; middle temporal gyrus, involved in speech processing and semantic memory, as well as in visual perception and multimodal sensory integration [
18]; anterior cingulate cortex having associative connections with the limbic system and prefrontal cortex, and thus playing an important role in the integration of affective and cognitive functions [
19].
2. Materials and Methods
2.1. Subjects
The study included 23 patients (18 men, 5 women, mean age 57±11 years) treated at the Konchalovsky Hospital, who met the following inclusion criteria: age 45-80 years; ischemic cerebral infarction of hemispheric localization, not affecting limbic structures; mild to moderate severity of IS; admission to hospital not later than 48 hours after an IS. Exclusion criteria were presence of stroke, craniocerebral trauma in the history with residual focal changes on computed tomography (CT)/ magnetic resonance imaging (MRI); presence of cognitive and depressive disorders in the anamnesis; acute and chronic somatic and hormonal diseases; alcohol or drug dependence.
Socio-demographic information, medical and life history were collected from patients after an IS during initial hospitalization.
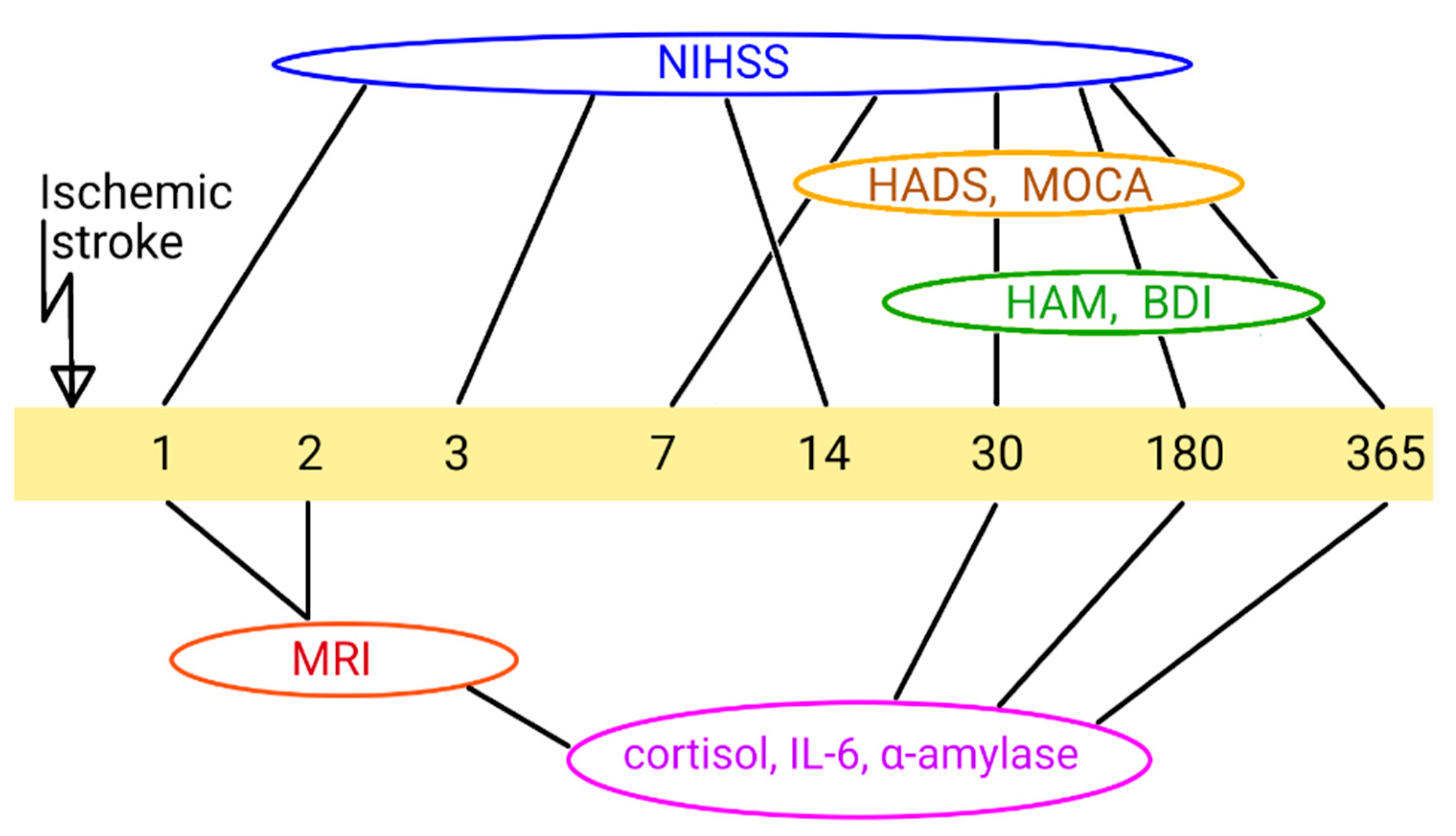
Patients were observed by a neurologist and psychiatrist for minimum 1 year. Neurological, psychiatric and cognitive indices of the subjects' condition were assessed on the 1st, 30th, 180th and 365th days after the onset of IS using the following scales: The National Institutes of Health Stroke Scale (NIHSS) [
20], Montreal Cognitive Assessment (MoCA) [
21]; Hospital Anxiety and Depression Scale (HADS) [
22]; Beck Depression Inventory (BDI) [
23]; Hamilton Rating Scale for Depression (HAM [
24]) (
Figure 1).
Informed consent to participate in the study was signed by each subject included in the study.
This study adhered to the tenets of the Declaration of Helsinki and had local ethics committee approval (#42, 23.08.2019) with informed consent obtained from all subjects.
2.2. Assessment of Biochemical Indices and Hormones
Blood was collected from the elbow vein in the morning hours on an empty stomach into S-Monovettes vacuum tubes with clotting activator to obtain serum, followed by centrifugation at 2000 g for 15 minutes at 4°C.
Salivary fluid was collected in SaliCap low-adhesion tubes (IBL, Sweden) in an amount of about 1 mL between 14 and 15 hours. Before salivary fluid collection, food intake and toothpaste use were excluded; the oral cavity was rinsed thoroughly. Salivary fluid samples were centrifuged at 2000 g for 15 minutes. The supernatant fraction was collected into a clean tube.
Hair samples were collected from the occipital scalp about 1 cm from the root (with cortisol levels corresponding to approximately 1 month prior to the study) and stored until cortisol extraction in separate airtight plastic containers. Cortisol extraction was performed as described in [
7].
The levels of cortisol and interleukin 6 (IL-6) in blood serum, cortisol in salivary fluid and cortisol in hair were evaluated on the 1st, 30th, 180th and 365th post-stroke days using Access2 immunoassay analyzer (BeckmanCoulter, USA). Salivary α-amylase activity was measured at the same time points using a kinetic colorimetric method on an ILAB Aries biochemical analyzer (Instrumentation Laboratory, USA).
2.3. MR Morphometry
MRI of the brain was performed on a scanner with a magnetic field induction of 1.5 Tesla ("SIGNA" HDxt, GEMedical Systems, USA) using pulse sequences: FLAIR T2 ax, FLAIRcor, T2 PROPELLER ax, T2* ax, DWI 1000bax, 3-plT2* FGRE, IR-FSPGR-3DT1 ax in the acute period and on the 30th day after an IS. IR-FSPGR-3DT1 ax sequence (TR=7 ms, TE= 3 ms, reconstruction matrix - 256×256, layer thickness 1 mm, FoV=90, flipangle=12, slice spacing -1mm) was used for anatomical image acquisition.
The morphometric characteristics of brain structures were determined using T1-weighted images using the FreeSurfer 7.2.0 software package.
During preprocessing and analysis, standard manipulations such as automatic transformation in Talairach space, reconstruction of the brain cortex, segmentation of white and gray matter of cortical and subcortical structures were performed.
Relative volumes of structures were calculated as follows: relative volume of structure (relative units) = (absolute volume of structure / total intracranial volume) × 1000.
2.4. Study Design
The general prospective study design is illustrated in
Figure 1. Post stroke time points are represented for different parameters measured.
2.5. Statistical Analysis
Statistical analysis was performed using the STATISTICA 10.0 (StatSoft Inc., Tulsa, OK, USA) and GraphPadPrism version 9.4.1. (GraphPadSoftware, Inc., SanDiego, CA, USA) software. Logistic regression method was used to assess the significance of factors influencing the development of post-stroke neuropsychiatric disorders. Normality of distribution was determined using Shapiro-Wilk test. Depending on the distribution, Student t-test or Mann-Whitney test were used to compare two unrelated samples, while Student t-test or Wilcoxon test were used to compare two related samples. The results are plotted as mean and standard error of the mean, or as median and range. Correlations were calculated using the Spearman test. At p<0.05, differences were considered significant; at p<0.1, differences were considered at the trend level of significance.
3. Results
3.1. Characteristics of the Patients
The study showed that in 65% of patients, the focus of IS was localized in the territory of middle cerebral artery, in 35% — of the posterior cerebral artery, predominantly on the right side (57%). Eighty seven per cent of patients had a history of concomitant arterial hypertension, and 17% had concomitant dyslipidemia. Subsequent post-stroke cognitive and/or affective impairment was found in 65% of subjects. When tetrachoric correlation was performed in patients with and without post-stroke cognitive and affective impairment with comorbid somatic diseases, positive significant correlations were found between the occurrence of post-stroke impairment and arterial hypertension (r=0. 62; p=0.049) and with dyslipidemia (r=0.84; p=0.03).
According to the MoCA scale and psychiatrist's evaluation, post-stroke cognitive impairment was detected in 57% of the examined patients. According to the HADS scale and psychiatrist's evaluation, post-stroke depressive disorder was observed in 26% of the examined patients as early as the 30th day after IS.
During the study, patients were conditionally divided into four subgroups: patients without PCI, patients with PCI, patients without PSD, and patients with PSD. The correctness of allocating patients to groups with and without PSD was additionally confirmed by the results of psychometric examination using the HAM and BDI scales. Assessment of the influence of sex, age, localization, and lateralization of the brain infarction performed out by logistic regression method, confirmed the absence of statistical significance of the effect of any of the above parameters in the cohort studied, either on the occurrence of PCI: sex (p=0. 77), age (p=0.31), stroke localization (p=1), stroke lateralization (p=0.69), or on the occurrence of PSD: gender (p=0.36), age (p=0.08), stroke localization (p=1), stroke lateralization (p=0.13).
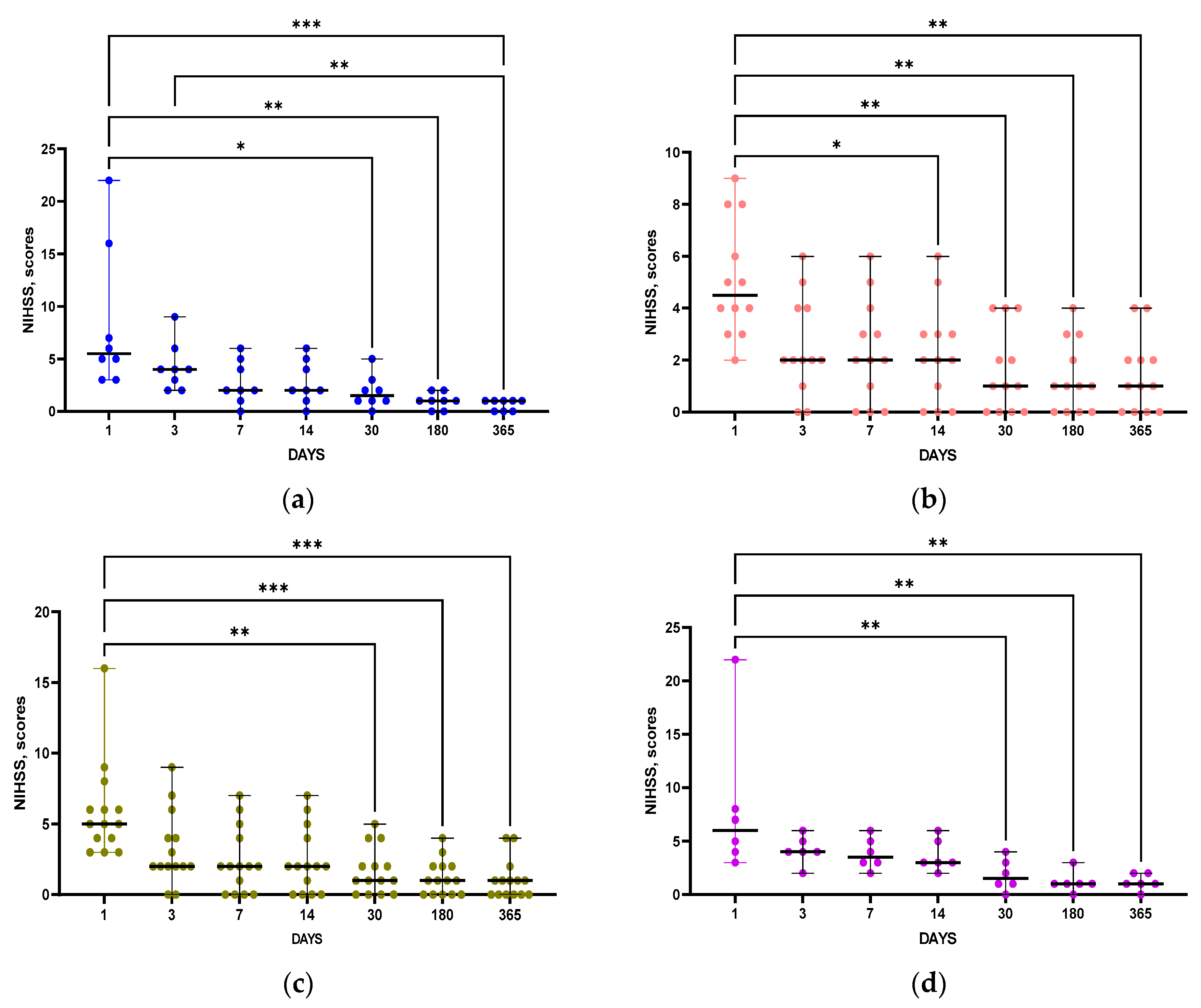
Importantly, NIHSS scores indicating neurological deficits were not statistically different between groups with and without post-stroke disorders at all time points (
Table 1). Notably, statistically significant positive dynamics of NIHSS score changes were observed in patients of all groups (
Figure 2 a-d)
3.2. Comparative Characteristics of Patients with and without PCI
3.2.1. Clinical Data
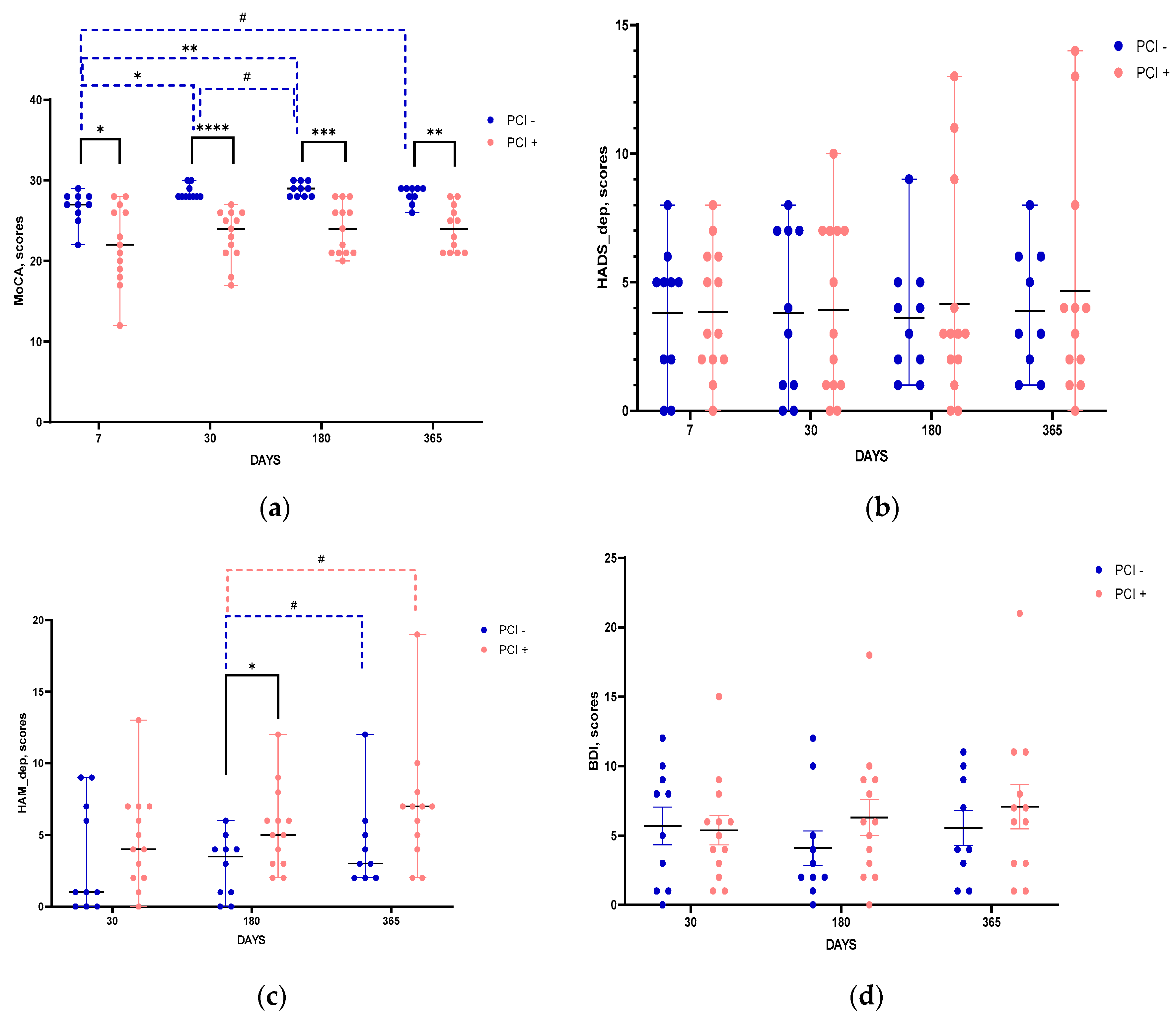
The results of psychometric testing indicated that MoCA scores in groups of patients with and without PCI differed starting from post stroke day 7 and remained stable throughout the entire follow-up period (
Figure 3a). In the group with PCI, MoCA scores did not show any dynamics within a year after IS, while in the group without PCI there was an improvement in cognitive status evident from post stroke day 30 (
Figure 3a). According to scores of neuropsychiatric scales assessing depressive disorders, HADS (
Figure 3b), HAM (
Figure 3c), and BDI (
Figure 3d), the groups did not differ significantly. The only exception was the HAM score on post stroke day 180, showing more expressed symptoms of depression in the group of patients with PCI (
Figure 3c).
3.2.2. Biochemical Data
Levels of salivary α-amylase, which indirectly characterizes the functional state of SAMS and reflects the level of norepinephrine [
25], did not differ significantly between the groups with and without PCI throughout the entire follow-up period. In patients of both groups, salivary α-amylase activity was low during the acute period, increased significantly by post stroke day 30 and remained unchanged thereafter (data not shown).
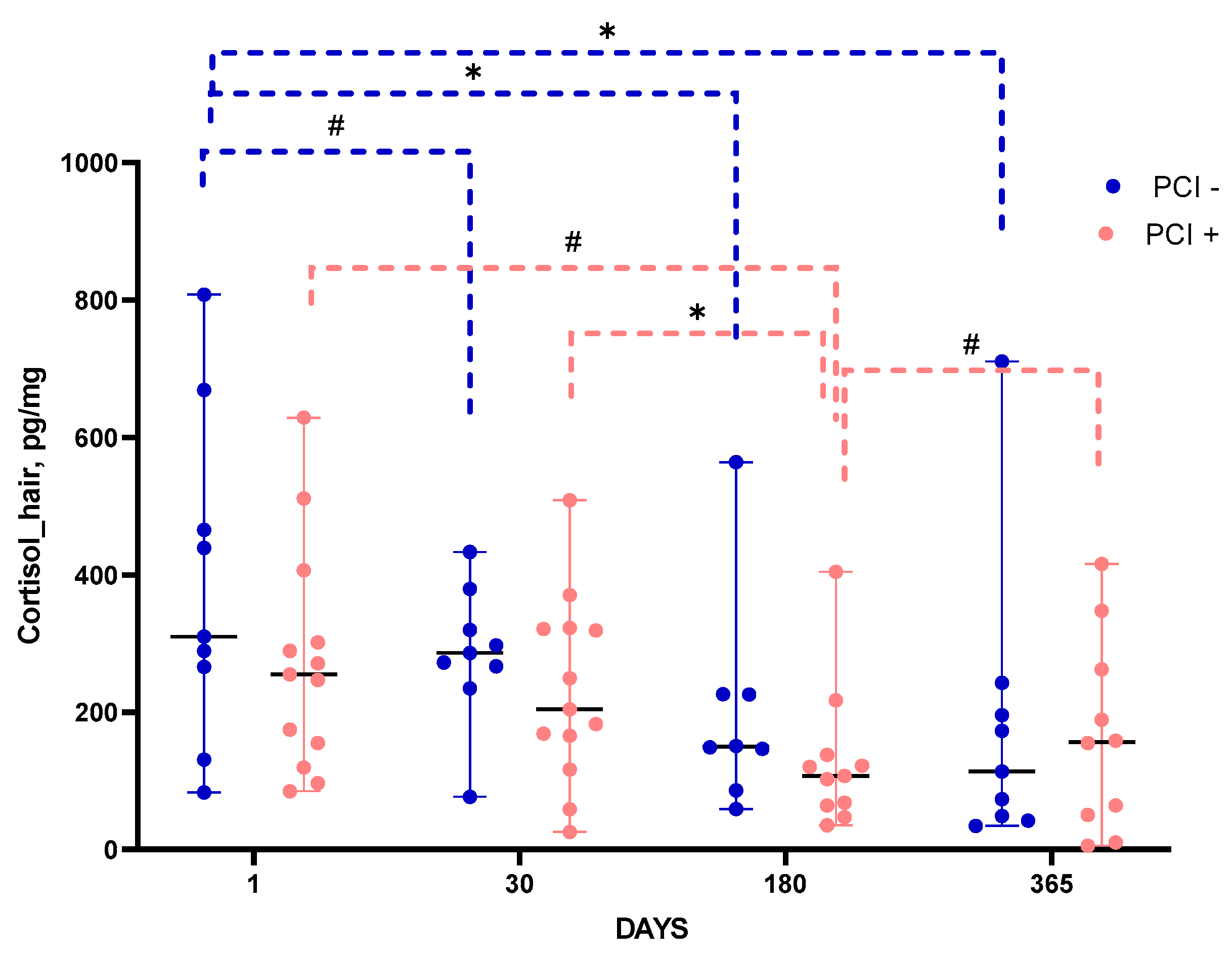
The level of cortisol in hair, which retrospectively characterizes the accumulated stress load [
5] demonstrated different time course in patients with and without PCI (
Figure 4). In both groups, the level of hair cortisol significantly decreased by post-stroke day 180 as compared with its level corresponding to a month before IS (post-stroke day 1). In patients without PCI, hair cortisol levels remained unchanged until the end of the follow-up period, while in patients with PCI they somewhat increased between post-stroke days 180 and 365.
3.2.3. Morphometric Data
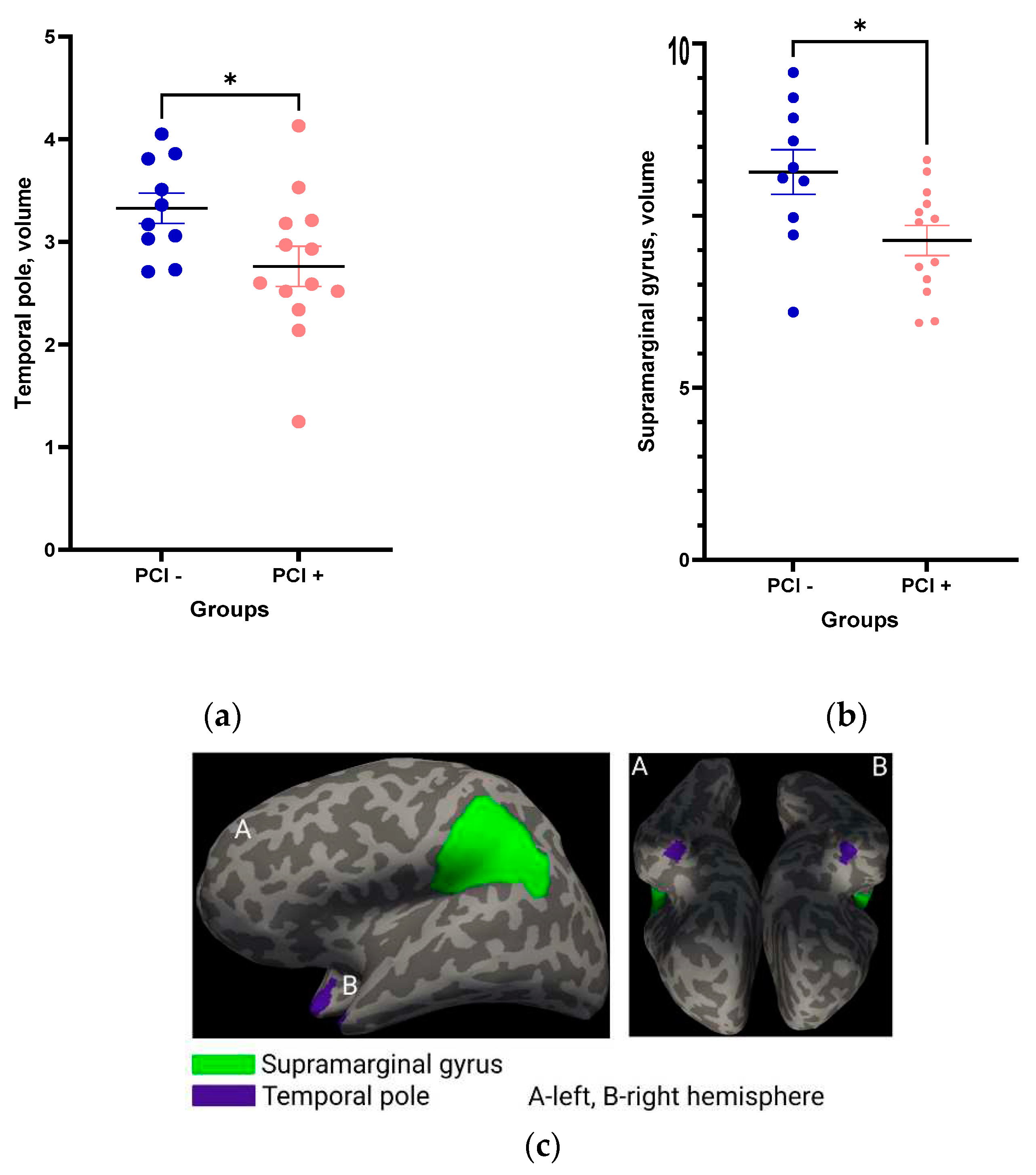
Comparison of the relative volumes of brain structures performed during the first post-stroke days indicated that the volumes of the temporal pole (
Figure 5a) and supramarginal gyrus (
Figure 5b,c) were significantly smaller in patients with PCI as compared to patients without evident PCI.
Positive correlations of supramarginal gyrus and temporal pole volumes with MoCA score were found both 30 days (R=0.49, p=0.02 and R=0.45, p=0.03, respectively) and 365 days post-stroke (R=0.42, p=0.06 and R=0.44, p=0.05, respectively).
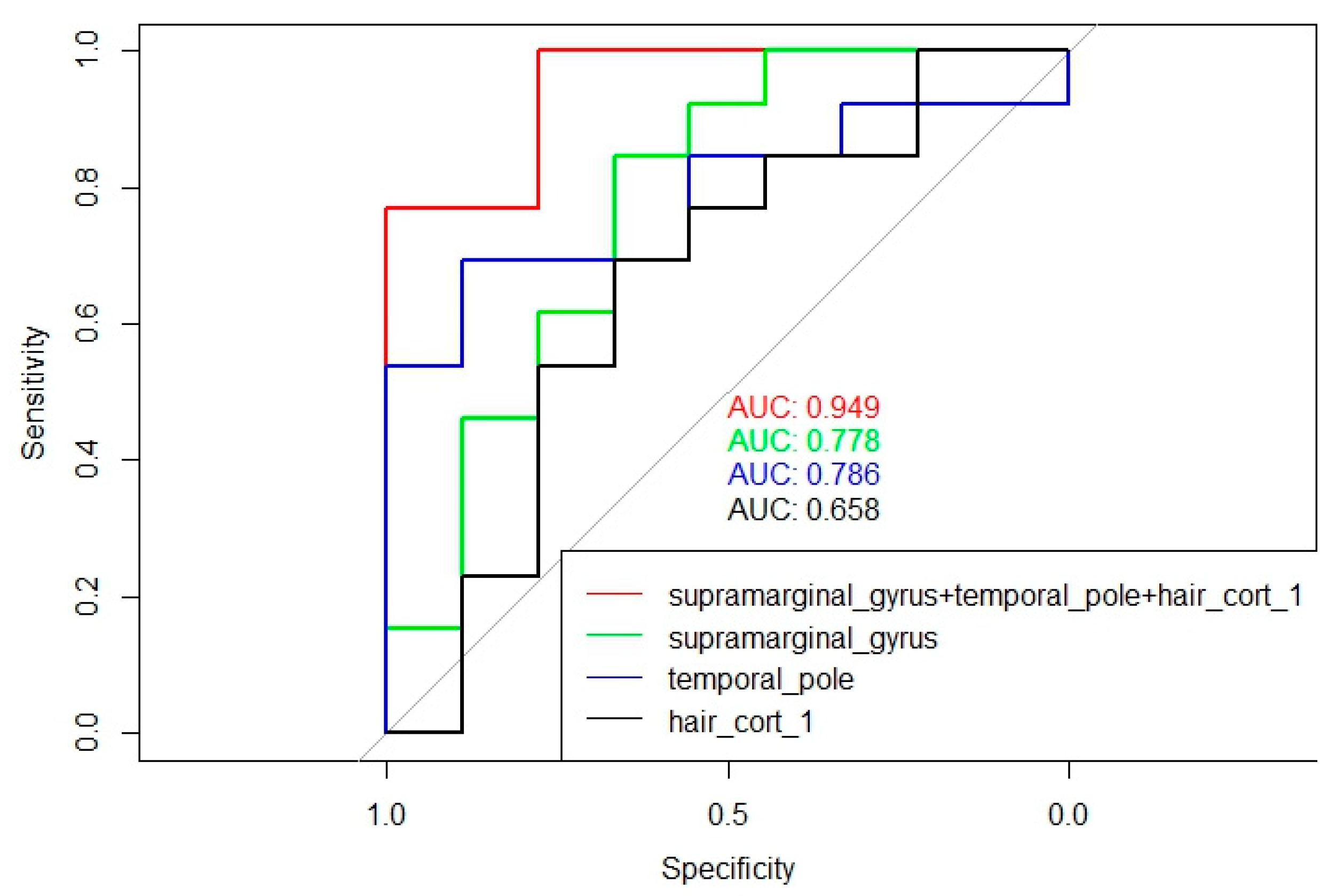
Multiple logistic regression analysis was performed, and the predictors that cooperatively had maximum influence on the development of PCI were included in the model (
Figure 6). The most effective predictors were: supramarginal gyrus volume (β0=3.41; β=-0.99; p=0.047), temporal pole volume (β0=3.41; β=-3.12; p=0.06), hair cortisol level on the 1st post-stroke day reflecting the level of stress/cortisol load during the last month before IS (β0=3.41; β=-0.05; p=0.08). For this model, pseudoR^2 was equal to 0.51.
3.2.4. Correlations of Brain Structure Volumes with Biochemical Parameters
The correlations of relative volumes of cortical and limbic brain structures with biochemical parameters are presented in
Table 2. In patients with PCI, a statistically significant negative correlation was found between relative temporal pole volume with IL-6 levels on the 1st and 30th post-stroke days (R=-0.62, p=0.04 and R=-0.66; p=0.02, respectively), as well as with α-amylase level a year after IS (R=-0.76, p=0.01). Patients without PCI showed a negative correlation of relative temporal pole volume with hair cortisol levels on the 1st and 30th post-stroke days (R=-0.68, p=0.04 and R=-0.7, p=0.04) and a positive correlation with salivary cortisol levels on the 30th post-stroke day (R=0.87, p=0.003).
3.3. Comparative Characteristics of Patients with and without PSD
3.3.1. Clinical Data
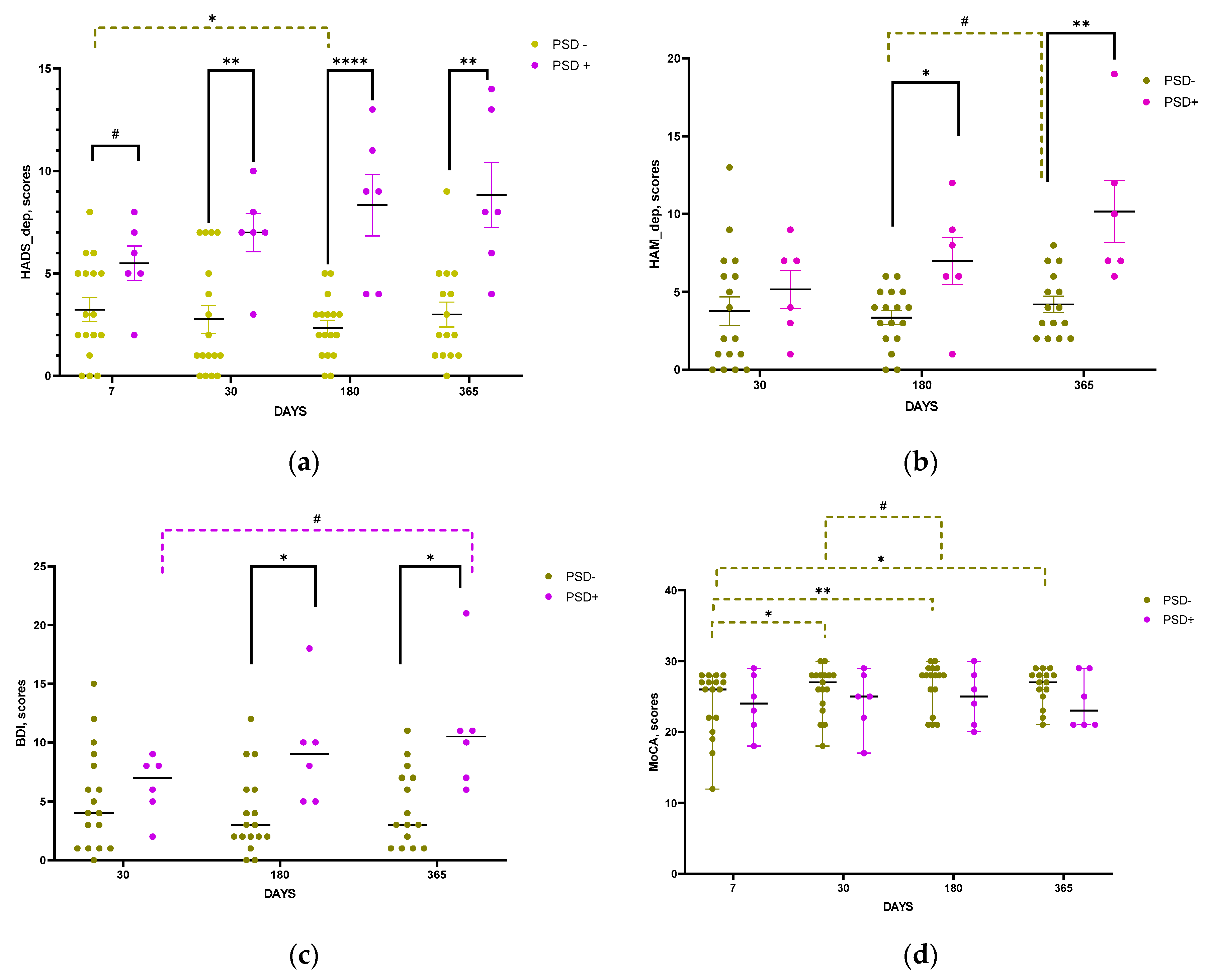
According to the HADS, HAM, and BDI scale scores, the psycho-emotional status of patients in the groups with and without PSD became significantly different from the 30th (HADS scale,
Figure 7a), and 180th day post-stroke (HAM and BDI scales,
Figure 7b, c). No significant differences between the groups were found according to MoCA scale, though positive dynamics in MOCA scores was observed in the group of patients without PSD starting from the 30th post-stroke day (
Figure 7d).
3.3.2. Biochemical Data
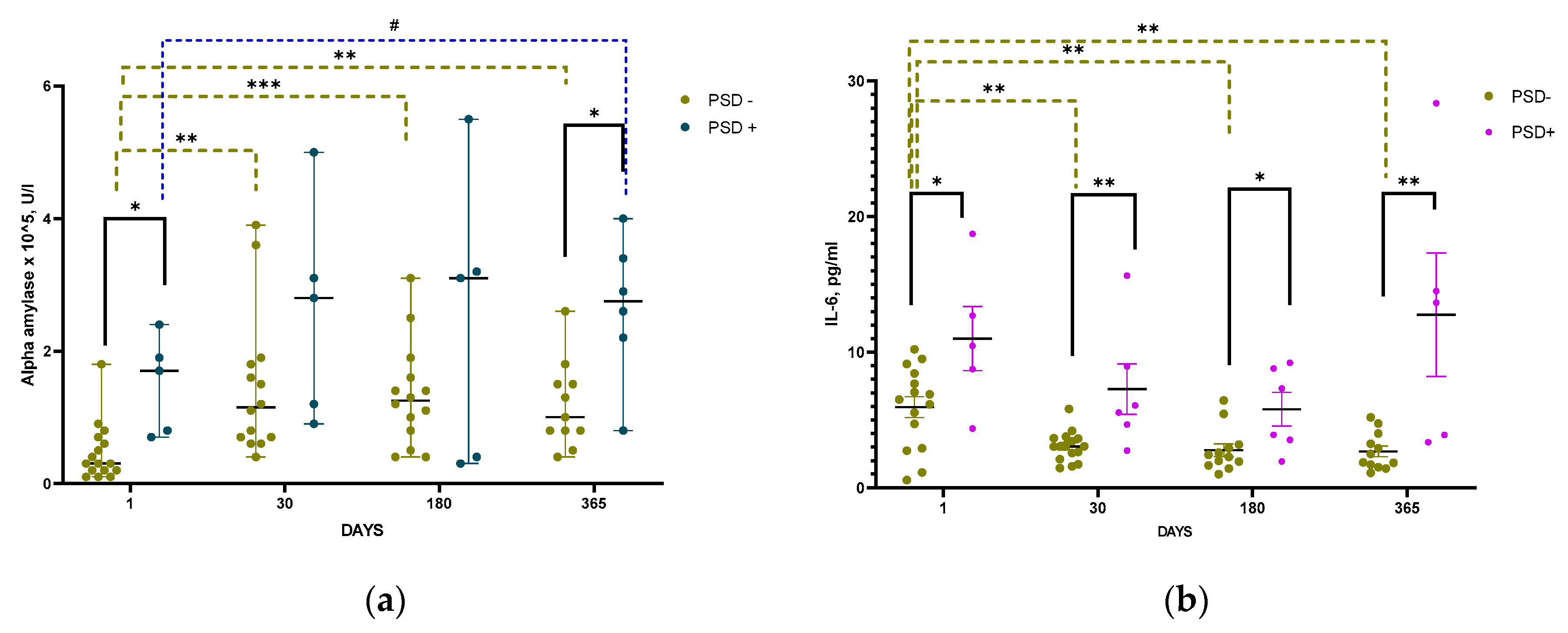
In patients with PSD, salivary α-amylase activity did not change significantly throughout the entire period of the study as compared with the first post-stroke. However, α-amylase activity was significantly higher in patients with PSD than in patients without PSD both on the 1st day and one year after IS. In patients without PSD, salivary α-amylase activity was lowest during the acute period of IS, significantly increased by the 30th post-stroke day after IS and did not change further (
Figure 8a).
The level of Il-6 in blood serum of patients with PSD was significantly higher than in patients without PSD during the entire follow-up period (
Figure 8b).
3.3.3. Morphometric Data
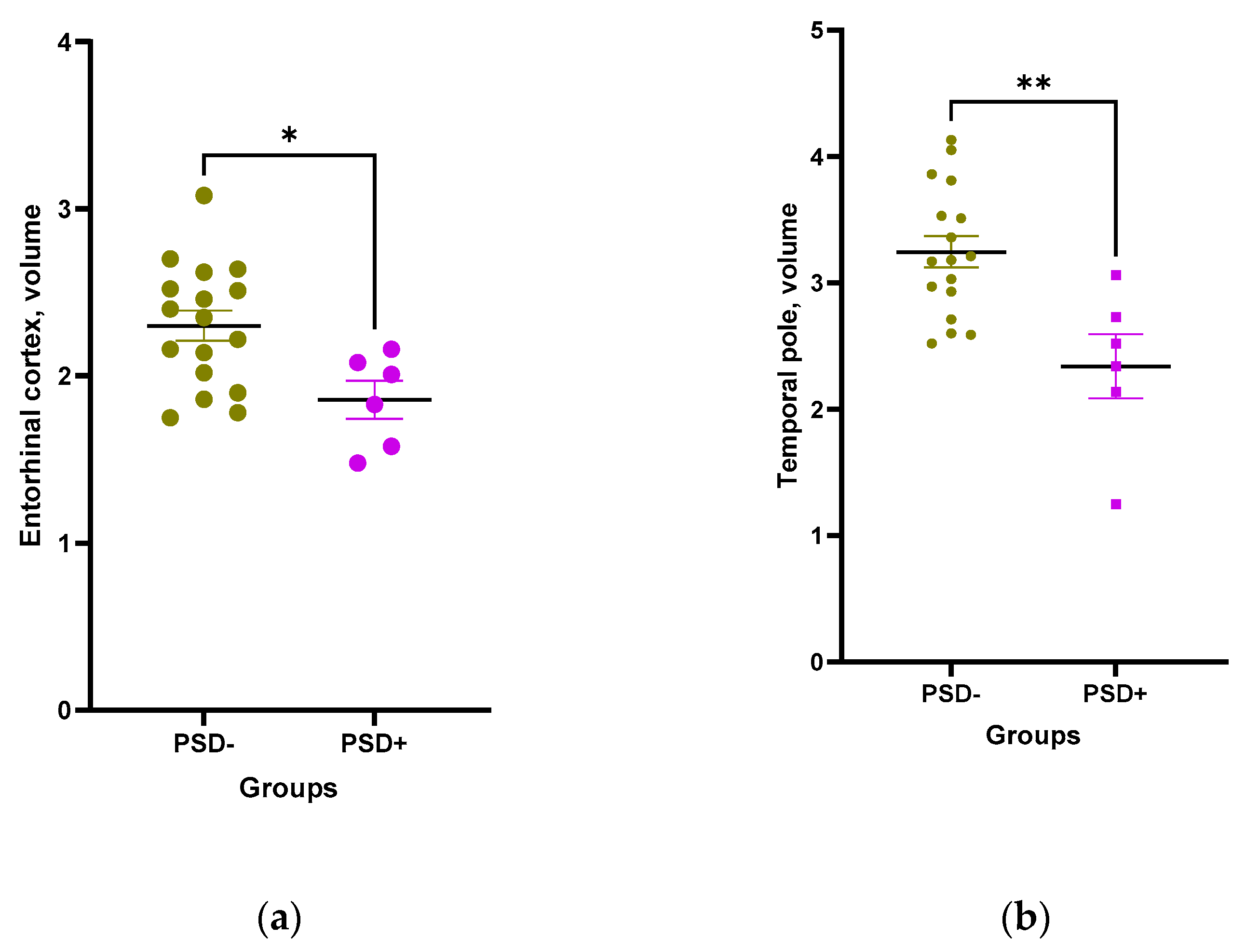
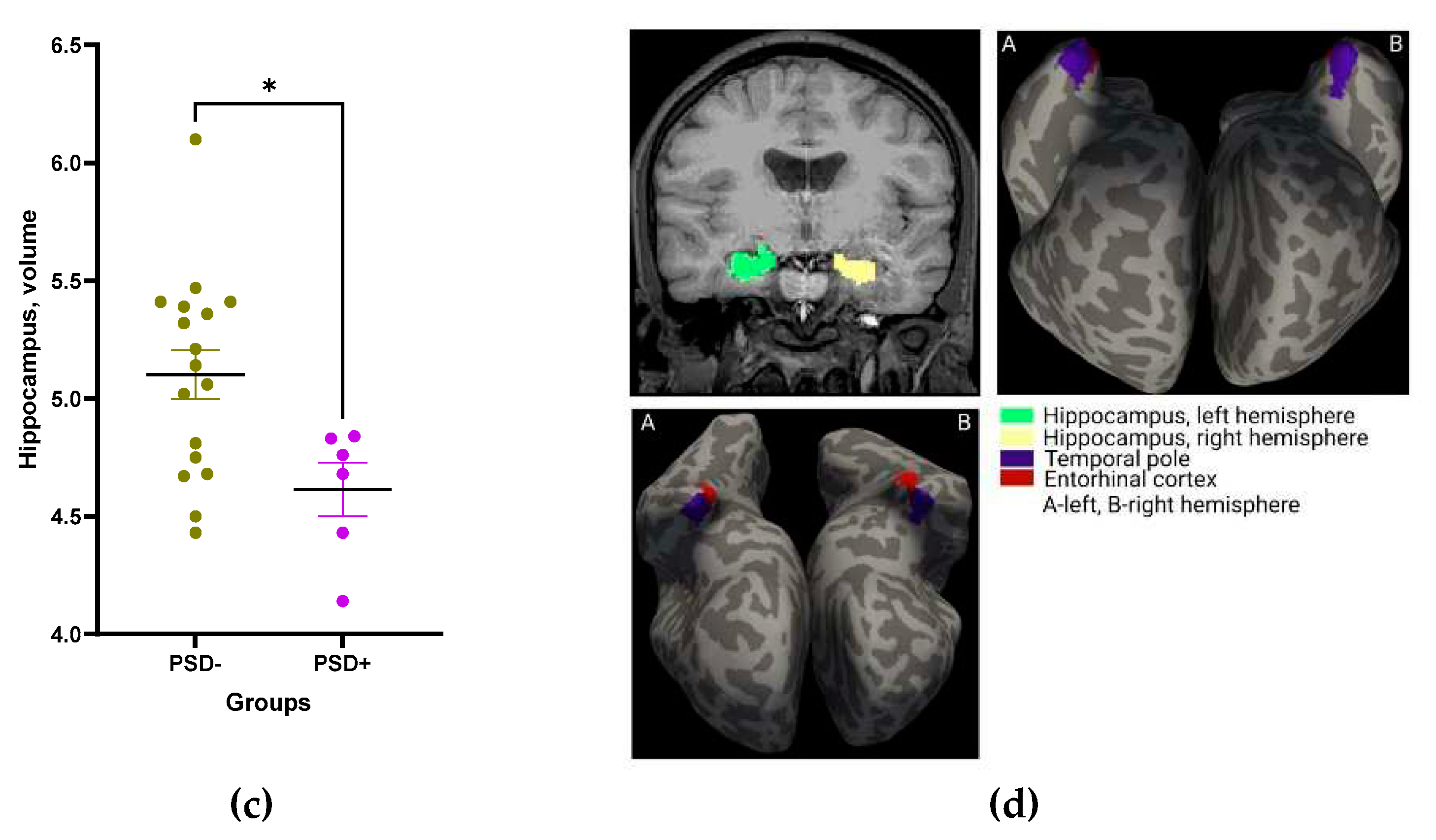
The relative volumes of entorhinal cortex, temporal pole, and hippocampus were significantly smaller in patients with PSD as compared to patients without PSD (
Figure 9 a-d).
Negative correlations of depression scores with temporal pole volume were found starting from 7th, with hippocampal volume from 30th, and with entorhinal cortex volume from 180th post-stroke days. Multiple logistic regression analysis showed that patients with reduced temporal pole volume (β0=10.9; β=-4.27; p=0.04; pseudoR2=0.42) and increased salivary α-amylase activity (β0=-3.55; β=2.68e-05; p=0.02, pseudoR2=0.4) had the highest probability of developing PSD (
Figure 10).
3.3.4. Correlations of Brain Structure Volumes with Biochemical Parameters
Table 3 represents the correlations of relative volumes of cortical and limbic structures of the brain with biochemical parameters studied. In the group of patients with PSD, a negative correlation between the relative volume of the entorhinal cortex and the level of cortisol in hair on day 1 post-stroke was revealed (R=-0.9; 0.04), while this correlation further disappeared and a positive correlation emerged a year after stroke (R=0.83; p=0.04). In the group of patients without PSD, a negative correlation was found between the relative volume of the entorhinal cortex and the level of hair cortisol on 30th post-stroke day (R=-0.64; p=0. 01); between relative temporal pole volume and salivary cortisol on day 1 (R=-0.5; p=0.049); and between relative hippocampal volume and hair cortisol levels on day 1 after SI (R=-0.48; p=0.0496). Positive correlations in this group of patients was also found between relative volume of the entorhinal cortex and α-amylase level on the 1st post-stroke day (R=0.51; 0.04) and with Il-6 a year after IS (R=0.67; p=0.02); and between relative volume of the temporal pole and α-amylase on the 1st post-stroke day (R=0.63; p=0.01) (
Table 3).
4. Discussion
4.1. Risk Factors for the Development of Post-Stroke Cognitive and Affective Disorders
It is well-known that there are many risk factors for the occurrence of an IS. Among them, major non-modifiable risk factors are age, gender, family history, ethnicity [
26,
27,
28], while major modifiable risk factors include arterial hypertension, cardiovascular disease, diabetes mellitus, psychological stress, smoking, etc. [
29,
30,
31,
32]. It has been repeatedly confirmed that modifiable risk factors are associated with systemic chronic inflammation [
33,
34], which can induce an increase in brain-blood barrier permeability [
35] and the development of subsequent neuroinflammation [
36,
37].
Systemic chronic inflammation is usually associated with activation of stress-realizing systems of the organism, HPA axis [
38] and SAMS [
39]. The hormones released upon HPA axis activation trigger multiple processes involved in inflammatory and immune interactions. For example, hypotalamic corticotropin releasing hormone induces activation of microglia; adrenocorticotropic hormone (ACTH) can inhibit immune cell activity and exert anti-inflammatory effects; glucocorticoids (cortisol, corticosterone) and mineralocorticoids bind to glucocorticoid and mineralocorticoid receptors, inducing signal transduction pathways involved in the central inflammatory response [
38]. Depending on the localization of these receptors in the brain, inflammation can be specifically triggered in selected brain regions [
40,
41], primarily affecting the most sensitive structures. Many studies have shown that the hippocampus, neocortex, amygdala, and hypothalamus are among selectively vulnerable to neuroinflammation brain regions easily affected by external signals related to peripheral systemic inflammation [
42,
43,
44].
The development of cognitive and affective disorders are closely related processes [
45,
46,
47]. Conditional allocating patients after IS to groups with PCI, without PCI, with PSD, and without PSD was performed to identify the most significant factors influencing the development of each pathology after IS. The morphometric analysis of cortical and limbic brain structures performed in the present study showed that patients with post-stroke cognitive and/or affective impairments had significantly reduced (as compared with IS patients without PCI or PSD) relative volumes of the temporal pole, hippocampus, entorhinal cortex, and submarginal gyrus in the first post-stroke days, soon after admission to the hospital. Neither the relative volumes of the structures, nor this difference are unlikely to be a consequence of IS per se due to the very short time period from the onset of IS when MRI was performed. Moreover, none of the structures included or was localized in the proximity of brain infarction area. The above considerations suggest that the volumes of selected structures were already reduced in patients by the time of IS onset in those patients which further developed either PCI or PSD. Comparative assessment of the levels and time course of biochemical indices reflecting the functioning of HPA axis, SAMS, and the inflammatory system in patients with and without PCI and PSD indicates different changes of these systems with time during post-stroke period. Correlations of the relative volumes of the cortical and limbic structures studied with the HPA axis, SAMS, and inflammation system indices revealed in this study may suggest their close relationship.
It was noted above that one of the leading comorbidities in the cohort studied was arterial hypertension (87%), which, on the one hand, is the most frequent risk factor of IS. On the other hand, hypertension is closely related to the development of systemic inflammation, which can alter the functioning of SAMS and HPA axis and indirectly favor neuroinflammation, primarily affecting cortical and limbic structures responsible for cognitive functions and emotions. The positive significant association of the development of PCI and PSD with arterial hypertension (r=0.62; p=0.049) revealed in this study using tetrachoric correlation suggests potential interconnections between brain mechanisms underlying hypertension and delayed post-stroke cognitive and affective disturbances.
The results of morphometric study of cortical and limbic brain structures together with the data on the level and changes in biochemical parameters during the follow-up period, as well as the correlations found between them suggest that dysregulation in the functioning of HPA axis, SAMS and inflammatory system appears to be more pronounced in patients with PCI and PSD.
4.1.1. Risk Factors for Cognitive Impairment Onset
The results obtained from the time course of hair cortisol changes suggested that dysregulation in HPA axis functioning was more pronounced in patients with subsequent PCI. Using the multiple logistic regression method, it was shown that the probability of developing PCI was higher in patients who had reduced volumes of the supramarginal gyrus (β0=3.41; β=-0.99; p=0.047) and temporal pole (β0=3. 41; β=-3.12; p=0.06), with increased hair cortisol levels on the 1st post-stroke day, which indirectly reflected the level of accumulated cortisol/stress load during the last month preceding the IS (β0=3.41; β=-0.05; p=0.08). This result confirms the data reported in [
6] that high hair cortisol concentrations predict worse cognitive outcome after IS. The positive correlation of the temporal pole volume with Il-6, found in patients with PCI, may indirectly indicate an involvement of inflammation in the onset of PCI.
4.1.2. Risk Factors for Post-Stroke Depression
Morphometric analysis showed that the relative volumes of the hippocampus, entorhinal cortex, and temporal pole were reduced in patients with subsequent PSD as compared with patients without post-stroke depression. The entorhinal cortex is the main input and output structure of the hippocampus, is involved in the regulation of subgranular neurogenesis [
12,
48]. The entorhinal cortex serves as one of the main sources of excitation in the dentate gyrus of the hippocampus, a major neurogenic niche providing for the generation and maturation of granule cells migrating to become part of the hippocampal circuits [
49]. Numerous studies indicate that impaired hippocampal neurogenesis is a hallmark of depression [
50,
51,
52]. A stimulation of the entorhinal cortex is accompanied by resetting of the hippocampal theta rhythm, which provides optimal induction of long-term potentiation contributing to fine coding of spatial information in the hippocampus [
53]. Thus, hippocampal neurogenesis and the functioning of the entorhinal cortex are closely related [
54]. Decline in their functions associated with the atrophy may increase the risk of developing depressive disorder [
55].
Application of the multiple logistic regression method demonstrated that patients with decreased temporal pole volume (β0=10.9; β=-4.27; p=0.04; pseudo R2=0.42) and increased salivary α-amylase activity (β0=-3.55; β=2.68e-05; p=0.02 pseudo R2=0.4) were more likely to develop PSD. Disorders in the temporal pole are associated with the development of a number of psychiatric and neurological diseases such as Alzheimer's disease, frontal temporal lobe epilepsy, schizophrenia, etc. [
56,
57,
58,
59]. The temporal pole is functionally connected with many areas of the cerebral cortex, thus allowing this pole to receive and process information from various sensory organs [
60]. A comparative analysis of the level and time course of biochemical parameters in patients with PSD revealed significantly increased salivary α-amylase activity, which indirectly indicates activation of SAMS, as well as Il-6 reflecting the inflammatory process. These results are consistent with our previously published data [
3].
4.2. Multiple Hit Hypothesis of PCI and PSD
A number of two-hit or multiple hit hypotheses of neurodegenerative diseases (Alzheimer's disease [
61,
62,
63,
64,
65,
66]; Parkinson's disease [
67,
68,
69]), depression [
70,
71], anxiety [
72], posttraumatic stress disorder [
73] and schizophrenia [
74,
75,
76,
77] suggest that a consecutive interaction between multiple (genetic and/or environmental) risk factors is needed to trigger the development of above diseases. Earlier events can prime the response to a successive negative challenge. For example, the two-hit model of depression hypothesizes that stress interacts with underlying (probably genetic) predispositions to produce a central nervous system that is primed to express psychopathology when faced stressful events later in life [
71]. Stress and infections early in life induce the activation of the hypothalamic-pituitary-adrenal (HPA) axis, alter the levels of neurotransmitters, neurotrophins and pro-inflammatory cytokines and affect the functions of microglia and oxidative stress. The multiple hit model of Parkinson's disease suggests that 'multiple hits' combining toxic stress (e.g. as a result of dopamine oxidation and/or mitochondrial dysfunction), accompanied by an inhibition of a neuroprotective response (e.g. loss of parkin function or stress-induced autophagy) may cause selective neuronal death [
68]. The multiple hit model of Alzheimer's disease explains how APOE4 induces vulnerable state of the brain and cerebral pathology through interconnected hits (neurodegeneration, neurovascular dysfunction, neuroinflammation, oxidative stress, endosomal trafficking destructions, disturbances in cellular metabolism, disrupted calcium homeostasis, impaired regulation of transcription) [
66].
Leveraging multi-hit approaches to the development of PCI and PSD we suggest that preceding IS multiple hits form the basis for post-stroke affective and cognitive disturbances. This first set of hits is, indeed, a continuum of endogenous and endogenous factors, forms a pre-pathological state of the brain. The hits, obviously involved in multiple crosstalk and acting in different combinations, leave the brain in a state where further insults will induce or exacerbate unfavorable processes. In this vulnerable status of the brain PCI and/or PSD may more easily take hold induced by even mild or moderate IS. Circulating hormones (including those related to HPA axis) are involved in orchestrating this crosstalk between the hits and their components. Pre-existing decreases in the volumes of cortical and limbic brain regions may reflect the effects of multiple hits that have formed the basis for the development of delayed post-stroke complications, PCI and PSD.
5. Conclusions
Based on the results obtained, it can be assumed that the development of cognitive and/or affective disorders in post-stroke patients is largely predetermined before the stroke by their potentially reduced adaptive response to stressors, leading to a more pronounced dysregulation of stress-realizing systems and the development of chronic inflammation damaging the most sensitive cortical and limbic structures of the brain. At the same time, the development of PCI is related to HPA dysregulation and associated with vulnerability of the temporal pole and submarginal gyrus. SAMS activation and pronounced inflammatory process leading to damage of the hippocampus, entorhinal cortex, and temporal pole may play a significant role in the development of PSD.
6. Limitations
One of the major limitations of this study is the rather small cohort of SI patients, in particular because of a prolonged follow-up period. Another limitation of the study is the absence of the MRI data for pre-frontal cortex which was caused by technical issues. The third important limitation is the assumption that the volumes of brain regions did not significantly change within several days between admission and MRI measurements. The impossibility of having the baseline (before the IS) MRI and laboratory data is an unavoidable limitation a prospective study design.
Author Contributions
Conceptualization, N.V.G.; methodology, T.A.D., E.E.V., and M.Y.Z.; validation, T.A.D., M.Y.Z., and N.V.I.; visualization, M.Y.Z., and N.V.I., formal analysis, M.Y.Z.; investigation, E.E.V., N.N.E., T.A.D., and N.V.I.; resources, A.B.G.; data curation, T.A.D.; writing—original draft preparation, T.A.D., and M.Y.Z.; writing—review and editing, N.V.G.; supervision, A.B.G., and N.V.G.; project administration, T.A.D.; funding acquisition, A.B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RSF grant # 21-75-20112.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Moscow Research and Clinical Centre for Neuropsychiatry (protocol #42 23.08.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Primary datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We greatly appreciate valuable advices and help provided by Irina Samotaeva.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
BDI, Beck Depression Inventory; HADS, Hospital Anxiety and Depression Scale; HAM, Hamilton Rating Scale for Depression; HPA axis, hypothalamic-pituitary-adrenal axis; IL-6, interleukin-6; IS, ischemic stroke; MoCA – Montreal Cognitive Assessment; MR, magnetic resonance; MRI, magnetic resonance imaging; NIHSS, The National Institutes of Health Stroke Scale; PCI, post-stroke cognitive impairment; PSD, post-stroke depressive disorder; SAMS , sympathoadrenal medullary system.
References
- He, A.; Wang, Z.; Wu, X.; Sun, W.; Yang, K.; Feng, W.; et al. Incidence of post-stroke cognitive impairment in patients with first-ever ischemic stroke: a multicenter cross-sectional study in China. Lancet Regional Health – Western Pacific 2023, 33, 100687. [Google Scholar] [CrossRef] [PubMed]
- Sexton, E.; McLoughlin, A.; Williams, D.J.; Merriman, N.A.; Donnelly, N.; et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur Stroke J. 2019, 4, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Zhanina, M.Y.; Druzhkova, T.A.; Yakovlev, A.A.; Vladimirova, E.E.; Freiman, S.V.; Eremina, N.N.; Guekht, A.B.; Gulyaeva, N.V. Development of post-stroke cognitive and depressive disturbances: associations with neurohumoral indices. Curr. Issues Mol. Biol. 2022, 44, 6290–6305. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V.; Onufriev, M.V.; Moiseeva, Y.V. Ischemic Stroke, Glucocorticoids, and Remote Hippocampal Damage: A Translational Outlook and Implications for Modeling. Front Neurosci. 2021, 15, 781964. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Biochemical Mechanisms and Translational Relevance of Hippocampal Vulnerability to Distant Focal Brain Injury: The Price of Stress Response. Biochemistry (Mosc). 2019, 84, 1306–1328. [Google Scholar] [CrossRef] [PubMed]
- Assayag, E.B.; Tene, O.; Korczyn, A.D.; Shopin, L.; Auriel, E.; et al. High hair cortisol concentrations predict worse cognitive outcome after stroke: Results from the TABASCO prospective cohort study. Psychoneuroendocrinol. 2017, 82, 133–139. [Google Scholar] [CrossRef]
- Pochigaeva, K.; Druzhkova, T.; Yakovlev, A.; Onufriev, M.; Grishkina, M.; Chepelev, A.; Guekht, A.B.; Gulyaeva, N.V. Hair cortisol as a marker of hypothalamic-pituitary-adrenal Axis activity in female patients with major depressive disorder. Metab. Brain Dis. 2017, 32, 577–583. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional neurochemistry of the ventral and dorsal hippocampus: stress, depression, dementia and remote hippocampal damage. Neurochem Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Glucocorticoids Orchestrate Adult Hippocampal Plasticity: GrowthPoints and Translational Aspects. Biochemistry (Mosc). 2023, 88, 565–589. [Google Scholar] [CrossRef]
- Patrick, K.L.; Bell, S.; Patrick, L.; Weindel, C.G.; Watson, R.O. Exploring the "Multiple-Hit Hypothesis" of Neurodegenerative Disease: Bacterial Infection Comes Up to Bat. Front. Cell. Infect. Microbiol. 2019, 9, 138. [Google Scholar] [CrossRef]
- Cattane, N.; Vernon, A.C.; Borsini, A.; Scassellati, C.; Endres, D.; Capuron, L.; Tamouza, R.; Benros, M.E.; Leza, J.C.; et al. European College of Neuropsychopharmacology (ECNP) ImmunoNeuroPsychiatry Thematic Working Group. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: Molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur. Neuropsychopharmacol. 2022, 58, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Witter, M.P.; Doan, T.P.; Jacobsen, B.; Nilssen, E.S.; Ohara, S. Architecture of the entorhinal cortex a review of entorhinal anatomy in rodents with some comparative notes. Front. Syst. Neurosci. 2017, 11, 2017. [Google Scholar] [CrossRef] [PubMed]
- Fransen, E. Functional role of entorhinal cortex in working memory processing. Neural Netw. 2005, 18, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Janal, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature. 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Gazzaniga, M.; Ivry, R.; Mangun, G. Cognitive Neuroscience: The Biology of the Mind. Norton Press, London. 2009, 768.
- Herfurth, K.; Kasper, B.; Schwarz, M.; Stefan, H.; Pauli, E. Autobiographical memory in temporal lobe epilepsy: role of hippocampal and temporal lateral structures. Epilepsy Behav. 2010, 19, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Piguet, O. The pivotal role of semantic memory in remembering the past and imagining the future. Front. Behav. Neurosci. 2013, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, T.; Shenton, M.E.; Salisbury, D.F.; Dickey, C.C.; Kasai, K.; Toner, S.K.; et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Amer. J.Psychiatry 2004, 161, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatry Clin Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Lyden, P. Using the National Institutes of Health Stroke Scale: A Cautionary Tale. Stroke. 2017, 48, 513–519. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960, 23(1), 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system; current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef]
- Arboix, A.; Garcia-Eroles, L.; Comes, E.; Oliveres, M.; Targa, C.; et al. Importance of cardiovascular risk profile for in-hospital mortality due to cerebral infarction. Rev. Esp. Cardiol. 2008, 61, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M. Genetics of ischaemic stroke. Lancet Neurol. 2007, 6, 149–161. [Google Scholar] [CrossRef]
- Furie, K.L.; Kasner, S.E.; Adams, R.J.; Albers, G.W.; Bush, R.L. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the americsn heart association/American stroke association. Practice Guideline. 2011, 42, 227–276. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G. Prognostic value of long-term blood pressure variability. Hypertension 2011, 57, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Milian, M.; Oliveres, M.; Garcia-Eroles, L.; Massons, J. Impact of female gender on prognosis in type 2 diabetic patients with ischemic stroke. Eur. Neurol. 2006, 56, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Alio, J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Alvarado, G.; Dominguez-Salazar, E.; Pavon, L.; Velazquez-Moctezuma, J.; Gomez-Gonzalez, B. Blood-Brain Barrier Disruption Induced by chronic sleep loss; Low-Grade inflammation may be the link. J. Immunol. Res. 2016, 4576012. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and cardiovascular diseases: The most recent findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Xiao, L.; Harrison, D.G. Inflammation in Hypertension. Can. J. Cardiol. 2020, 36, 635–647. [Google Scholar] [CrossRef]
- Elwood, E.; Lim, Z.; Naveed, H.; Galea, I. The effect of systemic inflammation on human brain barrier function. Brain. Behav. Immun. 2017, 62, 35–40. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef]
- Aktas, O.; Ullrich, O.; Infante-Duarte, C.; Nitsch, R.; Zipp, F. Neuronal damage in brain inflammation. Arch. Neurol. 2007, 64, 185–189. [Google Scholar] [CrossRef]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C.A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233(4764), 652–654. [Google Scholar] [CrossRef] [PubMed]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, F.; Hassan, A.M.; Farzi, A.; Jain, P.; Schuligoi, R.; Holzer, P. Dextran sulfate sodium-induced colits alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci. Rep. 2015, 5, 9970. [Google Scholar] [CrossRef]
- Riazi, K.; Galic, M.A.; Kentner, A.C.; Reid, A.Y.; Sharkey, K.A.; et al. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J. Neurosci. 2015, 35, 4942–4952. [Google Scholar] [CrossRef]
- Zonis, S.; Pechnick, R.N.; Ljubimov, V.A.; Mahgerefteh, M.; Wawrowsky, K.; et al. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflammation 2015, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Peppas, S.; Pansieri, C.; Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; et al. The Brain-Gut Axis: Psychological functioning and inflammatory bowel diseases. J. Clin. Med. 2021, 10, 377. [Google Scholar] [CrossRef]
- Babkair, L.A. Risk Factors for Poststroke Depression: An Integrative Review. J. Neurosci. Nurs. 2017, 49, 49,73–84. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta. 2016, 1862, 915–925. [Google Scholar] [CrossRef]
- Li, W.; Ling, S.; Yang, Y.; Hu, Z.; Davies, H.; Fang, M. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol. Lett. 2014, 35, 104–109. [Google Scholar]
- Li, Y.; Mu, Y.; Gage, F. H. Chapter 5 Development of neural circuits in the adult hippocampus. Current Topics in Development Biology, Academic Press. 2009, 87, 149–174. [CrossRef]
- Ge, S.; Sailor, K.A.; Ming, G.; Song, H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J. Physiol 2008, 586, 3759–3765. [Google Scholar] [CrossRef]
- Wang, H.; Warner-Schmidt, J.; Varela, S.; Enikolopov, G.; Greengard, P.; et al. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc. Natl. Acad. Sci. USA. 2015, 112, 9745–9750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Lee, M.M.; Reif, A.; Schmitt, A.G. Major depression: a role for hippocampal neurogenesis? Curr. Top. Behav. Neurosci. 2013, 14, 153–179. [Google Scholar] [CrossRef]
- Buzsaki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C. The entorhinal cortex and adult neurogenesis in major depression. Int. J. Mol. Sci. 2021, 22, 11725. [Google Scholar] [CrossRef]
- Kino, T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front. Physiol. 2015, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; et al. TDP-43 Pathology in Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Erp, T.G.M.; Walton, E.; Hibar, D.P.; Schmaal, L.; Jiang, W.; et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry 2018, 84, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, S.R.B.; Barbosa, J.H.O.; Rondinoni, C.; Dos Santos, A.C.; Salmon, C.E.G.; et al. Neuro-degeneration profile of Alzheimer’s patients: A brain morphometry study. Neuroimage 2017, 15, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Campo, P.; Poch, C.; Toledano, R.; Igoa, J.M.; Belinchon, M.; et al. Visual object naming in patients with small lesions centered at the left temporopolar region. Brain Struct Funct. 2016, 221(1), 473–485. [Google Scholar] [CrossRef] [PubMed]
- Herlin, B.; Navarro, V.; Dupont, S. The temporal pole: From anatomy to function – A literature appraisal. J. Chem. Neuroanat. 2021, 113, 101925. [Google Scholar] [CrossRef]
- Zhu, X.; Raina, A.K.; Perry, G.; Smith, M.A. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 2004, 3, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.G.; Perry, G.; Smith, M.A. Alzheimer disease, the two-hit hypothesis: an update. Biochim. Biophys. Acta 2007, 1772, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Bajić, V.P.; Spremo-Potparevic, B.; Casadesus, G.; Zhu, X.; Smith, M.A.; Lee, H.G. Review: cell cycle aberrations and neurodegeneration. Neuropathol. Appl. Neurobio 2010, 36, 157–163. [Google Scholar] [CrossRef]
- Moh, C.; Kubiak, J.Z.; Bajic, V.P.; Zhu, X.; Smith, M.; Lee, H.G. Cell cycle deregulation in the neurons of Alzheimer's disease. Results Probl. Cell. Differ. 2011, 53, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Custodia, A.; Ouro, A.; Romaus-Sanjurjo, D.; Pías-Peleteiro, J.M.; de Vries, H.E.; Castillo, J.; Sobrino, T. Endothelial Progenitor Cells and Vascular Alterations in Alzheimer's Disease. Front. Aging Neurosci 2022, 13, 811210. [Google Scholar] [CrossRef] [PubMed]
- Steele, O.G.; Stuart, A.C.; Minkley, L.; Shaw, K.; Bonnar, O.; Anderle, S.; Penn, A.C.; Rusted, J.; Serpell, L.; Hall, C.; et al. A multi-hit hypothesis for an APOE4-dependent pathophysiological state. Eur. J. Neurosci. 2022, 56, 5476–5515. [Google Scholar] [CrossRef]
- Carvey, P.M.; Punati, A.; Newman, M.B. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 2006, 15, 239–250. [Google Scholar] [CrossRef]
- Sulzer, D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007, 30, 244–250. [Google Scholar] [CrossRef]
- Cabezudo, D.; Baekelandt, V.; Lobbestael, E. Multiple-Hit Hypothesis in Parkinson's Disease: LRRK2 and Inflammation. Front. Neurosci. 2020, 14, 376. [Google Scholar] [CrossRef]
- Jacobs, R.H.; Orr, J.L.; Gowins, J.R.; Forbes, E.E.; Langenecker, S.A. Biomarkers of intergenerational risk for depression: a review of mechanisms in longitudinal high-risk (LHR) studies. J. Affect. Disord. 2015, 175, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Worlein, J.M. Nonhuman primate models of depression: effects of early experience and stress. ILAR J. 2014, 55, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Catuzzi, J.E.; Beck, K.D. Anxiety vulnerability in women: a two-hit hypothesis. Exp. Neurol. 2014, 259, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; James, L.M.; Christova, P.; Engdahl, B.E. A two-hit model of the biological origin of posttraumatic stress disorder (PTSD). J. Ment. Health Clin. Psychol. 2019, 2, 9–14. [Google Scholar] [CrossRef]
- Bayer, T.A.; Falkai, P.; Maier, W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the "two hit hypothesis". J. Psychiatr. Res. 1999, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, K.A.; Kusnecov, A.W.; Silverstein, S.M. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 2014, 38, 72–93. [Google Scholar] [CrossRef]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; et al. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neurosci.Biobehav.Rev. 2016, 65, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.; Barnes, S.A.; Markou, A.; Piercy, C.; Podda, G.; Neill, J.C. Postnatal Phencyclidine (PCP) as a Neurodevelopmental Animal Model of Schizophrenia Pathophysiology and Symptomatology: A Review. Curr. Top. Behav. Neurosci. 2016, 29, 403–428. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Design of the study. NIHSS, National Institutes of Health Stroke Scale; HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment; HAM, Hamilton Rating Scale for Depression; BDI, Beck Depression Inventory; MRI, Magnetic Resonance Imaging; 1-365, days after IS.
Figure 1.
Design of the study. NIHSS, National Institutes of Health Stroke Scale; HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment; HAM, Hamilton Rating Scale for Depression; BDI, Beck Depression Inventory; MRI, Magnetic Resonance Imaging; 1-365, days after IS.
Figure 2.
Time course of changes in NIHSS scores in groups of patients without PCI (a), with PCI (b), without PSD (c) and with PSD (d). Statistical differences between time points were assessed by Friedman test with post-hoc Dunn test: *p<0.05, **p<0.01, ***p<0.001. Data of graphs are presented as median with range.
Figure 2.
Time course of changes in NIHSS scores in groups of patients without PCI (a), with PCI (b), without PSD (c) and with PSD (d). Statistical differences between time points were assessed by Friedman test with post-hoc Dunn test: *p<0.05, **p<0.01, ***p<0.001. Data of graphs are presented as median with range.
Figure 3.
Time course of changes in the scores of psychometric scales. The groups with and without PCI are compared at each time point and the dynamics for each group is assessed. Scales used: MoCA (a), HADS (b), HAM (c), BDI (d). Statistical differences between groups were assessed by Mann-Whitney test for MoCA, HADS, HAM and by unpaired t-test for BDI: #p<0.1, *p<0.05, **p<0.01. Statistical differences of changes with time within the groups were assessed by Wilcoxon test for MoCA, HADS, HAM and paired t-test for BDI: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data on graphs a, b, and c are presented as median with range. Data of graph d are presented as mean with SEM.
Figure 3.
Time course of changes in the scores of psychometric scales. The groups with and without PCI are compared at each time point and the dynamics for each group is assessed. Scales used: MoCA (a), HADS (b), HAM (c), BDI (d). Statistical differences between groups were assessed by Mann-Whitney test for MoCA, HADS, HAM and by unpaired t-test for BDI: #p<0.1, *p<0.05, **p<0.01. Statistical differences of changes with time within the groups were assessed by Wilcoxon test for MoCA, HADS, HAM and paired t-test for BDI: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data on graphs a, b, and c are presented as median with range. Data of graph d are presented as mean with SEM.
Figure 4.
Time course of hair cortisol in groups with and without PCI. Statistical differences were assessed by Mann-Whitney test. #p<0.1, *p<0.05, **p<0.01.Data are presented as median with range.
Figure 4.
Time course of hair cortisol in groups with and without PCI. Statistical differences were assessed by Mann-Whitney test. #p<0.1, *p<0.05, **p<0.01.Data are presented as median with range.
Figure 5.
Relative volumes of the temporal pole (a) and supramarginal gyrus (b) in groups with and without PCI. Statistical differences between groups were assessed by unpaired t-test: *p<0.05. All data are presented as mean with SEM. Visualization of temporal pole and supramarginal gyrus (c).
Figure 5.
Relative volumes of the temporal pole (a) and supramarginal gyrus (b) in groups with and without PCI. Statistical differences between groups were assessed by unpaired t-test: *p<0.05. All data are presented as mean with SEM. Visualization of temporal pole and supramarginal gyrus (c).
Figure 6.
Multiple logistic regression and ROC analysis of predictors influencing the development of PCI.
Figure 6.
Multiple logistic regression and ROC analysis of predictors influencing the development of PCI.
Figure 7.
Time course of psycho-emotional status in groups with and without PSD according to HADS (a), HAM (b), BDI (c), and MoCA scores (d). Statistical differences between groups were assessed by unpaired t-test for HADS and HAM scales and by Mann-Whitney test for BDI and MoCA: #p<0.1, *p<0.05, **p<0.01, ****p<0.001. Statistical differences in the time course within same groups were assessed by paired t-test for HADS, HAM and by Wilcoxon test for BDI and MoCA scales: #p<0.1, *p<0.05, **p<0.01. Data on graphs a, b are presented as mean with SEM. Data on graphs c, d are presented as median with range.
Figure 7.
Time course of psycho-emotional status in groups with and without PSD according to HADS (a), HAM (b), BDI (c), and MoCA scores (d). Statistical differences between groups were assessed by unpaired t-test for HADS and HAM scales and by Mann-Whitney test for BDI and MoCA: #p<0.1, *p<0.05, **p<0.01, ****p<0.001. Statistical differences in the time course within same groups were assessed by paired t-test for HADS, HAM and by Wilcoxon test for BDI and MoCA scales: #p<0.1, *p<0.05, **p<0.01. Data on graphs a, b are presented as mean with SEM. Data on graphs c, d are presented as median with range.
Figure 8.
Time course of α-amylase in saliva (a) and Il-6 in blood serum (b). psycho-emotional status in groups with and without PSD: Statistical differences between groups were assessed by Mann-Whitney test for α-amylase in saliva(a) and cortisol in hair and by unpaired t-test for Il-6 in serum blood: *p<0.05, **p<0.01. Statistical differences of dynamics were assessed by Wilcoxon test for α-amylase in saliva and cortisol in hair and by paired t-test for Il-6 in serum blood: #p<0.1, *p<0.05, **p<0.01, ***p<0.001 Data on graphs a, b are presented as median with range. Data on graph c are presented as mean with SEM.
Figure 8.
Time course of α-amylase in saliva (a) and Il-6 in blood serum (b). psycho-emotional status in groups with and without PSD: Statistical differences between groups were assessed by Mann-Whitney test for α-amylase in saliva(a) and cortisol in hair and by unpaired t-test for Il-6 in serum blood: *p<0.05, **p<0.01. Statistical differences of dynamics were assessed by Wilcoxon test for α-amylase in saliva and cortisol in hair and by paired t-test for Il-6 in serum blood: #p<0.1, *p<0.05, **p<0.01, ***p<0.001 Data on graphs a, b are presented as median with range. Data on graph c are presented as mean with SEM.
Figure 9.
Relative volumes of the entorhinal cortex (a), temporal pole (b), and hippocampus (c) in groups of patients with and without PSD. Statistical differences between groups were assessed by unpaired t-test: *p<0.05, **p<0.01. All data are presented as mean with SEM. Visualization of the entorhinal cortex, temporal pole, and hippocampus (d).
Figure 9.
Relative volumes of the entorhinal cortex (a), temporal pole (b), and hippocampus (c) in groups of patients with and without PSD. Statistical differences between groups were assessed by unpaired t-test: *p<0.05, **p<0.01. All data are presented as mean with SEM. Visualization of the entorhinal cortex, temporal pole, and hippocampus (d).
Figure 10.
Multiple logistic regression and ROC analysis of the effect of changes in the volume of the temporal pole and level of α-amylase on the development of PSD.
Figure 10.
Multiple logistic regression and ROC analysis of the effect of changes in the volume of the temporal pole and level of α-amylase on the development of PSD.
Table 1.
NIHSS scores in patients with and without post-stroke cognitive impairment, with and without post-stroke depressive disorder.
Table 1.
NIHSS scores in patients with and without post-stroke cognitive impairment, with and without post-stroke depressive disorder.
| Days after IS |
Patients with PCI, Scores (Mean±SD), N =13 |
Patients without PCI, Scores (Mean±SD),
N =10 |
p-Value |
Patients with PSD, Scores (Mean±SD), N =6 |
Patients without PSD, Scores (Mean±SD),
N =17 |
p-Value |
| 1 |
5.2±2.2 |
7.3±6,5 |
0.93 |
8.2±7.0 |
4.4±1.7 |
0.33 |
| 3 |
2.9±2.2 |
4.0±2,2 |
0.23 |
4.2±1.3 |
2.6±2.1 |
0.15 |
| 7 |
2.7±2.3 |
2.6±2,1 |
0.98 |
3.8±1.5 |
2.4±2.3 |
0.1 |
| 14 |
2.6±2.3 |
2.6±2.1 |
0.98 |
3.7±1.5 |
2.4±2.3 |
0.1 |
| 30 |
1.5±1.6 |
2.0±1.8 |
0.52 |
1.8±1.5 |
1.8±1.9 |
0.75 |
| 180 |
1.2±1.4 |
1.0±0.8 |
0.97 |
1.2±0.9 |
1.1±1.4 |
0.69 |
| 365 |
1.4±1.4 |
0.6±0.5 |
0.20 |
1.2±0.8 |
0.8±1.2 |
0.37 |
Table 2.
Correlations between the relative volumes of morphological structures with biochemical parameters for patients with an without PCI.
Table 2.
Correlations between the relative volumes of morphological structures with biochemical parameters for patients with an without PCI.
| Variables |
Patients with PCI
(R; p-Value; n) |
Patients without PCI
(R; p-Value; n) |
| Temporal pole & Il-6 day 1 post-stroke |
-0.62; 0.04; 11 |
n.s. |
| Temporal pole& Il-6 day 30 post-stroke |
-0.66; 0.02; 12 |
n.s. |
| Temporal pole & α-amylase day 365 post-stroke |
-0.76; 0.01; 10 |
n.s. |
| Temporal pole & hair cortisol day 1 post-stroke |
n.s. |
-0.68; 0.04; 9 |
| Temporal pole & hair cortisol day 30 post-stroke |
n.s. |
-0.7; 0.04; 9 |
| Temporal pole & salivary cortisol day 30 post-stroke |
n.s. |
0.87; 0.003; 9 |
Table 3.
Correlations between the volumes of morphological structures of the with biochemical parameters (patients with an without PSD).
Table 3.
Correlations between the volumes of morphological structures of the with biochemical parameters (patients with an without PSD).
| Variables |
Patients with PSD
(R; p-Value; n) |
Patients without PSD
(R; p-Value; n) |
| Entorhinal cortex & hair cortisol day 1 post-stroke |
-0.9; 0.04; 5 |
n.s. |
| Entorhinal cortex & hair cortisol day 365 post-stroke |
0.83; 0.04; 6 |
n.s. |
| Entorhinal cortex & hair cortisol day 30 post-stroke |
n.s. |
-0.64; 0.01; 17 |
| Hippocampus & hair cortisol day 1 post-stroke |
n.s. |
-0.48; 0.0496; 17 |
| Temporal pole & salivary cortisol day 1 post-stroke |
n.s. |
-0.5; 0.049; 16 |
| Temporal pole & α- amylase day 1 post-stroke |
n.s. |
0.63; 0.01; 16 |
| Entorhinal cortex & α-amylase day 1 post-stroke |
n.s. |
0.51; 0.04; 16 |
| Entorhinal cortex & Il-6 day 365 post- stroke |
n.s. |
0.67; 0.02; 12 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).