1. Introduction
The modern science of nutrition, starting from its origins more than one century and a half ago, initially has been strictly confined to the concepts of dietary requirements. These included energy and single macronutrients, as well as micronutrients such as minerals, microelements, and vitamins. The main focus was directed towards the prevention and treatment of nutrient deficiency diseases such as scurvy because of inadequate Vitamin C intake. Determining nutrient reference intakes designed to promote optimal health and prevent chronic diseases across a diverse population group is a much more difficult question.
However, the landscape began to change in the late 1980s of the last century, other main factors entered nutrition science, such as functional nutrition and personalized nutrition entered the field. Additionally, obesity emerged as a significant concern, signaling the potential for future non-communicable disorders. Within a few years, obesity escalated into a global pandemic, whose causes cannot be simply related to the algebraic calculation of [energy input–energy output] [
1].
In the last decade, other factors have added complexity to the field of nutrition science. These include the rapid climate change connected to CO2 emissions and the necessity for policies that promote sustainability at both individual and global levels. The need for shorter dietary food supply chains has also become increasingly evident. This evolving scenario has been further dramatically deranged by the Covid-2 pandemic, further worsening the gap related to social inequalities [
2].
Within this complex setting, partly unexpected, the approach to nutrition science is also rapidly changing. Dietary needs are now being assessed in the context of local dietary patterns (sustainable, economically affordable, healthy on an epidemiologic point of view) [
3,
4].
The concept of the benefits (or damages) of components of the every-day diet has shifted from focusing on individual compounds to considering the food matrix as a whole [
5].
As a consequence, new evidence [correctly designed and interpreted], the revision of dietary guidelines in the light of “localized” dietary patterns, and using more comprehensive frameworks, are needed to elaborate messages able to reach consumers in a positive, and holistic, way [
6].
This manuscript serves to summarize the presentations made during the 10th N&G conference [
7], encapsulating key points and advancements in the field.
2. The Role of Evidence in Nutrition Research
Renowned researcher John P. Ioannidis from Stanford University School of Medicine has raised doubts about the reliability of most published research findings, suggesting that they are likely to be false [
8]. These concerns are further supported by a recent review published in BMJ [
9], which highlights the lack of independence and transparency in formula trials and the presence of biased outcomes resulting from selective reporting. Therefore, while nutrition research plays a vital role in shaping dietary guidelines, public health policies, and individual dietary choices, there is growing concern about the reliability and validity of research findings in this field. This section aims to explore some of the challenges in nutrition research and strategies to improve the quality of evidence.
2.1. Bias in Nutrition Research
One of the key challenges in nutrition research is the presence of bias. This term refers to any factor that can cause a study to produce results that are systematically different from the true effect of the intervention or exposure being studied [
10]. Biases can occur in various ways when studying healthcare effects. For example, there may be differences between the groups being compared (selection bias), variations in the care provided or exposure to other factors (performance bias), people dropping out or being excluded from the study (attrition bias), or biases in how outcomes are measured (detection bias). Other important biases include publication bias that skews results towards positive findings, and funding bias that can influence outcomes based on vested interests [
11].
Confounding factors also pose challenges in nutrition research. These factors are additional variables that can impact the relationship between an exposure and an outcome, leading to misleading or inaccurate conclusions [
12]. For instance, when studying the health effects of a specific nutrient, failing to account for other dietary or lifestyle factors that could confound the observed associations can result in erroneously attributing causation when there may only be a correlation. Maternal diet and childhood obesity provide an example where studies must consider confounders like genetics, postnatal feeding practices, and family lifestyle choices [
13]. Long-term follow-up and attrition rates present significant challenges in nutrition research due to their potential to introduce bias and affect the validity of results. Participant dropout during a study is inevitable and carries various consequences, including reduced statistical power, the introduction of bias, and limitations in the generalizability of findings. When it comes to long-term randomized controlled trials (RCTs) and prospective studies, there is an ongoing debate regarding the acceptable level of loss to follow-up. Some experts in Evidence-Based Medicine (EBM) suggest an 80% cut-off point [
14], indicating that studies with a higher attrition rate may be less reliable. However, others argue against using fixed benchmarks to determine acceptable follow-up rates, deeming it unnecessary and unhelpful as it may hinder future research in the field. Instead, it is crucial to approach attrition and its effects by considering the specific design and objectives of each study individually [
15,
16].
Ethical considerations also play a significant role in nutrition research. Randomized controlled trials are considered the gold standard for testing the safety and efficacy of novel nutritional interventions. However, ethical concerns arise when attempting to randomize infants to breastfeeding or formula-feeding groups [
17]. While exclusive breastfeeding is considered optimal, it is not possible or ethical to randomize infants in this manner [
18].
2.2. Improving the Quality of Evidence
Efforts to improve the quality of evidence in nutrition research can be supported through various strategies. One notable initiative in this regard is the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network [
19]. EQUATOR serves as an organization that promotes transparent and accurate reporting of research studies. A key aspect of their approach is advocating for the utilization of rigorous study designs and methodologies. This involves careful planning and execution of studies, employing robust methodologies that align with the specific clinical questions being investigated. One significant contribution of EQUATOR is the development of reporting guidelines. These standards aim to ensure that manuscripts encompass all essential details pertaining to the study's design, execution, and analysis. Authors are now required by editors to adhere to these standards when submitting their work for publication. Several noteworthy reporting guidelines have emerged as part of this effort.
One prominent set of guidelines is the CONSORT (Consolidated Standards of Reporting Trials) guidelines, which provide a checklist and flowchart specifically tailored for randomized controlled trials (RCTs) [
20]. CONSORT also offers extension statements for various types of RCTs, including structured abstracts, cluster RCTs, pragmatic trials, and non-inferiority and equivalence RCTs. Another significant standard is PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses), which provides a checklist and flowchart for conducting systematic reviews or meta-analyses of RCTs [
21]. STARD (Standards for the Reporting of Diagnostic Accuracy Studies) has also been established, offering a checklist and flowchart to evaluate diagnostic accuracy studies [
22]. Additionally, and STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) [
23] provides a checklist specifically designed for conducting observational trials. By diligently following these reporting guidelines and standards, researchers can significantly reduce the likelihood of a substantial portion of published research being flawed, thus improving the overall quality of evidence in the field of nutrition research.
The standardization of outcome measures and assessment methods is also of utmost importance. This allows for easier comparisons across different studies and facilitates the synthesis of evidence in systematic reviews. One initiative that addresses this issue is the Core Outcome Measures in Effectiveness Trials (COMET) Initiative [
24]. The goal of this initiative is to establish a set of standardized outcomes that should be measured and reported as a minimum requirement in all clinical trials within a specific area of health or healthcare. This approach helps tackle the problem of inconsistent outcome reporting and promotes greater consistency and transparency in nutrition research. In the context of pediatric nutrition research, there are specific examples of core outcome sets that may be useful. These include diarrhea [
25], infant colic [
26], or food allergy [
27]. Implementing these core outcome sets can contribute to a more consistent and comprehensive evaluation of pediatric nutrition interventions.
Collaboration among researchers, institutions, and funding agencies plays a crucial role in enhancing transparency and minimizing research bias. Transparent reporting involves registering and reporting all trial results, regardless of whether they support or refute the initial hypothesis [
28]. This practice serves as a preventive measure against selective reporting and publication bias, which tend to favor studies with positive results while neglecting those with negative or inconclusive findings. Additionally, the recent emphasis on data sharing ensures that research data is made openly accessible, enabling other researchers to analyze and validate the results independently [
29].
2.3. Evidence vs Recommendations
One example that illustrates how evidence can change recommendations is the Learning Early About Peanut Allergy (LEAP) study. Published in 2015 [
30], the LEAP study resulted in definitive changes in infant nutrition recommendations regarding the introduction of peanuts to prevent peanut allergy. Previously, it was widely believed that introducing peanuts to infants at an early age could increase the risk of developing allergies. However, the LEAP study revealed evidence to the contrary. This well designed and conducted RCT demonstrated a substantial reduction (up to 86%) in peanut allergy among children with eczema and/or egg allergy by introducing peanuts between 4 and 11 months of age, as opposed to avoiding peanuts entirely until the age of 5. These findings, along with subsequent research [
31,
32], led to a significant shift in infant feeding guidelines. Many organizations now recommend introducing peanuts early to prevent peanut allergies, especially in high-risk infants [
33,
34,
35]. This example demonstrates how robust evidence can challenge existing practices and directly impact recommendations and guidelines in the field of nutrition research.
The Role of Evidence in Nutrition Research: Synopsis
Evidence plays a crucial role in nutrition research and the development of evidence-based practices. However, challenges such as confounding factors, biases, heterogeneity, and limited data on specific populations can compromise the validity and reliability of research findings. By employing rigorous study design, transparent reporting, collaboration, and standardized outcome measures, we can improve the quality of evidence in nutrition research.
3. Evolution of Dietary Guidelines & The Evidence Base
Humans have survived on a wide range of diets, mostly reflecting access to food supply. Although protein needs are based on ideal body weight (0.8 g protein/kg body weight for adults), the amounts of carbohydrates and fats in healthy diets vary greatly [
36]. Traditional Eskimo diets contain 80% of calories as fat, whereas traditional African diets are 80% of calories as carbohydrates. The trick for good nutrition is to consume diets that contain the appropriate number of calories, adequate protein, and essential vitamins, minerals, and fluids for any given individual within the whole population. Food guides to assist consumers to choose the wide variety of foods to deliver nutrients vary across countries.
3.1. Accepted Nutrition Facts
Traditionally, nutrient recommendations were made to prevent deficiency diseases. In 1941, the National Academy of Sciences in the United States began issuing Recommended Dietary Allowances (RDAs), the quantity of nutrients a person needs to consume daily to ensure basic good health, proper growth, and reproductive success and to prevent nutrient deficiency diseases. The current nutrition standards for the United States and Canada are the 2002 Dietary Reference Intakes (DRIs). These standards include the Recommended Dietary Allowances (RDAs) for vitamins and minerals. RDAs are designed to meet the needs of 98% of the population. Average energy requirements across the lifecycle are also part of the RDA. Upper limits (UL) are quantities of nutrients that could cause potential harm. Dietary deficiency diseases have been virtually eliminated in the United States, thanks to the enrichment of refined grains with thiamin, riboflavin, niacin, and iron, the addition of Vitamin A and D to milk, and consumption of fortified products such as ready-to-eat breakfast cereals. A second universally accepted dietary principle is to maintain appropriate body weight by consuming only enough food to balance the amount of energy expended. This varies greatly across the lifecycle with energy needs increasing greatly during growth and development. Balancing energy intake with energy needs has become much more difficult as modern life has removed all needs for physical labor, and tasty foods are inexpensive and easily obtainable. We have had more success fighting obesity in pigs, perhaps because swine diets are normally balanced for all nutrients, which may be an important practice to prevent obesity in humans also [
37].
Dietary recommendations to prevent chronic disease have always been controversial. Dr. Alfred Harper, in his article “Killer French Fries: The Misguided Drive to Improve the American Diet” (1988) describes our ways of learning about nutrient deficiencies and describes how such a model will not work for chronic diseases such as heart disease and cancer [
38]. He points out misinformation in the early Dietary Guidelines. For example, fruits are listed as a source of “complex carbohydrates”, when in reality they are mostly a source of sugar and often are poor sources of nutrients including vitamins and minerals. “An apple a day keeps the doctor away” is a well-known phrase in the English language and an apple does provide 2 grams of dietary fiber, but 72% of dry matter in an apple is sugar and apples are high in fructose [
37]. Harper stated that “science cannot at this time ensure than an altered diet will provide protection from certain killer diseases such as heart disease and cancer.” And “clinical advice to change diet based on the need to lower serum cholesterol is much different than public health advice to suggest that all Americans should consume plant foods of low protein quality”. In 1980, “Nutrition and Your Health: Dietary Guidelines for Americans” was issued in response to the public’s desire for authoritative, consistent guidelines on diet and health [
39]. Public Law 101-445,
Section 3, requires publication of the Dietary Guidelines for Americans at least every 5 years. They represent federal nutrition policy established jointly by the US Department of Agriculture (USDA) and the Department of Health and Human Services (HHS). They are designed to provide science-based advice to help prevent chronic disease and promote health. They lay the foundation for federal nutrition program and nutrition education programs and serve as a basis for research gaps and priorities. The Dietary Guidelines for Americans have evolved over time, but have not gravitated far from their original 7 general recommendations:
Eat a variety of foods.
Maintain ideal weight.
Avoid too much fat, saturated fat, and cholesterol.
Eat foods with adequate starch and fiber.
Avoid too much sugar.
Avoid too much sodium.
If you drink alcohol, do so in moderation.
3.2. Controversies in Dietary Guidance
The process by which federal agencies and policy makers consult scientific research in developing proposed regulations and polices varies, and greatly impacts the nature of the ultimate recommendations [
40]. Efforts to micromanage the diet by imposing strict dietary rules are difficult to support with evidence-based nutrition science. Concepts such as added sugar and solid fats, which work well in computer-based food modeling, are not helpful to consumers attempting to select and consume a “healthier diet”. We eat foods, not nutrients, and cultural norms and traditions must be considered when determining dietary guidance.
Plant foods are universally promoted for their links to improved human health, yet carbohydrate-containing foods are often maligned based on isolated, reductionist methods that fail to assess carbohydrate foods as a matrix of nutrients and food components [
41]. Currently accepted positive carbohydrate quality indices include plant food whole-grain content, and dietary fiber content, while the need to reduce calorie intakes because of obesity has recommended a reduction of added sugar. Guidelines on dietary sugar do not meet the criteria for trustworthy recommendations and are based on low-quality evidence [
42]. Public health official (when promulgating these recommendations) and their public audience (when considering dietary behavior) should be aware of these limitations.
Efforts to measure “carbohydrate quality” have attempted to devise scoring systems to promote carbohydrate foods that are high in dietary fiber, low in free sugars, concentrated in whole grains, low in sodium, and significant sources of potassium, to align with dietary guidelines. Additionally, scoring systems attempt to consider culturally inclusive dietary patterns and consider food economics and food availability [
43].
Whereas past dietary guidelines placed limits on total fat intake especially saturated fats, recent studies indicate more complex links with health [
44]. Fat-free dairy products are recommended because of their nutrient density, not a link between their fat content and health or disease outcomes [
45]. Since fats contain 2.5 times the number of calories per grams as carbohydrate or protein, a low-calorie diet typically is low in fat. Thus, guidelines for fat intake must differ between regions of general poverty and malnutrition compared to regions that calories need to be reduced.
Plant-based diets, and more specifically plant-based proteins, have been the subject of growing interest from researchers and consumers because of their potential health benefits as well as their positive environmental impact [
46]. It is not possible to separate the health-promoting effects of dietary fiber, vitamins, minerals, and phytochemicals in plant foods from the plant protein they contain. Protein is not a “nutrient of concern” in the Dietary Guidelines for Americans, yet groups across the lifecycle, including children and the elderly, are at risk for protein deficiency because of food choices [
47]. Commonly consumed sources of dietary protein frequently contribute substantially to intakes of nutrients such as calcium, Vitamin D, potassium, dietary fiber, iron, and folate, which have been identified as nutrients of concern, i.e. intakes are often lower than recommended intakes. Yet dietary recommendations to reduce intakes of saturated fat and solid fats may result in dietary guidance to reduce intakes of commonly consumed food sources of protein, in particular animal-based protein. When we focus on removing nutritional “bad guys”, such as added sugars and solid fats, we may have the unintended consequences of reducing intakes of high-quality protein, and nutrients of concern such as calcium, iron, Vitamin D, potassium, dietary fiber, and folate. Clearly, once more, the critical subdivsion of nutrients (as in RDA) from foods in their whole food form (as in dietary patterns) is the critical challenge–and gap- in nutrition science.
3.3. Sustainable Diets vs Ultra-Processed Diets : Contrasting or Complementary?
With a growing global population, the demand for high-quality food to meet nutritional needs continues to increase. Our ability to meet those needs is challenged by a changing environment that includes constraints on land water resources and concerns about the impact of human activity including agricultural practices on the changing climate [
2,
48]. Animal source foods provide a wide array of nutrients, including high quality protein, but the impacts on the environment must be considered in the future of agricultural production. A case study of vegetable oils supports that sustainable nutrition should be considered beyond pure nutritional facts, at the light of soil preservation, local resources and human needs in terms of health, employment and socio-economic development [
49].
The 2025 Dietary Guidelines for American (DGA) Scientific Advisory Committee is addressing the relationship between dietary patterns with ultra-processed (UPF) and body composition and weight status [
50]. In a proof-of-concept study, we developed a list of foods that fit NOVA criteria for UPF, fit within dietary patterns in the 2020 DGA, and are commonly consumed by Americans. We then used these foods to develop a 7-day, 2000 kcalorie menu. In the ultra-processed DGA menu that was created, 91% of kcal were from UPF, or NOVA category 4. The Healthy Eating Index [
51] score was 86 out of a possible 100 points, mostly because of excess sodium and insufficient whole grains. The menu provided adequate amounts of all macro- and micronutrients except vitamin D, vitamin E, and choline. Healthy dietary patterns can include most of their energy from UPF and still receive a high diet quality score and contain adequate amounts of most macro- and micronutrients. Within the context of circular economy, consistent with either fewer CO2 emissions and the valorization of a short distribution chain, the development of newer UPF could lead to further improvements able to modulate the double burden of malnutrition while reducing the wasting of resources [
52].
Challenges of Dietary Guidance: Synopsis
Nutrition science relies on research tools that are most effective at isolating the effect of a particular nutrient, rather than tools that rely on either whole foods and food systems. Shifting from a nutrient focus to a whole foods focus is difficult as the body of scientific evidence to support consumption of whole foods compared to nutrients is limited [unless we consider dietary patterns historically developed in specific areas, such as the Mediterranean, Nordic, Japanese, and still others]. All dietary guidelines must provide nutrients across different age groups from birth to death, so nutrient dense foods like dairy, whole grains, fruits, vegetables, and protein foods will continue to be on the plate. Cost, sustainability, supply, culture, and convenience all impact food intake and must be considered in dietary guidance.
4. Benchmark of Dietary Guidelines
Grading the Evidence to Decision Making in Nutrition Research: a Public Health Perspective.
We have previously considered both advantages and limits of established dietary guidelines and related derived recommendations. Within this context, dietary guidelines and recommendations, usually developed by government bodies or large authoritative organizations, have major downstream effects on public policy, and should be based on the best evidence and be aligned with public values and preferences.
In order to support the development of evidence-informed dietary guidance the WHO Department of Nutrition and Food Safety in 2010 established the WHO Nutrition Guidance Expert Advisory Group (NUGAG) [
53], composed of experts in subjects such as nutrition science as well as public health or clinicians. The advisory group had the mandate of attending to certain elements essential to the development of effective guidelines, including 1) Scope of guidelines, 2) Selection and prioritization of important outcomes, 3) Examination and interpretation of the evidence. In formulating the recommendations, the NUGAG was asked to take into consideration the quality of evidence generated and compiled as well as diverse values, balance of benefits and harms, resource implications, priority of the problem, equity and human rights, acceptability, and feasibility.
In spite of this important effort, however, a growing body of evidence accumulated supporting the notion of serious deficiencies in the methods used so far to develop dietary guidelines. Such deficiencies included the failure to access or conduct comprehensive systematic reviews, the lack of systematic or rigorous evaluation of the quality of the evidence, the failure to acknowledge the limitations of the evidence-based underlying recommendations, and insufficiently stringent management of conflicts of interest [
54].
4.1. Grading the Evidence
In 2019, Zeraatkar et al. [
55] suggested to address this burning issue by adhering to international standards for guideline development, including adopting systematic review methodology, using rigorous systems to evaluate the certainty of the evidence, and moving from evidence to recommendations by adopting the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation). In fact, few years earlier Alonso-Coello et al. [
56] published the GRADE-Evidence to Decision Framework (EtD), that was developed to facilitate the process of moving from evidence to recommendations in a structured manner by building on the GRADE approach. The main purpose of the framework is to help groups of experts that constitute panels to use the evidence available in a structured and transparent way to inform decisions, guidelines and recommendations. Through this framework, decision makers can be informed about the relative benefits and harms of the options being considered, and ensure that all important factors are considered in the decision-making process. A number of applications of the GRADE-ETD framework in nutrition has been published since then [
57,
58].
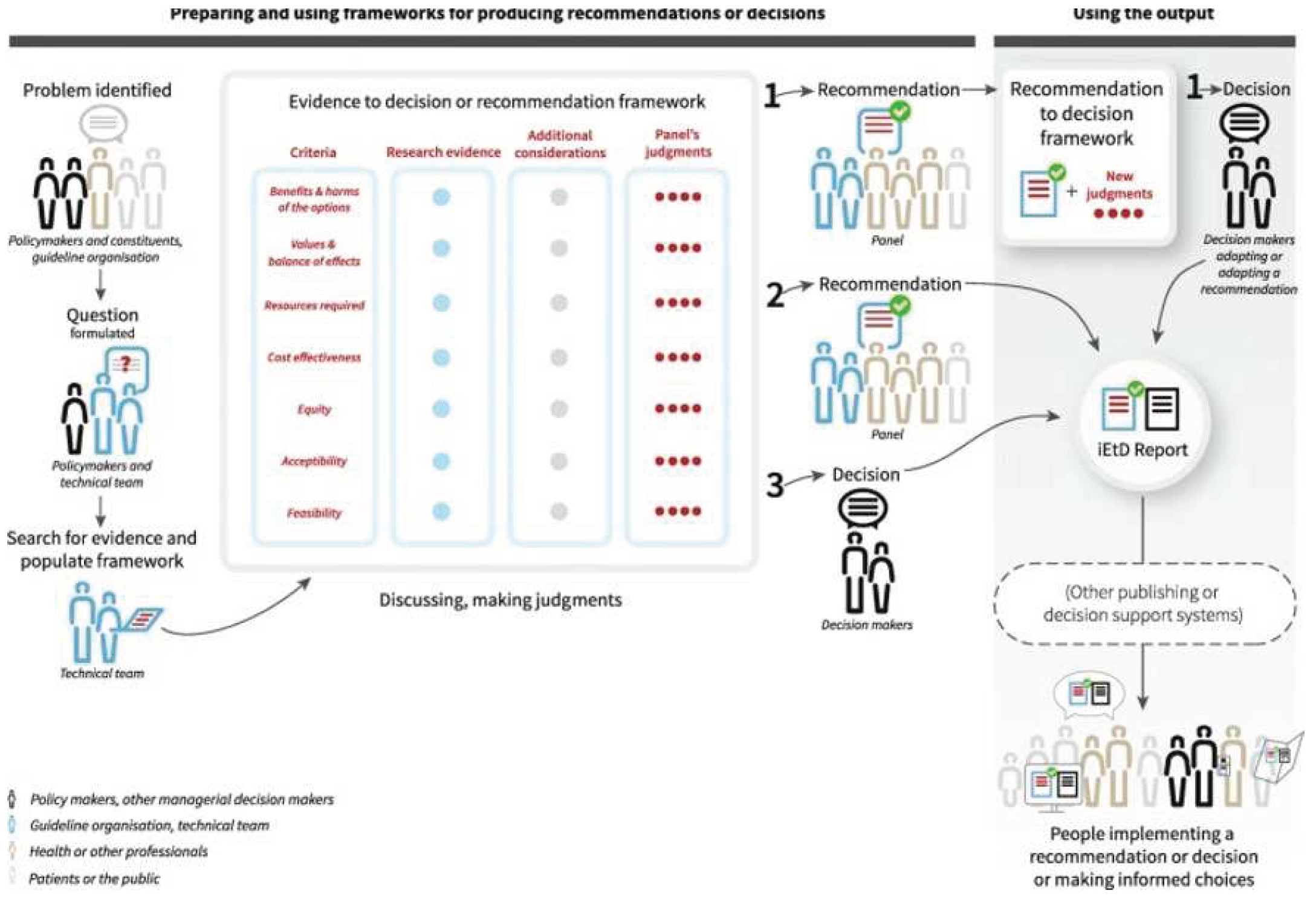
The process expected from the application of GRADE-EtD consists of three main parts depicted in
Figure 1:
Defining the question
Making the assessment
Making the recommendations.
In closer detail, the process starts with the setup of a panel composed by different stakeholders including policy makers, and a guideline organization (eg scientific society). The panel joins a technical team that will do the extensive work of collecting research evidence from the literature. The first step consists in formulating the objective of the recommendations/guidelines that usually is done through a narrative review and panel consultation. Immediately after, the technical team starts collecting the available evidence from the literature by following the criteria of the systematic literature review.
Beyond collecting the evidence on the main health outcomes, the peculiarity of the GRADE EtD is the inclusion of many criteria in the evidence gathering and assessment phase regarding the appropriateness of the intervention. In fact, in addition to the health effects associated with a certain diet or nutrient, dietary guidelines may also consider other criteria such as equity, feasibility, acceptability or economic evaluations. This largely depends on the perspective they assume (
Table 1 and
Figure 1). For example, dietary guidelines targeted at health professionals are less likely to consider costs or equity than guidelines with a societal perspective. Ideally, such additional considerations related to the implementation aspects, should be accompanied by a comprehensive search of the literature conducted with similar rigor when addressing systematic literature reviews for health outcomes. It is not surprising that the technical team usually do not achieve this degree of rigor, with most dietary guidelines limiting their scope to considering only health outcomes.
Once the available evidence is collected with reference to each criterion of the framework, an evaluation of the quality of the evidence is carried out according to the GRADE methodology. Based on the evidence collected for each criterion and the certainty of evidence produced by applying the GRADE methodology, the expert panel can make judgments that will then feed into a final recommendation (
Figure 1).
The recommendation can identify the intervention under study as strongly recommended, conditionally recommended (panel is less confident and therefore it also includes specific guidance on the conditions required for implementing) or against the recommendation. It should be noted that while the recommendation is developed independently from the panel members guided from the technical team and the guidelines organization that vote in favor or against each criteria and make the final judgment, the decision for implementation largely depends on the decision makers and this can be more political and not completely aligned with the conclusion of the constituent team and the panel.
Finally, although very strict in its implementation rules, there is by necessity a considerable amount of subjectivity involved in rating the certainty of evidence and grading the recommendations, and that two persons evaluating the same body of evidence might come reasonably to two different conclusions. However, the advantage of GRADE is that it provides a transparent framework through which these subjective judgments can be documented and communicated to stakeholders.
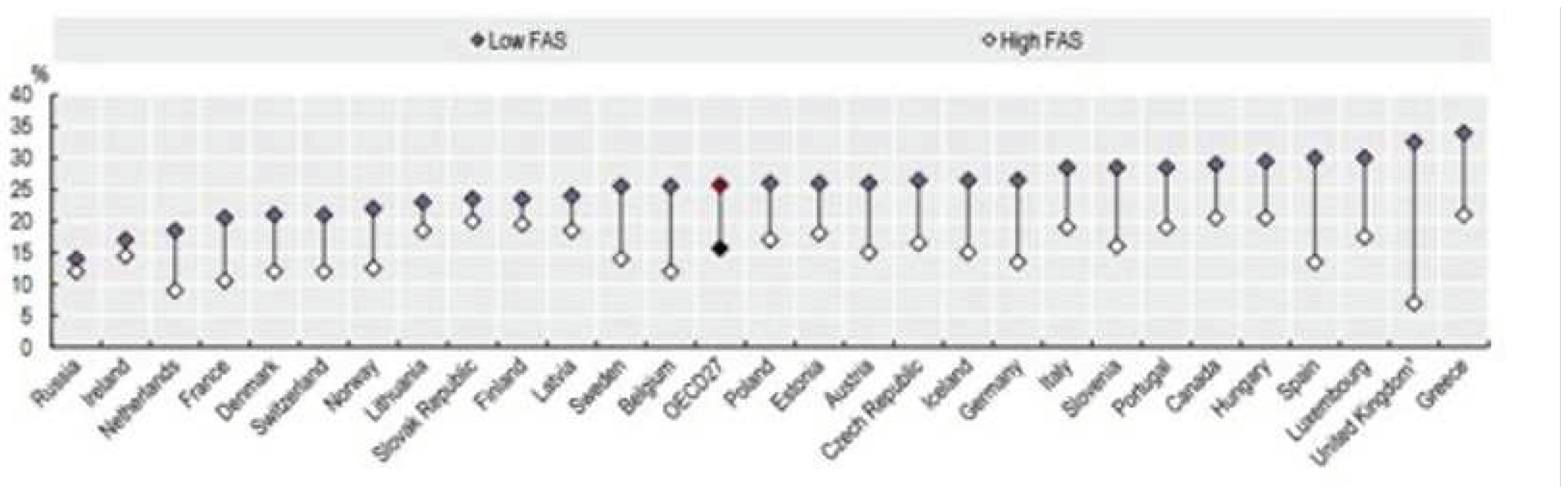
4.2. The Growing Issue of Health Equity
Guideline developers should consider the impact of guidelines on health equity. The latest report of the Health at Glance shows that high values of the Family Affluence Scale (a four-item measure of family wealth developed from the WHO Health Behaviour in School-aged Children Study) are associated with reduced overweight (
Figure 2). There is no doubt that that huge inequalites in diet exist and that such inequalities are associated with weight gain and obesity. There is also evidence, however, that higher cost diets tend to be more in line with the principles listed in institutional guidelines.
A systematic review published in 2010, suggested that dietary behaviors may contribute to socioeconomic inequalities in overweight/obesity in Europe [
59]. The study examined socioeconomic inequalities in intakes of dietary factors associated with weight gain, overweight/obesity among adults in Europe. The literature searches included studies published between 1990 and 2007 examining socioeconomic position (SEP) and the consumption of energy, fat, fiber, fruit, vegetables, energy-rich drinks and meal patterns. The most consistent evidence of dietary inequalities was for fruit and vegetable consumption, with lower socioeconomic groups less likely to consume fruit and vegetables. Differences in energy, fat and fiber intakes (when found) were small-to-moderate in magnitude; however, differences were moderate-to-large for fruit and vegetable intakes. There were no regional or gender differences in the direction and magnitude of the inequalities in the dietary factors examined. The question, however, is “do people consume unhealthy foods either because they do not know that they are unhealthy, or because they lack the motivation to stop consuming them? Or maybe because policies are not designed to address this issue (the higher costs of healthy foods, for instance)?
Evidence from a large systematic review indicates that provision of nutrition information is insufficient to change dietary behaviors, particularly among disadvantaged groups with fewer resources at their disposal [
60]. Policy makers should be aware that some healthy eating interventions targeted at healthy populations may have greater benefits for individuals of higher SEP (and subsequently increase inequalities), notably personalized nutritional education and dietary counselling interventions. On the other hand, a combination of taxes and subsidies may preferentially improve healthy eating outcomes for people of lower SEP (potentially reducing inequalities), as well as counseling activities aimed at improving knowledge to optimize the use low-cost healthy foods (cereals, dry legumes, nuts, milk, and others) (reference needed here). As noted, the majority of identified studies in the aforementioned review did not explore differential effects by SEP. When considering implementing a food policy at any level, those involved should consider the potential differential impact of these on health inequalities.
Benchmark of Dietary Guidelines. Synopsis
Improving the methods for developing dietary guidelines has the potential to improve public policy decision making about nutrition. To make this improvement, we must base all recommendations on methodologically rigorous systematic literature reviews and relying on high quality evidence across all the domains considered – not just those limited to single nutrients. If only low evidence is available, panels must acknowledge limitations and refrain from making recommendations. Decision-makers can make use of several resources that set standards for the development of guidelines, and GRADE-EtD represents one of the most comprehensive and rigorous instruments available.
5. Psycho-Social Factors Impacting Adherence to Dietary Guidelines
As previously underlined, although dietary guidelines are in search of solutions harmonized within more comprehensive frameworks, challenges still exist in transforming them in a real guidance for consumers’ behaviours [
62], at the light of next adjustments within more holistic views. Consumers, independently of their level of literacy about nutritional guidelines, often tend to not be compliant to them, and this become particularly challenging in the case of patients’ population, who’s disease course may depend also on their food choices and intake.
5.1. Individual demographics: The Need of a Behavioural Approach
Several factors impact on the level of people’ adherence to dietary guidelines. Undoubtfully levels of educations determine the extent to which consumers are better exposed to information about healthy models of nutrition and better able to comply to guidelines. On the contrary it is evident that a poor knowledge of healthy food selections could negatively influence consumers eating habits [
63]. Another fundamental factor is related to the economic wellness of the population, since, as already mentioned, financial aspects might also play a role in determining the consumer’s ability and willingness to consume healthier food [
64].
There are several evidences related to the increase of junk food choices in the most marginalized target of the population. Furthermore, ethics and cultural factors also influence consumers’ willingness (and ability) to comply with dietary regimes: traditions routed in a cultural group not only strongly shapes food consumption habits, but also orient (false) beliefs about the nutritional and healthy characteristics of specific food. This is also due to the peculiar characteristics of food, which is part of our cultural identity and personality [
65], and is often central in social gatherings [
66]. Moreover the psychological state of an individual may influence his/her food choices [
67]. Accordingly, poor mood promotes misbehaviors such as binge eating or snaking [
68] as a mean to provide a temporary lifting of mood [
69].
All those considerations, joint to the real-world evidence of challenges faced when traying to change individual’s food consumption preferences, claim for an enhanced consideration of learnings from behavioural sciences in order to orient educational and communication strategies better effective [
70]. In particular the perspective of consumer psychology may help to shed light on some aspects of consumer food choices and adherence to dietary guidelines. In the following paragraphs we shall discuss some of the psycho-social factors that mostly impact on consumers’ adherence to dietary guidelines.
5.2. Heuristics and Irrational Processes of Consumption Decision Making in the Average Consumer
A balanced, healthy diet comes with several benefits for consumers’ health [
71,
72]. As such, Health- and Food- related choices are often connected. However, when it comes to choosing, the average consumer has a hard time doing the “right choice” for health, as several irrational mechanisms take the upper hand in guiding purchase. When consumers choose their food, they don’t necessarily weight benefits over costs, nor they are generally capable of making an exact (or close to exact) estimation of the probabilities of a potential wrong choice. Instead, consumers tend to rely on a series of non-logical strategies when making decisions. Heuristics are a famous example of this [
73,
74]. Although necessary for our daily functioning, given their nature heuristics are not completely reliable mechanisms, especially when it comes down to complex decision-making or risk assessment, as they are responsible for most of the known cognitive biases, which in turn impact our ability to make sound decisions. Heuristics are short-cuts in our decision-making pathway, particularly easy and appealing when we engage in routinary and less involving choices, such as those related to our food habits. In contrast with one of the most established models which describe the consumer decision-making process from an economic perspective [
75], consumers’ choices are often poorly linear and rational. According to Engel and colleagues, indeed, the consumer journey as a linear pathway leading from recognizing a need to the purchase of the best (from a cost/benefit perspective) item that satisfied the need. On the contrary, a long tradition of consumer psychology studies has demonstrated that several irrational factors (i.e., not based on logical and sequential decision-making processes) play an important role in defining what a consumer desires and, ultimately, decides to buy and consume. This is particularly magnified in the setting of food consumption. First because consumers’ detection of a nutritional need cannot be relegated to the level of pure rational awareness of individuals. Complex emotional and motivational dynamics underlie the perception of our consumption needs and desires. And these dynamics are often unaware and difficult to control. But also, with regard to the information search stage related to the food options available on the mark, the dynamics of the psychological processes involved are decidedly more complex. Not only due to the growing complexity of the information stimuli to which we are subjected daily regarding consumption options (a complexity that puts a strain on our brain's ability to correctly process all the information), but also because the meaning we attribute to information depends from our previous experiences (memory), from our personality and from the historical-cultural context in which we live.
However, our mind follows abbreviated, chance-dictated and automatic decision-making processes, based on thought shortcuts and habits. Depending on the level of involvement towards the product to be purchased, or simply on how we feel in a certain place or moment, we can alternatively activate algorithmic logics of consideration of alternatives, or more abbreviated and adaptive thinking logics which, with less expenditure of energy mental, still lead us to make decisions quickly.
5.3. Emotions, Attitudes, Motivations
Emotions frame our everyday life and food consumption is not an exception. Even, often individuals recur to consumption and buying behaviours to deal with their emotions, particularly when negative. In this regard, some psychologists have theorized the existence of an “Emotion regulation consumption”, that is exactly the case when individual recur to consumption behaviours in order to manage their emotional status [
76]. The relationship between emotional state and food intake is rather established and accepted in literature [
67]. In particular, this relationship has been investigated starting, mostly, from clinically obese populations: early studies indeed were aimed at understanding how mood modulated the calorie intake of people with maladaptive nutritional behaviours such as binge eating, as food is a mean to reduce the perceived anxiety and distress [
77,
78]. Indeed, the relationship between poor mood (i.e., boredom, depression, or fatigue) and higher food consumption or binge eating is well known in literature [
68], although there is a stronger focus on clinical populations [
79]. Although recent studies also show that even positive mood might evoke a higher calorie intake [
80], it also seems that the search for sweet, comfort food is related more to poor mood events rather than to joy [
81]. Overall, literature seems to suggest that, when distressed or affected by negative emotions, human beings tend to prefer sweet and fatty food, resulting in an overall unhealthy diet. The use of such foods, high in carbohydrate, is probably a mean to provide a temporary lifting of mood. Several studies have demonstrated that there is a relationship between poor mood and the consume of “comfort food” [
68,
81,
82], and between binge eating and poor mood also in patients population [
83]. In this perspective, food choices should be regarded as a complex phenomenon, alimented by different motivations and complex decision-making processes.
Particularly, a factor often measured to assess the role of psychology in orienting consumers food choices is the construct of Food Involvement [
84,
85,
86]. This psychological construct describes and personalizes the consumers’ affective and behavioural commitment towards food.
5.4. The role of Patient Engagement and the Sense of Ownership
Food and health are strongly related, especially in groups affected by specific disorders. Indeed, asking people (healthy and clinical alike) to change their dietary behaviours in order to live a healthier life may actually require a huge commitment on their part, and might be compared (with the due differences) to asking a patient to adhere to another medical prescription,- like taking drugs, increasing physical activity, stop smoking, etc.- in terms of how this has the potential to impact their current lives, their habits, their sociality, and their perception of self. When it comes to asking a patient (in the broad sense of a person with a health need) to adhere to a prescription, be it a dietary change or a pill to take, patient engagement becomes a relevant psychological construct to be taken into account. Currently, there are several different definitions of patient engagement. Patients with a higher level of health engagement show improved clinical outcomes and reduced costs, health literacy, and -last but not least- adherence to medication and prescriptions [
87]. The interest towards this construct is increasing, and its measurement is becoming highly relevant in the approaches based on patient centred medicine (PCM). In this perspective, the patients’ expertise become a valuable asset in the decision-making process. However, this requires the patient to be capable and willing to play an active role, to be proactive in the relationship with the healthcare providers, and to be capable of autonomously navigate the whole healthcare system in which the patient is: for PCM to be effective, the patient needs to be engaged.
Recently the concept of engagement has also be extended to the idea of involving patients and stakeholders in crucial phases of guidelines development for healthy nutrition, in order to include people concerns and doubts about food choices and to improve acceptability of such guidelines [
88].
Psycho-Social Factors Impacting Adherence to Dietary Guidelines: Synopsis
More input is needed in studies aimed at casting light on all the different psycho-social factors which impact consumers’ adherence to nutritional guidelines. Further then examining the relevance of the different aspects that may impede behaviours change in a target population further effort is needed to better segment the target population in groups that share similar attitude towards compliance to health-related food issues (including dietary guidelines). The articulated analysis of psycho-social factors, thus, may be the basis for reaching personalized nutritional communications more effectively to impact individuals’ behaviours. Social marketing campaigns may be a good solution to create appealing messages finalized to this aim. Remarkably, behavioural sciences should mandatorily synergize with nutritional sciences for a cross-fertilization of insight that can benefit educational interventions aimed at target populations. Engaging citizens and patients to improve the approach to, and the knowledge about, nutrition and related dietary guidelines can be a further key to improve the rate of adherence, shifting from a “vicious circle” of food choices towards a “virtuous circle” for either preventive and therapeutic goals [
88,
89].
5. Conclusions
The panorama of nutrition science is rapidly changing. Animals need essential nutrients to survive and prosper and nutritionists must continue to devise nutritionally adequate diets to provide essential nutrients across the lifecycle [
90].
As dietary habits change and traditional foods are no longer consumed, nutritionists must continue to evaluate the nutrient content of the diet and support enrichment or fortification strategies to deliver the required nutrients to all consumers [
91].
Relationships between dietary exposures and risks of chronic disease are more difficult to discern and go beyond the need to deliver the required nutrients. Since the early 1980s, countries have adopted dietary guidelines that support that changes in food intake will decrease risk developing chronic diseases. Thus, usual dietary guidelines support fewer calories especially in the form of saturated fats and free sugars and less sodium. Food patterns that are recommended include more vegetables, fruits, whole grains, pulses, nuts, and seeds. Food patterns that deliver the essential nutrients, especially the nutrients of concern, dietary fiber, calcium, Vitamin D, and potassium in the US diet are still recommended and generally shown on visuals like myplate.gov [
92].
The traditional concept of dietary guidelines (based on technical terms such PRI, AR, AI) needs to be matched with new concepts to increase acceptance and reliability within the framework of sustainability [
93].
Collaboration rather than pure competition among researchers should lead to more homogenous study designs to derive evidence, frameworks, and algorithms that can be translated into every-day life and guide individual attitudes toward personalized food choices. This approach is transversal, merging abilities and knowledge among various branches of science, and should ultimately improve and promote the social advancement of population that are disadvantaged in terms of social inequalities and suffering from the development of either communicable or non-communicable diseases [
94].
We are crossing the Red Sea from globalization, with loss of individualities, to glocalization, with valorisation of local individuals and resources [
95].
Following these perspectives, nutrition science will not run the risk of limiting itself to theoretical numbers and indications, but will, on the contrary, expand within the emerging area of the exposome, “The cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behavior, and endogenous processes” [
96].
Author Contributions
Conceptualization, C.A, S.B, G.G, S.J. and H.S.; methodology, C.A, S.B, G.G, S.J. and H.S; writing—original draft preparation, C.A, S.B, G.G, S.J. and H.S.; writing—review and editing, C.A, S.B, G.G, S.J. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by a contribution from the Italian Ministry of Health (ricerca corrente).
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
Presented at the Symposium ‘The quality of diet: do we really have a science-based data bank?’ during the Nutrition & Growth Conference (London, 30 March 2023), sponsored by Soremartec Italia S.R.L. Its contents are solely the responsibility of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mozaffarian, D. Perspective: Obesity-an Unexplained Epidemic. Am. J. Clin. Nutr. 2022, 115, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Baglioni, M.; La Vecchia, A.; Molari, G.; Berti, C. Interlinkages between Climate Change and Food Systems: The Impact on Child Malnutrition-Narrative Review. Nutrients 2023, 15, 416. [Google Scholar] [CrossRef]
- Moreno, L.A.; Meyer, R.; Donovan, S.M.; Goulet, O.; Haines, J.; Kok, F.J.; Van’t Veer, P. Perspective: Striking a Balance between Planetary and Human Health-Is There a Path Forward? Adv. Nutr. 2022, 13, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, H.; Sharma, A. Global Food Production and Distribution Analysis Using Data Mining and Unsupervised Learning. Recent Adv. Food Nutr. Agric. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Ragalie-Carr, J.; Torres-Gonzalez, M. Perspective: Seeing the Forest through the Trees: The Importance of Food Matrix in Diet Quality and Human Health. Adv. Nutr. 2023, 14, 363–365. [Google Scholar] [CrossRef]
- Rossi, L.; Ferrari, M.; Ghiselli, A. The Alignment of Recommendations of Dietary Guidelines with Sustainability Aspects: Lessons Learned from Italy’s Example and Proposals for Future Development. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- 10th N&G Conference. Presented at the 10th N&G conference. London; 2022.
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Helfer, B.; Leonardi-Bee, J.; Mundell, A.; Parr, C.; Ierodiakonou, D.; Garcia-Larsen, V.; Kroeger, C.M.; Dai, Z.; Man, A.; Jobson, J.; et al. Conduct and Reporting of Formula Milk Trials: Systematic Review. BMJ 2021, 375, n2202. [Google Scholar] [CrossRef] [PubMed]
- &na; Statistics from the World Health Organization and the Centers for Disease Control. World Health Organization Global Statistics. AIDS 1992, 6, 1229. [Google Scholar] [CrossRef]
- Catalogue of Bias. Available online: https://catalogofbias.org (accessed on 10 March 2023).
- McNamee, R. Confounding and Confounders. Occup. Environ. Med. 2003, 60, 227–234; quiz 164, 234. [Google Scholar] [CrossRef]
- Patro, B.; Liber, A.; Zalewski, B.; Poston, L.; Szajewska, H.; Koletzko, B. Maternal and Paternal Body Mass Index and Offspring Obesity: A Systematic Review. Ann. Nutr. Metab. 2013, 63, 32–41. [Google Scholar] [CrossRef]
- Nunan, D.; Aronson, J.; Bankhead, C. Catalogue of Bias: Attrition Bias. BMJ Evid. Based Med. 2018, 23, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.S.; Domellöf, M.; Hojsak, I.; Hulst, J.M.; Kennedy, K.; Koletzko, B.; Mihatsh, W.; Stijnen, T. Attrition in Long-Term Nutrition Research Studies: A Commentary by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Early Nutrition Research Working Group. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.S.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.; Hadders-Algra, M.; Koletzko, B.; Lucas, A. How Much Loss to Follow-up Is Acceptable in Long-Term Randomised Trials and Prospective Studies? Arch. Dis. Child. 2008, 93, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.; Lee, M.K.; Kagawa, M. Ethical Challenges in Infant Feeding Research. Nutrients 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, K.; Helfer, B.; Eskander, M.; Crawley, H.; Trabulsi, J.; Caulfield, L.E.; Duffy, G.; Garcia-Larsen, V.; Hayward, D.; Hyde, M.; et al. Guidance for the Conduct and Reporting of Clinical Trials of Breast Milk Substitutes. JAMA Pediatr. 2020, 174, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Equator Network. Available online: https://www.equator-network.org (accessed on 10 March 2023).
- Schulz, K.F.; Altman, D.G.; Moher, D. ; CONSORT Group CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335. [CrossRef]
- Comet Initiative. Available online: https://www.comet-initiative.org (accessed on 10 March 2023).
- Karas, J.; Ashkenazi, S.; Guarino, A.; Lo Vecchio, A.; Shamir, R.; Vandenplas, Y.; Szajewska, H. Consensus Group on Outcome Measures Made in Paediatric Enteral Nutrition Clinical Trials (COMMENT) A Core Outcome Set for Clinical Trials in Acute Diarrhoea. Arch. Dis. Child. 2015, 100, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Steutel, N.F.; Benninga, M.A.; Langendam, M.W.; Korterink, J.J.; Indrio, F.; Szajewska, H.; Tabbers, M.M. Developing a Core Outcome Set for Infant Colic for Primary, Secondary and Tertiary Care Settings: A Prospective Study. BMJ Open 2017, 7, e015418. [Google Scholar] [CrossRef] [PubMed]
- Core Outcome Measures for Food Allergy. Available online: https://comfa.eu/ (accessed on 10 March 2023).
- All Trials Registered. Available online: https://www.alltrials.net/ (accessed on 10 March 2023).
- Taichman, D.B.; Sahni, P.; Pinborg, A.; Peiperl, L.; Laine, C.; James, A.; Hong, S.-T.; Haileamlak, A.; Gollogly, L.; Godlee, F.; et al. Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors. Lancet 2017, 389, e12–e14. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- de Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing Food Allergy in Infancy and Childhood: Systematic Review of Randomised Controlled Trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI Guideline: Preventing the Development of Food Allergy in Infants and Young Children (2020 Update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Watson, W.; Vander Leek, T.K.; Atkinson, A.; Primeau, M.-N.; Francoeur, M.-J.; McHenry, M.; Lavine, E.; Orkin, J.; Cummings, C.; et al. Dietary Exposures and Allergy Prevention in High-Risk Infants. Allergy Asthma Clin. Immunol. 2022, 18, 36. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Chan, E.S.; Venter, C.; Spergel, J.M.; Abrams, E.M.; Stukus, D.; Groetch, M.; Shaker, M.; Greenhawt, M. A Consensus Approach to the Primary Prevention of Food Allergy through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; And the Canadian Society for Allergy and Clinical Immunology. J. Allergy Clin. Immunol. Pract. 2021, 9, 22–43.e4. [Google Scholar] [PubMed]
- Slavin, J. Dietary Guidelines. Nutr. Today 2012, 47, 245–251. [Google Scholar] [CrossRef]
- van Kempen, T.A.T.G.; Zijlstra, R.T. Eat like a Pig to Combat Obesity. Metabolites 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E. Killer French Fries. Sciences (New York) 1988, 28, 21–27. [Google Scholar] [CrossRef]
- &na; Nutrition and Your Health Dietary Guidelines for Americans. Nutr. Today 1980, 15, 14–18.
- Slavin, J.L. The Challenges of Nutrition Policymaking. Nutr. J. 2015, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Slavin, J. Perspective: Defining Carbohydrate Quality for Human Health and Environmental Sustainability. Adv. Nutr. 2021, 12, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.; Sadeghirad, B.; Lytvyn, L.; Slavin, J.; Johnston, B.C. The Scientific Basis of Guideline Recommendations on Sugar Intake: A Systematic Review. Ann. Intern. Med. 2017, 166, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.B.; Drewnowski, A.; Papanikolaou, Y.; Jones, J.M.; Slavin, J.; Angadi, S.S.; Rodriguez, J. Application of a New Carbohydrate Food Quality Scoring System: An Expert Panel Report. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Meijaard, E.; Abrams, J.F.; Slavin, J.L.; Sheil, D. Dietary Fats, Human Nutrition and the Environment: Balance and Sustainability. Front. Nutr. 2022, 9, 878644. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Astrup, A.; Hill, J.O.; Slavin, J.L.; Allison, D.B.; Maki, K.C. Potential Cardiometabolic Health Benefits of Full-Fat Dairy: The Evidence Base. Adv. Nutr. 2020, 11, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of Plant Protein in Nutrition, Wellness, and Health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Fulgoni, V.L., 3rd; Heaney, R.P.; Nicklas, T.A.; Slavin, J.L.; Weaver, C.M. Commonly Consumed Protein Foods Contribute to Nutrient Intake, Diet Quality, and Nutrient Adequacy. Am. J. Clin. Nutr. 2015, 101, 1346S–1352S. [Google Scholar] [CrossRef]
- Raiten, D.J.; Allen, L.H.; Slavin, J.L.; Mitloehner, F.M.; Thoma, G.J.; Haggerty, P.A.; Finley, J.W. Understanding the Intersection of Climate/Environmental Change, Health, Agriculture, and Improved Nutrition: A Case Study on Micronutrient Nutrition and Animal Source Foods. Curr. Dev. Nutr. 2020, 4, nzaa087. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Jolliet, O.; Meijaard, E.; Slavin, J.; Rasetti, M.; Aleta, A.; Moreno, Y.; Agostoni, C. Sustainable Nutrition and the Case of Vegetable Oils to Match Present and Future Dietary Needs. Front. Public Health 2023, 11, 1106083. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.M.; Comeau, M.E.; Casperson, S.; Slavin, J.L.; Johnson, G.H.; Messina, M.; Raatz, S.; Scheett, A.J.; Bodensteiner, A.; Palmer, D.G. Dietary Guidelines Meet NOVA: Developing a Menu for A Healthy Dietary Pattern Using Ultra-Processed Foods. J. Nutr. 2023, 153, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Fouad, M.M.; Abd Elshafy, S.A.; Abdelfatah, D.; Lithy, R.M.; Abdelghani, A.; Ashoush, O.A. Beneficial Role of Healthy Eating Index-2015 Score & Physical Activity on COVID-19 Outcomes. BMC Nutr. 2023, 9, 113. [Google Scholar]
- Capozzi, F.; Magkos, F.; Fava, F.; Milani, G.P.; Agostoni, C.; Astrup, A.; Saguy, I.S. A Multidisciplinary Perspective of Ultra-Processed Foods and Associated Food Processing Technologies: A View of the Sustainable Road Ahead. Nutrients 2021, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Guidance Expert Advisory Group (NUGAG). Available online: https://www.who.int/groups/nutrition-guidance-expert-advisory-group-(nugag) (accessed on 3 October 2023).
- Blake, P.; Durão, S.; Naude, C.E.; Bero, L. An Analysis of Methods Used to Synthesize Evidence and Grade Recommendations in Food-Based Dietary Guidelines. Nutr. Rev. 2018, 76, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Johnston, B.C.; Guyatt, G. Evidence Collection and Evaluation for the Development of Dietary Guidelines and Public Policy on Nutrition. Annu. Rev. Nutr. 2019, 39, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Coello, P.; Schünemann, H.J.; Moberg, J.; Brignardello-Petersen, R.; Akl, E.A.; Davoli, M.; Treweek, S.; Mustafa, R.A.; Rada, G.; Rosenbaum, S.; et al. GRADE Evidence to Decision (EtD) Frameworks: A Systematic and Transparent Approach to Making Well Informed Healthcare Choices. 1: Introduction. BMJ 2016, 353, i2016. [Google Scholar] [CrossRef] [PubMed]
- Stadelmaier, J.; Rehfuess, E.A.; Forberger, S.; Eisele-Metzger, A.; Nagavci, B.; Schünemann, H.J.; Meerpohl, J.J.; Schwingshackl, L. Using GRADE Evidence to Decision Frameworks to Support the Process of Health Policy-Making: An Example Application Regarding Taxation of Sugar-Sweetened Beverages. Eur. J. Public Health 2022, 32, iv92–iv100. [Google Scholar] [CrossRef] [PubMed]
- Friesen, V.M.; Mbuya, M.N.N.; Wieringa, F.T.; Nelson, C.N.; Ojo, M.; Neufeld, L.M. Decisions to Start, Strengthen, and Sustain Food Fortification Programs: An Application of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Evidence to Decision (EtD) Framework in Nigeria. Curr. Dev. Nutr. 2022, 6, nzac010. [Google Scholar] [CrossRef] [PubMed]
- Giskes, K.; Avendano, M.; Brug, J.; Kunst, A.E. A Systematic Review of Studies on Socioeconomic Inequalities in Dietary Intakes Associated with Weight Gain and Overweight/Obesity Conducted among European Adults. Obes. Rev. 2010, 11, 413–429. [Google Scholar] [CrossRef] [PubMed]
- McGill, R.; Anwar, E.; Orton, L.; Bromley, H.; Lloyd-Williams, F.; O’Flaherty, M.; Taylor-Robinson, D.; Guzman-Castillo, M.; Gillespie, D.; Moreira, P.; et al. Erratum to: Are Interventions to Promote Healthy Eating Equally Effective for All? Systematic Review of Socioeconomic Inequalities in Impact. BMC Public Health 2015, 15, 894. [Google Scholar] [CrossRef]
- Moberg, J.; for the GRADE Working, Group; Oxman, A.D.; Rosenbaum, S.; Schünemann, H.J.; Guyatt, G.; Flottorp, S.; Glenton, C.; Lewin, S.; Morelli, A.; et al. The GRADE Evidence to Decision (EtD) Framework for Health System and Public Health Decisions. Health Res. Policy Syst. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Nayga, R.M., Jr.; Capps, O., Jr. US Consumers’ Perceptions of the Importance of Following the US Dietary Guidelines. Food Policy 1999, 24, 553–564. [Google Scholar] [CrossRef]
- El Ansari, W.; Suominen, S.; Samara, A. Eating Habits and Dietary Intake: Is Adherence to Dietary Guidelines Associated with Importance of Healthy Eating among Undergraduate University Students in Finland? Cent. Eur. J. Public Health 2015, 23, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C. Identifying Innovative Interventions to Promote Healthy Eating Using Consumption-Oriented Food Supply Chain Analysis. J. Hunger Environ. Nutr. 2009, 4, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Castellini, G.; Graffigna, G. “Food Is More than Just a Source of Nutrients”: A Qualitative Phenomenological Study on Food Involvement. Appetite 2022, 178, 106179. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.; Caplan, P. Food, Health and Identity. J. R. Anthropol. Inst. 1998, 4, 829. [Google Scholar] [CrossRef]
- Canetti, L.; Bachar, E.; Berry, E.M. Food and Emotion. Behavioural Processes 2002, 60, 157–164. [Google Scholar] [CrossRef] [PubMed]
- AlAmmar, W.A.; Albeesh, F.H.; Khattab, R.Y. Food and Mood: The Corresponsive Effect. Curr Nutr Rep 2020, 9, 296–308. [Google Scholar] [CrossRef]
- Macht, M.; Simons, G. Emotional Eating. In Emotion Regulation and Well-Being; Springer New York: New York, NY, 2011; pp. 281–295. ISBN 9781441969521. [Google Scholar]
- Rowe, S.; Alexander, N.; Almeida, N.; Black, R.; Burns, R.; Bush, L.; Crawford, P.; Keim, N.; Kris-Etherton, P.; Weaver, C. Food Science Challenge: Translating the Dietary Guidelines for Americans to Bring about Real Behavior Change. J. Food Sci. 2011, 76, R29–37. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, T.D.; Rahkovsky, I. Economic Costs and Benefits of Healthy Eating. Curr. Obes. Rep. 2013, 2, 225–234. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J. Current Evidence on Healthy Eating. Annu. Rev. Public Health 2013, 34, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux. 2011; ISBN 9780374275631. [Google Scholar]
- Tversky, A.; Kahneman, D. Judgment under Uncertainty: Heuristics and Biases. Science 1974, 185, 1124–1131. [Google Scholar] [CrossRef]
- Engel, J.; Kollat, D.; Blackwell, R. Consumer Behavior; Holt, Rinehart & Winston: New York, 1968. [Google Scholar]
- Kemp, E.; Kopp, S.W. Emotion Regulation Consumption: When Feeling Better Is the Aim. J. Consum. Behav. 2011, 10, 1–7. [Google Scholar] [CrossRef]
- Kaplan, H.I.; Kaplan, H.S. The Psychosomatic Concept of Obesity. Journal of Nervous and Mental Disease 1957, 125, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Schachter, S.; Goldman, R.; Gordon, A. Effects of Fear, Food Deprivation, and Obesity on Eating. J Pers Soc Psychol 1968, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cardi, V.; Leppanen, J.; Treasure, J. The Effects of Negative and Positive Mood Induction on Eating Behaviour: A Meta-Analysis of Laboratory Studies in the Healthy Population and Eating and Weight Disorders. Neuroscience & Biobehavioral Reviews 2015, 57, 299–309. [Google Scholar]
- Evers, C.; Adriaanse, M.; de Ridder, D.T.D.; de Witt Huberts, J.C. Good Mood Food. Positive Emotion as a Neglected Trigger for Food Intake. Appetite 2013, 68, 1–7. [Google Scholar] [CrossRef]
- van Strien, T.; Cebolla, A.; Etchemendy, E.; Gutiérrez-Maldonado, J.; Ferrer-García, M.; Botella, C.; Baños, R. Emotional Eating and Food Intake after Sadness and Joy. Appetite 2013, 66, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Leigh Gibson, E. Emotional Influences on Food Choice: Sensory, Physiological and Psychological Pathways. Physiology & Behavior 2006, 89, 53–61. [Google Scholar]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Andrews, J.M.; Bryant, R.V. Food Avoidance, Restrictive Eating Behaviour and Association with Quality of Life in Adults with Inflammatory Bowel Disease: A Systematic Scoping Review. Appetite 2021, 167, 105650. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, W.; Vackier, I. Profile and effects of consumer involvement in fresh meat. Meat Science 2004, 67, 159–68. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.N.S.; Getz, D. Food Enthusiasts and Tourism: Exploring Food Involvement Dimensions. Journal of Hospitality and Tourism Research 2016, 40, 432–55. [Google Scholar] [CrossRef]
- Castellini, G.; Graffigna, G. Assessing involvement with food: A systematic review of measures and tools. Food Qual Prefer 2022, 97, 104444. [Google Scholar] [CrossRef]
- Graffigna, G.; Barello, S.; Riva, G.; Corbo, M.; Damiani, G.; Iannone, P.; Bosio, A.C.; Ricciardi, W. Italian Consensus Statement on Patient Engagement in Chronic Care: Process and Outcomes. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Rueda, J.-D.; Gronseth, G.S.; Mullins, C.D. Framework for Enhancing Clinical Practice Guidelines through Continuous Patient Engagement. Health Expect. 2017, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, J.; Riddle, A.; Akl, E.A.; Khabsa, J.; Lytvyn, L.; Atwere, P.; Campbell, P.; Chalkidou, K.; Chang, S.M.; Crowe, S.; et al. Protocol for the Development of Guidance for Stakeholder Engagement in Health and Healthcare Guideline Development and Implementation. Syst. Rev. 2020, 9, 21. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Nishida, C.; James, W.P.T. A Life Course Approach to Diet, Nutrition and the Prevention of Chronic Diseases. Public Health Nutr. 2004, 7, 101–121. [Google Scholar] [CrossRef]
- Fatemi, S.F.; Irankhah, K.; Kruger, J.; Bruins, M.J.; Sobhani, S.R. Implementing Micronutrient Fortification Programs as a Potential Practical Contribution to Achieving Sustainable Diets. Nutr. Bull. 2023, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean Diet with Four Sustainable Benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Madlala, S.S.; Hill, J.; Kunneke, E.; Lopes, T.; Faber, M. Adult Food Choices in Association with the Local Retail Food Environment and Food Access in Resource-Poor Communities: A Scoping Review. BMC Public Health 2023, 23, 1083. [Google Scholar] [CrossRef]
- Prescott, S.L.; D’Adamo, C.R.; Holton, K.F.; Ortiz, S.; Overby, N.; Logan, A.C. Beyond Plants: The Ultra-Processing of Global Diets Is Harming the Health of People, Places, and Planet. Int. J. Environ. Res. Public Health 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Jones, D.P. The Nature of Nurture: Refining the Definition of the Exposome. Toxicol. Sci. 2014, 137, 1–2. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).