1. Introduction
Long-term health consequences are influenced by circumstances that occur during pregnancy. The confluence of the maternal and fetal circulations occurs at the placenta, which is the first organ to develop [1–4]. Placental pathology is relatively accurate in diagnosing intraamniotic inflammation [5]. Pathological changes of preterm placentas, including chronic chorioamnionitis and chronic deciduitis, suggest the cause of spontaneous preterm births and many perinatal pathologies, such as neonatal sepsis, asphyxia, bronchopulmonary dysplasia, and periventricular leukomalacia in preterm infants [6]. Many causes of spontaneous preterm births require a comprehensive understanding of the etiology, intensity, duration, characteristics and site of histological placental inflammation as predictors and determinants of the antenatal environment [7]. Microorganisms in the amniotic fluid were identified in case of clinical chorioamnionitis at term [8]. The inflammatory and microbial detections in the placenta can also predict parturition complications and explain adverse outcomes [9]. Maternal hyperthermia, leukocytosis, tachycardia, uterine tenderness, and preterm rupture of membranes are cardinal signs of clinical chorioamnionitis, which is less frequently observed than histologic chorioamnionitis [18,19].
In cases of intraamniotic infection or inflammation, the source of neutrophils in the amniotic fluid can originate from fetal or maternal origins, or a mixture of both, suggesting that both the fetus and the mother participate in the host defense mechanisms against intraamniotic infection [10]. While acute chorioamnionitis is evidence of a maternal host response, funisitis and chorionic vasculitis represent fetal inflammatory responses. Funisitis and chorionic vasculitis are the hallmarks of the fetal inflammatory response syndrome, and placental disease [11].
Inflammatory changes in the villous placenta in which maternal T cells infiltrate into chorionic villi by the induction of native T-cell chemokines could be an idiopathic occurrence. This occurrence is called villitis of unknown etiology (VUE). VUE poses a grave risk of neonatal neurodevelopmental abnormalities a few months after birth, which could manifest near the age of 2 years. Chronic chorioamnionitis is a predisposing factor for developing VUE. When chronic inflammatory infiltrates exist in the basal plate of the placenta, chronic deciduitis may develops [12]. Toll -like receptors (sTLR2) are part of the amniotic fluid innate immune system and participates in regulating the inflammatory response to microbial pathogens [13].
Placental chorioamnionitis is associated with ethnic disparity and premature birth before 35 weeks [14]. Chorioamnionitis was associated with increased levels of TNF, IL-1 and IL-6, while elevated IL-1, IL-6 and IL-8 concentrations [15]. High grade leukocyte infiltration in placenta tissues is associated with elevated levels of TNFα, IL-1β, IL-6, IL-8, p55, p75, IL-1RA and C-reactive protein in umbilical serum.
The presence of neonatal disease is associated with advanced chorioamnionitis, and highly elevated levels of both pro- and anti-inflammatory mediators in umbilical serum [13]. Chorioamnionitis can both injure and mature the fetal lung and cause immune nodulation. Postnatal care strategies also change how chorioamnionitis is related to clinical outcomes such as bronchopulmonary dysplasia [16].
VUE is a designation commonly used to describe the inflammatory infiltration of maternal T cells into the fetal chorionic villi, rendering devastating villous inflammation. T lymphocytes infiltrating chorionic villi demonstrate immunopositivity for CD3 and CD8. Hofbauer cells are positive for CD4 and CD14. Macrophages are positive for CD4 and CD68. Given the lack of diagnostic consensus on clinical identification of VUE cases, the histological examination shows a variety of microscopic pictures that are all subcategorized under the broad spectrum of VUE. The proliferative activity with areas of necrosis and granulation tissue formation is conspicuous when chronic villi are affected. The phenotypes are either distal, where terminal or mature intermediate villi are evident, or proximal when the stem villi are involved. The basal type consists of anchoring villi that lead to chronic deciduitis. Redline classified VUE into low-grade and high-grade VUE making with distinction as involvement of whether ten villi are affected per focus. Low grade VUE is defined as the presence of inflammation affecting fewer than 10 contiguous villi in any one focus, and more than one focus required for the diagnosis. High grade VUE is defined as the presence of multiple foci, on more than one section, at least one of which shows inflammation affecting more than 10 contiguous villi [23].
Chronic deciduitis is diagnosed in the presence of lymphoplasmacytic inflammation in the decidua or, in the absence of plasma cells, the presence of diffuse and intense (>50/HPF) non-perivascular lymphocytic inflammation [24]. VUE shows some extent of vasculitis or perivasculitis that may cause fetal vascular obliteration or thrombotic occlusions. Some cytologic features and immunohistochemical staining distinguish infectious villitis from VUE. The causative infectious organisms include syphilis, cytomegalovirus, parvovirus B19, or rubella. Thanks to the advances in vaccination, virally induced villitis is now of rare incidence.
This retrospective study aimed to re-examine placentas regarded as normal by the Obstetrics and Gynecology Department in our institution so that the grading and staging of any evident inflammatory response could be evaluated according to the gender of newborns. This study also aimed to find the relationship between inflammatory pathological aspects of the placenta
2. Materials and Methods
2.1. Study Design
This study was conducted at King Saud University Medical City in Riyadh, Saudi Arabia, during January and August 2019.This study focused on the placentas considered as normal by the Obstetrics and Gynecology Department which were not sent to the Histopathology Department. This research was approved by the institutional review board (IRB: E-17-2729). The consent of the women participating in this study including the description of their medical reports and the collection of placental tissue.
2.2. Placental Samples
Eighty-four full-term placentas were collected directly after delivery. Placentas from women with chronic diseases, non-Saudi women, and twin deliveries were excluded. Measurements of fresh placenta including weight, length, and width were recorded for morphological examination. The length, coiling, and diameter of the umbilical cord were also measured.
2.3. Histological Study
Full thicknesses of placenta samples were obtained from the fetal side to maternal side. The placenta samples were taken from two regions from placenta disc at the central and marginal regions. Samples of placenta were fixed in neutral buffered formalin (10%) and then dehydrated by passing them on an upward series of ethanol, then clearing in xylene, and embedding in paraffin wax. The blocks were sectioned into 3–5 µm and stained with hematoxylin–eosin stain. The standards suggested by the Amsterdam Placental Workshop Group were used to rate inflammation in placenta included maternal inflammatory response, fetal inflammatory response, villitis of an unknown etiology, and chronic deciduitis.
The different sections of the placental tissues were chosen for microscopic examination depending on the extent of the inflammation. The Olympus BX63 microscope with DP80 digital camera and connected to Cellsens Entry imaging software were used for examination and imaging of tissue sections for placentas.
2.4. Immunohistochemistry Staining
Paraffin wax blocks in each of the studied placentas were evaluated with immunohistochemistry (IHC) using murine monoclonal anti-CD8 (C8/144B – IHC – Prediluted [NBP2-45325] Novus Biologicals, USA), and murine monoclonal anti-CD68 (PG-M1;[M0876], Dako, Carpinteria, CA, USA; 1:20) to detect the inflammatory cells. Blocks were cut to 3 µl in thickness and remove the paraffin by passing tissue sections on xylene twice for 10 minutes each time. The tissue sections were hydrate with a descending series of ethanol and then immersed in distilled water for 5 minutes each stage. The tissue sections were incubated with peroxidase then with a protein block for 5 min each. The antibodies were diluted according to the manufacturer’s instructions and the tissue sections were incubated with primary antibody for overnight at 4ᵒ C. The primary antibodies were removed then the tissue sections were washed. The tissue sections following treatment for 30 minutes with secondary antibodies at room temperature. The manufacturer'’s instructions were followed in order to complete these procedures. At 400× magnification power, the hotspots were assessed by two pathologists and by histomorphometry. The morphological evaluation of IHC expression was conducted by ImageJ software to measure the area fraction computationally.
2.5. Statistical Analysis
Data were presented as mean ± standard deviations and percentages. The descriptive analysis, independent-sample t-tests, Pearson’s chi-square test and, phi correlation coefficient were performed using SPSS program version 25 (IBM Inc., Armonk, NY). Differences were considered significant at p < 0.05.
3. Results
3.1. Anthropometric of Population Study and Placenta Inflammation
The characteristics of pregnant women who participated in this study and their offspring are shown in
Table 1. Pregnant women on average were 30 years old, weighed 79 kg on average, stood 158 cm height, and had a body mass index of 32 kg/m2. Babies in this research were measured at 3179 g and 49.7 cm in length, respectively. The average weight of the placenta was 446 g.
After examining the placental sample with light microscopy and immunohistochemistry, representative photomicrographs were captured for histomorphometric analysis. The clinical findings were charted to be correlated with the histological pictures. The maternal inflammatory response was positive in seventeen cases (20.2%) from 84 status. The staging of the positive cases was 8 (9.5%) in Stage 1, seven cases (8.3%) in Stage 2, and two cases (2.4%) in Stage 3. The grading was as follows: Grade 1, eight case (9.6%), and Grade 2, nine cases (10.7%). For the fetal inflammatory response, only ten cases were positive (11.9%) from 84 status. Of these positive responses, ten cases were in Stage 1 (11.9%). No cases were detected in other stages. The grading of stage 1 cases was as follows: Grade 1 (8 cases) while Grade 2 was only seen in two cases (
Table 2).
Figure 1A–F displays normal and maternal inflammatory responses in placenta. Fetal inflammatory responses are illustrated in
Figure 2B–D.
Table 3 demonstrates the number and percentage of the diagnosed VUE and chronic deciduitis in the maternal placenta. Villitis of unknown etiology and deciduits were illustrated in
Figure 3. Immunohistochemistry was utilized to confirm the presence of inflammatory cells (plasma cells and T lymphocyte) in chorionic villi, and the results were positive for the antibodies employed CD8 and CD86 (
Figure 4).
3.2. Anthropometric of Population Study and Placenta Inflammation According to the Gender of Babies
Based on the gender of the newborn, differences in the study population were examined. Out of the 84 pregnant women who participated in this study, 37 gave birth to boys, and 47 gave birth to girls. The findings revealed that there were no significant variations in the measurements of the mothers, newborns, or placenta between male and female newborns, with the exception of the mothers’ taller, where mothers of female were higher than mothers of male (
Table 4).
The prevalence of fetal and maternal inflammatory responses were greater in the placentas of mothers of males than in placentas of female mothers (
Table 5). However there is no significant difference in VUE or chronic deciduitis percentage in the placentas of mothers of both genders (
Table 6).
Table 7 compares the study of population’s characteristics, based on the presence of inflammation model in the placenta. The placentas of mothers who were less obese showed signs of maternal and fetal inflammation. The VUE has also been seen in placentas with lower weight.
Table 8 reveals the correlation between inflammatory signs of the placenta, indicating that there is a positive correlation between maternal inflammatory response and the fetal inflammatory response. The results also indicate that there is a positive correlation between chronic deciduitis with maternal and fetal inflammatory response. The correlation between maternal inflammatory response and fetal inflammatory response is strongly positive. The coexistence of chronic chorioamnionitis with VUE was not detected in the positive cases of the current study
4. Discussion
Because mother-to-fetus nutrition occurs through the placenta, successful pregnancy and fetal growth depend on the functionality of the normal placenta and maternal circulation. Maternal growth factors required for placental development and their corresponding mechanisms of action were reviewed by Forbes et al. [17]. In the past ten years, research on the developmental causes of health and disease has concentrated its attention to the placenta [25]. The placenta was considered to be the "center of the chronic disease universe" in a recent article [26]. In the investigation of the risk of chronic disease, placental inflammation is a sub-focus, especially in consideration of the worldwide obesity pandemic and low-level [4]. Numerous factors can cause placental inflammation, including maternal autoimmune diseases, genetic risk factors, obesity, and the immune system'’s response to an infection [1] with bacteria [27–29], viruses [30], other infectious organisms.
Previous study indicated that the VUE can appear in 5–15 % of placentas [31]. This is consistent with the current study. The molecular explanation of inclining to develop VUE is yet to be explored. Although Perforin-1 and Granzyme B are reported to play a pivotal role in triggering some cell-mediated immune responses that trigger cascade of caspases to initiate cytolysis and apoptosis execution, this molecular evidence is not applauded among pathologists. Other studies suggest that the activation of C5 initiates the inflammatory reaction [32]. Cole et al [33] concluded that stimulating MAIT cells by IL-7, IL-12, IL-15, or IL-18 trigger the secretion of interferon-γ, tumor necrosis factor-α, and IL-17, and in addition, mediate cytotoxic effects via granzyme B (GrzB) and perforin. Degrading granzyme B reduces the efficacy of NK cell-mediated lysis, minimizing sensitivity and stronger immune escape ability [34]. Chronic chorioamnionitis shows histologically inflammatory infiltrates extending into the chorioamniotic membranes or the chorionic plate. The immunoreactivity of these cells is usually patchy or diffuse immunopositivity for maternal CD8+ T cells. Trophoblast damage by CD8+ T cells in the form of apoptosis can be demonstrated using double immunofluorescence staining with antibodies against CD8+ lymphocytes and M30. The detection of lymphocytes and plasma cells in the basal plate of the placenta is suggestive of chronic deciduitis. This inflammatory cell population is proposed to migrate to the basal plate owing to microbial or immunological etiopathogenesis. In our study, the frequency of chronic deciduitis and VUE were more remarkable that the previously reported findings in the literature of placental pathology [35].
Grading placental inflammation usually predicts the degree of maternal anti-fetal cellular rejection. It is possible to screen for anti-fetal antibody-mediated rejection by identifying of maternal serum antibodies against fetal HLA and determing whether they are specific for the fetus in the index pregnancy. Although immunohistochemical workup using CD68 is the gold standard, CD163, perforin, granzyme B, granzyme K, and C5b-9 seem to be key proteins in the pathogenesis of placental pathology.
According to the findings of this study, the fetal and Maternal inflammatory responses were more frequently in the placentas of male mothers compared to females. Similar studies have demonstrated an increased cytokine response in plasma of male infant compared to female at birth [36,37]. Cytokine antagonists are upregulated to maximize the labor-inducing inhibition of the production of cytokine that could contribute to parturition [15]. Cytokines participate in the placental paracrine or autocrine regulatory networks during the second and third gestation trimester to protect the fetus from pathological organisms. They also contribute to fetus expulsion by uterine contractions, membrane rupture, and cervix dilation [38]. Although extraplacental membranes change during normal term parturition, the labor-associated changes in villous placenta are more remarkable [38]. Interleukin-6 is a diagnostic marker of intra-amniotic inflammation, and is predictive of those at risk for impending preterm delivery [39]. The increased incidence of pregnancy complications in male fetuses compared to female fetuses, including spontaneous abortions, preterm birth, and preterm premature rupture of membranes [40–42], may be explained by the gender-specific difference in inflammatory response noted in the placenta in this study.
The maternal and fetal inflammatory response tends to appear in the placentas of pregnant women with lower body mass, and the VUE tend to occur in low-weight placentas as shown by the results of this study. Goldstein et al indicated that pregnant women with a high BMI tend to have chronic inflammation [1]. Additionally, it has been shown in other earlier investigations that fetal growth restriction, preterm delivery, and low birth weight are all linked to increased placental inflammation [12,31,43]. This may be due to differences in lifestyles in the study populations.
The findings of this study showed correlations between inflammatory aspects in the placenta. This result agreed with previous studies that indicate a relationship between inflammatory aspects in the placenta [29,44,45].
5. Conclusions
Grading placental inflammation usually predicts the degree of maternal anti-fetal cellular rejection. It is possible to screen for anti-fetal antibody-mediated rejection by identifying maternal serum antibodies against fetal HLA, and determining whether they are specific to the fetus in the index pregnancy. There are differences in the placental inflammatory response based on the sex of the newborn, which may explain some of the complications that occur in mothers of males. Because placental pathologies were undiagnosed, many cases of fetal inflammatory response syndrome went unnoticed in the studied population. Therefore, it may be better to expand the types of placental samples that need to be sent for microscopic examination and study their relationship to maternal and newborn measurements in an attempt to discover the causes of chronic diseases.
Author Contributions
Alwasel, S. and Aljerian K. disgned this experiment, Aldahmash, W. performed the experiment and write the article. Alwasel, S., and Aljerian, K. reviewed this manuscript.
Funding
This research was funded by Researchers Supporting Program number (RSPD2023R1080), in King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (IRB: E-17-2729) in King Saud University Medical City.
Informed Consent Statement
The consent of the women participating in this study including the description of their medical reports and the collection of placental tissue.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Program number (RSPD2023R1080), in King Saud University, Riyadh, Saudi Arabia, for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R., and Gernand, A.D. Maternal-Fetal Inflammation in the Placenta and the Developmental Origins of Health and Disease. Frontiers in Immunology, 2020. 11. [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S., and Swanson, J.M. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin Reprod Med, 2009. 27(05): p. 358-368. [CrossRef]
- Norris, S.A.; Daar, A.; Balasubramanian, D.; Byass, P.; Kimani-Murage, E.; Macnab, A.; Pauw, C.; Singhal, A.; Yajnik, C.; Akazili, J., et al. Understanding and acting on the developmental origins of health and disease in Africa would improve health across generations. Global Health Action, 2017. 10(1): p. 1334985. [CrossRef]
- Goldstein, J.A.; Norris, S.A., and Aronoff, D.M. DOHaD at the intersection of maternal immune activation and maternal metabolic stress: a scoping review. Journal of Developmental Origins of Health and Disease, 2017. 8(3): p. 273-283. [CrossRef]
- Pettker, C.M.; Buhimschi, I.A.; Magloire, L.K.; Sfakianaki, A.K.; Hamar, B.D., and Buhimschi, C.S. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstetrics & Gynecology, 2007. 109(3): p. 739-749. [CrossRef]
- Faye-Petersen, O. The placenta in preterm birth. Journal of clinical pathology, 2008. 61(12): p. 1261-1275. [CrossRef]
- Redline, R.W. Inflammatory responses in the placenta and umbilical cord. in Seminars in Fetal and Neonatal Medicine. 2006. Elsevier. [CrossRef]
- Romero, R.; Miranda, J.; Kusanovic, J.P.; Chaiworapongsa, T.; Chaemsaithong, P.; Martinez, A.; Gotsch, F.; Dong, Z.; Ahmed, A.I., and Shaman, M. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine, 2015. 43(1): p. 19-36. [CrossRef]
- Redline, R.W. Placental inflammation. in Seminars in Neonatology. 2004. Elsevier. [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Leng, Y.; Garcia-Flores, V.; Miller, D.; Jacques, S.M.; Hassan, S.S.; Faro, J., and Alsamsam, A. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? American journal of obstetrics and gynecology, 2017. 217(6): p. 693. e1-693. e16. [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H., and Kim, Y.M. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology, 2015. 213(4): p. S29-S52. [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P., and Kim, J.-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. American journal of obstetrics and gynecology, 2015. 213(4): p. S53-S69. [CrossRef]
- Dulay, A.T.; Buhimschi, C.S.; Zhao, G.; Oliver, E.A.; Mbele, A.; Jing, S., and Buhimschi, I.A. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. The Journal of Immunology, 2009. 182(11): p. 7244-7253. [CrossRef]
- Holzman, C.; Lin, X.; Senagore, P., and Chung, H. Histologic chorioamnionitis and preterm delivery. American journal of epidemiology, 2007. 166(7): p. 786-794. [CrossRef]
- Arntzen, K.J.; Kjøllesdal, A.M.; Halgunset, J.; Vatten, L., and Austgulen, R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. 1998. [CrossRef]
- Jobe, A.H. Effects of chorioamnionitis on the fetal lung. Clinics in perinatology, 2012. 39(3): p. 441-457. [CrossRef]
- Forbes, K. and Westwood, M. Maternal growth factor regulation of human placental development and fetal growth. Journal of Endocrinology, 2010. 207(1): p. 1-16. [CrossRef]
- Redline, R.W. Inflammatory response in acute chorioamnionitis. in Seminars in Fetal and Neonatal Medicine. 2012. Elsevier. [CrossRef]
- Galinsky, R.; Polglase, G.R.; Hooper, S.B.; Black, M.J., and Moss, T.J. The consequences of chorioamnionitis: preterm birth and effects on development. Journal of pregnancy, 2013. 2013. [CrossRef]
- Menon, R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Frontiers in immunology, 2014. 5: p. 567. [CrossRef]
- Menon, R.; Behnia, F.; Polettini, J.; Saade, G.R.; Campisi, J., and Velarde, M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY), 2016. 8(2): p. 216. [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.-È.; Duval, C.; Chemtob, S., and Girard, S. Sterile inflammation and pregnancy complications: a review. Reproduction, 2016. 152(6): p. R277-R29. [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M., and Gillan, J.E. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Archives of pathology & laboratory medicine, 2016. 140(7): p. 698-713. [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Gordijn, S.J.; Morgan, T.K., and Nikkels, P.G. Introduction: An Approach to Placental Pathology. Pathology of the Placenta: A Practical Guide, 2019: p. 3-8. [CrossRef]
- Burton, G.J.; Fowden, A.L., and Thornburg, K.L. Placental Origins of Chronic Disease. Physiological Reviews, 2016. 96(4): p. 1509-1565. [CrossRef]
- Thornburg, K.L. and Marshall, N. The placenta is the center of the chronic disease universe. American Journal of Obstetrics and Gynecology, 2015. 213(4, Supplement): p. S14-S20. [CrossRef]
- Roberts, D.J.; Celi, A.C.; Riley, L.E.; Onderdonk, A.B.; Boyd, T.K.; Johnson, L.C., and Lieberman, E. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One, 2012. 7(3): p. e31819. [CrossRef]
- Pankuch, G.A.; Appelbaum, P.C.; Lorenz, R.P.; Botti, J.J.; Schachter, J., and Naeye, R.L. Placental microbiology and histology and the pathogenesis of chorioamnionitis. Obstetrics & Gynecology, 1984. 64(6): p. 802-806.
- Romero, R.; Salafia, C.M.; Athanassiadis, A.P.; Hanaoka, S.; Mazor, M.; Sepulveda, W., and Bracken, M.B. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. American Journal of Obstetrics and Gynecology, 1992. 166(5): p. 1382-1388. [CrossRef]
- Ernst, L.M.; Bockoven, C.; Freedman, A.; Wang, V.; Pellerite, M.; Wylie, T.N., and Wylie, K.M. Chronic villitis of unknown etiology: Investigations into viral pathogenesis. Placenta, 2021. 107: p. 24-30. [CrossRef]
- Redline, R.W. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Human Pathology, 2007. 38(10): p. 1439-1446. [CrossRef]
- Peng, T.; Hao, L.; Madri, J.A.; Su, X.; Elias, J.A.; Stahl, G.L.; Squinto, S., and Wang, Y. Role of C5 in the development of airway inflammation, airway hyperresponsiveness, and ongoing airway response. J Clin Invest, 2005. 115(6): p. 1590-600. [CrossRef]
- Cole, S.; Murray, J.; Simpson, C.; Okoye, R.; Tyson, K.; Griffiths, M.; Baeten, D.; Shaw, S., and Maroof, A. Interleukin (IL)-12 and IL-18 Synergize to Promote MAIT Cell IL-17A and IL-17F Production Independently of IL-23 Signaling. Front Immunol, 2020. 11: p. 585134. [CrossRef]
- Hirst, C.E.; Buzza, M.S.; Sutton, V.R.; Trapani, J.A.; Loveland, K.L., and Bird, P.I. Perforin-independent expression of granzyme B and proteinase inhibitor 9 in human testis and placenta suggests a role for granzyme B-mediated proteolysis in reproduction. Molecular human reproduction, 2001. 7(12): p. 1133-1142. [CrossRef]
- Ito, Y.; Matsuoka, K.; Uesato, T.; Sago, H.; Okamoto, A.; Nakazawa, A., and Hata, K. Increased expression of perforin, granzyme B, and C5b-9 in villitis of unknown etiology. Placenta, 2015. 36(5): p. 531-537. [CrossRef]
- Majetschak, M.; Christensen, B.; Obertacke, U.; Waydhas, C.; Schindler, A.E.; Nast-Kolb, D., and Schade, F.U. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma, 2000. 48(5): p. 832-9; discussion 839-40. [CrossRef]
- Kim-Fine, S.; Regnault, T.R.; Lee, J.S.; Gimbel, S.A.; Greenspoon, J.A.; Fairbairn, J.; Summers, K., and de Vrijer, B. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Matern Fetal Neonatal Med, 2012. 25(11): p. 2470-4. [CrossRef]
- Bowen, J.; Chamley, L.; Keelan, J., and Mitchell, M. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta, 2002. 23(4): p. 257-273. [CrossRef]
- Chaemsaithong, P.; Romero, R.; Korzeniewski, S.J.; Martinez-Varea, A.; Dong, Z.; Yoon, B.H.; Hassan, S.S.; Chaiworapongsa, T., and Yeo, L. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. The Journal of Maternal-Fetal & Neonatal Medicine, 2016. 29(3): p. 349-359. [CrossRef]
- Ingemarsson, I. Gender aspects of preterm birth. Bjog, 2003. 110 Suppl 20: p. 34-8. [CrossRef]
- Cooperstock, M. and Campbell, J. Excess males in preterm birth: interactions with gestational age, race, and multiple birth. Obstet Gynecol, 1996. 88(2): p. 189-93. [CrossRef]
- Hassold, T.; Quillen, S.D., and Yamane, J.A. Sex ratio in spontaneous abortions. Ann Hum Genet, 1983. 47(1): p. 39-47. [CrossRef]
- Kovo, M.; Ganer Herman, H.; Gold, E.; Bar, J., and Schreiber, L. Villitis of unknown etiology – prevalence and clinical associations. The Journal of Maternal-Fetal & Neonatal Medicine, 2016. 29(19): p. 3110-3114. [CrossRef]
- Jacques, S.M. and Qureshi, F. Chronic chorioamnionitis: A clinicopathologic and immunohistochemical study. Human Pathology, 1998. 29(12): p. 1457-1461. [CrossRef]
- Kim, C.J.; Romero, R.; Kusanovic, J.P.; Yoo, W.; Dong, Z.; Topping, V.; Gotsch, F.; Yoon, B.H.; Chi, J.G., and Kim, J.-S. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Modern Pathology, 2010. 23(7): p. 1000-1011. [CrossRef]
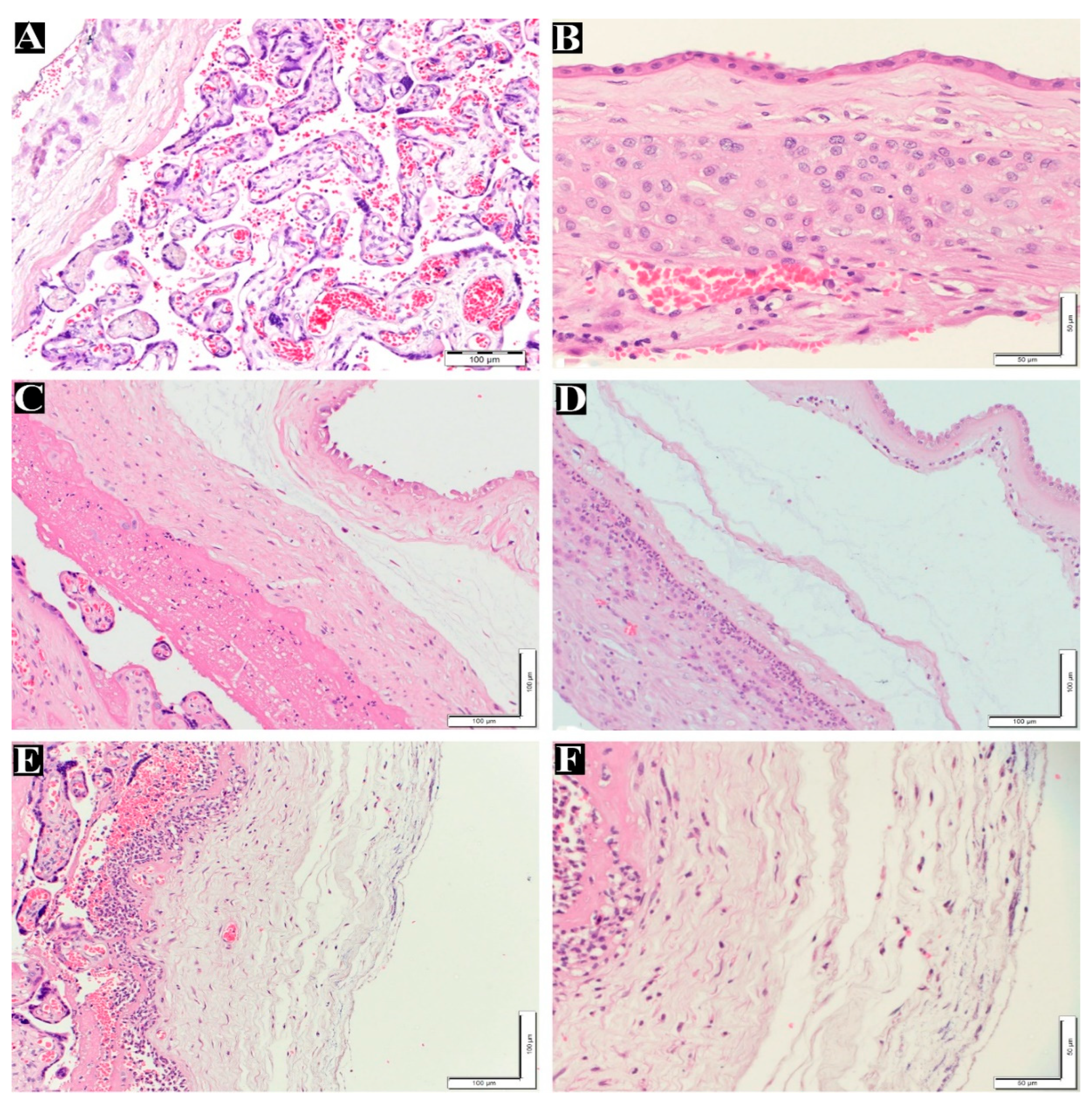
Figure 1.
Maternal inflammatory response stages and grades in placenta. (A) normal villi and decidua layer without inflammatory cells (200x). (B) Normal membrane without inflammatory cells in chorion and amnion layer (400x). (C) Maternal inflammatory response stage 1 (chorionitis), grade1 200x. (D) Maternal inflammatory response stage 2, grade 2 (200x). (E) Maternal inflammatory response stage 3, Grade 2 (200x). (F) High magnification for picture(E) Maternal inflammatory response stage 3, Grade 2 (400x). H&E staining.
Figure 1.
Maternal inflammatory response stages and grades in placenta. (A) normal villi and decidua layer without inflammatory cells (200x). (B) Normal membrane without inflammatory cells in chorion and amnion layer (400x). (C) Maternal inflammatory response stage 1 (chorionitis), grade1 200x. (D) Maternal inflammatory response stage 2, grade 2 (200x). (E) Maternal inflammatory response stage 3, Grade 2 (200x). (F) High magnification for picture(E) Maternal inflammatory response stage 3, Grade 2 (400x). H&E staining.
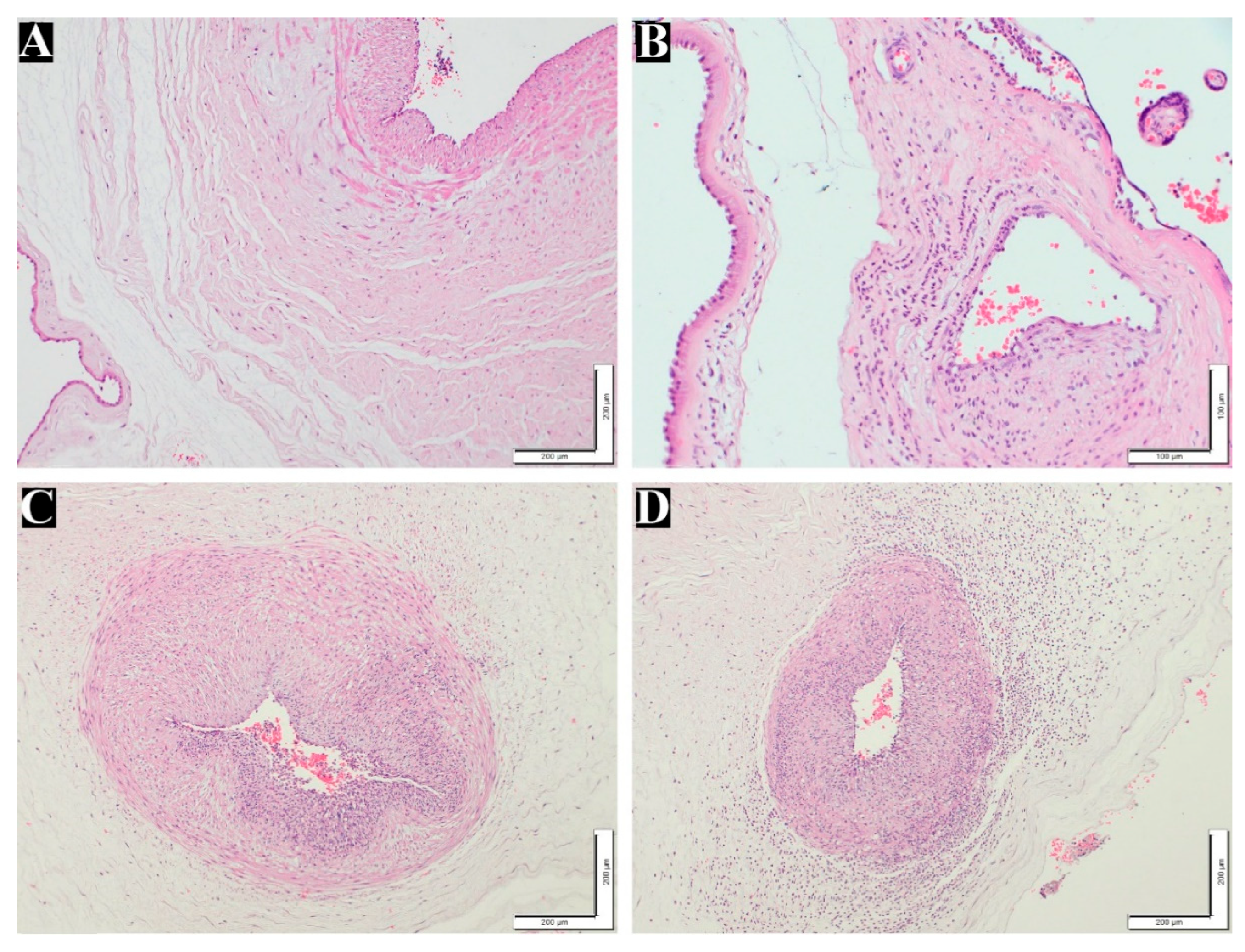
Figure 2.
Fetal inflammatory response stages and grades in placenta. (A) normal umbilical artery (100x). (B) Fetal inflammatory response stage 1, grade 1 (200x). (C) Fetal inflammatory response stage 2, grade 2 (100x). (D) Fetal inflammatory response stage 3, grade 2 (100x). H&E staining.
Figure 2.
Fetal inflammatory response stages and grades in placenta. (A) normal umbilical artery (100x). (B) Fetal inflammatory response stage 1, grade 1 (200x). (C) Fetal inflammatory response stage 2, grade 2 (100x). (D) Fetal inflammatory response stage 3, grade 2 (100x). H&E staining.
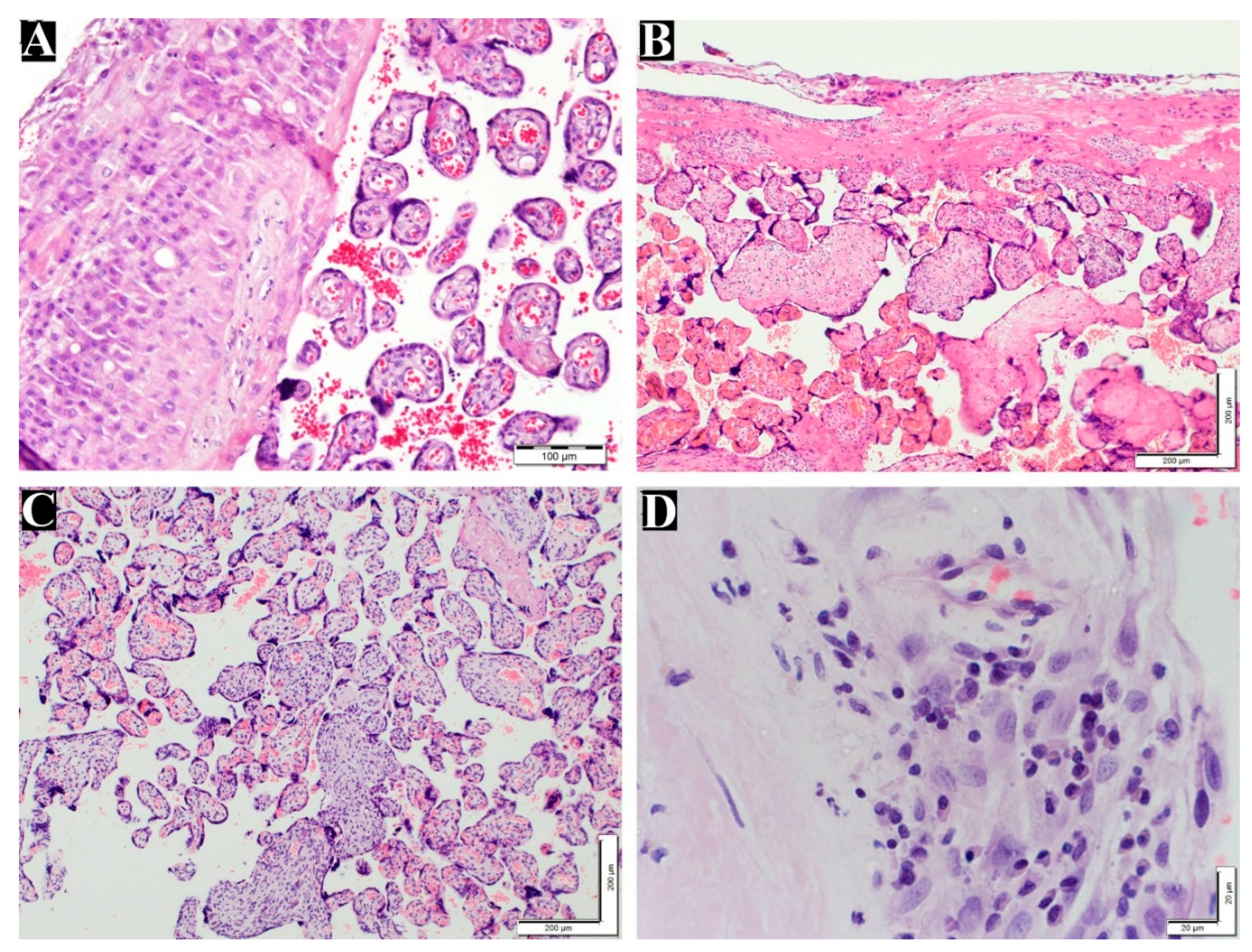
Figure 3.
Grade of villitis unknown etiology and chronic deciduitis in placenta. (A) normal chorionic villi and decidua layer (200x). (B) Low grade VUE (100x). (C) High grade VUE (100x). (D) Chronic deciduitis with plasma cells (600x). H&E staining.
Figure 3.
Grade of villitis unknown etiology and chronic deciduitis in placenta. (A) normal chorionic villi and decidua layer (200x). (B) Low grade VUE (100x). (C) High grade VUE (100x). (D) Chronic deciduitis with plasma cells (600x). H&E staining.
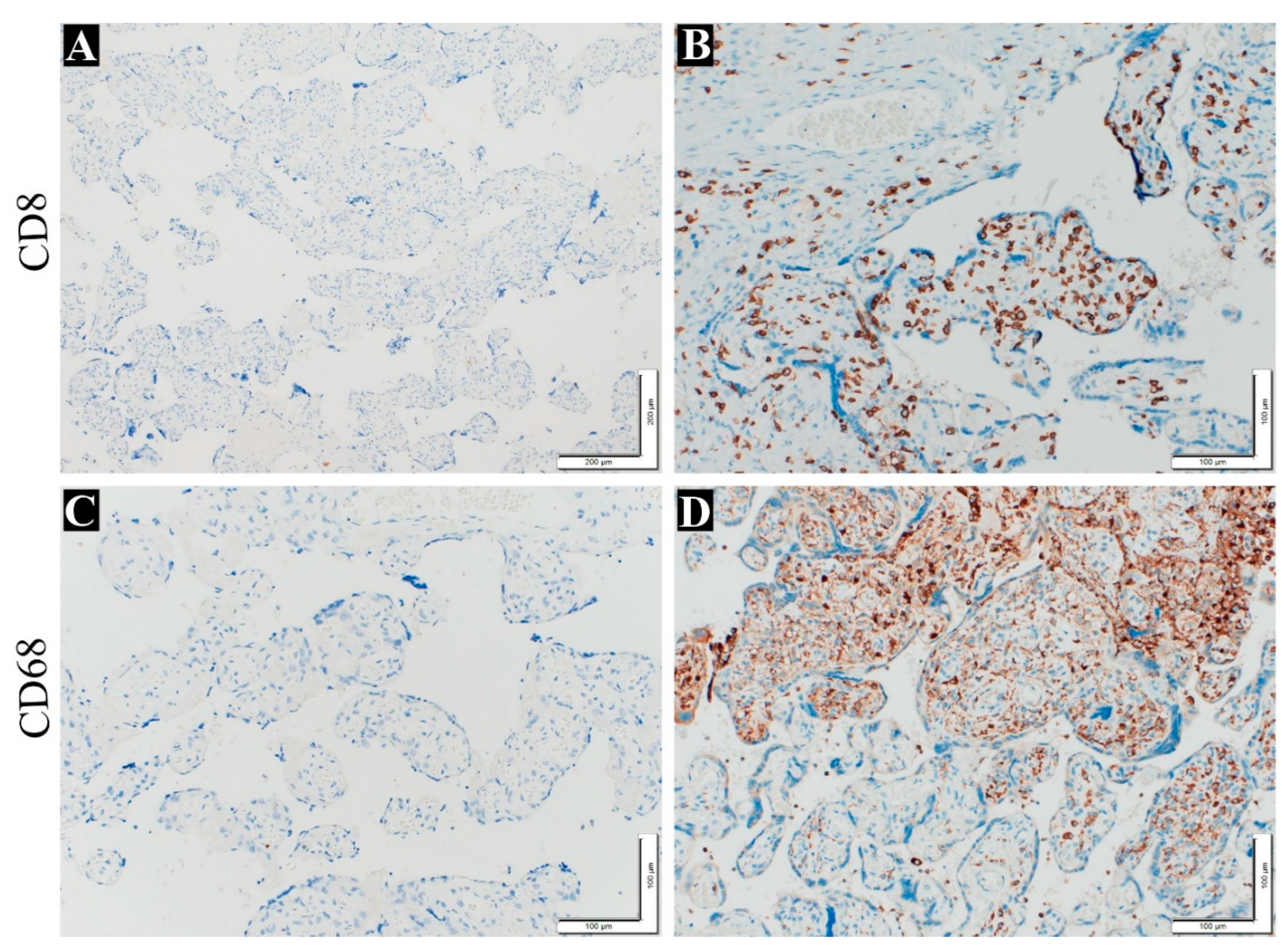
Figure 4.
Immunohistochemistry staining to detect VUE in the placenta. (A) show negative control slide without anti-CD8 (100x), (B) show T cell stain with anti-CD8 (200x), (C) show negative control slide without anti-CD68 (200x), (D) show macrophage cell stain with anti CD68 (200x).
Figure 4.
Immunohistochemistry staining to detect VUE in the placenta. (A) show negative control slide without anti-CD8 (100x), (B) show T cell stain with anti-CD8 (200x), (C) show negative control slide without anti-CD68 (200x), (D) show macrophage cell stain with anti CD68 (200x).
Table 1.
Anthropometrics of the study population.
Table 1.
Anthropometrics of the study population.
| |
|
Mean |
Std. Deviation |
| Mothers |
Age (Year) |
30.4 |
5.62 |
| |
Height (cm) |
157.96 |
4.96 |
| |
Weight (kg) |
79.47 |
14.57 |
| |
Parity |
1.66 |
1.72 |
| |
Body mass Index (kg/m2) |
31.76 |
5.57 |
| Babies |
Gestational age (week) |
38.6 |
1.45 |
| |
Birth weight (g) |
3179 |
442 |
| |
Length(cm) |
49.7 |
2.2 |
| Placenta |
Weight (g) |
445.6 |
86.5 |
| |
Length (cm) |
19.7 |
3.2 |
| |
Width (cm) |
16.2 |
1.8 |
| Umbilical Cord |
Length (cm) |
53.1 |
11.5 |
| |
Diameter (cm) |
1.2 |
0.2 |
| |
No. Coiling |
3.65 |
0.96 |
Table 2.
Fetal and maternal inflammatory response changes in the placenta.
Table 2.
Fetal and maternal inflammatory response changes in the placenta.
| |
|
|
No (%) |
| Maternal inflammatory response |
Positive |
|
17 (20.2) |
| |
Stage |
1 |
8 (9.5) |
| |
|
2 |
7 (8.3) |
| |
|
3 |
2 (2.4) |
| |
Grade |
1 |
8 (9.6) |
| |
|
2 |
9 (10.7) |
| Fetal inflammatory response |
Positive |
|
10 (11.9) |
| |
Stage |
1 |
10 (11.9) |
| |
|
2 |
0 (0) |
| |
|
3 |
0 (0) |
| |
Grade |
1 |
8 (9.5) |
| |
|
2 |
2 (2.4) |
Table 3.
Villitis of unknown etiology and chronic deciduitis in the placenta.
Table 3.
Villitis of unknown etiology and chronic deciduitis in the placenta.
| |
|
|
N (%) |
| VUE |
Positive |
|
8 (9.5) |
| |
Grade |
Low |
4 (4.8) |
| |
|
High |
4 (4.8) |
| Chronic deciduitis |
Positive |
|
55 (65.5) |
| |
|
Lymphocytes > 50 |
31 (36.9) |
| |
|
Plasma cells |
24 (28.6) |
Table 4.
Anthropometrics for study population compare between male and female babies.
Table 4.
Anthropometrics for study population compare between male and female babies.
| |
|
Male (n=37) |
Female (n=47) |
| |
|
Mean ± SD |
Mean ± SD |
p. value |
| Mothers |
Age (Years) |
29.3 ± 5.01 |
31.2 ± 5.96 |
0.1 |
| |
Height (cm) |
156.4 ± 3.8 |
159.1 ± 5.4 |
0.02 |
| |
Weight (kg) |
76 ± 13 |
82 ± 15.2 |
0.06 |
| |
Parity |
1.25 ± 1.5 |
1.98 ± 1.8 |
0.055 |
| |
Body mass Index (kg/m2) |
30.8 ± 5.3 |
32.5 ± 5.7 |
0.2 |
| Babies |
Gestational age (week) |
38.5 ± 1.7 |
38.7 ± 1.3 |
0.5 |
| |
Birth weight (g) |
3152 ± 444 |
3199 ± 444 |
0.6 |
| |
Length (cm) |
49.9 ± 2.3 |
49.5 ± 2.2 |
0.4 |
| |
Head Cir (cm) |
34 ± 1.3 |
34.3 ± 1.4 |
0.2 |
| |
Chest Cir (cm) |
33.7 ± 2.7 |
33 ± 2 |
0.2 |
| |
Thigh Cir (cm) |
16.4 ± 1.8 |
16 ± 1.7 |
0.3 |
| Placenta |
Weight (g) |
428.5 ± 78 |
458.7 ± 90 |
0.1 |
| |
Length (cm) |
19.4 ± 3.2 |
19.9 ± 3.3 |
0.5 |
| |
Width (cm) |
16 ± 1.7 |
16.4 ± 1.8 |
0.3 |
| Umbilical cord |
Length (cm) |
54.7 ± 10.7 |
51.8 ± 12 |
0.2 |
| |
Diameter (cm) |
1.2 ± 0.2 |
1.1 ± 0.2 |
0.1 |
| |
No. Coiling |
3.4 ± 0.9 |
3.8 ± 1 |
0.1 |
Table 5.
Fetal and maternal inflammation response in the placenta according to male and female babies.
Table 5.
Fetal and maternal inflammation response in the placenta according to male and female babies.
| |
|
|
Male
(n=37) |
|
Female (n=47) |
p. value |
| |
|
|
N (%) |
|
N (%) |
|
| Maternal inflammatory response |
Positive |
|
12 (32.4) |
|
5 (10.6) |
0.01 |
| |
Stage |
1 |
7 (18.9) |
|
1 (2.1) |
|
| |
|
2 |
3 (8.1) |
|
4 (8.5) |
|
| |
|
3 |
2 (5.4) |
|
0 (0) |
|
| |
|
|
|
|
|
|
| |
Grade |
1 |
5 (13.5) |
|
3 (6.4) |
|
| |
|
2 |
7 (18.9) |
|
2 (4.3) |
|
| |
|
|
|
|
|
|
| Fetal inflammatory response |
Positive |
|
8 (21.6) |
|
2 (4.3) |
0.01 |
| |
Stage |
1 |
8 (21.6) |
|
2(4.3) |
|
| |
|
2 |
0 |
|
0 |
|
| |
|
3 |
0 |
|
0 |
|
| |
|
|
|
|
|
|
| |
Grade |
1 |
7 (18.9) |
|
1 (2.1) |
|
| |
|
2 |
1 (2.7) |
|
1 (2.1) |
|
Table 6.
VUE and chronic deciduitis in the placenta according to gender of babies.
Table 6.
VUE and chronic deciduitis in the placenta according to gender of babies.
| |
|
|
Male (n=37) |
Female (n=47) |
p. value |
| |
|
|
N (%) |
N (%) |
| VUE |
Positive |
|
6 (16.2) |
2 (4.2) |
0.06 |
| |
Grade |
Low |
3 (8.1) |
1 (2.1) |
|
| |
|
High |
3 (8.1) |
1 (2.1) |
|
| |
|
|
|
|
|
| Chronic deciduitis |
Positive |
|
21 (56.8) |
34 (72.3) |
0.3 |
| |
|
Lymphocytes > 50 |
11 (29.7) |
20 (42.6) |
|
| |
|
Plasma cells |
10 (27.1) |
14 (29.8) |
|
Table 7.
A comparison between populations anthropometrics based on the appearance of placental inflammatory.
Table 7.
A comparison between populations anthropometrics based on the appearance of placental inflammatory.
| |
Maternal inflammation |
Fetal inflammation |
VUE |
|
Chronic deciduitis |
| |
|
|
|
|
|
|
|
|
| |
Negative |
Positive |
p. value |
Negative |
Positive |
p. value |
Negative |
Positive |
p. value |
Negative |
Positive |
p. value |
| Mother BMI (kg/m2) |
32.6 |
28.3 |
0.01 |
32.3 |
27.6 |
0.02 |
31.9 |
30.2 |
0.36 |
30.5 |
32.4 |
0.21 |
| Baby weight (g) |
3135 |
3351 |
0.07 |
3170 |
3248 |
0.60 |
3188 |
3095 |
0.57 |
3224 |
3155 |
0.49 |
| Placental weight (g) |
440 |
469 |
0.22 |
445 |
452 |
0.81 |
452 |
387 |
0.04 |
454 |
441 |
0.53 |
| Gestational age (week) |
38.5 |
39.2 |
0.09 |
38.6 |
39.2 |
0.20 |
38.6 |
39 |
0.47 |
38.8 |
38.5 |
0.45 |
Table 8.
Correlations between inflammatory features of the placenta.
Table 8.
Correlations between inflammatory features of the placenta.
| |
Maternal inflammatory response |
Fetal inflammatory response |
VUE |
| Fetal inflammatory response |
0.73***
|
|
|
| VUE |
0.06 |
0.01 |
|
| Chronic deciduitis |
0.26*
|
0.27*
|
0.06 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).