Introduction
Regenerative medicine is a multidisciplinary field that focuses on emerging or reinforcing functioning organs, tissues, and cells while replacing/repairing injured ones [
1,
2]. Regenerative medicine offers a solution to many health problems, such as organ failure, traumatic injuries, and progressive conditions that don't reply well to conventional medicine [
3]. Regenerative medicine has already made some noteworthy advances, such as the use of skin grafts and stem cells to support the recovery of severe burns [
3].
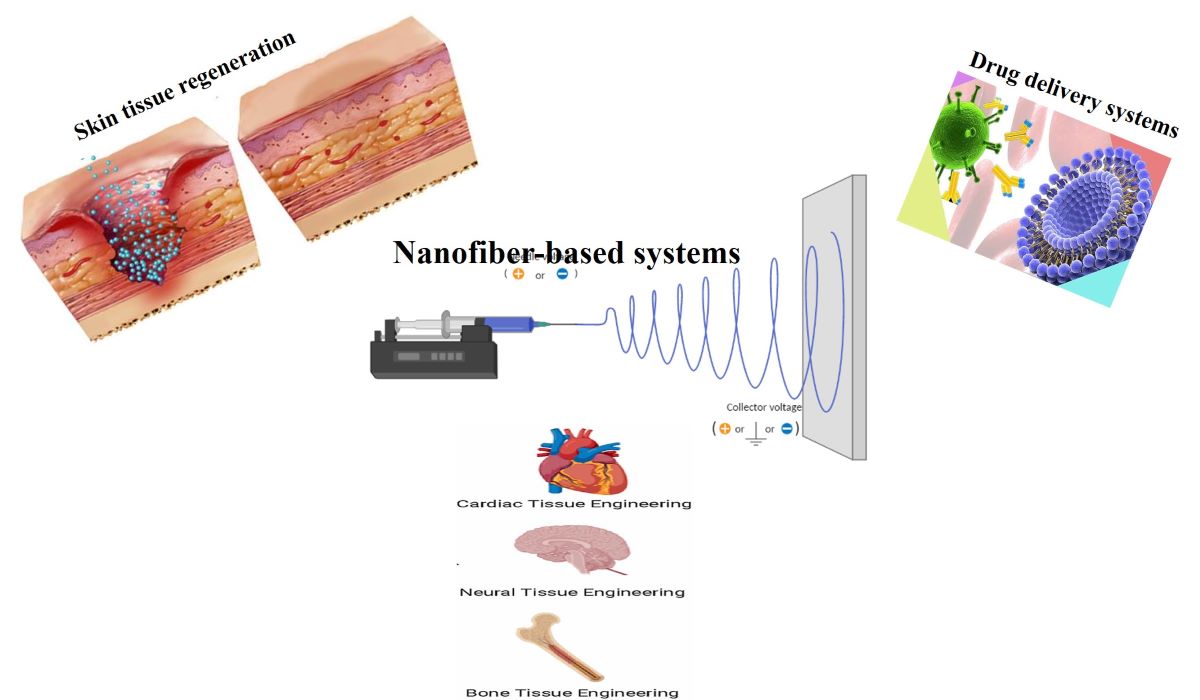
Nanofiber-based systems provide highly consistent, biocompatible, and functional scaffolds for growing complex biological tissues (
Figure 1) which are a critical component for mimicking native tissue structure and function [
4,
5]. In regenerative medicine, the natural tissue structure is decisive for finding the proper function of planned tissue, and the use of nanofiber-based systems gives us this opportunity to mimic the physical, mechanical, and biological functions to a higher degree [
5].
Technical Approach of the Nanofiber-based Systems
Progress functional and biocompatible nanofibers to attend as scaffolds for regenerative medicine [
6,
7]. Polymers, hydrogels, ceramics, or composites that are capable of developing nano-size fibers [
7]. Electrospinning, self-assembly, phase separation, or 3D printing with nano-ink have been widely applied as the main fabrication techniques in this field [
8,
9]. After fabrication, scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and mechanical testing (
Table 1) are commonly applied for characterization of obtained nanofibers [
9].
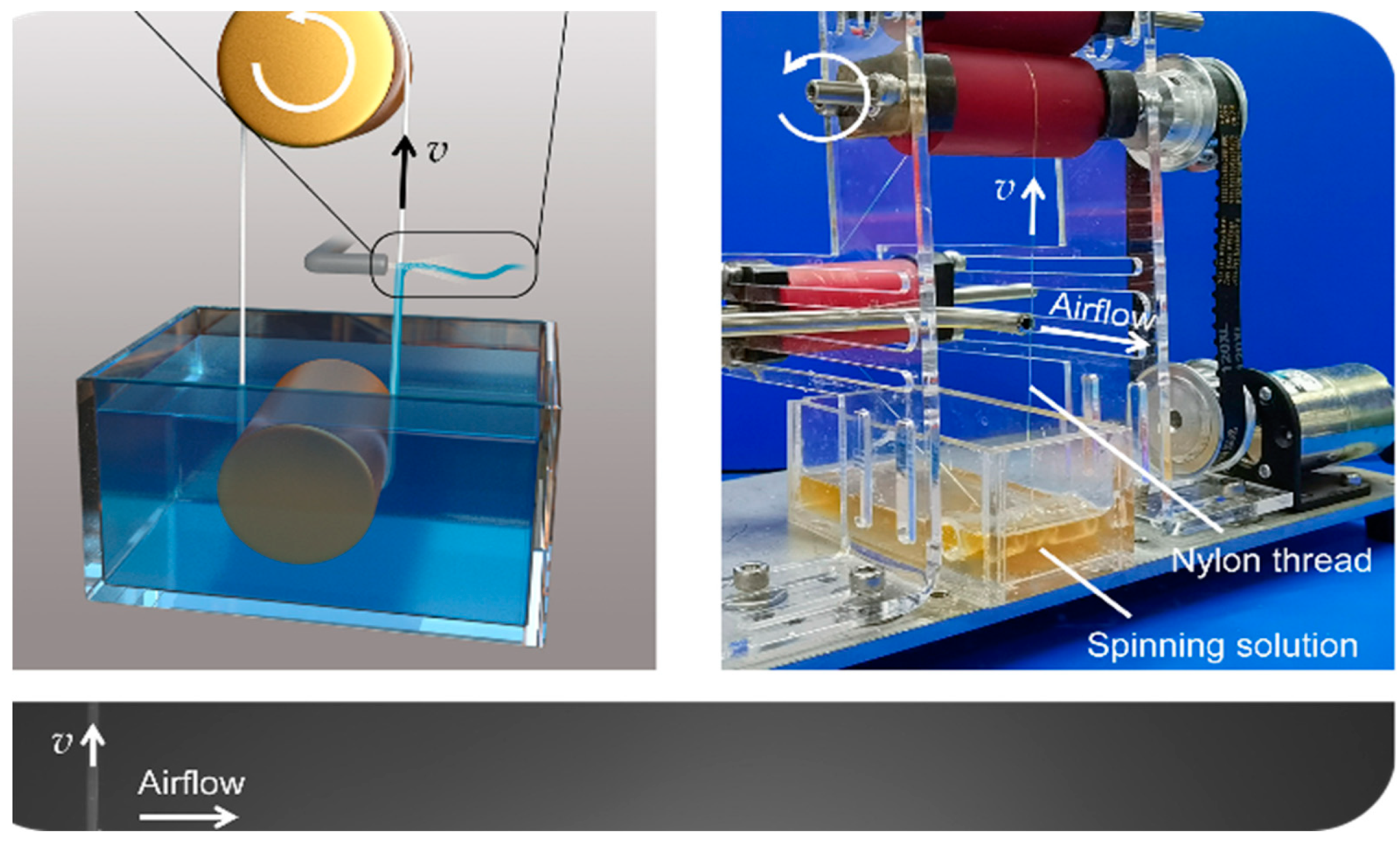
The production of nanofiber-based systems typically involves the use of a biodegradable polymer solution, which is electrospun into fibers with diameters ranging from tens to hundreds of nanometers [
10]. These fibers are then collected, frequently rolled into sheets, and sterilized before use (
Figure 2).
Potential Applications and Benefits
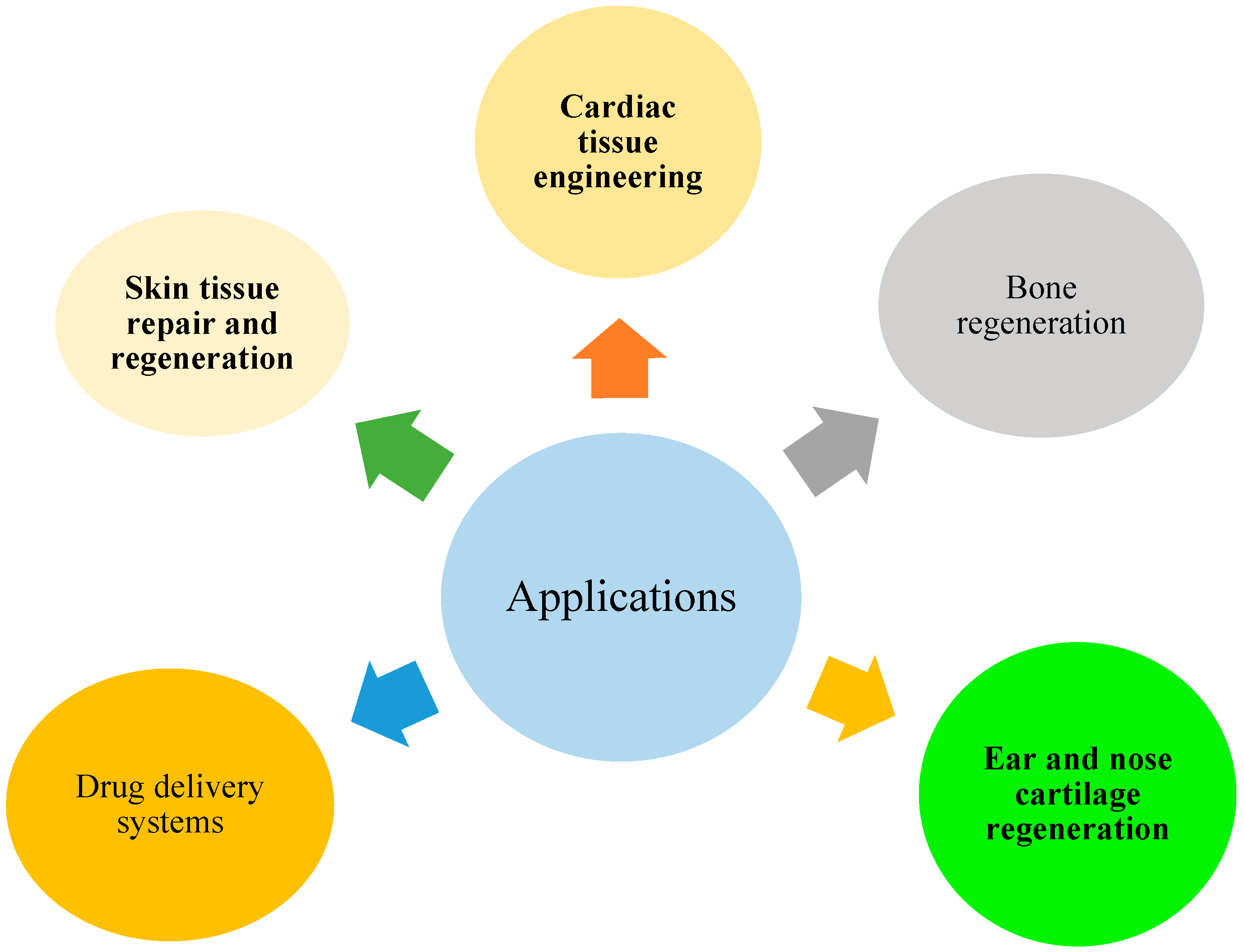
Nanofiber-based scaffolds provide the perfect setup for cells to regenerate damaged tissues [
11]. These scaffolds mimic the natural extracellular matrix in our body and afford structural support, enabling cells to obey and breed. Whether it's bone, cartilage, skin, or even organs, nanofiber-based scaffolds offer a promising key for tissue regeneration (
Figure 3). Nanofiber-based systems can also glint as drug delivery carriers [
11]. Thanks to their high surface area and customizable properties, these nanofibers can be loaded with therapeutic agents/drugs and targeted to specific parts of the body. It's like having a tiny superhero courier that delivers healing powers directly to the site of injury.
Nanofiber systems can support cell proliferation and differentiation, mimic the extracellular matrix of native tissue, provide an extremely controlled and biocompatible environment for cells, and promote an accurate operation of engineered tissue.
Challenges, Limitations, and Future Perspectives
While nanofiber-based systems hold great promise, scaling up their production remains a challenge. Production of these complex structures on a bulky scale requires advanced techniques and specific control. Overwhelming scalability barriers will be essential to conveying nanofiber-based systems into mainstream regenerative medicine.
As remarkable as nanofiber-based systems are, receiving those to effortlessly integrate with host tissues can be a bit tricky. Our bodies are multifarious, and presenting foreign agents can occasionally lead to immune responses or rejection. Ensuring compatibility and promoting tissue integration are key obstacles to address to maximize the success of nanofiber-based systems in regenerative medicine.
Hold on to your lab coats because the future of nanofiber-based systems in regenerative medicine is brimming with exciting possibilities. Researchers are constantly developing new materials, fabrication techniques, and even integrating advanced technologies like 3D printing and bioengineering. These advances will drive nanofiber-based systems to new heights, enabling even more precise control and appropriate solutions for tissue regeneration.
As nanofiber-based systems continue to improve, their impact on clinical practice is set to be revolutionary. From regenerating damaged tissues to delivering targeted therapies, these tiny fibers have the potential to reshape the way we approach regenerative medicine.
In conclusion, nanofiber-based systems hold incredible promise in the field of regenerative medicine, offering a technical approach that can transform the landscape of tissue engineering and regenerative therapies. With their ability to mimic native tissues, facilitate cell growth and tissue regeneration, and enable targeted drug delivery, nanofiber-based systems have the potential to develop the field. However, further research and development are needed to address challenges such as scalability, combination with host tissues, and manufacturing limitations. As advancements continue to unfold, it is clear that nanofiber-based systems will play a key role in the future of regenerative medicine, paving the way for innovative treatments and enhanced patient outcomes.
Declaration of interests
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research received no external funding.
Informed Consent Statement
Not available.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chowdhury, S.R., Keshavan, N., Basu, B.: Urinary bladder and urethral tissue engineering, and 3D bioprinting approaches for urological reconstruction. J. Mater. Res. 36, 3781–3820 (2021). [CrossRef]
- Jiang, Y., Fu, X.: Traditional Medicine and Tissue Repair and Regeneration BT - Regenerative Medicine in China. Presented at the (2021).
- Vijayavenkataraman, S., Yan, W.-C., Lu, W.F., Wang, C.-H., Fuh, J.Y.H.: 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 132, 296–332 (2018). [CrossRef]
- Fernandez-Yague, M.A., Abbah, S.A., McNamara, L., Zeugolis, D.I., Pandit, A., Biggs, M.J.: Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 84, 1–29 (2015). [CrossRef]
- Wu, S., Liu, X., Yeung, K.W.K., Liu, C., Yang, X.: Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Reports. 80, 1–36 (2014). [CrossRef]
- Pina, S., Oliveira, J.M., Reis, R.L.: Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 27, 1143–1169 (2015). [CrossRef]
- Arslan, E., Garip, I.C., Gulseren, G., Tekinay, A.B., Guler, M.O.: Bioactive Supramolecular Peptide Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 3, 1357–1376 (2014). [CrossRef]
- Su, C., Chen, Y., Tian, S., Lu, C., Lv, Q.: Natural Materials for 3D Printing and Their Applications, (2022).
- Vedadghavami, A., Minooei, F., Mohammadi, M.H., Khetani, S., Rezaei Kolahchi, A., Mashayekhan, S., Sanati-Nezhad, A.: Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 62, 42–63 (2017). [CrossRef]
- Wang, C., Wang, J., Zeng, L., Qiao, Z., Liu, X., Liu, H., Zhang, J., Ding, J.: Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies, (2019).
- Wang, X., Ding, B., Li, B.: Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today. 16, 229–241 (2013). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).