1. Introduction
Fungi with pathogenic potential for humans have been considered as etiologically relevant for severe infections in an increasing number of both immunocompromised and immunocompetent patients in recent years. Primarily, this increase was attributed to a rise in patients with acquired immunosuppression due to agents like the human immunodeficiency virus (HIV) and in those who develop neutropenia in response to chemotherapy [
1]. Although antifungal resistance is already a well-recognized issue of concern, most attention and public resources remain focused on researching and developing new drugs against multidrug-resistant bacteria [
2].
Nevertheless, the global emergence of multidrug-resistant fungi, such as certain
Candida auris lineages, has raised alertness in clinical settings [
3]. In particular,
C. auris’ ability of spreading rapidly among sick patients and on critical care units as well as its propensity of showing resistance to the main classes of antifungal agents, including azoles (fluconazole, FLU), polyenes (amphotericin B, AmB), and echinocandins, makes its clinical management challenging and calls for strict infection control enforcement in case of its diagnostic detection [
4,
5,
6].
Naphthoquinones are molecules in the quinone class with two aromatic rings as the chemical backbone. They are synthesized by various plant families (Bignoniaceae, Ebenaceae, Droseraceae, Juglandaceae, Plumbaginaceae, Boraginaceae, among others). Additionally, they can be found as secondary metabolites in various algae, fungi, bacteria, and even some animals [
7]. These molecules exhibit important biological activities, such as antibacterial, antiviral, antioxidant, antiparasitic, cytotoxic, and antifungal properties [
8]. The antifungal potential of semi-synthetic naphthoquinones was evaluated against 89 fungal isolates, and a compound named IVS320 was demonstrated to be promising. In detail, it showed the best minimum inhibitory concentration (MIC) values for all tested cultures, primarily for
Candida species and dermatophytes [
9].
The study presented here aimed at evaluating the antifungal potential of four naphthoquinones against fungal reference isolates (Candida spp., Sporothrix spp., Trichophyton spp., and Fusarium spp.), at determining the cytotoxic profile in MRC-5 human fibroblast cells, at assessing toxicity using Artemia salina, and at examining phytotoxicity in tomato (Solanum lycopersicum) and arugula (Eruca sativa) seeds. Additionally, the study investigated the mechanism of naphthoquinone action in C. albicans ATCC 60193 for the substance with the best antifungal profile.
2. Materials and Methods
2.1. Naphthoquinones
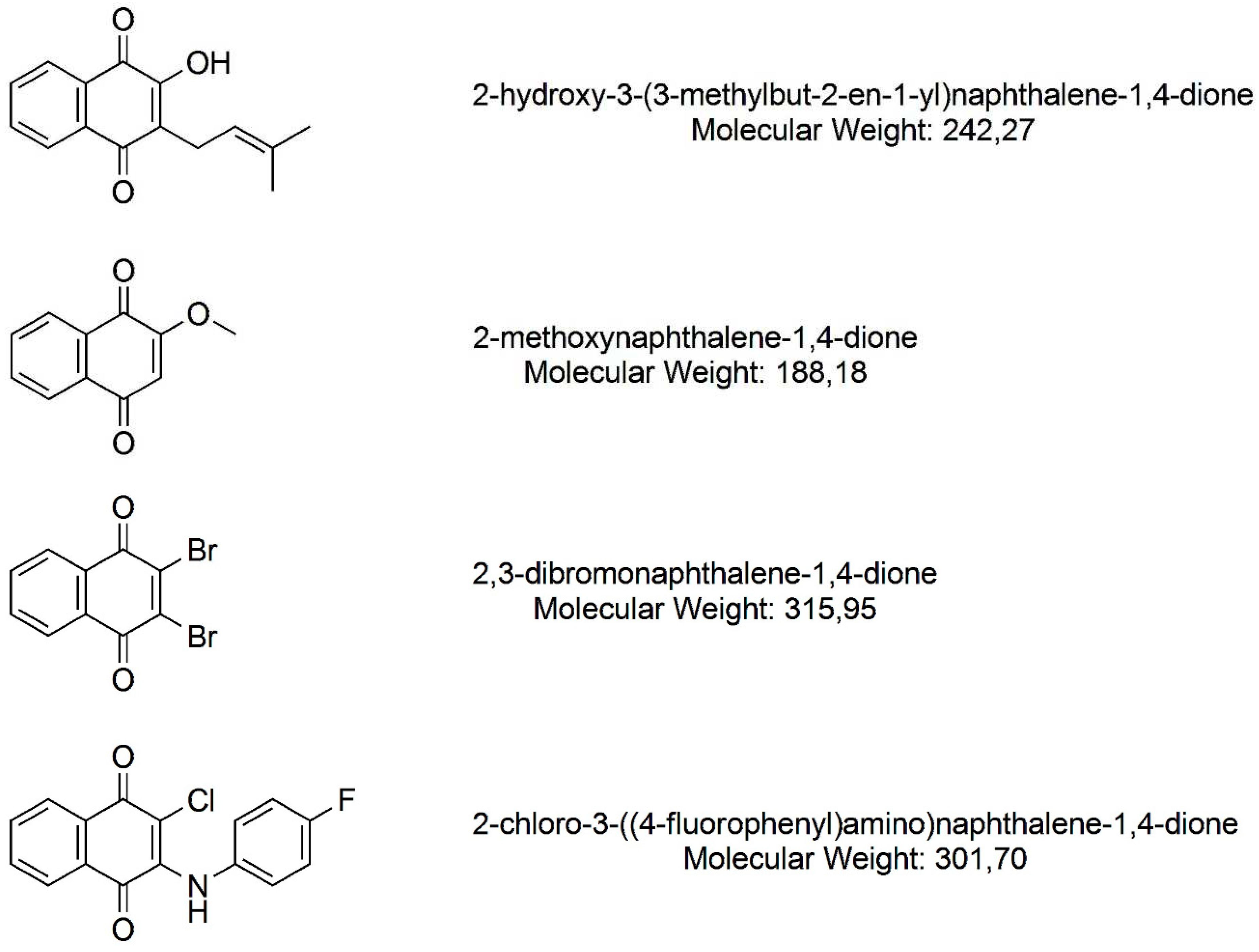
The substances used in the present study comprised 2-hydroxy-3-(methyl-2-butenyl)-1,4-naphthoquinone (lapachol), 2-methoxy-1,4-naphthoquinone (2-MNQ), 2,3-dibromo-1,4-naphthoquinone (2,3-DBNQ), and 2-chloro-3-(4-fluoroanilino)-1,4-naphthoquinone (2-ClFNQ) (
Figure 1). The substances were obtained from Sigma-Aldrich (St. Louis, Missouri, USA), and stock solutions (3.2 mg/mL) of each of them were prepared and subsequently diluted in RPMI-(Roswell Park Memorial Institute-)1640 medium (Sigma-Aldrich) to obtain the necessary concentrations for the assays (1.56 - 800 µg/mL).
2.2. Microorganisms
Eleven reference strains were used in this study (
Table 1), belonging to the Collection of Microorganisms of Medical Interest of the National Institute of Amazonian Research -INPA (Instituto Nacional de Pesquisas da Amazônia). Subcultures were performed in Sabouraud dextrose medium to ensure purity and viability until the test was conducted.
2.3. Antifungal activity assays
The assays applied to determine the minimum inhibitory concentration (MIC) values were based on the broth microdilution technique as described by the CLSI (Clinical and Laboratory Standards Institute) [
10,
11]. Briefly, 100 µL of the test substance solutions diluted in RPMI-1460 broth were added to 96-well microplates, with final concentrations ranging from 1.56 - 800 µg/mL (naphthoquinone (NQ)), 0.125 - 64 µg/mL (fluconazole (FLU)), and 0.0313 - 16 µg/mL (amphotericin B (AmB)). Next, 100 µL of the inoculum containing 2.5x10³ cells/mL of yeasts or 2.5x10⁴ cells/mL of dermatophytes and opportunistic filamentous agents, respectively, were added. The plates were incubated at 35°C for 24 hours for yeasts and for 96 hours for dermatophytes and opportunistic filamentous agents, with visual readings performed following the incubation periods. The MIC was defined as the lowest concentration of naphthoquinones necessary to cause 100% fungal growth inhibition compared to the control assay (performed without antifungal substances). After determining the MIC, 10 µL aliquots were taken from any wells without visible fungal growth and inoculated onto Sabouraud dextrose medium for the determination of the minimum fungicidal concentration (MFC) [
12].
2.4. Antifungal mechanism of action
Sorbitol protection assay: The MIC of 2,3-DBNQ against
C. albicans ATCC 60193 was determined following the CLSI guidelines (1.56 – 800 µg/mL), in the absence and presence of 0.8 M sorbitol (Sigma-Aldrich), a substance used as an osmotic stabilizer. The MICs were determined after 24 hours (h) of incubation at 35°C [
9].
Ergosterol effect assay: The MIC of 2,3-DBNQ against
C. albicans ATCC 60193 was determined following the CLSI guidelines as previously described, both in the absence and in the presence of different concentrations (200 – 1600 µg/mL) of ergosterol (Sigma-Aldrich) added to the assay medium. Amphotericin B was used as the standard antifungal substance in this assay. The MICs were determined after 24 h of incubation at 35°C [
9].
Extravasation assay for substances absorbing in the 260 nm-spectrum:C. albicans ATCC 60193 cells were cultured under agitation at 35°C until the early stationary growth phase (18 h of growth) in RPMI medium. After incubation, they were washed and resuspended in 3-(N-Morpholino)propanesuflonic acid (MOPS) buffer (0.16M, pH 7.0). Microtubes (final volume 1,500 µL) containing the inoculum (3 x 10
7 cells/mL) and 2,3-DBNQ (at 1x and 4x MIC) were incubated for 2, 4, and 24 h. After the incubation periods, the microtubes were centrifuged at 3,000g for 5 minutes applying a MiniSPin Microcentrifuge device (Eppendorf, Hamburg, Germany) and the absorbance of the supernatants (100 µL) was read at 260 nm using a Gene Quant DNA/RNA spectrophotometer (Eppendorf, Hamburg, Germany). In this assay, 100% extravasation was considered as the absorbance measured with cells treated with SDS (sodium dodecyl sulfate, 2%) [
13].
2.5. Toxicity assays
Due to the pronounced antifungal potential exhibited by 2,3-DBNQ and 2-MNQ in this study (details in the results section), their toxicity in the microcrustacean Artemia salina was further evaluated. The phytotoxic/cytostatic potential of 2,3-DBNQ was additionally assessed in Solanum lycopersicum (tomato) and Eruca sativa (arugula) seeds. All four NQs (naphthoquinones) were investigated in cell viability assays using the MRC-5 human fibroblast cell line.
MRC-5 cell line toxicity assay: The assay was conducted in line with the protocol provided by Ansar Ahmed et al. [
14], aiming at analyzing cell viability in MRC-5 (fibroblast) cells after 24-hour exposure to the substance of interest. In 96-well plates, MRC-5 cells were inoculated at a concentration of 0.5 x 10
4 cells/well. After 24 hours of incubation and cell adhesion, they were treated with the chosen naphthoquinones at various concentrations (1.56 – 100 µM). After the treatment period, 10 µL of alamarBlue® Cell Viability Reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA) (0.4% stock solution 1:20 in culture medium) was added. Following a 3-hour resazurin metabolization period, fluorescence reading was performed on a microplate reader (spectrophotometer) to quantify viable cells, as resazurin is a fluorescent redox indicator that shows reducing chemical activity in proliferating cells. The oxidized form is blue (non-fluorescent/non-viable), and the reduced form is pink (fluorescent/viable).
Artemia salina toxicity assay: The assay was conducted adhering with the protocol proposed by Meyer et al. [
15].
A. salina cysts were incubated in sterilized synthetic seawater with 36 g/L marine salt, i.e., Ocean Tech Reef Salt (Ocean Technologies Group, London, England, United Kingdom), under the condition of constant illumination at 28°C. After 48 hours of incubation, nauplii (first larval stages) were collected and transferred to 24-well plates. In this assay, the naphthoquinones 2,3-DBNQ and 2-MNQ were used due to their promising MIC values in the antifungal assay (see the results section for details). Stock solutions of the naphthoquinones were prepared at 1 mg/mL in 5% DMSO and synthetic seawater. Subsequently, the samples were serially diluted (1000, 500, 300, 100, and 50 µg/mL) and tested in triplicate. Ten nauplii were used per well, 5% DMSO and synthetic seawater served as positive controls. After 24 hours, the number of surviving nauplii was counted to determine the LC
50, using the PoloPlus, version 1.0, software (LeOra Software LLC, Parma, MO, USA).
Germination inhibition assay in tomato and arugula seeds: For tomato seeds, the protocol as proposed by Sánchez Perera et al. [
16] was used with modifications as follows. In Petri dishes containing sterile filter paper, five seeds each were covered with different concentrations of 2-3-DBNQ naphthoquinone (0 – 800 µg/mL) diluted in 10% DMSO and sterile distilled water with a final volume of 3 mL. For arugula seeds, the protocol described by Chouychai et al. [
17] was used. In Petri dishes containing 50g of soil, five seeds each were covered with different concentrations of 2,3-DBNQ naphthoquinone (0 – 600 µg/mL) diluted in 10% DMSO and sterile distilled water, with a final volume of 3 mL. For both approaches, the plates were sealed to maintain humidity and incubated for 168 h at 25 – 27°C. After the incubation period, seed germination and root length were observed, and the proportion of germination was calculated using the following formula:
2.6. Statistical analysis
The results were reported as mean ± standard deviation (SD) from three independent experiments, each performed in triplicate. Statistical differences (p < 0.05) in cytotoxicity tests were determined using analysis of variance (ANOVA), followed by Tukey's or Bonferroni's post-tests in GraphPad Prism 6.0 for Windows (GraphPad, San Diego, CA).
3. Results
3.1. Antifungal Activity
In order to assess the antifungal potential of naphthoquinones, we investigated the MIC of the compounds lapachol, 2-MNQ, 2,3-DNBQ, 2-CIFNQ with 11 well-characterized reference strains of fungi with etiological relevance for human patients, including opportunistic yeasts, dermatophytes, subcutaneous pathogenic fungi, and opportunistic filamentous fungi (
Table 1 above). The substances 2-MNQ, 2,3-DNBQ, 2-CIFNQ showed antifungal activity, with 2,3-DBNQ being associated with the most pronounced antifungal activity and particularly convincing effects against
Candida species (MIC <1.56 – 6.25 µg/mL) and dermatophytes (MIC <1.56 µg/mL) (
Table 2).
We investigated whether the tested substance 2,3-DBNQ had fungicidal and/or fungistatic properties. Indeed, 2,3-DBNQ displayed a fungicidal profile for seven out of the 10 tested strains (Candida krusei, C. tropicalis, C. parapsilosis, Sporothrix brasiliensis, S. schenckii, Trichophyton mentagrophytes, and Fusarium oxysporum). 2-MNQ and 2-ClFNQ proved to be fungicidal for four and six strains, respectively.
3.2. Mechanisms of Action 2,3-DBNQ
Due to the demonstrated antifungal potential of 2,3-DBNQ, it was selected for the evaluation of possible mechanisms of biological action. The assessment of the biological mechanism included assays that evaluated the interference/interaction of the antifungal substance with the organism's cell wall, its cell wall ergosterol, and with potential cellular leakage.
The "sorbitol protection" assay was conducted to determine whether or not 2,3-DBNQ has an influence on the integrity of the fungal cell wall. In this assay, MIC assessments of 2,3-DBNQ against C. albicans ATCC 60193 were performed in parallel, both in the presence and absence of sorbitol (0.8M), an osmotic protectant used for stabilizing fungal protoplasts. It is known that a compound that negatively interferes with the fungal cell wall will shift the MIC to a higher value in the presence of osmotic stabilizers. The MIC of 2,3-DBNQ did not change in the presence of sorbitol (6.25 µg/mL) after 24 hours of incubation, suggesting that 2,3-DBNQ does not act by inhibiting mechanisms controlling the synthesis or integrity of the fungal cell wall.
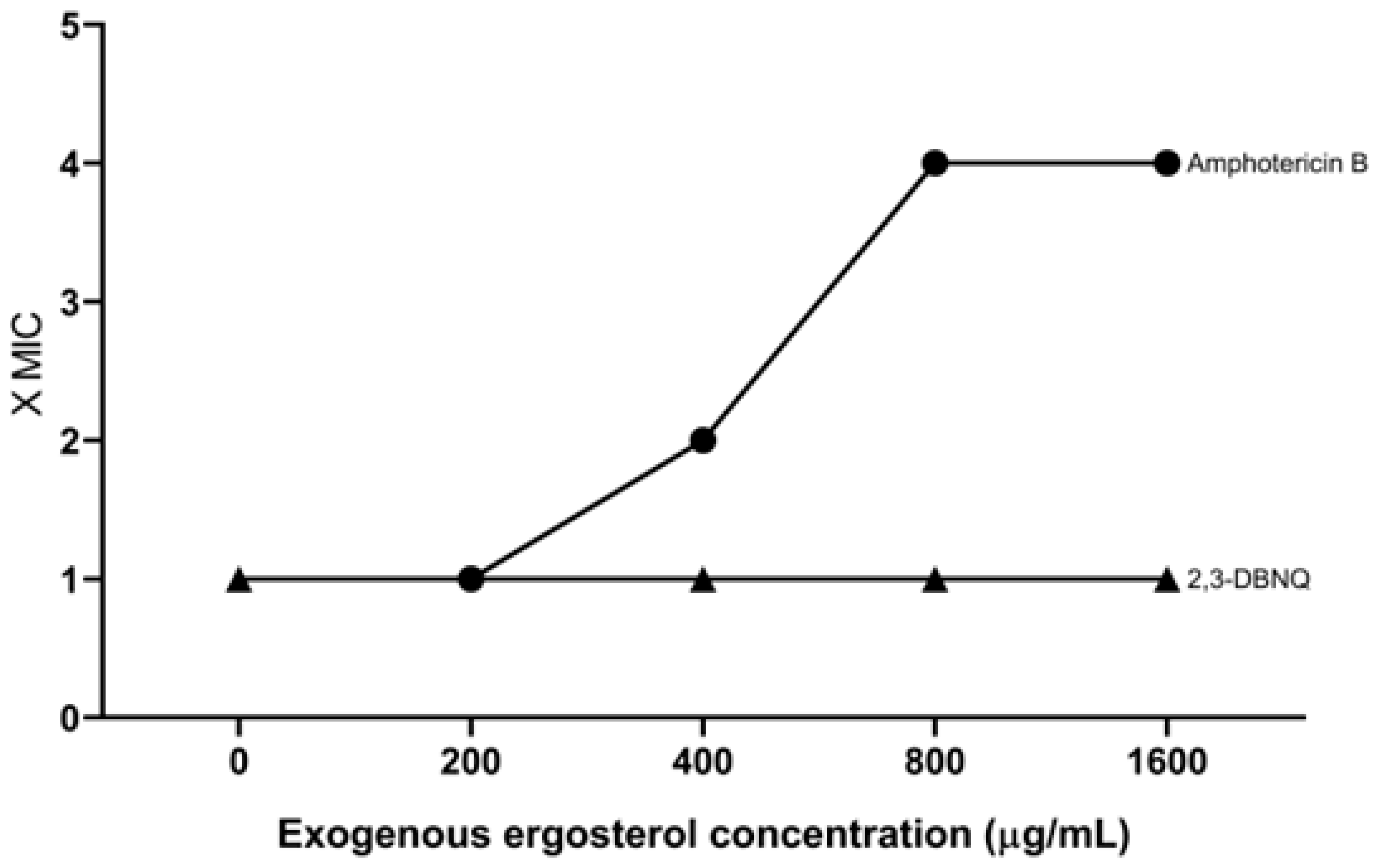
In order to assess whether 2,3-DBNQ may have caused changes in membrane ergosterol, the ergosterol assay was conducted. This test aims at identifying whether or not a compound interacts with the ergosterol present in the fungal cell membrane by providing exogenous ergosterol. Compounds with some affinity for ergosterol rapidly form complexes with exogenous ergosterol, thus preventing complexation with the ergosterol in the fungal cell membrane. As a direct consequence, there is an increase in MIC. The results (
Figure 2) showed that the MIC of 2,3-DBNQ when tested against
Candida albicans ATCC 60193 cells remained unchanged in the presence of different concentrations (200 to 1600 µg/mL) of exogenous ergosterol. Accordingly, these data suggest that this naphthoquinone does not relevantly interact with ergosterol. In comparison, a 4x increase in the MIC of amphotericin B, a standard substance known to interact with ergosterol, was observed in the assay.
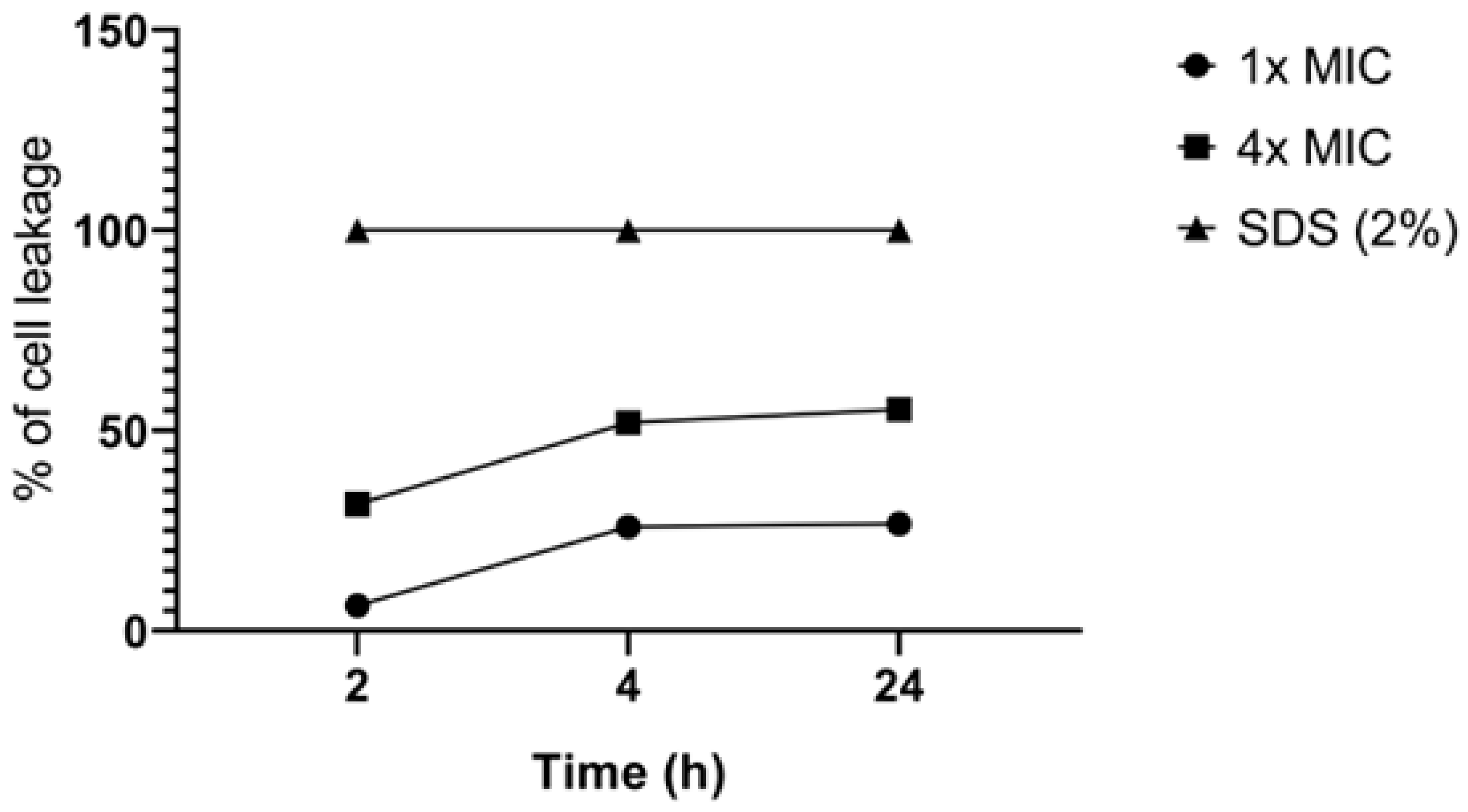
Finally, we investigated whether 2,3-DBNQ induces cellular leakage, resulting in the efflux of substances absorbing at 260 nm from
C. albicans ATCC 60193 cells (
Figure 3). We observed that a concentration of 3.25 µg/mL (1x MIC) 2,3-DBNQ caused leakage in dimensions of 6%, 25.9%, and 26.6% after 2, 4, and 24 hours, respectively, compared to the positive control. At a concentration of 12.5 µg/mL, the substance resulted in leakage in dimensions of 31.6%, 51.9%, and 55.2% after 2, 4, and 24 hours, respectively.
3.3. Toxicity assays
We selected 2-MNQ and 2,3-DBNQ as naphthoquinones with notable antifungal activity in order to evaluate their toxicity with a human fibroblast cell line (MRC-5), the microcrustacean A. salina, and seeds of Solanum lycopersicum and Eruca sativa.
Regarding toxicity as observed with the human fibroblast cell line (MRC-5), the naphthoquinones 2-MNQ, 2,3-DBNQ, and 2-ClFNQ showed IC50 values at concentrations of 11.9 µM, 15.4 µM, and 29.2 µM, respectively. The compound lapachol exhibited low toxicity under experimental conditions with MRC-5 cells in a dimension that its IC50 value could not even be calculated.
In addition, we investigated the toxicity of 2-MNQ and 2,3-DBNQ with A. salina. At a concentration of 50 µg/mL, we observed 63% and 100% mortality caused by 2-MNQ and 2,3-DBNQ, respectively.
Last not least, we examined the toxicity of 2,3-DBNQ with seeds of S. lycopersicum and E. sativa. The compound 2,3-DBNQ resulted in a 64.1% inhibition of S. lycopersicum germination at a concentration of 400 µg/mL, while it caused a 94.1% inhibition of E. sativa germination at a concentration of 200 µg/mL.
4. Discussion
The results of this study confirm the antifungal potential of naphthoquinones against fungi with etiological relevance for human patients, including opportunistic yeasts, dermatophytes, subcutaneous pathogenic fungi, and opportunistic filamentous fungi. Among the tested compounds, 2,3-DBNQ showed the most pronounced antifungal activity, particularly against Candida species and dermatophytes. Moreover, the compound displayed a fungicidal profile for several tested strains. Our findings suggest that naphthoquinones in general and 2,3-DBNQ in particular could be a promising source for the development of new antifungal agents.
However, antifungal activity of naphthoquinones in general has already been extensively reported in the scientific literature [
18,
19]. These compounds are known to possess a broad spectrum of biological activities, including antifungal, antibacterial, antiviral, and antiparasitic properties [
8]. The antifungal activity of naphthoquinones is thought to be associated with their redox properties, which allow them to interact with essential cellular components, such as enzymes and fungal DNA [
20]. The structure-activity relationship of naphthoquinones is complex and varies depending on the substituents on the naphthoquinone ring [
21]. Insofar, our finding that 2,3-DBNQ showed the most pronounced antifungal activity is well in line with previous reports which suggested the importance of the 2,3-disubstitution pattern for antifungal activity [
22].
The molecular mechanisms of naphthoquinone activity against fungi are not well understood, but are believed to involve multiple targets, including the fungal cell wall, the cell membrane, and intracellular components [
23,
24]. In our study, we investigated potential mechanisms of antifungal activity of 2,3-DBNQ. Our results suggest that 2,3-DBNQ does not act by inhibiting mechanisms controlling the synthesis or integrity of the fungal cell wall, nor does it interact with ergosterol, a key component of the fungal cell membrane, in a relevant dimension. Instead, our results indicate that 2,3-DBNQ is able to induce cellular leakage in
C. albicans ATCC 60193 cells. The precise molecular mechanism by which 2,3-DBNQ causes cellular leakage requires further investigation in the near future. Of note, previous studies have suggested that naphthoquinones may disrupt the membrane permeability barrier, leading to the leakage of intracellular components [
9,
19]. Our research confirms this hypothesis as useful for future molecular assessments.
As a potential obstacle for the clinical use of these substances, toxicity of naphthoquinones has been extensively reported in the literature [
25,
26]. In more detail, these compounds have been shown to exhibit a wide range of toxic effects, including cytotoxicity, genotoxicity, and mutagenicity [
27]. In our study, we evaluated the toxicity of naphthoquinones with a human fibroblast cell line,
A. salina, and seeds of
S. lycopersicum and
E. sativa. Our findings suggest that 2-MNQ and 2,3-DBNQ exhibit moderate cytotoxicity as observed with the human fibroblast cell line. Furthermore, 2,3-DBNQ killed
A. salina in a dose-dependent manner and inhibited the germination of
S. lycopersicum and
E. sativa seeds. The toxicity of naphthoquinones is thought to be associated with their redox properties and their ability of generating reactive oxygen species, which in turn can cause oxidative damage to cellular components [
28].
In spite of these obstacles, the promising antifungal potential of naphthoquinones, especially of 2,3-DBNQ, provides a solid foundation for further investigations into their potential applications in treating fungal infections. Future research should focus on elucidating the precise molecular mechanism underlying the cellular leakage induced by 2,3-DBNQ, as well as on identifying potential synergistic interactions with existing antifungal agents. Additionally, evaluating the efficacy of naphthoquinones in in vivo models of infection and exploring their safety profile in animal models will be essential steps towards the development of novel antifungal therapeutics based on these compounds.
5. Conclusions
In summary, this study highlights considerable antifungal properties of naphthoquinones, with 2,3-DBNQ demonstrating the most pronounced activity against fungi with potential etiological relevance in human patients, including Candida species and dermatophytes. Our findings contribute to the growing body of evidence supporting the development of naphthoquinone-based antifungal agents, as they exhibit a broad spectrum of biological activity and are based upon multiple potential modes of action. Further research into the precise molecular antifungal mechanisms and the optimal applications of these compounds is warranted, because they could represent a valuable addition to the arsenal of antifungal therapies available to combat the ongoing threat of drug-resistant fungal infections.
Author Contributions
Conceptualization, J.V.B.d.S.; methodology, J.V.B.d.S.; validation, R.S.K.F. and A.C.A.C.; formal analysis, J.D.R.d.A., J.V.B.d.S. and É.S.d.S.; investigation, J.D.R.d.A., E.S.L., J.G.d.S.O., R.S.K.F. and A.C.A.C.; resources, J.D.R.d.A., J.V.B.d.S. and É.S.d.S.; data curation, J.D.R.d.A., J.V.B.d.S. and É.S.d.S.; writing—original draft preparation, J.D.R.d.A., J.V.B.d.S. and É.S.d.S.; writing—review and editing, J.G.d.S.O., J.V.B.d.S., É.S.d.S. and H.F.; supervision, J.V.B.d.S. and É.S.d.S.; project administration, J.V.B.d.S. and É.S.d.S.; funding acquisition, J.V.B.d.S. and É.S.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) for the funding of the research (EDITAL N. 010/2021- CT&I ÁREAS PRIORITÁRIAS and EDITAL N. 006/2019 - UNIVERSAL AMAZONAS and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (Finance code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author
Acknowledgments
The authors would like to recognize funding received from Fundação de Amparo à Pesquisa do Estado do Amazonas, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of generative ai and ai-assisted technologies in the writing process
The authors only used conventional softwares for checking grammar, spelling and references. We have nothing to disclose.
References
- Ozturk I, Tunçel A, Yurt F, Biyiklioglu Z, Ince M, Ocakoglu K. Antifungal photodynamic activities of phthalocyanine derivatives on Candida albicans. Photodiagnosis Photodyn Ther 2020;30. [CrossRef]
- Hendrickson JA, Hu C, Aitken SL, Beyda N. Antifungal Resistance: a Concerning Trend for the Present and Future. Curr Infect Dis Rep 2019;21:1–8. [CrossRef]
- CDC C for D and CP. Candida auris | Candida auris | Fungal Diseases | CDC. Candida auris: A Drug-Resistant Yeast That Spreads in Healthcare Facilities 2018. https://www.cdc.gov/fungal/candida-auris/ (accessed August 2, 2022).
- Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, et al. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 2019;57:1–12. [CrossRef]
- Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. Journal of Antimicrobial Chemotherapy 2018;73:891–9. [CrossRef]
- Lockhart SR. Candida auris and multidrug resistance: Defining the new normal. Fungal Genetics and Biology 2019;131:103243. [CrossRef]
- Labbozzetta M, Poma P, Occhipinti C, Sajeva M, Notarbartolo M. Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia. Molecules 2022;27:4203. [CrossRef]
- Aminin D, Polonik S. 1,4-Naphthoquinones: Some biological properties and application. Chem Pharm Bull (Tokyo) 2020;68:46–57. [CrossRef]
- Ferreira M do PSBC, Cardoso MF do C, da Silva F de C, Ferreira VF, Lima ES, Souza JVB. Antifungal activity of synthetic naphthoquinones against dermatophytes and opportunistic fungi: Preliminary mechanism-of-action tests. Ann Clin Microbiol Antimicrob 2014;13:1–6. [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts; CLSI-M27. 4th ed. Wayne, USA: Clinical and Laboratory Standards Institute; 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; CLSI-M38. 3rd ed. Wayne, USA: Clinical and Laboratory Standards Institute; 2017.
- Bona EAM De, Pinto FG da S, Fruet TK, Jorge TCM, Moura AC de. Comparação de métodos para avaliação da atividade antimicrobiana e determinação da concentração inibitória mínima (cim) de extratos vegetais aquosos e etanólicos. Arq Inst Biol (Sao Paulo) 2014;81:218–25. [CrossRef]
- Escalante A, Gattuso M, Pérez P, Zacchino S. Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J Nat Prod 2008;71:1720–5. [CrossRef]
- Ansar Ahmed S, Gogal RM, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 1994;170:211–24. [CrossRef]
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 1982;45:31–4. [CrossRef]
- Sánchez Perera LM, Mancebo Dorvigny B, Regalado Veloz AI. Inhibition of seed germination, toxicity on Artemia salina and phytochemical prospecting with from Cuban plants as indicator of antitumo activity. Macedonian Pharmaceutical Bulletin 2018;63:29–36. [CrossRef]
- Chouychai W, Thongkukiatkul A, Upatham S, Lee H, Pokethitiyook P, Kruatrachue M. Phytotoxicity assay of crop plants to phenanthrene and pyrene contaminants in acidic soil. Environ Toxicol 2007;22:597–604. [CrossRef]
- Vaezi A, Moghadaszadeh M, Nasri E, Gharibi S, Diba K, Matkowski A, et al. In vitro activity of juglone (5-hydroxy-1,4-naphthoquinone) against both fluconazole-resistant and susceptible Candida isolates. Rev Iberoam Micol 2022;39:50–3. [CrossRef]
- Yang J, Xia X, Guo M, Zhong L, Zhang X, Duan X, et al. 2-Methoxy-1,4-naphthoquinone regulated molecular alternation of Fusarium proliferatum revealed by high-dimensional biological data. RSC Adv 2022;12:15133–44. [CrossRef]
- López V, Les F. Fungal Quinones: Benzo-, Naphtho-, and Anthraquinones. Natural Secondary Metabolites, Cham: Springer International Publishing; 2023, p. 607–26. [CrossRef]
- Futuro DO, Ferreira PG, Nicoletti CD, Borba-Santos LP, Da Silva FC, Rozental S, et al. The antifungal activity of naphthoquinones: An integrative review. An Acad Bras Cienc 2018;90:1187–214. [CrossRef]
- Sánchez-Calvo JM, Barbero GR, Guerrero-Vásquez G, Durán AG, Macías M, Rodríguez-Iglesias MA, et al. Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: a structure–activity relationship study. Medicinal Chemistry Research 2016;25:1274–85. [CrossRef]
- Chen X, Li W, Chen J, Zhang X, Zhang W, Duan X, et al. Transcriptomics Integrated with Metabolomics Reveals 2-Methoxy-1, 4-Naphthoquinone-Based Carbon Dots Induced Molecular Shifts in Penicillium italicum. Journal of Fungi 2022;8:420. [CrossRef]
- Guo M, Liu J, Xu Z, Wang J, Li T, Lei H, et al. 2-Methoxy-1,4-naphthoquinone Induces Metabolic Shifts in Penicillium Digitatum Revealed by High-Dimensional Biological Data. J Agric Food Chem 2020;68:9697–706. [CrossRef]
- de Almeida PDO, dos Santos Barbosa Jobim G, dos Santos Ferreira CC, Rocha Bernardes L, Dias RB, Schlaepfer Sales CB, et al. A new synthetic antitumor naphthoquinone induces ROS-mediated apoptosis with activation of the JNK and p38 signaling pathways. Chem Biol Interact 2021;343:109444. [CrossRef]
- Mahalapbutr P, Leechaisit R, Thongnum A, Todsaporn D, Prachayasittikul V, Rungrotmongkol T, et al. Discovery of Anilino-1,4-naphthoquinones as Potent EGFR Tyrosine Kinase Inhibitors: Synthesis, Biological Evaluation, and Comprehensive Molecular Modeling. ACS Omega 2022;7:17881–93. [CrossRef]
- Lima Bezerra JJ, Johanes I, Vieira Pinheiro AA. Anticancer potential and toxicity of the genus Handroanthus Mattos (Bignoniaceae): A systematic review. Toxicon 2022;217:131–42. [CrossRef]
- Emadi A, Ross AE, Cowan KM, Fortenberry YM, Vuica-Ross M. A Chemical Genetic Screen for Modulators of Asymmetrical 2,2′-Dimeric Naphthoquinones Cytotoxicity in Yeast. PLoS One 2010;5:e10846. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).