Introduction
The improvement and development of multimodal treatment and diagnostic methods for various malignant neoplasms is significantly prolonging the life of patients with malignant neoplasms; therefore, prevention of side effects of anti-tumour therapy is one of the main problems in oncological care [
1,
2]. Most patients are treated with radiotherapy in addition to chemotherapy. Extracorporeal pelvic radiotherapy (ETLT) is more commonly used for neoplasms of the gastrointestinal, urinary and reproductive systems. ETLT is used as neoadjuvant therapy before surgery, together with chemotherapy, or as a stand-alone treatment [
3]. Radiation has both a direct effect on cells, damaging DNA and causing cell death, and an indirect effect through ionisation of intracellular molecules [
4]. The cytotoxic effect is manifested not only in atypical cells, but also in the paratumoral region and in the surrounding normal tissue. If a tumour of one or more pelvic organs is irradiated, post-irradiation damage may occur in any of these organs due to their anatomical proximity.
The intestine is one of the most radiosensitive organs [
5]. Firstly, the intestinal epithelium is one of the most frequently renewed and rapidly dividing tissues. Secondly, due to its long length, there is a risk of extensive damage. Stem cells are the most sensitive to ionising radiation due to their high mitotic activity [
6,
7].
Radiation-induced intestinal lesions are common complications in oncology. For example, post-radiation colitis [
8].
One of the most promising forms of radiotherapy is electron beam irradiation, which, according to a number of researchers, has a less damaging effect on paratumoral tissues, thus preserving the structural and functional status of the colon in particular, and also providing prevention of post-radiation damage. Therefore, to assess the functional status of the colon, it is necessary to study the molecular mechanisms of epitheliocyte proliferation and apoptosis.
Aim of the study: Morphological evaluation of apoptosis and proliferation of colonic epithelium after local irradiation with electrons at doses 2 Gy and 25 Gy.
Material and Methods of Research
Animals - sexually mature Wistar rats (n=60; body weight 200 ± 10 g) were divided into two groups: I - control group (n=10); II- experimental group (n = 20, local single electron irradiation at a dose of 2 Gy), III - experimental group (n=30), local fractional irradiation with electrons at local fractional electron irradiation at summary dose 25 Gy. The irradiation dose was selected according to the "Practical guidelines for drug treatment of colorectal cancer" (2020). Anaesthesia was administered before the procedure.
The rats were placed in a special "stretching" apparatus. The animals were irradiated in the Department of Radiation Biophysics of A.F. Tsyba MRSC (Obninsk, Russia) using a linear accelerator "NOVAC-11" (dose rate 1 Gy/min, energy 10 MeV and frequency 9 Hz, field size Ø 40 mm).
Animals of the experimental group were removed from the experiment on the 5th day after the last irradiation.
All manipulations were performed according to the "International Recommendations for Biomedical Research Using Animals" (EEC, Strasbourg, 1985) and the Declaration of Helsinki of the World Medical Association.
Animal experiments were conducted in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of National Medical Research Radiological Centre of the Ministry of Health of the Russian Federation. The animal experimental protocols used were approved by the Ethics Committee on Animal Experimentation (Permit Number: AP-153334), Minutes No. 7 of 08.12.21.
After opening the abdominal cavity, the colon was carefully removed and fixed in buffered formalin, embedded in paraffin blocks, sectioned at 3 μm on a microtome and stained with haematoxylin and eosin. The number of crypts was assessed on the circumference of the colon.
Microscopic analyses were performed using a video microscopy system (Leica DM2000 microscope, Germany; Leica ICC50 HD camera).
Morphological changes were scored according to the following parameters
A) Degenerative changes: 0 - absent, 1 - mild, 2 - moderate, 3 - severe;
B) Dystrophic changes of the epithelium: 0 - absent, 1 - hypertrophy of cells, 2 - vacuolization of cytoplasm up to 50% of cells, 3 - degree of vacuolization of cytoplasm of all cells;
C) Number of goblet cells: 0 - absent, 1 - mild reduction, 2 - moderate reduction, 3 - severe reduction;
D) Number of smooth muscle cells: 0 - absent, 1 - degree of reduction / weak, 2 - degree of reduction / moderate, 3 - degree of reduction / severe.
E) Inflammation: 0 - absent, 1 - mild (within the mucosa), 2 - moderate (erythema, ulceration), 3 - severe (deep ulceration);
E) Macrophage infiltration, cell density per 1 mm2 was calculated by
G) Vascularisation and full blood vessels: 0 - absent, 1 - congestion of up to 3 blood vessels, 2 - congestion of 3 to 5 blood vessels, 3 - congestion of more than 5 blood vessels;
The number of goblet cells, smooth muscle cells and macrophages were counted per 1 mm2 and the results are reflected in the scores.
Morphometric analysis of haematoxylin and eosin-stained microspecimens was performed in 10 randomly selected fields of view of a light microscope at ×400 magnification using a video microscopy system (Leica DM2000 microscope) with Leica Application Suite, version 4.9.0. Computer morphometry was performed using ImageJ computer image analysis system.
Each section of the colon, representing a segment, was carefully examined under the light microscope, taking into account the following histomorphometric parameters: organ length; wall thickness; diameters; depth of intestinal crypts; diameter of the largest lymphoid follicle at maximum thickness. Diameters (external and internal) were calculated using the formulas D = C/π, A = rD2 /4 wall thickness = serosal-mucosal diameter/2, taking into account the values of external (serosal) circumference and internal (mucosal) circumference. The above parameters were calculated in microns: mean luminal diameter, mean lymphoid follicle diameter and mean serosal-mucosal circumference (wall thickness).
Immunohistochemistry was performed according to the standard protocol in automatic mode in the Bond-Max immunohistostainer (Leica, Germany). Primary antibodies: against caspase-3 (Invitrogen/ Thermo Fisher Scientific; 74T2, 1:50) and Ki-67 (Abcam; ab15580, 1:100); secondary - universal antibodies (HiDef Detection™ HRP polymer system, Cell Marque", USA). The number of immunopositive cells was counted in 10 fields of view at ⋅400 magnification. To estimate the proliferation index based on Ki-67, the percentage of stained cells per crypt was calculated.
The data obtained were processed using the SPSS 10.0 for Windows statistical software package (IBM Analytics, USA) and the arithmetic mean, mean error and Student's criterion were calculated. Differences between means were considered significant at p≤0.05.
Results
Macroscopic examination of the colon of the experimental group after local single electron irradiation by 2 Gy showed no signs of inflammatory process, tumour growth and destructive changes. Histological examination after local single electron irradiation by 2 Gy of the colon slides on day 5th showed a slight reduction in goblet cells with a tendency towards recovery.
Macroscopic examination of the colon of the experimental group on the fifth day after local fractional electron irradiation by 25 Gy showed hyperemia of the mucosa without pronounced destructive change.
Microscopic examination on the 5th day after local electron irradiation at local fractional electron irradiation at summary dose 25 Gy revealed moderate degenerative changes of the colonic mucosa: hypertrophy and dystrophic changes of some epitheliocytes with reduction of mitotic figures in comparison with the control group. A reduction of goblet cells (63.17 ± 1.87 vs. 21.64 ± 1.37) was observed over a large length. The wall thickness and diameter of the colon were also reduced. The thickness of the muscular lamina of the mucosa and muscularis was slightly reduced due to reduction and dystrophic changes of some smooth muscle cells. A moderate cellular inflammatory infiltration, mainly of lymphocytes and macrophages, was found in the stromal component. The vascularisation index was increased, with full blood flow and congestion in some blood vessels (
Table 1).
It should be emphasised that the disruption of the colonic histoarchitecture caused by electron irradiation, accompanied by damage to intestinal crypts with a high regenerative potential, made it difficult to count cell populations.
According to the quantitative analysis carried out, the colon has a moderate radioresistance, which is partly in agreement with studies by other authors using other irradiation methods [
16].
The morphometric study of the colon after single local electron irradiation at dose 2 Gy showed highly insignificant histomorphometric changes in some parameters (
Table 2).
The morphometric study of the colon after fractional irradiation with electrons at local fractional electron irradiation at summary dose 25 Gy showed insignificant histomorphometric changes in some parameters (
Table 2). For example, when comparing the thickness of the colon wall and the internal diameter, which reflects the width of the organ lumen, we found an insignificant decrease in the parameters. The intraluminal diameter of the colon after electron irradiation decreased by 13-20% compared to the control group (p < 0.05). At the same time, measurements of colon length and outer diameter showed no statistically significant difference (p > 0.05).
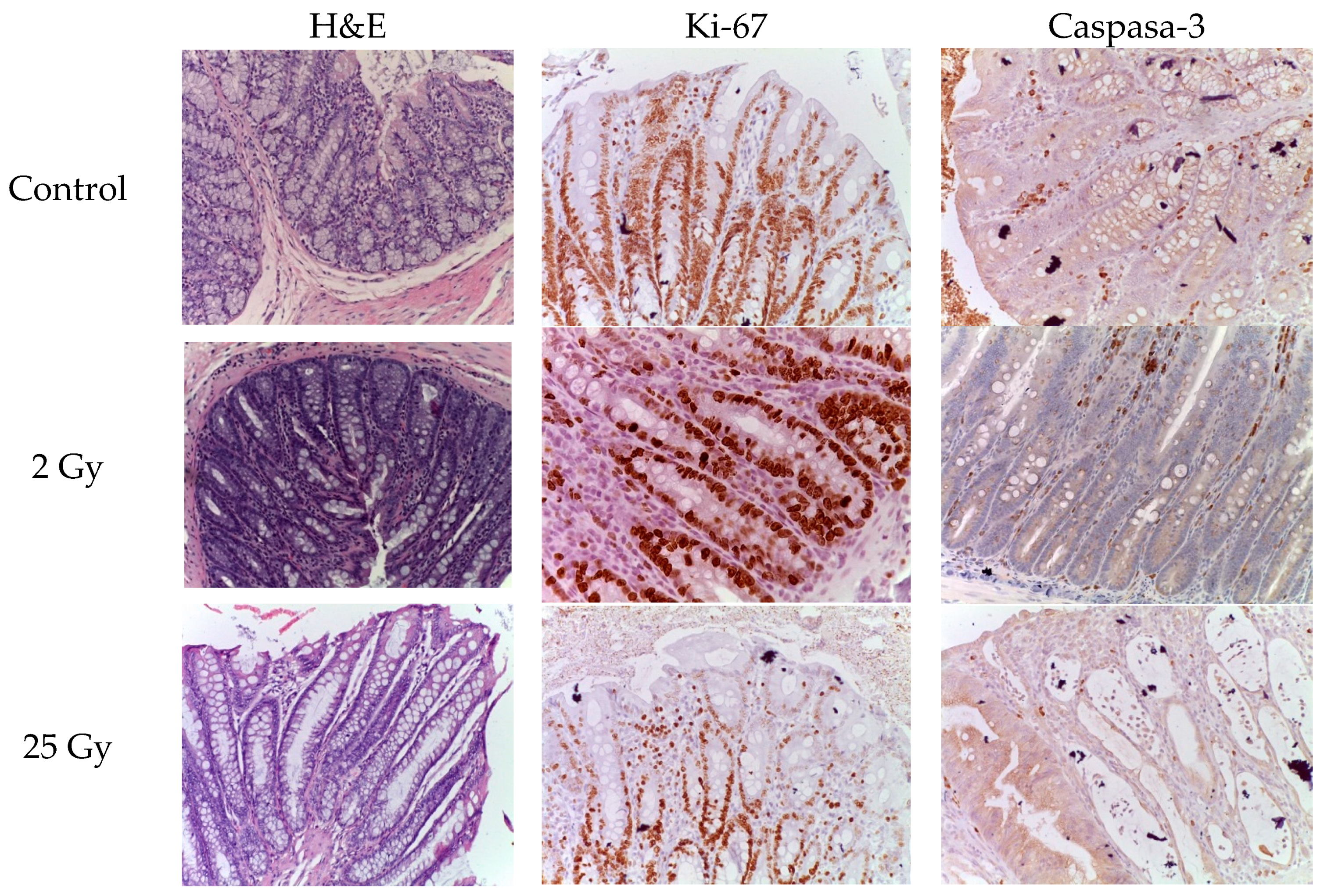
The number of caspase-3- and Ki-67-positive cells was examined by immunohistochemistry.
On the 5th day after a single electron irradiation at a dose of 2 Gy, the number of caspase-3- and Ki-67-positive cells almost approached the control values. No statistically significant difference was observed, p>0.05 (
Table 3).
After irradiation with 25 Gy the number of caspase-3-positive epithelial cells increased 1.97-fold compared to control values (
Table 3). At the same time, normal histoarchitecture of the colon crypts and a few caspase-3-positive cells were observed in the colon samples of the control group. The proliferation index (Ki-67) in the intestinal crypts of the colon after local irradiation with electrons at a local fractional electron irradiation at summary dose 25 Gy was reduced by 1.74 times compared to the control group (
Table 3).
The data obtained indicate that the damage to the intestinal crypts of the colon induced by irradiation with electrons at a local fractional electron irradiation is accompanied by a decrease in the number of progenitor cells and, as a consequence, leads to a decrease in the "survival rate" of the crypts (
Figure 1).
Discussion
The present work is devoted to the study of the effect of fractional local irradiation with electrons at a local fractional electron irradiation at single dose of 2 Gy, and summary dose 25 Gy on the colonic epithelium of experimental animals.
The present work is devoted to the study of morphofunctional changes in the crypt and the features of proliferation and apoptosis of the colon epithelium after electron irradiation at a dose of 2 Gy. Comparative analysis was performed at two time points, taking into account cycles of colon epithelium proliferation [
11,
12].
Against the background of local fractional irradiation with local fractional electron irradiation in the colon there are: degenerative changes of the mucous membrane, decrease in the number of epitheliocytes, reduction of goblet cells and smooth myocytes, inflammatory infiltration, haemodynamic changes leading to a violation of local homeostasis. A morphometric study of transverse sections of the colon showed a slight decrease in organ length, wall thickness, and external and internal diameters. When comparing the depth and extent of the pathomorphological changes detected, they were less pronounced in contrast to other types of irradiation [
16,
17].
The development of radiation-induced colitis depends on a number of factors, including those responsible for the regulation of the cell cycle of the colonic epithelium, such as: caspases, Ki-67, PSNA, p53, PUMA, etc., as well as a number of other factors.
To assess the regenerative potential after electron exposure, immunohistochemical study of actively dividing cells of intestinal crypts was performed by determining the DNA of the M-phase of mitosis using biomarkers Ki-67, as well as the terminal stage of apoptosis - caspase-3. The difference of radiosensitivity of stem and progenitor cells related to DNA damage pathways and cell cycle regulation was taken into account.
According to some authors, the majority of active stem cells undergo apoptosis in the first day after 12 Gy of X-ray radiation, and then, as a rule, survive due to the effective ability to regenerate after 48 hours [
17].
The observed increase in the number of caspase-3-positive epitheliocytes was attributed to the cytotoxic action of electrons. However, these changes were weaker compared to other types of irradiation [
17]. It should be emphasised that the expected rapid recovery of the pool of epitheliocytes and progenitor cells did not occur, as the fractionation mode was used.
The increase in the activity of the terminal effector caspase-3 is due to the release of cytochrome from the mitochondria, which leads to the multimerisation of Bax and the initiation of the apoptotic cascade, which is implemented through the intrinsic and extrinsic pathways [
10,
11].
One of the mechanisms of crypt recovery after exposure to electrons is related to their ablative effect on the epithelium, in contrast to other types of irradiation, which allows us to assign epitheliocytes to radioresistant populations. In addition, damage to progenitor cells results in delayed repopulation of intestinal crypts.
Activation of either of these mechanisms can lead to a marked decrease in Ki-67 expression and correlate with apoptosis - a dramatic increase in caspase-3.
The regenerative process after electron irradiation, like the effects in other species, appears to consist of three phases: apoptotic (2-4 days), proliferative (2-4 days) and normalisation (4-7 days) [
17].
Another equally important aspect of the study was to assess the degree of intoxication caused by fractional irradiation with local fractional electron irradiation at summary dose 25 Gy. It was found that, unlike other types of irradiation, exposure to electrons does not lead to pronounced changes in the functional status of the colon (maintenance of water-electrolyte balance, etc.) and, consequently, reduces the risk of intoxication and death. It should be noted that preserving the functional status of the intestine is one of the main tasks in coloproctology and oncology - since cancer patients, especially in severe stages with metastases, are usually accompanied by cachexia.
Separately, it should be emphasised that local fractional irradiation with local fractional electron irradiation at summary dose 25 Gy is available and controlled, has shown its efficacy and can be used as a model for more detailed in vivo studies.
In conclusion, according to the results of morphological and immunohistochemical studies, the evaluation of the regenerative potential of the colonic epithelium and the status of progenitor cells in comparison with literature data, electron irradiation is one of the promising types, one of the advantages of which is a less damaging effect on the tissues.
Conclusion
Local single electron irradiation of the colon at a dose of 2 Gy resulted in a slight reduction in the number of goblet cells while maintaining their regenerative potential. On the other hand, local fractional electron irradiation of the colon with a total dose of 25 Gy caused mild degenerative-dystrophic changes in epithelial cells and a greater decrease in the number of goblet cells while retaining their regenerative potential. Injury to intestinal crypts results in a rise in caspase-3-positive epitheliocytes and a reduction in Ki-67 levels.
Ethical Statement
All manipulations were performed according to the “International Recommendations for Biomedical Research Using Animals”; (EEC, Strasburg, 1985) and the Declaration of Helsinki of the World Medical Association.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Competing Interests
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
Animal experiments were conducted in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of National Medical Research Radiological Centre of the Ministry of Health of the Russian Federation. The animal experimental protocols used were approved by the Ethics Committee on Animal Experimentation (Permit Number: AP-153334) №22; December 4, 2021 y.
Conflicts of Interest
the authors declare that there is no conflict of interest on the submitted article.
References
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol. 2007, 46, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, J.; Knopf, A.; Eiben, B.; McClelland, J.; Grimwood, A.; Harris, E.; Menten, M.; Poulsen, P.; Nguyen, D.T.; Keall, P.; Oelfke, U. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys Med Biol. 2019, 64, 15TR01. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, C.; Valdagni, R.; Rancati, T.; Sanguineti, G. Dose–volume effects for normal tissues in external radiotherapy: Pelvis. Radiother Oncol. 2009, 93, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.N. Gastrointestinal Problems after Pelvic Radiotherapy: the Past, the Present and the Future. Clin Oncol. 2007, 19, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, W.; Chen, L.; Su, Q.; Wang, Y.; Guo, Z.; Lu, Y.; Liu, B.; Qin, S. Radiation-induced intestinal damage: latest molecular and clinical developments. Future Oncol. 2019, 15, 4105–4118. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J Pathol. 2020, 250, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ahmed, R. Radiation in Gastroenterology. Gastroenterol Res. 2022, 15, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Prieto, S.P.; Reed, C.L.; James, H.M.; Quinn, K.P.; Muldoon, T.J. Differences in colonic crypt morphology of spontaneous and colitis-associated murine models via second harmonic generation imaging to quantify colon cancer development. BMC Cancer. 2019, 19, 428. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Jiao, S.; Duan, K.; Wang, Y.X.; Petralia, R.S.; Li, Z. The BAD-BAX-Caspase-3 Cascade Modulates Synaptic Vesicle Pools via Autophagy. J Neurosci Off J Soc Neurosci. 2021, 41, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Kanth, P.; Rajan, T. Chromogranin A and Ki67 Marker in Normal Colon, Serrated Polyp and Colorectal Tubular Adenoma: 1470. Off J Am Coll Gastroenterol ACG 2011, 106, S562. [Google Scholar] [CrossRef]
- Akedo, I.; Ishikawa, H.; Ioka, T.; Kaji, I.; Narahara, H.; Ishiguro, S.; Suzuki, T.; Otani, T. Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2001, 10, 925–930. [Google Scholar]
- Andrés-Sánchez, N.; Fisher, D.; Krasinska, L. Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. 2022, 135, jcs258932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Dinjens, W.N.; Bosman, F.T. Proliferation and apoptosis in proliferative lesions of the colon and rectum. Virchows Arch. 1997, 431, 111–117. [Google Scholar] [CrossRef] [PubMed]
Authors
Demyashkin Grigory – PhD, MD; Head of the Laboratory of Histology and Immunohistochemistry, Institute of Translational Medicine and Biotechnology, Sechenov University, e-mail: dr.dga@mail.ru; ORCID ID:
https://orcid.org/0000-0001-8447-2600
Tarusova Natalia – student of the N.V. Sklifosovsky Institute of Clinical Medicine of the Sechenov University, e-mail: tarusova.natasha@mail.ru, ORCID ID:
https://orcid.org/0009-0003-7200-916X
Guseinova Amina – student of the N.V. Sklifosovsky Institute of Clinical Medicine of the Sechenov University, e-mail: Aminariiguseinova@mail.ru, ORCID ID:
https://orcid.org/0009-0001-0066-083X
Saakian Susanna – postgraduate student of the Department of Pathomorphology of National Medical Research Centre of Radiology, Ministry of Health of Russia, e-mail: drsaakyan@icloud.com, ORCID ID:
https://orcid.org/0000-0001-8606-8716
Karakayeva Elza – postgraduate student of the Department of Pathomorphology of National Medical Research Centre of Radiology, Ministry of Health of Russia, e-mail: kchr09@mail.ru, ORCID ID:
https://orcid.org/0000-0001-9833-3433
Gotovtsev Konstantin – student of the N.V. Sklifosovsky Institute of Clinical Medicine of the Sechenov University, e-mail: kostya.gotovtsev@gmail.com, ORCID ID:
https://orcid.org/0009-0008-6862-5900
Dmitrii Atiakshin – PhD, MD; Director of Research and Educational Resource Center for Immunophenotyping, Digital Spatial Profiling and Ultrastructural Analysis Innovative Technologies, RUDN University, e-mail: atyakshin-da@rudn.ru; ORCID ID:
https://orcid.org/0000-0002-8347-4556
Shegai Petr – PhD, oncologist; Head of the Centre of Innovational Radiological and Regenerative Technologies of National Medical Research Radiological Centre; e-mail: dr.shegai@mail.ru, ORCID ID:
https://orcid.org/0000-0001-9755-1164
Kaprin Andrei – PhD, MD, oncologist; General Director of National Medical Research Radiological Centre; Head of the Department of Oncology; Аcademician of the Russian Academy of Sciences; e-mail: kaprin@mail.ru, ORCID ID:
https://orcid.org/0000-0001-8784-8415
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).