Introduction
Systemic lupus erythematous (SLE) is an autoimmune, episodic, and chronic disease that can affect multiple organs in the body, in particular skin, kidneys, liver, lungs, heart, joints, nervous system, and hematopoietic organs [
1,
2]. Consequently, it can present with variety of sign and symptoms. SLE’s pathogenetic mechanism consists of polyclonal b cell activation, autoantibodies’ production and deposition of immune complexes. SLE also causes an imbalance in both humoral and cellular immunity, and like other autoimmune diseases SLE patients have higher risk of malignancy, especially lymphoma [
3]. Studies have demonstrated an increased risk of lymphoma to be 4-7 times greater in SLE patients compared to the general population [
3].
Hypercalcemia without primary hyperparathyroidism has been rarely reported in the context of SLE, and may be caused by other underlying conditions [
4,
5]. Furthermore, hypercalcemia has been shown to be associated with poor prognosis in lymphoma patients [
6]. In this case report, we present a patient with SLE flare up and hypercalcemia alongside B-cell lymphoma.
Case Presentation
We report a 41-year-old man which initially presented with thrombosis in left femoral and popliteal veins ten years ago. He was administered warfarin (5 milligram daily) and was relatively well till one year after anticoagulant tapering, another thrombosis was formed in his right leg, which was also accompanied with fatigue, photosensitivity, malar rash, thrombocytopenia, low complement level and positive anti-phospholipid antibodies (APL), anti-double stranded DNA (anti-ds-DNA) and FANA antibodies. He was diagnosed as a case of SLE and antiphospholipid syndrome and was administered 10 mg prednisolone, 100 mg azathioprine and 400mg hydroxychloroquine daily.
He was relatively well till three months prior to admission, when he developed severe malaise and fatigue, for which his prednisolone and azathioprine dosage was increased to 15 mg and 150 mg daily, respectively. After two months, not only didn’t his condition improve but also developed dyspnea, which 1gr of rituximab was added to his treatment regimen. However, no improvement was achieved in his condition during the next two weeks, and he was subsequently admitted to our center for further evaluation.
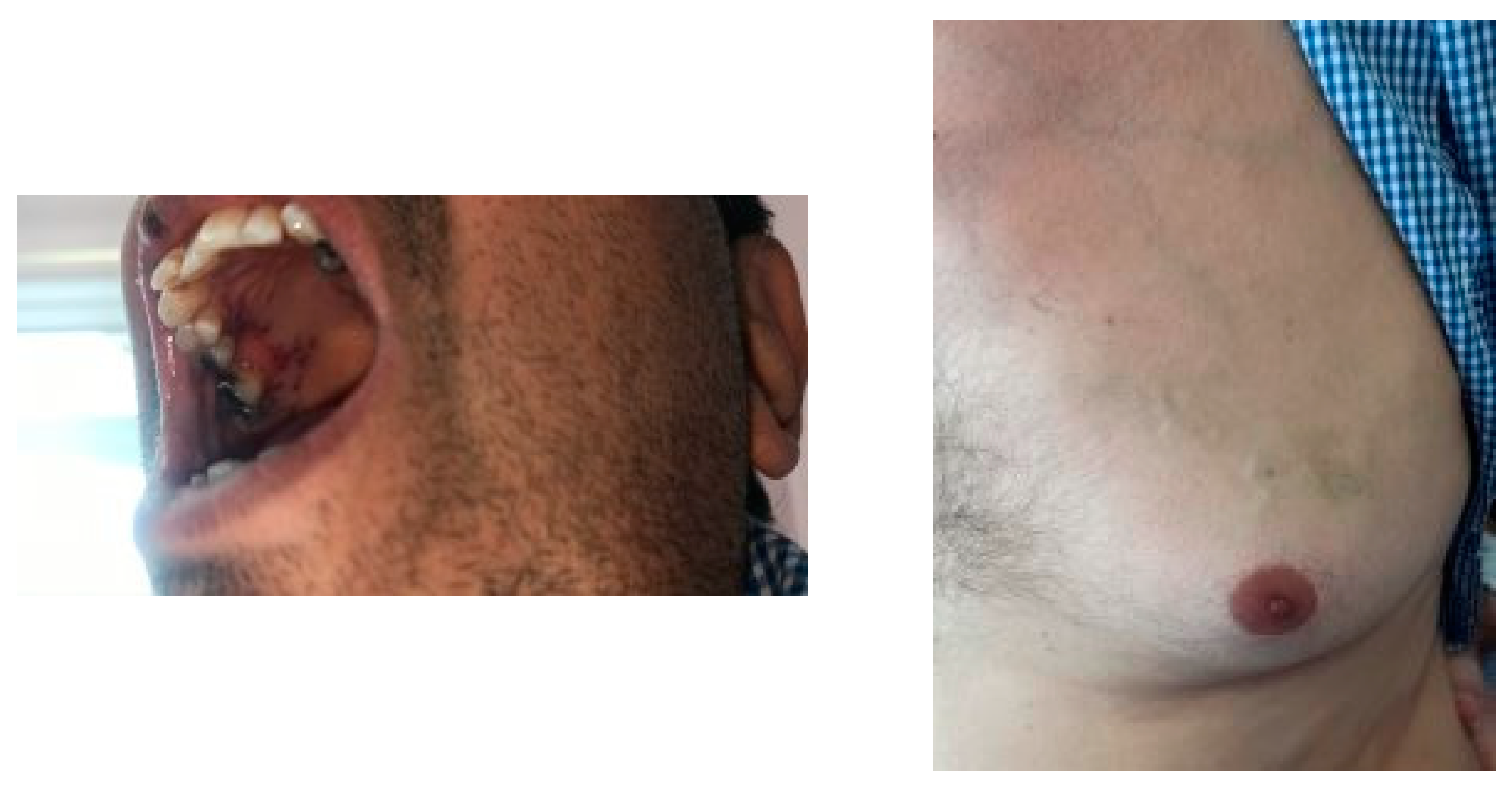
In our evaluation, the patient was alert and had stable vital signs. He complained of fatigue, pigmented lesions and a painless ulcer (1*2 cm) on his hard palate and fever. Physical examinations showed no significant respiratory distress, a temperature of 38°C, painless oral lesions, swelling of both wrists with no limitation in range of motion, palpable spleen, normal distal pulses, or lymphadenopathy (
Figure 1). The patient also had enlarged tortuous veins on his left breast from two years ago (
Figure 1).
Initial laboratory evaluation demonstrated a white blood cell (WBC) count of 1900/microliter with neutrophil 52%, lymphocyte 10% and eosinophil 5%, platelet (PLT) 26000/microliter, erythrocyte sedimentation rate (ESR) 102 mm/h (normal range <20) , CRP 187 mg/L (normal range <10) , brain natriuretic peptide test (Pro BNP) 786 pg./mL (normal range <250), C3 of 34 mg/dL (normal range 90-180) , C4 of 5mg/dL (normal range 10-40), anti-ds DNA of 23.5 (normal range <12). The patient’s full laboratory data are presented in
Table 1.
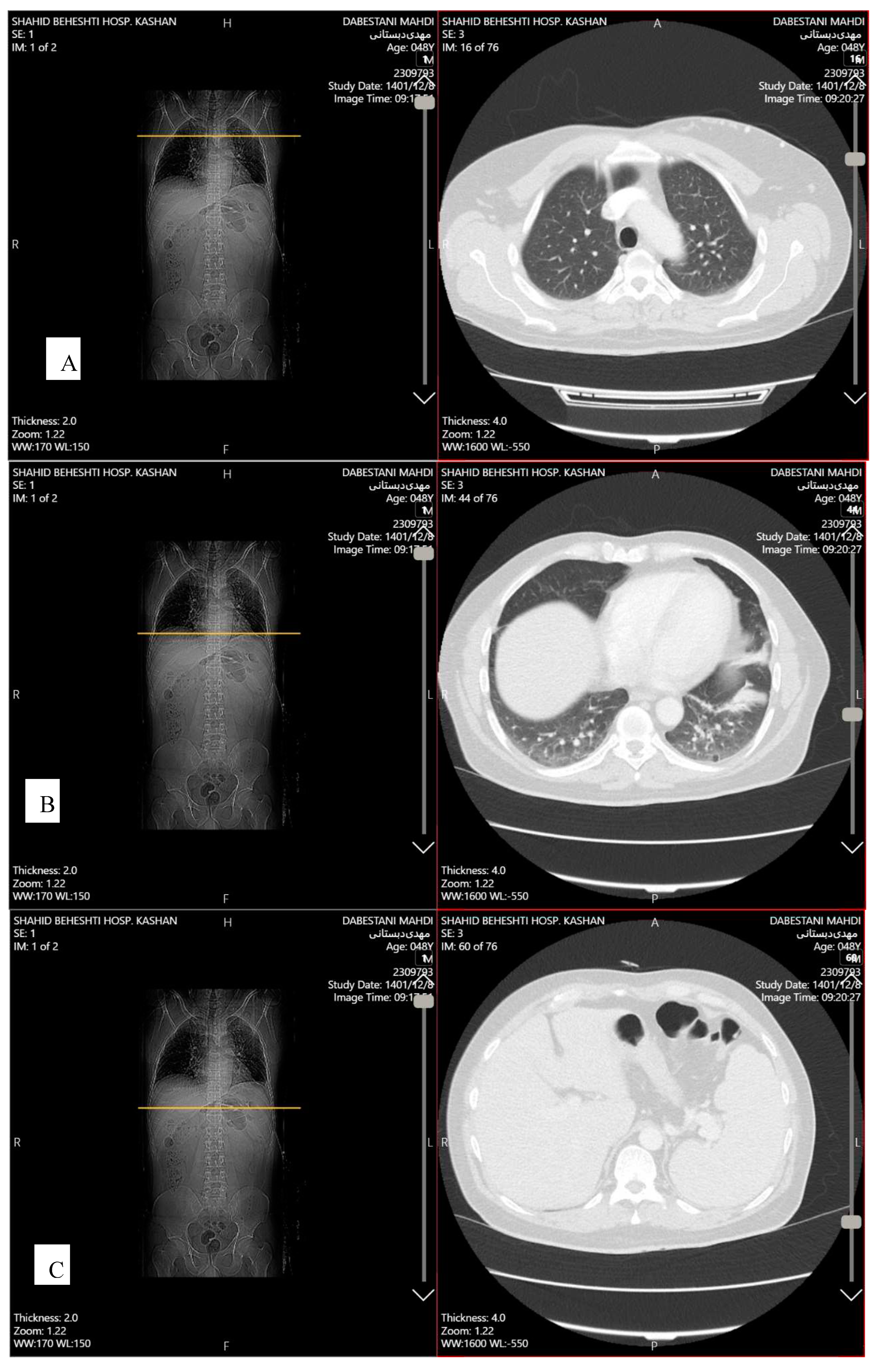
Transthoracic echocardiography (TTE) showed mild pericardial effusion, and mild diastolic dysfunction. Abdominopelvic spiral computed tomography (CT) scan with contrast demonstrated splenomegaly (135 mm). Chest spiral CT scan with contrast showed patchy collapse with features of air bronchogram in the right middle lobe, left hemidiaphragm elevation, diffuse discoid atelectasis in both lung fields with precedence in the left lower lobe and left upper lobe, multiple varicoid vessels on the left breast, aneurysmal vessels (7*12 mm) in lateral of left breast due to Mondor's disease, and mild fibrotic changes in the base of both lungs (
Figure 2). Breast sonography was unremarkable.
Based on the patient’s oral lesions, mild pericardial effusion, low complement levels, and high ESR, anti-ds DNA, and pro-BNP levels, SLE flare-up was suspected, and a daily dose of 1gr methylprednisolone was initiated for a period of three days. Furthermore, broad-spectrum intravenous antibiotics (including one-gram meropenem every 8 hours, 400mg ciprofloxacin every 12 hour, 1gram vancomycin every 12 hours) was given to the patient as an infection was presumed to trigger his flare-up, based on the patients high procalcitonin and fever.
After these three days, the patient’s general condition improved as his oral lesions decreased, his fever resolved, and pancytopenia converted to bicytopenia with low levels of red blood cells (RBC) and PLT. Afterwards, methylprednisolone was discontinued and prednisolone was started with dose of 1mg/kg for him.
Despite this progress, the patient remained bicytopenic. Bone densitometry were performed, which showed signs of osteopenia. Initially, based on the patient hypercalcemia, he received three pulses of corticosteroids with the suspicion of lupus flare-up, in which calcium levels returned to normal after the pulse; however, two days after the administration of pulse, calcium levels increased again to 12-13, in which intravenous pamidronate was administered, consequently decreasing calcium levels. However, after one week, a rerise in the calcium level was observed. Despite the absence of parathormone suppression and based on the persistent hypercalcemia, sestemibi scan was performed to evaluate the parathyroid glands, which was unremarkable (regarding hypercalcemia, PTH level was not suppressed). Therefore, based on the resistant hypercalcemia and lack of improvement in blood parameters and progressive fatigue and weakness, bone marrow malignancy was suspected and the patient underwent bone marrow aspiration and biopsy, as well as flow cytometry.
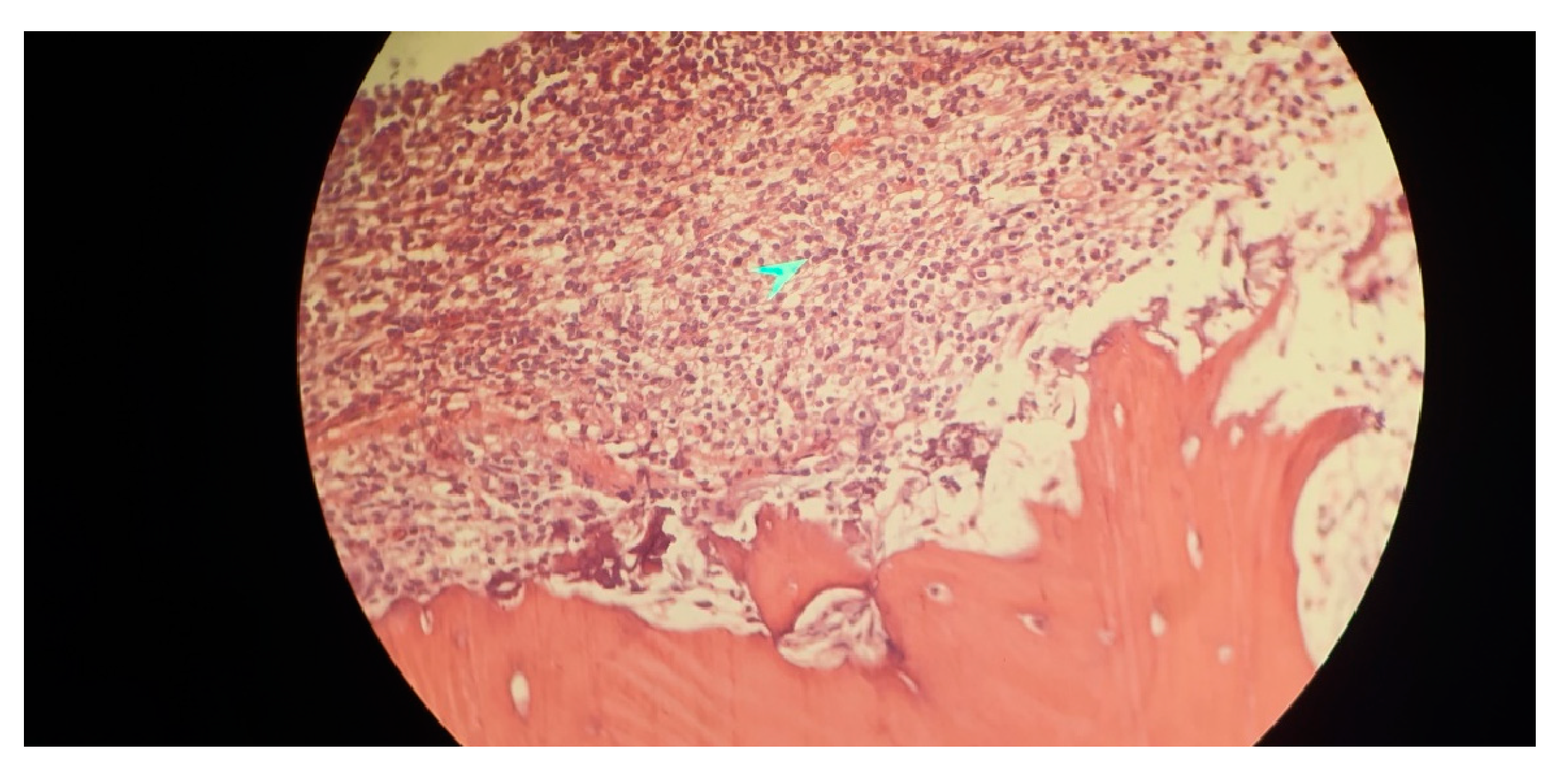
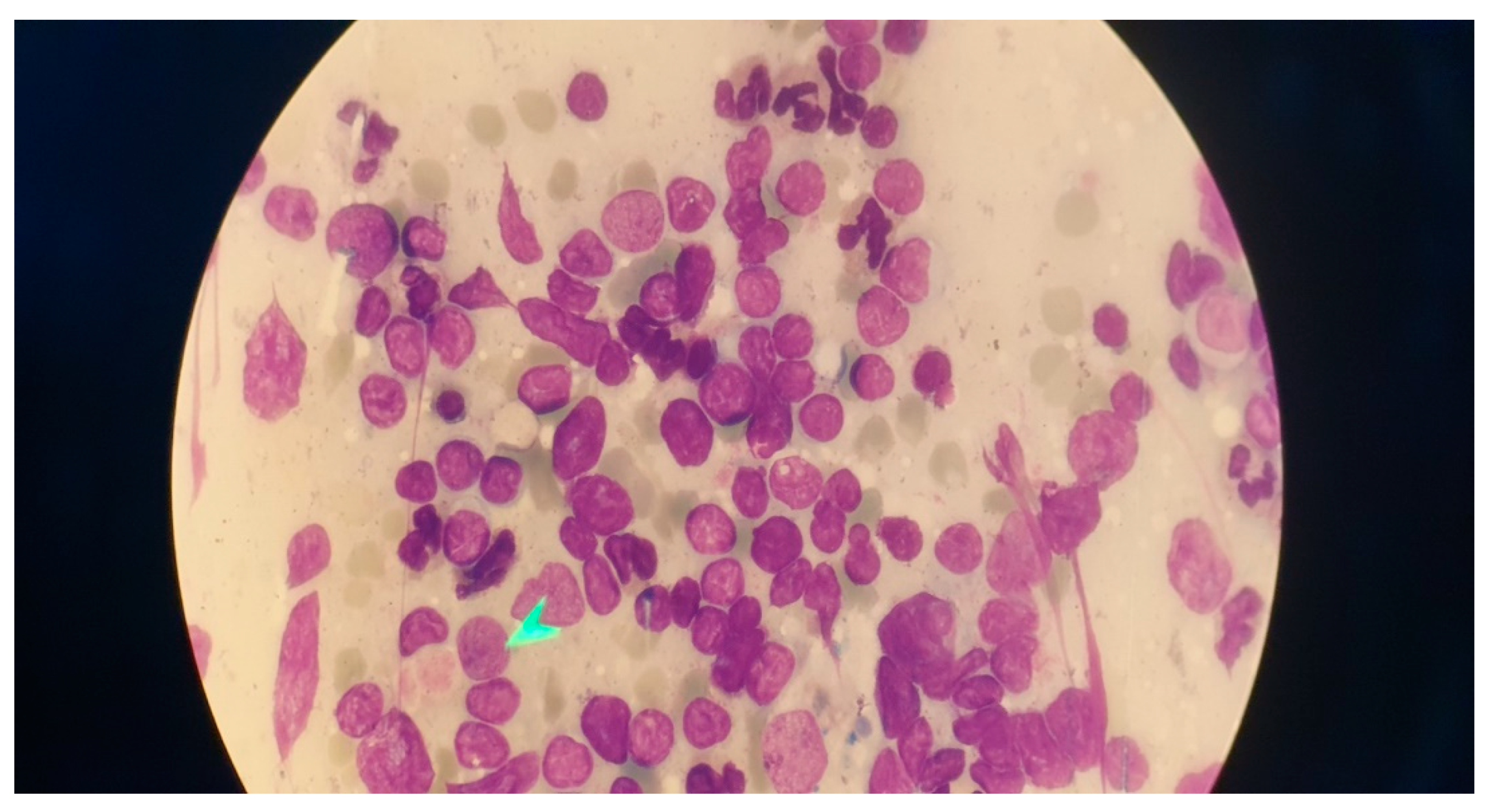
Immunophenotyping results of bone marrow aspiration by flow cytometry revealed a lymphoid cell population of about 52% of the total cell analyzed, mostly composed of mature B cells that express CD45-CD19- CD20-CD22 - surface kappa light chain and negative for CD5- CD10- CD11c-CD23-CD25- CD103-FMC7-CD34-TdT and T cell markers. Bone marrow biopsy showed fragments of bone trabecula and marrow spaces with about 70% cellularity, megakaryocytes were decreased, and no granuloma was identified. Hematopoietic elements were replaced by neoplastic lymphoid cells having round nuclei and clear cytoplasm arranged diffusely, all of which suggested marginal B cell lymphoma as the ultimate diagnosis (
Figure 3).
He was started on R-CHOP chemotherapy regimen, and patient’s follow up shows relative respond to the treatment; the reduction of blood’s cell lines and patient’s general condition improved and he only complaint of mild fatigue.
Discussion
We report a case of SLE flare up, whom developed hypercalcemia and during our exploration, was diagnosed as a case of B-cell Lymphoma. Although hypercalcemia itself can be a rare complication of SLE flare up as mentioned in multiple case reports[
7], this disturbance in calcium levels can have a wide variety of causes such as, hyperparathyroidism, malignancies especially solid tumors, and lymphoproliferative diseases. Also, other infrequent etiologies such as D hypervitaminosis, granulomatous diseases, some drugs, and endocrine diseases can be accounted responsible[
1]. our case is the first report of concurrent SLE flare up with hypercalcemia and lymphoma as the ultimate diagnosis. The definite reason for hypercalcemia in this case couldn’t be specified to solemnly one of the etiologies mentioned above. Therefore, clinicians should take into consideration the possibility of lymphoma among the etiologies to be the cause of hypercalcemia in a patient with SLE flare-up, aside from other endocrinology or rheumatology-based diseases.
SLE was confirmed based on the patient’s photosensitivity, malar rash, thrombocytopenia, and positive anti-ds DNA, which fulfilled 4/11 of the ACR classification criteria for definite diagnosis of SLE [
8]. The patient also developed hypercalcemia, which has been reported to rarely occur among SLE patients. Since hypercalcemia didn’t resolve after administering treatment for SLE flare up, we came to the conclusion that patient’s hypercalcemia has another underlying etiology.
The pathophysiology of lymphoma causing hypercalcemia can be summarized in 3 different ways; secretion of PTHrP also called humoral hypercalcemia malignancy, osteolytic metastases, and increased production of calcitriol. Typical laboratory data findings in patients with humoral hypercalcemia malignancy are suppressed PTH, high PTHrP, and variable calcitriol level[
9]. Osteolytic metastasis as a pathophysiology for lymphoma to cause hypercalcemia is rather uncommon. Furthermore, typical findings include low PTH, low calcitriol, low PTHrP level, and extensive skeletal metastases[
10]. In our case, the patient turned out to have multiple conditions with overlapping sign and symptoms, and each one can be a consequence of the other. Features such as resistant cytopenia and splenomegaly alongside hypercalcemia raised our attention for evaluating causes of malignancies. Therefore, physicians should be wary of concurrent signs and symptoms in favor of underlying malignancies, such as lymphoma, in acute phase patients with rheumatologic diseases.
SLE, similar to other autoimmune diseases, is associated with increased risk of developing lymphoma when compared to the general population. Diffuse large B cell lymphoma followed by Hodgkin lymphoma are the most common encountered subtypes. Lymphoma's pathogenesis in SLE is still poorly understood. Neither prolonged disease activity nor immunosuppressive therapy is correlated with the development of lymphoma [
11] Both SLE and lymphoma more frequently exhibit elevated levels of certain cytokines and proteins known to be linked to cell survival and proliferation, such as BAFF, APRIL, IL6, and BCL2, which likely have an impact on pathogenesis [
3]. Aside from the pathogenesis of the diseases, a high level of suspicious for malignancies should be considered in SLE patients when presenting with features unresponsive to treatment, or unjustified with SLE presentations.
Hyperparathyroidism is the most common cause of hypercalcemia in the general population[
4]. We considered this entity in our patient based on his hypercalcemia and low phosphorus, however was ruled out by Sestemibi scan and normal unsuppressed PTH level. SLE can cause polyclonal overactivation of b cell lymphocytes that can consequently produce anti-PTH receptor autoantibodies which binds to and activate PTH receptors and results in hypercalcemia. However, this phenomenon causes a low PTH level in the blood, and so can’t be the reason of hypercalcemia due to normal level of PTH in our patient[
4,
5]. Renal insufficiency can cause tertiary hyperparathyroidism and consequently hypercalcemia,[
12]; however, in our patient urinalysis showed only mild proteinuria, normal plasma creatinine level, with normal sized kidneys in the ultrasound and therefore renal insufficiency was ruled out. Furthermore, our patient didn’t have any sign or symptoms supporting granulomatosis diseases or sarcoidosis as an etiology for hypercalcemia[
13]. Ultimately, the patient’s lack of response to the treatment and pancytopenia led us to the diagnosis of lymphoma which was confirmed by bone marrow aspiration and biopsy.
As both lymphoma and SLE flare up can present with cytopenia and hypercalcemia and other signs that have overlap with one another, it is important to be aware of the fact that both of these conditions can mimic each other’s presentation[
14]. Specialists and physicians should be aware of this possible concurrence, to avoid misapprehension and overlooking other etiologies based on routine management of SLE flare ups. Although the presentation of hypercalcemia is rare in the context of SLE flare up, this presentation ultimately led us to further assessment of the patient and achieving a diagnosis of lymphoma. On the other hand, lymphoma could present itself as a possible cause of SLE flare up, similar to other etiologies such as infection. Therefore, thorough examination and reassessment of the patient should be considered in the context of SLE flare up, especially when presenting with uncommon manifestations.
Conclusion
SLE flare up could manifest with various signs and symptoms, which may mimic other entities; However, misapprehension of the cause SLE flare up could cloud the diagnosis of possible concurrent diseases such as lymphoma. In our case, we couldn’t entirely justify the high calcium level with SLE flare up or other etiologies, which led to the ultimate diagnosis of a lymphoma. Although lymphoma itself could also trigger SLE flare up, the presentation could cause challenges in diagnosis, especially in cases with uncommon presentation which mimic other entities. Hypercalcemia in SLE patients should be investigated accordingly, and in cases with normal parathyroid hormone levels, malignancies should be suspected, especially when accompanied with other evidences of malignancy, such as resistant cytopenia or organomegaly. Physicians should be aware of the fact that patients with SLE flare up can present with manifestations mimicking concomitant conditions, which require further exploration.
Funding
No financial support was received for this report.
Ethics approval and consent to participate
Written informed consent was obtained from the patient in our study. The purpose of this research was completely explained to the patients and they were assured that their information will be kept confidential by the researcher.
Consent for publication
Written informed consent was obtained from the patient for the publication of this report and the related images. A copy of the written consent is available for review by the Editor of this journal.
Availability of data and materials
All data regarding this study has been reported in the manuscript. Please contact the corresponding author if you are interested in any further information.
Acknowledgements
The authors would like to thank the assistance of all members and professors of Shariati hospital in the management of this case, and also valuable comments.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Del Porto, F.; Proietta, M.; Koverech, A.; Trappolini, M.; Aliberti, G. Hypercalcaemia in systemic lupus erythematosus. Lupus 2011, 20, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Kiper Yilmaz, H.T.; Tosun Tasar, P.; Carlioglu, A. Hypercalcemic Crisis in Systemic Lupus Erythematosus. Acta Endocrinol (Buchar) 2018, 14, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Polliack, A.; Gafter-Gvili, A. Systemic lupus erythematosus and lymphoma: Incidence, pathogenesis and biology. Leuk Res 2018, 75, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, H.B.; Hamaz, S.; Bachir, H.; Eloumri, A.A.; Berrimi, M.; Bouayad, A.; Serraj, K. Concomitant primary hyperparathyroidism and systemic lupus erythematosus: coincidence or not? A new case report. Pan Afr Med J 2020, 37, 228. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, J.; Pu, R.; Yue, Z.; Mo, Y.; Jiang, X. Lupus Nephritis With Mild Asymptomatic Hypercalcemia in Children: A Case Report and Literature Review. Front Pediatr 2019, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Vallet, N.; Ertault, M.; Delaye, J.B.; Chalopin, T.; Villate, A.; Drieu La Rochelle, L.; Lejeune, J.; Foucault, A.; Eloit, M.; Barin-Le Guellec, C.; et al. Hypercalcemia is associated with a poor prognosis in lymphoma a retrospective monocentric matched-control study and extensive review of published reported cases. Ann Hematol 2020, 99, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, L.; Zhang, X. Systemic lupus erythematosus-related hypercalcemia with ectopic calcinosis. Rheumatol Int 2016, 36, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.J.; Tedesco, M.B.; Sereika, S.M.; Hollis, B.W.; Garcia-Ocana, A.; Stewart, A.F. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 2003, 88, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. N Am J Med Sci 2015, 7, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Polliack, A.; Gafter-Gvili, A. Rheumatoid arthritis and lymphoma: Incidence, pathogenesis, biology, and outcome. Hematol Oncol 2018, 36, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin J Am Soc Nephrol 2017, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Shepard, M.M.; Smith, J.W. , 3rd. Hypercalcemia. Am J Med Sci 2007, 334, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, R.; Sin, W.Y. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep 2014, 2014, bcr2013201802. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).