1. Introduction
In recent years, there has been a growing recognition of the importance of promoting health-protective behaviors and preventing health-compromising behaviors such as physical exercise (e.g., [
1,
2,
3,
4]). This is because many of the leading causes of morbidity and mortality, such as cardiovascular diseases (CVDs), cancer, and diabetes as non-communicable diseases are related to lifestyle factors such as physical inactivity, poor nutrition, and smoking. Health-protective behaviors refer to actions that individuals can take to maintain and improve their health and well-being, such as regular physical activity, self-care maintenance, and seeking preventive healthcare. These behaviors can help reduce the risk of chronic diseases and other health problems [
1,
4]. On the other hand, health-compromising behaviors refer to actions that can negatively impact an individual’s health, such as highly sedentary behavior. These behaviors can increase the risk of chronic diseases, and premature death [
1]. Promoting health-protective behaviors and preventing health-compromising behaviors require a comprehensive approach [
3].
Currently, there are more than 6 million new cases of CVD in the European Union (EU) every year. With almost 49 million people living with CVD, the economic impact in the EU is high at €210 billion a year. CVD is the leading cause of death in Europe, accounting for 2.2 million deaths in females and 1.9 million deaths in males. Thereby ischemic heart disease accounts for 38% of CVD deaths in female and 44% in male patients [
1].
The prevention of acute cardiovascular events is of utmost importance: For instance, a study in Germany from 2015 shows that inpatient treatment costs in the acute phase of CVD account for the largest share within the first year, while medication is one of the least cost-intensive components [
3]. However, physical exercise is also effective in reducing cardiovascular mortality, total mortality, and myocardial infarction [
4]. This emphasizes the potential for secondary and tertiary prevention in cardiovascular risk management.
Reducing the risk profile for cardiovascular co-morbidity and mortality is a lifelong task and requires patients to have – or acquire – strong self-management abilities. Cardiac secondary and tertiary prevention as well as medical rehabilitation as described in evidence-based guidelines, therefore, includes providing health education and strengthening health literacy. However, strengthening health literacy also implies increased self-efficacy. The theoretical construct of self-efficacy (also known as self-care confidence) can be considered an element of health literacy. According to this, a person is health literate if they have the confidence and willingness to apply health information to themselves and consequently convert it into action [
5]. Furthermore, ensuring reliable lifestyle-related measures such as no smoking, low to moderate alcohol drinking, regular physical activity, a healthy diet, and optimal body weight is important [
6]. The implementation of mobile rehabilitation approaches in China demonstrates that mHealth is evidence-based and effective, but for Germany, there is little evidence in comparison (e.g., [
7]).
Syntheses of randomized controlled trials report successful improvement of blood pressure control via increases in self-management in patients with hypertension [
8], and a significant reduction in mortality risks in patients with coronary heart disease [[9]. The described measures of cardiac treatment are effective and, thus, are the evidence-based standard of care. However, a large survey by the European Society of Cardiology found that majority of coronary heart disease patients do not adhere to the recommendations for secondary prevention of cardiovascular disease [[11]. According to a meta-analysis, average medication adherence is low for secondary prevention of cardiovascular disease – with only 66% [
12].
Strategies to improve adherence to therapy guidelines are urgently needed and digital behavioral interventions such as eHealth provides much evidence [
13]. In 2020, Germany passed a policy allowing digital health applications for the treatment of diseases to be prescribed by physicians and to be covered by health insurance. Digital applications hold the potential to support patients with CVD in their daily therapy routine, while being cost-effective and providing coverage for best practice measures [
14,
15]. International studies on the effect of smartphone applications to support the monitoring and strengthening of adherence of patients with CVD indicate their benefit in terms of significantly improved medication adherence [
16,
17,
18] and significant reduction of systolic blood pressure [
19,
20,
21]. Health psychology can help to improve such intervention in healthcare or also in addition to the healthcare system.
A theoretical basis from
health psychology for such approaches [
22] can be the Compensatory Carry-Over Action Model (CCAM,
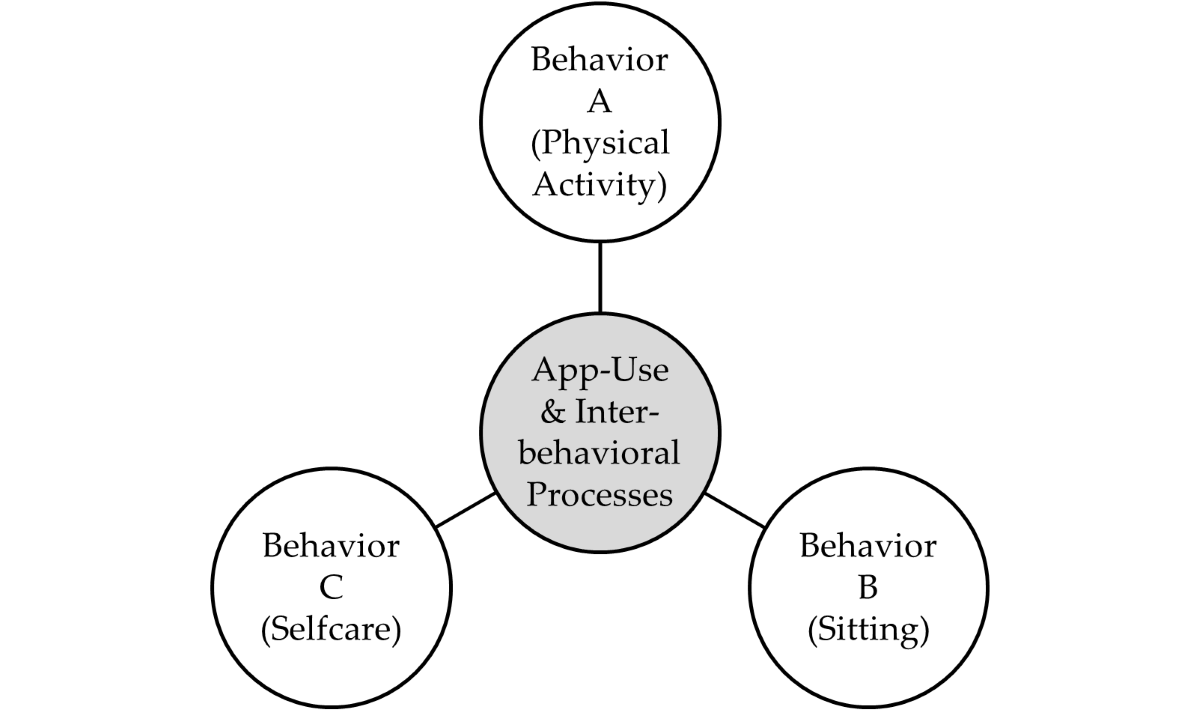
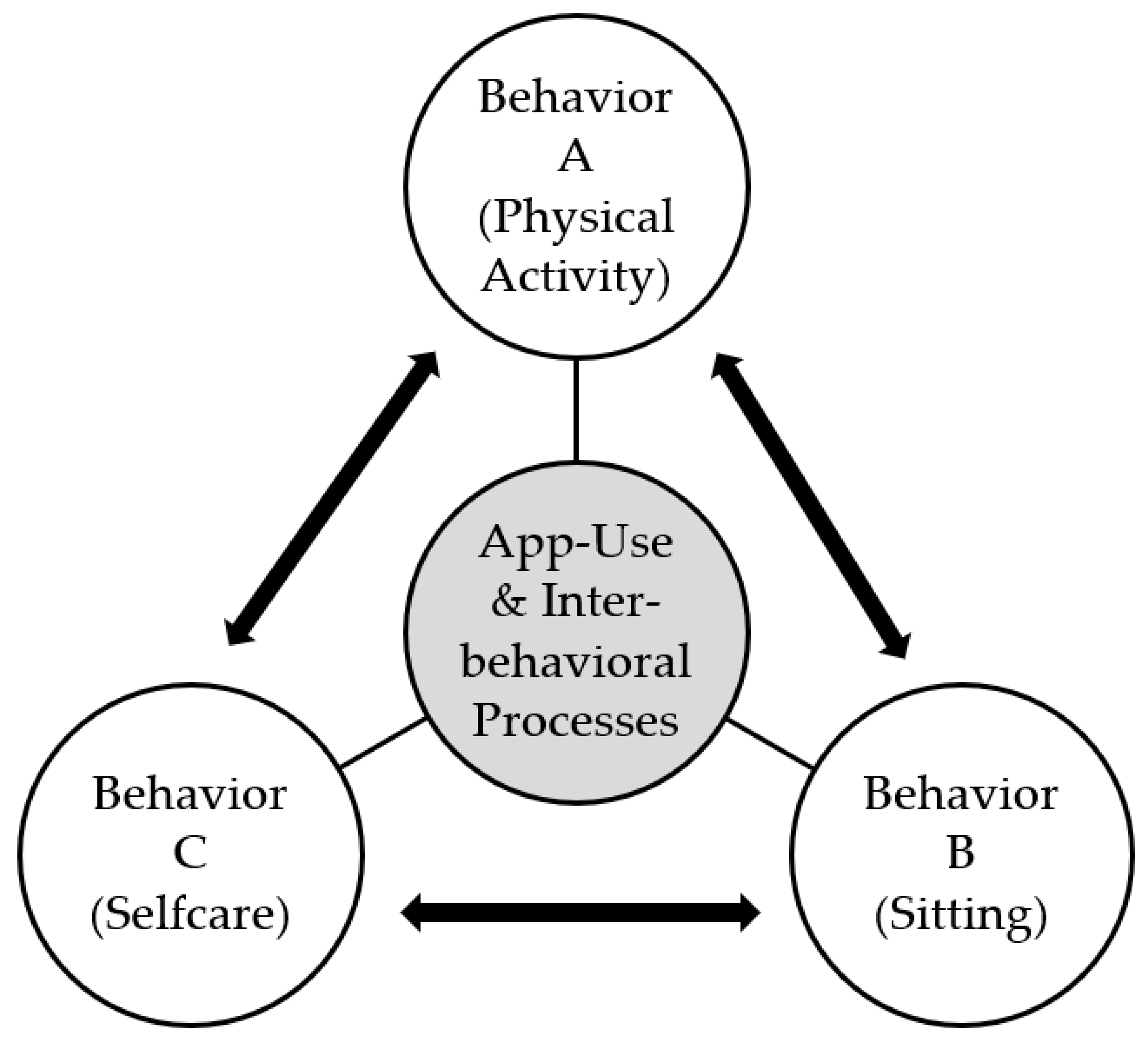
Figure 1 [
24]), which explains how positive behavior changes in one domain can lead to compensatory behaviors in another domain. Concretely, self-care and physical activity are behaviors that should correlate positively with each other and negatively with sitting. The model has important implications for understanding how behavior change interventions can be designed to promote sustainable behavior change across multiple domains of life.
So far, none of the evaluated smartphone applications for strengthening adherence of cardiovascular patients were certified as medical devices in Germany after being tested in a randomized controlled trial. Especially as there are few cardiovascular self-management smartphone applications registered as a medical device and none has been systematically evaluated with clinical patients in the German healthcare setting, this calls for according action. Moreover, little is known about the interrelations of different behaviors over time and with each other. Therefore, this study integration of science and practice tested this with the following objectives.
The aim of the study with the study design of a randomized controlled trial was to explore the effects of the reCardial smartphone application (reCardial app) on disease self-management and health behavior change. It was hypothesized that the use of the reCardial app in addition to standard care shows superiority compared to its non-use (i.e., standard care alone) after 12 weeks (T1) with regards to the increase of therapy guideline adherence in patients with hypertension, and concomitant chronic ischemic heart disease and/or heart failure. The secondary aim was to investigate treatment effects on patient-reported self-efficacy, physical activity, and self-measured blood pressure. The following hypotheses were tested.
H1: Health behaviors (physical activity and sitting) and disease self-management indicators (self-care maintenance, self-care confidence) are closely interrelated.
H2: The change in health behaviors and disease self-management indicators over time (T0 to T1) is more pronounced in the use of the reCardial app in addition to standard care compared to using standard care alone.
H3: In the intervention group, changes in systolic and diastolic blood pressure indicate a positive trajectory.
2. Materials and Methods
Design
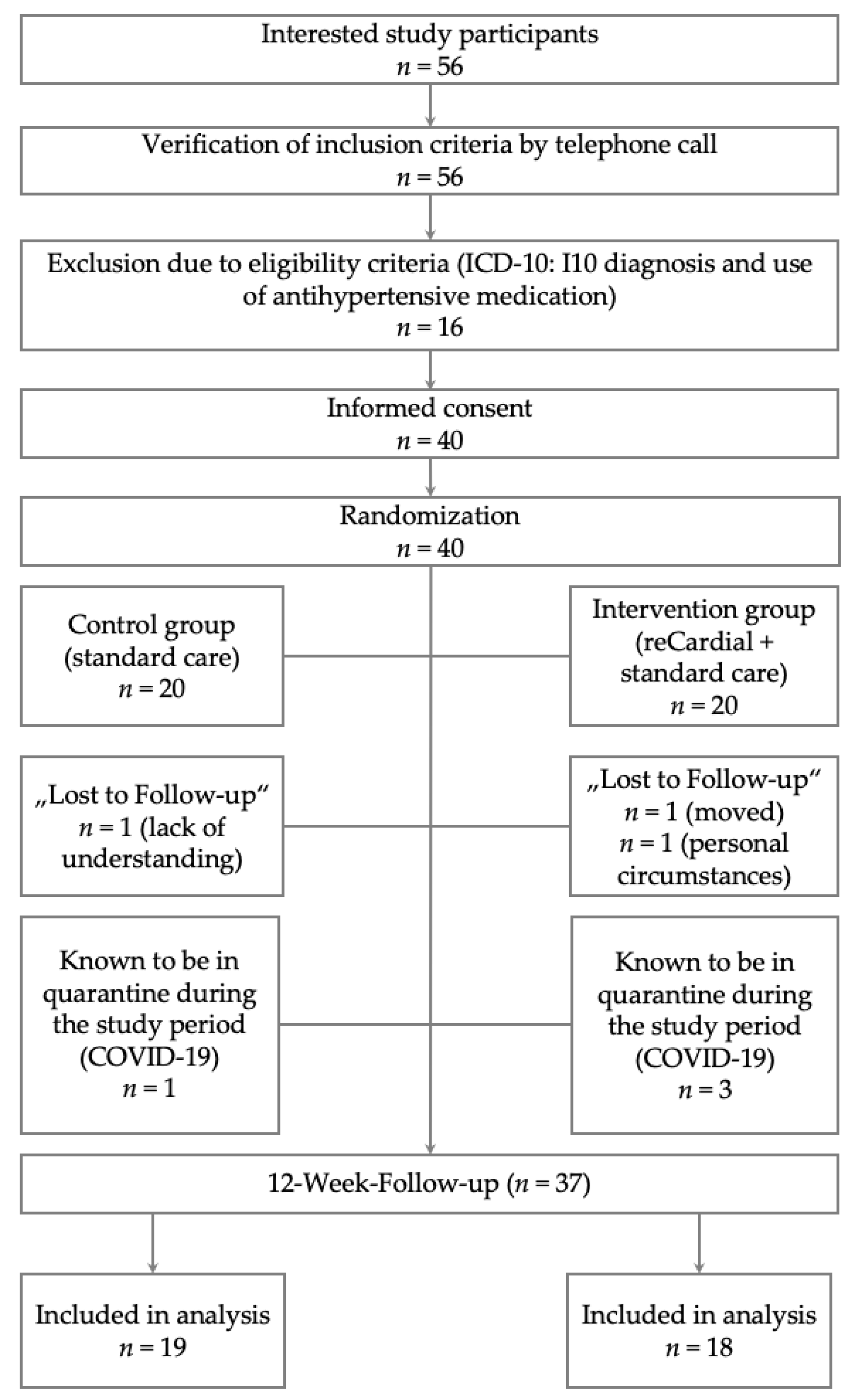
This is a national, randomized controlled and superiority trial with a pilot study methodology. Eligible participants (
n=40) were randomly assigned to one of two parallel groups: a control group (
n=20) or the intervention group (
n=20) using a 1:1 allocation (see
Figure 2).
The intervention group received access to the reCardial app in addition to standard care for 12 weeks. The control group received only standard care without the smartphone application. A flow diagram of the study design is shown in
Figure 2. After the 12-week follow-up assessment, the control group received access to the reCardial app as well.
The behavioral intervention in the form of access to a smartphone application to support the self-management of CVD took place in the patient's daily care routine at home. The control group received the standard care as recommended by currently valid guidelines or as realized in disease management programs (without receiving the smartphone application in addition).
Recruitment and Eligibility Criteria
Study participants were recruited by general practitioners and specialists in the Bremen region who distributed flyers directing their interested patients to the study website. Further, recruitment also took place through cardiac sports groups, social media calls, and media press releases.
Subjects needed to have been diagnosed with essential (primary) hypertension (I10 code according to the International Classification of Diseases 10th Revision [ICD-10]) to be included in the study. Subjects with concomitant diagnosed chronic ischemic heart disease (ICD-10 code: I25), and/or subjects with concomitant diagnosed mild to moderate heart failure (ICD-10 code: I50; New York Heart Association, NYHA, Functional Classification of stages I to III) were included as well. Further inclusion criteria were the patient’s informed consent; being at least 18 years of age; the ability to read and understand the German language; regularly taking antihypertensive medication; owning a smartphone and being able to use it.
Patients were excluded if any of the following applied: having had an acute cardiovascular event (e.g., a myocardial infarction) within the previous four weeks; pregnancy; active psychosis or severe dementia (in accordance with the instructions for use of the reCardial app); prior use of the reCardial app; and simultaneous participation in another study.
In addition, a set of contraindications is defined for the use of the reCardial app, which also led to exclusion from the study (see
Table A1 in the supplementary material
Appendix A).
Behavioral Intervention
To accurately portray the German standard healthcare, the comparator (i.e., the control group) was chosen to receive the evidence-based standard care in accordance with therapy guidelines. This provided information for general practice care in accordance with the guidelines. Usually, control visits took place on a quarterly basis.
The reCardial app in addition to standard care was defined as the intervention in this randomized controlled trial. It was developed by a multidisciplinary group of experts of medical practice and health psychology research integrating also stakeholders needs by a co-design strategy. The app was designed in accordance with the mentioned therapy guidelines and applies health psychological theories [
22,
24] and behavior change strategies [
25] to support patients in the everyday management of their disease [
8,
26,
27,
28]. Essential hypertension, heart failure, and chronic ischemic heart disease are lifelong conditions and are highly dependent on lifestyle. Accordingly, they require a high level of health literacy and self-management skills. This includes that patients continuously monitor and plan relevant parameters, such as regular medication intake, physical activities, and measurement of blood pressure and/or body weight. Thus, the reCardial app was designed to provide additional support in parallel to standard care by means of enhancing self-management skills and strengthening adherence to therapy guidelines.
The reCardial app is a self-management tool for the daily monitoring and prevention of cardiovascular risk factors and aims to support the implementation and adherence to therapy guidelines for the treatment and secondary/tertiary prevention of CVD. The digital health application includes the following main features: reminder function for scheduled medications, trainings, and vital data (such as blood pressure) measurements; self-monitoring of recommended health behaviors and health measurements; as well as health and self-management education, as this demonstrated to be effective [
29,
30,
31,
32]. It is available for iOS and Android.
In consultation with the attending physician, the medication plan and target values for exercise and training, as well as weight and blood pressure, are defined. In the reCardial app, patients can then select the relevant modules they want to focus on ("steps", "medication" and "training") and enter the target values they defined with their physicians. Patients are instructed to manually document and/or synchronize their values and activities via wearable devices (such as activity trackers, smart watches, or blood pressure monitors) every day using the reCardial app. Under “progress”, the development of daily steps and vital data is graphically displayed, and users can track their adherence to behavioral goals regarding planned medications and training. Reminders in the form of push notifications provide additional support for taking medication and adhering to planned physical activities and blood pressure measurements.
In addition, users receive theory-based information and recommendations in the form of texts and videos which are tailored to the duration of use (see e.g., [
21,
23]). The first twelve-week phase of using the reCardial app includes a structured health education course that belongs to the “knowledge” module and covers twelve weekly topics. The course content is based on psychological theories and behavior change strategies and has been developed by a multidisciplinary team of medical specialists (e.g., cardiologists), health scientists, and behavioral psychologists. During the first weeks, the relevant cardiovascular risk factors, risk behaviors as well as corresponding health consequences are explained to address individuals who are not yet motivated to adhere to the therapy guidelines. In subsequent weeks, advice on therapy guideline adherence is given to increase intention to change the behavior and to enhance the perceived self-efficacy [
31]. Towards the end of the twelve-week course, patients are educated on implementation and planning methods to support habit formation and perceived self-efficacy for maintaining the newly adopted healthy lifestyle in the long term. The patient receives further regular information with helpful advice based on personal preferences. The monitoring implemented in the app continues as usual. Furthermore, it is important that what has been learned is internalized and implemented.
In order to monitor and support the therapy, the reCardial app makes use of behavior change techniques [
22,
23,
24], which are designed to help promote relevant predictors of behavior change by initiating so-called mechanisms of action [
25]. For example, patients are encouraged to set goals, define barriers to health behavior change, create if-then plans, and regularly monitor and critically assess their progress. They are supported by app features such as reminders, visual feedback, biofeedback (display of vital signs), highlighting of health consequences, and praise (e.g., see [
19]). In addition, at the beginning of each week, users receive a “task of the week”, encouraging them to implement behavior change strategies such as setting goals, seeking social support, trying out a stress management method, or restructuring their environment. With quizzes, patients can test their health knowledge and self-management skills on a weekly basis. Weekly short surveys serve to prompt the participants to evaluate their health behavior, satisfaction with quality of life, health, stress, and sleep, or social-cognitive determinants of promoting health-protective behaviors and preventing health-compromising behaviors i.e., levels of self-efficacy, intention, and planning.
The “locations” module additionally displays rehabilitation clinics, hospitals with diagnostic and therapy units for the care of patients with acute chest pain (Chest Pain Unit, CPU) as well as nearby supervised cardiac sports groups [
31]. As part of the randomized controlled trial, all participants received a blood pressure monitor and instructions to measure their blood pressure regularly [
32] and – if they belonged to the intervention group – instructions to synchronize the results with the reCardial app.
Participation in the study was terminated at the request of the study participants. No criteria for deterioration of the medical condition are defined in advance, as this was monitored during the study. No intervention modifications were planned. As incentives, all study participants received a blood pressure monitor that could be kept at the end of the study and a small financial amount, under the condition the study participants complete the 12-week follow-up online questionnaire.
Additionally, within the reCardial app, various indicators of usage and adherence were collected. These included: monitoring of blood pressure (average number of synchronized blood pressure readings per week, average systolic and diastolic blood pressure per week); number and percentage of medications marked as taken and not marked as taken per week; number and percentage of planned workouts marked as done and not marked as done per week; average daily steps per week; number of articles read per week in the “knowledge” module; and number of correct and incorrect answers to weekly quiz questions.
Users receive push notifications if one of the indicators of adherence has not been logged for seven consecutive days. A further strategy to improve adherence was the implementation of gamification features such as streaks for continuous monitoring and meeting of targets and collecting “heart points” for using and adhering to app features.
All study participants were treated with standard care according to evidence-based guidelines. Patients were required to refrain from undergoing alternative treatment options which required simultaneous participation in another study. Patients were advised to consult their physician before undergoing any concomitant care which was not defined as standard care or as a certified disease management program.
Outcomes
Primary outcome
The primary endpoint was the change in the adherence to therapy guidelines after 12 weeks, which is operationalized using the Self-Care of Hypertension Inventory (SC-HI) score – section A (Self-Care Maintenance), Version 2.0 [
33] with eleven items, covering recommended self-care activities, measured on a four-point Likert scale. The SC-HI has also already been applied to a Polish sample [
34] and was analyzed using the standardized score with values potentially ranging from 0 to 100 (higher values indicating higher self-care). A difference of eight points and a standard deviation (SD) of 16 was considered clinically relevant [
35,
36].
Secondary outcomes
The following parameters were investigated as secondary endpoints:
Self-Care Confidence/Self-efficacy at baseline and after 12 weeks measured by the SC-HI score – section C, Version 2.0 [
33] with 6 items, measured on a four-point Likert scale and using the standardized score ranging from 0 to 100.
Physical activity at baseline and after 12 weeks measured by the short version of the International Physical Activity Questionnaire (IPAQ-S). The short version contains seven items with open-ended questions about the physical activities performed in the last seven days as well as about sedentary behavior [
37,
38]. The total minutes spent walking (ten minutes at least per week) and moderate and vigorous activities per week, were multiplied by the metabolic equivalent of task (MET) to calculate the total physical activity in MET minutes per week. Sitting behavior was determined separately as hours spent sitting per day [
38]. The IPAQ-S is applied not only to rather healthy populations but also to populations suffering from chronic diseases [
39]. European studies can be found in which patients with diagnosed hypertension, coronary heart disease, and/or heart failure were included [
39,
40,
41]. A difference of 200 MET minutes (SD: 300) is considered clinically relevant [
42].
Self-measured systolic and diastolic blood pressure in mmHg at baseline and after 12 weeks within the intervention group using Omron M400 Intelli IT, a calibrated blood pressure monitor (sphygmomanometer, see e.g., [
43]). In addition to the above-mentioned endpoints, the baseline questionnaire also recorded sex, date of birth, and the highest level of education. Patients were also asked to indicate which of the included ICD-10 diagnoses (chronic ischemic heart disease, I25, and/or heart failure, I50) applied to them.
Data Analyses
Statistical analyses were performed with R statistical software (version 4.2.2.). To test H1, correlation analyses were performed (Spearman). To test H2, analyses were run to test the superiority of the intervention over the control condition. Given the repeated measures structure of the data, MANOVAs were used to examine time and intervention effects, as well as the time*intervention interaction in relation to changes in the dependent variables (self-care maintenance, self-care confidence, physical activity, and sitting), adjusted for sex. Further, univariate ANOVAs and t-tests were calculated within descriptive analyses. To test H3, analyses were run to test changes in the intervention group only over time. To do so, t-tests were used to examine time effects in the dependent variables (systolic blood pressure in mmHg and diastolic blood pressure in mmHg).
For precision of effect sizes, the 95% confidence interval (95% CI) was calculated and a two-sided p-value <0.05 was regarded statistically significant. No significant tests were performed for the baseline indicators because they are accounted for by randomization and by inclusion in the MANOVA.
3. Results
3.1 Study population characteristics
In total, n=37 (92.5%) participants completed this study (
Figure 2). A description of the study population at baseline is given in
Table 1. A total of 25% (control group) and 30% (intervention group) of the study participants were ≥70 years old at baseline. The median age was 61.0 years (IQR: 55.0-69.8) in the control group and 63.0 years (IQR: 56.0-70.8) in the intervention group. The proportion of female study participants was 45% in the control group and 35.0% in the intervention group. In the control group, 45% had another concomitant CVD, while in the intervention group, 70.0% were comorbid (
Table 1).
3.2. Interrelation of health behaviors and disease self-management indicators
To test H1 (i.e., that health behaviors and disease self-management indicators are closely interrelated in users of the reCardial app), we conducted intercorrelation analyses. The results displayed in
Table 2 indicate that cross-sectionally (only at T0), self-care maintenance, and confidence were significantly correlated with physical activity. The same pattern emerged at T1 (self-care maintenance and confidence were significantly correlated with physical activity).
Sitting was significantly negatively correlated with physical activity, but only in the way that the patients with more sitting time at T0 adopted less physical activity at T1 and those sitting more at T1 also adopted less physical activity than the ones with less pronounced sedentary behavior.
The highest correlations between health behaviors and disease self-management indicators emerged between self-care maintenance and physical activity (rt0=0.59), self-care confidence and physical activity (rt1=0.52) as well as sitting and physical activity (rt1=-0.54).
Summarizing regarding H1: The health behaviors and disease self-management indicators self-care maintenance, self-care confidence as well as sitting and physical activity were interrelated in the total sample.
3.3. Change in health behaviors and disease self-management indicators over time
To test H2 (i.e., that the change in health behaviors and disease self-management indicators over time (T0 to T1) is more pronounced in the use of the reCardial app in addition to standard care compared to using standard care alone), we ran MANOVAs. The results show only marginally significant differences (Eta²=0.21;
p=0.06) in the test variables after twelve weeks between the groups (see
Table 3). The effect sizes are in the medium range, which shows clinical importance.
Taken together, the mean SC-HI-A value in the intervention group was
M=73.9 (
SD=16.0) and the mean score in the control group was
M=54.1 (
SD=14.6). This is implying a significant mean difference of 19.8 between the groups (
t(35) = 3.95;
p <0.001). Within the intervention group, the score significantly differed with 57.6 (
SD=17.7) at baseline and
M=73.9 (
SD=16.0) after twelve weeks (clinical difference of
M=18.0 [
SD=24.2;
p <0.01]) (see
Table 5). Also, the clinical difference was higher than described in the literature by Riegel et al. 2009 [
35]. In contrast to the ANOVA,
t-test results for sitting did not show significant results (see
Table B3,
Table C3, and
Table 5).
Regarding the evaluation of the IPAQ-S, no significant differences can be shown, neither in relation to total physical activity nor in relation to the individual components (walking, moderate and vigorous activities,
Table B4 and
Table C4). The mean differences for the total physical activity (MET in minutes per week) within the groups were 604.7 (
SD=2311.8) and 1640.1 (
SD=4842.6) in the control group/intervention group, respectively. Between the groups, the differences were 1517.8 and 1308.1 at the baseline time point and after twelve weeks. Despite no statistical significance, the values were higher than the recommended values from Stenman et al. [
42].
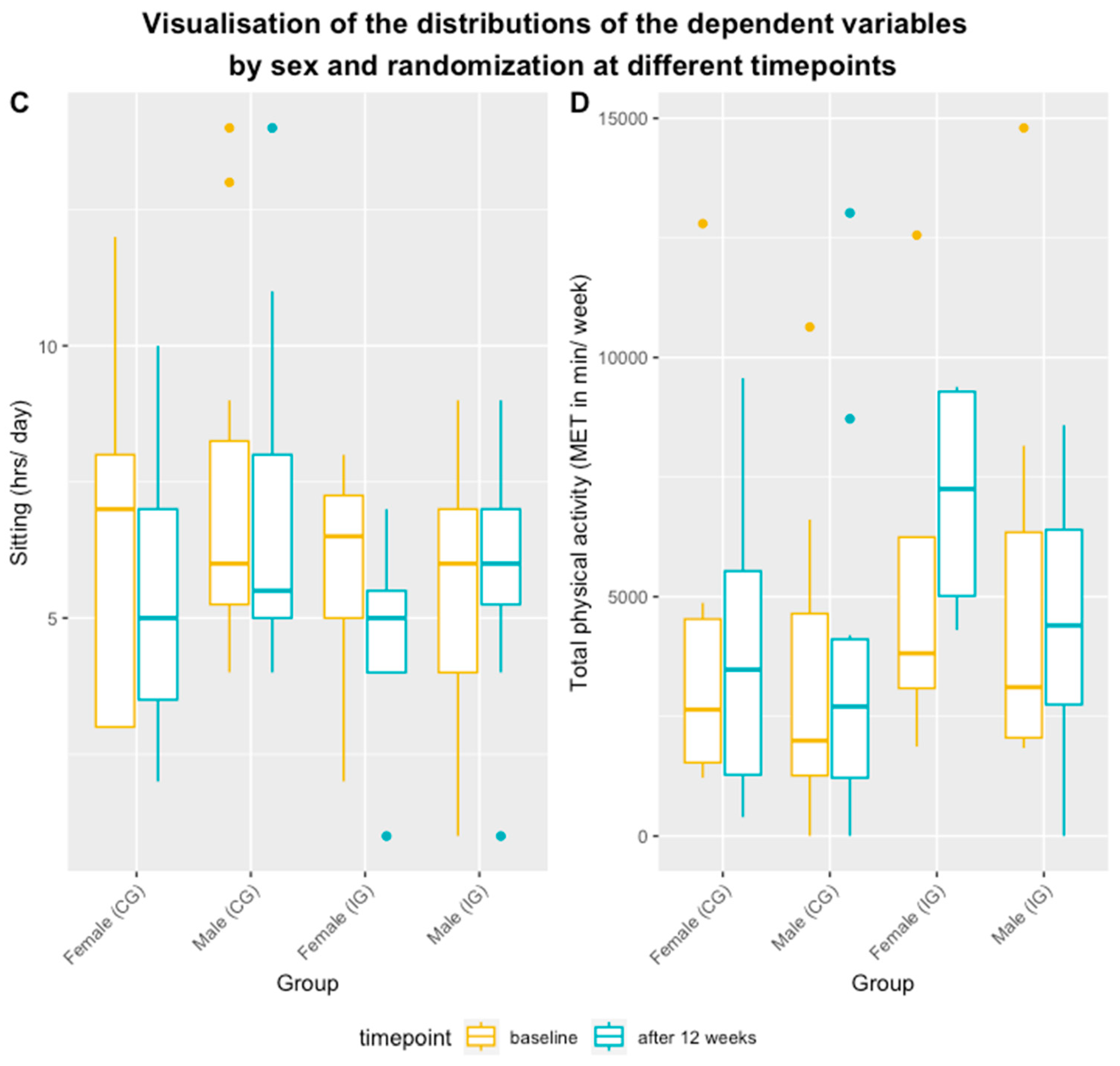
Figure 3 and
Figure 4 display the mean scores, ranges, and standard deviations (
SD) by group and sex over time for sitting and physical activity.
Summarizing, a significant difference between the groups after twelve weeks (
t(35)=2.05;
p <0.05) could be observed with regard to the SC-HI-C: while the mean score in the intervention group was at
M=75.0 (
SD=17.3), the mean score in the control group was
M=60.2 (
SD= 25.5), implying a mean difference of 14.8 points (see
Table 5). This effect size of
d = 0.314 was statistical significant at
Z = -1.9842,
p = 0.04724 validated with a Mann-Whitney-U-Test clearly speaking for the effects of app use.
Table 5.
Mean differences of the health behaviors and disease self-management indicators at baseline (T0) and after 12 weeks (T1).
Table 5.
Mean differences of the health behaviors and disease self-management indicators at baseline (T0) and after 12 weeks (T1).
| |
Control group |
Intervention group |
Mean difference between groups |
| M (SD) |
Difference within groups
M (SD)
|
M (SD) |
Difference within groups
M (SD)
|
| T0 |
T1 |
T0 |
T1 |
T0 |
T1 |
| Self-care Maintenance |
55.9 (12.3);
n=20 |
54.1 (14.6);
n=19 |
3.0 (10.4); t(18)=1.27; p=0.220 |
57.6 (17.7);
n=20 |
73.9 (16.0);
n=18 |
-18.0 (24.2); t(17)=-3.16; p=0.006 |
1.7; t(38)=0.35; p=0.731 |
19.8; t(35)=3.95; p=0.0004 |
| Self-care Confidence |
68.3 (20.9);
n=20 |
60.2 (25.5);
n=19 |
8.8 (29.7); t(18)=1.29; p=0.213 |
68.6 (20.5);
n=20 |
75.0 (17.3);
n=18 |
-6.8 (25.0); t(17)=-1.15; p=0.264 |
0.3; t(38)=0.04; p=0.966 |
14.8; t(35)=2.05; p=0.048 |
| Total physical activity |
3809.0 (3796.9);
n=14 |
3843.3 (3656.7);
n=17 |
604.7 (2311.8); t(12)=0.94; p=0.433 |
5326.9(4538.4); n=11 |
5151.4 (2887.2);
n=13 |
1640.1 (4842.6); t(6)=0.90; p=0.499 |
1517.8 t(23)=0.91;
p=0.372 |
1308.1; t(28)=1.06; p=0.298 |
| Vigorous activity |
1422.9 (1629.0);
n=14 |
2054.1 (2519.9);
n=17 |
-73.9 (1684.0); t(12)=-0.16; p=0.877 |
2160.0 (2562.5); n=11 |
2215.4 (1466.9);
n=13 |
754.3 (3176.2); t(6)= 0.62; p=0.553 |
737.1; t(23)=0.88;
p=0.390 |
161.3; t(28)=0.21;
p=0.840 |
Moderate
activity
|
1182.9 (1211.8);
n=14 |
889.4 (1200.8);
n=17 |
360.0 (832.8); t(12)=1.56; p=0.145 |
1236.4 (1586.9); n=11 |
1473.9 (1169.7);
n=13 |
-468.6 (1776.0); t(6)=0.70; p=0.511 |
53.5; t(23)=0.10;
p=0.925 |
584.4; t(28)=1.34;
p=0.192 |
Walking
activity
|
1203.3 (1448.8);
n=14 |
899.7 (1080.8);
n=17 |
318.6 (737.6); t(12)=1.56; p=0.145 |
1930.5 (1102.6); n=11 |
1462.2 (774.2);
n=13 |
-417.2 (737.5); t(6)=-1.50; p=0.185 |
727.2; t(23)=1.38;
p=0.181 |
562.4; t(28)=1.59; p=0.124 |
| Sitting |
6.9 (3.5);
n=17 |
6.3 (3.2);
n=15 |
0.8 (1.7); t(14)=1.86 p=0.084 |
5.6 (2.3);
n=15 |
5.4 (2.5);
n=14 |
0.2 (1.3); t(11)=0.44; p=0.670 |
1.3; t(30)=1.19; p=0.243 |
0.9; t(27)=0.84;
p=0.408 |
Concluding regarding H2 (i.e, that the change in health behaviors and disease self-management indicators over time from T0 to T1 is more pronounced in the use of the reCardial app in addition to standard care compared to using standard care alone), support was found as expected but not at a statistically meaningful level.
H3 (i.e, that the intervention group reveals improvements in the self-measured systolic blood pressure in mmHg and diastolic blood pressure in mmHg), was tested in the study participants in the intervention group, who monitored their values using the reCardial app during study participation. The monitored values included systolic and diastolic blood pressure, the number of steps per day, and weight checks. Similarly, the knowledge section was accessed by all participants.
Table 5 shows the blood pressure readings of the intervention group for week 1 and week 12. In the intervention group, the mean systolic blood pressure was
M=137 mmHg (
SD=15.8 mmHG) in week 1 and
M=135.3 mmHg (
SD=17.7 mmHg) in week 12. The difference between both measurement periods was not significantly different (
t(5)=0.83;
p=0.444).
Table 5.
Results regarding systolic and diastolic blood pressure in mmHg after 12 weeks, self-assessed by the study participants and recorded in the reCardial app.
Table 5.
Results regarding systolic and diastolic blood pressure in mmHg after 12 weeks, self-assessed by the study participants and recorded in the reCardial app.
| Intervention group |
T0, M (SD) |
T1, M (SD) |
Difference within group M (SD) |
| Systolic blood pressure in mmHg |
137.0 (15.8); n=15 |
135.3 (17.7); n=17 |
2.7 (7.9); t(5)=0.83 (p=0.444) |
| Diastolic blood pressure in mmHg |
80.9 (10.3); n=15 |
81.8 (8.7); n=17 |
-1.3 (7.9); t(5)=-0.42 (p=0.695) |
Concluding on H3, we only found on a descriptive level that the intervention group improved in the objective measure of systolic blood pressure in mmHg as hypothesized but not in the diastolic blood pressure in mmHg. Due to the small sample size, the statistic of Δ 2.7 did not become significant (
Table 5,
d= 0.101).
The same holds true for the diastolic blood pressure in mmHg but in the unfavorite direction (d = 0.094). Both effect sizes are small but only the one for systolic blood pressure in mmHg clearly indicates the effect of intervention even if only of small effect size.
4. Discussion
This study from health psychology aiming at integration of science and practice was theory-based and investigated the interrelations of different behaviors and the effect of using a medical app (reCardial app) in 37 patients. The patients were diagnosed with (primary) hypertension (ICD-10 code: I10) or concomitant diagnosed chronic ischemic heart disease (ICD-10 code: I25), and/or mild to moderate heart failure (ICD-10 code: I50). It was found that health behaviors and disease self-management indicators were closely interrelated. Changes in health behaviors and disease self-management indicators over time (T0 to T1) were more pronounced in users of the reCardial app in addition to standard care compared to using standard care alone.
Scientific evaluations of digital health applications in the area of CVD self-management and adherence to therapy guidelines are scarce and have thus far rarely been reported in the German healthcare context. However, evidence does support the assumption that digital health applications can provide valuable and clinically relevant improvements in patient-relevant health indicators [
14,
15,
16,
17,
18,
19,
20,
21,
26,
28,
46]. In a systematic review [
47] on the effectiveness of mobile applications for self-management of CVD, only two of the eight included studies were randomized controlled trials of a smartphone application. However, the review reported that mobile self-management applications can reduce hospital admissions, cholesterol levels and blood pressure, and can enhance disease-specific knowledge as well as psychological well-being. In addition, Coorey et al. (2018) reported that there is evidence for positive effects on daily physical activity, and smoking cessation [
47]. While the mHealth measure of text messaging alone appeared to be promising in improving medication adherence for secondary prevention of cardiovascular disease, the evidence is highly heterogeneous and of low quality, according to a review [
48]. The worldwide changes through the Covid19 pandemic situation and the implications on healthcare systems led to increased attention on digital health technologies. Different authors have emphasized the useful potential of digital health applications even in older or frail patients in a published consensus statement (e.g., [
46]). Our data accordingly adds to the evidence that digital health applications can help with the secondary and tertiary prevention of CVD. Also, the use of a theory backing the understanding of multiple behavior change and relating to physiological outcomes was demonstrated as calls for demonstrating that health and the promotion of health-protective behaviors and the prevention of health-compromising behaviors were pronounced [
22,
23,
24,
25,
26,
27,
28,
29,
30].
Targeting modifiable risk factors and promoting behavioral changes, and thus preventing recurrent cardiac events, is therefore of high clinical importance and addressing health literacy in a broader sense can help [
5]. Health psychology is a discipline which can make the behavioral effects better observable and with that impacting physiologically determining. Appreciating behavioral effects is accordingly of elevated importance. Furthermore, a recent study found the complexity of medication regimens to be inversely associated with medication adherence and blood pressure management in patients with hypertension [
46,
47,
48,
49]. The advantages of digital and mHealth tools, such as sending regular reminders, having all relevant information accessible anytime, and concisely visualizing the medication plan, could provide the needed guidance to increase adherence to recommended health and disease management behaviors. Investigating more social-cognitive variables of the different behaviors could help to identify further targets for interventions [
49].
The originality of this randomized controlled pilot trial was particularly characterized by its research focus, as it was conducted as part of the fast-track procedure associated with the preliminary inclusion of the reCardial app in the list of digital therapeutics (DTx) under the Digital Health Care Act, which has come into force in 2020. Despite its novelty and relevance accompanied by strong methodology and evidence-based development, which are clear strengths of this study, the authors must acknowledge potential study limitations and discuss the associated possibility of bias. The small sample size and therefore reduced statistical power of the study could have led to an underestimation of clinically relevant effects.
A further limitation is that blinding of patients and personnel conducting the study was not possible. A lack of blinding of the study personnel could possibly lead, for example, to a performance bias: the study support could provide persons in the intervention group with further accompanying measures, bringing about biased results [
47]. Yet, this does not apply in the case of this study, as the behavioral intervention was not carried out by study staff or an investigator but consisted of the use of a smartphone application. A potential selection bias due to lack of blinding during randomization does not apply either, as this was performed automatically by the online questionnaire tool. Finally, an observer bias or detection bias could occur, i.e., the study personnel could unconsciously adjust the observations to the expectations by subjective endpoint collection or evaluation [
50]. A social desirability bias is also conceivable in this study, as well as a recall bias. The latter was mainly due to the application of the IPAQ-S by querying the activities in the last seven days (due to the inclusion criteria) from a relatively older population [
51].
According to a study, a lack of blinding in the evaluation of patient-reported outcomes can lead to an overestimation of the effect; however, such an association is only significant in bias-prone randomization or allocation methods [
52]. The randomization which was applied in this study cannot be influenced either by the study personnel, nor by the patients. Moreover, this potential bias is prevented by the prospective specification of all analyses in the statistical analysis plan. Despite the randomized controlled trial design, it should be emphasized that an adjustment was nevertheless made in the model analyses because baseline differences cannot be excluded.
It should also be pointed out that the majority of measures in this study were subjective. For example, physical activity was measured by a self-reported questionnaire without objective measurement for validation. This addresses the well-known problem of mapping actual movement behavior (gap between self-reported measures and objective measurements per accelerometer) [
53].
Future research directions are to also evaluate effects of the app on multiple behavior change as so far only the effects on the single behaviors were evaluated. In a study with more study participants replicating the findings of this pilot test further analyses should also include the evaluation of mechanisms, e.g., if more behavior change also ensures better physiological outcomes. However, due to the nature of this study and the limited number of participants, such analyses could not be run. Moreover, in the future, closer cooperation with stakeholders with co-creative, participatory health research should ensure the impact of this study for the target group [
54]. Additionally, including healthcare providers of the patients should be included as stakeholders, too, to ensure the fit with general services and effectiveness of healthcare provisions.
Extending the view to the digital media field for behavior change is important for healthcare providers and app developers [
55]. While evidence for the effectiveness of digital behavioral interventions in changing behavior is limited this study set out an example of rigorous research even with a small sample size. We face the need for more rigorous intervention studies and future studies should exploring the full functionality of digital devices to maximize opportunities for behavior change. The long-term effects of social media interventions should built on theories of multiple behavior change in this context as the current study demonstrated. The importance of formative research to evaluate the feasibility and acceptability of projects is important, along with the need for a more rigorous application of program evaluation principles [
55]. Also other authors have identified health literacy as a crucial dimension, and making digital media interventions more sensitive to health literacy needs [
55] can be done like in this study outlining and evaluating the app. Apps can not only help patients: The harbor the potential to help also practitioners to work from home in helping their patients to manage adherence [
56]. This should be also taken into account with future policy building and refinements: Allowing healthcare providers to prescribe such interventions, supporting patients in a dynamic, adapting manner, and appreciating the behavioral effects might high importance.
5. Conclusions
This study provides implications for strengthening the evidence-based improvement of healthcare regarding secondary and tertiary prevention of CVD. At the same time, the behavioral intervention can support patients with hypertension, chronic ischemic heart disease, and heart failure in the form of a digital health application. Besides the call for replication with a lager study sample and objective measures in the control group, there are some practical implications for theory and practice.
Theories integrating different behaviors stemming for instance from health psychology should further investigate various behaviors and their interrelations. This can make behavioral interventions more effective. It is also of high importance for daily practice considering the lack of resources in the healthcare system. Providing behavioral changes with digital health technology can close the gap between regular healthcare professional contact and sufficient disease self-management for patients. There is an unmet need for encouraging patients to regularly use digital applications, and for health professionals in healthcare in prescribing them, too.
Author Contributions
Conceptualization, S.L., T.R. and A.F.; Methodology, T.R. and A.F.; Software, J.H. and C.O.; Validation, S.L. and V.A.K.; Formal Analysis, L.K.; Investigation, T.R. and L.K.; Resources, J.H. and C.O.; Data Curation, V.A.K.; Writing—Original Draft Preparation, S.L.; Writing—Review and Editing, T.R., L.K. and V.A.K.; Visualization, L.K. and S.L.; Supervision, J.H. and T.R.; Project Administration, L.K. and C.O.; Funding Acquisition, J.H. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the application manufacturer apprevent GmbH and partially funded by the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement 956501.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Ethikkommission der Ärztekammer Bremen (protocol code with the ethical approval code 762 October, 15, 2021). The randomized controlled trial has been prospectively registered in the German Clinical Trials Registry (identification number: DRKS00026136). The study had the title „reCardial - Smartphone-Applikation zur Stärkung der Therapieleitlinien-Adhärenz bei Patienten mit Hypertonie, chronischen ischämischen Herzkrankheiten oder Herzinsuffizienz“[reCardial - smartphone application to strengthen therapy guideline adherence in patients with hypertension, chronic ischemic heart disease or heart failure].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data is of a sensitive nature as it includes personal health information. Thus, participants were assured that their raw data remains confidential and is not to be shared. The final pseudonymized trial dataset is to be only available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge the support with proofreading and formatting this manuscript by Melody Marie Pogoy Lawas. The authors are grateful to Prof. Dr. Viviane Scherenberg and Prof. Dr. Harm Wienbergen for providing comments and suggestions on earlier drafts of the study protocol. Further, we thank the whole apprevent GmbH team for the collaboration.
Conflicts of Interest
The reCardial study is initiated and funded by the app manufacturer apprevent GmbH, which is owned by Jan Oltmans and Jan Homoth. The funder was involved in participant management, and interpretation of data; in the writing of the manuscript and in the decision to publish the results. The apprevent GmbH has no involvement in study design. Tiara Ratz was a former employee of the "reCardial" project at apprevent GmbH. Andreas Fach is associated with the apprevent GmbH. Luisa Korte works as a research associate within apprevent GmbH. Sonia Lippke and Vinayak Anand Kumar declare that they do not have any conflict of interest.
Appendix A
Table A1.
Information on medical contraindications of the digital health application.
Table A1.
Information on medical contraindications of the digital health application.
| ICD-10 Code |
Description of ICD-10 Code |
| A41.0 |
Sepsis due to Staphylococcus aureus |
| E05 |
Thyrotoxicosis [hyperthyroidism] |
| I24.9 |
Acute ischemic heart disease, unspecified |
| I26.0 |
Pulmonary embolism with acute cor pulmonale |
| I33 |
Acute and subacute endocarditis |
| I40 |
Acute myocarditis |
| I50.05 |
Congestive heart failure with symptoms at rest |
| I50.14 |
Left ventricular failure: With symptoms at rest |
| I71 |
Aortic aneurysm and dissection |
| I80 |
Phlebitis and thrombophlebitis |
| Z73 |
Problems related to life-management difficulty |
Appendix B
Table B1.
Maintenance Self-Care of Hypertension Inventory (SC-HI) at baseline (T0) and after 12 weeks (T1) by sex and treatment (see
Table C1 for the results of the univariate ANOVAs with Maintenance Self-Care of Hypertension Inventory, SC-HI).
Table B1.
Maintenance Self-Care of Hypertension Inventory (SC-HI) at baseline (T0) and after 12 weeks (T1) by sex and treatment (see
Table C1 for the results of the univariate ANOVAs with Maintenance Self-Care of Hypertension Inventory, SC-HI).
| |
|
Min |
Max |
M (SD) |
| |
|
T0 |
T1 |
T0 |
T1 |
T0 |
T1 |
| Control group |
Female |
39.4 |
24.2 |
81.8 |
75.8 |
55.9 (14.7) |
54.5 (17.9) |
| Male |
33.3 |
36.4 |
69.7 |
72.7 |
55.9 (10.6) |
53.6 (12.0) |
| Intervention group |
Female |
15.2 |
51.5 |
78.8 |
97.0 |
49.4 (21.2) |
71.7 (18.2) |
| Male |
27.3 |
51.5 |
84.8 |
100.0 |
62.0 (14.6) |
75.0 (15.5) |
Table B2.
Confidence Self-Care of Hypertension Inventory (SC-HI) at baseline (T0) and after 12 weeks (T1) by sex and treatment (see
Table C2 for the results of the univariate ANOVAs with Confidence Self-Care of Hypertension Inventory, SC-HI).
Table B2.
Confidence Self-Care of Hypertension Inventory (SC-HI) at baseline (T0) and after 12 weeks (T1) by sex and treatment (see
Table C2 for the results of the univariate ANOVAs with Confidence Self-Care of Hypertension Inventory, SC-HI).
| |
|
Min |
Max |
M (SD) |
| |
|
T0 |
T1 |
T0 |
T1 |
T0 |
T1 |
| Control group |
Female |
38.9 |
0.0 |
100.0 |
88.9 |
67.9 (18.0) |
56.2 (27.7) |
| Male |
27.8 |
27.8 |
100.0 |
94.4 |
68.7 (23.9) |
63.9 (24.2) |
| Intervention group |
Female |
27.8 |
50.0 |
94.4 |
100.0 |
66.7 (27.4) |
78.7 (18.1) |
| Male |
33.3 |
44.4 |
88.9 |
100.0 |
69.7 (16.9) |
73.1 (17.4) |
Table B3.
Sitting at baseline (t0) and after 12 weeks (t1) by sex and treatment (see
Table C3 for the results of the univariate ANOVAs: Sitting, hrs/ day).
Table B3.
Sitting at baseline (t0) and after 12 weeks (t1) by sex and treatment (see
Table C3 for the results of the univariate ANOVAs: Sitting, hrs/ day).
| |
|
Min |
Max |
M (SD) |
| |
|
T0 |
T1 |
T0 |
T1 |
T0 |
T1 |
| Control group |
Female |
3.0 |
2.0 |
12.0 |
10.0 |
6.3 (3.5) |
5.4 (2.8) |
| Male |
4.0 |
4.0 |
14.0 |
14.0 |
7.4 (3.5) |
7.1 (3.5) |
| Intervention group |
Female |
2.0 |
1.0 |
8.0 |
7.0 |
5.8 (2.6) |
4.5 (2.5) |
| Male |
1.0 |
1.0 |
9.0 |
9.0 |
5.6 (2.4) |
6.0 (2.4) |
Table B4.
PA at baseline (T0) and after 12 weeks (T1) by sex and treatment.
Table B4.
PA at baseline (T0) and after 12 weeks (T1) by sex and treatment.
| |
|
Min |
Max |
M (SD) |
| |
|
T0 |
T1 |
T0 |
T1 |
T0 |
T1 |
| Control group |
Female |
1215.0 |
396.0 |
12798.0 |
9570.0 |
4269.8 (4408.5) |
3866.9 (3274.0) |
| Male |
0.0 |
0.0 |
10638.0 |
13022.0 |
3463.5 (3545.7) |
3826.9 (4077.4) |
| Intervention group |
Female |
1866.0 |
4302.0 |
12558.0 |
9386.0 |
5514.0 (4792.4) |
7048.3 (2651.2) |
| Male |
1836.0 |
0.0 |
14799.0 |
8586.0 |
5220.0 (4775.7) |
4308.4 (2696.2) |
Appendix C
Table C1.
Results of the univariate ANOVAs: Maintenance Self-Care of Hypertension Inventory (SC-HI).
Table C1.
Results of the univariate ANOVAs: Maintenance Self-Care of Hypertension Inventory (SC-HI).
| |
df |
Sum Sq |
Mean Sq |
F value |
p (>F) |
| (Intercept) |
1 |
186493 |
186493 |
923.0909 |
< 0.001 |
| Sex |
1 |
11 |
11 |
0.0548 |
0.816136 |
| Treatment |
1 |
1634 |
1634 |
8.0862 ** |
0.007066 |
| Time |
1 |
124 |
124 |
0.6114 |
0.438972 |
| Sex*Treatment |
1 |
213 |
213 |
1.0523 |
0.311302 |
| Sex*Time |
1 |
39 |
39 |
0.1908 |
0.664662 |
| Treatment*Time |
1 |
679 |
679 |
3.3633 |
0.074305 |
| Sex*Treatment*Time |
1 |
293 |
293 |
1.4506 |
0.235692 |
| Residuals |
39 |
7879 |
202 |
|
|
Table C2.
Results of the univariate ANOVAs: Confidence Self-Care of Hypertension Inventory (SC-HI).
Table C2.
Results of the univariate ANOVAs: Confidence Self-Care of Hypertension Inventory (SC-HI).
| |
df |
Sum Sq |
Mean Sq |
F value |
p (>F) |
| (Intercept) |
1 |
234048 |
234048 |
574.3697 |
< 0.001 |
| Sex |
1 |
288 |
288 |
0.7070 |
0.4056 |
| Treatment |
1 |
589 |
589 |
1.4449 |
0.2366 |
| Time |
1 |
189 |
189 |
0.4640 |
0.4998 |
| Sex*Treatment |
1 |
25 |
25 |
0.0612 |
0.8060 |
| Sex*Time |
1 |
84 |
84 |
0.2069 |
0.6517 |
| Treatment*Time |
1 |
3 |
3 |
0.0067 |
0.9350 |
| Sex*Treatment*Time |
1 |
147 |
147 |
0.3609 |
0.5515 |
| Residuals |
39 |
15892 |
407 |
|
|
Table C3.
Results of the univariate ANOVAs: Sitting (hrs/ day).
Table C3.
Results of the univariate ANOVAs: Sitting (hrs/ day).
| |
df |
Sum Sq |
Mean Sq |
F value |
p (>F) |
| (Intercept) |
1 |
1979.26 |
1979.26 |
231.2236 |
< 0.001 |
| Sex |
1 |
1.72 |
1.72 |
0.2008 |
0.65652 |
| Treatment |
1 |
38.42 |
38.42 |
4.4883 * |
0.04055 |
| Time |
1 |
16.04 |
16.04 |
1.8744 |
0.17881 |
| Sex*Treatment |
1 |
3.74 |
3.74 |
0.4364 |
0.51276 |
| Sex*Time |
1 |
5.79 |
5.79 |
0.6768 |
0.41569 |
| Treatment*Time |
1 |
0.52 |
0.52 |
0.0604 |
0.80708 |
| Sex*Treatment*Time |
1 |
3.68 |
3.68 |
0.4298 |
0.51596 |
| Residuals |
39 |
333.84 |
8.56 |
|
|
Table C4.
Results of the univariate ANOVAs: Total physical activity (MET in min/ week).
Table C4.
Results of the univariate ANOVAs: Total physical activity (MET in min/ week).
| |
df |
Sum Sq |
Mean Sq |
F value |
p (>F) |
| (Intercept) |
1 |
955605898 |
955605898 |
68.7968 |
< 0.001 |
| Sex |
1 |
5857107 |
5857107 |
0.4217 |
0.5199 |
| Treatment |
1 |
6440704 |
6440704 |
0.4637 |
0.4999 |
| Time |
1 |
3655057 |
3655057 |
0.2631 |
0.6109 |
| Sex*Treatment |
1 |
135551 |
135551 |
0.0098 |
0.9218 |
| Sex*Time |
1 |
2154192 |
2154192 |
0.1551 |
0.6959 |
| Treatment*Time |
1 |
472288 |
472288 |
0.0340 |
0.8547 |
| Sex*Treatment*Time |
1 |
36083404 |
36083404 |
2.5977 |
0.1151 |
| Residuals |
39 |
541720486 |
13890269 |
|
|
References
- Townsend, N. , Kazakiewicz, D., Lucy Wright, F. et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Zhang, Y. , Liu, J., Zhang, Y., Ke, L., & Liu, R. Interactive Compensation Effects of Physical Activity and Sleep on Mental Health: A Longitudinal Panel Study among Chinese College Students during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health 2022, 19, 12323. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T. Costs of treating cardiovascular events in Germany: a systematic literature review. Health Economics Review 2015, 5, 27. [Google Scholar] [CrossRef]
- Abell, B. , Glasziou, P., & Hoffmann, T. The contribution of individual exercise training components to clinical outcomes in randomised controlled trials of cardiac rehabilitation: a systematic review and meta-regression. Sports medicine-open 2017, 3, 1–31. [Google Scholar] [CrossRef]
- Liu C, Wang D, Liu C, Jiang J, Wang X, Chen H, Ju X, Zhang X. What is the meaning of health literacy? A systematic review and qualitative synthesis. Fam Med Com Health 2020, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. B., Pan, X. F., Lu, Q., Wang, Y. X., Geng, T. T., Zhou, Y. F., ... & Pan, A. Association of Combined Healthy Lifestyles With Cardiovascular Disease and Mortality of Patients With Diabetes: An International Multicohort Study. In Mayo Clinic Proceedings 2023, 98, 60–74). Elsevier. [CrossRef]
- Duan Y, Liang W, Shang B, Li X, Guo L, Lippke S (2022) A WeChat mini program-based intervention for physical activity, fruit and vegetable consumption among Chinese cardiovascular patients in home-based rehabilitation: a study protocol. Frontiers in Public Health | www.frontiersin.org. [CrossRef]
- Shahaj O, Denneny D, Schwappach A, Pearce G, Epiphaniou E, Parke HL, Taylor SJC, Pinnock H. Supporting self-management for people with hypertension: a meta-review of quantitative and qualitative systematic reviews. J Hypertens 2019, 37, 264–279. [Google Scholar] [CrossRef]
- Du L, Cheng Z, Zhang Y, Li Y, Mei D. The impact of medication adherence on clinical outcomes of coronary artery disease: a meta-analysis. European Journal of Preventive Cardiology 2017, 24, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Salzwedel A, Jensen K, Rauch B, Doherty P, Metzendorf M-I, Hackbusch M, Völler H, Schmid J-P, Davos CH. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the Cardiac Rehabilitation Outcome Study (CROS-II). European Journal of Preventive Cardiology 2020, 27, 1756–1774. [Google Scholar] [CrossRef]
- Kotseva K, Wood D, de Bacquer D, et al. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. European Journal of Preventive Cardiology 2016, 23, 636–648. [Google Scholar] [CrossRef]
- Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. American Journal of Medicine 2012, 125, 882–887. [Google Scholar] [CrossRef]
- Yang, M. , Duan, Y. , Liang, W., Peiris, D. L. I. H. K., & Baker, J. S. Effects of Face-to-Face and eHealth Blended Interventions on Physical Activity, Diet, and Weight-Related Outcomes among Adults: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health 2023, 20, 1560. [Google Scholar] [CrossRef] [PubMed]
- 14. Hamilton SJ, Mills B, Birch EM, Thompson SC. Smartphones in the secondary prevention of cardiovascular disease: a systematic review. BMC Cardiovascular Disorders. [CrossRef]
- Xiong S, Berkhouse H, Schooler M, Pu W, Sun A, Gong E, Yan LL. Effectiveness of mHealth interventions in improving medication adherence among people with hypertension: a systematic review. Current Hypertension Reports 2018, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Gong K, Yan Y-L, Li Y, Du J, Wang J, Han Y, Zou Y, Zou X, Huang H, She Q. Mobile health applications for the management of primary hypertension. Medicine 2020, 99, e19715. [Google Scholar] [CrossRef] [PubMed]
- Santo K, Singleton A, Rogers K, Thiagalingam A, Chalmers J, Chow CK, Redfern J. Medication reminder applications to improve adherence in coronary heart disease: a randomised clinical trial. Heart 2019, 105, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Márquez Contreras E, Márquez Rivero S, Rodríguez García E, López-García-Ramos L, Carlos Pastoriza Vilas J, Baldonedo Suárez A, Gracia Diez C, Gil Guillén V, Martell Claros N. Specific hypertension smartphone application to improve medication adherence in hypertension: a cluster-randomized trial. Current Medical Research and Opinion 2019, 35, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Persell SD, Peprah YA, Lipiszko D, Lee JY, Li JJ, Ciolino JD, Karmali KN, Sato H. Effect of home blood pressure monitoring via a smartphone hypertension coaching application or tracking application on adults with uncontrolled hypertension. JAMA Network Open 2020, 3, e200255. [Google Scholar] [CrossRef] [PubMed]
- Chandler J, Sox L, Kellam K, Feder L, Nemeth L, Treiber F. Impact of a culturally tailored mHealth medication regimen self-management program upon blood pressure among hypertensive Hispanic adults. International Journal of Environmental Research and Public Health 2019, 16, 1226. [Google Scholar] [CrossRef] [PubMed]
- Karhula T, Vuorinen A-L, Rääpysjärvi K, et al. Telemonitoring and mobile phone-based health coaching among Finnish diabetic and heart disease patients: randomized controlled trial. Journal of Medical Internet Research 2015, 17, e153. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (HAPA). Rehabilitation Psychology 2011, 56, 161–170. [Google Scholar] [CrossRef]
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Lippke, S. , Dahmen, A., Gao, L., Guza, E., & Nigg, C. R. To what extent is internet activity predictive of psychological well-being? Psychology research and behavior management 2021, 2021, 207–219. [Google Scholar]
- Connell LE, Carey RN, de Bruin M, Rothman AJ, Johnston M, Kelly MP, Michie S. Links between behavior change techniques and mechanisms of action: an expert consensus study. Annals of Behavioral Medicine 2018, 53, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Storm V, Dörenkämper J, Reinwand DA, Wienert J, de Vries H, Lippke S (2016) Effectiveness of a web-based computer-tailored multiple-lifestyle intervention for people interested in reducing their cardiovascular risk: a randomized controlled trial. Journal of Medical Internet Research. [CrossRef]
- Fleig L, Lippke S, Pomp S, Schwarzer R. Intervention effects of exercise self-regulation on physical exercise and eating fruits and vegetables: a longitudinal study in orthopedic and cardiac rehabilitation. Preventive Medicine 2011, 53, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Duan YP, Liang W, Guo L, Wienert J, Si GY, Lippke S. Evaluation of a web-based intervention for multiple health behavior changes in patients with coronary heart disease in home-based rehabilitation: pilot randomized controlled trial. J Med Internet Res 2018, 20, e12052. [Google Scholar] [CrossRef] [PubMed]
- Lippke S, Fleig L, Wiedemann AU, Schwarzer R. A computerized lifestyle application to promote multiple health behaviors at the workplace: testing its behavioral and psychological effects. Journal of Medical Internet Research 2015, 17, e225. [Google Scholar] [CrossRef] [PubMed]
- Fleig L, Pomp S, Schwarzer R, Lippke S. Promoting exercise maintenance: how interventions with booster sessions improve long-term rehabilitation outcomes. Rehabilitation Psychology 2013, 58, 323–333. [Google Scholar] [CrossRef]
- Ganeshan, S. , Jackson, H., Grandis, D. J., Janke, D., Murray, M. L., Valle, V., & Beatty, A. L. Clinical outcomes and qualitative perceptions of in-person, hybrid, and virtual cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 2022, 42, 338–346. [Google Scholar] [CrossRef]
- Sangeethalakshmi, K. , Preethi, U., & Pavithra, S. Patient health monitoring system using IoT. Materials Today: Proceedings 2023, 80, 2228–2231. [Google Scholar] [CrossRef]
- Dickson V, v. , Lee C, Yehle KS, Abel WM, Riegel B. Psychometric testing of the Self-care of Hypertension Inventory: conceptual challenges to collaborative care. Journal of Cardiovascular Nursing 2017, 32, 431–438. [Google Scholar] [CrossRef]
- Świątoniowska-Lonc N, Polański J, Jankowska-Polańska B. Psychometric properties of the Polish version of the Self-care of Hypertension Inventory. Journal of Cardiovascular Nursing 2021, 36, 437–445. [Google Scholar] [CrossRef]
- Riegel B, Lee CS, Dickson V v. , Carlson B. An update on the self-care of heart failure index. Journal of Cardiovascular Nursing 2009, 24, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life. Medical Care 2003, 41, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Patterson E (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms (05). 20 November.
- Kambic T, Šarabon N, Hadžić V, Lainscak M. Objectively measured physical activity in patients with coronary artery disease: a cross-validation study. Biosensors (Basel) 2021, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Arija V, Villalobos F, Pedret R, Vinuesa A, Jovani D, Pascual G, Basora J. Physical activity, cardiovascular health, quality of life and blood pressure control in hypertensive subjects: randomized clinical trial. Health and Quality of Life Outcomes 2018, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Saran T, Owoc J, Bojar I. Use of the IPAQ questionnaire in the form of a mobile application in monitoring physical activity of patients with cardiovascular diseases. Annals of Agricultural and Environmental Medicine 2018, 25, 395–402. [Google Scholar] [CrossRef]
- Stenman E, Leijon ME, Calling S, Bergmark C, Arvidsson D, Gerdtham U-G, Sundquist K, Ekesbo R. Study protocol: a multi-professional team intervention of physical activity referrals in primary care patients with cardiovascular risk factors-the Dalby lifestyle intervention cohort (DALICO) study. BMC Health Services Research 2012, 12, 173. [Google Scholar] [CrossRef]
- Duschek, S. , Hoffmann, A., Bair, A., Del Paso, G. A. R., & Montoro, C. I. Cerebral blood flow modulations during proactive control in chronic hypotension. Brain and Cognition 2018, 125, 135–141. [Google Scholar] [CrossRef]
- Dickson V, v. , Lee CS, Yehle KS, Mola A, Faulkner KM, Riegel B. Psychometric testing of the Self-Care of Coronary Heart Disease Inventory (SC-CHDI). Research in Nursing and Health 2017, 40, 15–22. [Google Scholar] [CrossRef]
- Audette LM, Hammond MS, Rochester NK. Methodological issues with coding participants in anonymous psychological longitudinal studies. Educational and Psychological Measurement 2020, 80, 163–185. [Google Scholar] [CrossRef]
- Guasti, L. , Dilaveris, P., Mamas, M. A., Richter, D., Christodorescu, R., Lumens, J.,... & Cowie, M. R. Digital health in older adults for the prevention and management of cardiovascular diseases and frailty. A clinical consensus statement from the ESC Council for Cardiology Practice/Taskforce on Geriatric Cardiology, the ESC Digital Health Committee and the ESC Working Group on e-Cardiology. ESC Heart Failure, 2822. [Google Scholar] [CrossRef]
- Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. European Journal of Preventive Cardiology 2018, 25, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Adler AJ, Martin N, Mariani J, Tajer CD, Owolabi OO, Free C, Serrano NC, Casas JP, Perel P (2017) Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews Art. No. 0118.
- Duan, Y. , Lippke, S., Liang, W., Shang, B., Keller, F. M., Wagner, P.,... & He, J. Association of social-cognitive factors with individual preventive behaviors of Covid-19 among a mixed-sample of older adults from China and Germany. International Journal of Environmental Research and Public Health 2022, 19, 6364. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, Thomas J, Chandler J et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. Chichester (UK): John Wiley & Sons, 2019.
- Cleland C, Ferguson S, Ellis G, Hunter RF (2018) Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Medical Research Methodology. [CrossRef]
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ 2008, 336, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M (2008) A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity. [CrossRef]
- Verloigne, M. , Altenburg, T., Cardon, G., Chinapaw, M., Dall, P., Deforche, B.,... & Chastin, S. Making co-creation a trustworthy methodology for closing the implementation gap between knowledge and action in health promotion: the Health CASCADE project. Perspectives in Public Health 2022, 211, 107193. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.D.; Abroms, L.C.; Broniatowski, D.; Napolitano, M.A.; Arnold, J.; Ichimiya, M.; Agha, S. Digital Media for Behavior Change: Review of an Emerging Field of Study. Int. J. Environ. Res. Public Health 2022, 19, 9129. [Google Scholar] [CrossRef]
- Neidlinger, S.M.; Felfe, J.; Schübbe, K. Should I Stay or Should I Go (to the Office)?—Effects of Working from Home, Autonomy, and Core Self–Evaluations on Leader Health and Work–Life Balance. Int. J. Environ. Res. Public Health 2023, 20, 6. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).