Submitted:
11 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics statement
2.2. Study population
2.3. Study parameters
2.4. Statistical analysis
3. Results
3.1. Patient characteristics, clinical presentation, and outcome
3.2. Injury severity and injury pattern
3.3. Predicting factors of shock episode
3.4. Overall morbidities
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eastridge, B.J.; Starr, A.; Minei, J.P.; O’Keefe, G.E.; Scalea, T.M. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions. J Trauma. 2002, 53, 446–450; discussion 450–451. [CrossRef]
- Ciriano Hernández, P.; Moreno Hidalgo, A.; Grao Torrente, I.; Ruiz Moreno, C.; Seisdedos Rodrigez, L.; Kayser Mata, S.; Echenagusia Boyra, M.J.; González Leyte, M.; Pérez Díaz, M.D.; Turégano Fuentes, F. Pelvic fractures with associated retroperitoneal hematoma: time until angioembolization and results. Cir Esp (Engl). 2019, 97, 261–267. Fracturas de pelvis con hematoma retroperitoneal asociado: tiempo hasta la angioembolización y resultados. [CrossRef]
- Matsushima, K.; Piccinini, A.; Schellenberg, M.; Cheng, V.; Heindel, P.; Strumwasser, A.; Benjamin, E.; Inaba, K.; Demetriades, D. Effect of door-to-angioembolization time on mortality in pelvic fracture: every hour of delay counts. J Trauma Acute Care Surg. 2018, 84, 685–692. [Google Scholar] [CrossRef]
- Gustavo Parreira, J.; Coimbra, R.; Rasslan, S.; Oliveira, A.; Fregoneze, M.; Mercadante, M. The role of associated injuries on outcome of blunt trauma patients sustaining pelvic fractures. Injury. 2000, 31, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, D.; Karaiskakis, M.; Velmahos, G.C.; Alo, K.; Murray, J.; Chan, L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003, 54, 1146–1151; discussion 1151. [CrossRef]
- Charbit, J.; Millet, I.; Martinez, O.; Roustan, J.P.; Merigeaud, S.; Taourel, P.; Capdevila, X. Does the size of the hemoperitoneum help to discriminate the bleeding source and guide therapeutic decisions in blunt trauma patients with pelvic ring fracture? J Trauma Acute Care Surg. 2012, 73, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Liao, C.A.; Liao, C.H.; Kang, S.C.; Wang, S.Y.; Hsu, Y.P.; Lin, B.C.; Yuan, K.C.; Kuo, I.M.; Ouyang, C.H. Intra-abdominal injury is easily overlooked in the patients with concomitant unstable hemodynamics and pelvic fractures. Am J Emerg Med. 2014, 32, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Chan, S.Y.; Wang, S.Y.; Hsieh, C.H.; Liao, C.H.; Huang, J.F.; Hsu, Y.P.; Kang, S.C. The effect of angioembolization for life-threatening retroperitoneal hemorrhage in patients with pelvic fracture. Am J Emerg Med. 2019, 37, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, R.B.; Bruns, B.R.; Narayan, M.; Dubose, J.; Guliani, S.S.; Brenner, M.L.; Boswell, S.; Stein, D.M.; Scalea, T.M. Angiographic embolization for hemorrhage following pelvic fracture: is it “time” for a paradigm shift? J Trauma Acute Care Surg. 2017, 82, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Inoue, F.; Takakura, Y.; Hoshida, T. Statistical analysis of fatal bleeding pelvic fracture patients with severe associated injuries. J Orthop Sci. 2008, 13, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Thorson, C.M.; Ryan, M.L.; Otero, C.A.; Vu, T.; Borja, M.J.; Jose, J.; Schulman, C.I.; Livingstone, A.S.; Proctor, K.G. Operating room or angiography suite for hemodynamically unstable pelvic fractures? J Trauma Acute Care Surg. 2012, 72, 364–370; discussion 371–372. [CrossRef]
- Hsieh, T.M.; Cheng Tsai, T.; Liang, J.L.; Che Lin, C. Non-operative management attempted for selective high grade blunt hepatosplenic trauma is a feasible strategy. World J Emerg Surg. 2014, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, S.M.; Brownstein, M.; Watts, D.D.; Baker, C.C.; Oller, D. Relatively short diagnostic delays (<8 hours) produce morbidity and mortality in blunt small bowel injury: an analysis of time to operative intervention in 198 patients from a multicenter experience. J Trauma. 2000, 48, 408–414; discussion 414–415. [CrossRef]
- Malinoski, D.J.; Patel, M.S.; Yakar, D.O.; Green, D.; Qureshi, F.; Inaba, K.; Brown, C.V.; Salim, A. A diagnostic delay of 5 hours increases the risk of death after blunt hollow viscus injury. J Trauma. 2010, 69, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassani, A.; Tuma, M.; Mahmood, I.; Afifi, I.; Almadani, A.; El-Menyar, A.; Zarour, A.; Mollazehi, M.; Latifi, R.; Al-Thani, H. Dilemma of blunt bowel injury: what are the factors affecting early diagnosis and outcomes. Am Surg. 2013, 79, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsieh, F.J.; Chen, C.C.; Cheng, C.T.; Ooyang, C.H.; Hsieh, C.H.; Yang, S.J.; Fu, C.Y. The prognosis of blunt bowel and mesenteric injury-the pitfall in the contemporary image survey. J Clin Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, S.M.; Allawi, A.; Ferguson, P.L.; Michetti, C.P.; Newcomb, A.B.; Liu, C.; Brownstein, M.R. ; EAST small bowel perforation (SBP) Multi-Center Study Group. Blunt small bowel perforation (SBP): an Eastern Association for the Surgery of Trauma multicenter update 15 years later. J Trauma Acute Care Surg. 2019, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.M.; Granieri, S.; Gupta, S.; Altomare, M.; Cioffi, S.P.B.; Sammartano, F.; Cimbanassi, S.; Chiara, O. Traumatic hollow viscus and mesenteric injury: role of CT and potential diagnostic-therapeutic algorithm. Update Surg. 2021, 73, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, S.J.; Fu, C.Y.; Liao, C.H.; Kang, S.C.; Hsu, Y.P.; Lin, B.C.; Yuan, K.C.; Wang, S.Y. The risk factors of concomitant intraperitoneal and retroperitoneal hemorrhage in the patients with blunt abdominal trauma. World J Emerg Surg. 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Yamazaki, S.; Fukuma, S.; Matsushima, K.; Yamashiro, T.; Fukuhara, S. Comparison between laparotomy first versus angiographic embolization first in patients with pelvic fracture and hemoperitoneum: a nationwide observational study from the Japan Trauma Data Bank. Scand J Trauma Resusc Emerg Med. 2013, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.M.; Chuang, P.C.; Liu, C.T.; Wu, B.Y.; Liu, Y.W.; Hsieh, C.H. Protective role of obesity on trauma impact: A retrospective analysis of patients with surgical blunt bowel mesenteric injury due to road traffic accidents. Risk Manag Healthc Policy. 2022, 15, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Aragón, L.E.; Guevara-Torres, L.; Vaca-Pérez, E.; Belmares-Taboada, J.A.; Ortiz-Castillo, G.; Sánchez-Aguilar, M. Primary closure in colon trauma. Cir Cir. 2009, 77, 359–364. [Google Scholar] [PubMed]
- Faria, G.R.; Almeida, A.B.; Moreira, H.; Barbosa, E.; Correia-da-Silva, P.; Costa-Maia, J. Prognostic factors for traumatic bowel injuries: killing time. World J Surg. 2012, 36, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Okishio, Y.; Ueda, K.; Nasu, T.; Kawashima, S.; Kunitatsu, K.; Kato, S. Surgical intervention for blunt bowel and mesenteric injury: indications and time intervals. Eur J Trauma Emerg Surg. 2021, 47, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Kim, S.H.; Kim, K.H. Blunt isolated small bowel perforation intervention: does a delay in management matter? Emerg Med Int. 2020, 2020, 7478485. [Google Scholar] [CrossRef]
- Loftus, T.J.; Morrow, M.L.; Lottenberg, L.; Rosenthal, M.D.; Croft, C.A.; Smith, R.S.; Moore, F.A.; Brakenridge, S.C.; Borrego, R.; Efron, P.A.; et al. The impact of prior laparotomy and intra-abdominal adhesions on bowel and mesenteric injury following blunt abdominal trauma. World J Surg. 2019, 43, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Juern, J.S.; Milia, D.; Codner, P.; Beckman, M.; Somberg, L.; Webb, T.; Weigelt, J.A. Clinical significance of computed tomography contrast extravasation in blunt trauma patients with a pelvic fracture. J Trauma Acute Care Surg. 2017, 82, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, D.; Zegg, M.; Jeske, C.; El Attal, R.; Blauth, M.; Rieger, M. Hemorrhage after low-energy pelvic trauma. J Trauma Acute Care Surg. 2012, 72, 437–442. [Google Scholar] [CrossRef] [PubMed]
- ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS working group. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg: ninth edition. 2013, 74, 1363–1366. [CrossRef]
- Grimm, M.R.; Vrahas, M.S.; Thomas, K.A. Pressure-volume characteristics of the intact and disrupted pelvic retroperitoneum. J Trauma. 1998, 44, 454–459. [Google Scholar] [CrossRef]
|
Overall (N=158) |
Non-shock (n=88) |
Shock (n=70) |
P value | |
| Age | 46.5 (30-59) | 48.5 (28-60) | 44.5 (33-58) | 0.805 |
| Male sex | 128 (81%) | 75 (85.2%) | 53 (75.7%) | 0.130 |
| ISS | 16 (9-25) | 9 (9-18) | 21.5 (16-29) | <0.001 |
| ISS≥16 | 85 (53.8%) | 30 (34.1%) | 55 (78.6%) | <0.001 |
| ISS≥25 | 41 (25.9%) | 9 (10.2%) | 32 (45.7%) | <0.001 |
| NISS | 18 (9-27) | 13 (9-22) | 27 (17-34) | <0.001 |
| TRISS | 0.99 (0.938-0.735) | 0.99 (0.968-0.994) | 0.97 (0.994-0.938) | <0.001 |
| RTS | 7.84 (7.108-7.84) | 7.84 (7.84-7.84) | 7.108 (6.17-7.84) | <0.001 |
| ED vital sign | ||||
| SBP (mm/Hg) | 116(91-134) | 124(112-140) | 86 (73-123) | <0.001 |
| HR (/min) | 96 (80-117) | 91 (79-104) | 104 (83-126) | 0.020 |
| RR (/min) | 20 (18-20) | 20 (18-20) | 20 (17-22) | 0.671 |
| GCS | 15 (15-15) | 15 (15-15) | 15 (6-15) | <0.001 |
| Mechanism | ||||
| Motorcycle (%) | 80 (50.6%) | 41 (46.6%) | 39 (55.7%) | 0.515 |
| Car (%) | 42 (26.6%) | 22 (25%) | 20 (28.6%) | |
| Fall (%) | 3 (1.9%) | 3 (3.4%) | 0 (0%) | |
| High fall (%) | 6 (3.8%) | 4 (4.5%) | 2 (2.9%) | |
| Pedestrian (%) | 8 (5.1%) | 4 (4.5%) | 4 (5.7%) | |
| Assault (%) | 5 (3.2%) | 4 (4.5%) | 1 (1.4%) | |
| Bicycle (%) | 7 (4.4%) | 5 (5.7%) | 2 (2.9%) | |
| Impact (%) | 7 (4.4%) | 5 (5.7%) | 2 (2.9%) | |
| Clinical presentation | ||||
| ED hemoglobin g/dL | 12.6 (10.7-14.2) | 13.4 (12-14.8) | 11.5 (9-12.7) | <0.001 |
| ED Intubation (%) | 34 (21.5%) | 4 (4.5%) | 30 (42.9%) | <0.001 |
| Chest tube (%) | 31 (19.6%) | 11 (12.5%) | 20 (28.6%) | 0.012 |
| Blood transfusion | ||||
| Blood transfusion at ED (%) | 85 (53.8%) | 22 (25%) | 63 (90%) | <0.001 |
| ED Pack RBC (U) | 2 (0-4) | 0 (0-1) | 4 (2-7) | <0.001 |
| ED FFP (U) | 0 (0-2) | 0 (0-0) | 2 (0-4) | <0.001 |
| 24 HR Pack RBC (U) | 4 (0-12) | 0 (0-4) | 12 (8-21) | <0.001 |
| 24 HR FFP (U) | 2 (0-8) | 0 (0-2) | 9 (4-16) | <0.001 |
| Massive transfusion (%) | 49 (31%) | 3 (3.4%) | 46 (65.7%) | <0.001 |
| OR Pack RBC (U) | 2 (0-6) | 0 (0-2) | 7 (4-12) | <0.001 |
| OR FFP (U) | 0 (0-4) | 0 (0-0) | 4 (2-8) | <0.001 |
| Ward pack RBC (U) | 0 (0-4) | 0 (0-0) | 2 (0-6) | <0.001 |
| Ward FFP (U) | 0 (0-4) | 0 (0-0) | 2 (0-10) | <0.001 |
| Operative finding: | ||||
| Isolated bowel injury (%) | 41 (25.9%) | 38 (43.2%) | 3 (4.3%) | <0.001 |
| Isolated colon injury (%) | 18 (11.4%) | 15 (17%) | 3 (4.3%) | 0.012 |
| Isolated mesentery injury (%) | 48 (30.4%) | 15 (17%) | 33 (47.1%) | <0.001 |
| Combined injury (%) | 51 (32.3%) | 20 (22.7%) | 31 (44.3%) | 0.004 |
| OP blood loss (ml) | 500 (100-2000) | 100 (50-400) | 2000 (1000-3500) | <0.001 |
| Delayed OP | 18 (11.4%) | 15 (17%) | 3 (4.3%) | 0.012 |
| Outcome | ||||
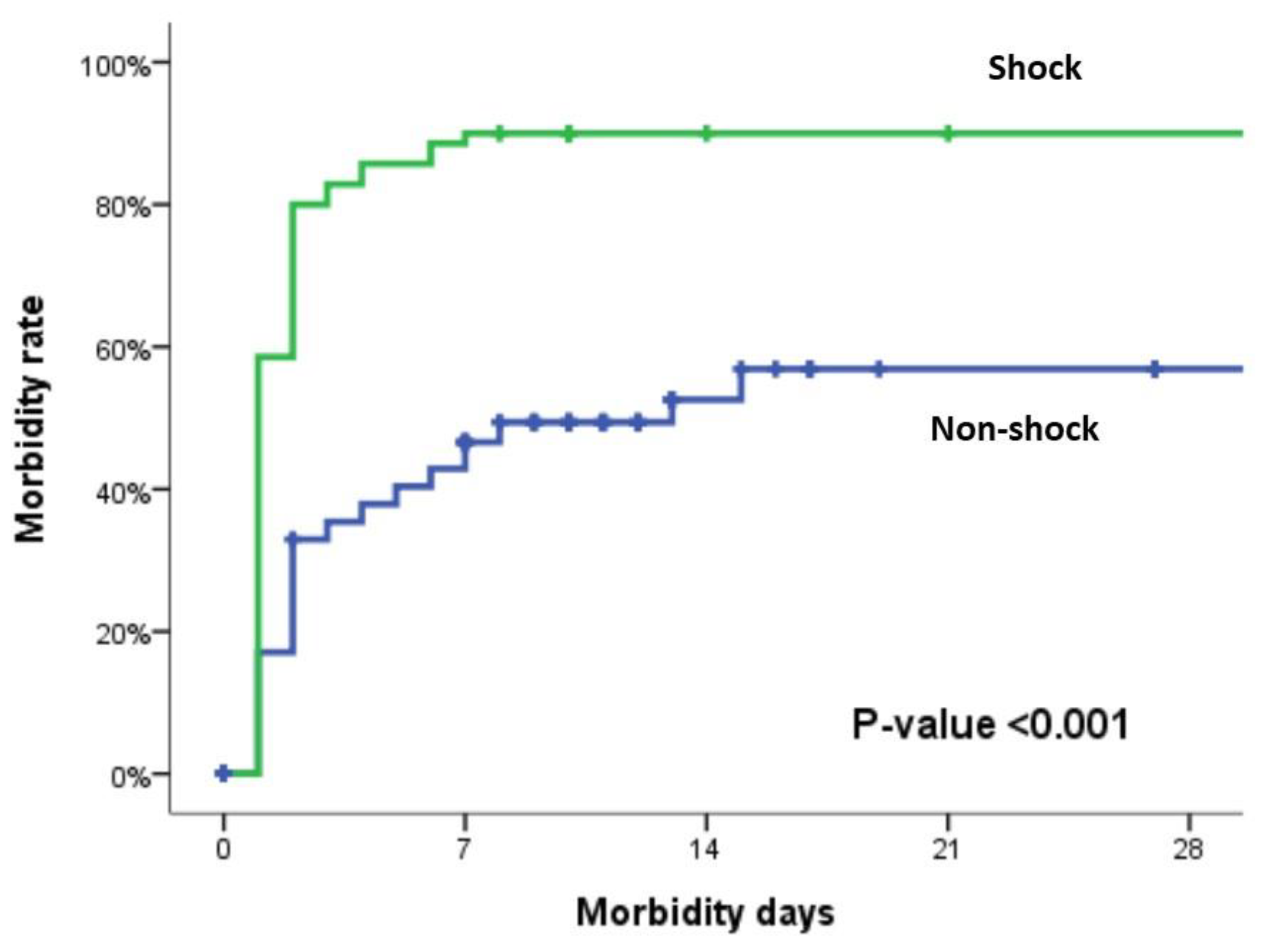
| Morbidity (%) | 106 (67.1%) | 43 (48.9%) | 63 (90%) | <0.001 |
| Mortality (%) | 21 (13.3%) | 2 (2.3%) | 19 (27.1%) | <0.001 |
| 24 hours mortality (%) | 7 (4.4%) | 1 (1.1%) | 6 (8.6%) | 0.045 |
| Bowel related mortality (%) | 5 (3.2%) | 2 (2.3%) | 3 (4.3%) | 0.656 |
| Exsanguination mortality (%) | 11 (7%) | 0 (0%) | 11 (15.7%) | <0.001 |
| ICU length of stay (day) | 3 (2-7) | 2 (0-5) | 4 (2-14) | <0.001 |
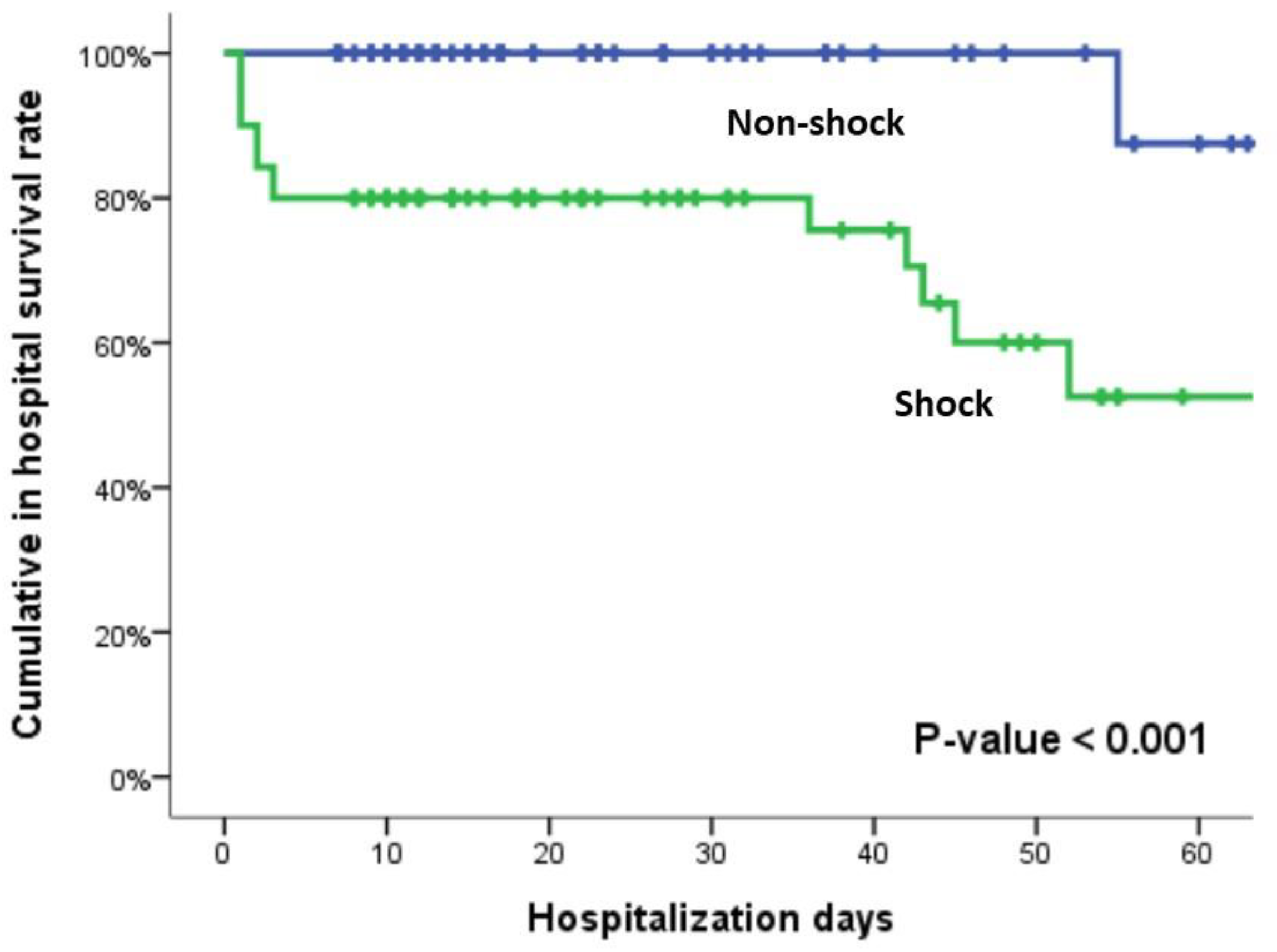
| Hospitalization LOS (day) | 17 (11-31) | 16.5 (11-30) | 18 (9-36) | 0.704 |
|
Overall (N=158) |
Shock (-) (n=88) |
Shock (+) (n=70) |
P value | |
| AIS head | 0 (0-0) | 0 (0-0) | 0 (0-2) | <0.001 |
| AIS face | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.413 |
| AIS chest | 0 (0-1) | 0 (0-0) | 0 (0-3) | 0.05 |
| AIS abdomen | 3 (3-4) | 3 (3-3) | 3 (3-4) | <0.001 |
| AIS extremities | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0.014 |
| AIS head ≥2 | 21 (13.3%) | 1 (1.1%) | 20 (28.6%) | <0.001 |
| AIS head ≥3 | 15 (9.5%) | 1 (1.1%) | 14 (20%) | <0.001 |
| AIS face ≥2 | 10 (6.3%) | 4 (4.5%) | 6 (8.6%) | 0.340 |
| AIS face ≥3 | 0 (0%) | 0 (0%) | 0 (0%) | ─ |
| AIS chest≥2 | 38 (24.1%) | 17 (19.3%) | 21 (30%) | 0.119 |
| AIS chest≥3 | 35 (22.2%) | 14 (15.9%) | 21 (30%) | 0.034 |
| AIS abdomen ≥2 | 158 (100%) | 88 (100%) | 70 (100%) | ─ |
| AIS abdomen ≥3 | 144 (91.1%) | 79 (89.8%) | 65 (92.9%) | 0.498 |
| AIS extremities ≥2 | 58 (36.7%) | 24 (27.3%) | 34 (48.6%) | 0.006 |
| AIS extremities ≥3 | 28 (17.7%) | 12 (13.6%) | 16 (22.9%) | 0.132 |
|
Overall (N=158) |
Shock (-) (n=88) |
Shock (+) (n=70) |
P value | |
| Spleen | 13 (8.2%) | 6 (6.8%) | 7 (10%) | 0.470 |
| Liver | 29 (18.4%) | 13 (14.8%) | 16 (22.9%) | 0.192 |
| Pancreas | 9 (5.7%) | 4 (4.5%) | 5 (7.1%) | 0.511 |
| Urinary bladder | 2 (1.3%) | 2 (2.3%) | 0 (0%) | 0.503 |
| Kidney | 10 (6.3%) | 3 (3.4%) | 7 (10%) | 0.109 |
| Diaphragm | 6 (3.8%) | 3 (3.4%) | 3 (4.3%) | 1.000 |
| Vessel | 23 (14.6%) | 7 (8%) | 16 (22.9%) | 0.008 |
| Intracerebral hemorrhage | 18 (11.4%) | 2 (2.3%) | 16 (22.9%) | <0.001 |
| Skull fracture | 4 (2.5%) | 0 (0%) | 4 (5.7%) | 0.037 |
| Facial bone fracture | 15 (9.5%) | 6 (6.8%) | 9 (12.9%) | 0.275 |
| C-spine | 4 (2.5%) | 2 (2.3%) | 2 (2.9%) | 1.000 |
| Lung contusion | 18 (11.4%) | 7 (8%) | 11 (15.7%) | 0.127 |
| Rib fracture | 27 (17.1%) | 6 (6.8%) | 21 (30%) | <0.001 |
| Clavicle fracture | 10 (6.3%) | 2 (2.3%) | 8 (11.4%) | 0.023 |
| Scapula | 3 (1.9%) | 2 (2.3%) | 1 (1.4%) | 1.000 |
| Hemopneumothorax | 27 (17.1%) | 12 (13.6%) | 15 (21.4%) | 0.196 |
| Thoracic spine fracture | 3 (1.9%) | 2 (2.3%) | 1 (1.4%) | 1.000 |
| Lumbar spine fracture | 8 (5.1%) | 5 (5.7%) | 3 (4.3%) | 1.000 |
| Pelvis fracture | 20 (12.7%) | 5 (5.7%) | 15 (21.4%) | 0.003 |
| Upper limb fracture | 25 (15.8%) | 14 (15.9%) | 11 (15.7%) | 1.000 |
| Lower limb fracture | 28 (17.7%) | 10 (11.4%) | 18 (25.7%) | 0.019 |
| Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P value | AOR (95% CI) | P value | |
| Age | 1.00 (0.96-1.02) | 0.786 | ─ | ─ |
| Male sex | 0.54 (0.24-1.21) | 0.133 | ─ | ─ |
| Liver | 1.71 (0.76-3.85) | 0.195 | ─ | ─ |
| Kidney | 3.15 (0.78-12.66) | 0.106 | ─ | ─ |
| Vessel | 3.43 (1.32-8.89) | 0.011 | 1.10 (0.32-3.72) | 0.882 |
| ICH | 12.74 (2.82-57.61) | 0.001 | 10.87 (1.70-69.75) | 0.012 |
| Lung contusion | 2.16 (0.79-5.90) | 0.134 | ─ | ─ |
| Rib fracture | 5.86 (2.21-15.51) | <0.001 | 5.94 (1.06-33.45) | 0.043 |
| Hemopneumothorax | 1.73 (0.75-3.98) | 0.199 | ─ | ─ |
| Pelvic fracture | 4.53 (1.56-13.17) | 0.006 | 2.94 (0.66-13.13) | 0.157 |
| Low limb fracture | 2.70 (1.16-6.31) | 0.022 | 1.66 (0.51.41) | 0.405 |
| Operation finding (compare to Isolated bowel injury) | ||||
| Isolated colon injury | 2.53 (0.46-13.98) | 0.286 | ─ | ─ |
| Isolated mesentery injury | 27.86 (7.41-104.78) | <0.001 | 23.50 (4.61-119.88) | <0.001 |
| Combined injury | 19.63 (5.34-72.25) | <0.001 | 25.43 (4.89-132.28) | <0.001 |
|
Overall (N=158) |
Non-shock (n=88) |
Shock (n=70) |
P value | |
| Sepsis | 25 (15.8%) | 12 (13.6%) | 13 (18.6%) | 0.398 |
| Pumonia | 24 (15.2%) | 9 (10.2%) | 15 (21.4%) | 0.051 |
| Septic shock | 10 (6.3%) | 3 (3.4%) | 7 (10%) | 0.091 |
| Unplanned ventilator | 33 (20.9%) | 8 (9.1%) | 25 (35.7%) | <0.001 |
| Intraabdominal abscess | 17 (10.8%) | 8 (9.1%) | 9 (12.9%) | 0.448 |
| Leakage | 9 (5.7%) | 5 (5.7%) | 4 (5.7%) | 0.993 |
| Coagulopathy | 63 (39.9%) | 14 (15.9%) | 49 (70%) | <0.001 |
| Acute renal failure | 57 (36.1%) | 21 (23.9%) | 36 (51.4%) | <0.001 |
| Acidosis | 46 (29.1%) | 11 (12.5%) | 35 (50%) | <0.001 |
| Urinary tract infection | 26 (16.5%) | 11 (12.5%) | 15 (21.4%) | 0.133 |
| Stroke | 4 (2.5%) | 0 (0%) | 4 (5.7%) | 0.023 |
| Pulmonary embolism | 2 (1.3%) | 1 (1.1%) | 1 (1.4%) | 1.000 |
| ARDS | 5 (3.2%) | 1 (1.1%) | 4 (5.7%) | 0.171 |
| Pleural effusion | 25 (15.8%) | 12 (13.6%) | 13 (18.6%) | 0.398 |
| Enterocutaneous fistula | 2 (1.3%) | 2 (2.3%) | 0 (0%) | 0.503 |
| Wound infection | 33 (20.9%) | 18 (20.5%) | 15 (21.4%) | 0.881 |
| Wound dehiscence | 8 (5.1%) | 2 (2.3%) | 6 (8.6%) | 0.140 |
| Abdomen compartment | 7 (4.4%) | 1 (1.1%) | 6 (8.6%) | 0.045 |
| tracheostomy | 3 (1.9%) | 1 (1.1%) | 2 (2.9%) | 0.585 |
| ECMO | 4 (2.5%) | 0 (0%) | 4 (5.7%) | 0.037 |
| Return to OR | 24 (15.2%) | 9 (10.2%) | 15 (21.4%) | 0.051 |
| Hemodialysis | 3 (1.9%) | 1 (1.1%) | 2 (2.9%) | 0.585 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





