Submitted:
13 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dysferlinopathies: Clinical Landscape and Phenotypic Variability
| Dysferlinopathies phenotypes | |||||
|---|---|---|---|---|---|
| Features | Limb-girdle muscular dystrophy recessive type 2 | Miyoshi myopathy | Distal myopathy with anterior tibial onset | Dysferlin-deficient proximo-distal phenotype | Asymptomatic hyperCKemia |
| Age of onset | Late teen to thirties | Late teen to thirties | Early adulthood | Variable, usually early twenties to early thirties | Variable, usually >50 years |
| Type of muscular dystrophy | Proximal | Distal | Distal (anterior tibial) | Proximal and distal combined | – |
| Atrophy | Present | Present | Present | Present | No |
| Initial symptoms | Weakness and atrophy of the proximal muscles, especially the gluteus maximus (buttock) and quadriceps (thigh) muscles | Weakness and atrophy of the distal muscles, especially the gastrocnemius (calf) and soleus (lower leg) muscles | Weakness and atrophy of the distal muscles, especially the anterior tibialis (shin) and extensor digitorum longus (toe extensor) muscles | Weakness and atrophy of both proximal and distal muscles, with variable distribution and severity | Only elevated serum CK levels with no or insignificant muscle weakness or other symptoms |
| Progression | Slowly progressive, spreading to other proximal muscles and eventually affecting distal muscles; usually symmetrical | Slowly progressive (may be faster than LGMDR2), spreading to other distal muscles and eventually affecting proximal muscles; usually symmetrical | Slowly progressive (may be faster than LGMDR2 and MM), spreading to other distal muscles and sometimes affecting proximal muscles; usually symmetrical | Slowly (variable compared to LGMDR2, MM, and DMAT) progressive, affecting both proximal and distal muscles in a symmetrical or asymmetrical pattern; may have focal or regional involvement | Stable or fluctuating CK levels with no or insignificant muscle involvement |
| CK levels (times normal) |
Very high (50–200) |
Very high (50–200) |
High (20–70) |
High (10–50) |
High (5–10) |
| Muscle biopsy findings | No/very low levels of dysferlin in muscle fibers; muscle damage with degeneration and regeneration of muscle fibers; inflammation with cells that invade the muscle tissue; scar tissue formation and fat deposits that replace muscle tissue; these changes can vary in severity and distribution among different types of dysferlinopathy | No/low levels of dysferlin in muscle fibers; mild or no changes in muscle fibers; no inflammation, scar tissue, or fat deposits | |||
| Cardiac involvement | Rare, 3–10% patients may develop cardiac dysfunction or arrhythmias; may require cardiac monitoring and treatment if present | ||||
| Respiratory involvement | Uncommon, 20–30% patients may develop respiratory impairment and/or sleep apnea; may require respiratory monitoring and treatment if present | ||||
| Life expectancy | Not significantly impacted unless cardiac/respiratory involvement is observed | Not affected, as there is no muscle involvement or other complications | |||
| Quality of life | Causes significant disability; may result in complete loss of ambulation; may impact psychosocial well-being; may require multidisciplinary care and support to cope with the challenges and improve the function | May not be affected; some patients may experience stress and anxiety due to elevated CK levels and potential future risk of muscle weakness | |||
2.1. LGMDR2
2.2. MM
2.3. DMAT
2.4. Dysferlin-deficient proximo-distal phenotype
2.5. Asymptomatic hyperCKemia
2.6. Dysferlin-associated congenital phenotype
3. Dysferlin: A Mosaic of Uncharted Roles and Functionalities
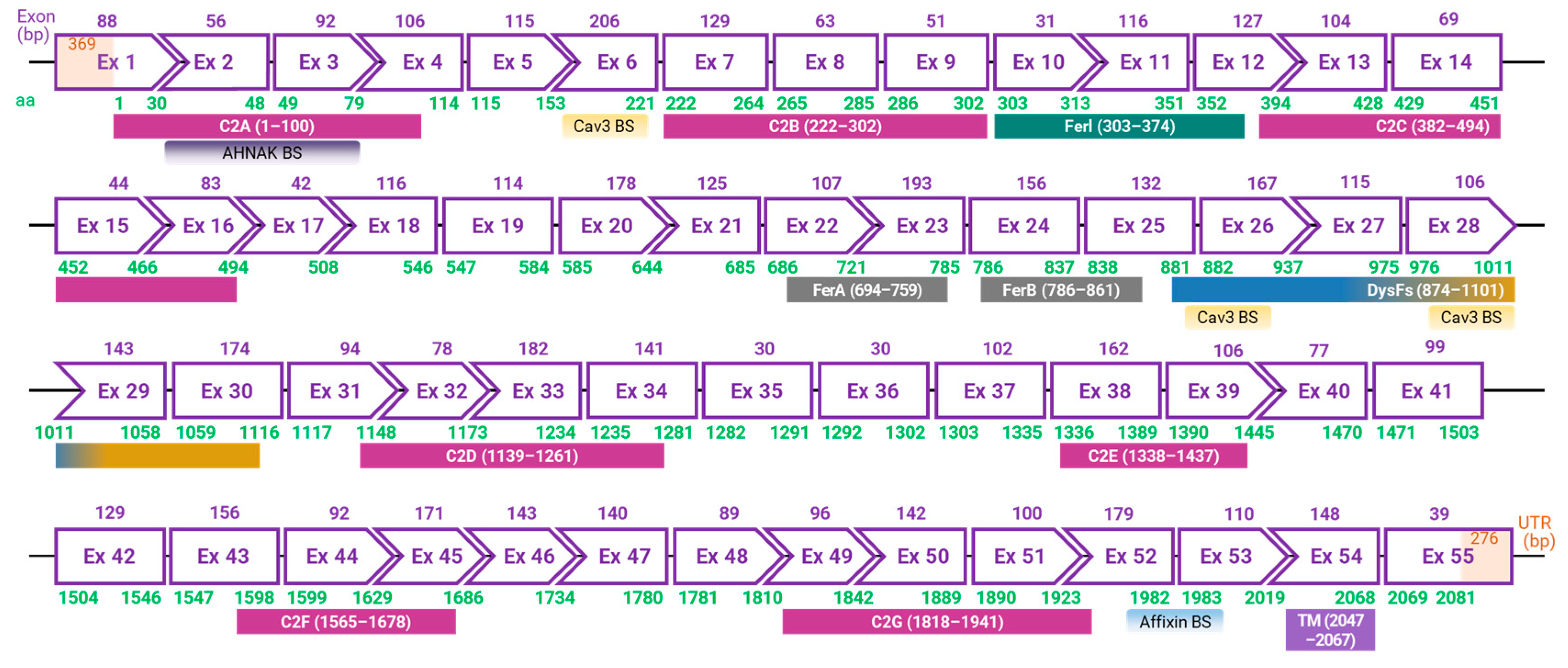
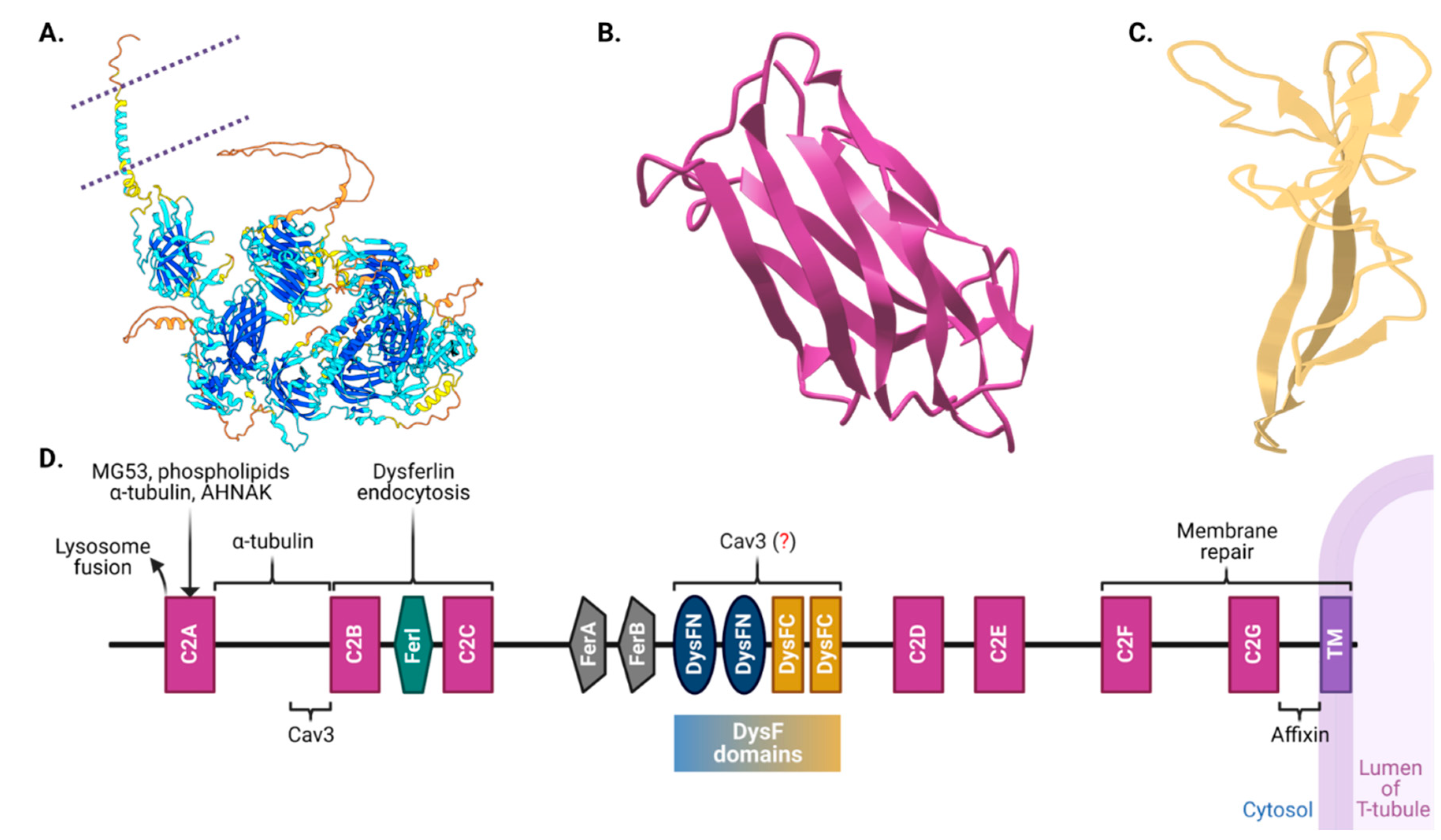
3.1. Into the Structural Intricacies of Dysferlin
3.2. Multifaceted Functional Aspects of Dysferlin
3.2.1. Dysferlin in Membrane Repair and Integration
3.2.2. Calcium Regulation
3.2.3. Dysferlin in Immune and Inflammatory Response
3.2.4. Dysferlin in Cholinergic Signaling
3.2.5. Dysferlin Beyond Skeletal Muscle
3.2.6. The Dysferlin Interactome
3.3. Dysferlin in the Pathobiology of Dysferlinopathies
4. DYSF mutations: genotype-phenotype correlations and the role of dysferlin partner proteins
4.1. Genotype-Phenotype Correlations in Dysferlinopathies
4.2. The Impacts of Pathogenic DYSF Mutations
4.3. The Role of Disease Modifiers
5. Animal models for Studying Dysferlinopathies
6. Therapeutic Approaches for Dysferlinopathies
6.1. Symptomatic Treatments
6.2. Pharmacological Approaches
6.2.1. Proteasome Inhibitors
6.2.2. Galectin-1 Treatment
6.2.3. Blockade of Hemichannels
6.2.4. N-Acetylcysteine (NAC)
6.2.5. Diltiazem
6.2.6. Halofuginone
6.3. Molecular and Genetic Therapies
6.3.1. Gene Replacement and Gene Editing Therapies
6.3.2. Antisense-Mediated Exon Skipping
6.3.3. Membrane Repair and Stabilization
6.4. Cell-based and Tissue Engineering Approaches
6.5. Dietary and Metabolic Approaches
6.5.1. Ketogenic Diet
6.5.2. AMPK Complex Activation
6.6. Clinical Trials for dysferlinopathies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A Gene Related to Caenorhabditis Elegans Spermatogenesis Factor Fer-1 Is Mutated in Limb-Girdle Muscular Dystrophy Type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Aoki, M.; Illa, I.; Wu, C.; Fardeau, M.; Angelini, C.; Serrano, C.; Andoni Urtizberea, J.; Hentati, F.; Hamida, M. Ben; et al. Dysferlin, a Novel Skeletal Muscle Gene, Is Mutated in Miyoshi Myopathy and Limb Girdle Muscular Dystrophy. Nat. Genet. 1998, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pajusalu, S.; Lake, N.J.; Zhou, G.; Ioannidis, N.; Mittal, P.; Johnson, N.E.; Weihl, C.C.; Williams, B.A.; Albrecht, D.E.; et al. Estimating Prevalence for Limb-Girdle Muscular Dystrophy Based on Public Sequencing Databases. Genet. Med. 2019, 21, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Guglieri, M.; Magri, F.; D’Angelo, M.G.; Prelle, A.; Morandi, L.; Rodolico, C.; Cagliani, R.; Mora, M.; Fortunato, F.; Bordoni, A.; et al. Clinical, Molecular, and Protein Correlations in a Large Sample of Genetically Diagnosed Italian Limb Girdle Muscular Dystrophy Patients. Hum. Mutat. 2008, 29, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Shilling, C.J.; Westra, S.; Wall, C.; Wicklund, M.P.; Stolle, C.; Brown, C.A.; Michele, D.E.; Piccolo, F.; Winder, T.L.; et al. Limb-Girdle Muscular Dystrophy in the United States. J. Neuropathol. Exp. Neurol. 2006, 65, 995–1003. [Google Scholar] [CrossRef]
- Aoki, M.; Liu, J.; Richard, I.; Bashir, R.; Britton, S.; Keers, S.M.; Oeltjen, J.; Brown, H.E.V.; Marchand, S.; Bourg, N.; et al. Genomic Organization of the Dysferlin Gene and Novel Mutations in Miyoshi Myopathy. Neurology 2001, 57, 271–278. [Google Scholar] [CrossRef]
- Nguyen, K.; Bassez, G.; Bernard, R.; Krahn, M.; Labelle, V.; Figarella-Branger, D.; Pouget, J.; Hammouda, E.H.; Béroud, C.; Urtizberea, A.; et al. Dysferlin Mutations in LGMD2B, Miyoshi Myopathy, and Atypical Dysferlinopathies. Hum. Mutat. 2005, 26, 165. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective Membrane Repair in Dysferlin-Deficient Muscular Dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Bansal, D.; Campbell, K.P. Dysferlin and the Plasma Membrane Repair in Muscular Dystrophy. Trends Cell Biol. 2004, 14, 206–213. [Google Scholar] [CrossRef]
- Kerr, J.P.; Ziman, A.P.; Mueller, A.L.; Muriel, J.M.; Kleinhans-Welte, E.; Gumerson, J.D.; Vogel, S.S.; Ward, C.W.; Roche, J.A.; Bloch, R.J. Dysferlin Stabilizes Stress-Induced Ca2+ Signaling in the Transverse Tubule Membrane. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 20831–20836. [Google Scholar] [CrossRef]
- Klinge, L.; Laval, S.; Keers, S.; Haldane, F.; Straub, V.; Barresi, R.; Bushby, K. From T-tubule to Sarcolemma: Damage-induced Dysferlin Translocation in Early Myogenesis. FASEB J. 2007, 21, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.; Bersch, K.; Büssenschütt, R.; Drzymalski, M.; Liebetanz, D.; Nikolaev, V.O.; Wagner, S.; Maier, L.S.; Gärtner, J.; Klinge, L.; et al. Dysferlin Mediates Membrane Tubulation and Links T-Tubule Biogenesis to Muscular Dystrophy. J. Cell Sci. 2017, 130, 841–852. [Google Scholar] [CrossRef]
- Therrien, C.; Fulvio, S. Di; Pickles, S.; Sinnreich, M. Characterization of Lipid Binding Specificities of Dysferlin C2 Domains Reveals Novel Interactions with Phosphoinositides. Biochemistry 2009, 48, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.B.; Doherty, K.R.; Delmonte, A.J.; McNally, E.M. Calcium-Sensitive Phospholipid Binding Properties of Normal and Mutant Ferlin C2 Domains. J. Biol. Chem. 2002, 277, 22883–22888. [Google Scholar] [CrossRef]
- Lennon, N.J.; Kho, A.; Bacskai, B.J.; Perlmutter, S.L.; Hyman, B.T.; Brown, R.H. Dysferlin Interacts with Annexins A1 and A2 and Mediates Sarcolemmal Wound-Healing. J. Biol. Chem. 2003, 278, 50466–50473. [Google Scholar] [CrossRef]
- Azakir, B.A.; Di Fulvio, S.; Therrien, C.; Sinnreich, M. Dysferlin Interacts with Tubulin and Microtubules in Mouse Skeletal Muscle. PLoS One 2010, 5, e10122. [Google Scholar] [CrossRef]
- Cai, C.; Weisleder, N.; Ko, J.K.; Komazaki, S.; Sunada, Y.; Nishi, M.; Takeshima, H.; Ma, J. Membrane Repair Defects in Muscular Dystrophy Are Linked to Altered Interaction between MG53, Caveolin-3, and Dysferlin. J. Biol. Chem. 2009, 284, 15894–15902. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Hayashi, Y.K.; Ogawa, M.; Aoki, M.; Murayama, K.; Nishino, I.; Nonaka, I.; Arahata, K.; Brown, R.H. The Sarcolemmal Proteins Dysferlin and Caveolin-3 Interact in Skeletal Muscle. Hum. Mol. Genet. 2001, 10, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Laval, S.H.; Remoortere, A.; Baudier, J.; Benaud, C.; Anderson, L.V.B.; Straub, V.; Deelder, A.; Frants, R.R.; Dunnen, J.T.; et al. AHNAK a Novel Component of the Dysferlin Protein Complex, Redistributes to the Cytoplasm with Dysferlin during Skeletal Muscle Regeneration. FASEB J. 2007, 21, 732–742. [Google Scholar] [CrossRef]
- Therrien, C.; Dodig, D.; Karpati, G.; Sinnreich, M. Mutation Impact on Dysferlin Inferred from Database Analysis and Computer-Based Structural Predictions. J. Neurol. Sci. 2006, 250, 71–78. [Google Scholar] [CrossRef]
- Blandin, G.; Marchand, S.; Charton, K.; Danièle, N.; Gicquel, E.; Boucheteil, J.B.; Bentaib, A.; Barrault, L.; Stockholm, D.; Bartoli, M.; et al. A Human Skeletal Muscle Interactome Centered on Proteins Involved in Muscular Dystrophies: LGMD Interactome. Skelet. Muscle 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Smirnikhina, S.; Lavrov, A. Dysferlinopathies: Clinical and Genetic Variability. Clin. Genet. 2022, 102, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, J.P. Portrait of Dysferlinopathy: Diagnosis and Development of Therapy. J. Clin. Med. 2023, 12, 6011. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.V.B.; Davison, K.; Moss, J.A.; Young, C.; Cullen, M.J.; Walsh, J.; Johnson, M.A.; Bashir, R.; Britton, S.; Keers, S.; et al. Dysferlin Is a Plasma Membrane Protein and Is Expressed Early in Human Development. Hum. Mol. Genet. 1999, 8, 855–861. [Google Scholar] [CrossRef] [PubMed]
- De Morreé, A.; Flix, B.; Bagaric, I.; Wang, J.; Van Den Boogaard, M.; Moursel, L.G.; Frants, R.R.; Illa, I.; Gallardo, E.; Toes, R.; et al. Dysferlin Regulates Cell Adhesion in Human Monocytes. J. Biol. Chem. 2013, 288, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Cheng, Q.; Chen, X.; Yu, Q.; Li, Z. Abnormal Expression of Dysferlin in Blood Monocytes Supports Primary Dysferlinopathy in Patients Confirmed by Genetic Analyses. Front. Neurol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- BioGPS GeneAtlas U133A, Gcrma. Available online: http://biogps.org/#goto=genereport&id=8291 (accessed on 21 September 2023).
- Mahjneh, I.; Bushby, K.; Passos-Bueno, M.-R.; Zatz, M.; Nasher, L.; Bashir, R.; Strachan, T.; Marconi, G. The Phenotype of Chromosome 2P-Linked Limb-Girdle Muscular Dystrophy. Neuromuscul. Disord. 1996, 6, S7. [Google Scholar] [CrossRef]
- Illa, I.; Serrano-Munuera, C.; Gallardo, E.; Lasa, A.; Rojas-García, R.; Palmer, J.; Gallano, P.; Baiget, M.; Matsuda, C.; Brown, R.H. Distal Anterior Compartment Myopathy: A Dysferlin Mutation Causing a New Muscular Dystrophy Phenotype. Ann. Neurol. 2001, 49, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Rowland, L.P.; Hays, A.P.; Dimauro, S.; Hopkins, L.C. High Serum Levels of Creatine Kinase: Asymptomatic Prelude to Distal Myopathy. Muscle Nerve 1987, 10, 346–350. [Google Scholar] [CrossRef]
- Aoki, M.; Takahashi, T. Dysferlinopathy; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; Washington, Seattle, 1993. [Google Scholar]
- Krahn, M.; Béroud, C.; Labelle, V.; Nguyen, K.; Bernard, R.; Bassez, G.; Figarella-Branger, D.; Fernandez, C.; Bouvenot, J.; Richard, I.; et al. Analysis of the DYSF Mutational Spectrum in a Large Cohort of Patients. Hum. Mutat. 2009, 30. [Google Scholar] [CrossRef]
- Xi, J.; Blandin, G.; Lu, J.; Luo, S.; Zhu, W.; Béroud, C.; Pécheux, C.; Labelle, V.; Lévy, N.; Urtizberea, J.A.; et al. Clinical Heterogeneity and a High Proportion of Novel Mutations in a Chinese Cohort of Patients with Dysferlinopathy. Neurol. India 2014, 62, 635–639. [Google Scholar] [CrossRef]
- Nguyen, K.; Bassez, G.; Krahn, M.; Bernard, R.; Laforêt, P.; Labelle, V.; Urtizberea, J.A.; Figarella-Branger, D.; Romero, N.; Attarian, S.; et al. Phenotypic Study in 40 Patients with Dysferlin Gene Mutations: High Frequency of Atypical Phenotypes. Arch. Neurol. 2007, 64, 1176–1182. [Google Scholar] [CrossRef]
- Barthélémy, F.; Wein, N.; Krahn, M.; Lévy, N.; Bartoli, M. Translational Research and Therapeutic Perspectives in Dysferlinopathies. Mol. Med. 2011, 17, 875–882. [Google Scholar] [CrossRef]
- Miyoshi, K.; Saijo, K.; Kuryu, T.; Tada, Y.; Otsuka, Y.; Oshima, Y.; Nakano, N.; Kawai, H.; Miyake, M.; Okazawa, T.; et al. Four Cases of Distal Myopathy in Two Families. Jpn. J. Hum. Genet. 1967, 12. [Google Scholar]
- Sasaki, K.; Mori, H.; Takahashi, K.; Nakamura, H. Distal Myopathy--Report of Four Cases. Clin. Neurol. 1969, 9, 627–637. [Google Scholar]
- Ideta, T.; Shikai, T.; Uchino, M.; Okajima, T.; Akatsuka, M. Distal Myopathy-Report of 4 Cases in Two Families. Rinsho Shinkeigaku 1973, 13, 579–586. [Google Scholar]
- Klinge, L.; Dean, A.F.; Kress, W.; Dixon, P.; Charlton, R.; Müller, J.S.; Anderson, L. V.; Straub, V.; Barresi, R.; Lochmüller, H.; et al. Late Onset in Dysferlinopathy Widens the Clinical Spectrum. Neuromuscul. Disord. 2008, 18, 288–290. [Google Scholar] [CrossRef]
- Illarioshkin, S.N.; Ivanova-Smolenskaya, I.A.; Tanaka, H.; Vereshchagin, N. V.; Markova, E.D.; Poleshchuk, V. V.; Lozhnikova, S.M.; Sukhorukov, V.S.; Limborska, S.A.; Slominsky, P.A.; et al. Clinical and Molecular Analysis of a Large Family with Three Distinct Phenotypes of Progressive Muscular Dystrophy. Brain 1996, 119, 1895–1909. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuzaki, T.; Suehara, M.; Kanzato, N.; Takashima, H.; Higuchi, I.; Matsumura, T.; Goto, K.; Arahata, K.; Osame, M. Phenotypic Variation in a Large Japanese Family with Miyoshi Myopathy with Nonsense Mutation in Exon 19 of Dysferlin Gene. J. Neurol. Sci. 2001, 184, 15–19. [Google Scholar] [CrossRef]
- Umakhanova, Z.R.; Bardakov, S.N.; Mavlikeev, M.O.; Chernova, O.N.; Magomedova, R.M.; Akhmedova, P.G.; Yakovlev, I.A.; Dalgatov, G.D.; Fedotov, V.P.; Isaev, A.A.; et al. Twenty-Year Clinical Progression of Dysferlinopathy in Patients from Dagestan. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Urtizberea, Ja.; Bassez, G.; Leturcq, F.; Nguyen, K.; Krahn, M.; Levy, N. Dysferlinopathies. Neurol. India 2008, 56, 289. [Google Scholar] [CrossRef]
- Miyoshi, K.; Kawai, H.; Iwasa, M.; Kusaka, K.; Nishino, H. Autosomal Recessive Distal Muscular Dystrophy as a New Type of Progressive Muscular Dystrophy: Seventeen Cases in Eight Families Including an Autopsied Case. Brain 1986, 109, 31–54. [Google Scholar] [CrossRef]
- Rowin, J.; Meriggioli, M.N.; Cochran, E.J.; Sanders, D.B. Prominent Inflammatory Changes on Muscle Biopsy in Patients with Miyoshi Myopathy. Neuromuscul. Disord. 1999, 9, 417–420. [Google Scholar] [CrossRef]
- Izumi, R.; Takahashi, T.; Suzuki, N.; Niihori, T.; Ono, H.; Nakamura, N.; Katada, S.; Kato, M.; Warita, H.; Tateyama, M.; et al. The Genetic Profile of Dysferlinopathy in a Cohort of 209 Cases: Genotype–Phenotype Relationship and a Hotspot on the Inner DysF Domain. Hum. Mutat. 2020, 41, 1540–1554. [Google Scholar] [CrossRef]
- Wang, N.; Han, X.; Hao, S.; Han, J.; Zhou, X.; Sun, S.; Tang, J.; Lu, Y.; Wu, H.; Ma, S.; et al. The Clinical, Myopathological, and Molecular Characteristics of 26 Chinese Patients with Dysferlinopathy: A High Proportion of Misdiagnosis and Novel Variants. BMC Neurol. 2022, 22, 398. [Google Scholar] [CrossRef]
- Contreras-Cubas, C.; Barajas-Olmos, F.; Frayre-Martínez, M.I.; Siordia-Reyes, G.; Guízar-Sánchez, C.C.; García-Ortiz, H.; Orozco, L.; Baca, V. Dysferlinopathy Misdiagnosed with Juvenile Polymyositis in the Pre-Symptomatic Stage of HyperCKemia: A Case Report and Literature Review. BMC Med. Genomics 2022, 15, 139. [Google Scholar] [CrossRef]
- Aoki, M. Dysferlinopathy. In GeneReviews® [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Eds.; University of Washington: Seattle, 1993. [Google Scholar]
- Urtizberea, J.A.; Bassez, G.; Leturcq, F.; Nguyen, K.; Krahn, M.; Levy, N. Dysferlinopathies. Neurol. India 2008, 56, 289–297. [Google Scholar] [CrossRef]
- Blandin, G.; Beroud, C.; Labelle, V.; Nguyen, K.; Wein, N.; Hamroun, D.; Williams, B.; Monnier, N.; Rufibach, L.E.; Urtizberea, J.A.; et al. UMD-DYSF, a Novel Locus Specific Database for the Compilation and Interactive Analysis of Mutations in the Dysferlin Gene. Hum. Mutat. 2012, 33. [Google Scholar] [CrossRef]
- Shen, J.Y.; Prasad, K.; Goh, L.L.; Angkodjojo, S.; Khoo, C.Y.; Umapathi, T. Dysferlinopathy, with Mild Cardiac Involvement, from a Novel Mutation of DYSF Gene. QJM An Int. J. Med. 2023, 116, 453–454. [Google Scholar] [CrossRef]
- Diaz-Manera, J.; Fernandez-Torron, R.; LLauger, J.; James, M.K.; Mayhew, A.; Smith, F.E.; Moore, U.R.; Blamire, A.M.; Carlier, P.G.; Rufibach, L.; et al. Muscle MRI in Patients with Dysferlinopathy: Pattern Recognition and Implications for Clinical Trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1071–1081. [Google Scholar] [CrossRef]
- Alharbi, N.; Matar, R.; Cupler, E.; Al-Hindi, H.; Murad, H.; Alhomud, I.; Monies, D.; Alshehri, A.; Alyahya, M.; Meyer, B.; et al. Clinical, Neurophysiological, Radiological, Pathological, and Genetic Features of Dysferlinopathy in Saudi Arabia. Front. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Moore, U.; Fernandez-Torron, R.; Jacobs, M.; Gordish-Dressman, H.; Diaz-Manera, J.; James, M.K.; Mayhew, A.G.; Harris, E.; Guglieri, M.; Rufibach, L.E.; et al. Cardiac and Pulmonary Findings in Dysferlinopathy: A 3-year, Longitudinal Study. Muscle Nerve 2022, 65, 531–540. [Google Scholar] [CrossRef]
- Ueyama, H.; Kumamoto, T.; Horinouchi, H.; Fujimoto, S.; Aono, H.; Tsuda, T. Clinical Heterogeneity in Dysferlinopathy. Intern. Med. 2002, 41, 532–536. [Google Scholar] [CrossRef]
- Klinge, L.; Aboumousa, A.; Eagle, M.; Hudson, J.; Sarkozy, A.; Vita, G.; Charlton, R.; Roberts, M.; Straub, V.; Barresi, R.; et al. New Aspects on Patients Affected by Dysferlin Deficient Muscular Dystrophy. J. Neurol. Neurosurg. Psychiatry 2010, 81, 946–953. [Google Scholar] [CrossRef]
- Harris, E.; Bladen, C.L.; Mayhew, A.; James, M.; Bettinson, K.; Moore, U.; Smith, F.E.; Rufibach, L.; Cnaan, A.; Bharucha-Goebel, D.X.; et al. The Clinical Outcome Study for Dysferlinopathy. Neurol. Genet. 2016, 2, e89. [Google Scholar] [CrossRef]
- Takahashi, T.; Aoki, M.; Suzuki, N.; Tateyama, M.; Yaginuma, C.; Sato, H.; Hayasaka, M.; Sugawara, H.; Ito, M.; Abe-Kondo, E.; et al. Clinical Features and a Mutation with Late Onset of Limb Girdle Muscular Dystrophy 2B. J. Neurol. Neurosurg. Psychiatry 2013, 84, 433–440. [Google Scholar] [CrossRef]
- Diaz-Manera, J.; Fernandez-Torron, R.; Llauger, J.; James, M.K.; Mayhew, A.; Smith, F.E.; Moore, U.R.; Blamire, A.M.; Carlier, P.G.; Rufibach, L.; et al. Muscle MRI in Patients with Dysferlinopathy: Pattern Recognition and Implications for Clinical Trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1071–1081. [Google Scholar] [CrossRef]
- Reyngoudt, H.; Smith, F.E.; Caldas de Almeida Araújo, E.; Wilson, I.; Fernández-Torrón, R.; James, M.K.; Moore, U.R.; Díaz-Manera, J.; Marty, B.; Azzabou, N.; et al. Three-Year Quantitative Magnetic Resonance Imaging and Phosphorus Magnetic Resonance Spectroscopy Study in Lower Limb Muscle in Dysferlinopathy. J. Cachexia. Sarcopenia Muscle 2022, 13, 1850–1863. [Google Scholar] [CrossRef]
- Socoliuc, C.G.; Oprişan, A.; Dobrescu, A.; Manole, E.; Bastian, A.E. THE CHALLENGING DIAGNOSIS OF DYSFERLINOPATHY – A CASE REPORT. Rom. J. Neurol. 2021, 20, 264–270. [Google Scholar] [CrossRef]
- Fanin, M.; Angelini, C. Progress and Challenges in Diagnosis of Dysferlinopathy. Muscle and Nerve 2016, 54, 821–835. [Google Scholar] [CrossRef]
- Ho, M.; Gallardo, E.; McKenna-Yasek, D.; De Luna, N.; Illa, I.; Brown, R.H. A Novel, Blood-Based Diagnostic Assay for Limb Girdle Muscular Dystrophy 2B and Miyoshi Myopathy. Ann. Neurol. 2002, 51, 129–133. [Google Scholar] [CrossRef]
- Ankala, A.; Nallamilli, B.R.; Rufibach, L.E.; Hwang, E.; Hegde, M.R. Diagnostic Overview of Blood-Based Dysferlin Protein Assay for Dysferlinopathies. Muscle and Nerve 2014, 50, 333–339. [Google Scholar] [CrossRef]
- Sinclair, A. Genetics 101: Detecting Mutations in Human Genes. C. Can. Med. Assoc. J. 2002, 167, 275–279. [Google Scholar]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A Gene Related to Caenorhabditis Elegans Spermatogenesis Factor Fer-1 Is Mutated in Limb-Girdle Muscular Dystrophy Type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Bashlr, R.; Strachan, T.; Keers, S.; Stephenson, A.; Mahjneh, I.; Marconi, G.; Nashef, L.; Bushby, K.M.D. A Gene for Autosomal Recessive Limb-Girdle Muscular Dystrophy Maps to Chromosome 2p. Hum. Mol. Genet. 1994, 3, 455–457. [Google Scholar] [CrossRef]
- Weiler, T.; Greenberg, C.R.; Nylen, E.; Halliday, W.; Morgan, K.; Eggertson, D.; Wrogemann, K. Limb-Girdle Muscular Dystrophy and Miyoshi Myopathy in an Aboriginal Canadian Kindred Map to LGMD2B and Segregate with the Same Haplotype. Am. J. Hum. Genet. 1996, 59, 872–878. [Google Scholar]
- Passos-Bueno, M.R.; Vainzof, M.; Moreira, E.S.; Zatz, M. Seven Autosomal Recessive Limb-Girdle Muscular Dystrophies in the Brazilian Population: From LGMD2A to LGMD2G. Am. J. Med. Genet. 1999, 82, 392–398. [Google Scholar] [CrossRef]
- McNally, E.M.; Ly, C.; Rosenmann, H.; Rosenbaum, S.M.; Jiang, W.; Anderson, L.V.B.; Soffer, D.; Argov, Z. Splicing Mutation in Dysferlin Produces Limb-Girdle Muscular Dystrophy with Inflammation. Am. J. Med. Genet. 2000, 91, 305–312. [Google Scholar] [CrossRef]
- Illa, I.; De Luna, N.; Domínguez-Perles, R.; Rojas-García, R.; Paradas, C.; Palmer, J.; Márquez, C.; Gallano, P.; Gallardo ERufibach, L.E.; Lek, M. Symptomatic Dysferlin Gene Mutation Carriers: Characterization of Two Cases. Neurology 2007, 68, 1284–1289. [Google Scholar] [CrossRef]
- Spuler, S.; Carl, M.; Zabojszcza, J.; Straub, V.; Bushby, K.; Moore, S.A.; Bähring, S.; Wenzel, K.; Vinkemeier, U.; Rocken, C. Dysferlin-Deficient Muscular Dystrophy Features Amyloidosis. Ann. Neurol. 2008, 63, 323–328. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Numitone, G.; Aurino, S.; Caserta, I.R.; Fanin, M.; Politano, L.; Minetti, C.; Ricci, E.; Piluso, G.; Angelini, C.; et al. Muscular Dystrophy with Marked Dysferlin Deficiency Is Consistently Caused by Primary Dysferlin Gene Mutations. Eur. J. Hum. Genet. 2011, 19, 974–980. [Google Scholar] [CrossRef]
- Ro, L.S.; Lee-Chen, G.J.; Lin, T.C.; Wu, Y.R.; Chen, C.M.; Lin, C.Y.; Chen, S.T. Phenotypic Features and Genetic Findings in 2 Chinese Families with Miyoshi Distal Myopathy. Arch. Neurol. 2004, 61, 1594–1599. [Google Scholar] [CrossRef]
- Yamanouchi, Y.; Ozawa, E.; Nonaka, I. Autosomal Recessive Distal Muscular Dystrophy: Normal Expression of Dystrophin, Utrophin and Dystrophin-Associated Proteins in Muscle Fibers. J. Neurol. Sci. 1994, 126, 70–76. [Google Scholar] [CrossRef]
- Barohn, R.J.; Amato, A.A.; Griggs, R.C. Overview of Distal Myopathies: From the Clinical to the Molecular. Neuromuscul. Disord. 1998, 8, 309–316. [Google Scholar] [CrossRef]
- Barohn, R.J.; Miller, R.G.; Griggs, R.C. Autosomal Recessive Distal Dystrophy. Neurology 1991, 41, 1365–1370. [Google Scholar] [CrossRef]
- Linssen, W.H.J.P.; Notermans, N.C.; Van Der Graaf, Y.; Wokke, J.H.J.; Van Doorn, P.A.; Höweler, C.J.; Busch, H.F.M.; De Jager, A.E.J.; De Visser, M. Miyoshi-Type Distal Muscular Dystrophy. Clinical Spectrum in 24 Dutch Patients. Brain 1997, 120, 1989–1996. [Google Scholar] [CrossRef]
- Vilchez, J.J.; Gallano, P.; Gallardo, E.; Lasa, A.; Rojas-García, R.; Freixas, A.; De Luna, N.; Calafell, F.; Sevilla, T.; Mayordomo, F.; et al. Identification of a Novel Founder Mutation in the DYSF Gene Causing Clinical Variability in the Spanish Population. Arch. Neurol. 2005, 62, 1256–1259. [Google Scholar] [CrossRef]
- Paradas, C.; Llauger, J.; Diaz-Manera, J.; Rojas-García, R.; De Luna, N.; Iturriaga, C.; Márquez, C.; Usón, M.; Hankiewicz, K.; Gallardo, E.; et al. Redefining Dysferlinopathy Phenotypes Based on Clinical Findings and Muscle Imaging Studies. Neurology 2010, 75, 316–323. [Google Scholar] [CrossRef]
- Umakhanova, Z.R.; Bardakov, S.N.; Mavlikeev, M.O.; Chernova, O.N.; Magomedova, R.M.; Akhmedova, P.G.; Yakovlev, I.A.; Dalgatov, G.D.; Fedotov, V.P.; Isaev, A.A.; et al. Twenty-Year Clinical Progression of Dysferlinopathy in Patients from Dagestan. Front. Neurol. 2017, 8, 77. [Google Scholar]
- Fischer, D.; Walter, M.C.; Kesper, K.; Petersen, J.A.; Aurino, S.; Nigro, V.; Kubisch, C.; Meindl, T.; Lochmüller, H.; Wilhelm, K.; et al. Diagnostic Value of Muscle MRI in Differentiating LGMD2I from Other LGMDs. J. Neurol. 2005, 252, 538–547. [Google Scholar] [CrossRef]
- Paradas, C.; González-Quereda, L.; De Luna, N.; Gallardo, E.; García-Consuegra, I.; Gómez, H.; Cabello, A.; Illa, I.; Gallano, P. A New Phenotype of Dysferlinopathy with Congenital Onset. Neuromuscul. Disord. 2009, 19, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Vandré, D.D.; Ackerman IV, W.E.; Kniss, D.A.; Tewari, A.K.; Mori, M.; Takizawa, T.; Robinson, J.M. Dysferlin Is Expressed in Human Placenta but Does Not Associate with Caveolin. Biol. Reprod. 2007, 77, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Brown, R.H. Dysferlin in Membrane Trafficking and Patch Repair. Traffic 2007, 8, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, G.; Serafini, P.R.; Myers, J.A.; Alexander, M.S.; Kunkel, L.M. Characterization of Zebrafish Dysferlin by Morpholino Knockdown. Biochem. Biophys. Res. Commun. 2011, 413, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Harsini, F.M.; Chebrolu, S.; Fuson, K.L.; White, M.A.; Rice, A.M.; Sutton, R.B. FerA Is a Membrane-Associating Four-Helix Bundle Domain in the Ferlin Family of Membrane-Fusion Proteins. Sci. Rep. 2018, 8, 10949. [Google Scholar] [CrossRef] [PubMed]
- Harsini, F.M.; Bui, A.A.; Rice, A.M.; Chebrolu, S.; Fuson, K.L.; Turtoi, A.; Bradberry, M.; Chapman, E.R.; Sutton, R.B. Structural Basis for the Distinct Membrane Binding Activity of the Homologous C2A Domains of Myoferlin and Dysferlin. J. Mol. Biol. 2019, 431, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Sula, A.; Cole, A.R.; Yeats, C.; Orengo, C.; Keep, N.H. Crystal Structures of the Human Dysferlin Inner DysF Domain. BMC Struct. Biol. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Li, C.; Fernandez, I.; Zhang, X.; Südhof, T.C.; Rizo, J. Synaptotagmin-Syntaxin Interaction: The C2 Domain as a Ca2+-Dependent Electrostatic Switch. Neuron 1997, 18, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Padmanarayana, M.; Marty, N.J.; Johnson, C.P. Quantitation of the Calcium and Membrane Binding Properties of the C2 Domains of Dysferlin. Biophys. J. 2014, 106, 382–389. [Google Scholar] [CrossRef]
- Lek, A.; Evesson, F.J.; Lemckert, F.A.; Redpath, G.M.I.; Lueders, A.K.; Turnbull, L.; Whitchurch, C.B.; North, K.N.; Cooper, S.T. Calpains, Cleaved Mini-Dysferlinc72, and L-Type Channels Underpin Calcium-Dependent Muscle Membrane Repair. J. Neurosci. 2013, 33, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Redpath, G.M.I.; Woolger, N.; Piper, A.K.; Lemckert, F.A.; Lek, A.; Greer, P.A.; North, K.N.; Cooper, S.T. Calpain Cleavage within Dysferlin Exon 40a Releases a Synaptotagmin-like Module for Membrane Repair. Mol. Biol. Cell 2014, 25, 3037–3048. [Google Scholar] [CrossRef] [PubMed]
- Fuson, K. Alternate Splicing of Dysferlin C2A Confers Ca2+-Dependent and Ca2+-Independent Binding for Membrane Repair. Biophys. J. 2014, 106, 503a. [Google Scholar] [CrossRef]
- Patel, P.; Harris, R.; Geddes, S.M.; Strehle, E.-M.; Watson, J.D.; Bashir, R.; Bushby, K.; Driscoll, P.C.; Keep, N.H. Solution Structure of the Inner DysF Domain of Myoferlin and Implications for Limb Girdle Muscular Dystrophy Type 2B. J. Mol. Biol. 2008, 379, 981–990. [Google Scholar] [CrossRef]
- Ponting, C.P.; Mott, R.; Bork, P.; Copley, R.R. Novel Protein Domains and Repeats in Drosophila Melanogaster: Insights into Structure, Function, and Evolution. Genome Res. 2001, 11, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Muriel, J.; Lukyanenko, V.; Kwiatkowski, T.; Bhattacharya, S.; Garman, D.; Weisleder, N.; Bloch, R.J. The C2 Domains of Dysferlin: Roles in Membrane Localization, Ca2+ Signalling and Sarcolemmal Repair. J. Physiol. 2022, 600, 1953–1968. [Google Scholar] [CrossRef]
- Wang, Y.; Tadayon, R.; Santamaria, L.; Mercier, P.; Forristal, C.J.; Shaw, G.S. Calcium Binds and Rigidifies the Dysferlin C2A Domain in a Tightly Coupled Manner. Biochem. J. 2021, 478, 197–215. [Google Scholar] [CrossRef]
- Kwok, E.; Otto, S.C.; Khuu, P.; Carpenter, A.P.; Codding, S.J.; Reardon, P.N.; Vanegas, J.; Kumar, T.M.; Kuykendall, C.J.; Mehl, R.A.; et al. The Dysferlin C2A Domain Binds PI(4,5)P2 and Penetrates Membranes. J. Mol. Biol. 2023, 435, 168193. [Google Scholar] [CrossRef]
- Lek, A.; Evesson, F.J.; Sutton, R.B.; North, K.N.; Cooper, S.T. Ferlins: Regulators of Vesicle Fusion for Auditory Neurotransmission, Receptor Trafficking and Membrane Repair. Traffic 2012, 13, 185–194. [Google Scholar] [CrossRef]
- Dominguez, M.J.; McCord, J.J.; Sutton, R.B. Redefining the Architecture of Ferlin Proteins: Insights into Multi-Domain Protein Structure and Function. PLoS One 2022, 17, e0270188. [Google Scholar] [CrossRef]
- Llanga, T.; Nagy, N.; Conatser, L.; Dial, C.; Sutton, R.B.; Hirsch, M.L. Structure-Based Designed Nano-Dysferlin Significantly Improves Dysferlinopathy in BLA/J Mice. Mol. Ther. 2017, 25, 2150–2162. [Google Scholar] [CrossRef] [PubMed]
- Lostal, W.; Richard, I. Therapeutic Approaches for Dysferlinopathy in Animal Models. In Muscle Gene Therapy; Duan, D., Mendell, J.R., Eds.; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-03094-0. [Google Scholar]
- Tidball, J.G.; Welc, S.S.; Wehling-Henricks, M. Immunobiology of Inherited Muscular Dystrophies. Compr. Physiol. 2018, 8, 1313–1356. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Cohen, T. V.; Ampong, B.; Francia, D.; Henriques-Pons, A.; Hoffman, E.P.; Nagaraju, K. Inflammasome Up-Regulation and Activation in Dysferlin-Deficient Skeletal Muscle. Am. J. Pathol. 2010, 176, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Campbell, K.P. Dysferlin and Muscle Membrane Repair. Curr. Opin. Cell Biol. 2007, 19, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.R.; Cave, A.; Davis, D.B.; Delmonte, A.J.; Posey, A.; Early, J.U.; Hadhazy, M.; McNally, E.M. Normal Myoblast Fusion Requires Myoferlin. Development 2005, 132, 5565–5575. [Google Scholar] [CrossRef]
- Davis, D.B.; Delmonte, A.J.; Ly, C.T.; McNally, E.M. Myoferlin, a Candidate Gene and Potential Modifier of Muscular Dystrophy. Hum. Mol. Genet. 2000, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- de Luna, N.; Gallardo, E.; Soriano, M.; Dominguez-Perles, R.; de la Torre, C.; Rojas-García, R.; García-Verdugo, J.M.; Illa, I. Absence of Dysferlin Alters Myogenin Expression and Delays Human Muscle Differentiation “in Vitro. ” J. Biol. Chem. 2006, 281, 17092–17098. [Google Scholar] [CrossRef] [PubMed]
- Flix, B.; De La Torre, C.; Castillo, J.; Casal, C.; Illa, I.; Gallardo, E. Dysferlin Interacts with Calsequestrin-1, Myomesin-2 and Dynein in Human Skeletal Muscle. Int. J. Biochem. Cell Biol. 2013, 45, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Ampong, B.N.; Imamura, M.; Matsumiya, T.; Yoshida, M.; Takeda, S. Intracellular Localization of Dysferlin and Its Association with the Dihydropyridine Receptor. Acta Myol. 2005, 24, 134–144. [Google Scholar]
- Roche, J.A.; Ru, L.W.; O'Neill, A.M.; Resneck, W.G.; Lovering, R.M.; Bloch, R.J. Unmasking Potential Intracellular Roles For Dysferlin through Improved Immunolabeling Methods. J. Histochem. Cytochem. 2011, 59, 964–975. [Google Scholar] [CrossRef]
- Kerr, J.P.; Ward, C.W.; Bloch, R.J. Dysferlin at Transverse Tubules Regulates Ca2+ Homeostasis in Skeletal Muscle. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Hofhuis, J.; Bersch, K.; Büssenschütt, R.; Drzymalski, M.; Liebetanz, D.; Nikolaev, V.O.; Wagner, S.; Maier, L.S.; Gärtner, J.; Klinge, L.; et al. Dysferlin Mediates Membrane Tubulation and Links T-Tubule Biogenesis to Muscular Dystrophy. J. Cell Sci. 2017, 130, 841–852. [Google Scholar] [CrossRef]
- Klinge, L.; Harris, J.; Sewry, C.; Charlton, R.; Anderson, L.; Laval, S.; Chiu, Y.H.; Hornsey, M.; Straub, V.; Barresi, R.; et al. Dysferlin Associates with the Developing T-Tubule System in Rodent and Human Skeletal Muscle. Muscle and Nerve 2010, 41, 166–173. [Google Scholar] [CrossRef]
- LB, W.; FA, L.; XF, Z.; Tran, J.; FJ, E.; JM, H.; Lek, A.; NE, S.; Lin, P.; NF, C.; et al. Dysferlin, Annexin A1, and Mitsugumin 53 Are Upregulated in Muscular Dystrophy and Localize to Longitudinal Tubules of the T-System with Stretch. J. Neuropathol. Exp. Neurol. 2011, 70, 302–313. [Google Scholar]
- Demonbreun, A.R.; Rossi, A.E.; Alvarez, M.G.; Swanson, K.E.; Deveaux, H.K.; Earley, J.U.; Hadhazy, M.; Vohra, R.; Walter, G.A.; Pytel, P.; et al. Dysferlin and Myoferlin Regulate Transverse Tubule Formation and Glycerol Sensitivity. Am. J. Pathol. 2014, 184, 248–259. [Google Scholar] [CrossRef]
- Di Maio, A.; Karko, K.; Snopko, R.M.; Mejía-Alvarez, R.; Franzini-Armstrong, C. T-Tubule Formation in Cardiacmyocytes: Two Possible Mechanisms? J. Muscle Res. Cell Motil. 2007, 28, 231–241. [Google Scholar] [CrossRef]
- Nagaraju, K.; Rawat, R.; Veszelovszky, E.; Thapliyal, R.; Kesari, A.; Sparks, S.; Raben, N.; Plotz, P.; Hoffman, E.P. Dysferlin Deficiency Enhances Monocyte Phagocytosis: A Model for the Inflammatory Onset of Limb-Girdle Muscular Dystrophy 2B. Am. J. Pathol. 2008, 172, 774–785. [Google Scholar] [CrossRef]
- Rayavarapu, S.; Van der meulen, J.H.; Gordish-Dressman, H.; Hoffman, E.P.; Nagaraju, K.; Knoblach, S.M. Characterization of Dysferlin Deficient SJL/J Mice to Assess Preclinical Drug Efficacy: Fasudil Exacerbates Muscle Disease Phenotype. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Q.; Chen, T.; Niu, J.; Ban, R.; Liu, J.; Mao, Y.; Pu, C.Q. CD4+ Cells, Macrophages, MHC-I and C5b-9 Involve the Pathogenesis of Dysferlinopathy. Int. J. Clin. Exp. Pathol. 2015, 8, 3069–3075. [Google Scholar]
- Wenzel, K.; Zabojszcza, J.; Carl, M.; Taubert, S.; Lass, A.; Harris, C.L.; Ho, M.; Schulz, H.; Hummel, O.; Hubner, N.; et al. Increased Susceptibility to Complement Attack Due to Down-Regulation of Decay-Accelerating Factor/CD55 in Dysferlin-Deficient Muscular Dystrophy. J. Immunol. 2005, 175, 6219–6225. [Google Scholar] [CrossRef]
- Han, R.; Frett, E.M.; Levy, J.R.; Rader, E.P.; Lueck, J.D.; Bansal, D.; Moore, S.A.; Ng, R.; De Bernabé, D.B.V.; Faulkner, J.A.; et al. Genetic Ablation of Complement C3 Attenuates Muscle Pathology in Dysferlin-Deficient Mice. J. Clin. Invest. 2010, 120, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Henning, A.; Han, R. Dysferlin-Deficient Muscular Dystrophy and Innate Immune Activation. FEBS J. 2013, 280, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Radley-Crabb, H.G.; Iwasaki, T.; Lemckert, F.A.; Arthur, P.G.; Grounds, M.D. Oxidative Stress and Pathology in Muscular Dystrophies: Focus on Protein Thiol Oxidation and Dysferlinopathies. FEBS J. 2013, 280, 4149–4164. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, D.; Senguttuvan, S.; Alexander, M.; Oommen, A. Involvement of Oxidative Stress, Nuclear Factor Kappa B and the Ubiquitin Proteasomal Pathway in Dysferlinopathy. Life Sci. 2014, 108, 54–61. [Google Scholar] [CrossRef] [PubMed]
- De Luna, N.; Gallardo, E.; Illa, I. In Vivo and in Vitro Dysferlin Expression in Human Muscle Satellite Cells. J. Neuropathol. Exp. Neurol. 2004, 63, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Starostina, I.G.; Solovyeva, V. V.; Yuryeva, K.S.; Shevchenko, K.G.; Fedotov, V.P.; Rizvanov, A.A.; Deev, R. V.; Isaev, A.A. Modeling and Gene Therapy of Dysferlinopathy. Cell. Transplant. Tissue Eng. 2013, 8, 61–70. [Google Scholar]
- Chiu, Y.H.; Hornsey, M.A.; Klinge, L.; Jørgensen, L.H.; Laval, S.H.; Charlton, R.; Barresi, R.; Straub, V.; Lochmüller, H.; Bushby, K. Attenuated Muscle Regeneration Is a Key Factor in Dysferlin-Deficient Muscular Dystrophy. Hum. Mol. Genet. 2009, 18, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Krajacic, P.; Pistilli, E.E.; Tanis, J.E.; Khurana, T.S.; Lamitina, S.T. FER-1/Dysferlin Promotes Cholinergic Signaling at the Neuromuscular Junction in C. Elegans and Mice. Biol. Open 2013, 2, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Redpath, G.M.; Sophocleous, R.A.; Turnbull, L.; Whitchurch, C.B.; Cooper, S.T. Ferlins Show Tissue-Specific Expression and Segregate as Plasma Membrane/Late Endosomal or Trans-Golgi/Recycling Ferlins. Traffic 2016, 17, 245–266. [Google Scholar] [CrossRef]
- Gallardo, E.; de Luna, N.; Diaz-Manera, J.; Rojas-García, R.; Gonzalez-Quereda, L.; Flix, B.; de Morrée, A.; van der Maarel, S.; Illa, I. Comparison of Dysferlin Expression in Human Skeletal Muscle with That in Monocytes for the Diagnosis of Dysferlin Myopathy. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Goswamia, D.; Tannetta, D.S.; Magee, L.A.; Fuchisawa, A.; Redman, C.W.G.; Sargent, I.L.; von Dadelszen, P. Excess Syncytiotrophoblast Microparticle Shedding Is a Feature of Early-Onset Pre-Eclampsia, but Not Normotensive Intrauterine Growth Restriction. Placenta 2006, 27, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.P.; Moll, S.J.; Jones, C.J.P.; Baker, P.N.; Crocker, I.P. Formation of Syncytial Knots Is Increased by Hyperoxia, Hypoxia and Reactive Oxygen Species. Placenta 2007, 28. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.T.; Markham, K.B.; Behrendt, N.J.; Suarez, A.A.; Samuels, P.; Vandre, D.D.; Robinson, J.M.; Ackerman IV, W.E. Placental Dysferlin Expression Is Reduced in Severe Preeclampsia. Placenta 2009, 30, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Vandré, D.D.; Ackerman IV, W.E. Placental Proteomics: A Shortcut to Biological Insight. Placenta 2009, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Aoki, M.; Hayashi, Y.K.; Ho, M.F.; Arahata, K.; Brown, R.H. Dysferlin Is a Surface Membrane-Associated Protein That Is Absent in Miyoshi Myopathy. Neurology 1999, 53, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Cacciottolo, M.; Belcastro, V.; Laval, S.; Bushby, K.; Di Bernardo, D.; Nigro, V. Reverse Engineering Gene Network Identifies New Dysferlin-Interacting Proteins. J. Biol. Chem. 2011, 286, 5404–5413. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Deviez, D.J.; Martin, S.; Laval, S.H.; Lo, H.P.; Cooper, S.T.; North, K.N.; Bushby, K.; Parton, R.G. Aberrant Dysferlin Trafficking in Cells Lacking Caveolin or Expressing Dystrophy Mutants of Caveolin-3. Hum. Mol. Genet. 2006, 15, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, R.; Magri, F.; Toscano, A.; Merlini, L.; Fortunato, F.; Lamperti, C.; Rodolico, C.; Prelle, A.; Sironi, M.; Aguennouz, M.; et al. Mutation Finding in Patients with Dysferlin Deficiency and Role of the Dysferlin Interacting Proteins Annexin A1 and A2 in Muscular Dystrophies. Hum. Mutat. 2005, 26, 283. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Kameyama, K.; Tagawa, K.; Ogawa, M.; Suzuki, A.; Yamaji, S.; Okamoto, H.; Nishino, I.; Hayashi, Y.K. Dysferlin Interacts with Affixin (β-Parvin) at the Sarcolemma. J. Neuropathol. Exp. Neurol. 2005, 64, 334–340. [Google Scholar] [CrossRef]
- Anderson, L.V.B.; Harrison, R.M.; Pogue, R.; Vafiadaki, E.; Pollitt, C.; Davison, K.; Moss, J.A.; Keers, S.; Pyle, A.; Shaw, P.J.; et al. Secondary Reduction in Calpain 3 Expression in Patients with Limb Girdle Muscular Dystrophy Type 2B and Miyoshi Myopathy (Primary Dysferlinopathies). Neuromuscul. Disord. 2000, 10, 553–559. [Google Scholar] [CrossRef]
- Huang, Y.; de Morrée, A.; van Remoortere, A.; Bushby, K.; Frants, R.R.; Dunnen, J.T.; van der Maarel, S.M. Calpain 3 Is a Modulator of the Dysferlin Protein Complex in Skeletal Muscle. Hum. Mol. Genet. 2008, 17, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Capanni, C.; Sabatelli, P.; Mattioli, E.; Ognibene, A.; Columbaro, M.; Lattanzi, G.; Merlini, L.; Minetti, C.; Maraldi, N.M.; Squarzoni, S. Dysferlin in a HyperCKaemic Patient with Caveolin 3 Mutation and in C2C12 Cells after P38 MAP Kinase Inhibition. Exp. Mol. Med. 2003, 35, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.C.; Braun, C.; Vorgerd, M.; Poppe, M.; Thirion, C.; Schmidt, C.; Schreiber, H.; Knirsch, U.I.; Brummer, D.; Müller-Felber, W.; et al. Variable Reduction of Caveolin-3 in Patients with LGMD2B/MM. J. Neurol. 2003, 250, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Azakir, B.A.; Fulvio, S. Di; Therrien, C.; Sinnreich, M. Dysferlin Interacts with Tubulin and Microtubules in Mouse Skeletal Muscle. PLoS One 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Lankes, W.T.; Furthmayr, H. Moesin: A Member of the Protein 4.1-Talin-Ezrin Family of Proteins. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 8297–8301. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Tsukita, S.; Tsukita, S. Direct Involvement of Ezrin/Radixin/Moesin (ERM)-Binding Membrane Proteins in the Organization of Microvilli in Collaboration with Activated ERM Proteins. J. Cell Biol. 1999, 145, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Vernengo, L.; Carrasco, L.; Angelini, C.; Rodriguez, M. Dysferlinopathies. J. Genet. Syndr. Gene Ther. 2013, 4, 134. [Google Scholar] [CrossRef]
- Philippi, S.; Bigot, A.; Marg, A.; Mouly, V.; Spuler, S.; Zacharias, U. Dysferlin-Deficient Immortalized Human Myoblasts and Myotubes as a Useful Tool to Study Dysferlinopathy. PLoS Curr. 2012, 4, RRN1298. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Kiyosue, K.; Nishino, I.; Goto, Y.; Hayashi, Y.K. Dysferlinopathy Fibroblasts Are Defective in Plasma Membrane Repair. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef]
- Wein, N.; Avril, A.; Bartoli, M.; Beley, C.; Chaouch, S.; Laforêt, P.; Behin, A.; Butler-Browne, G.; Mouly, V.; Krahn, M.; et al. Efficient Bypass of Mutations in Dysferlin Deficient Patient Cells by Antisense-Induced Exon Skipping. Hum. Mutat. 2010, 31, 136–142. [Google Scholar] [CrossRef]
- Lostal, W.; Bartoli, M.; Roudaut, C.; Bourg, N.; Krahn, M.; Pryadkina, M.; Borel, P.; Suel, L.; Roche, J.A.; Stockholm, D.; et al. Lack of Correlation between Outcomes of Membrane Repair Assay and Correction of Dystrophic Changes in Experimental Therapeutic Strategy in Dysferlinopathy. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Towler, M.C.; Kaufman, S.J.; Brodsky, F.M. Membrane Traffic in Skeletal Muscle. Traffic 2004, 5, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Schindler, T.; Muller, B.; Porter, J.D.; Ruegg, M.A.; Langen, H. Identification of Proteins Interacting with Dysferlin Using the Tandem Affinity Purification Method. Open Cell Dev. Biol. J. 2008, 1, 17–23. [Google Scholar] [CrossRef]
- Han, R. Muscle Membrane Repair and Inflammatory Attack in Dysferlinopathy. Skelet. Muscle 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Suzuki, N.; Ishiguro, H.; Hirota, K.; Itoyama, Y.; Takahashi, T.; Aoki, M. Distal Anterior Compartment Myopathy with Early Ankle Contractures. Muscle and Nerve 2007, 36, 525–527. [Google Scholar] [CrossRef]
- Argov, Z.; Sadeh, M.; Mazor, K.; Soffer, D.; Kahana, E.; Eisenberg, I.; Mitrani-Rosenbaum, S.; Richard, I.; Beckmann, J.; Keers, S.; et al. Muscular Dystrophy Due to Dysferlin Deficiency in Libyan Jews. Clinical and Genetic Features. Brain 2000, 123, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Aoki, M.; Tateyama, M.; Kondo, E.; Mizuno, T.; Onodera, Y.; Takano, R.; Kawai, H.; Kamakura, K.; Mochizuki, H.; et al. Dysferlin Mutations in Japanese Miyoshi Myopathy: Relationship to Phenotype. Neurology 2003, 60, 1799–1804. [Google Scholar] [CrossRef]
- Weiler, T.; Bashir, R.; Anderson, L.V.B.; Davison, K.; Moss, J.A.; Britton, S.; Nylen, E.; Keers, S.; Vafiadaki, E.; Greenberg, C.R.; et al. Identical Mutation in Patients with Limb Girdle Muscular Dystrophy Type 2B or Miyoshi Myopathy Suggests a Role for Modifier Gene(S). Hum. Mol. Genet. 1999, 8, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Leshinsky-Silver, E.; Argov, Z.; Rozenboim, L.; Cohen, S.; Tzofi, Z.; Cohen, Y.; Wirguin, Y.; Dabby, R.; Lev, D.; Sadeh, M. Dysferlinopathy in the Jews of the Caucasus: A Frequent Mutation in the Dysferlin Gene. Neuromuscul. Disord. 2007, 17, 950–954. [Google Scholar] [CrossRef]
- Cagliani, R.; Fortunato, F.; Giorda, R.; Rodolico, C.; Bonaglia, M.C.; Sironi, M.; D’Angelo, M.G.; Prelle, A.; Locatelli, F.; Toscano, A.; et al. Molecular Analysis of LGMD-2B and MM Patients: Identification of Novel DYSF Mutations and Possible Founder Effect in the Italian Population. Neuromuscul. Disord. 2003, 13, 788–795. [Google Scholar] [CrossRef]
- Charnay, T.; Blanck, V.; Cerino, M.; Bartoli, M.; Riccardi, F.; Bonello-Palot, N.; Pécheux, C.; Nguyen, K.; Lévy, N.; Gorokhova, S.; et al. Retrospective Analysis and Reclassification of DYSF Variants in a Large French Series of Dysferlinopathy Patients. Genet. Med. 2021, 23, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Hong, Y. Bin; Hong, J.M.; Yun, U.K.; Kim, S.W.; Shin, H.Y.; Kim, S.M.; Choi, Y.C. Null Variants in DYSF Result in Earlier Symptom Onset. Clin. Genet. 2021, 99, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sinnreich, M.; Therrien, C.; Karpati, G. Lariat Branch Point Mutation in the Dysferlin Gene with Mild Limb-Girdle Muscular Dystrophy. Neurology 2006, 66, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, K.; Carl, M.; Perrot, A.; Zabojszcza, J.; Assadi, M.; Ebeling, M.; Geier, C.; Robinson, P.N.; Kress, W.; Osterziel, K.J.; et al. Novel Sequence Variants in Dysferlin-Deficient Muscular Dystrophy Leading to MRNA Decay and Possible C2-Domain Misfolding. Hum. Mutat. 2006, 27, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Kouroku, Y.; Isoai, A.; Kumagai, H.; Misutani, A.; Matsuda, C.; Hayashi, Y.K.; Momoi, T. Two Endoplasmic Reticulum-Associated Degradation (ERAD) Systems for the Novel Variant of the Mutant Dysferlin: Ubiquitin/Proteasome ERAD(I) and Autophagy/Lysosome ERAD(II). Hum. Mol. Genet. 2007, 16, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.; Ravindran, A.; Casarotto, M.G. AHNAK: The Quiet Giant in Calcium Homeostasis. Cell Calcium 2021, 96. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Nonneman, R.J.; Llanga, T.; Dial, C.F.; Riddick, N. V.; Hampton, T.; Moy, S.S.; Lehtimäki, K.K.; Ahtoniemi, T.; Puoliväli, J.; et al. Hip Region Muscular Dystrophy and Emergence of Motor Deficits in Dysferlin-deficient Bla/J Mice. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Lostal, W.; Bartoli, M.; Bourg, N.; Roudaut, C.; Bentaïb, A.; Miyake, K.; Guerchet, N.; Fougerousse, F.; McNeil, P.; Richard, I. Efficient Recovery of Dysferlin Deficiency by Dual Adeno-Associated Vector-Mediated Gene Transfer. Hum. Mol. Genet. 2010, 19, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.C.; Wicker, L.S.; Urba, W.J. Genetic Control of Susceptibility to Cryptococcus Neoformans in Mice. Infect. Immun. 1980, 29, 494–499. [Google Scholar] [CrossRef]
- Ho, M.; Post, C.M.; Donahue, L.R.; Lidov, H.G.W.; Bronson, R.T.; Goolsby, H.; Watkins, S.C.; Cox, G.A.; Brown, R.H. Disruption of Muscle Membrane and Phenotype Divergence in Two Novel Mouse Models of Dysferlin Deficiency. Hum. Mol. Genet. 2004, 13, 1999–2010. [Google Scholar] [CrossRef]
- Weller, A.H.; Magliato, S.A.; Bell, K.P.; Rosenberg, N.L. Spontaneous Myopathy in the SJL/J Mouse: Pathology and Strength Loss. Muscle and Nerve 1997, 20, 72–82. [Google Scholar] [CrossRef]
- Bittner, R.E.; Anderson, L.V.B.; Burkhardt, E.; Bashir, R.; Vafiadaki, E.; Ivanova, S.; Raffelsberger, T.; Maerk, I.; Höger, H.; Jung, M.; et al. Dysferlin Deletion in SJL Mice (SJL-Dysf) Defines a Natural Model for Limb Girdle Muscular Dystrophy 2B [2]. Nat. Genet. 1999, 23, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Malcher, J.; Heidt, L.; Goyenvalle, A.; Escobar, H.; Marg, A.; Beley, C.; Benchaouir, R.; Bader, M.; Spuler, S.; García, L.; et al. Exon Skipping in a Dysf-Missense Mutant Mouse Model. Mol. Ther. - Nucleic Acids 2018, 13, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Heidt, L.; Bader, M.; Spuler, S.; Schoewel, V. Dysferlinopathy Caused by Protein Misfolding: The Novel Murine Animal Model Dysf-MMex38. Neuromuscul. Disord. 2014, 24, 902–903. [Google Scholar] [CrossRef]
- Ballouhey, O.; Chapoton, M.; Alary, B.; Courrier, S.; Da Silva, N. Da; Krahn, M.; Lévy, N.; Weisleder, N.; Bartoli, M. A Dysferlin Exon 32 Nonsense Mutant Mouse Model Shows Pathological Signs of Dysferlinopathy. Biomedicines 2023, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Straub, V.; Bushby, K. Therapeutic Possibilities in the Autosomal Recessive Limb-Girdle Muscular Dystrophies. Neurotherapeutics 2008, 5, 619–626. [Google Scholar] [CrossRef] [PubMed]
- White, Z.; Theret, M.; Milad, N.; Tung, L.W.; Chen, W.W.H.; Sirois, M.G.; Rossi, F.; Bernatchez, P. Cholesterol Absorption Blocker Ezetimibe Prevents Muscle Wasting in Severe Dysferlin-Deficient and Mdx Mice. J. Cachexia. Sarcopenia Muscle 2022, 13, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Rao, D.; Pachman, L.M. Clarifying the Boundaries between the Inflammatory and Dystrophic Myopathies: Insights from Molecular Diagnostics and Microarrays. Rheum. Dis. Clin. North Am. 2002, 28, 743–757. [Google Scholar] [CrossRef]
- Walter, M.C.; Reilich, P.; Thiele, S.; Schessl, J.; Schreiber, H.; Reiners, K.; Kress, W.; Müller-Reible, C.; Vorgerd, M.; Urban, P.; et al. Treatment of Dysferlinopathy with Deflazacort: A Double-Blind, Placebo-Controlled Clinical Trial. Orphanet J. Rare Dis. 2013, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- De Luna, N.; Gallardo, E.; Sonnet, C.; Chazaud, B.; Dominguez-Perles, R.; Suarez-Calvet, X.; Gherardi, R.K.; Illa, I. Role of Thrombospondin 1 in Macrophage Inflammation in Dysferlin Myopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 643–653. [Google Scholar] [CrossRef]
- Fernández-Simón, E.; Lleixà, C.; Suarez-Calvet, X.; Diaz-Manera, J.; Illa, I.; Gallardo, E.; de Luna, N. Proteasome Inhibitors Reduce Thrombospondin-1 Release in Human Dysferlin-Deficient Myotubes. BMC Musculoskelet. Disord. 2020, 21, 784. [Google Scholar] [CrossRef] [PubMed]
- Van Ry, P.M.; Wuebbles, R.D.; Key, M.; Burkin, D.J. Galectin-1 Protein Therapy Prevents Pathology and Improves Muscle Function in the Mdx Mouse Model of Duchenne Muscular Dystrophy. Mol. Ther. 2015, 23, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo-Zúniga, M.L.; Rathgeber, M.F.; Poulson, P.D.; Hayes, S.; Luddington, J.S.; Gill, H.N.; Teynor, M.; Kartchner, B.C.; Valdoz, J.; Stowell, C.; et al. Treatment with Galectin-1 Improves Myogenic Potential and Membrane Repair in Dysferlin-Deficient Models. PLoS One 2020, 15, e0238441. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Fernández, G.; Arias-Bravo, G.; Castillo-Ruiz, M.; Escamilla, R.; Brañes, M.C.; Sáez, J.C. Blockade of Hemichannels Normalizes the Differentiation Fate of Myoblasts and Features of Skeletal Muscles from Dysferlin-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 6025. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The Antioxidant Action of N-Acetylcysteine: Its Reaction with Hydrogen Peroxide, Hydroxyl Radical, Superoxide, and Hypochlorous Acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]
- García-Campos, P.; Báez-Matus, X.; Jara-Gutiérrez, C.; Paz-Araos, M.; Astorga, C.; Cea, L.A.; Rodríguez, V.; Bevilacqua, J.A.; Caviedes, P.; Cárdenas, A.M. N-Acetylcysteine Reduces Skeletal Muscles Oxidative Stress and Improves Grip Strength in Dysferlin-Deficient Bla/J Mice. Int. J. Mol. Sci. 2020, 21, 4293. [Google Scholar] [CrossRef] [PubMed]
- Begam, M.; Collier, A.F.; Mueller, A.L.; Roche, R.; Galen, S.S.; Roche, J.A. Diltiazem Improves Contractile Properties of Skeletal Muscle in Dysferlin-Deficient BLAJ Mice, but Does Not Reduce Contraction-Induced Muscle Damage. Physiol. Rep. 2018, 6, e13727. [Google Scholar] [CrossRef] [PubMed]
- Barzilai-Tutsch, H.; Dewulf, M.; Lamaze, C.; Butler Browne, G.; Pines, M.; Halevy, O. A Promotive Effect for Halofuginone on Membrane Repair and Synaptotagmin-7 Levels in Muscle Cells of Dysferlin-Null Mice. Hum. Mol. Genet. 2018, 27, 2817–2829. [Google Scholar] [CrossRef]
- Pines, M.; Spector, I. Halofuginone - The Multifaceted Molecule. Molecules 2015, 20, 573–594. [Google Scholar] [CrossRef]
- Azakir, B.A.; Di Fulvio, S.; Salomons, S.; Brockhoff, M.; Therrien, C.; Sinnreich, M. Modular Dispensability of Dysferlin C2 Domains Reveals Rational Design for Mini-Dysferlin Molecules. J. Biol. Chem. 2012, 287, 27629–27636. [Google Scholar] [CrossRef]
- Pryadkina, M.; Lostal, W.; Bourg, N.; Charton, K.; Roudaut, C.; Hirsch, M.L.; Richard, I. A Comparison of AAV Strategies Distinguishes Overlapping Vectors for Efficient Systemic Delivery of the 6.2 Kb Dysferlin Coding Sequence. Mol. Ther. - Methods Clin. Dev. 2015, 2, 15009. [Google Scholar] [CrossRef]
- Potter, R.A.; Griffin, D.A.; Sondergaard, P.C.; Johnson, R.W.; Pozsgai, E.R.; Heller, K.N.; Peterson, E.L.; Lehtimäki, K.K.; Windish, H.P.; Mittal, P.J.; et al. Systemic Delivery of Dysferlin Overlap Vectors Provides Long-Term Gene Expression and Functional Improvement for Dysferlinopathy. Hum. Gene Ther. 2018, 29, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pichavant, C.; du Bois, H.; Bhakta, M.; Calos, M.P. DNA-Mediated Gene Therapy in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Mol. Ther. - Methods Clin. Dev. 2017, 7, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bittel, D.C.; Sreetama, S.C.; Chandra, G.; Ziegler, R.; Nagaraju, K.; Van der Meulen, J.H.; Jaiswal, J.K. Secreted Acid Sphingomyelinase as a Potential Gene Therapy for Limb Girdle Muscular Dystrophy 2B. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef]
- Porto, E.M.; Komor, A.C.; Slaymaker, I.M.; Yeo, G.W. Base Editing: Advances and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2020, 19, 839–859. [Google Scholar] [CrossRef] [PubMed]
- Godbout, K.; Tremblay, J.P. Prime Editing for Human Gene Therapy: Where Are We Now? Cells 2023, 12. [Google Scholar] [CrossRef]
- Anwar, S.; Mir, F.; Yokota, T. Enhancing the Effectiveness of Oligonucleotide Therapeutics Using Cell-Penetrating Peptide Conjugation, Chemical Modification, and Carrier-Based Delivery Strategies. Pharmaceutics 2023, 15, 1130. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; van Ommen, G.-J.B. Antisense-Mediated Exon Skipping: A Versatile Tool with Therapeutic and Research Applications. RNA 2007, 13, 1609–1624. [Google Scholar] [CrossRef]
- Wilton-Clark, H.; Yokota, T. Recent Trends in Antisense Therapies for Duchenne Muscular Dystrophy. Pharmaceutics 2023, 15, 778. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Singh, K.H.K.; Fokkema, I.F.A.C.; Ginjaar, I.B.; van Ommen, G.-J.; Dunnen, J.T. den; van der Maarel, S.M. Therapeutic Exon Skipping for Dysferlinopathies? Eur. J. Hum. Genet. 2010, 18, 889–894. [Google Scholar] [CrossRef]
- Anwar, S.; He, M.; Lim, K.R.Q.; Maruyama, R.; Yokota, T. A Genotype-Phenotype Correlation Study of Exon Skip-Equivalent In-Frame Deletions and Exon Skip-Amenable Out-of-Frame Deletions across the DMD Gene to Simulate the Effects of Exon-Skipping Therapies: A Meta-Analysis. J. Pers. Med. 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Yokota, T. Golodirsen for Duchenne Muscular Dystrophy. Drugs Today (Barc). 2020, 56, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.; Maruyama, R.; Yokota, T. Eteplirsen in the Treatment of Duchenne Muscular Dystrophy. Drug Des. Devel. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020. [Google Scholar] [CrossRef] [PubMed]
- Wilton-Clark, H.; Yokota, T. Casimersen for Duchenne Muscular Dystrophy. Drugs of Today 2021, 57, 707. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, F.; Blouin, C.; Wein, N.; Mouly, V.; Courrier, S.; Dionnet, E.; Kergourlay, V.; Mathieu, Y.; Garcia, L.; Butler-Browne, G.; et al. Exon 32 Skipping of Dysferlin Rescues Membrane Repair in Patients’ Cells. J. Neuromuscul. Dis. 2015, 2, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.A.; Maruyama, R.; Duddy, W.; Sakurai, H.; Yokota, T. Identification of Novel Antisense-Mediated Exon Skipping Targets in DYSF for Therapeutic Treatment of Dysferlinopathy. Mol. Ther. - Nucleic Acids 2018, 13, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Gushchina, L. V.; Bhattacharya, S.; McElhanon, K.E.; Choi, J.H.; Manring, H.; Beck, E.X.; Alloush, J.; Weisleder, N. Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Mol. Ther. 2017, 25, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, Y.; Nagino, T.; Sasa, K.; Oikawa, T.; Miyake, K.; Kume, A.; Fukuda, M.; Fuse, H.; Tozawa, R.; Sakurai, H. Phenotypic Drug Screening for Dysferlinopathy Using Patient-Derived Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2019, 8, 1017–1029. [Google Scholar] [CrossRef]
- Leriche-Guérin, K.; Roy, B.; Goulet, M.; Tremblay, J.P.; Anderson, L.V.B.; Wrogemann, K.; Wrogemann, K. Dysferlin Expression after Normal Myoblast Transplantation in SCID and in SJL Mice. Neuromuscul. Disord. 2002, 12, 167–173. [Google Scholar] [CrossRef]
- Escobar, H.; Schöwel, V.; Spuler, S.; Marg, A.; Izsvák, Z. Full-Length Dysferlin Transfer by the Hyperactive Sleeping Beauty Transposase Restores Dysferlin-Deficient Muscle. Mol. Ther. - Nucleic Acids 2016, 5, e277. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The Ketogenic Diet Preserves Skeletal Muscle with Aging in Mice. Aging Cell 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Suzuki, N.; Kanno, S.; Kawahara, G.; Izumi, R.; Takahashi, T.; Kitajima, Y.; Osana, S.; Nakamura, N.; Akiyama, T.; et al. AMPK Complex Activation Promotes Sarcolemmal Repair in Dysferlinopathy. Mol. Ther. 2020, 28, 1133–1153. [Google Scholar] [CrossRef] [PubMed]
- Azakir, B.A.; Di Fulvio, S.; Kinter, J.; Sinnreich, M. Proteasomal Inhibition Restores Biological Function of Mis-Sense Mutated Dysferlin in Patient-Derived Muscle Cells. J. Biol. Chem. 2012, 287, 10344–10354. [Google Scholar] [CrossRef] [PubMed]
- Azakir, B.A.; Erne, B.; Di Fulvio, S.; Stirnimann, G.; Sinnreich, M. Proteasome Inhibitors Increase Missense Mutated Dysferlin in Patients with Muscular Dystrophy. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, P.C.; Griffin, D.A.; Pozsgai, E.R.; Johnson, R.W.; Grose, W.E.; Heller, K.N.; Shontz, K.M.; Montgomery, C.L.; Liu, J.; Clark, K.R.; et al. AAV.Dysferlin Overlap Vectors Restore Function in Dysferlinopathy Animal Models. Ann. Clin. Transl. Neurol. 2015, 2, 256–270. [Google Scholar] [CrossRef]
- Grose, W.E.; Clark, K.R.; Griffin, D.; Malik, V.; Shontz, K.M.; Montgomery, C.L.; Lewis, S.; Brown, R.H.; Janssen, P.M.L.; Mendell, J.R.; et al. Homologous Recombination Mediates Functional Recovery of Dysferlin Deficiency Following AAV5 Gene Transfer. PLoS One 2012, 7, e39233. [Google Scholar] [CrossRef]
| Mutation | Associated phenotype | Impact on protein | Pathobiological implications |
|---|---|---|---|
| c. 573-574TG>AT (p. Val67Asp) |
MM, proximo-distal, LGMDR2 | Affects calcium-binding | Altered muscle contraction |
| c.1867C>T (p.Gln623Ter) |
MM | Nonsense mutation causing premature truncation | Diverse clinical manifestations |
| c.2372C>G (p.Pro791Arg) |
LGMDR2, mild distal myopathy (similar to MM), asymmetric hypertrophy, mild proximal muscle weakness | Unclear; probable protein instability and/or malfunctionality | Diverse clinical manifestations; founder effect observed in a Canadian Aboriginal population |
| c. 1566C>G (p.Tyr522X) |
LGMDR2, MM (MM is more common) | Leads to mRNA instability | Earlier disease onset |
| c.2997G>T (p. Trp999Cys) |
LGMDR2, MM (LGMDR2 is more common) | Unclear; probable protein instability and/or malfunctionality | Late onset and a milder course of the disease |
| c. 3373del (p.Glu1125LysfsX9) |
MM, LGMDR2 (sporadic) | Unclear; probable protein instability and/or malfunctionality | Diverse clinical manifestations |
| c.3946A>G (p.Ile1316Val) |
MM< LGMDR2, DMAT | Unclear; probable protein instability and/or malfunc-tionality | Diverse clinical manifestations |
| c. 6135G>A (p.Trp2045X) |
MM | Unclear; probable protein instability and/or malfunctionality | Diverse clinical manifestations |
| c.1234G>T (p.Glu412X) |
LGMDR2 | Loss of protein function | Severe muscle wasting |
| c.1609G>A (p.Gly537Arg) |
MM | Unclear; probable protein instability and/or malfunc-tionality | May result in late onset milder manifestation |
| c.1927G>T (p.Asp643Tyr) |
LGMDR2 | Unclear; probable nonfunc-tional protein | Associated with late onset, progressive fatigue, increased serum CK levels, and fatty infiltrations in the lower limb muscles |
| c.3497-33A>G | LGMDR2 | Intronic mutation resulting in the in-frame large deletion of exon 32, resulting in a significantly reduced production of the protein | May be associated with a milder manifestation |
| c.4567del (p.Ser1523ValfsX9) |
LGMDR2, MM | Premature truncation | Muscle degeneration |
| c.7890C>A (p.Tyr2630X) |
LGMDR2 | Loss of protein function | Muscle atrophy and weakness |
| c. 2345C>T (p.Arg785X) |
MM | Leads to mRNA instability | Muscle weakness |
| c. 9876G>A (p.Trp3286X) |
LGMDR2 | Loss of protein function | Severe muscle degeneration |
| c.IVS12+7delAGTGCGTG (c.1180+7delAGTGCGTG) |
MM, proximo-distal phenotype, LGMDR2 | Intronic mutation resulting in abnormal splicing | Diverse clinical manifestations; founder effect observed in a Portuguese population |
| c.2779delG (p.Ala927LeufsX21) |
MM, proximo-distal phenotype, LGMDR2, asymptomatic hyperCKemia, congenital phenotype | Unclear; frameshift mutation possibly leading to a truncated, likely nonfunctional protein | Diverse clinical manifestations; founder effect observed in Caucasian Jewish population |
| c.2875C>T (p.Arg959Trp) |
May result in a milder phenotype | Unclear; probable protein instability and/or malfunctionality | Diverse clinical manifestations; founder effect observed in an Italian population |
| c.3191G>A (p.Arg1064His) |
MM | Unclear; probable protein instability and/or malfunc-tionality | Associated with early onset and significantly higher CK levels |
| c.4989_4993delinsCCCC (p.Glu1663fs) |
May result in severer phenotypes | Complex mutation involving deletion and insertion, leading to a frameshift and truncated protein | Likely associated with severe manifestation of the disease; founder effect observed in Lebanese Jewish population |
| c.5156_5174+4dup 23-bp ins |
LGMDR2 | Tandem duplication resulting from replication slippage, and was predicted to result in frameshift and premature termination | Diverse clinical manifestations |
| c.5174+5G>A | LGMDR2 | Intronic mutation resulting in abnormal splicing | Associated with elevated CK levels, presence of inflammatory process in histopathology |
| c.5492G>A (p.Gly1831Arg) |
LGMDR2, MM, extreme hypertrophy, asymptomatic hyperCKemia, cardiac arrhythmia | Unclear; possible altered mRNA splicing | Diverse clinical manifestations; founder effect observed in a Portuguese population |
| c.5713C>T (p.Arg1905X) |
MM, LGMDR2, DMAT, proximo-distal phenotype | Nonsense mutation leading to premature protein termination | Likely associated with severe phenotypes; founder effect observed in a Spanish population |
| c.6241C>T (p.Arg2081Cys) | MM, LGMDR2 | Unclear; probable protein instability and/or malfunc-tionality | Diverse clinical manifestations |
| Strain | Background | Genetic makeup | Clinico-pathological manifestation |
|---|---|---|---|
| BLA/J (B6.A-Dysfprmd/GeneJ) |
C57BL/6J | Spontaneous ETn retrotransposon insertion in intron 4; no dysferlin expression | Dystrophic features by 4-5 months; loss of muscle mass, lipid deposition by 8 months; slow progression; limb girdle, psoas, quad most affected. |
| A/J | Inbred A/J | Spontaneous ETn retrotransposon insertion in intron 4 resulting no expression of dysferlin protein | Similar to BLA/J, but initial proximal bias; rapid abdominal muscle wasting; mild cardiomyopathy at ~10 months; hearing loss; lung adenomas; C5 deficiency; susceptibility to infections. |
| SJL/J | Wild-derived Swiss mice | Splice site mutation in exon 45; ~15% normal dysferlin expression | Dystrophic features by 2-4 months; pronounced histopathology by 6-8 months; enhanced inflammation; faster progression; aggression; high lymphoma incidence; susceptibility to autoimmune diseases and infections. |
| 129-Dysftm1Kcam/J B6-Dysftm1Kcam/J |
129 C57BL/6J |
Neomycin resistance gene replacement causing deletion of the last three coding exons or transmembrane domain | Dystrophic features by 2 months; pronounced histopathology by 8 months; psoas most affected; mild cardiomyopathy with fibrosis from 12-14 months, worsens with cardiac stress exercise |
| MMex38 | 129 | Introduction of the missense c.4079T > C mutation in exon 38 of murine Dysf; this mutation is analogous to a clinically relevant mutation (p.Leu1341Pro ) in human | Dystrophic features by 12 weeks of age; exhibits a progressive dystrophic pattern, amyloid formation, and defects in membrane repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





