Submitted:
09 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
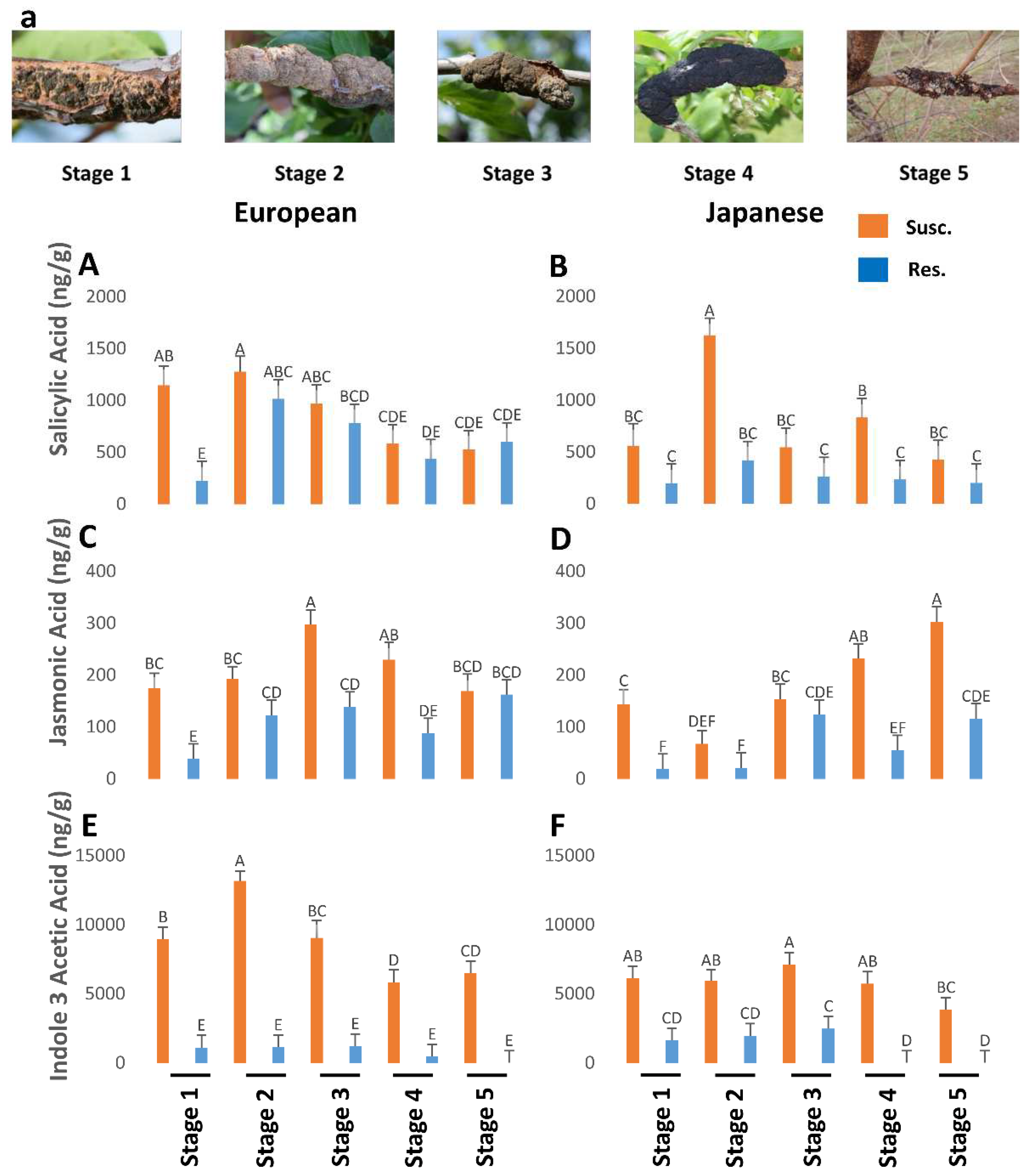
2. Results
Salicylic Acid
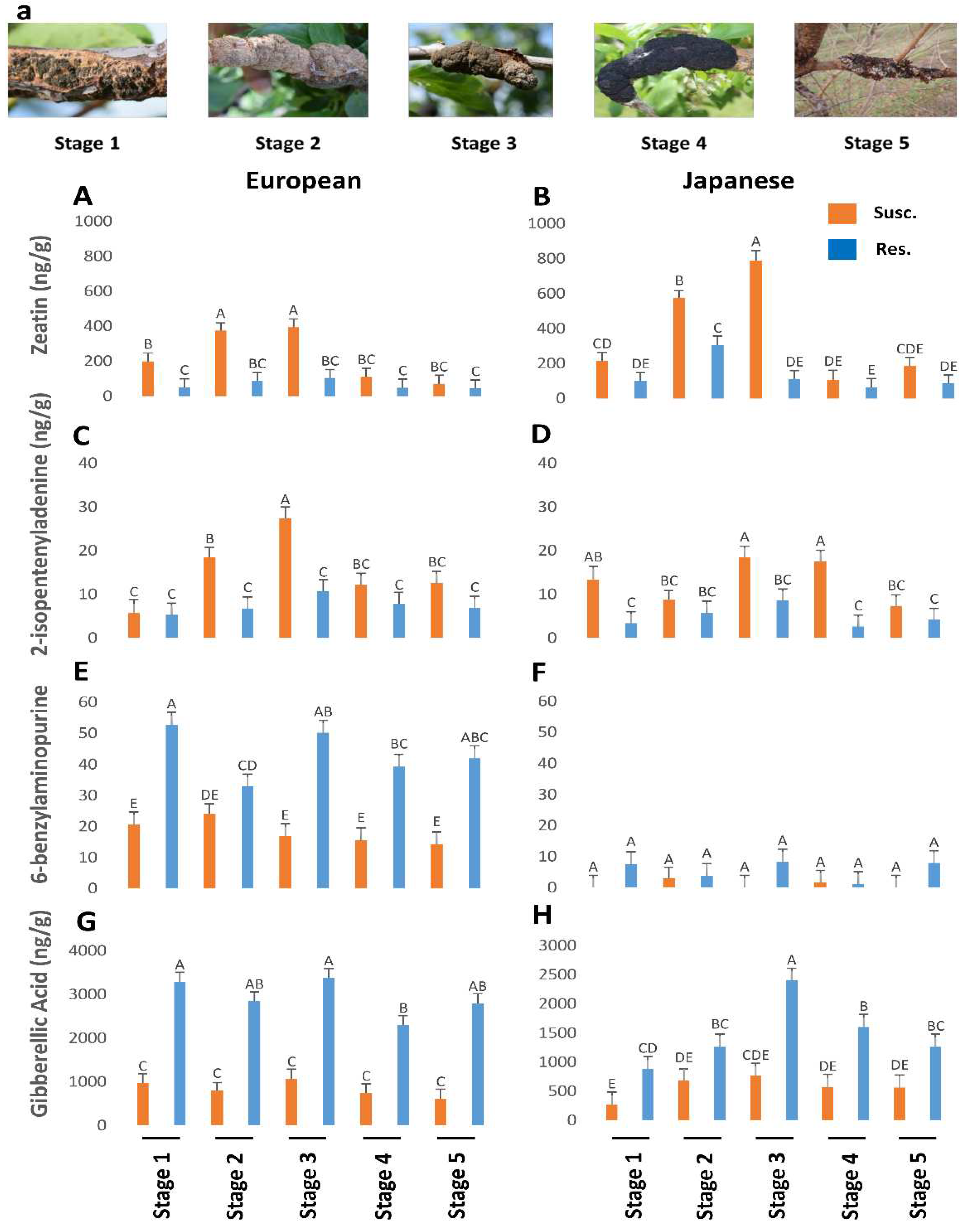
Jasmonic Acid
Principal Component Analysis
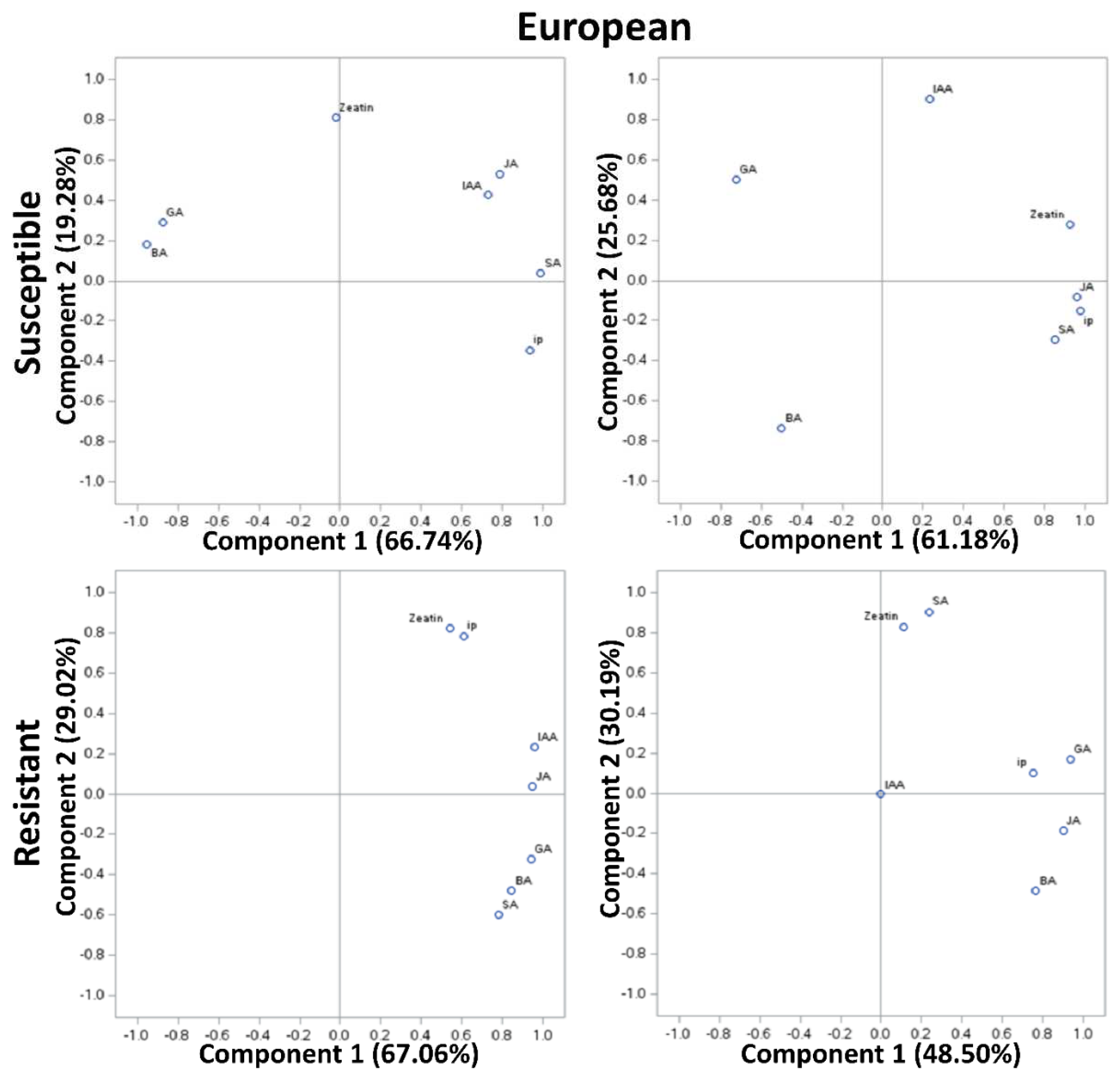
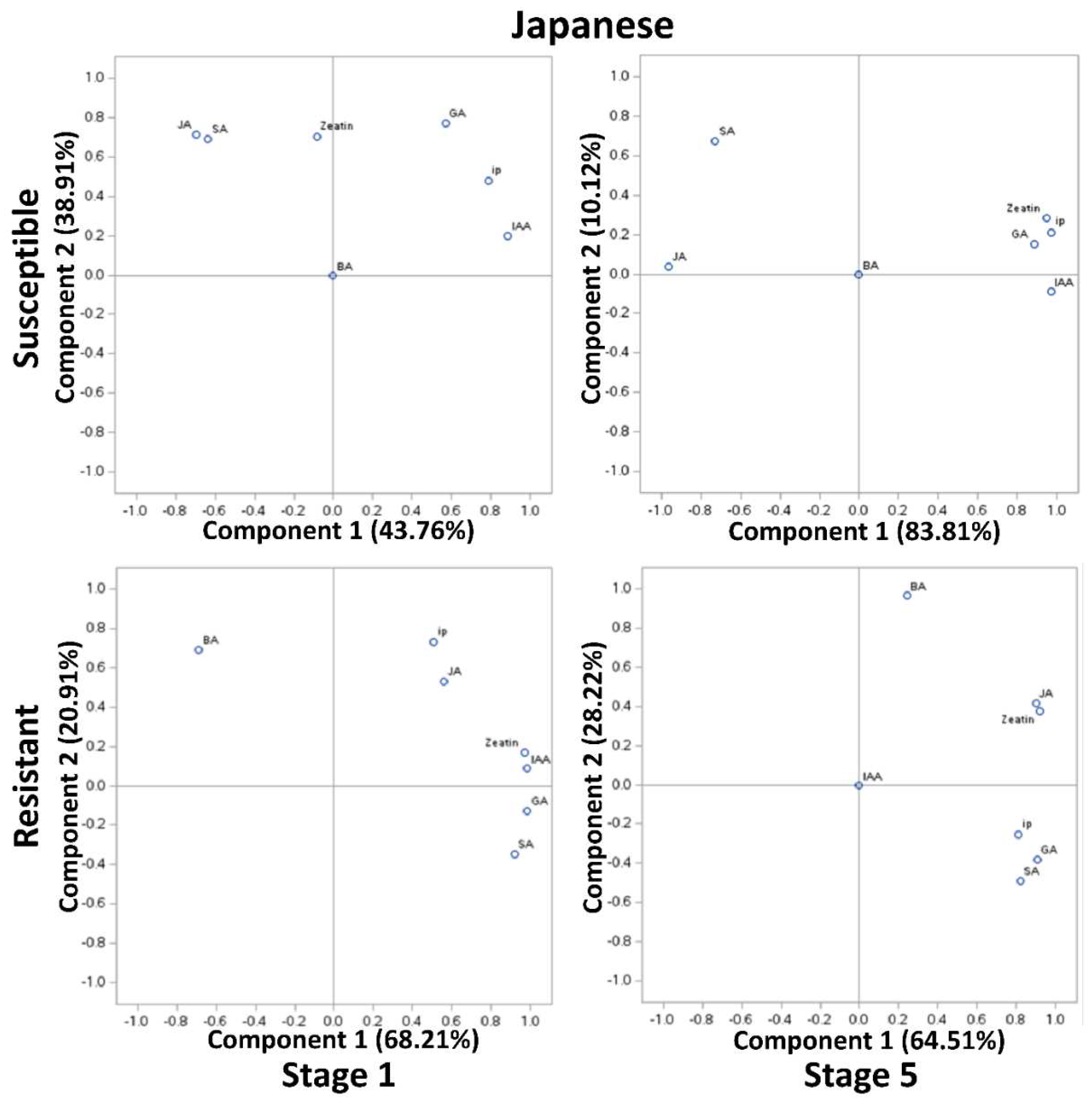
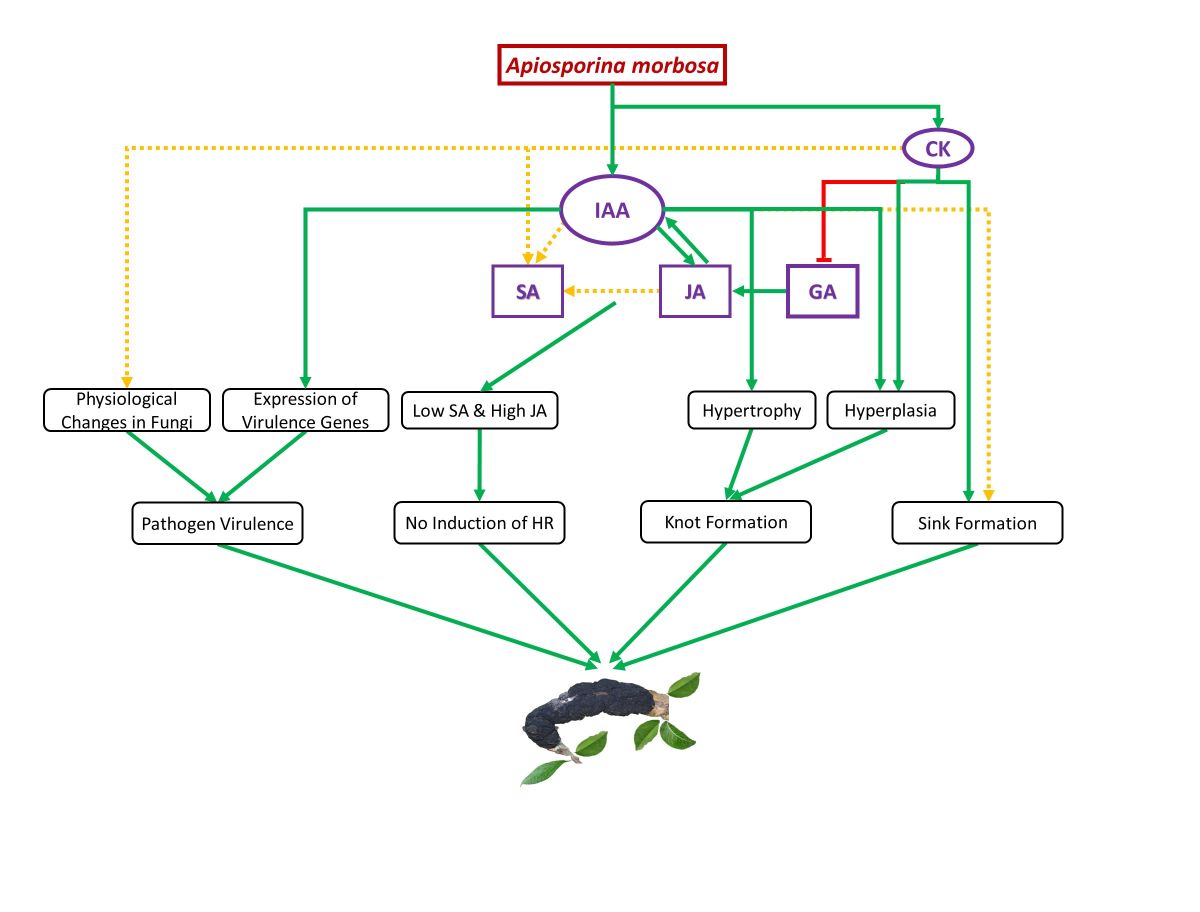
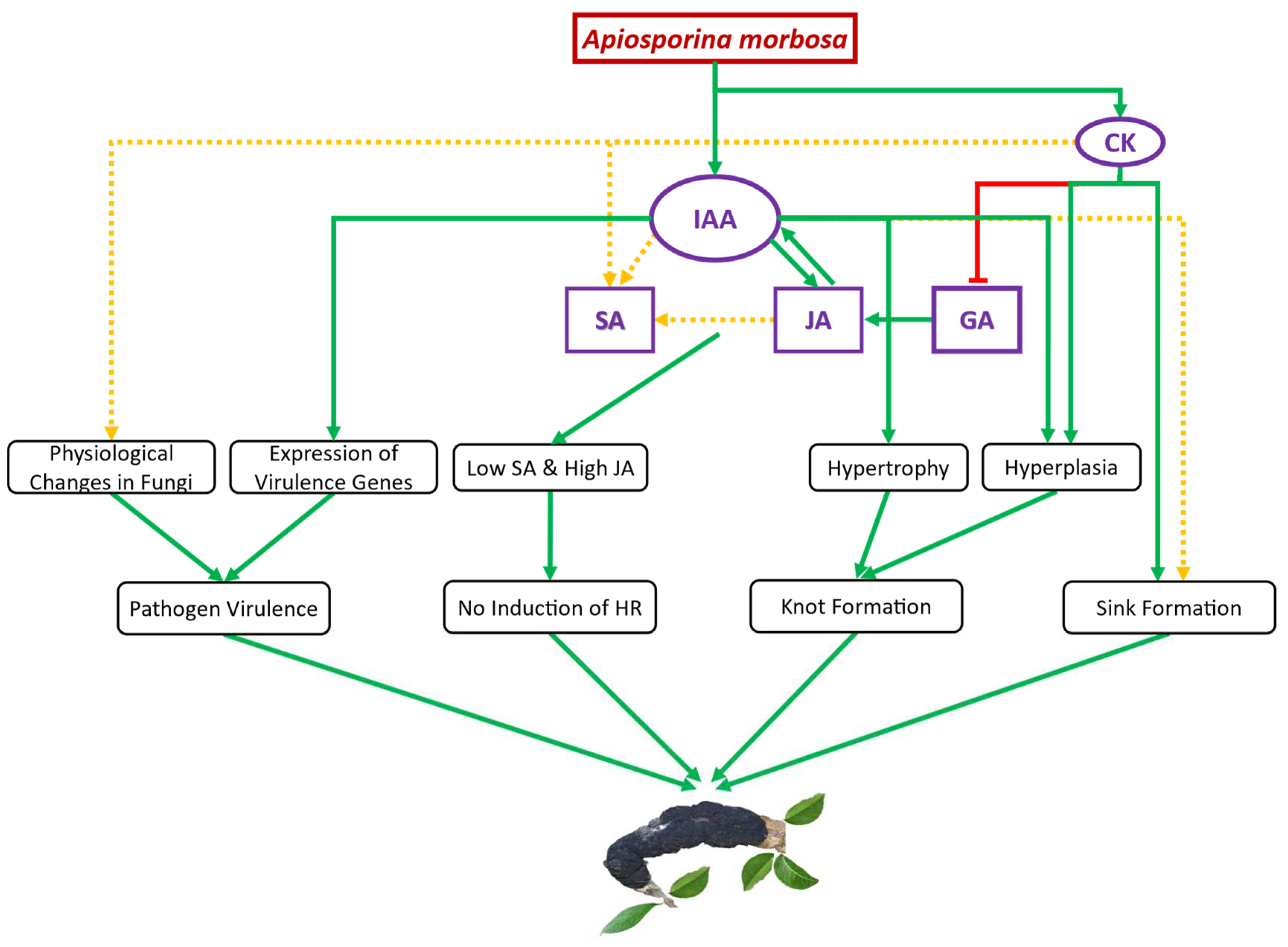
3. Discussion
- Auxin vs SA-JA in BK Disease Progression
- 2.
- Cytokinins vs SA-JA in BK Disease Progression
- 3.
- GA vs SA-JA in BK Disease Progression
- 4.
- JA and SA in BK Disease Progression
4. Materials and Methods
4.1. Sample Collection
4.2. Freeze Drying and Grinding
4.3. Hormone Extraction, Identification, and Quantification
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mengiste, T. Plant Immunity to Necrotrophs. Annual Review of Phytopathology, 2012, 50. [CrossRef]
- Agrios, G.N. Plant Pathology; Elsevier, 2005.
- Glazebrook, J. Contrasting Mechanisms of Defense against Biotrophic and Necrotrophic Pathogens. Annual Review of Phytopathology, 2005, 43. [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature, 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ghozlan, M.H.; EL-Argawy, E.; Tokgöz, S.; Lakshman, D.K.; Mitra, A. Plant Defense against Necrotrophic Pathogens. Am J Plant Sci, 2020, 11. [CrossRef]
- Katagiri, F.; Tsuda, K. Understanding the Plant Immune System. Molecular Plant-Microbe Interactions, 2010, 23. [CrossRef]
- Ma, K.W.; Ma, W. Phytohormone Pathways as Targets of Pathogens to Facilitate Infection. Plant Mol Biol, 2016, 91, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Zhou, J.M. Phytopathogen Effectors Subverting Host Immunity: Different Foes, Similar Battleground. Cell Host and Microbe, 2012, 12. [CrossRef]
- Kunkel, B.N.; Johnson, J.M.B. Auxin Plays Multiple Roles during Plant-Pathogen Interactions. Cold Spring Harbor perspectives in biology, 2021, 13. [CrossRef]
- Laird, T.S.; Flores, N.; Leveau, J.H.J. Bacterial Catabolism of Indole-3-Acetic Acid. [CrossRef]
- Kayal, W. el; Chamas, Z.; El-Sharkawy, I.; Subramanian, J. Comparative Anatomical Responses of Tolerant and Susceptible European Plum Varieties to Black Knot Disease. Plant Dis, 2021, 105. [CrossRef]
- Wilcox, W.F. Black Knot of Plums. 1992.
- Stewart, S.A.; Weber, D.J. Environmental Site Characteristics and Incidence of Chokecherry Black Knot in Utah. Great Basin Naturalist, 1984, 44, 6. [Google Scholar]
- Scorza, R.; Demuth, M. Black Knot [Apiosporina Morbosa (Schw.)] Resistance in Imported and Domestic Prunus domestica L. Germplasm and Cultivars. Journal of the American Pomological Society. 2015, 69, 45–50. [Google Scholar]
- Shinde, R.; Ayyanath, M.-M.; Shukla, M.; El Kayal, W.; Saxena, P.; Subramanian, J. Hormonal Interplay Leading to Black Knot Disease Establishment and Progression in Plums. Plants, 2023, 12. [CrossRef]
- Shinde, R.; Shum, C.; Gill, R. ; Subramanian Jayasankar. Phenotyping Black Knot Resistance in Plum Germplasm.; Guelph, 2023.
- Kayal, W. el; Chamas, Z.; El-Sharkawy, I.; Subramanian, J. Comparative Anatomical Responses of Tolerant and Susceptible European Plum Varieties to Black Knot Disease. Plant Dis, 2021, 105. [CrossRef]
- Liqin, G.; Jianguo, Z.; Xiaoxia, L.; Guodong, R. Polyploidy-Related Differential Gene Expression between Diploid and Synthesized Allotriploid and Allotetraploid Hybrids of Populus. Molecular Breeding, 2019, 39. [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic Characterization of Worldwide Prunus domestica (Plum) Germplasm Using Sequence-Based Genotyping. Hortic Res, 2019, 6. [CrossRef]
- Cui, F.; Wu, S.; Sun, W.; Coaker, G.; Kunkel, B.; He, P.; Shan, L. The Pseudomonas syringae Type III Effector AvrRpt2 Promotes Pathogen Virulence via Stimulating Arabidopsis Auxin/Indole Acetic Acid Protein Turnover. Plant Physiol, 2013, 162, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic Acid Inhibits Pathogen Growth in Plants through Repression of the Auxin Signaling Pathway. Current Biology, 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- McClerklin, S.A.; Goo Lee, S.; Harper, C.P.; Nwumeh, R.; Jez, J.M.; Kunkel, B.N. Indole-3-Acetaldehyde Dehydrogenase-Dependent Auxin Synthesis Contributes to Virulence of Pseudomonas Syringae Strain DC3000. 2018. [CrossRef]
- Djami-Tchatchou, A.T.; Harrison, G.A.; Harper, C.P.; Wang, R.; Prigge, M.J.; Estelle, M.; Kunkel, B.N. Dual Role of Auxin in Regulating Plant Defense and Bacterial Virulence Gene Expression During Pseudomonas syringae PtoDC3000 Pathogenesis. 2020, 33. [CrossRef]
- Kidd, B.N.; Kadoo, N.Y.; Dombrecht, B.; Tekeoğlu, M.; Gardiner, D.M.; Thatcher, L.F.; Aitken, E.A.B.; Schenk, P.M.; Manners, J.M.; Kazan, K. Auxin Signaling and Transport Promote Susceptibility to the Root-Infecting Fungal Pathogen Fusarium oxysporum in Arabidopsis. / 733 MPMI, 2011, 24, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. Linking Development to Defense: Auxin in Plant-Pathogen Interactions. Trends in Plant Science, 2009, 14. [CrossRef]
- Yuan, H.M.; Liu, W.C.; Lu, Y.T. CATALASE2 Coordinates SA-Mediated Repression of Both Auxin Accumulation and JA Biosynthesis in Plant Defenses. Cell Host Microbe, 2017, 21. [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.C.; Korzelius, J.P.; van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Brown, R.; Kazan, K.; van Loon, L.C.; Dong, X.; Pieterse, C.M.J. NPR1 Modulates Cross-Talk between Salicylate- and Jasmonate-Dependent Defense Pathways through a Novel Function in the Cytosol. Plant Cell, 2003, 15. [CrossRef]
- Zhang, H.; Tan, X.; Li, L.; He, Y.; Hong, G.; Li, J.; Lin, L.; Cheng, Y.; Yan, F.; Chen, J.; Sun, Z. Suppression of Auxin Signalling Promotes Rice Susceptibility to Rice Black Streaked Dwarf Virus Infection. Mol Plant Pathol, 2019, 20. [CrossRef]
- Lahey, K.A.; Yuan, R.; Burns, J.K.; Ueng, P.P.; Timmer, L.W.; Chung, K.R. Induction of Phytohormones and Differential Gene Expression in Citrus Flowers Infected by the Fungus Colletotrichum acutatum. Molecular Plant-Microbe Interactions, 2004, 17. [CrossRef]
- Vinutha, T.; Vanchinathan, S.; Bansal, N.; Kumar, G.; Permar, V.; Watts, A.; Ramesh, S. v.; Praveen, S. Tomato Auxin Biosynthesis/Signaling Is Reprogrammed by the Geminivirus to Enhance Its Pathogenicity. Planta, 2020, 252. [CrossRef]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.C.; Hutchison, C.E.; Schaller, G.E.; Dangl, J.L.; Kieber, J.J. Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity. PLoS Genet, 2012, 8. [CrossRef]
- Reusche, M.; Klásková, J.; Thole, K.; Truskina, J.; Novák, O.; Janz, D.; Strnad, M.; Spíchal, L.; Lipka, V.; Teichmann, T. Stabilization of Cytokinin Levels Enhances Arabidopsis Resistance against Verticillium longisporum. Molecular Plant-Microbe Interactions, 2013, 26. [CrossRef]
- Pogány, M.; Koehl, J.; Heiser, I.; Elstner, E.F.; Barna, B. Juvenility of Tobacco Induced by Cytokinin Gene Introduction Decreases Susceptibility to Tobacco Necrosis Virus and Confers Tolerance to Oxidative Stress. Physiol Mol Plant Pathol, 2004, 65. [CrossRef]
- Darke, S.F.; Burritt, D.J.; Jameson, P.E.; Guy, P.L. Effects of Plant Hormones on White Clover Mosaic Potexvirus Double-Stranded RNA. Plant Pathol, 2000, 49. [CrossRef]
- Shanks, C.M.; Rice, J.H.; Zubo, Y.; Schaller, G.E.; Hewezi, T.; Kieber, J.J. The Role of Cytokinin during Infection of Arabidopsis Thaliana by the Cyst Nematode Heterodera schachtii. Molecular Plant-Microbe Interactions, 2016, 29. [CrossRef]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The Cytokinin-Activated Transcription Factor ARR2 Promotes Plant Immunity via TGA3/NPR1-Dependent Salicylic Acid Signaling in Arabidopsis. Dev Cell, 2010, 19. [CrossRef]
- Gupta, R.; Pizarro, L.; Leibman-Markus, M.; Marash, I.; Bar, M. Cytokinin Response Induces Immunity and Fungal Pathogen Resistance, and Modulates Trafficking of the PRR LeEIX2 in Tomato. Mol Plant Pathol, 2020, 21. [CrossRef]
- Spallek, T.; Gan, P.; Kadota, Y.; Shirasu, K. Same Tune, Different Song — Cytokinins as Virulence Factors in Plant–Pathogen Interactions? Current Opinion in Plant Biology, 2018, 44, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Argueso, C.T. Should I Fight or Should I Grow Now? The Role of Cytokinins in Plant Growth and Immunity and in the Growth-Defence Trade-Off. Annals of Botany, 2017, 119. [CrossRef]
- Großkinsky, D.K.; Naseem, M.; Abdelmohsen, U.R.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novák, O.; Strnad, M.; Pfeifhofer, H.; van der Graaff, E.; Simon, U.; Roitsch, T. Cytokinins Mediate Resistance against Pseudomonas syringae in Tobacco through Increased Antimicrobial Phytoalexin Synthesis Independent of Salicylic Acid Signaling. Plant Physiol, 2011, 157. [CrossRef]
- Babosha, A. v. Regulation of Resistance and Susceptibility in Wheat–Powdery Mildew Pathosystem with Exogenous Cytokinins. J Plant Physiol, 2009, 166, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Molecular Plant, 2014, 7. [CrossRef]
- Belkhadir, Y.; Yang, L.; Hetzel, J.; Dangl, J.L.; Chory, J. The Growth-Defense Pivot: Crisis Management in Plants Mediated by LRR-RK Surface Receptors. Trends in Biochemical Sciences, 2014, 39. [CrossRef]
- Naseem, M.; Philippi, N.; Hussain, A.; Wangorsch, G.; Ahmed, N.; Dandekara, T. Integrated Systems View on Networking by Hormones in Arabidopsis Immunity Reveals Multiple Crosstalk for Cytokinin. Plant Cell, 2012, 24. [CrossRef]
- Sun, T.P.; Gubler, F. Molecular Mechanism of Gibberellin Signaling in Plants. Annual Review of Plant Biology, 2004, 55. [CrossRef]
- Grant, M.R.; Jones, J.D.G. Hormone (Dis)Harmony Moulds Plant Health and Disease. Science, 2009, 324. [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D.G. DELLAs Control Plant Immune Responses by Modulating the Balance of Jasmonic Acid and Salicylic Acid Signaling. Current Biology, 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Saloua, N.; Mourad, S.M.; Ammar, O. In Vitro and in Vivo Evaluating the Efficacy of Salicylic Acid and Gibberellic Acid Against Fusariumverticillioides in Garlic (Allium sativum L). World Journal of Environmental Biosciences, 2018, 7, 1–8. [Google Scholar]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; Dong, X. NPR3 and NPR4 Are Receptors for the Immune Signal Salicylic Acid in Plants. Nature, 2012, 486. [CrossRef]
- de Vleesschauwer, D.; Gheysen, G.; Höfte, M. Hormone Defense Networking in Rice: Tales from a Different World. Trends in Plant Science, 2013, 18. [CrossRef]
- Rostás, M.; Winter, T.R.; Borkowski, L.; Zeier, J. Copper and Herbivory Lead to Priming and Synergism in Phytohormones and Plant Volatiles in the Absence of Salicylate-Jasmonate Antagonism. Plant Signal Behav, 2013, 8. [CrossRef]
- Sun, N.; Kong, X.; Liu, Y.; Gong, T.; Gu, X.; Liu, L. The THO/TREX Complex Active in Alternative Splicing Mediates Plant Responses to Salicylic Acid and Jasmonic Acid. Int J Mol Sci, 2021, 22. [CrossRef]
- Betsuyaku, S.; Katou, S.; Takebayashi, Y.; Sakakibara, H.; Nomura, N.; Fukuda, H. Salicylic Acid and Jasmonic Acid Pathways Are Activated in Spatially Different Domains around the Infection Site during Effector-Triggered Immunity in Arabidopsis thaliana. Plant Cell Physiol, 2018, 59. [CrossRef]
- Lemarié, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol, 2015, 56. [CrossRef]
- Chen, Q.; Zhang, R.; Li, D.; Wang, F. Integrating Transcriptome and Coexpression Network Analyses to Characterize Salicylic Acid- and Jasmonic Acid-Related Genes in Tolerant Poplars Infected with Rust. Int J Mol Sci, 2021, 22, 5001. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Schmidt, A.; Reichelt, M.; Tsai, C.J.; Gershenzon, J. Lack of Antagonism between Salicylic Acid and Jasmonate Signalling Pathways in Poplar. New Phytologist, 2022, 235, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Sherif, S.M.; Shukla, M.R.; Murch, S.J.; Bernier, L.; Saxena, P.K. Simultaneous Induction of Jasmonic Acid and Disease-Responsive Genes Signifies Tolerance of American Elm to Dutch Elm Disease. Sci Rep, 2016, 6. [CrossRef]
- Silverman, P.; Seskar, M.; Kanter, D.; Schweizer, P.; Métraux, J.P.; Raskin, I. Salicylic Acid in Rice: Biosynthesis, Conjugation, and Possible Role. Plant Physiol, 1995, 108. [CrossRef]
- Likić, S.; Šola, I.; Ludwig-Müller, J.; Rusak, G. Involvement of Kaempferol in the Defence Response of Virus Infected Arabidopsis Thaliana. Eur J Plant Pathol, 2014, 138. [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front Plant Sci, 2015, 6. [CrossRef]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; Feussner, K.; Meinicke, P.; Stierhof, Y.D.; Schwarz, H.; MacEk, B.; Mann, M.; Kahmann, R. Metabolic Priming by a Secreted Fungal Effector. Nature, 2011, 478. [CrossRef]
- Altmann, M.; Altmann, S.; Rodriguez, P.A.; Weller, B.; Elorduy Vergara, L.; Palme, J.; Marín-de la Rosa, N.; Sauer, M.; Wenig, M.; Villaécija-Aguilar, J.A.; Sales, J.; Lin, C.W.; Pandiarajan, R.; Young, V.; Strobel, A.; Gross, L.; Carbonnel, S.; Kugler, K.G.; Garcia-Molina, A.; Bassel, G.W.; Falter, C.; Mayer, K.F.X.; Gutjahr, C.; Vlot, A.C.; Grill, E.; Falter-Braun, P. Extensive Signal Integration by the Phytohormone Protein Network. Nature, 2020, 583. [CrossRef]
- Navarrete, F.; Gallei, M.; Kornienko, A.E.; Saado, I.; Khan, M.; Chia, K.S.; Darino, M.A.; Bindics, J.; Djamei, A. TOPLESS Promotes Plant Immunity by Repressing Auxin Signaling and Is Targeted by the Fungal Effector Naked1. Plant Commun, 2022, 3. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




