Submitted:
13 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO/CBC
2.3. Characterization
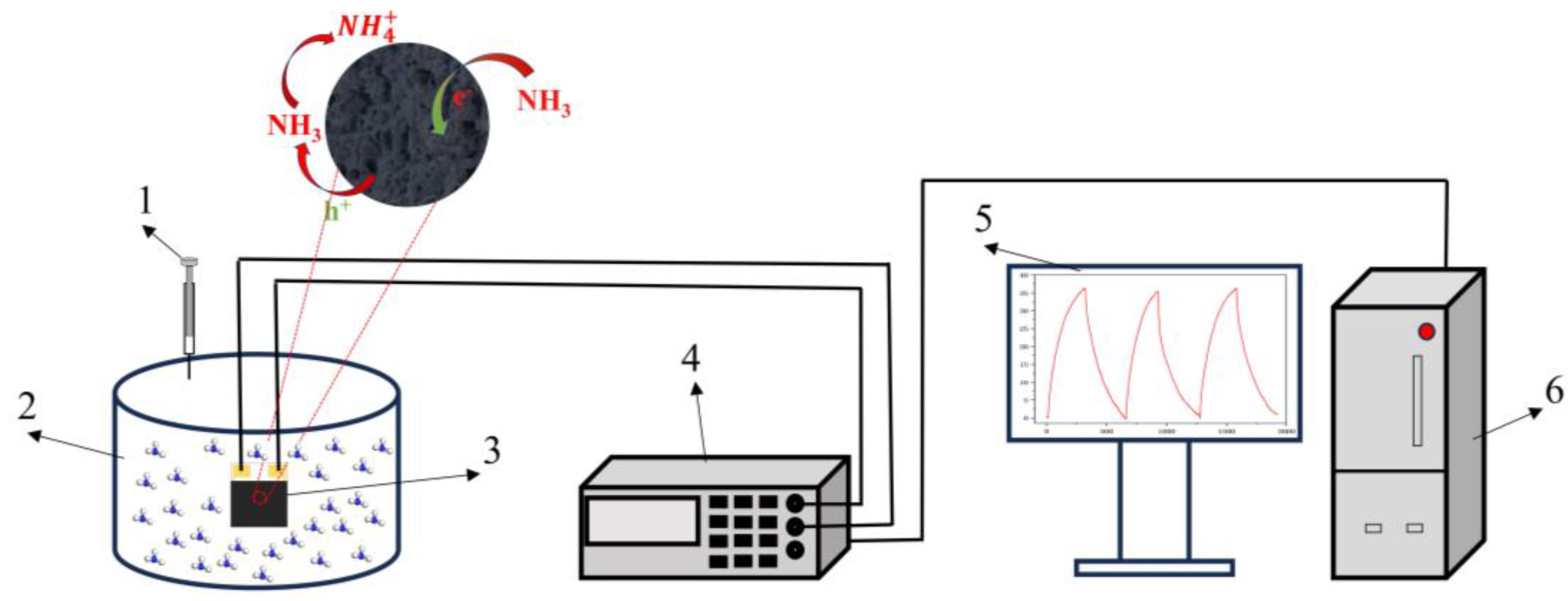
2.4. Fabrication of gas sensing device
3. Results
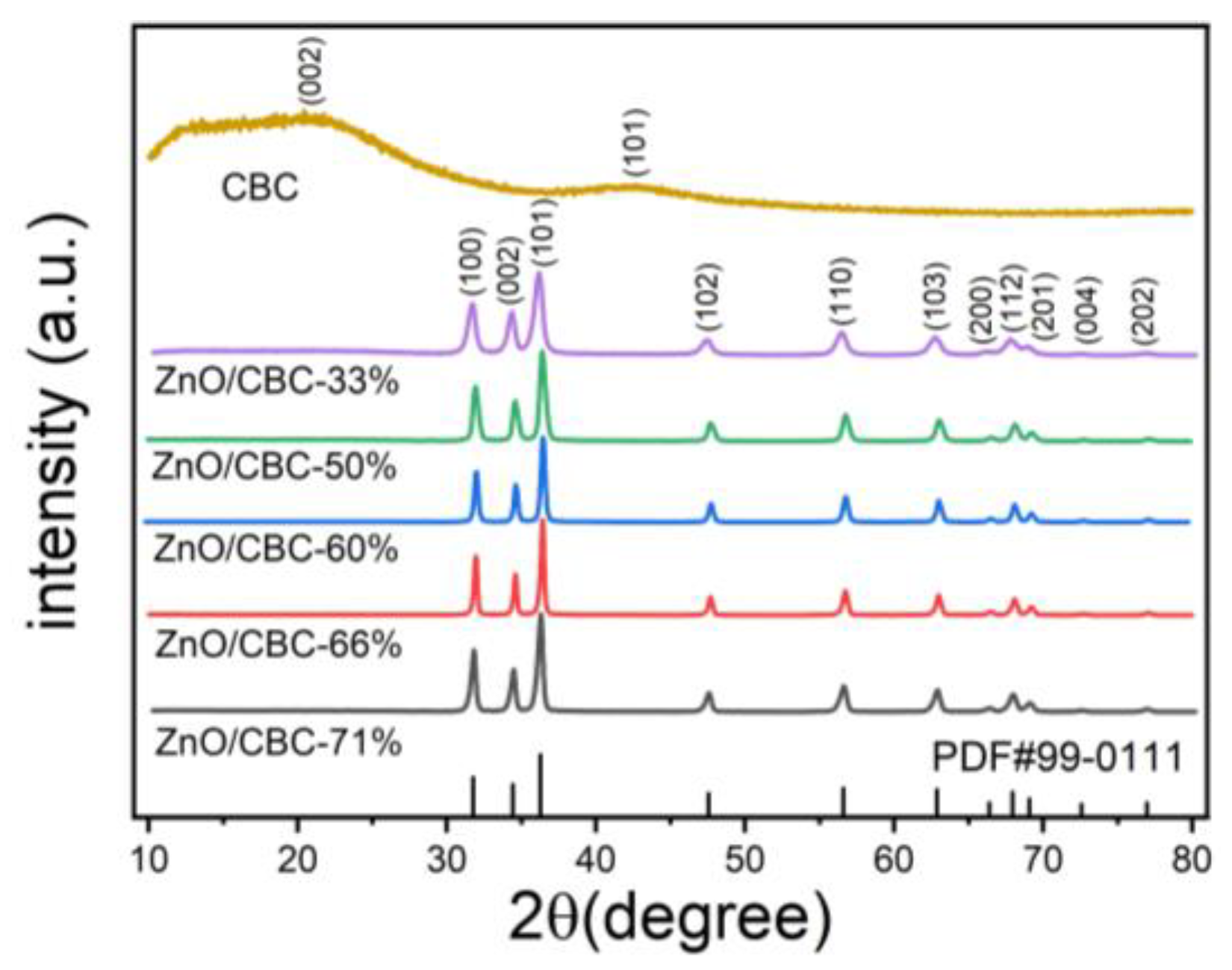
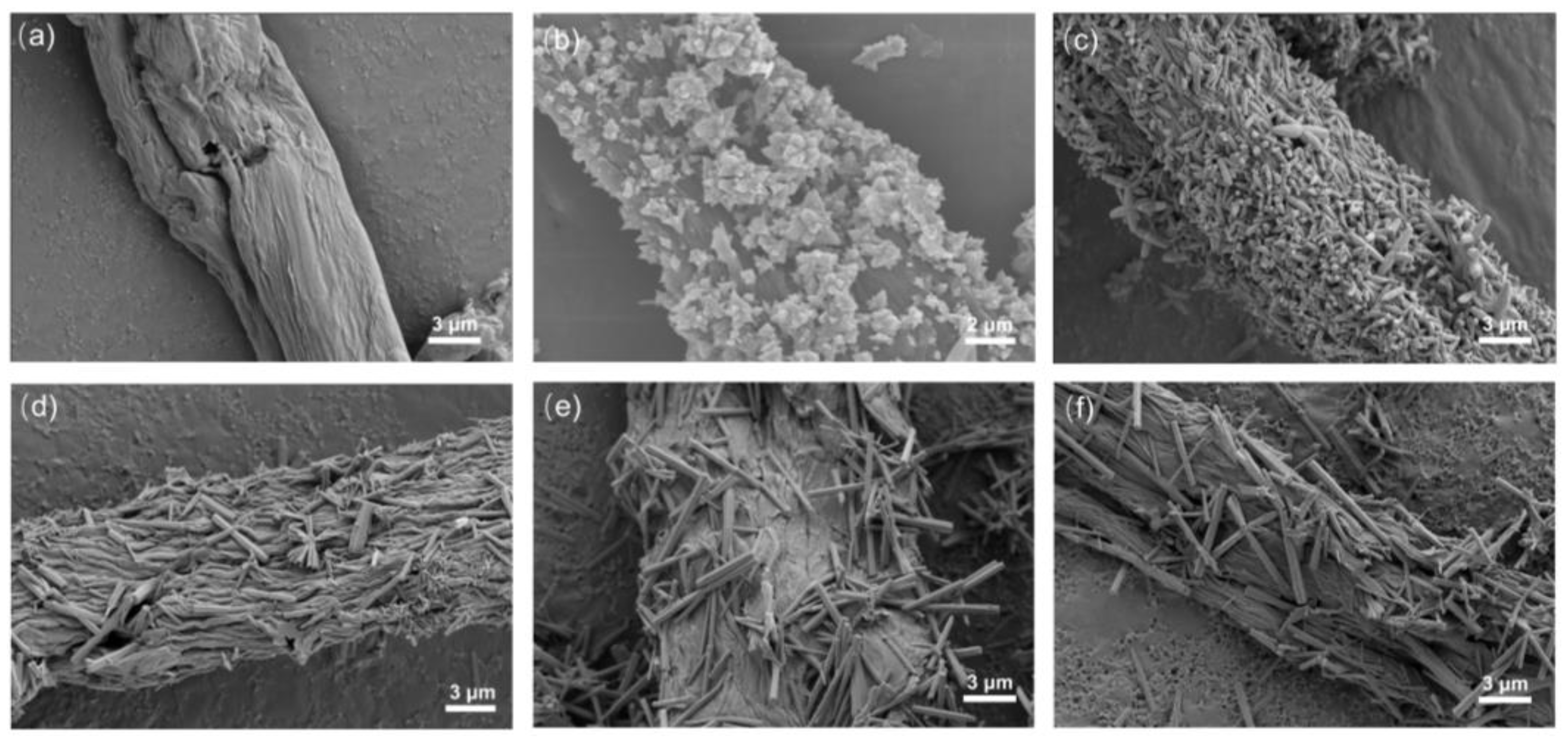
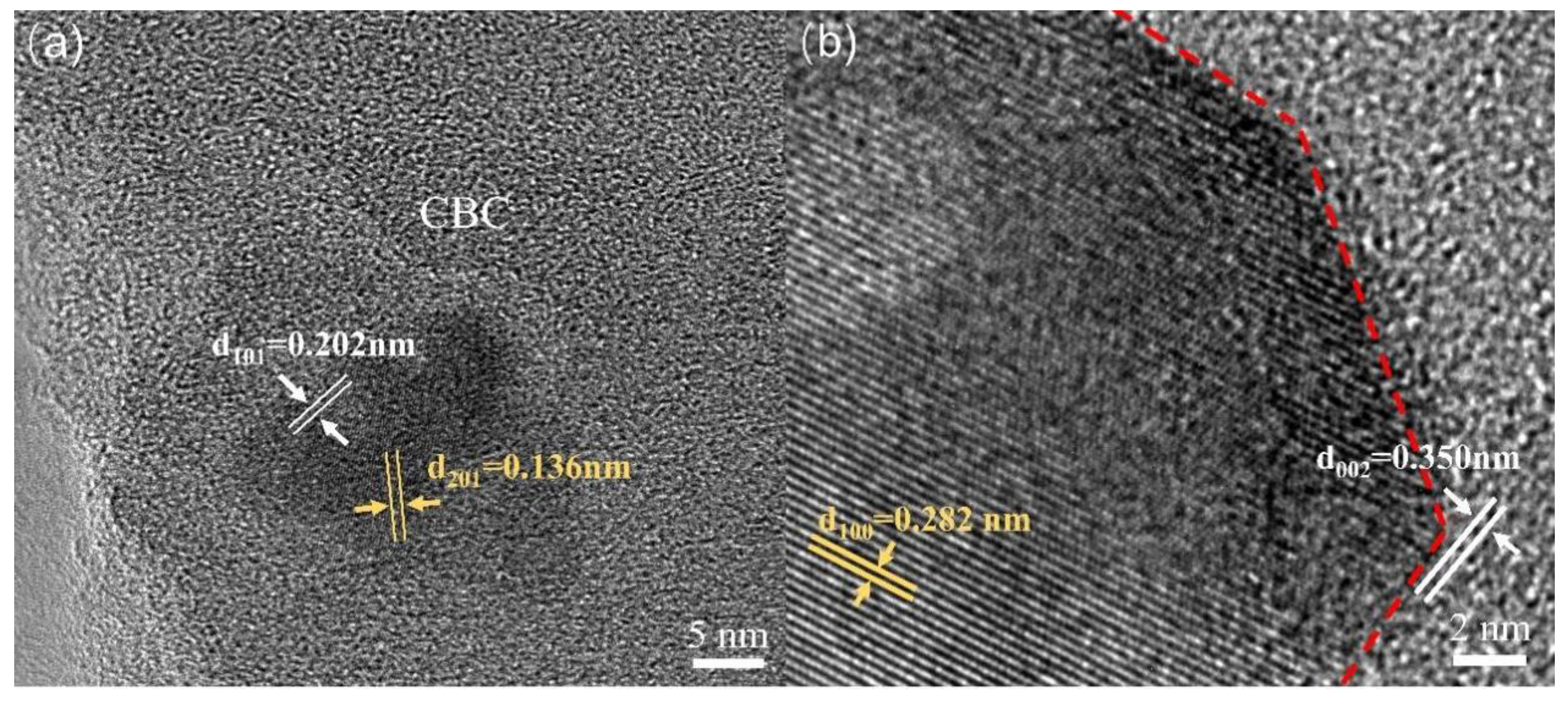
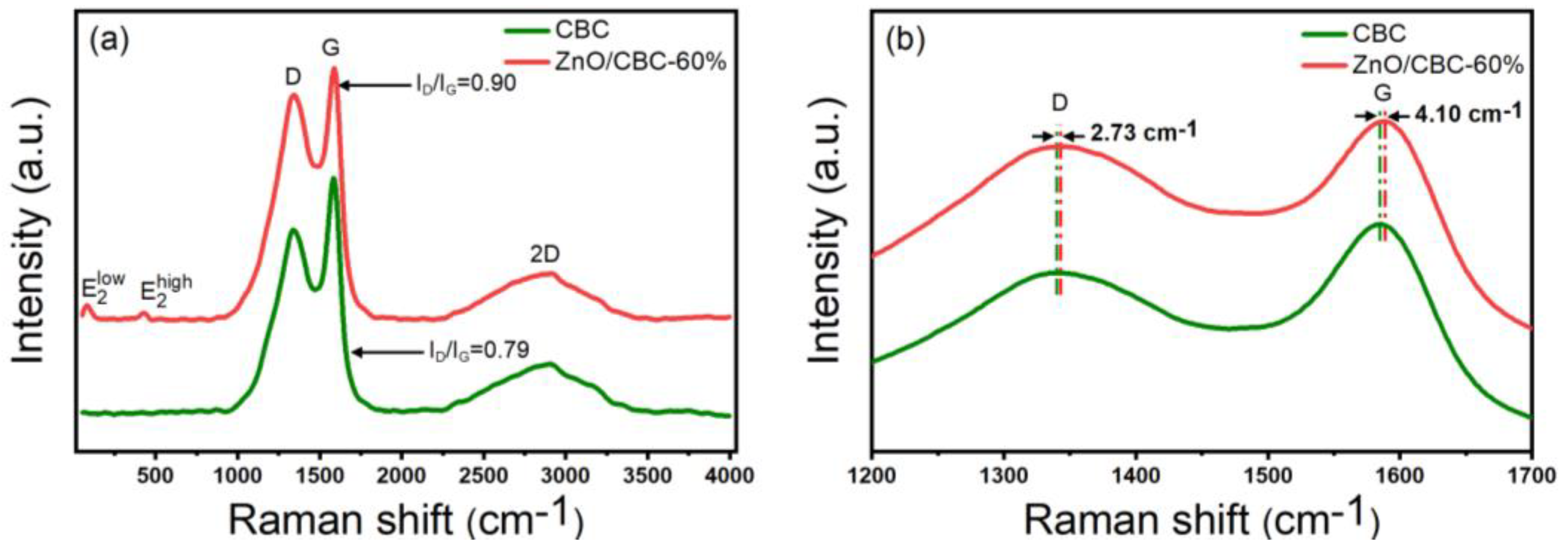
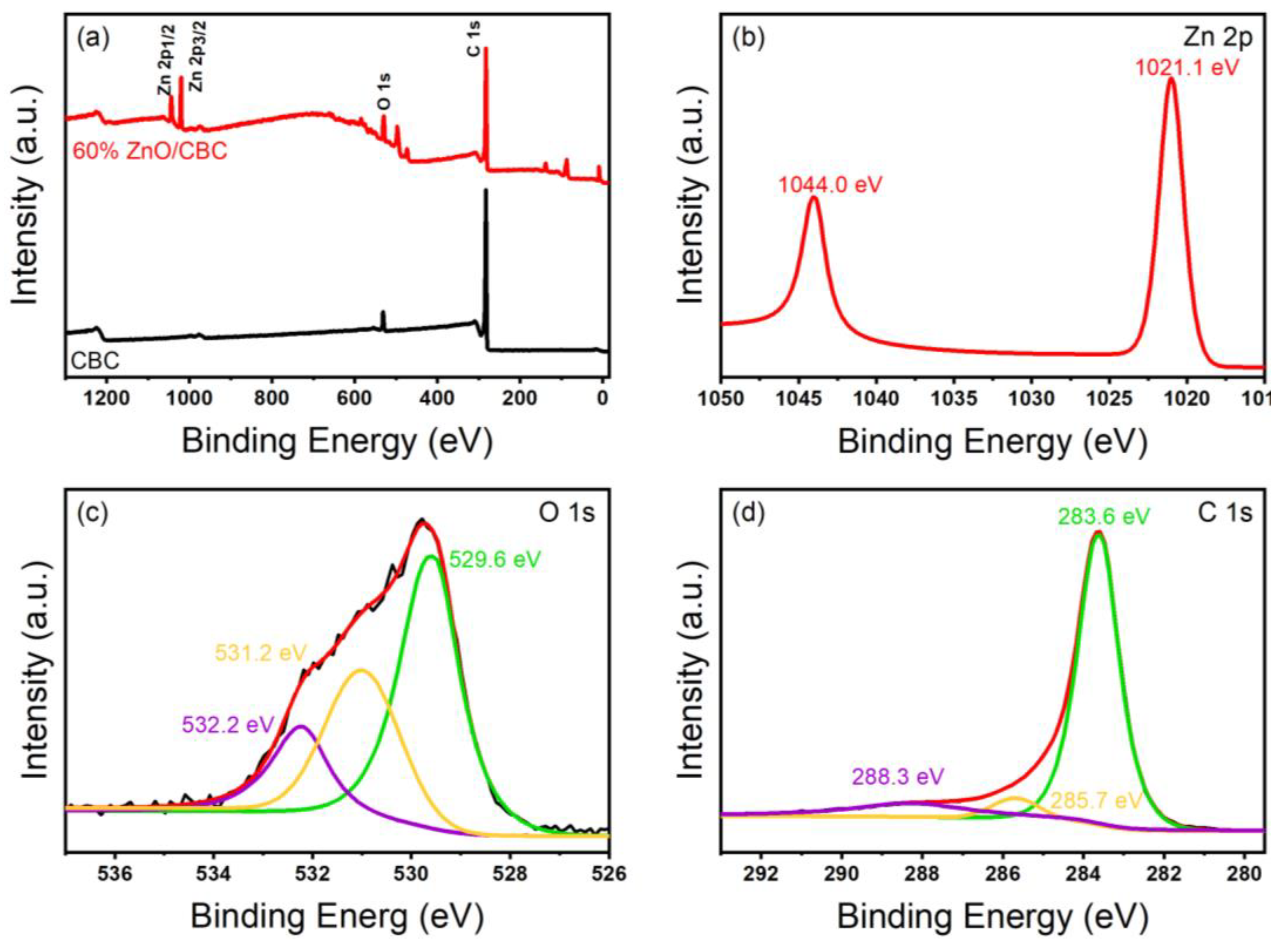
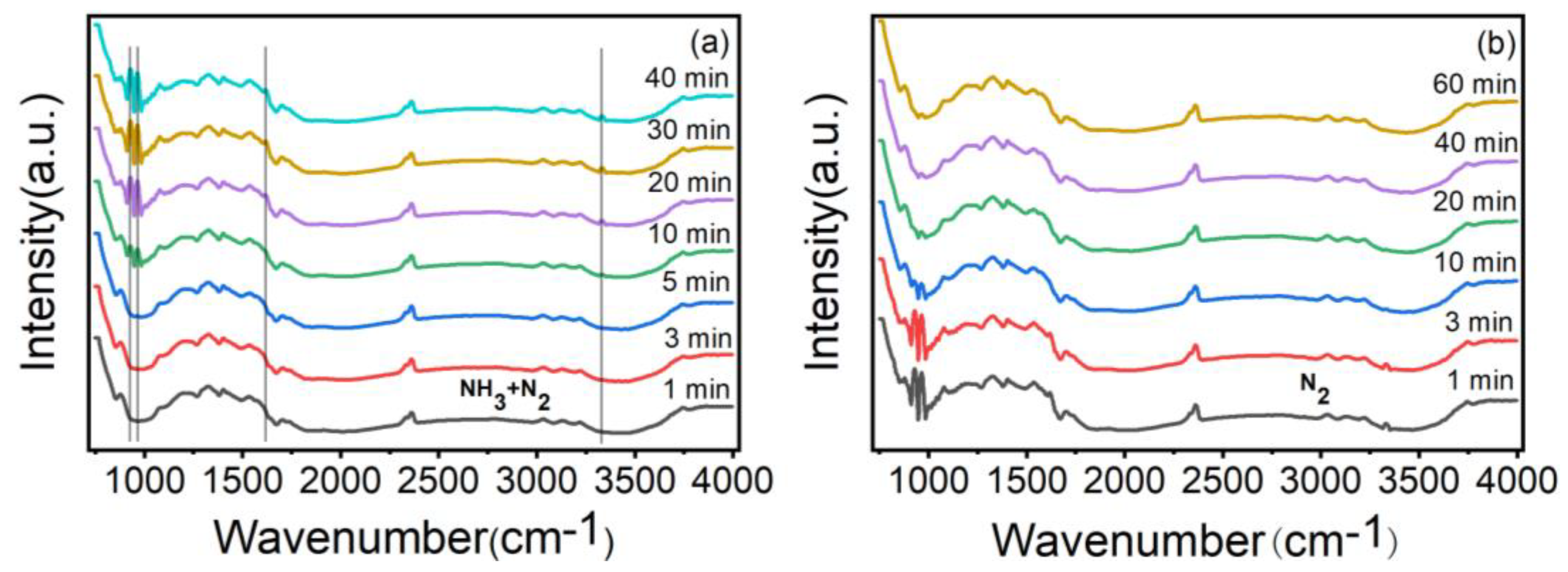
3.1. Characterization of ZnO/CBC
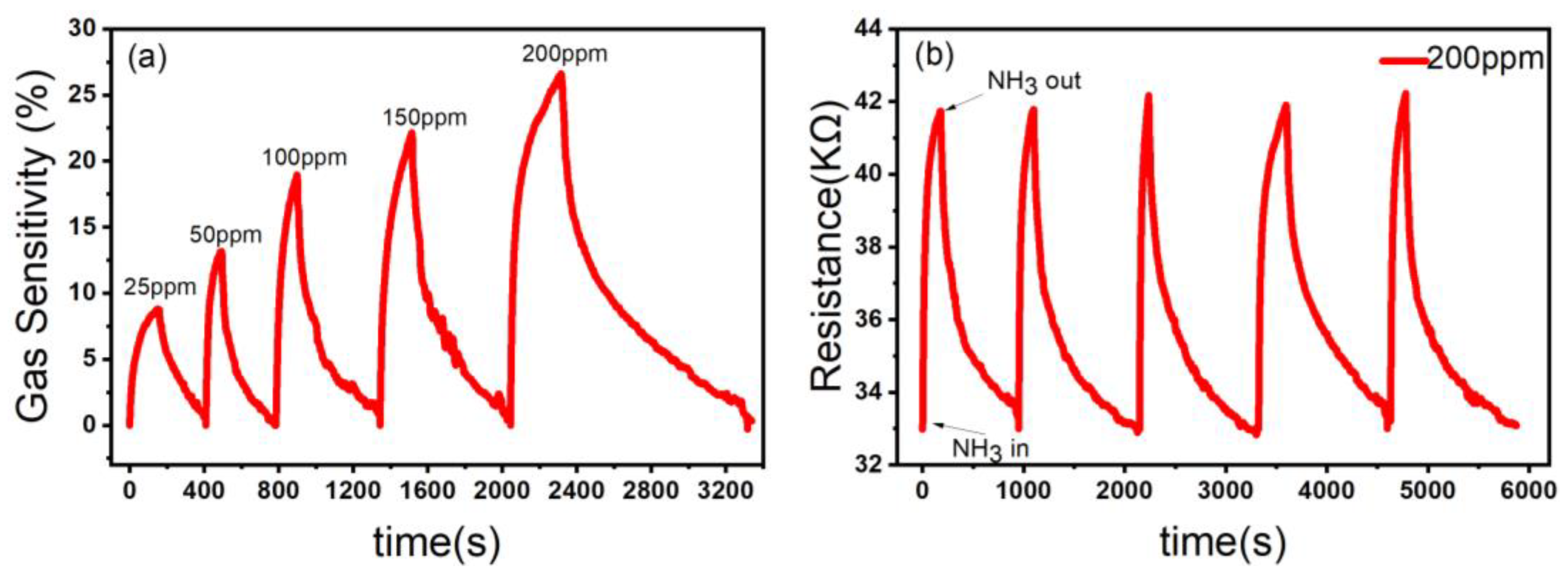
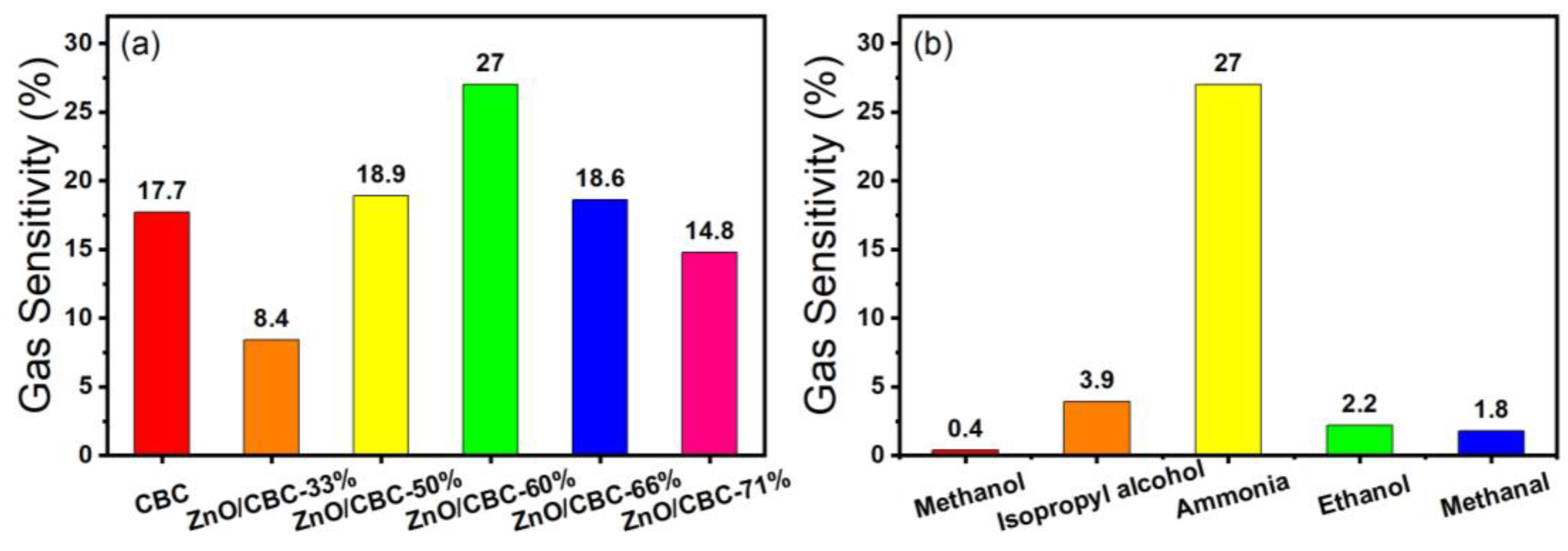
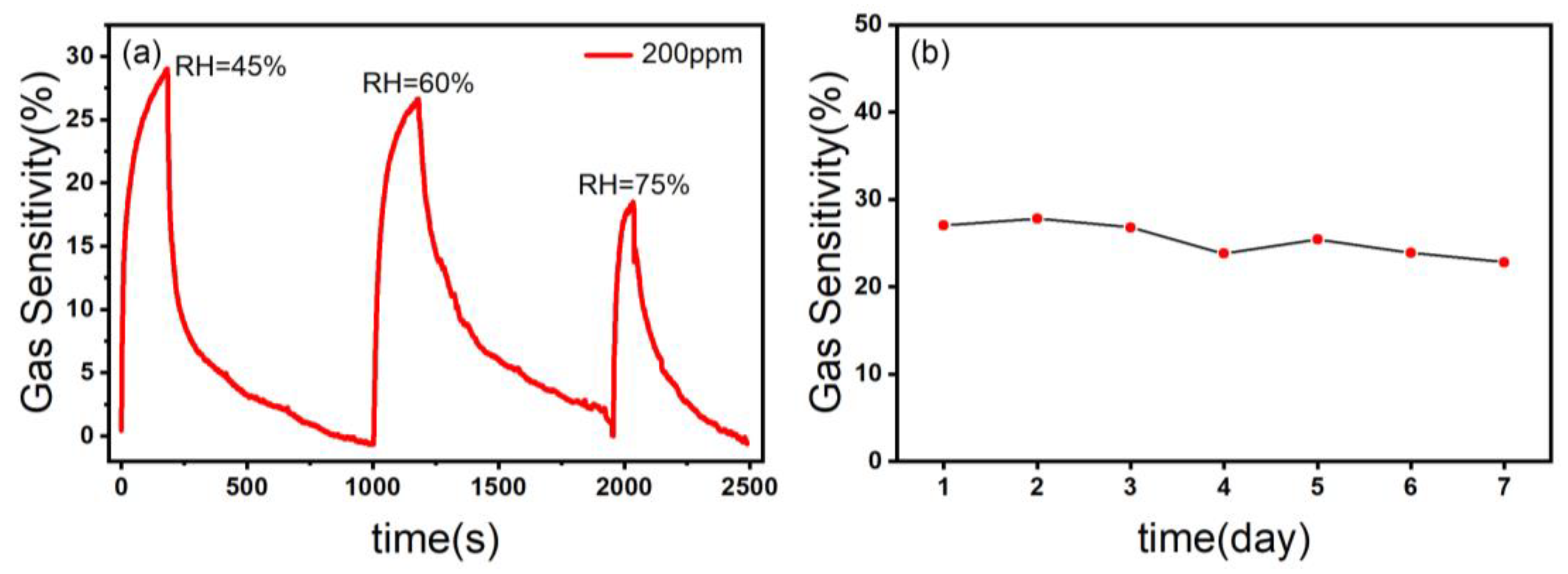
3.2. Results of Sensing Tests
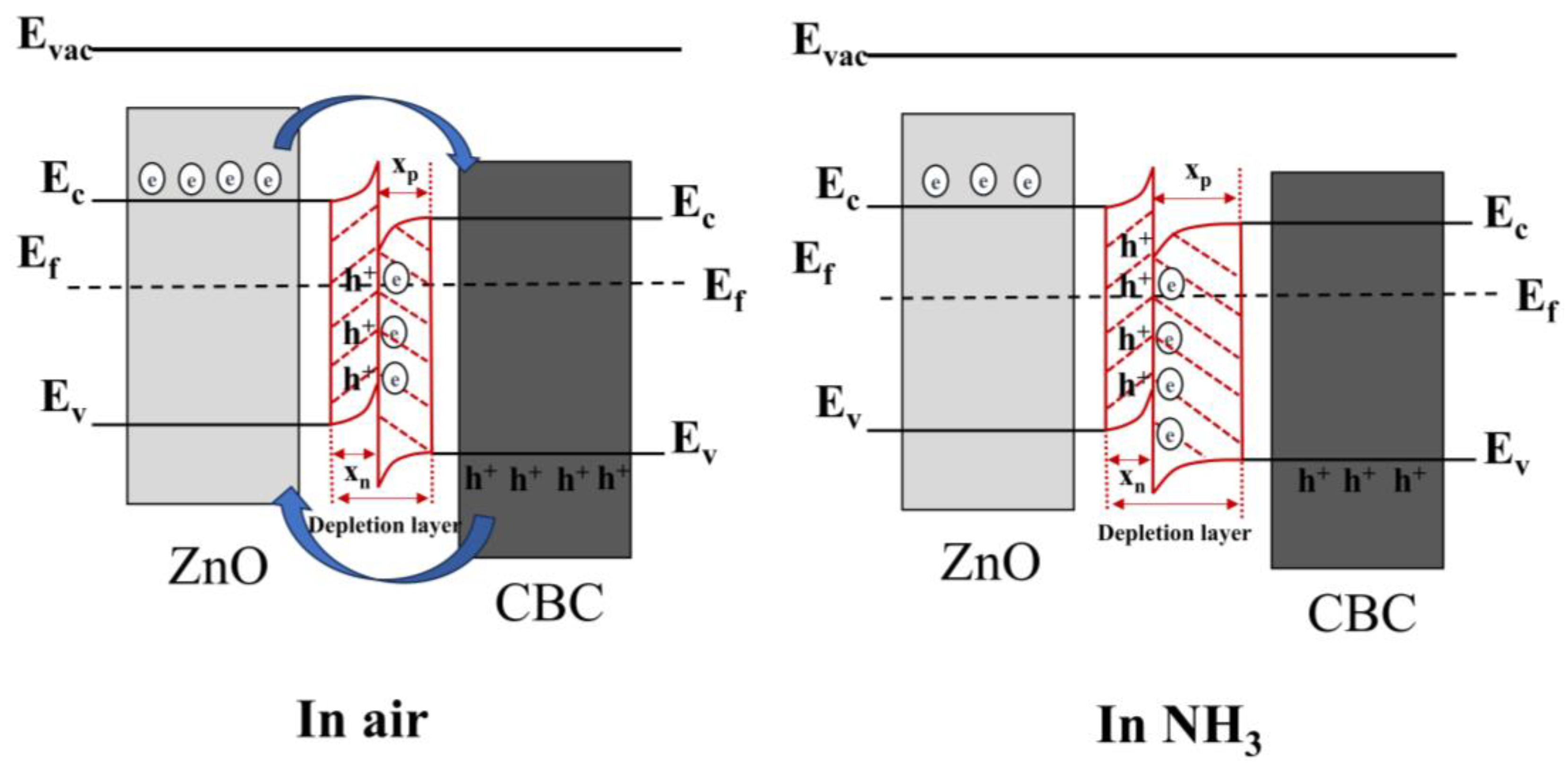
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Danasa, A.S.; Soesilo, T.E.B.; Martono, D. N.; Sodri, A.; Hadi, A.S.; Chandrasa, G.T. The ammonia release hazard and risk assessment: A case study of urea fertilizer industry in Indonesia. IOP conference series: Earth and environmental science, 2019; IOP Publishing: 2019; pp. 012087. [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R. K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Si, X.; Wei, Y.; Wang, C.; Li, L.; Ding, Y. A sensitive electrochemical sensor for ofloxacin based on a graphene/zinc oxide composite film. Anal. Methods 2018, 10, 1961–1967. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sensor Acturt B-Chem 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Young, S.J.; Liu, Y.H.; Shiblee, M. N. I.; Ahmed, K.; Lai, L.T.; Nagahara, L.; Thundat, T.; Yoshida, T.; Arya, S.; Furukawa, H. Flexible ultraviolet photodetectors based on one-dimensional gallium-doped zinc oxide nanostructures. ACS Appl. Electron. Mater 2020, 2, 3522–3529. [Google Scholar] [CrossRef]
- Yu, S.; Chen, C.; Zhang, H.; Zhang, J.; Liu, J. Design of high sensitivity graphite carbon nitride/zinc oxide humidity sensor for breath detection. Sensor Acturt B-Chem 2021, 332, 129536. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Tripathi, R.; Kumar, A. Advancing of zinc oxide nanoparticles for cosmetic applications. In Handbook of consumer Nanoproducts, Springer: Singapore, 2022; pp. 1–16. [CrossRef]
- Kanaparthi, S.; Singh, S. G. Chemiresistive sensor based on zinc oxide nanoflakes for CO2 detection. ACS Appl. Nano Mater 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Hao, J.; Lin, L.; Zeng, W.; Peng, X.; Wang, Z. Enhancement of NH3 sensing performance in flower-like ZnO nanostructures and their growth mechanism. Appl. Surf. Sci 2015, 357, 31–36. [Google Scholar] [CrossRef]
- Ganesh, R. S.; Durgadevi, E.; Navaneethan, M.; Patil, V.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.; Hayakawa, Y. Tuning the selectivity of NH3 gas sensing response using Cu-doped ZnO nanostructures. Sensor Actuat A: Phy 2018, 269, 331–341. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Reddy, M. R. Improved gas sensing performance of Al doped ZnO/CuO nanocomposite based ammonia gas sensor. Mater Sci Eng B-Adv 2018, 227, 61–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, H.; Wei, Z.; Xue, Y.; Sun, Y.; Zhang, W.; Li, P.; Gong, W.; Zhuiykov, S.; Hu, J. NH3 sensor based on 3D hierarchical flower-shaped n-ZnO/p-NiO heterostructures yields outstanding sensing capabilities at ppb level. Sensors-Basel 2020, 20, 4754. [Google Scholar] [CrossRef]
- Abdulsattar, M. A.; Jabbar, R. H.; Abed, H. H.; Abduljalil, H. M. The sensitivity of pristine and Pt doped ZnO nanoclusters to NH3 gas: A transition state theory study. Optik 2021, 242, 167158. [Google Scholar] [CrossRef]
- Ramesh, A.; Gavaskar, D.; Nagaraju, P.; Duvvuri, S.; Vanjari, S. R. K.; Subrahmanyam, C. Mn-doped ZnO microspheres prepared by solution combustion synthesis for room temperature NH3 sensing. Appl. Surf. Sci.Adv 2022, 12, 100349. [Google Scholar] [CrossRef]
- Chao, J.; Chen, Y.; Xing, S.; Zhang, D.; Shen, W. Facile fabrication of ZnO/C nanoporous fibers and ZnO hollow spheres for high performance gas sensor. Sensor Acturt B-Chem 2019, 298, 126927. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Zhang, M.; Qin, Z.; Cao, S.; Zhong, F.; Li, S.; Duan, H. M.; Zhang, J. Improved Gas-Sensitive Properties by a Heterojunction of Hollow Porous Carbon Microtubes Derived from Sycamore Fibers. ACS Sustainable Chem. Eng 2021, 43, 9. [Google Scholar] [CrossRef]
- Hu, J.; Yin, C.; Cheng, M.; Wei, T.; Liu, Q.; Li, W.; Ling, Y.; Zhang, Y.; Liu, B. Facile synthesis of N-doped carbon sheets-ZnO hybrids for NO2 sensing at ppb level. J. Alloys Compd 2022, 892, 162243. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent progress in high-strength and robust regenerated cellulose materials. Adv. Mater 2021, 33, 2000682. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr (VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res 2020, 185, 109414. [Google Scholar] [CrossRef] [PubMed]
- Dou, T.; Zang, L.; Zhang, Y.; Sun, Z.; Sun, L.; Wang, C. Hybrid g-C3N4 nanosheet/carbon paper membranes for the photocatalytic degradation of methylene blue. Mater. Lett 2019, 244, 151–154. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Sol–gel synthesis of carbon-doped TiO2 nanoparticles based on microcrystalline cellulose for efficient photocatalytic degradation of methylene blue under visible light. Environ. Technol 2019, 41, 3233–3247. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Xu, L.; Chen, M.; Wang, L.; Liu, J.; Han, S.; Mei, C.; Zhong, Q. The fabrication of bio-renewable and recyclable cellulose based carbon microspheres incorporated by CoFe2O4 and the photocatalytic properties. J. Cleaner Prod 2018, 196, 594–603. [Google Scholar] [CrossRef]
- John, R. A. B.; Shruthi, J.; Reddy, M. R.; Kumar, A. R. Manganese doped nickel oxide as room temperature gas sensor for formaldehyde detection. Ceram. Int 2022, 48, 17654–17667. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, S.; Xu, X.; Jiao, H.; Zhang, G.; Liu, L.; Wang, P.; Gengzang, D.; Yao, H. Optimization ethanol detection performance manifested by gas sensor based on In2O3/ZnS rough microspheres. Sensor Acturt B-Chem 2018, 264, 263–278. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A.; Reka, U. Hydrothermal synthesis and characterization of Zinc Oxide nanoparticles of various shapes under different reaction conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Bagheri, F.; Haratizadeh, H. UV-activated CO2 sensor based on ZnO nanoparticles at low temperatures. Mater. Sci. Semicond. Process 2022, 141, 106422. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y. X.; Xu, Y. S.; Meng, Q. S.; Gao, J. C.; Sun, Y. G.; Hu, Y. S.; Chang, B. B.; Liu, C. T.; Cao, A. M. Pitch-derived soft carbon as stable anode material for potassium ion batteries. Adv. Mater 2020, 32, 2000505. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Cheng, H.; Hu, M.; Zhang, S. Classification and carbon structural transformation from anthracite to natural coaly graphite by XRD, Raman spectroscopy, and HRTEM. Spectrochim Acta A 2021, 249, 119286. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, X.; Pan, F.; Deng, B.; Liu, Z.; Zhang, X.; Huang, C.; Xiang, Z.; Lu, W. Mace-like carbon fiber/ZnO nanorod composite derived from Typha orientalis for lightweight and high-efficient electromagnetic wave absorber. Adv. Compos. Hybrid Mater 2021, 4, 1002–1014. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Zhang, Y.; He, W.; Zhu, M.; Wang, B.; Yan, H. , Hydrothermal synthesis of zinc oxide powders with controllable morphology. Ceram Int 2004, 30, 93–97. [Google Scholar] [CrossRef]
- Chang, X.; Li, K.; Qiao, X.; Xiong, Y.; Xia, F.; Xue, Q. ZIF-8 derived ZnO polyhedrons decorated with biomass derived nitrogen-doped porous carbon for enhanced acetone sensing. Sensor Actuat B: Chem 2021, 330, 129366. [Google Scholar] [CrossRef]
- Chen, J.; Lv, H.; Bai, X.; Liu, Z.; He, L.; Wang, J.; Zhang, Y.; Sun, B.; Kan, K.; Shi, K. Synthesis of hierarchically porous Co3O4/Biomass carbon composites derived from MOFs and their highly NO2 gas sensing performance. Microporous Mesoporous Mater 2021, 321, 111108. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, Z.; Sun, Q.; Sun, J.; Zhang, M.; Shaymurat, T.; Lv, C.; Duan, H. , Biomimetic gas sensor derived from disposable bamboo chopsticks for highly sensitive and selective detection of NH3. Chem. Eng. J 2023, 462, 142203. [Google Scholar] [CrossRef]
- Nada, A. A.; Orimolade, B. O.; El-Maghrabi, H. H.; Koiki, B. A.; Rivallin, M.; Bekheet, M. F.; Viter, R.; Damberga, D.; Lesage, G.; Iatsunskyi, I. Photoelectrocatalysis of paracetamol on Pd–ZnO/N-doped carbon nanofibers electrode. Appl. Mater. Today 2021, 24, 101129. [Google Scholar] [CrossRef]
- e Silva, R. L. d. S.; Franco Jr, A. Raman spectroscopy study of structural disorder degree of ZnO ceramics. Mater. Sci. Semicond. Process 2020, 119, 105227. [Google Scholar] [CrossRef]
- Hussain, M. Z.; Pawar, G. S.; Huang, Z.; Tahir, A. A.; Fischer, R. A.; Zhu, Y.; Xia, Y. Porous ZnO/Carbon nanocomposites derived from metal organic frameworks for highly efficient photocatalytic applications: A correlational study. Carbon 2019, 146, 348–363. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Li, R.; Xing, Y. MOF derived ZnO/C nanocomposite with enhanced adsorption capacity and photocatalytic performance under sunlight. J. Hazard. Mater 2020, 385, 121599. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Q.; Cai, W.; Shi, X.; Yang, D.-P.; Lin, H.; Qiu, E. C-doped ZnO Nanocomposites Molecularly Imprinted Photoelectrochemical Sensor for Ultrasensitive and Selective Detection of Oxytetracycline in Milk. Food Chem 2023, 426, 136535. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, Z.; Zhang, B.; Zhao, Y.; Yuan, Z.; Zhang, Y.; Wu, Y.; Jiang, Y.; Tai, H. Local Gaussian process regression with small sample data for temperature and humidity compensation of polyaniline-cerium dioxide NH3 sensor. Sensor Actuat B: Chem 2023, 378, 133113. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, T.; Wang, X.; Li, X.; Zhao, Y.; Li, F.; Zhao, G.; Xu, X. Investigation of pn sensing transition and related highly sensitive NH3 gas sensing behavior of SnPx/rGO composites. Chem. Eng. J 2023, 471, 144499. [Google Scholar] [CrossRef]
- Li, Q.; Hou, Y.; Wang, J.; Liu, Y.; Xiang, N.; Huang, Z. Superiority of raw biomass and potassium hydroxide in preparation of ultrahigh nitrogen doping of carbon for NH3-SCR reaction. ACS Sustainable Chem. Eng 2020, 8, 11308–11316. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Zhang, Y.; Zhang, Z.; Wang, X. Low-temperature SCR of NO with NH3 over activated semi-coke composite-supported rare earth oxides. Appl. Surf. Sci 2014, 309, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Zhang, L.; Fan, J.; Yu, J. 0D/2D CdS/ZnO composite with nn heterojunction for efficient detection of triethylamine. J. Colloid Interface Sci 2021, 600, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, P.; Feng, Y.; Li, D. Novel ultrathin mesoporous ZnO-SnO2 nn heterojunction nanosheets with high sensitivity to ethanol. Sensor Actuat B: Chem 2020, 309, 127801. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Xiao, Y.; Chen, T.; Xiao, X.; Wang, Y. Pt/MoS2/Polyaniline Nanocomposite as a Highly Effective Room Temperature Flexible Gas Sensor for Ammonia Detection. ACS Appl. Mater. Interfaces 2023, 15, 9604–9617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




