Submitted:
14 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
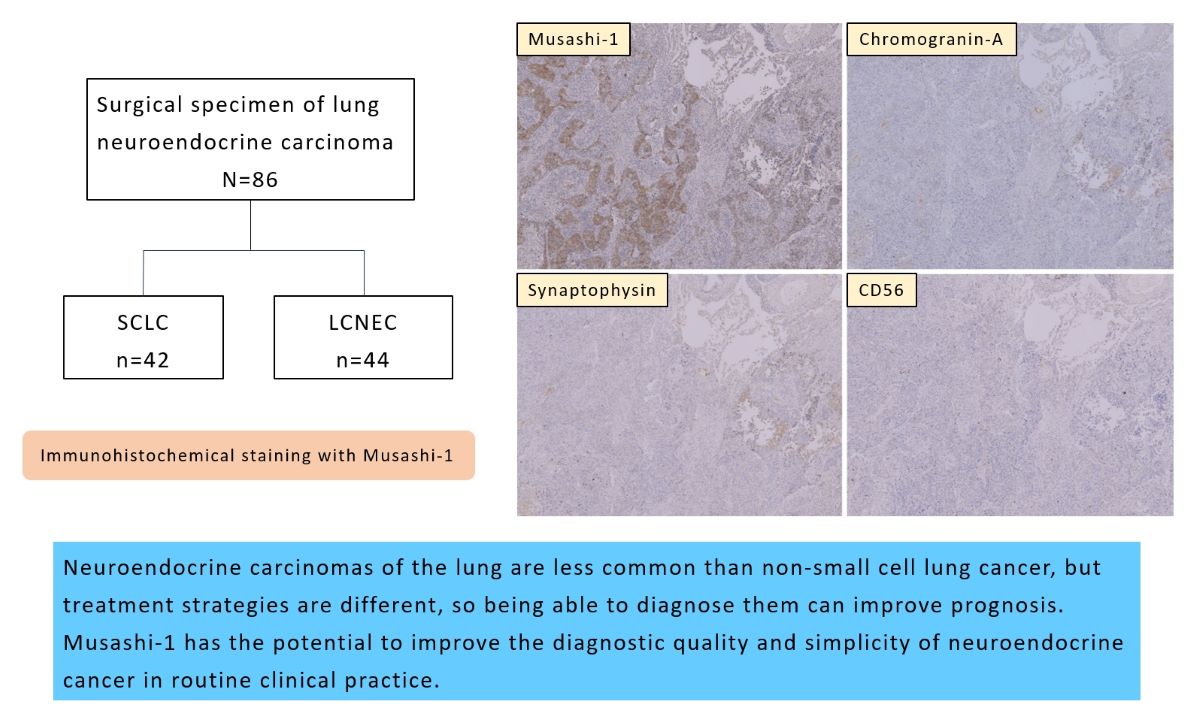
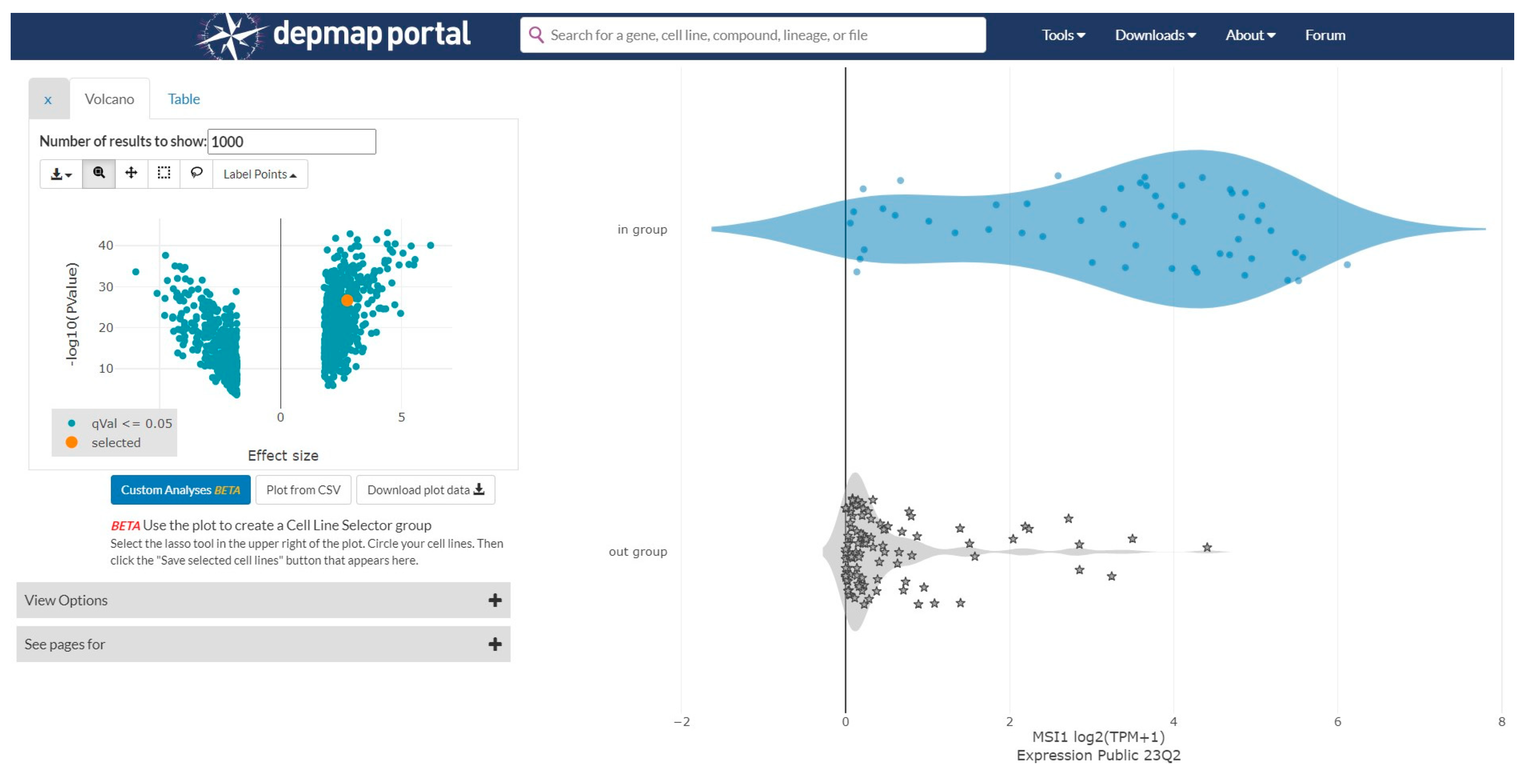
2.1. Gene Expression Data Analysis from the Cancer Dependency Map
2.2. Patients and Histological Samples
2.3. Immunohistochemical Procedures and Evaluation of Musashi-1 Expression
3. Results
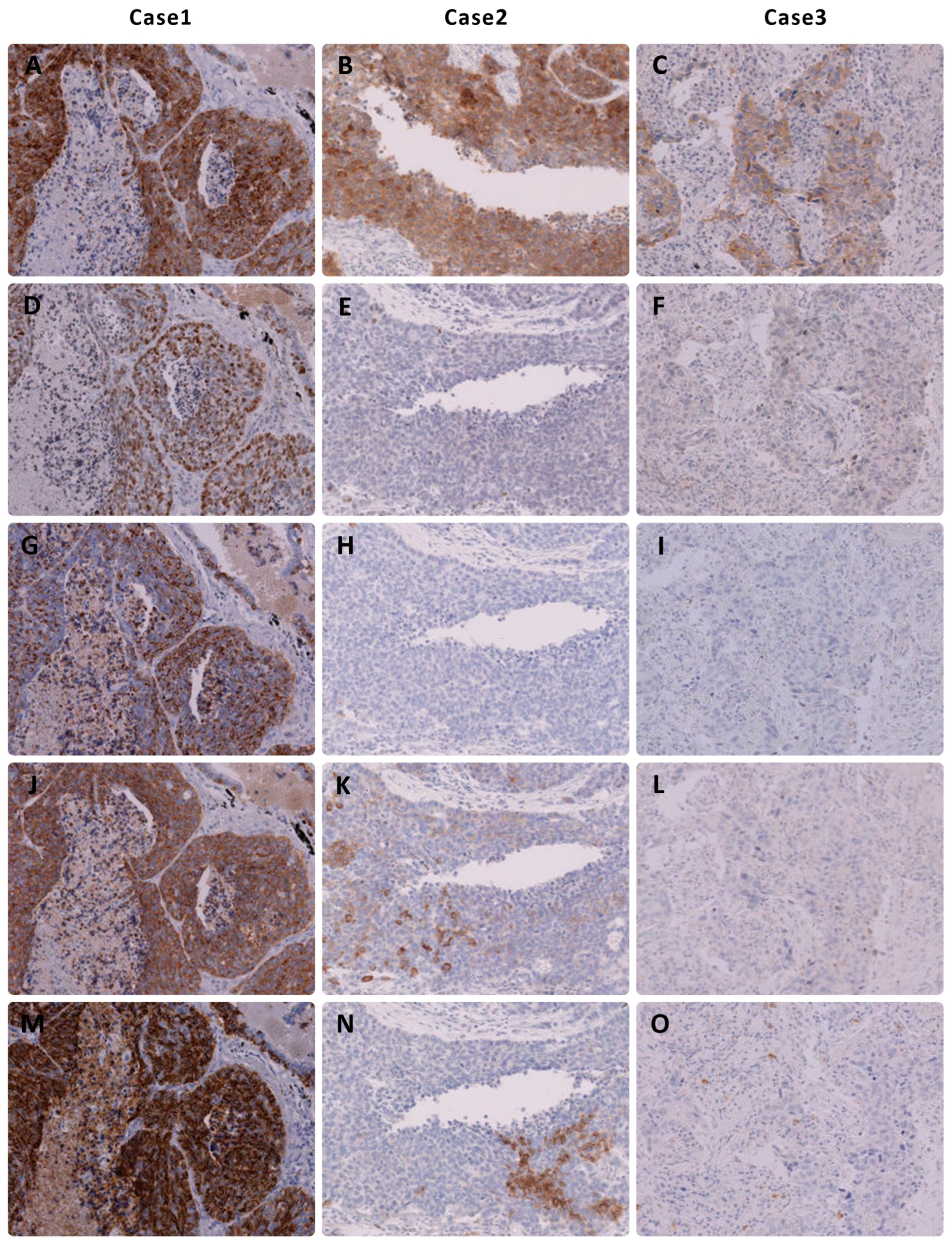
3.1. Immunohistochemical Results of NEC
3.2. Immunohistochemical Results of NSCLC
3.3. Results with a Cutoff of >10% Staining for Each Marker
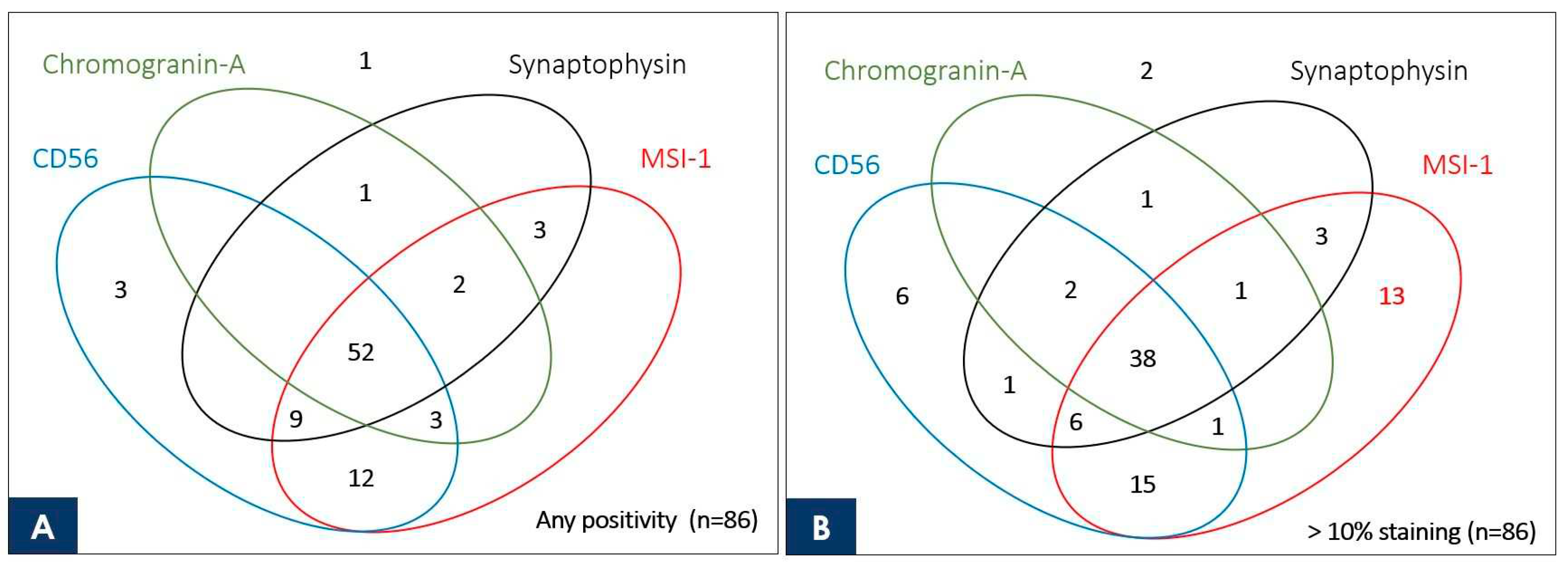
3.4. Venn Diagram Showing the Number of Duplicate Staining Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CANCER STATISTICS IN JAPAN - 2021, Foundation for Promotion of Cancer Research. Edited by Research FfPoC. Tokyo, Japan. ISSN 2433-3212.
- Society TJLC: Guidelines for Diagnosis and Treatment of the Lung Cancer 2022: Kanehara & Co., Ltd, 2022; ISBN 978-4-307-20456-9.
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nature Reviews Disease Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board (Ed.) WHO Classification of Tumours. In Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; ISBN 978-92-832-4506-3.
- Thunnissen, E.; Borczuk, A.C.; Flieder, D.B.; Witte, B.; Beasley, M.B.; Chung, J.-H.; Dacic, S.; Lantuejoul, S.; Russell, P.A.; den Bakker, M. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. An international reproducibility study in a demanding set of cases. Journal of thoracic oncology 2017, 12, 334-346.
- Rekhtman, N. Neuroendocrine tumors of the lung: an update. Archives of Pathology and Laboratory Medicine 2010, 134, 1628–1638. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dermawan, J.K.; Lanigan, C.P.; Farver, C.F. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Modern Pathology 2019, 32, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Yasufuku, K.; Kudoh, S.; Motooka, Y.; Sato, Y.; Wakimoto, J.; Kubota, I.; Suzuki, M.; Ito, T. INSM1 is the best marker for the diagnosis of neuroendocrine tumors: comparison with CGA, SYP and CD56. Int J Clin Exp Pathol 2017, 10, 5393–5405. [Google Scholar]
- Rooper, L.M.; Sharma, R.; Li, Q.K.; Illei, P.B.; Westra, W.H. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. The American journal of surgical pathology 2017, 41, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Švajdler, M.; Mezencev, R.; Šašková, B.; Ondič, O.; Mukenšnábl, P.; Michal, M. Triple marker composed of p16, CD56, and TTF1 shows higher sensitivity than INSM1 for diagnosis of pulmonary small cell carcinoma: proposal for a rational immunohistochemical algorithm for diagnosis of small cell carcinoma in small biopsy and cytology specimens. Human pathology 2019, 85, 58–64. [Google Scholar]
- Kaneko, Y.; Sakakibara, S.; Imai, T.; Suzuki, A.; Nakamura, Y.; Sawamoto, K.; Ogawa, Y.; Toyama, Y.; Miyata, T.; Okano, H. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Developmental neuroscience 2000, 22, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, S.; Imai, T.; Hamaguchi, K.; Okabe, M.; Aruga, J.; Nakajima, K.; Yasutomi, D.; Nagata, T.; Kurihara, Y.; Uesugi, S. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Developmental biology 1996, 176, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, T.; Røsland, G.V.; Sakariassen, P.O.; Kavalar, R.; Lah, T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surgical neurology 2007, 68, 133–143. [Google Scholar] [CrossRef]
- Chiou, G.-Y.; Yang, T.-W.; Huang, C.-C.; Tang, C.-Y.; Yen, J.-Y.; Tsai, M.-C.; Chen, H.-Y.; Fadhilah, N.; Lin, C.-C.; Jong, Y.-J. Musashi-1 promotes a cancer stem cell lineage and chemoresistance in colorectal cancer cells. Scientific reports 2017, 7, 2172. [Google Scholar] [CrossRef]
- Choi, J.E.; Bae, J.S.; Lee, J.H.; Jang, K.Y.; Chung, M.J.; Moon, W.S. Musashi-1 expression and clinicopathological significance in young gastric cancer patients: A matched case-control study. International Journal of Oncology 2014, 44, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-X.; Kameya, T.; Shoji, M.; Dobashi, Y.; Shinada, J.; Yoshimura, H. Large cell neuroendocrine carcinoma of the lung: a histologic and immunohistochemical study of 22 cases. The American journal of surgical pathology 1998, 22, 526–537. [Google Scholar] [CrossRef]
- Yang, L.; Fan, Y.; Lu, H. Pulmonary Large Cell Neuroendocrine Carcinoma. Pathology and Oncology Research 2022, 28, 1610730. [Google Scholar] [CrossRef] [PubMed]
- Baine, M.K.; Hsieh, M.-S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. Journal of Thoracic Oncology 2020, 15, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Miyata, Y.; Hirano, S.; Kimura, S.; Irisuna, F.; Ikeda, K.; Kushitani, K.; Tsutani, Y.; Ueda, D.; Tsubokawa, N. Therapeutic strategies and genetic profile comparisons in small cell carcinoma and large cell neuroendocrine carcinoma of the lung using next-generation sequencing. Oncotarget 2017, 8, 108936. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Pelosi, G.; Rekhtman, N. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. Journal of thoracic oncology 2019, 14, 377–407. [Google Scholar] [CrossRef]
- Tsai, H.K.; Hornick, J.L.; Vivero, M. INSM1 expression in a subset of thoracic malignancies and small round cell tumors: rare potential pitfalls for small cell carcinoma. Modern Pathology 2020, 33, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Kawahara, H.; Toriya, M.; Nakao, K.; Shibata, S.; Imai, T. Function of RNA-binding protein Musashi-1 in stem cells. Experimental cell research 2005, 306, 349–356. [Google Scholar] [CrossRef]
- Nickerson, P.; Myers, T.; Clarke, D.; Chow, R. Changes in Musashi-1 subcellular localization correlate with cell cycle exit during postnatal retinal development. Experimental eye research 2011, 92, 344–352. [Google Scholar] [CrossRef]
- Payne, C.; Hadfield, J.; Stovin, P.; Barker, V.; Heard, B.; Stark, J. Diagnostic accuracy of cytology and biopsy in primary bronchial carcinoma. Journal of Clinical Pathology 1981, 34, 773–778. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Chou, T.Y. Pulmonary neuroendocrine tumors: study of 90 cases focusing on clinicopathological characteristics, immunophenotype, preoperative biopsy, and frozen section diagnoses. Journal of surgical oncology 2014, 109, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.L.; Dingemans, A.M.C.; van Suylen, R.J.; den Bakker, M.A.; Damhuis, R.A.; van den Broek, E.C.; Speel, E.J.; Thunnissen, E. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology 2019, 74, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Seldenrijk, K.; de Bruin, P.; van Swieten, H.; van den Bosch, J. Pulmonary large-cell neuroendocrine carcinoma (LCNEC). European journal of cardio-thoracic surgery 2003, 23, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Abi-Raad, R.; Baldassarri, R.; Adeniran, A.J.; Cai, G. Expression of insulinoma-associated protein 1 in non–small cell lung cancers: A diagnostic pitfall for neuroendocrine tumors. Human Pathology 2021, 115, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, G.; Duan, Y.; Huo, Z. Prognostic value and non-neuroendocrine role of INSM1 in small cell lung cancer. Pathology-Research and Practice 2022, 229, 153693. [Google Scholar] [CrossRef]
- Brominska, B.; Gabryel, P.; Jarmołowska-Jurczyszyn, D.; Janicka-Jedyńska, M.; Trojanowski, M.; Sawicka-Gutaj, N.; Czepczyński, R.; Gut, P.; Bromiński, G.; Dyszkiewicz, W. Clinical significance of nestin and its association with survival in neuroendocrine lung tumours. Polish Journal of Pathology 2017, 68, 291–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Antibody to | Clone | Source | Dilution | Antigen Retrieval |
|---|---|---|---|---|
| Musashi-1 | EP-1302 | Abcam | 1:250 | CC1 -30 min |
| INSM1 | C-8 | Santa Cruz | 1:100 | CC1 -60 min |
| Chromogranin-A | LK2H10 | Ventana-Roche | prediluted | CC1 -60 min |
| Synaptophysin | MRQ-40 | Ventana-Roche | prediluted | CC1 -60 min |
| CD56 | MRQ-42 | Ventana-Roche | prediluted | CC1 -30 min |
| SCLC | Total Cases |
Number of Positive Cases | Immunohistochemical Score | |||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |||
| Musashi-1 | 42 | 41 (98%) | 1 | 1 | 6 | 34 |
| INSM1 | 42 | 40 (95%) | 2 | 4 | 2 | 34 |
| Chromogranin-A | 42 | 35 (83%) | 7 | 7 | 6 | 22 |
| Synaptophysin | 42 | 38 (90%) | 4 | 7 | 0 | 31 |
| CD56 | 42 | 40 (95%) | 2 | 0 | 9 | 31 |
| LCNEC | Total Cases |
Number of Positive Cases | Immunohistochemical score | |||
| 0 | 1+ | 2+ | 3+ | |||
| Musashi-1 | 44 | 40 (91%) | 4 | 6 | 6 | 28 |
| INSM1 | 44 | 30 (68%) | 14 | 10 | 6 | 14 |
| Chromogranin-A | 44 | 23 (52%) | 21 | 8 | 1 | 14 |
| Synaptophysin | 44 | 29 (66%) | 15 | 11 | 1 | 17 |
| CD56 | 44 | 39 (89%) | 5 | 10 | 6 | 23 |
| NSCLC | Total Cases |
Number of Positive Cases | Immunohistochemical score | |||
| 0 | 1+ | 2+ | 3+ | |||
| Musashi-1 | 80 | 14 (18%) | 66 | 10 | 3 | 1 |
| INSM1 | 80 | 10 (13%) | 70 | 10 | 0 | 0 |
| Chromogranin-A | 80 | 3 (4%) | 77 | 3 | 0 | 0 |
| Synaptophysin | 80 | 15 (19%) | 66 | 10 | 4 | 1 |
| CD56 | 80 | 15 (19%) | 65 | 11 | 4 | 0 |
| Any positivity | > 10% Positive cells | |||||
|---|---|---|---|---|---|---|
| sensitivity | specificity | sensitivity | specificity | |||
| SCLC n = 42 |
LCNEC n = 44 |
n = 80 |
SCLC n = 42 |
LCNEC n = 44 |
n = 80 |
|
| Musashi-1 | 41 (98%) | 40 (91%) | 66 (83%) | 40 (95%) | 34 (77%) | 76 (95%) |
| INSM1 | 40 (95%) | 30 (68%) | 70 (87%) | 36 (86%) | 20 (45%) | 80 (100%) |
| Chromogranin-A | 35 (83%) | 23 (52%) | 77 (96%) | 28 (67%) | 15 (34%) | 80 (100%) |
| Synaptophysin | 38 (90%) | 29 (66%) | 65 (81%) | 31 (73%) | 18 (41%) | 75 (94%) |
| CD56 | 40 (95%) | 39 (89%) | 65 (81%) | 40 (95%) | 29 (66%) | 76 (95%) |
| Case | MSI-1 | Syn | CD56 | ChrA | ISNM1 | type | pStage | smoke (BI) | rec |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3+ | 0 | 2 | 0 | 1 | Ad (pap) | IA2 | +(1600) | - |
| 2 | 2+ | 0 | 0 | 0 | 0 | Sq | IA2 | +(1000) | - |
| 3 | 2+ | 0 | 0 | 0 | 1 | Sq | IB | +(860) | - |
| 4 | 2+ | 1 | 0 | 0 | 0 | Sq | IB | +(600) | - |
| 5 | 1+ | 0 | 0 | 0 | 1 | Sq | IIB | +(3000) | - |
| 6 | 1+ | 0 | 2 | 0 | 0 | Sq | IIIB | +(2700) | + |
| 7 | 1+ | 0 | 1 | 0 | 0 | Sq | IB | +(1920) | - |
| 8 | 1+ | 0 | 1 | 0 | 1 | Sq | IB | +(1800) | - |
| 9 | 1+ | 1 | 0 | 0 | 0 | Sq | IIIA | +(1380) | + |
| 10 | 1+ | 0 | 0 | 0 | 1 | Ad (aci) | IB | +(1320) | - |
| 11 | 1+ | 0 | 1 | 0 | 0 | Sq | IB | +(1200) | + |
| 12 | 1+ | 0 | 0 | 1 | 1 | Ad (pap) | IB | +(1075) | - |
| 13 | 1+ | 0 | 0 | 0 | 0 | Sq | IB | +(660) | - |
| 14 | 1+ | 0 | 0 | 0 | 0 | Sq | IA2 | +(600) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





