Submitted:
14 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chang Gung Research Database (CGRD)
2.2. Patient Selection
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Clinical Manifestations
3.2. Radiologic and Laboratory Diagnosis
3.3. Treatment Strategies and Complications
4. Discussion
4.1. The Previous Studies
4.2. Our IFSD Score
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Grammatico, L., et al., Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002-2003. Epidemiol Infect, 2008. 136(5): p. 653-60. [CrossRef]
- Beronius, M., B. Bergman, and R. Andersson, Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis, 2001. 33(7): p. 527-32. [CrossRef]
- Babic, M. and C.S. Simpfendorfer, Infections of the Spine. Infect Dis Clin North Am, 2017. 31(2): p. 279-297. [CrossRef]
- Kim, C.W., et al., Fungal infections of the spine. Clin Orthop Relat Res, 2006. 444: p. 92-9. [CrossRef]
- Geisler Crone, C., et al., Clinical characteristics of pyogenic vertebral osteomyelitis, and factors associated with inadequate treatment response. Int J Infect Dis, 2021. 108: p. 487-493. [CrossRef]
- Gouliouris, T., S.H. Aliyu, and N.M. Brown, Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother, 2010. 65 Suppl 3: p. iii11-24. [CrossRef]
- Hadjipavlou, A.G., et al., Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976), 2000. 25(13): p. 1668-79. [CrossRef]
- Parry, M.F., et al., Candida osteomyelitis and diskitis after spinal surgery: an outbreak that implicates artificial nail use. Clin Infect Dis, 2001. 32(3): p. 352-7. [CrossRef]
- Tay, B.K., J. Deckey, and S.S. Hu, Spinal infections. J Am Acad Orthop Surg, 2002. 10(3): p. 188-97. [CrossRef]
- Wu, M.-H., et al., Treatment outcomes of fungal vertebral osteomyelitis: A case series study and literature review. Formosan Journal of Musculoskeletal Disorders, 2015. 6: p. 89-97. [CrossRef]
- Talha, K.M., et al., Native Vertebral Osteomyelitis in Patients with Staphylococcus Aureus Bacteremia. Am J Med Sci, 2022. 363(2): p. 140-146. [CrossRef]
- Cobar, A., et al., Journal of Spine Fungal Spondylodiscitis: Review. Journal of Spine, 2016. 5. [CrossRef]
- Frazier, D.D., et al., Fungal infections of the spine. Report of eleven patients with long-term follow-up. J Bone Joint Surg Am, 2001. 83(4): p. 560-5.
- Caldera, G., et al., Fungal Spondylodiscitis: Review. Journal of Spine, 2016. 2016: p. 1-6. [CrossRef]
- Ganesh, D., et al., Fungal Infections of the Spine. Spine, 2015. 40(12): p. E719-E728. [CrossRef]
- Ascioglu, S., et al., Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis, 2002. 34(1): p. 7-14. [CrossRef]
- Peter Donnelly, J., Consensus definitions for invasive fungal disease: Strengths, limitations, and revisions. Med Mycol, 2006. 44(Supplement_1): p. S285-s288. [CrossRef]
- De Pauw, B., et al., Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis, 2008. 46(12): p. 1813-21. [CrossRef]
- Donnelly, J.P., et al., Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis, 2020. 71(6): p. 1367-1376. [CrossRef]
- Tsitsikas, D.A., et al., Impact of the revised (2008) EORTC/MSG definitions for invasive fungal disease on the rates of diagnosis of invasive aspergillosis. Med Mycol, 2012. 50(5): p. 538-42. [CrossRef]
- Richaud, C., et al., Candida vertebral osteomyelitis (CVO) 28 cases from a 10-year retrospective study in France. Medicine (Baltimore), 2017. 96(31): p. e7525. [CrossRef]
- Tsai, M.S., et al., Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed J, 2017. 40(5): p. 263-269. [CrossRef]
- Shao, S.C., et al., The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf, 2019. 28(5): p. 593-600. [CrossRef]
- Nesković, V., Preoperative assesment of the immunocompromised patient. Acta Chir Iugosl, 2011. 58(2): p. 185-92. [CrossRef]
- Miller, D.J. and G.C. Mejicano, Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis, 2001. 33(4): p. 523-30. [CrossRef]
- Issa, K., et al., Delay in Diagnosis of Vertebral Osteomyelitis Affects the Utility of Cultures. Surg Technol Int, 2016. 29: p. 379-383.
- Grados, F., et al., Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine, 2007. 74(2): p. 133-9. [CrossRef]
- Boody, B.S., et al., Vertebral Osteomyelitis and Spinal Epidural Abscess: An Evidence-based Review. J Spinal Disord Tech, 2015. 28(6): p. E316-27. [CrossRef]
- Cornett, C.A., et al., Bacterial Spine Infections in Adults: Evaluation and Management. J Am Acad Orthop Surg, 2016. 24(1): p. 11-8. [CrossRef]
- Enoch, D.A., et al., Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol, 2008. 61(6): p. 750-3. [CrossRef]
- Chew, F.S. and M.J. Kline, Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology, 2001. 218(1): p. 211-4. [CrossRef]
- Diehn, F.E., Imaging of spine infection. Radiol Clin North Am, 2012. 50(4): p. 777-98. [CrossRef]
- Zimmerli, W., Clinical practice. Vertebral osteomyelitis. N Engl J Med, 2010. 362(11): p. 1022-9. [CrossRef]
- Foreman, S.C., et al., MR and CT Imaging to Optimize CT-Guided Biopsies in Suspected Spondylodiscitis. World Neurosurg, 2017. 99: p. 726-734.e7. [CrossRef]
- Gathe, J.C., Jr., et al., Candida osteomyelitis. Report of five cases and review of the literature. Am J Med, 1987. 82(5): p. 927-37. [CrossRef]
- Treglia, G., et al., The role of nuclear medicine in the diagnosis of spondylodiscitis. Eur Rev Med Pharmacol Sci, 2012. 16 Suppl 2: p. 20-5.
- Sethi, S., et al., Aspergillus vertebral osteomyelitis in immunocompetent patients. Indian J Orthop, 2012. 46(2): p. 246-50. [CrossRef]
- Sapico, F.L. and J.Z. Montgomerie, Vertebral osteomyelitis. Infect Dis Clin North Am, 1990. 4(3): p. 539-50. [CrossRef]
- Waldvogel, F.A., G. Medoff, and M.N. Swartz, Osteomyelitis: a review of clinical features, therapeutic considerations, and unusual aspects. N Engl J Med, 1970. 282(4): p. 198-206. [CrossRef]
- Bonakdar-pour, A. and V.D. Gaines, The radiology of osteomyelitis. Orthop Clin North Am, 1983. 14(1): p. 21-37. [CrossRef]
- Golimbu, C., H. Firooznia, and M. Rafii, CT of osteomyelitis of the spine. AJR Am J Roentgenol, 1984. 142(1): p. 159-63. [CrossRef]
- Lew, D.P. and F.A. Waldvogel, Osteomyelitis. N Engl J Med, 1997. 336(14): p. 999-1007. [CrossRef]
- Kang, M., et al., CT-guided fine-needle aspiration biopsy of spinal lesions. Acta Radiol, 1999. 40(5): p. 474-8. [CrossRef]
- Broner, F.A., D.E. Garland, and J.E. Zigler, Spinal infections in the immunocompromised host. Orthop Clin North Am, 1996. 27(1): p. 37-46. [CrossRef]
- Gerometta, A., F. Bittan, and J.C. Rodriguez Olaverri, Postoperative spondilodiscitis. Int Orthop, 2012. 36(2): p. 433-8. [CrossRef]
- Kushwaha, V.P., et al., Musculoskeletal coccidioidomycosis. A review of 25 cases. Clin Orthop Relat Res, 1996(332): p. 190-9.
- Almekinders, L.C. and W.B. Greene, Vertebral Candida infections. A case report and review of the literature. Clin Orthop Relat Res, 1991(267): p. 174-8.
- Bross, J., et al., Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. Am J Med, 1989. 87(6): p. 614-20. [CrossRef]
- Williams, R.L., et al., Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol, 1999. 20(3): p. 381-5.
- Tang, T.J., et al., Aspergillus osteomyelitis after liver transplantation: conservative or surgical treatment? Eur J Gastroenterol Hepatol, 2000. 12(1): p. 123-6.
- Hummel, M., et al., Aspergillosis with Aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J Heart Lung Transplant, 1993. 12(4): p. 599-603.
- Rex, J.H., et al., Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis, 2000. 30(4): p. 662-78. [CrossRef]
- Dellinger, R.P., et al., Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med, 2013. 41(2): p. 580-637. [CrossRef]
- Devran, O., et al., C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip Respir Med, 2012. 7(1): p. 47. [CrossRef]
- WHO, Hemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity. World Health Organization, Geneva, 2011.
- Nahm, F.S., Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol, 2022. 75(1): p. 25-36. [CrossRef]
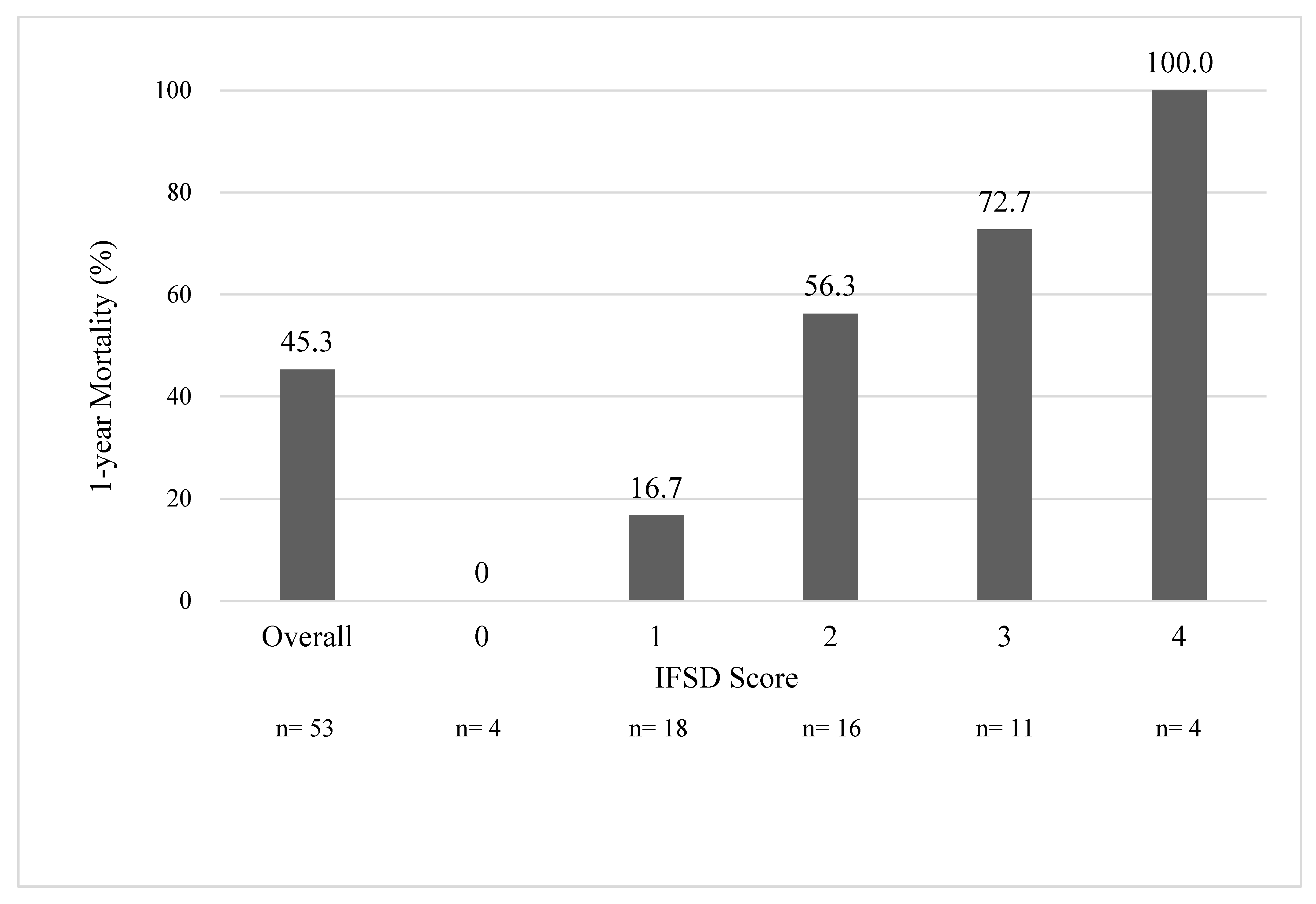
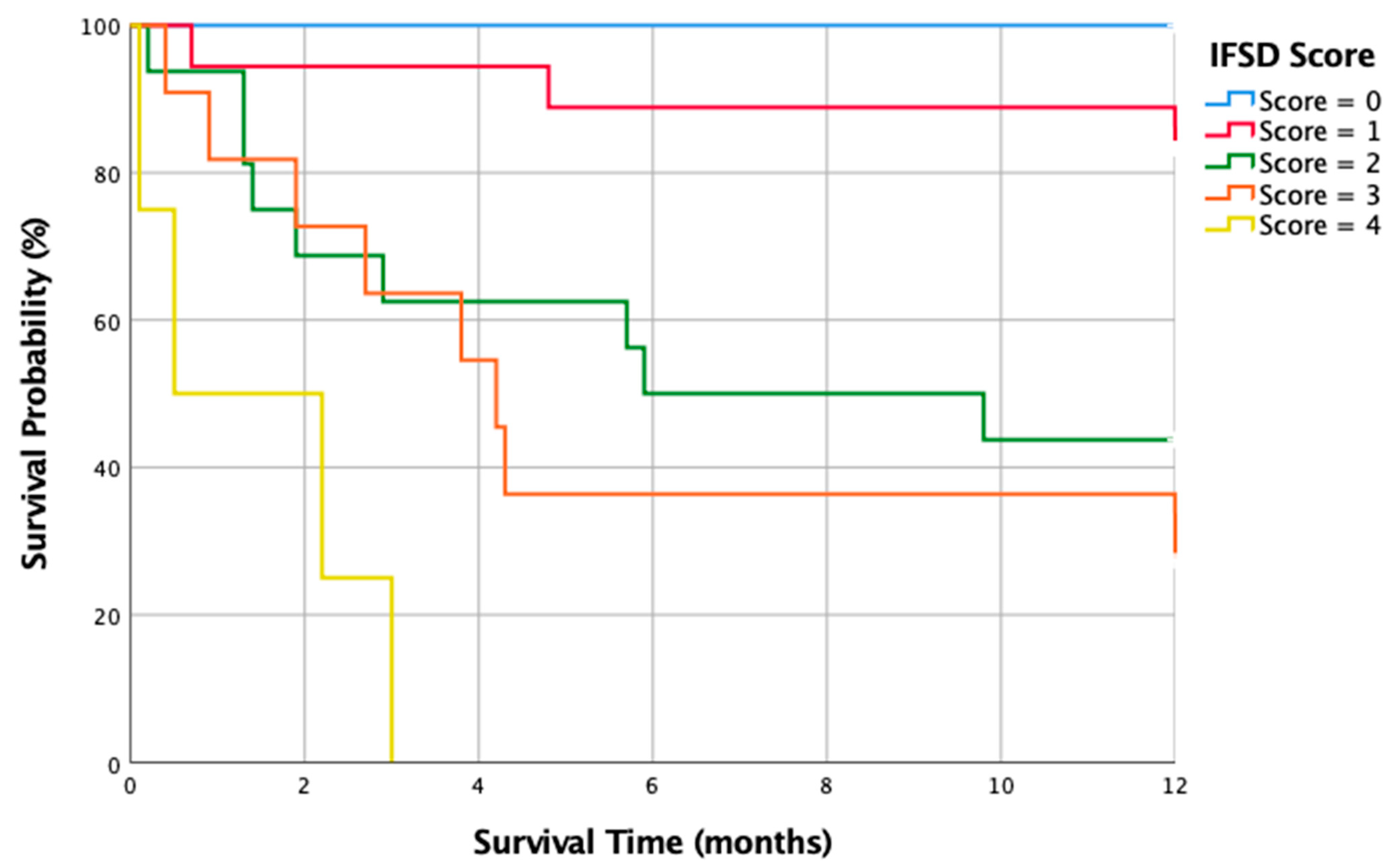
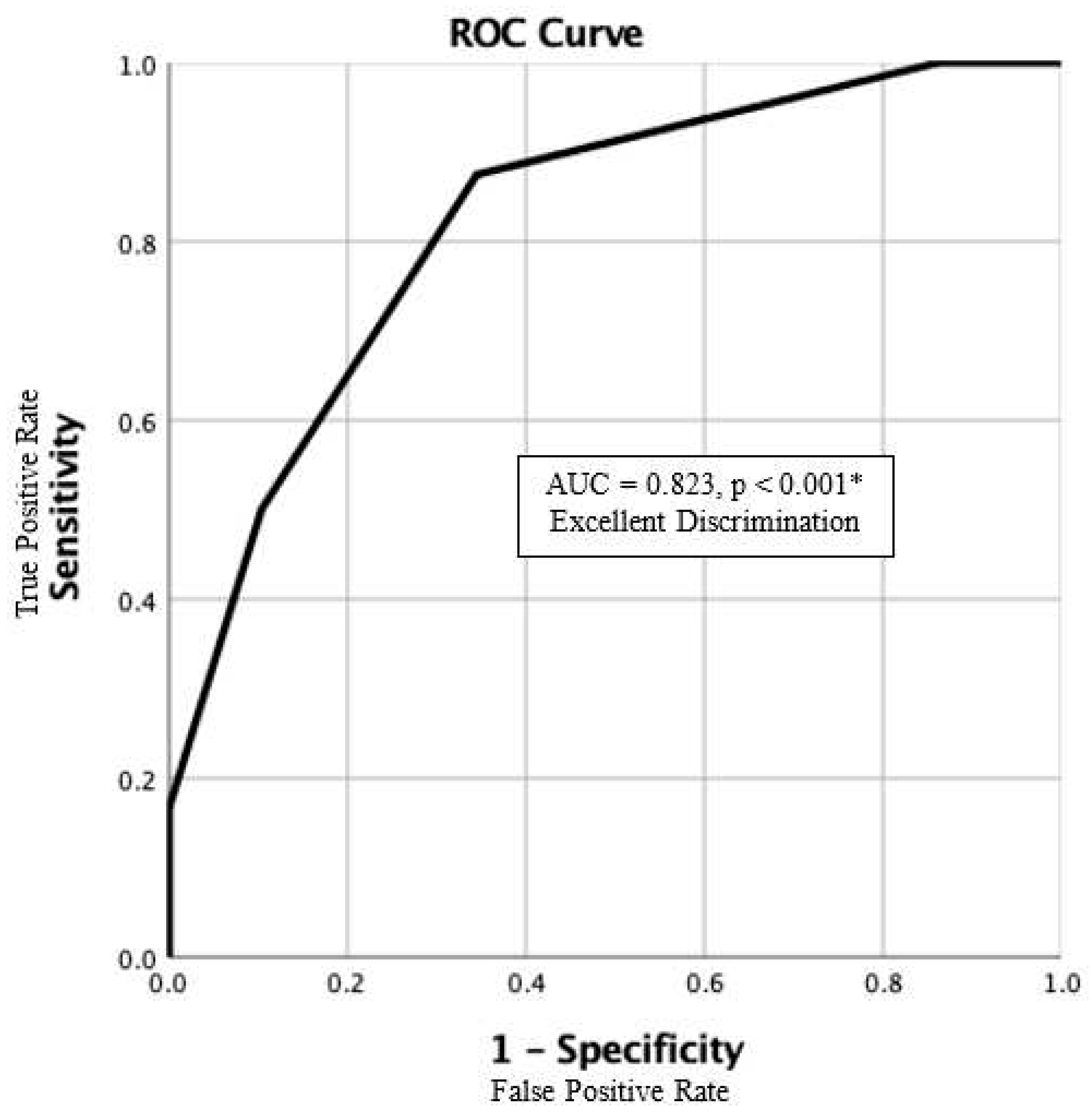
| All | 1-year Mortality | |||
| Dead (n=24) | Survivors (n=29) | p value | ||
|
Age, years, median, interquartile range |
65.6 (56.1-74.0) | 67.5 (59.1-74.8) | 64.0 (49.4-73.8) | 0.090 |
| Male, n, % | 35 (66.0) | 16 (66.7) | 19 (65.5) | 0.930 |
| Predisposing Immunocompromised State, n, % | 17 (32.1) | 12 (50.0) | 5 (17.2) | 0.011 |
| Initial Clinical Manifestations | ||||
| Fever, n, % | 30 (56.6) | 15 (62.5) | 15 (51.7) | 0.431 |
| Radicular pain, n, % | 33 (62.3) | 15 (62.5) | 18 (62.1) | 0.974 |
| Muscular weakness, n, % | 23 (43.4) | 11 (45.8) | 12 (41.4) | 0.745 |
| Myelopathy, n, % | 7 (13.2) | 4 (16.7) | 3 (10.3) | 0.499 |
| Onset time to diagnosis, weeks, median, interquartile range |
3.0 (2.0-5.0) | 3.0 (1.8-4.0) | 3.0 (2.0-8.0) | 0.578 |
| Initial Radiological Findings | ||||
| X-rays | ||||
| Disc space narrowing, n, % | 35 (79.5) | 12 (75.0) | 23 (82.1) | 0.572 |
| Endplate erosion, n, % | 30 (68.2) | 9 (56.3) | 20 (71.4) | 0.307 |
| VB collapse, n, % | 10 (22.7) | 2 (12.5) | 8 (28.6) | 0.221 |
| CTs | ||||
| Endplate erosion, n, % | 23 (88.5) | 12 (85.7) | 10 (91.7) | 0.867 |
| VB collapse, n, % | 15 (57.7) | 9 (64.3) | 6 (50.0) | 0.462 |
| Paraspinal tissue abscess, n, % | 23 (88.5) | 11 (78.6) | 11 (100.0) | 0.356 |
| Epidural abscess, n, % | 17 (65.4) | 7 (50.0) | 16 (83.3) | 0.006* |
| MRIs | ||||
| Loss of intradiscal key sign, n, % | 44 (93.6) | 18 (94.7) | 26 (92.9) | 0.796 |
| VB signal change T2 edema, n, % | 38 (80.9) | 15 (78.9) | 23 (82.1) | 0.785 |
| Cord/Sac compression, n, % | 35 (79.5) | 12 (75.0) | 23 (82.1) | 0.572 |
| Root compression, n, % | 30 (68.2) | 9 (56.3) | 20 (71.4) | 0.307 |
| Paraspinal tissue abscess, n, % | 10 (22.7) | 10 (22.7) | 8 (28.6) | 0.221 |
| Bone scans | ||||
| Positive for spondylodiscitis, n, % | 13 (76.5) | 5 (55.6) | 8 (100.0) | 0.001* |
| Inflammatory scans | ||||
| Positive for spondylodiscitis, n, % | 19 (86.4) | 6 (66.7) | 13 (100.0) | 0.069 |
| Pre-treatment Lab Findings | ||||
| WBC, 103/uL, median, IQ range | 10.5 (7.1-13.7) | 12.4 (8.9-14.2) | 11.0 (7.6-12.5) | 0.040* |
| Hb, g/dL, median, IQ range | 9.7 (8.6-10.9) | 9.0 (8.2-10.1) | 10.2 (9.4-11.2) | 0.034* |
| Platelet, 103/uL, median, IQ range | 257 (134.0-350.0) | 169 (101.5-293.5) | 291 (179.0-407.0) | 0.021* |
| ESR, mm/h, median, IQ range | 86 (56.5-103) | 95 (58.0-112.3) | 82 (57.0-101.0) | 0.585 |
| CRP, mg/dL, median, IQ range | 94 (41.0-162.8) | 150 (91.3-195.5) | 50 (25.0-112.0) | 0.002* |
| Candidemia, n, % | 25 (47.2) | 16 (66.7) | 9 (31.0) | 0.010* |
| Fungal Species | ||||
| Aspergillus unspecified, n, % | 1 (1.9) | 1 (4.2) | 0 (0.0) | - |
| Candida albicans, n, % | 32 (60.4) | 11 (45.8) | 21 (72.4) | 0.049* |
| Candida glabrata, n, % | 3 (5.7) | 2 (8.3) | 1 (3.4) | 0.444 |
| Candida krusei, n, % | 1 (1.9) | 0 (0.0) | 1 (3.4) | - |
| Candida parapsilosis, n, % | 4 (7.5) | 2 (8.3) | 2 (6.9) | 0.844 |
| Candida tropicalis, n, % | 7 (13.2) | 4 (16.7) | 3 (10.3) | 0.499 |
| Canda lusitaniae, n, % | 1 (1.9) | 1 (4.2) | 0 (0.0) | - |
| Candida unspecified, n, % | 1 (1.9) | 1 (4.2) | 0 (0.0) | - |
| Cryptococcus neoformans, n, % | 1 (1.9) | 1 (4.2) | 0 (0.0) | - |
| Debaryomyces hansenii | 1 (1.9) | 1 (4.2) | 0 (0.0) | - |
| Fonsecaea pedrosoi | 1 (1.9) | 0 (0.0) | 1 (3.4) | - |
| Treatment Strategies | ||||
| Non-surgical treatment, n, % | 18 (34.0) | 13 (54.2) | 5 (17.2) | 0.005* |
| Surgical intervention, n, % | 35 (66.0) | 11 (45.8) | 24 (82.8) | - |
| Debridement Only, n, % | 11 (31.4) | 4 (36.4) | 7 (37.5) | 0.011* |
| Debridement + Instrumentation n, % | 24 (68.6) | 9 (63.6) | 16 (62.5) | - |
| Multiple-stage OP, n, % | 25 (71.4) | 8 (80.0) | 16 (64.0) | 0.002* |
| 1-stage OP, n, % | 10 (28.6) | 2 (20.0) | 9 (36.0) | - |
| Surgical Related Complications | ||||
| Superficial wounds infection, n, % | 5 (9.4) | 1 (4.2) | 4 (13.8) | 0.233 |
| Reoperation for debridement, n, % | 7 (13.2) | 3 (12.5) | 4 (13.8) | 0.890 |
| General Complications | ||||
| Respiratory failure, n, % | 19 (35.8) | 15 (62.5) | 3 (13.8) | <0.001* |
| Hospital acquired pneumonia, n, % | 17 (32.1) | 14 (58.3) | 3 (10.3) | <0.001* |
| Acute kidney injury, n, % | 13 (24.5) | 11 (45.8) | 2 (6.9) | 0.001* |
| Urinary tract infection, n, % | 13 (24.5) | 7 (29.2) | 6 (20.7) | 0.475 |
| Electrolyte imbalance, n, % | 18 (34.0) | 12 (50.0) | 6 (20.7) | 0.025* |
| Acute coronary syndrome, n, % | 3 (5.7) | 2 (8.3) | 1 (3.4) | 0.444 |
| Metabolic encephalopathy, n, % | 10 (18.9) | 9 (37.5) | 1 (3.4) | 0.002* |
| UGI bleeding, n, % | 10 (18.9) | 7 (29.2) | 3 (10.3) | 0.081 |
| Pressure sore, n, % | 4 (7.5) | 1 (4.2) | 3 (10.3) | 0.397 |
| Hazard ratio | 95.0% Confidence interval | p value | ||
| Lower | Upper | |||
| Age > 65 | 1.53 | 0.63 | 3.69 | 0.346 |
| Immunocompromised | 3.01 | 1.15 | 7.84 | 0.024* |
| BT > 38.3 or < 36 | 2.44 | 0.84 | 7.11 | 0.103 |
| Radiculopathy or Myelopathy | 4.04 | 1.37 | 11.93 | 0.012* |
| WBC > 1.2 or < 0.4 | 2.83 | 1.00 | 7.95 | 0.049* |
| Hemoglobin < 8 | 4.93 | 1.39 | 17.50 | 0.014* |
| Platelet < 100 | 0.86 | 0.24 | 3.17 | 0.826 |
| CRP > 100 | 1.90 | 0.68 | 5.29 | 0.218 |
| Candidemia | 2.78 | 0.99 | 7.77 | 0.052 |
| The IFSD Score | ||
| Component | IFSD Score Points | |
| Immune State | ||
| Immunocompromised | 1 | |
| Immunocompetent | 0 | |
| Neurological Deficits | ||
| Radiculopathy or Myelopathy | 1 | |
| None | 0 | |
| WBC Count (103/uL) | ||
| > 12.0 or < 0.4 | 1 | |
| 0.4 to 12.0 | 0 | |
| Hemoglobin (g/dL) | ||
| <8 | 1 | |
| ≧8 | 0 | |
| Evidence of Candidemia | ||
| Yes | 1 | |
| None | 0 | |
| Overall IFSD score | Sum of the points above (0-5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





