1. Introduction
The British Association of Dermatologists states that Basal Cell Carcinoma (BCC) is the predominant malignancy among non–melanoma skin cancers in Caucasians, arising from epidermal cell origins. Its occurrence in the nasal region presents intricate challenges for surgeons due to anatomical, functional, and aesthetic issues [
1,
2,
3,
4]. The prevalence of this heterogeneous group of tumors, encompassing a spectrum of histopathological and clinical traits spanning from superficial lesions to extensive, destructive manifestations, has demonstrated a consistent upward trend on a global scale in recent decades, notably among the elderly population [
1,
5,
6,
7]. In European countries, including Lithuania, the average life expectancy of the population is on the rise. This demographic shift is accompanied by an annual increase of approximately 5 % in the incidence of BCC cases, accounting for 80 % of the total prevalence in the facial region, with 31.3 % specifically located on the nose [
3,
4,
8,
9]. The geographic distribution of this tumor exhibits variability attributed to ethnic disparities and the diverse range of skin phenotypes. The highest reported incidence rates for BCC are observed in Australia, ranging from 726 to 1000 cases per 100,000 person–years, while the lowest incidence is found in Africa, with fewer than one case per 100,000 person–years. In European countries such as Ireland, Great Britain, Finland, Lithuania, Germany, Switzerland, Italy, France, and Spain, the incidence rates range from 44.6 to 157 cases per 100,000 individuals annually [
4,
10]. As a result, early diagnosis and effective treatment of such tumors are becoming increasingly pertinent in the routine clinical practice of physicians. According to the National Comprehensive Cancer Network (NCCN) guidelines, basal cell carcinoma is stratified into low– and high–risk tumors, as well as locally advanced and metastatic disease, contingent on histopathological subtypes and diverse anatomical localizations. This classification necessitates distinct treatment approaches, leading to notable disparities in therapeutic algorithms for each category [
11,
12]. Upon scrutiny of the most recent treatment guidelines for BCC, it was identified that in 2019, a multidisciplinary European consensus introduced a novel classification, stratifying BCC into categories of 'easy' and 'difficult' to manage oncological conditions. The therapeutic approach for 'difficult–to–treat' BCC encompasses the use of Hedgehog inhibitors such as vismodegib or sonidegib, immunotherapy involving PD–1 antibodies, and radiotherapy [
13,
14]. The first–line strategy for 'easy–to–treat' BCC is radical tumor excision, considered a pivotal quality factor in the management of skin cancer [
6,
13,
15]. Recent literature indicates that incomplete tumor excision amplifies the risk of recurrence [
6,
12,
16]. These figures vary based on the data provided by different authors, but on average, the occurrence of non–radical surgery falls within a range of 6 % to 50 %, while recurrence rates span from 3 % to 38 %. Notably, surgical excision of BCC on the nose exhibited a recurrence rate 2.5 times higher than that observed in skin cancer at other anatomical sites [
8,
17,
18,
19]. Indeed, given that re–excision of BCC and subsequent treatment demands more intricate surgical considerations and entails higher costs, this amplifies the burden on both the healthcare system and the affected individuals. Consequently, the successful treatment of BCC continues to be a crucial healthcare concern [
13]. In this research study, we seek to assess the age, gender, and tumor characteristics of patients who received treatment at the Department of Plastic and Reconstructive Surgery, Hospital of Lithuanian University of Health Sciences, Kaunas Clinics, and underwent surgical procedures for the removal of nasal basal cell carcinoma. The secondary aim of this study is to analyze the level of surgical radicality achieved through the employed treatment methods, and subsequently, investigate the potential correlations between radicality and the recurrence rate.
2. Materials and Methods
A retrospective study was conducted on patients with Basal Cell Carcinoma (BCC) in the nasal region at the Department of Plastic and Reconstructive Surgery, Hospital of Lithuanian University of Health Sciences Kaunas Clinics. Ethical approval for this study was obtained from Kaunas Regional Biomedical Research Ethics Committee (16 December 2022, No. BEC–MF–123). Scientific research focused on patients who underwent standard surgical BCC excisions of the nose between January 1, 2019, and December 31, 2022. The study involved the analysis of patients' medical records, which were accessed through the Hygiene Institute Health Information Centre, utilizing the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD–10–CM) code: C44.3. After a comprehensive review of 572 case histories, 343 eligible patients were identified and included in the study. Patients were only included if they met all the study’s inclusion criteria: they were older than 18 years old during inclusion in the study, possess a confirmed diagnosis of basal cell carcinoma confined to the nasal region, as validated by the ICD–10–AM code: C44.3. Additionally, they were mandated to provide informed written consent prior to their participation in the research. The exclusion criteria precluded individuals falling within the following categories: those below 18 years of age, patients with incomplete medical records lacking precise information regarding the localization, size, or type of surgical intervention for basal cell carcinoma, and individuals who expressed dissent towards participation in the research. Throughout this study, subjects' confidentiality was meticulously safeguarded. All patient health data were anonymized and encoded to prevent direct identification. Dissemination of medical information to third parties or institutions only occurred in compliance with legal mandates. Retrospectively, information pertaining to age, gender, mean size of the basal cell carcinoma in the nasal region, surgical modality, histopathological assessments, surgical radicality, and recurrence frequency in the study cohort was gathered. Subsequently, the acquired data underwent statistical analysis, enabling comparisons and the elucidation of the aforementioned criteria integral to the research evaluation. The data were collected and organized utilizing Microsoft Office Excel 2019. Statistical analyses were conducted using the IBM Statistical Package for Social Sciences (SPSS) 27.0 software. The normality assumption of continuous variables was assessed employing the Kolmogorov–Smirnov test. In the realm of analytical statistics, for bivariate data analyses, the Chi–square (χ2) criterion, Student's t–test (applied for normally distributed variables), and Mann–Whitney U test (utilized for non–normally distributed variables) were employed to assess the distribution of phenomena across groups. Normally distributed data were expressed as mean ± standard deviation, while non–normally distributed data were presented as median or as both median and mean in the event of identical medians. Statistically significant disparities and associations between features were considered valid when the calculated p–value was less than the predetermined significance level (α = 0.05).
3. Results
Throughout the course of this research endeavor, a total of 572 surgical interventions were conducted for the removal of basal cell carcinoma in the nasal region. Of these, 343 patients were deemed to meet the established inclusion criteria, comprising 252 females (73.5 %) and 91 males (26.5 %). In this heterogeneous cohort, there was a statistically significant predominance of females over males (p < 0.001, χ2 = 75.571). The mean age of the participating patients was 75.18 ± 10.22 years, with the youngest individual being 43 years old and the eldest – 98 years old.
3.1. Characteristics of Nasal Basal Cell Carcinoma
The research aimed to investigate the distribution of BCC localization within the nasal region. An observable trend revealed a statistically significant preponderance of tumor formations in regions that were directly exposed to sunlight, particularly in Zone H, which encompasses the left sidewall of the nose and the dorsum. These areas accounted for 87 cases (25.4 %) and 85 cases (24.8 %), respectively. Conversely, the least frequent occurrence of BCC was noted on the tip of the nose, with only 40 cases (11.7 %) identified. The observed differences in BCC localization were found to be highly significant (p < 0.001, χ2 = 23.166) (
Table 1).
Another characteristic parameter of BCC examined in this study was tumor size. To facilitate a precise evaluation of tumor diameter, subjects were stratified by gender, and the measurement of this parameter was conducted separately for each group. Analysis of the results (
Table 2) revealed that there was no statistically significant difference in BCC size between female and male patients (0.79 ± 0.38 cm and 0.82 ± 0.44 cm, respectively) (p > 0.05). The smallest recorded tumor diameter in females was 0.2 cm, whereas in males it was 0.3 cm. The largest observed diameters were 2.5 cm in females and 3.5 cm in males.
According to the presented data (
Table 3), the infiltrative histopathological subtype of BCC was the most prevalent, observed in 243 cases (70.8 %), whereas the sclerosing morphological subtype was the least common, occurring in only 13 cases (3.8 %). The application of the Goodness–of–fit Chi–square test revealed a statistically significant disparity in the distribution of data within the group, indicating heterogeneity among the groups (p < 0.001, χ2 = 565,79).
3.2. Surgical Management of Nasal Basal Cell Carcinoma: Methods, Findings, and Long-Term Outcomes
Following the statistical examination of the dataset, the prevalence of selected surgical treatment modalities among the sampled patients was computed. The findings of this study reveal that, for the excision of BCC in various nasal regions, the most employed surgical approach was standard surgical excision (SSE) with fasciocutaneous plastic, implemented in 262 cases (76.4 %). Conversely, the least frequently employed technique was excision coupled with nasal reconstruction, utilized in only 6 cases (1.7 %) (p < 0.001, χ2 = 306.9).
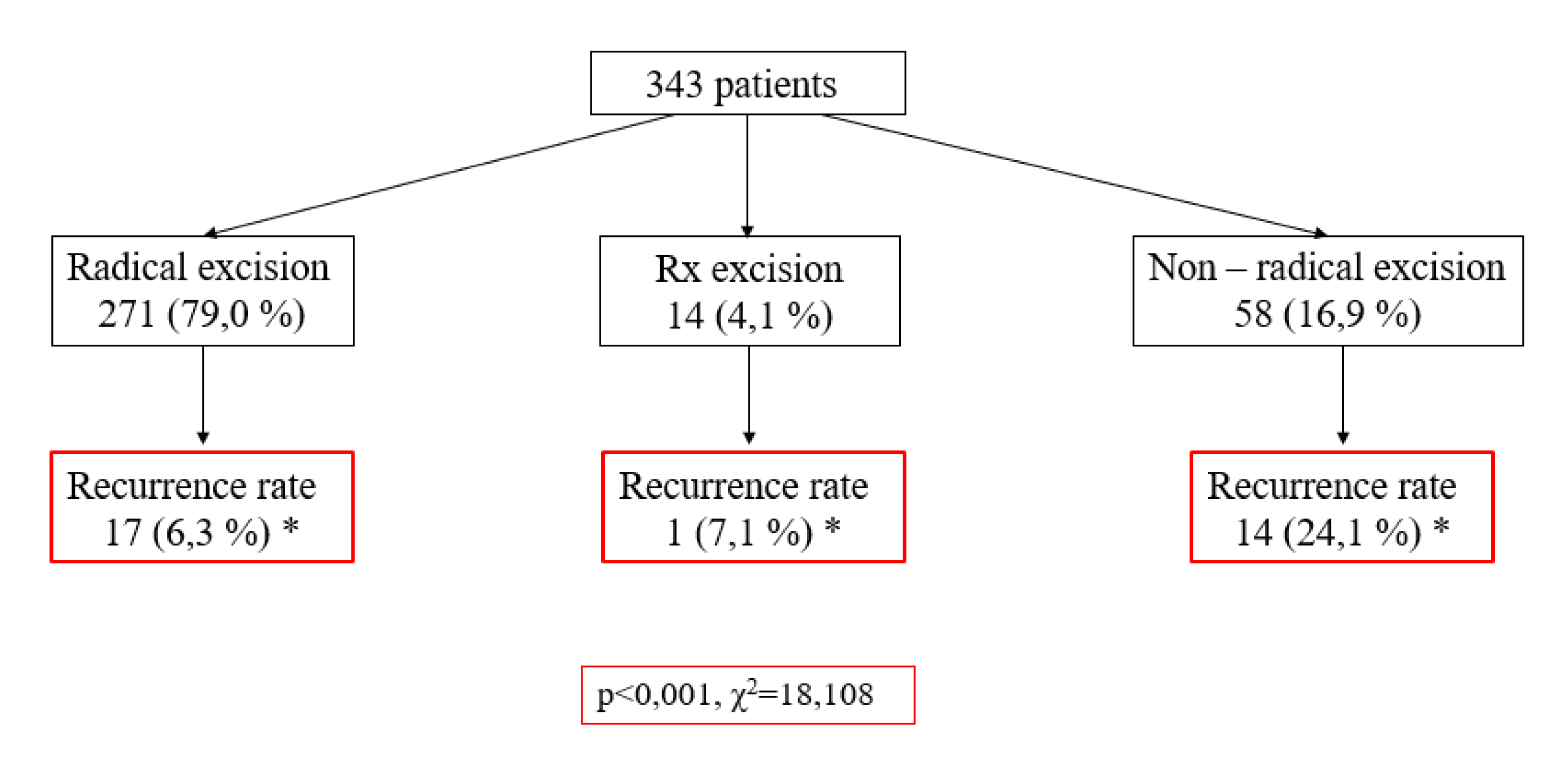
This study also aimed to ascertain the level of radicality in the surgical management of patients with BCC and to assess its correlation with recurrence frequency. According to the data of the scheme (
Figure 1), 271 cases (79.0 %) underwent radical excisions, while 58 cases (16.9 %) were subjected to non–radical removal. A notable association was observed, wherein non–radical excisions exhibited a higher recurrence rate (24.1 %) compared to radical excisions, which demonstrated a recurrence rate of only 6.3 % (p < 0.001, χ2 = 18.108). Subsequent evaluation of the influence of variables such as gender, tumor size, and morphological subtype on BCC recurrence did not yield statistically significant differences (p > 0.05).
Upon analyzing the selection of subsequent treatment strategies for patients, considering the radicality of the initial operation, it was observed that in cases where tumors were non–radically removed (R1), further treatment involved a recommendation for re–excision when recurrence becomes apparent, constituting 23 cases (39.7 %). Additionally, re–excision within 0 – 6 months was pursued in 17 cases (29.3 %) (p < 0.001, χ2 = 310.99). Comparatively, the less frequently employed strategies encompassed re–excision after more than 12 months and targeted re–excision, accounting for 8.6 % and 10.3 % respectively. No consultation with a dermatologist was deemed necessary for this subgroup. Within the category of radically excised tumor formations (R0), a statistically significant distribution of data was observed. Remarkably, the predominant approach applied to the vast majority of subjects, encompassing 92.6 %, involved a dermatologist's consultation after a span of 2 – 3 months. In this group, repeated surgical treatment was rarely necessary, occurring in only 0.7 % of cases (p < 0.001, χ2 = 310.99) (
Table 4).
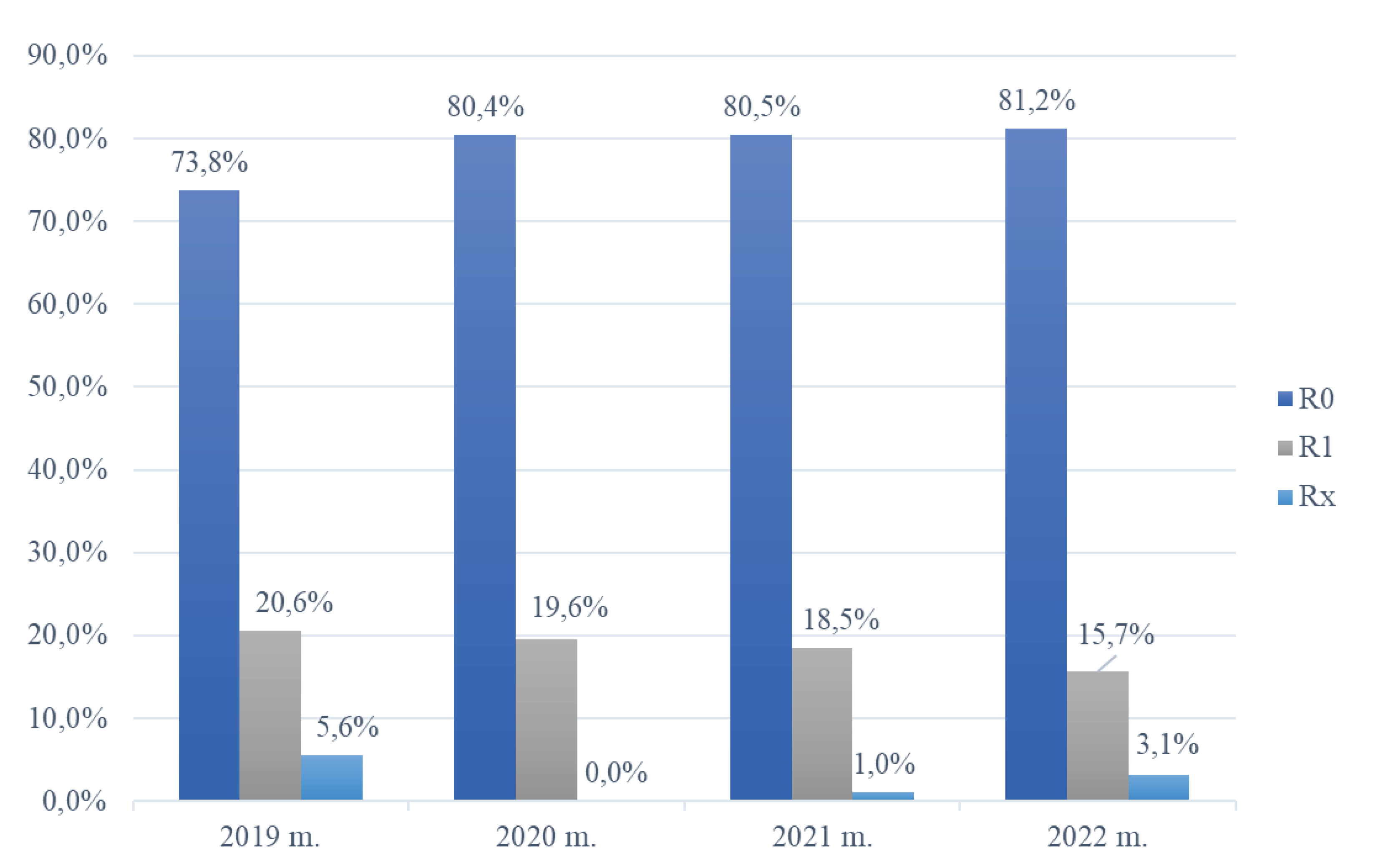
In the conducted data analysis, the frequency distribution of surgical radicality in patients undergoing treatment for BCC in the nasal region at the Department of Plastic and Reconstructive Surgery, Hospital of Lithuanian University of Health Sciences Kaunas Clinics, was assessed from 2019 to 2022. As depicted in
Figure 2, a discernible trend was noted, with the proportion of radical (R0) excisions increasing from 73.8 % in 2019 to 81.2 % in 2022. Conversely, the incidence of incompletely removed tumor formations (R1) exhibited an inverse trajectory, decreasing from 20.6 % in 2019 to 15.7 % in 2022. Following the application of the non–parametric Chi–square test for independence of attributes in the statistical analysis, it was determined that the distribution of data within the R1 group exhibited a statistically significant difference, primarily stemming from a notable reduction in the number of R1 excisions over the course of these four years (
Figure 2).
4. Discussion
Lultschik et al. conducted a retrospective study in Canada in 2023 to examine the distribution of basal cell carcinoma based on age, gender, and recurrence rates. The study sample comprised 155 (55.8 %) females and 123 (44.2 %) males, with an average participant age of 57.2 years. The statistical analysis revealed that the results did not exhibit significant differences in the age and gender groups [
20]. While the distribution of patients by gender aligns with our study, a notable disparity emerged in the evaluation of age metrics. In our research, participants were notably older, with an average age differing by approximately 18 years compared to the referenced study. Devine et al. (2017) similarly identified a younger sample in Great Britain, with a mean age of 72.5 years [
21]. We also reviewed other studies where, in contrast to our data, the majority of subjects were male [
3,
22,
23,
24]. Ghanadan et al. (2014) attributed this gender distribution to factors such as women's higher usage of SPF face protection products and men's increased outdoor exposure leading to greater direct UV radiation exposure [
25].
In a 2019 retrospective cohort single–center study authored by De Nicolo et al. and published in Italy, the primary focus centered on the comprehensive evaluation of histopathological classifications, anatomical localization, and size distribution of BCC within facial regions. The study's objective was to delineate potential correlations between these attributes and the likelihood of recurrence. The findings exhibited a preponderance of tumors predominantly located in the nasal region (31.8 %), with 11.4 % situated on the dorsum and an equivalent 8.0 % present on both the right and left nasal sidewalls. The prevalent histopathological manifestations of BCC were noted as nodular and sclerodermiform subtypes, accounting for 65.9 % and 15.9 % respectively. The calculated average dimensions of the tumors, determined through the length–to–width ratio, amounted to 0.9 × 0.7 cm, yielding an area of 0.83 cm² [
15]. In comparison to our own study, notable distinctions emerged. Specifically, our investigation identified the primary site of BCC occurrence to be the left nasal sidewall (25.4 %). Furthermore, the nodular morphological subtype ranked second in prevalence, representing only 12.8 %. In stark contrast, the infiltrative subtype prevailed as the most common histological manifestation, encompassing a substantial 70.8 %. Additionally, our assessment of average tumor size was predicated exclusively on the mean diameter dimensions, resulting in a smaller measurement of 0.8 cm, in contrast to the reported analysis. It is essential to acknowledge that De Nicolo et al. did not report any instances of BCC recurrence, underscoring the necessity to consult additional scientific sources for a more comprehensive evaluation of the interplay between these attributes and recurrence rates. Morgan et al. conducted a comprehensive analysis of 496 cases of BCC, stratifying their cohort based on tumor size, distinguishing between those exceeding and falling below a 2 cm diameter threshold. The investigation determined that larger tumors, surpassing 2 cm, exhibited a notably heightened recurrence risk, quantified at 8.9 %, in stark contrast to the 0.8 % recurrence rate observed in tumors measuring less than 2 cm [
26]. Additionally, in a study undertaken by Kondo et al., the more aggressive micronodular and sclerodermiform subtypes accounted for 17.24 % and demonstrated a statistically significant association with recurrence compared to other subtypes (p = 0.0001) [
27]. Based on our study's findings, it is reasonable to infer that tumor attributes such as anatomical localization, morphological classification, and size exerted negligible influence on the occurrence of BCC recurrences. This inference is drawn from the absence of a discernible statistical correlation between the recurrence rate and these specific characteristics.
We conducted an analysis of a prospective randomized controlled trial that compared the radicality and recurrence rate between surgical excision with serial section histology and 3D–histology in the management of facial basal cell carcinoma, as reported by Kofler et al. in Germany in 2021. This investigation encompassed 569 BCC cases, comprising 59 subjects (20.9 %) who underwent serial section histology and 113 (39.4 %) who underwent 3D–histology. The statistical findings from this study reveal that the frequency of non–radical operations was 30.2 %, and the recurrence rate with serial section histology stood at 8.4 %. In contrast, the use of 3D–histology yielded a significantly lower recurrence rate of 3.5 % among subjects, resulting in an overall recurrence frequency of 6.0 % [
28]. In our own study, which exclusively employed standard surgical excisions with serial section histology, the incidence of non–radical operations was notably lower compared to the aforementioned study (16.9 %). Nevertheless, it is worth noting that scientific literature suggests that this indicator generally falls within the range of 1 to 40 % worldwide [
19,
29,
30,
31,
32]. To obtain a more precise estimation of the prevalence of incompletely removed tumors, we conducted a literature review. Wollina et al. (2014) performed a retrospective study in Germany and found that the rate of non–radical (R1) excisions was 30.5 % in BCC treated with SSE [
8]. Szewczyk et al. (2022) reported a frequency of 19.6 % for R1 excisions [
33]. In a retrospective study by Ürün et al. (2022), they found that the rate of non–radical (R1) excisions was 18.9 %, and the recurrence rate was 9.6 % in BCC treated with SSE. Their findings also demonstrated a statistically significant higher recurrence rate for incompletely removed tumors compared to those removed with radical excision (p < 0.05) [
5]. In our research, a similar trend of statistical dependence was observed, with BCC recurrence after R1 operations being 14.5 % higher, while the overall recurrence was 3.2 % lower. However, in other studies, this parameter exhibits a wider range of variation (3.2 – 46.0 %) [
32]. We also explored publications with lower percentages for the analyzed criteria. Veronese et al. (2012) reported that non–radical surgery in their study accounted for only 7.2 %, of which 4.2 % recurred, while radically removed tumors recurred in only 3.4 % of the study participants. The overall recurrence rate was 8.3 % [
34]. According to the results of our study, the overall recurrence rate of BCC in the nasal area (6.4 %) possibly increased because the majority of the study sample consisted of the aggressive and, according to literature sources, more prone to relapse infiltrative BCC morphological subtype.
This research paper exhibits several methodological limitations. Primarily, a retrospective study design was chosen, relying on data extracted from patients' medical records. However, the assessment and documentation of objective symptoms are inherently subjective, potentially introducing inaccuracies during re–evaluation. It is essential to acknowledge that this scientific investigation was confined to a single medical institution, which may impact the overall accuracy of the data, especially if the studied patients sought care in other healthcare facilities or refrained from consulting at all due to recurrent skin oncological conditions outside the scope of this study. Additionally, when comparing our data analysis with international studies, it becomes evident that the research sample size was relatively limited, and certain surgical treatment methods considered as the gold standard in other countries are not available in Lithuania due to financial constraints and complex techniques. Consequently, it is imperative to acknowledge the constraints of this research, potential sources of bias, and approach the results with circumspection.
5. Conclusions
The findings derived from this study necessitate cautious interpretation and call for substantiation through subsequent research efforts. Nonetheless, they suggest a predominant focus on surgical intervention for Basal Cell Carcinoma (BCC) among elderly female patients. The primary tumor localization exhibited notable prominence on the left sidewall of the nose and the dorsum. Additionally, there was no statistically significant disparity in the size of BCC between the genders, with the infiltrative morphological type being the most prevalent. The preferred surgical modality entailed standard surgical excision with fasciocutaneous plastic reconstruction. Radical excisions of BCC, surpassing non–radical procedures by a factor of four, exhibited an association with reduced recurrence rates. It is deduced that nasal reconstruction mandates a comprehensive repertoire of surgical techniques to precisely tailor the procedures in accordance with factors such as tumor localization, size, depth, and the specific requirements of individual patients. Despite the meticulous adherence of surgeons to visual guidelines for basal cell carcinoma excision, the occurrence of non–radical procedures cannot be entirely eradicated.
Author Contributions
Conceptualization, E.Z. and A.P.; methodology, K.B., E.Z. and L.P.; software, K.B.; validation, E.Z., K.B. and A.P.; formal analysis, A.P.; investigation, K.B..; resources, E.Z. and K.B.; data curation, L.P.; writing—original draft preparation, K.B.; writing—review and editing, E.Z. and A.P.; visualization, K.B.; supervision, L.P.; project administration, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for this study was obtained from the Kaunas Regional Biomedical Research Ethics Committee (16 December 2022, No. BEC-MF-123).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Konopnicki, S.; Hermeziu, O.; Bosc, R.; Abd Alsamad, I.; Meningaud, J.P. Nasal basal cell carcinomas. Can we reduce surgical margins to 3mm with complete excision? Ann. Chir. Plast. Esthet. 2016, 61, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Vatamanesku, I.; Parasca, S.V.; Parasca, O.M.; Vaida, F.A.; Mehedinţi, M.C.; Grosu, F.; Ciurea, M.E. Basal cell carcinoma of the nasal pyramid excision margins: A retrospective study. Rom. J. Morphol. Embryol. 2019, 60, 1261–1268. [Google Scholar] [PubMed]
- Derebaślnlloǧlu, H.; Özkaya, N.K. Analysis of Basal Cell Carcinoma and Squamous Cell Carcinoma according to Nasal Subunit Location. Facial Plast. Surg. 2021, 37, 407–410. [Google Scholar] [CrossRef]
- Verkouteren, J.A.C.; Ramdas, K.H.R.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gürsel Ürün, Y.; Can, N.; Bağış, M.; Sarıkaya Solak, S.; Ürün, M. Adequacy of surgical margins, re-excision, and evaluation of factors associated with recurrence: a retrospective study of 769 basal cell carcinomas. An. Bras. Dermatol. 2023, 98, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.B.; de Carvalho Fantini, B.; dos Santos, C.A.; Melo, R.V.G.; Rosan, I.; Chahud, F.; da Silva Souza, C. Attributes and risk factors of positive margins on 864 excisions of basal cell carcinomas: a single-center retrospective study. J. Dermatolog. Treat. 2020, 31, 589–596. [Google Scholar] [CrossRef] [PubMed]
- COJOCARU, A.; MARINESCU, E.-A.; NICA, O.; ILINOIU, E.; NEGRILA, A.; CIUREA, M.-E. Basal Cell Carcinoma and its Impact on Different Anatomical Regions. Curr. Heal. Sci. J. 2021, 47, 75. [Google Scholar] [CrossRef]
- Wollina, U.; Bennewitz, A.; Langner, D. Basal Cell Carcinoma of the Outer Nose: Overview on Surgical Techniques and Analysis of 312 Patients. J. Cutan. Aesthet. Surg. 2014, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Hogan, S.; Leonardi-Bee, J.; Williams, H.C.; Bath-Hextall, F.J. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar]
- Basset-Seguin, N.; Herms, F. Update on the management of basal cell carcinoma. Acta Derm. Venereol. 2020, 100, 284–290. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Basal Cell Skin Cancer. 2023 2–5.
- Pinna, G.; Dell’Antonia, M.; Atzori, L.; Ferreli, C.; Casula, L.; Minerba, L.; Faa, G.; Rongioletti, F.; Pilloni, L. Recurrent nasal basal cell carcinoma treated with standard surgery excision: evaluation of volume ratio. Ital. J. dermatology Venereol. 2023, 158, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V. del; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Pabst, F.; Krönert, C.; Schorcht, J.; Haroske, G.; Klemm, E.; Kittner, T. High-risk basal cell carcinoma: an update. Expert Rev. Dermatol. 2010, 5, 357–368. [Google Scholar] [CrossRef]
- Bertozzi, N.; Simonacci, F.; Grieco, M.P.; Grignaffin, E.; Raposio, E. Single center evidence for the treatment of basal cell carcinoma of the head and neck. Acta Bio Medica Atenei Parm. 2019, 90, 77. [Google Scholar] [CrossRef]
- Palmer, V.M.; Wilson, P.R. Incompletely excised basal cell carcinoma: Residual tumor rates at mohs re-excision. Dermatologic Surg. 2013, 39, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Yun, B.M.; Kang, J.W. Unusual Basal Cell Carcinoma in the Nasal Vestibule Treated with Excision and a Full-Thickness Skin Graft. Ear. Nose. Throat J. 2022. [Google Scholar] [CrossRef]
- Kappelin, J.; Nielsen, K.; Nilsson, F.; Bjellerup, M.; Ahnlide, I. Surgical treatment of basal cell carcinoma: a case series on factors influencing the risk of an incomplete primary excision. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2518–2525. [Google Scholar] [CrossRef]
- Bozan, A.; Gode, S.; Kaya, I.; Yaman, B.; Uslu, M.; Akyildiz, S.; Apaydin, F.; Ceylan, C.; Ozturk, G. Long-term Follow-up of Positive Surgical Margins in Basal Cell Carcinoma of the Face. Dermatol. Surg. 2015, 41, 761–767. [Google Scholar] [CrossRef]
- Lultschik, S.; Tran, J.; Sapra, S.; Sharma, K.; Dong, K. Non-Invasive Treatment of Basal Cell Carcinoma: Photodynamic Therapy Following Curettage. J. Drugs Dermatol. 2023, 22, 481–485. [Google Scholar] [CrossRef]
- Devine, C.; Srinivasan, B.; Sayan, A.; Ilankovan, V. Epidemiology of basal cell carcinoma: a 10-year comparative study. Br. J. Oral Maxillofac. Surg. 2018, 56, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Pazdrowski, J.; Pabiszczak, M.; Więckowska, B.; Dańczak-Pazdrowska, A.; Golusiński, W. Local recurrence risk in head and neck basal cell carcinoma. Otolaryngol. Pol. = Polish Otolaryngol. 2022, 76, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Agozzino, M.; Cannavó, P.S.; Dianzani, C.; di Meo, N.; Nardello, C.; Neagu, N.; Guarneri, F.; Zalaudek, I. Basal cell carcinoma and dermal nevi of the face: comparison of localization and dermatoscopic features. Int. J. Dermatol. 2021, 60, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, R.; Kavoussi, H.; Ebrahimi, A.; Salari, N.; Madani, S.H. Outcome of staged excision with pathologic margin control in high-risk basal cell carcinoma of the head region. An. Bras. Dermatol. 2020, 95, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ghanadan, A.; Abdollahi, P.; Rabet, M.; Naraghi, Z.; Abbasi, M.A.; Moslehi, H.; Abbasi, A. Different Anatomical Distribution of Basal Cell Carcinoma Subtypes in Iranian Population: Association between Site and Subtype. Ann. Dermatol. 2014, 26, 559. [Google Scholar] [CrossRef] [PubMed]
- Morgan, F.C.; Ruiz, E.S.; Karia, P.S.; Besaw, R.J.; Neel, V.A.; Schmults, C.D. Factors predictive of recurrence, metastasis, and death from primary basal cell carcinoma 2 cm or larger in diameter. J. Am. Acad. Dermatol. 2020, 83, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.N.; Gon, A.D.S.; Junior, R.P. Recurrence rate of basal cell carcinoma in patients submitted to skin flaps or grafts. An. Bras. Dermatol. 2019, 94, 442. [Google Scholar] [CrossRef]
- Kofler, L.; Breuninger, H.; Schreiber, R.H.; Eichner, M.; Häfner, H.M.; Schnabl, S.M. Three-dimensional histology vs. serial section histology in the treatment of primary basal cell carcinoma: a randomized, prospective, blinded study of 569 tumours. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Inatomi, Y.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Furue, M.; Uchi, H. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: an analysis of 288 lesions in Japan. J. Eur. Acad. Dermatology Venereol. 2015, 29, 1828–1831. [Google Scholar] [CrossRef]
- Codazzi, D.; Van Der Velden, J.; Carminati, M.; Bruschi, S.; Bocchiotti, M.A.; Di Serio, C.; Barberis, M.; Robotti, E. Positive compared with negative margins in a single-centre retrospective study on 3957 consecutive excisions of basal cell carcinomas. Associated risk factors and preferred surgical management. J. Plast. Surg. Hand Surg. 2014, 48, 38–43. [Google Scholar] [CrossRef]
- Masud, D.; Moustaki, M.; Staruch, R.; Dheansa, B. Basal cell carcinomata: Risk factors for incomplete excision and results of re-excision. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, M.C.; Stelini, R.F.; Staffa, L.P.; Moraes, A.M. de; Magalhães, R.F. Basal cell carcinoma with compromised margins: retrospective study of management, evolution, and prognosis. An. Bras. Dermatol. 2021, 96, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Pazdrowski, J.; 1f, P.; Więckowska, B.; Dańczak-Pazdrowska, A.; Golusiński, W.; Roszak, M.; Adamski, Z. Local recurrence risk in head and neck basal cell carcinoma. Polish J. Otolaryngol. 2022, 76, 30–35. [Google Scholar] [CrossRef]
- Veronese, F.; Farinelli, P.; Zavattaro, E.; Zuccoli, R.; Bonvini, D.; Leigheb, G.; Colombo, E. Basal cell carcinoma of the head region: therapeutical results of 350 lesions treated with Mohs micrographic surgery. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 838–843. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).