1. Introduction
Alkylphenols belong to a group of endocrine disruptors (ED) and xenoestrogen, which are exogenous substances disrupting the endocrine system. They can disrupt the hormonal structure of the body and have adverse effects on human health. The growing attention regarding the health of the next generation is devoted to the effect of endocrine disrupting chemicals on human health [
1,
2,
3,
4]. Nonylphenols are used for the production of nonylphenol polyethoxylates (NPEOs), the most often used nonionic surfactans in industrial and household products, personal care products, detergents, emulsifiers, additives in cosmetic products, water-based paints, textiles, in production of plastics, resins, paints, cleaners and pesticides etc. [
5]. Nonylphenol is a mixture of different isomers (ortho-, meta- or para-), which are located by the arrangement of the carbon chain, whereas the most common isomer is the para isomer (4-NP). 4-NP is a derivative of phenol, where a carbon chain with nine carbon atoms is attached to its aromatic ring. It is a potential threat to the environment because it is toxic to aquatic organisms and can have harmful effects on the ecosystems of waterways and seas [
6]. In some legislation [
7], measures have been taken to limit the use of NP and its compounds in some products and industrial processes in order to minimize the risks associated with this substance.
NP and NPEOs are regulated in the European Union under Directive 2003/53/EC, which was adopted on June 18, 2003 and became effective on January 17, 2005 [
8]. This directive sets restrictions on the use of these substances in various products. According to this directive, it is prohibited to market and use textile products that contain NP and NPEOs in concentrations higher than 0.1 percent by weight. In addition, the use of these substances in washing preparations, detergents and cleaning agents is prohibited if it leads to the emulsification of hazardous substances in water. However, it is possible that the rules and regulations can be changed and supplemented. Therefore, it is important to follow current information and updates from the European Union and important countries regarding the use of NP [
9,
10,
11].
Recently, the high occurrence of persistent endocrine disruptors in environment can caused growing concern about their impact on human health. Infants are particularly sensitive to hormonal influences of environmental chemicals. Due to their presence in human milk, concerns for mother´s and children´s health are increasing [
12,
13]. Since the lipophilic character, ED can be accumulated in maternal body fat, can pass through the placental barrier along with residual contaminants and can be transferred from plasma into breast milk during lactation period [
5,
14].
Breast milk is among the body fluids most suitable for monitoring the effect of alkylphenol exposure on both mother and child [
15]. Breast milk is a complex and variable fluid that evolved to nourish the infant and protect it from disease while its immune system continues to develop. The composition of human breast milk varies depending on many factors that correspond to the needs of the child according to its age and other factors [
16,
17,
18]. It is an irreplaceable form of nutrition for newborns and its consumption provides important benefits for the newborn immune system, growth, psychological development. Breastfed babies have a more stable and less diverse intestinal microflora than formula-fed babies, but they have more than twice the number of bacterial cells. This may be partly due to changes at the level of the intestinal mucosa due to bioactive substances in breast milk. Moreover, breastfeeding reduced the risk of developing different allergies, infections and other chronic diseases, decreased mortality and morbidity in infants as well as occurrence of cancer and osteoporosis in mothers [
19]. However, breastfeeding, which is the only source of nutrition for infants and babies, can also be a route of contamination for children. Breast milk can serve as a marker to measure human exposure to these chemicals through different routes of exposure. The main routes of human exposure is through chemicals residue in food, water and air [
6,
20,
21,
22]. NP is excreted by the organism and therefore its concentration in breast milk can be affected by various factors such as diet, environment and individual differences (e.g. in the rate of metabolism) between people. However, it is important to emphasize that the presence of NP in breast milk varies between regions and women. It is important to remember that the benefits of breastfeeding for the baby outweigh the potential risks associated with exposure to NP.
Chromatographic methods are mostly used to determine alkylphenols. It can be gas chromatography (GC) with mass spectrometry (MS) [
23,
24,
25,
26] high performance liquid chromatography (HPLC) with fluorescence detection (FSC) [
27,
28] or liquid chromatography with mass spectrometry (LC-MS) [
29,
30,
31,
32,
33,
34].
In our study, the high-performance liquid chromatography with fluorescence detection was used for determination of 4-nonylphenol in breast milk. Considering the complexity of the breast milk samples and the low concentration levels of nonylphenol (µg/L), HPLC analysis of these substances requires the sample pretreatment. During this phase, together with the concentration of the analyte, all potential interferences that can affect the sensitivity and accuracy of the method are eliminated. The most frequently used technique for sample treatment is solid phase extraction (SPE) based on the affinity of analytes to the sorbent [
12,
28,
35,
36,
37].
Different types of SPE sorbents (C18, Oasis HLB, Strata-X etc.) are available for extraction of target analytes. Breast milk samples were first subjected to liquid-liquid extraction and then each extract was processed by SPE.
In the present study, an HPLC-FSC applying Chromabond C18ec SPE sorbent was used for the determination of 4-nonylphenol in breast milk samples.
2. Materials and Methods
2.1. Characteristics of mothers and children
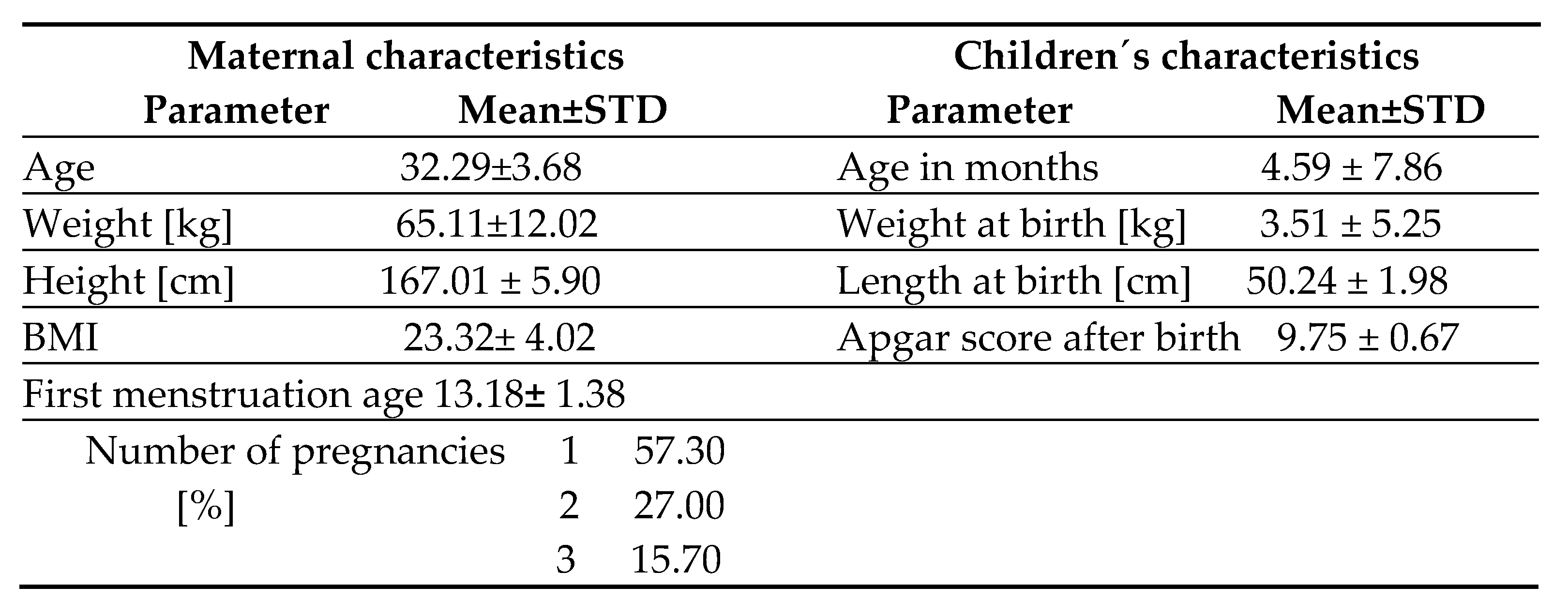
Initially, the 110 nursing mothers were approached, of which 89 mothers willing to collect breast milk were included in the study, while 21 mothers refused to provide a breast milk sample without giving a reason. Mothers had university degree (89.90%) or secondary (10.10%) education. The characteristics of mothers and children are presented in
Table 1. Nursing mothers who were expected to give birth spontaneously without complications were included in the study. All participants came from Bratislava and its surroundings, they were subjectively healthy and willing to participate in the trial.
2.2. Milk sample collection and storage
The nursing mothers were from the gynecology clinic of the University Hospital in Bratislava. Each nursing mother from study donated 15 mL of freshly sucked breast milk at the gynecologic clinic. Samples were taken twice, in the first month and in the fourth month of breastfeeding in the morning between 8 and 10 a.m. The samples were transported on dry ice to the laboratory, divided into 1 mL microtubes and stored at -80°C until analysis. Trained nurse from the gynecology clinic was responsible for transporting the samples. 4-nonylphenol concentrations in breast milk were determined by the HPLC method.
2.3. Reagents and Chemicals
4-nonylphenol and 4-octylphenol were supplied by Sigma Aldrich (Steinheim, Germany). Formic acid (98%), methanol (HPLC gradient grade), acetonitrile (HPLC gradient grade) and water were purchased from Merck (Darmstad, Germany).
The Chromabond C18ec (100 mg/1 mL, 45 µm) was obtained from Macherey-Nagel (Germany). SPE experiments were performed using a Visiprep TM SPE Vacuum Manifold DL (Sigma Aldrich, Germany). After elution, samples were evaporated under the nitrogen and residues were dissolved in mobile phase.
2.4. Sample pretreatment
Firstly, breast milk samples were incubated at 37 ºC, sonicated in order to disrupt the fat milk layers and then centrifuged at 15,000× g for 15 min. Subsequently, 0.5 mL of 0.1% (v/v) formic acid in methanol was added to 0.5 mL skimmed milk, sample was vortexed for 3 min and centrifuged at 14,000 for 10 min. Later, 0.5 mL of methanol was added to supernatant and sample was centrifuged for 10 min. Then, 50 μL of 4-octylphenol as internal standard was added to supernatant (450 μL) to obtain the final volume of 0.5 mL, which was further pretreated by solid-phase extraction. Individual steps of SPE procedure are summarized in
Table 2.
2.5. HPLC analysis
All analysis were performed using the HPLC (HP 1200, Agilent, Munich, Germany) chromatograph with fluorescence detection. Chromatographic separation was achieved on a Nucleodur C18ec column (150 x 45.6 mm, 5 μm, Macherey-Nagel, Germany) with a precolumn C18 (10 x 4.6 mm, 5 μm). Mobile phase consisted of acetonitrile (A) and water (B). The linear gradient was following: 0-3.9 min 30% B; 4 min 70% B; 4.2-10 min 100% B; 10.1 min 30% B; 13.5 min stop. Flow rate of mobile phase was 1 ml/min. Sample injected volume was 25 µl.
2.6. Validation
In general, a validated analytical method gives reliable and reproducible results and clearly defined variable parameters (FDA, 2001). The present method was validated according to linearity, extraction recovery, limit of detection and quantification.
Linearity
Seven calibration standards were used to determine linearity on 10 separate days and each calibration standard was analyzed three times. Calibration curves were prepared from 0.5 to 100 ng/mL for 4-nonylphenol (NP) in breast milk and 10 ng/mL for octylphenol as internal standard. Coefficients of determination (R2) obtained by regression analysis was higher than 0.99.
Limit of detection and quantification
The limit of detection (LOD) was defined as the value exceeding the signal to noise ratio (S/N) by factor 3. LOD for 4- NP was 0.27 ng/mL. The limit of quantification (LOQ) was defined at signal to noise ratio > 10. LOQ for 4-NP was 0.91 ng/mL.
2.7. Questionnaire
The study used a self-constructed questionnaire that contained 50 questions. The questions dealt with the education and accommodation of the nursing mother, the health status of the baby and nursing mother, socioeconomic status, use of cosmetics and cleaning products, as well as eating habits.
2.8. Ethics committee and informed consent
The Research Ethics Committee of the Slovak Health University in Bratislava approved the project in accordance with the rules and guidelines for human research before the start of research in 2022 (n.13/2022). Participants were informed about the development of the research and those who agreed to participate signed an informed consent form, in two copies, one of which was given to the nursing mother.
2.9. Statistical analysis
Data were analyzed using SPSS (Statistical Package for the Social Science, Chicago,IL, USA) for Windows, version 19. The final model was tested by structural equation modeling (SEM) was used for statistical. This software provides extensive possibilities for manipulation, analysis and presentation of data. Descriptive statistics were performed - means, medians, variances, standard deviations and percentile values.
3. Results
In our questionnaire, we asked the birth weight, length and Apgar score of the child. The Apgar score is a simple method that is used to evaluate the health status of a newborn in the first minutes of life (
Table 1). It is determined concerning five criteria - heart rate, breathing, muscle tone, response to irritation, skin color. This score provides a quick and objective value of the newborn’s health status. We found that the measured concentrations of nonylphenol in breast milk do not affect any of the given parameters in the newborn, for example neither the birth weight, nor the birth length, nor the Apgar score of the child. In the questionnaire, we identified what kind of water did mothers drink (
Table 3). All food came from local stores and/or sources. Each nursing mother included in the study consumed homemade food, no fast food or highly processed food products. During breastfeeding, they ate meat 3 times per week and fish once a week.
The lowest and highest concentration of NP in breast milk was 0.97 ng/mL and 4.37 ng/mL, respectively. The average concentration ±STD was 3.4±0,27 ng/mL.
Higher concentrations were observed in mothers consuming pork (3.31 ng/ml) and fish (3.14 ng/mL) compared to non-consumers (3.03 and 2. 89 ng/mL, respectively). This parameter was statistical significance for pork consumption (p= 0.048) as well as for fish consumption (p= 0.041) in relation to 4-NP concentration. Results are presented in
Table 4.
In addition, it was shown that mothers with gel nails have higher concentration of 4-NP (p=0.06). At the border of statistical significance was beef consumation (p= 0.06) and vitamin supplemetation with (p=0.06). Findings are presented in
Table 5 and
Table 6. Other monitored factors had not a significant influence on nonylphenol values.
Table 3.
Statistical evaluation of water drinking in nursing mothers.
Table 3.
Statistical evaluation of water drinking in nursing mothers.
| Kind of water |
N |
Mean±STD |
Std.error |
95% confidence interval for mean |
Minimum |
Maximum |
| |
|
|
|
Lower Uperbound bound |
|
|
| Bottled |
22 |
2.28±0.29 |
0.06 |
2.05 2.40 |
0.38 |
1.69 |
| Tap |
53 |
1.16±0,31 |
0.04 |
1.07 1.24 |
0.67 |
2.27 |
| Other |
14 |
2.08±±0.23 |
0.06 |
1.95 2.21 |
0.57 |
1.47 |
| Total |
89 |
1.15±0.29 |
0.03 |
1.09 2.31 |
0.38 |
2.27 |
Table 4.
Statistical evaluation of meat consumption in nursing mothers.
Table 4.
Statistical evaluation of meat consumption in nursing mothers.
| Consumption of fish |
N |
Mean±STD |
STD error mean |
|
|
| NP yes |
80 |
3.14±0.30 |
0.03 |
p = 0.041 |
|
| no |
9 |
2.89±0.21 |
0.07 |
|
|
| Consumption of pork |
N |
Mean±STD |
STD error mean |
|
|
| NP yes |
60 |
3.31±0.32 |
0.04 |
p = 0.048 |
|
| no |
29 |
3.03±0.21 |
0.04 |
|
|
| Consumption of beef |
N |
|
|
|
|
| NP yes |
49 |
3.23±0.35 |
0.05 |
p = 0.06 |
|
| no |
40 |
2.98±0.21 |
0.03 |
|
|
In
Table 5 and
Table 6 we can see the influence of gloves using, nail polish, vitamins and medicines in nursing mothers on nonylphenol concentration.
Table 5.
Statistical evaluation of use of gloves and nail polish in nursing mothers.
Table 5.
Statistical evaluation of use of gloves and nail polish in nursing mothers.
| Using gloves for cleaning |
N |
Mean±STD |
STD error
mean |
|
| NP yes |
20 |
2..83±0.28 |
0.06 |
p = 0.709 |
| no |
69 |
2.26±0.29 |
0.04 |
|
| Using nail polish |
N |
Mean±STD |
STD error
mean |
|
| NP yes |
19 |
2.23±0.28 |
0.07 |
p = 0.729 |
| no |
70 |
2.15±0.29 |
0.04 |
|
| Gel nails |
N |
|
|
|
| NP yes |
19 |
4.21±0.28 |
0.07 |
p = 0.06 |
| no |
70 |
4.14±0.29 |
0.04 |
|
Table 6.
Statistical evaluation of maternal vitamins and medication use in nursing mothers.
Table 6.
Statistical evaluation of maternal vitamins and medication use in nursing mothers.
| Vitamins supplements |
N |
Mean±STD |
STD error mean |
|
|
| NP yes |
60 |
3.48 ± 0.29 |
0.04 |
p = 0.06 |
|
| no |
27 |
3.06 ± 0.29 |
0.06 |
|
|
| Medication |
N |
Mean±STD |
STD error mean |
|
|
| NP yes |
23 |
2.94 ± 0.34 |
0.07 |
p = 0.093 |
|
| no |
65 |
2.72 ± 0.27 |
0.03 |
|
|
4. Discussion
Breast milk remains the best source of infant nutrition but constant surveillance is needed to keep it pure. Breastfeeding offers many advantages to neonates and infants and provides a range of short- and long-term benefits for growth, immunity, cognitive and psychological development, as well as protection against infection, allergies, and other chronic diseases. The composition of human milk varies at different stages of lactation, distinct times of the day, during each feed, and even between breasts; it contains powerful growth- and immune-enhancing factors; and breastfeeding is considered as the best and only source of nutrition necessary for the infant during the first 6 months of life. Chemical contaminants in milk, if the level is high enough or if the infant is sensitive enough, interact at many possible physiological levels. Numerous studies have associated breastfeeding with potential medical and social benefits, which include decreased mortality and morbidity in infants from infectious and other diseases, influence on brain development, increased resistance to chronic diseases, and decreased incidence of cancer and osteoporosis in the mothers. Some mothers avoid breastfeeding on personal or social grounds; but the reasons can be associated with complications including secretion, breast pain, engorgement, or mastitis [
38]. The adverse events associated with drug exposure via lactation occur most often in neonates younger than 2 months and rarely in infants older than 6 months [
39].
Nonylphenol (NP) is a chemical used as an additive in some industrial processes and products, and it can enter the environment. Therefore, it is possible that it can also occur in the home environment. Another factor may include environmental pollution. If the nursing mother’s accommodation is near industrial zones, waste dump or other sources of pollution, there is a higher probability that NP can get into her body and subsequently into her breast milk. If a nursing mother consumes food or water containing NP, it is possible that this substance can accumulate in her body and pass into her breast milk. But also the use of products containing NP, such as use of cleaning agents, cosmetics or other products, can contribute to higher concentrations of this substance in breast milk. Nursing mothers who live in the village had increased concentrations of nonylphenol, but these findings were not statistically significant. The assumption was that they could live near an industrial zone or in a polluted environment despite the fact that they live in a village.
Maternal exposure to insecticides or to other chemical pollutants is rarely a contraindication to nursing unless the exposure is excessive. Sweden and Germany have systematic breast milk monitoring programs that have tested considerable numbers of women over time; however, most countries have done little monitoring for metal, pesticides, or industrial chemicals in breast milk. In the United States the objectives and goals of a breast milk monitoring program for women are as follows: Information should be obtained on women from diverse geographic regions of the United States and from different socioeconomic and demographic backgrounds. Samples should be collected from both rural and urban locations. Previous studies should be extended by testing for an increased number of environmental chemicals in breast milk (e.g., certain heavy metals as well as other chemicals with significant lipid solubility and long biological elimination half-life). Longitudinal information should be obtained during the course of lactation so that the decrease in concentration of the chemical over time can be assessed. Lactating women should be enrolled in the study on a longitudinal basis, donating samples on a monthly basis and then every 2-3 months if lactation continues. Recruitment of participants may be aided by lactation consultants. Harmonization of sampling and analysis protocols should be promoted to improve the comparability of the results [
40].
NP is a family of close relatives organic compounds called alkylphenols. They are used in the production of industrial antioxidants, additives to lubricating oils, laundry and dish detergents, emulsifiers and solubilizers. These compounds are also precursors of commercially important nonionic surfactants alkylphenol ethoxylates and NPethoxylates, which are used in cleaning products, paints, pesticides, personal care products and plastics. Considering the prevalence of NP in the environment and its potential roles as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity. NPs act as endocrine disruptors similar to xenoestrogens they bind to estrogen receptors and competitively inhibit natural estrogens. NPs have been shown to mimic natural hormone 17b-estradiol and compete with the endogenous hormone to bind to the estrogen receptors ERα and ER. The sidewalk analysis with NP was studied in spermatozoa tubule because NP is known to act on the testis [
6].
Xenobiotics in milk, in high levels or when the child is sensitive they interact sufficiently on many possible physiological levels. Huge benefits of breastfeeding offset the risks of most xenobiotics used during breastfeeding. Unfortunately, there is a lack of epidemiological data on the probability of adverse effects in breastfed newborns and infants, despite existing information on xenobiotics in breast milk. The finding of toxic chemicals in breast milk creates important questions for pediatric practice, for the field of public health and the environment health research community. Fat levels in human milk are a good indicator of potential future public health and environmental problems. Exposure varies greatly depending on local conditions chemical substances and the eating habits of the population in the studies. This measure is also relevant to the developmental exposure of unborn children. Although the research yielded information about different types of chemicals found in breast milk and with regard to the toxicological aspects of many of these chemicals, there is little data on infant parameters exposure through breastfeeding, including those with a time-dependent nature. Great concerns were expressed about the presence environmental contaminants such as pesticides, heavy metals and persistent organic pollutants (POPs) in breast milk and their possible effects on the infant health and development. In some cases, mothers with known or suspected high levels of contaminants in breast milk due to acute or chronic exposure recommended to reduce or stop breastfeeding.
Breast milk is the basis of healthy development of an individual. In our study, we dealt with the occurrence of nonylphenol in human milk. 4-nonylphenol is a chemical substance that was used until 2012 [
8,
9,
10,
11,
41,
42]. From this year, nonylphenol was only accessible to industry. Since it is a lipophilic substance, we were interested in whether the given substance binds to breast milk and passes into the child’s organism in nursing mothers.
So far, there is no study in Slovakia that would investigate the relationship between eating habits and the concentration of alkylphenols in breast milk. In the present study, pork and fish consumption were significantly associated with 4-NP concentration.
Tolerable Daily Intake (TDI) of 5 kg body weight (bw) for NP was proposed by the Danish Institute of Safety and Toxicology [
43]. From obtained NP concentration, it is possible to calculate the daily intake. Using average NP concentration 3,4 ng/mL, the daily intake for Slovak infants (with 5 kg bw) consuming 500 ml of breast milk was calculated as 0.26 μg/kg/d. This value is much lower than those obtained in the Taiwanese study (4.47 μg/kg/d) [46]; Japanese study (0.65-1.4 μg/kg/d) [47]; Italian study (3.94 μg/kg/d) [
12]. In Taiwanese study, authors examined dietary habits of 59 mothers. They observed significant asssociation (p<0.05) between frequency of cooking oil and fish oil capsules (p<0.01) consumation and OP concentration.
A similar value of TDI as in our study (0.26 μg/kg/d) was published in German study (0.3 μg/kg/d) [48], where NPs in 60 different food samples, including breast milk, were analyzed. It was assumed that due to the lipophilic properties of NPs high concentrations of NPs will be obtained in fatty food (e.g. butter, liver sausage etc.). But high NP concentrations were also observed in nonfatty foods like fruits.
Nonylphenols are persistent endocrine disruptors that are priority hazardous substances of the European Union Water Framework Directive. Their presence in the environment raises growing concerns about their impact on human health. Recent studies have shown that nonylphenol is ubiquitous in commercially available foods and is also present in human blood. Günter et al. [
4] determined 4-nonylphenol in 44 infant formula samples. They found that the distribution of nonylphenol isomers depends on the analyzed food. Although some isomeric groups predominate, different distributions are common. Variations are even found in the same food group. Nonylphenol is a complex mixture of isomers, and the estrogenic potentials of each of these isomers are very different. Consequently, an isomer-specific approach is needed to determine the potential toxicological impact of nonylphenol in foods [49].
NPs, octylphenol (OP), NP monoethoxylate (NP1EO), and two octylphenol ethoxylates (OPEOs) (namely, OP1EO and OP2EO) have been detected in human breast milk of Italian women [
12]. NP was the contaminant found at the highest levels (32 ng/mL), about two orders of magnitude higher than OP (0.08 ng/mL), OP1EO (0.07 ng/mL), and OP2EO (0.16 ng/mL). In this study group, a positive correlation between fish consumption and the levels of NP in the milk was observed, in accordance with the evidence that seafood represents one of the most important sources of exposure to this group of contaminants in Italy. The high concentrations of alkylphenols can be linked to the environmental contamination or to the bioaccumulation in food of animal source. On the basis of the concentrations found in the breast milk samples, a maximum NP daily intake of 3.94 mg/kg/day can be calculated, which is close to the TDI of 5 mg/kg b.w.
5. Conclusions
Human milk is one way removing the mother’s environmental toxic chemicals burden. Assessment of presence and quantity environmental toxic chemicals in breast milk, provide information on maternal toxic load and serves as a marker of prenatal fetal exposure this chemical.
The Tolerable daily intake for Slovak infants (with 5 kg bw) consuming 500 ml of breast milk was calculated as 0.26 μg/kg/d and it was comparable to the value from German study.
In this study, the relationship between eating habits and the concentration of 4- nonylphenol in breast milk were investigated. Following parameters: pork and fish consumption were statistical significance in relation to 4-NP concentration. Parameters such as beef consumption, vitamin supplementation and using gel nails appeared at the border of statistical significance.
Based on the obtained results, we can evaluate that breast milk contains concentrations of nonylphenol in reference values and thus does not affect human health. This confirms that nonylphenol is not present in high doses in the mother’s body, and therefore does not cause health problems, and breast milk is a safe food for the child. It is very important that such studies are carried out in order to better understand the potential risks to the health of children and mothers.
Risk management should therefore focus on reduce lifetime exposure, especially during the prenatal period and pregnancy, since toxic chemicals accumulate late before pregnancy and are released during pregnancy and lactation.
Author Contributions
“Conceptualization, A.R., Z.S. and M.V.; methodology, Cs. M. and M.M.; software, Cs.M.; validation, M.V.; Cs.M.; M.M.; formal analysis, Cs. M. and L.Ž.; investigation, J.H., Cs. M. and M.V.; resources, J.H.; writing—original draft preparation, Cs.M., Z.S.; M.M. and M.V.; writing—review and editing, Cs. M., Z.S. and M.V.; visualization, Cs.M. L.Ž and M.V.; supervision, A.R., Cs. M. and M.V.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.”.
Funding
“This research was funded by project SVG_07/2021 of the Slovak Medical University in Bratislava.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data collected during this study is available on request from the corresponding author.
Acknowledgments
The authors are grateful to the mothers.
References
- Raecker,T.; Thiele, B.; Boehme, R.M.; Guenther, K. Endocrine disrupting nonyl- and octylphenol in infant food in Germany: Considerable daily intake of nonylphenol for babies. Chemosphere, 2011, 82, 1533-1540. [CrossRef]
- Acir,I.-H.; Guenther, K. Endocrine-disrupting metabolites of alkylphenol ethoxylates – A critical review of analytical methods, environmental occurrences, toxicity, and regulation. Sci. Total Environ., 2018, 635, 1530–1546. [CrossRef]
- Yi, B.; Kim, C.; Park, M.; Han, Y.; Park, J.Y.; Yang, M. Association between Endocrine Disrupting Phenols in Colostrums and Maternal and Infant Health. Int, J. Endocrin. 2013, 2013, 1-7. [CrossRef]
- Günther, K.; Racker, T.; Bohme, R. An Isomer-Specific Approach to Endocrine-Disrupting Nonylphenol in Infant Food. J.Agric. Food Chem. 2017, 65, 1247−1254. [CrossRef]
- Osimitza, T.G.; Droegea, W.; Driverb, J.H. Human Risk Assessment for Nonylphenol. Human Ecol. Risk Assess. An Inter. J. 2015, 21, 1903-1919. [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the envi-ronment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033-49. [CrossRef]
- Commission Regulation (EU) 2016/26 of 13 January 2016 amending Annex XVII to Regulation of the European Parliament and of the Council (EC) No. 1907/2006 on the Registration, Evaluation, Authorization and Restriction of Chemicals ("REACH") as regards nonylphenol ethoxylates.
- Directive 2003/53/EC of the European Parliament and of the Council of 18 June 2003 amending for the 26th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (nonylphenol, nonylphenol ethoxylate and cement): DIRECTIVE 2003/53/EC, 2003.
- European Union. Environmental Quality Standards Directive; 2008.
- European Commission. Tris(nonylphenyl)phosphite_Cosmetic Ingredient Database (CosIng). https://ec.europa.eu/growth/toolsdatabases/cosing/index.cfm?fuseaction=search.details_v2&id=38769.web.
- European Union Risk Assessment Report: 4-Nonylphenol (Branched) and Nonylphenol: EUR 20387 EN; European Commission.
- Ademollo, N.; Ferrara, F.; Delise, M.; Fabietti, F.; Funari, E. Nonylphenol and octylphenol in human breast milk. Environ. Int., 2008, 34, 984–987. [CrossRef]
- Hudson, R.E.; Metz, T.D.; Ward, R.M.; McKnite, A.M.; Enioutina, E.Y.; Sherwin, C.M.; Watt, K.M.; Job, K.M. Drug exposure during pregnancy: Current understanding and approaches to measure maternal-fetal drug exp.
- osure. Front. Pharmacol. 2023, 14, 1111601.
- Huang, Y.-F.; Wang, P.-W.; Huang, L.-W.; Yang, S.-H.; Chiu, H.-H.; Chen, M.-L. Nonylphenol in pregnant women and their matching fetuses: Placental transfer and potential risks o infants. Environ. Res. 2014, 34, 143-148. [CrossRef]
- Ringbeck, B.; Bury, D.; Lee, I.; Lee, G.; Alakeel, R.; Alrashed, M.; Tosepu, R.; Jayadipraja, E.A.; Tantrakarnapa, K.; Kliengchuay, W.; Brüning, T.; Choi, K.; Koch, H.M. Biomarker-Determined Nonylphenol Exposure and Associated Risks in Children of Thailand, Indonesia, and Saudi Arabia. Environ. Sci. Technol., 2022, 56,10229−10238. [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 2013, 60, 49-74. [CrossRef]
- Lu, Y.Y.; Chen, M.L.; Sung, F.C.; Paulus, S.-G.W.; Mao, I.F. Daily intake of 4- nonylphenol in Taiwanese. Environ. Int. 2007, 33, 903–10. [CrossRef]
- Nickerson, K. Environmental contaminants in breast milk. J Midwifery Womens Health 2006, 51, 26-34. [CrossRef]
- Noorimotlagh, Z.; Haghighi, N.J.; Ahmadimoghadam, M., Rahim, F. An updated systematic review on the possible effect of nonylphenol on male fertility. Environ. Sci. Pollut. Res., 2017, 24, 3298–3314. [CrossRef]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [CrossRef]
- Gyllenhammar, I.; Glynn, A.; Darnerud, P.O.; Lignell, S.; van Delft, R.; Aune, M. Nonylphenol and bisphenol A in Swedish food and exposure in Swedish nursing women. Environ Int. 2012, 43, 21–28. [CrossRef]
- Hong, Y.; Feng1, Ch.; Yan, Z.; Wang, Y.; Liu, D.; Liao1, W.; Bai1, Y. Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ. Chem. Lett. 2020, 18, 2095–2106.
- Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S.; Lekkas, T.D. Simultaneous determination of the endocrine disrupting compounds nonylphenol, nonylphenol ethoxylates, triclosan and bisphenol A in wastewater and sewage sludge by gas chromatography–mass spectrometry. J. Chromatogr. A. 2007, 1138, 32-41. [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols,phenylphenols, bisphenol A and triclosan in human urine, blood andbreast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [CrossRef]
- Caban, M.; Stepnowski, P. The quantification of bisphenols and their analogues in wastewaters and surface water by an improved solid-phase extraction gas chromatography/mass spectrometry method. Environ. Sci. Polutt. 2020, 27, 28829–28839. [CrossRef]
- Lee, S.M.; Cheong, D.; Kim, M.; Kim, Y.-S. Analysis of Endocrine Disrupting Nonylphenols in Foods by Gas Chromatography-Mass Spectrometry. Foods 2023, 12, 269-282. [CrossRef]
- Núňez, L.; Turiel, E.; Tadeo, J.L. Determination of nonylphenol and nonylphenol ethoxylates in environmental solid samples by ultrasonic-assisted extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A. 2007, 1146, 157-163. [CrossRef]
- Sise, S.; Uguz, U. Nonylphenol in Human Breast Milk in Relation to Sociodemo-graphic Variables, Diet, Obstetrics Histories and Lifestyle Habits in a Turkish Population. Iran J. Public Health, 2017, 46, 491-499.
- Al Rashed, N.; Guenther, K. Determination of Endocrine-Disrupting Nonylphenols and Nonylphenol Carboxylates by High-Performance Liquid Chromatography-Tandem Mass Spectrometry: Levels in German Food after Restriction. Anal. Lett. 2021, 55, 634−647. [CrossRef]
- Mottier, P.; Frank, N.; Dubois, M.; Tarres, A.; Bessaire, T.; Romero, R.; Delatour, T. LC- MS/MS analytical procedure to quantify tris(nonylphenyl)phosphite, as a source of the endocrine disruptors 4-nonylphenols, in food packaging materials. Food Addit. Contam. Part A, 2014, 31, 962-972. [CrossRef]
- Lara-Martín, P.A.; González-Mazo, E.; Brownawell, B.J. Environmental analysis of alcohol ethoxylates and nonylphenol ethoxylate metabolites by ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2359–2368. [CrossRef]
- Shan, X.M.; Shen, D.H.; Wang, B.S.; Lu, B.B.; Huang, F.Y. Simultaneous Determination of Bisphenols and Alkylphenols in Water by Solid Phase Extraction and Ultra Performance Liquid Chromatography-tandem Mass Spectrometry. Biomed. Environ. Sci. 2014, 27, 471-474. [CrossRef]
- Shao, B.; Han, H.; Li, D.; Zhao, R.; Meng, J.; Ma, Y. Analysis of Nonylphenol, Octylphe-nol and Bisphenol A in Animal Tissues by Liquid Chromatography-Tandem Mass Spec-trometry with Accelerated Solvent Extraction. Se Pu, 2005, 23, 362-365.
- Shao, B.; Han, H.; Tu, X.; Huang, L. Analysis of alkylphenol and bisphenol A in eggs and milk by matrix solid phase dispersion extraction and liquid chromatography with tandem mass spectrometry. J. Chromatogr. B., 2007, 850, 412-416. [CrossRef]
- Otaka, H.; Yasuhara, A.; Morita, M. Determination of bisphenol A and 4-nonylphenol in human milk using alkaline di-gestion and cleanup by solid-phase extraction. Anal. Sci. 2003, 19, 1663-1666. [CrossRef]
- Lee, T.; Park, K.-Y.; Pyo, D. Simultaneous determination of bisphenol A, chlorophenols and alkylphenols by solid-phase extraction and HPLC. Anal. Sci. & Technol. 2017, 30, 20-25. [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Koupparis, M.A. Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol. Lett. 2012, 210, 141– 154. [CrossRef]
- Stuebe, A. The risks of not breastfeeding for mothers and infants. Rev. Obstet. Gynecol. 2009, 2 (4), 222e231.
- Anderson, P.O., Pochop, S.L., Manoguerra, A.S. Adverse drug reaction in breastfed infants: less than imagined. Clin. Pediatr. 2003, 42, 325e340. [CrossRef]
- LaKind, J.S., Berlin, C.M., Naiman, D.Q. Infant exposure to chemicals in breast milk in the United States: what we need to learn from a breast milk monitoring program. Environ. Health Perspect. 2001, 109, 75e88. [CrossRef]
- Kim, S.H.; Nam, K.H.; Hwang, K.A.; Choi, K.C. Influence of hexabromocyclododecane and 4-nonylphenol on the regulation of cell growth, apoptosis and migration in prostatic cancer cells. Toxicol. In Vitro. 2016, 32, 240-247. [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk,D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, Ch.; Grasl-Kraupp, B., Hogstrand, Ch.; Hoogenboom, L., Leblanc, J.-Ch.; Nebbia, C.S.; Nielsen, E.; Ntzani, E.; Petersen, A.; Sand, S.; Schwerdtle, T.; Wallace, H.; Benford, D.; Fürst, P.; Rose, M.; Ioannidou, S., Nikolič, M.; Bordajandi, L.R.; Vleminckx, Ch. Update of the risk assessment of hexabromocyclododecanes (HBCDDs) in food. EFSA J. 2021, 19, e06421. [CrossRef]
- Nielsen, E.; Østergaard, G.; Thorup, I.;, Ladefoged, O.; Jelnes, O.; Jelnes, J.E. Toxicological evaluation and limit values for nonylphenol, nonylphenol ethoxylates, tricresyl, phosphates and benzoic acid. Environmental Project Copenaghen, DK: Danish. Environ. Protection Agency. 2000, p. 43.
- Lin,W.C.; Wang, S.L.; Cheng, C.Y.; Ding, W.H. Determination of alkylphenol residues in breast and commercial milk by solid-phase extraction and gas chromatography–mass spectrometry. Food Chem. 2009, 114, 753-757. [CrossRef]
- Chen, G.W.; Ding, W.H.; Ku, H.Y.; Chao, H.R.; Chen, H.Y.; Huang, M.C.; Wang, S.L. Alkylphenols in human milk and their relations to dietary habits in central Taiwan. Food Chem Toxicol. 2010, 48, 1939-1944. [CrossRef]
- Otaka, H., Yasuhara, A., Morita, M. Determination of bisphenol A and 4-nonylphenol in human milk using alkaline digestion and cleanup by solid-phase extraction. Anal. Sci. 2003, 19, 1663-1666. [CrossRef]
- Guenther, K.; Heinke, V.; Thiele, B.; Kleist, E.; Prast, H.; Raecker, T. Endocrine Disrupting Nonylphenols are ubiquitous in food. Environ. Sci. Technol. 2002, 36, 1676-1680. [CrossRef]
- Chung, S.W.C. The development of isomer-specific analysis of branched 4-nonylphenol in food for dietary exposure - a critical review of analytical methods and occurrence in foodstuffs. Food Addit Contam. Part A Chem Anal. Control Expo Risk Assess. 2021, 38, 842-855. [CrossRef]
Table 1.
Description of mothers and children.
Table 1.
Description of mothers and children.
Table 2.
Solid-phase extraction procedure using C18ec sorbent for target analytes.
Table 2.
Solid-phase extraction procedure using C18ec sorbent for target analytes.
| Extraction steps |
Chromabond C18ec |
| Conditioning |
1 mL methanol
1 mL water |
| Sample load |
0,5 L with ISTD |
| Cleaning |
1 mL 5% methanol |
| Drying |
under vacuum 1-2 min |
| Elution of analytes |
1 mL acetonitrile |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).