1. Introduction
Antibiotic-resistant bacteria such as methicillin-resistant
S. aureus (MRSA) or extended-spectrum beta-lactamase (ESBL) secreting Gram-negative bacilli (GNB) have emerged and have spread from the hospital into the community. Inappropriate antibiotic (AB) use in human and veterinary medicine is the most important preventable cause of antimicrobial resistance (AMR) in both hospital-acquired infection (HAI) and community-acquired infections (CAI). Infections with resistant pathogens are associated with increased morbidity, mortality, and costs and in addition, represent an important patient safety issue [
1,
2].
Emergency Departments (EDs) are found at the interface between community and hospital and are an important setting concerning the approach of inappropriate antimicrobial prescribing practices, given their frequent use in these areas. ED clinicians routinely prescribe antimicrobials to patients for a wide variety of infections: skin and soft tissue, urinary tract, bloodstream, upper and lower respiratory tract, etc.
Practitioners in this sector have the unique opportunity to have a positive impact on the management of AB in both inpatient and outpatient settings, with important implications for both sectors. There are, for example, observational studies conducted in the ED, reporting significant rates of AB overprescribing in acute bronchitis (over 75% of prescriptions being for broad-spectrum AB, despite a certain improvement in clinical status) [
3]. Reducing unnecessary AB administration is imperative, not only for lowering AMR rates in the community, but also for individual patient safety, given the increased rate of allergic reactions and the development of secondary infections associated with antibiotic administration, such as
C. difficile infection [
4,
5].
The literature data underline the importance of correct AB management in the ED and provides practical recommendations drawn from the existing evidence for the application of different strategies and tools that could be implemented in the ED: development of clinical guidelines, clinical decision support systems or implementation of rapid diagnostic methods [
6,
7].
Antimicrobial stewardship comprises a collection of strategies, policies, and guidelines that aim to provide training and evaluation and collectively result in the optimization of antibiotic prescribing practices. It has been found that when Antimicrobial Stewardship Programs (ASP) are effectively implemented and monitored, they provide a measurable impact across multiple clinical departments: reducing drug costs, duration of treatment, adverse events to antibiotics, and local resistance. However, to date, ASPs have been targeted primarily at the hospital setting and there is a lack of literature data on antimicrobial stewardship strategies in EDs [
8,
9].
However, implementing ASP in the ED is a challenge, if we think about diagnostic uncertainty, patient fluctuation or even patient satisfaction concerns [
10].
The purpose of this study was to evaluate the etiology and AMR pattern of the community-acquired pathogens, as well as the epidemiological characteristics of patients admitted through ED, within the largest tertiary emergency hospital in the Western part of the country, in order to guide appropriate antibiotic therapy.
2. Materials and Methods
A retrospective observational study was carried out in the Emergency Department (ED) of the "Pius Brinzeu "Clinical County Emergency Hospital Timișoara (SCJUPBT), over a period of 6 months, from 01.01.2021 to 30.06.2021. This institution is a tertiary teaching hospital, affiliated to the university, with 1174 beds, providing medical care for the western region of Romania. The study aimed to describe the demographic profile of 657 patients hospitalized through ED and to characterize the etiological and AMR spectrum of microorganisms isolated from the 767 clinical samples collected from these patients.
We considered the community-acquired infection as an infection acquired after exposure to microbial agents in the community/home environment, unrelated to health care delivery.
Inclusion criteria were as follows: all patients who presented to the ED-SCJUPBT and were subsequently admitted to hospital wards, whose samples were collected for microbiological diagnosis as part of diagnostic and inpatient paraclinical investigations.
We have used the following exclusion criteria: samples collected more than 3 days after presentation, samples for which isolated microorganisms were considered contaminants, patients with a negative microbiological result, multiple samples from the same patient with the same identified pathogen, patients transferred to other hospitals within 1-3 days of presentation to the ED, patients operated on no more than 30 days before the current admission (90 days in case of implant for possible wound infections), and patients from chronic care units or elderly care units.
Pathogens identification were diagnosed with CAI, causing or associated with inpatient illness, for which treatment was instituted according to microbiological diagnosis and antibiotic susceptibility testing (AST), minimum inhibitory concentration (MIC) determination were performed according to the protocols of the Microbiology Department of the Clinical Laboratory of the SCJUPBT, using MALDI-TOF-Bruker and VITEK® 2C systems and interpretation of AST according to CLSI standards corresponding to the study period.
For the clinically significant bacteria, the following classifications have been used, depending on their acquired antibiotic resistance phenotypes:
Methicillin-resistant S. aureus (MRSA): S. aureus with MIC ≥ 4 to oxacillin
Multidrug-resistant (MDR) bacteria: with resistance to at least one antibiotic from three or more classes of antibiotics active for a given species.
Extensively drug-resistant bacteria (XDR): with resistance to at least one agent from all antimicrobial classes except one or two classes
Extended-spectrum beta-lactamase (ESBL) secreting Gram-negative bacilli (GNB): with resistance to all penicillins/cephalosporins.
Carbapenem-resistant GNB (CR-GNB): enterobacteria with MIC ≥ 4 to imipenem, meropenem and non-fermentative GNB with MIC ≥ 8 to imipenem, meropenem
Difficult-to-treat resistance (DTR): bacteria resistant to all first-line antibiotics, represented by: carbapenems (imipenem, meropenem, ertapenem/doripenem), extended-spectrum cephalosporins (those relevant to the respective pathogens), fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin).
Statistical analysis of the data was performed using the EPI INFO vers. 7.2.50. Category variables have been defined by value and percentage and the comparison has been done with the contingency table and the application of the hi2 test (Fisher exact test).
3. Results
During the period under study, a total of 25676 patients presented to the ED, of which 7661 were admitted through the ED, i.e., 29.83% of those presented. The total number of patients admitted to the SCJUPB was 12777, so patients admitted through the ED accounted for 59.96% of the total admissions.
Evaluation of the proportion of transfer wards of ED admissions showed that the most requested specialties were Surgery (SUR), Neurology (NEUR) and Gastroenterology (GE) (16.88%/ 14.25%/10.31%), followed by Vascular Surgery (VS), Cardiology (CD) and Urology (URO) (
Table 1).
In terms of the incidence of ED admissions to total wards admissions it was almost, 100% for Cardiology (CD) (99.54%), Vascular Surgery (VS) (99.54%) and Nephrology (NEF) (99.54%) and over 80% for Neurology (NEUR) (99.54%), Neurosurgery (NSUR) (99.54%), Gastroenterology (GE) (84.67%), Diabetes and Nutrition (DN) (83.25%) respectively.
From the study group of 7661 ED inpatients, 657 (8.57%) were diagnosed with CAI, causing or associated with inpatient illness, for which treatment was instituted according to microbiological diagnosis and AST. ED patients with an infectious diagnosis accounted for 37.69% of all patients with infections admitted during this period.
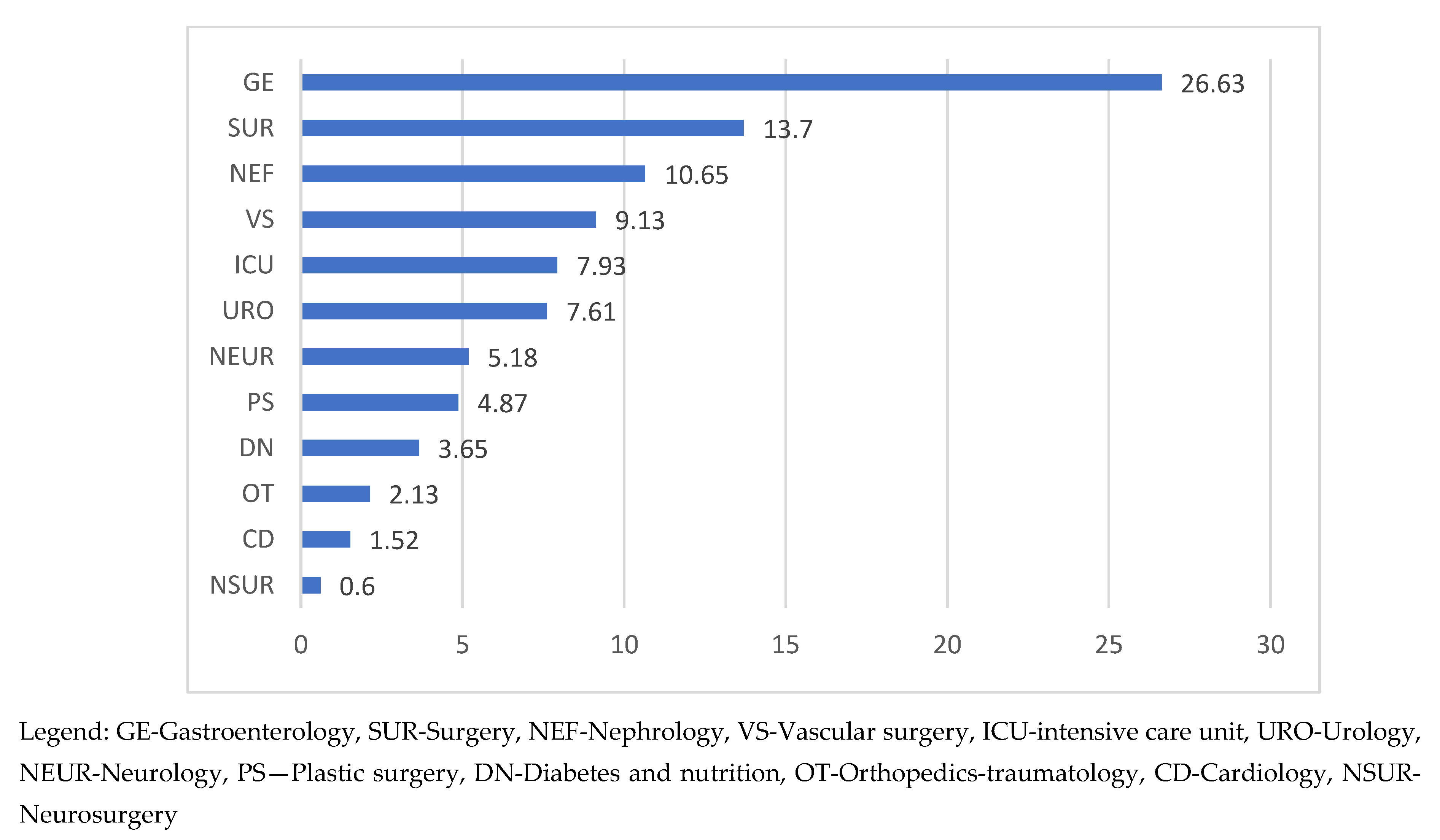
In terms of the distribution of ED-CAI admissions by ward, the data obtained showed that the Gastroenterology (GE) was the most requested ward for ED admissions, hospitalizing more than ¼ of the total of this group of patients (26.79%). Diagnoses with high frequency were acute angiocolitis (26.13%), cirrhosis (29.54%), hepatic neoplasm (20.45%), and pancreatitis (15.34%). At a distance, registering half of the GE frequencies was Surgery (13.7%), followed by Nephrology (10.65%) (
Figure 1).
Surgery admissions were indicated for diagnosis of abscess/phlegmon/gangrene/plague (36.67%), peritonitis (21.12%), appendicitis (15.56%), intestinal occlusions (7,78%) while on the Nephrology ward patients were transferred with sepsis with renal starting point (48.57%), chronic kidney disease (47.14%), acute kidney injury (34.28%), acute pyelonephritis (18.57%).
A special group of ED inpatients are the group of burn patients (N = 28) and the patients with trauma of various etiologies (N = 67), road traffic accidents, accidents at work or domestic accidents.
Of the group of patients with burns, 54% (N = 15) were major burns, transferred to the Functional Burns Unit (FBU), while 46% (N = 13), with limited injuries, admitted to the Plastic Surgery ward (PS). Burn patients accounted for 0.36% of all ED admissions. Burn patients with infected burn injuries (23) represented 3.5% of all ED-CAI patients, being admitted to the PS (60%) and FBU (40%) wards.
The number of patients with severe trauma was 67, representing 0.87% of the total ED admissions. They were subsequently transferred to different wards: ICU, Neurosurgery (NSUR), Orthopedics-Traumatology (OT), and Plastic Surgery (PS), depending on the severity and complexity of the injuries.
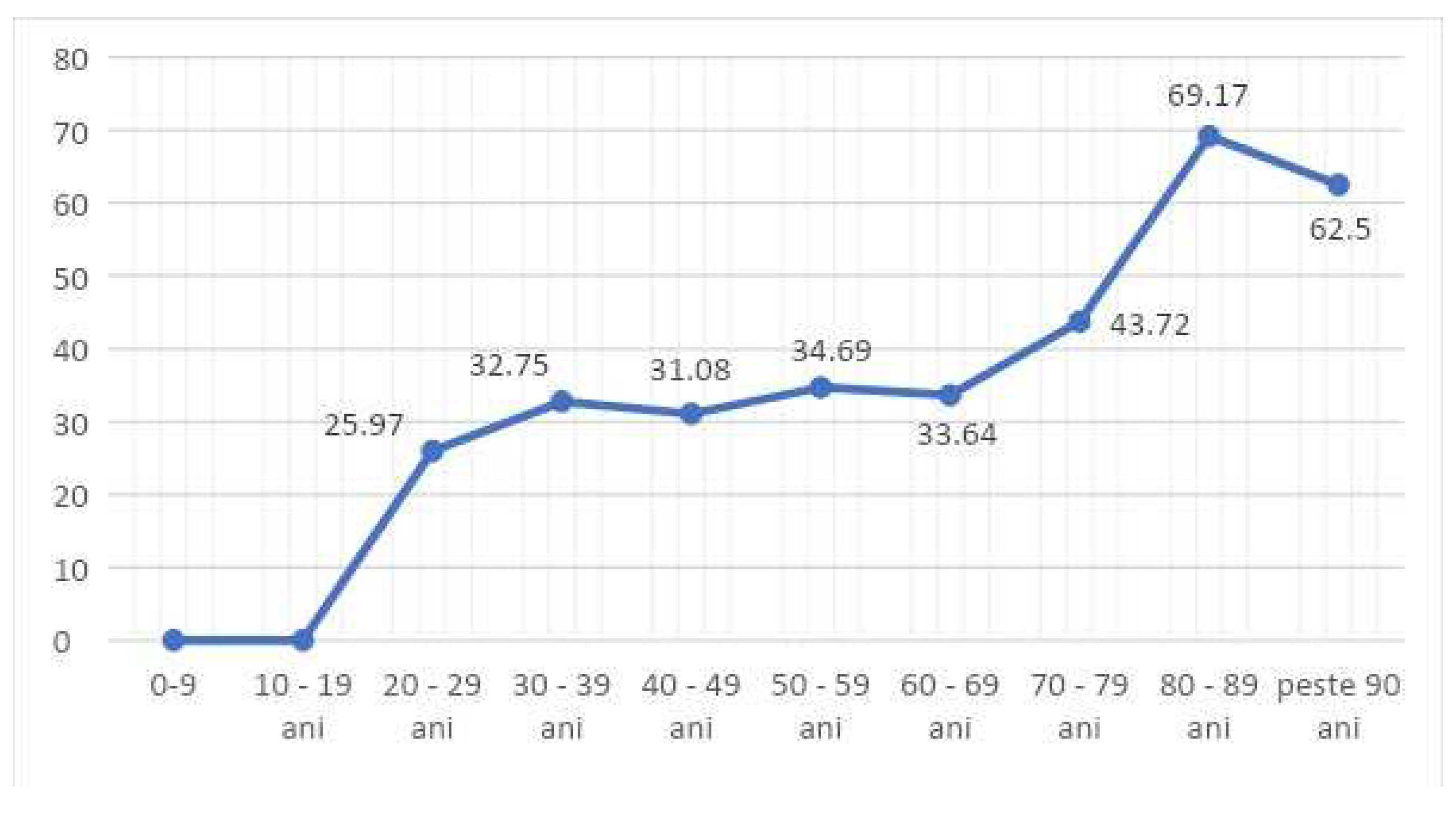
In terms of age decade distribution, of the 657 ED-CAI patients, 80.42% (456) patients were in the 50-80 age decade. No ED- CAI were recorded in patients under 20 years of age.
Patients aged 30-70 years with CAI constituted 32-34% of all patients with infections in the wards, for each decade. Those aged over 80 years represented 69.17% (
Figure 2).
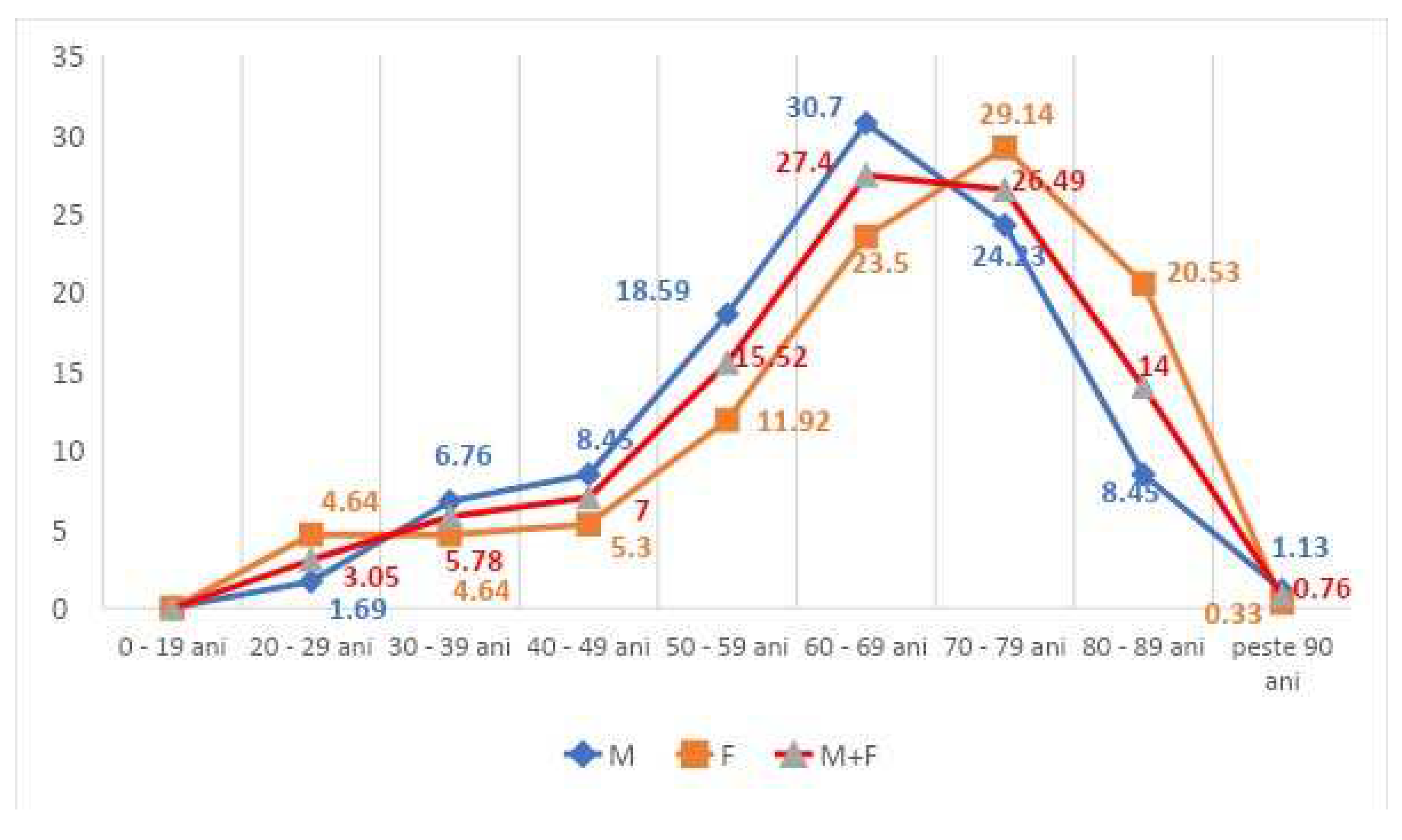
The gender distribution was roughly equal, with a slight predominance of men (54%). The frequency curve showed that women's referral to ED services increases from age 50 onwards and peaks in the 70-79 age range. In the case of men, referral increases from 40 years of age and peaks between 60-69 years of age. In the whole study group of ED patients diagnosed with CAI, there is a significant increase in frequencies between 40 and 60 years, from 7.00% to 27.40% (
Figure 3).
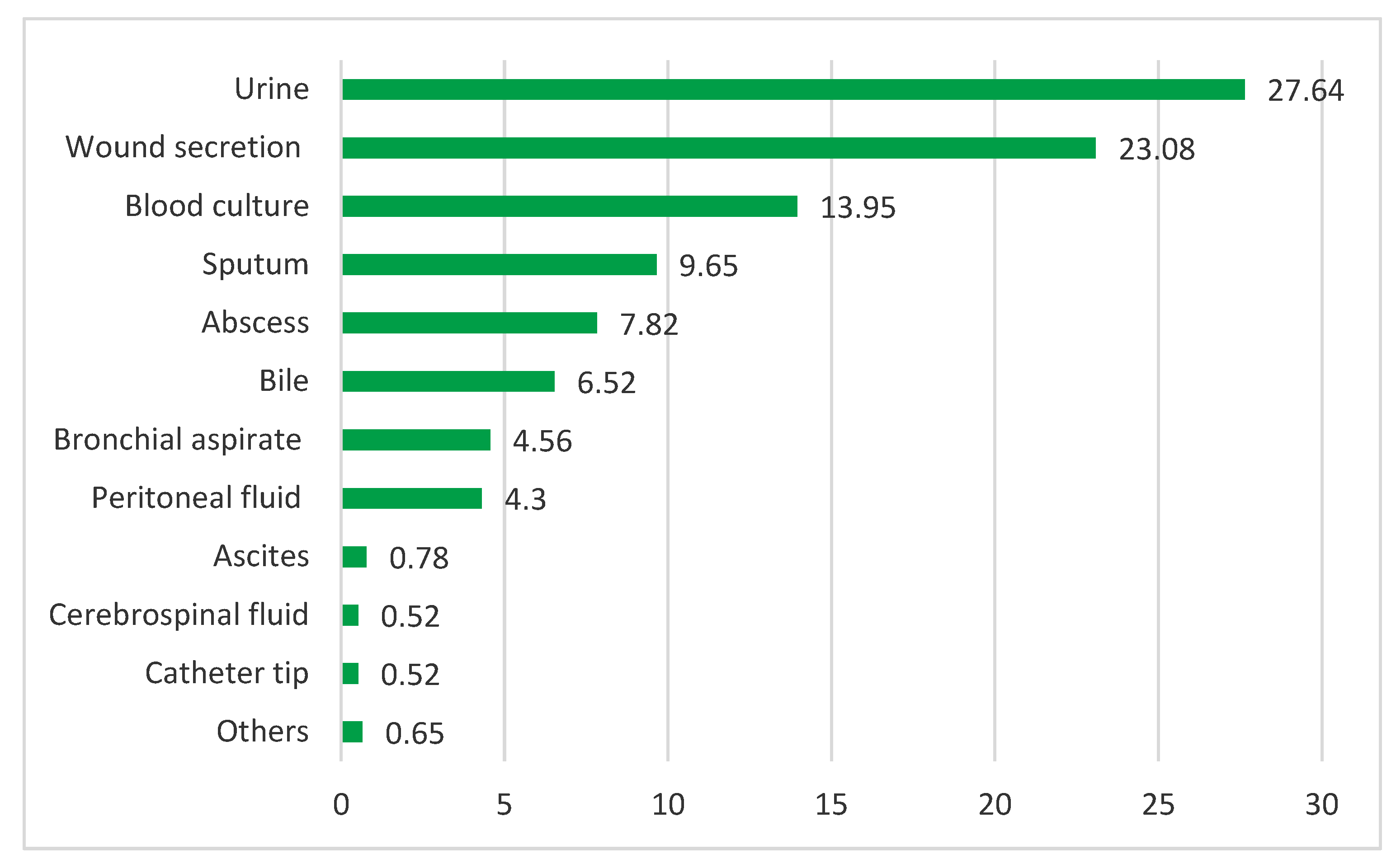
Study of ED-CAI infections. From 657 ED-CAI patients, 767 clinical samples were collected for bacteriological diagnosis. The most numerous were urine cultures, wound secretions and blood cultures (27.64%, 23.08%, 13.95%). Internal fluids together account for a significant percentage of 11.60% (
Figure 4).
Of the 767 samples analysed, 903 microbial isolates were identified, of which 48.95% were Enterobacterales, 10.08% non-fermentative GNB, 34.22% Gram-positive cocci (GPC) and 4.98% fungi.
The bacterial species most represented were
E. coli, accounting for approximately ¼ of the total strains isolated (24.25%), followed by
Klebsiella spp. (13.73%) and
S. aureus (10.63%) (
Table 2).
The distribution of isolates in clinical samples and the distribution of AMR phenotypes are shown in
Table 3 and
Table 4.
Nearly 50% of the identified
E. coli strains (47.03%) were from urine cultures, followed by wound secretion (12.32%) and internal fluids: bile fluid, peritoneal fluid, blood cultures (9.59%, 8.68%, 7.76%) (
Table 3). In terms of AMR, 25% of
E. coli strains showed resistance to trimethoprim/sulfamethoxazole (SXT) and 17.8% showed resistance to fluoroquinolones (FQ); 6.4% recorded ESBL phenotype, and 9% fell into the MDR category (
Table 4).
The second most common isolate in ED-CAI was
Klebsiella spp. The highest number of
Klebsiella isolates was in urine cultures (24.20%), followed by sputum (16.93%) and abscess cultures (10.48%) (Tab. 3). AMR was marked by the identification of ESBL (11.30%) and CR (7.28%) type strains, respectively SXT (14.51%) and FQ (15.32%) resistant strains. As a result, 12.09% of strains were MDR type, 7.28% DTR type, and 2.50% were XDR strains (
Table 4)
Among Enterobacterales species, Klebsiella spp. had statistically significantly higher percentages for XDR, DTR, CRE but lower for SXT vs. E.coli resistance.
Non-fermentative GNB species had a low representation, with a frequency of 6.87% for Pseudomonas spp. and 1.88% for Acinetobacter spp. respectively.
Pseudomonas spp. had the highest frequencies in cultures from wound secretions (40.32%), followed by cultures from bile fluids (12,9%), and urine cultures (12.90%) (Tab 3). 19.35% were CR resistant strains, 17.74% showed resistance to FQ, and 11.29% resistance to AG; 24.19% of strains were identified in the MDR and 11.29% in the DTR category, while the frequency of strains with extremely limited therapeutic options, XDR, was 6.45% (
Table 4).
Acinetobacter spp. had the highest frequencies in cultures from wound secretions (58.83%). The remaining strains were present in bronchial aspirates and peritoneal fluid samples (
Table 3). AMR studies reported high incidences of acquired resistance phenotypes of
Acinetobacter spp. strains: over 50% CR and AG resistance phenotype. Accordingly, 52.94% of isolates fell into the DTR, 58.82% in the MDR category and 23.15% were XDR strains.
Among non-fermentative isolates,
Acinetobacter spp. showed significantly higher percentages for DTR, MDR, ESBL, CR, R-AG, R-FQ vs.
Pseudomonas aeruginosa. (
Table 4).
Among the GPC, the highest frequency was recorded by
Staphylococcus aureus (SA) strains. The majority of SA positive cultures were taken from wounds (53.12%), followed by sputum and bronchial aspirates (10.42%, 10.42%) (
Table 3). Phenotypic analysis showed that SA strains from CAI are resistant to antibiotics commonly used for the treatment of these infections. Thus 52% were beta-lactamase-secreting strains, 32.3% were MRSA and 30.2% were identified with macrolide-lincosamide-streptogramin (MLSB) resistance phenotype, respectively. Consequently, the frequency of MDR strains was 37.5%. Penicillin resistance of
S. aureus was significantly higher versus enterococci p < 0.001 and macrolide resistance did not differ p=1.00.
4. Discussion
The present study was conducted during the period January-June 2021, which numbered 181 days. The year 2021 was a pandemic year, located in the middle of the interval dominated by SARS COV-2 infection (February 2020—March 2022), in which hospital admissions were carried out according to well-established protocols, with limitations aimed at preventing intra-hospital transmission of the virus. The SCJUPBT ED was requested by an average of 142 patients/day (25676/181 days), of which, an average of 42 patients/day were admitted, out of an average total of 71 admissions/day/hospital, a flow that represented a percentage of approximately 60% ED admissions out of total admissions in the entire hospital.
The pathology of ED-CAI patients was split between surgical and medical specialties, but it was noted that surgical pathology had a higher frequency of cases than medical pathology (53.64% versus 46.35%). In comparison, for the total number of ED admissions in the same period, ED patients admitted to surgical wards were about 20% more numerous than ED patients admitted to medical wards (59.45% versus 40.54%).
In the large group of ED patients, ED-CAIs represent a distinct segment due to the challenging diagnosis and a critical patient approach, given the potential for worsening of the disease state that can be induced by the infectious condition. This potential correlates with a number of patient-specific factors: age, gender, location of infection, level of immunocompetence, type of pathogens identified, their pathogenic potential, bacterial associations, and AMR.
Moreover, patients with ED-CAI represent an important gateway for pathogens to enter the healthcare institution, as well as a significant source of infection, especially when their incidence is high. In the present study, ED- CAI patients accounted for 37.69% of the total number of patients admitted to wards with a positive bacteriological diagnosis.
Most of the antibiotic treatments initiated in the ED for these patients are empirical. Antibiotic administration is profoundly influenced by patient demographic variables as well as diagnosis. High prescription rate use of antimicrobial treatments, regardless of these variables, however, has been observed in ED low-resource settings, highlighting the importance of surveillance in order to implement targeted intervention [
11,
12].
In the present study, the majority of ED-CAI patients were over 50 years of age, but about 55% were between 60-79 years of age, similar to other studies [
13,
14]. ED-CAI patients over 80 years old were lower in number (15%), but they represented more than 2/3 of all patients admitted with at least one positive bacteriological diagnosis, signaling that in these cases, the diagnostic protocol in ED should include bacteriological sampling to obtain an appropriate therapeutic indication as soon as possible.
The gender distribution of ED patients was not significant. However, the age-frequency graph of ED-CAI males was placed one decade ahead of that of ED-CAI females, i.e. the disease state and need for hospital care was present earlier for males (peak frequency 30.7%, decade 60-69 years p =0.043 vs women from the same decade) than for women. In contrast, in the interval 80-89 years, p<0.001 shows the preponderance of the female sex, explained by the lower average life expectancy of men. In Romania it is 71.5 years vs. 79.3 years for women.
The strategy for dealing with the ED-CAI infectious patient depends on the framing of the disease—medical or surgical pathology. In the present study, ED-CAI patients with surgical pathology were 7.29% more common than those with medical diagnosis.
Among surgical patients, general surgery and vascular surgery account for about 23% of all infectious admissions. It is noted that renal patients as a whole (NEF+URO) achieve a significant frequency of ED patients with CAI (18.26%).
The problem of burn and trauma patients is a great challenge for the ED, and transfer wards (BFU, respectively ICU, OT, NSUR, PS), both in terms of medical treatment, length of stay and cost of care. For the 28 burn patients admitted through the ED, 294 positive bacterial cultures were recorded throughout the admission, which means an average of 10.50 positive samples/patient and an average length of stay of 30 days, with a maximum of 156 days in isolation [
15]. These figures show the importance of sampling for the diagnosis of microbial biofilms in these infections, as the biofilm is known to impair the patient's immune response and delay the healing of burn wounds even with optimal treatment instituted early [
16,
17]. The same problem of biofilm detection is posed for all types of wounds, catheter tip cultures, but also for respiratory tract cultures (sputum and bronchial aspirate), as
Pseudomonas spp. strains are known to form biofilms in secretions of pulmonary patients, especially those with cystic fibrosis.
The most common bacterial species in wound biofilms (burn, ischemic, gangrenous) are
S. aureus [
18,
19] and
P. aeruginosa [
20,
21] with resistance to local and general treatments. Consequently, in these cases genotypic investigation and biofilmography should be viewed as routine investigations for the institution of treatment as far as these patients are concerned (burns, diabetic with gangrene, venous ulcers).
The implementation of ASP—ED strategies for the management of CAI patients must consider the peculiarities of ED functioning—rapid treatment decision-making in the context of time-constrained bacteriological investigation and access to rapid diagnostic tools. In this regard Nauclér [
22] has illustrated that rapid initiation of antibiotic treatment is considered to be crucial regarding/for patients with severe infections. He concluded that the literature supports prompt administration of effective antibiotics for septic shock and bacterial meningitis, but there is no clear evidence that delayed initiation of therapy is associated with a worse outcome for less severe infectious syndromes. For patients in whom bacterial infections are suspected, suspending antibiotic therapy until microbiological diagnostic results are available (e.g. up to 4-8 hours) seems acceptable in most cases, except in the situation where septic shock or bacterial meningitis is suspected. This approach promotes the use of ecologically favourable antibiotics in the ED, reducing the risks of side effects and resistance selection.
To initiate AB treatment in ED, May [
7] shows that the most important questions related to AMS to be answered at this stage are: Is there a rationale for starting antimicrobial treatment for this diagnosis? Should I treat now? Or can I wait? Currently, the answer to initiating antibiotic therapy in ED is facilitated by the provision of rapid diagnostics through molecular techniques for the diagnosis of viral infections and the presence of biomarkers needed to differentiate bacterial from viral infections. Incorporating these findings into the clinical diagnostic process in ED has the potential to significantly reduce unnecessary antibiotic use [
10,
23,
24].
The use of procalcitonin as a biomarker has been considered when decision-making in chronic lung disease. There are studies that have shown a reduction in total antibiotic exposure (days of administration) and adverse effects based on values of this biomarker [
25,
26].
Multiplex PCR investigation on platforms such as rapid syndromic testing is another method that would allow rapid identification of pathogens and detection of resistance genes. Thus, Sun L. (2021) [
27] has showed that in his study the overall sensitivity and specificity of Unyvero at detecting bacteria in lower respiratory tract infections was high (84.0% and 98.0%.) The overall concordance between Unyvero and routine culture was 69/84 (82.1%). In addition, Unyvero showed good performance for antibiotic-resistant bacteria, except for
Pseudomonas aeruginosa.
Similar agreements were published by Collins et al [
28], who compared the performance of the Unyvero LRT panel with routine bacterial culture methods on 175 bronchoalveolar lavage (BAL) samples and reported a sensitivity of 96.5% and a specificity of 99.6% among microbial targets. For antibiotic resistance markers in the LRT BAL lower respiratory tract panel, a positive predictive value (PPV) of 100% was reported. In another recent publication, Pickens et al. [
29] reported a sensitivity of 85.7% and a specificity of 98.4% for 620 respiratory samples (395 bronchoscopic or none bronchoscopic BAL samples, 225 aspirates) using the Unyvero LRT panel.
Regarding GNB resistance genes, Klein [
30] has shown that the detection of a resistance gene does not necessarily link it to the host bacteria. However, for GNB, there were strong genotypic and phenotypic correlations of Unyvero results with the corresponding isolates. The reporting of resistance genes may provide a clue over the presence of an underlying resistant organism, which may have implications regarding infection prevention and control (e.g., if blaKPC, blaNDM, blaVIM or blaOXA-48 is detected), even if the species with which the gene is associated is unknown.
Such an approach was adopted for some patients in the present study, so that multiplex PCR investigation on the Unyvero platform of an ED-CAI patient with polytrauma identified in blood culture the association of A. baumannii and P. aeruginosa, with the presence of NDM , OXA-24, SUL1, gyrA83 Pseu resistance genes, and of an ED-CAI patient transferred GE, Unyvero investigation of a bronchial aspirate identified A. baumannii and P. aeruginosa and SUL1, gyrA83 Pseu resistance genes.
In the present study, the identification of germs responsible for ED-CAI infections has shown that the most frequently isolated strains were
E. coli (24.25%), followed by
Klebsiella species (13.73%) and
S. aureus (10.63%). Ria Benkő [
31] showed in the ED study conducted in 2020 that she identified germs in the same hierarchy (
E. coli (44.10%),
Klebsiella spp. (13.40%),
S. aureus (11.30%)), with the caveat that in this study
E. coli isolates had almost double the frequency, tending to account for half of the total strains in the study.
Most ED-CAI patients with
E. coli strains were admitted to GE wards (31.96%), kidney disease wards (NEF+URO, 21.46%) and SUR wards (18.26%). Isolates were identified in urine cultures and internal fluids (biliary, peritoneal, blood) and posed no treatment problems, a small number being MDR respectively DTR strains. However, resistance to sulfonamides (R-S) and fluoroquinolones (R-FQ) is noted, which argues for the frequent use of these antibiotics in community infectious pathology [
32] and draws attention to their use in empiric therapy in ED.
Klebsiella spp. strains (13.73%) were isolated from urine cultures, RT cultures (sputum and bronchial aspirate) and wounds/abscesses of ED-CAI patients transferred to the GE, NEF+URO, SUR and ICU wards (35.92%, 23.30%, 14.56%, 11.65%).
Klebsiella spp. strains showed a higher percentage of MDR (12.9%), with acquired beta-lactam resistance phenotypes (ESBL and CR) and high frequencies of R-FQ, a fact present in other studies [
10]. The difficult problem of establishing treatment regards the XDR strains (2.5%), with treatment options limited to at most two classes of antibiotics, which were identified in GE and SUR patients.
As for
S. aureus strains, they were mostly identified in wound samples respectively RT samples of patients who were transferred to SUR, GE, NEF+URO, and ICU wards (48.14%, 14.81%, 11.12%, 8.64%). The incidence of SA-MDR was high, (37.5%), explained by the frequency of MRSA and MLSB strains. A much lower rate of MRSA (only 0.4%) was identified in a study that set out to determine AMR in microorganisms causing community-onset bacteremia [
33].
Non-fermentative GNBs were represented by Pseudomonas spp. and Acinetobacter spp. known to have high AMR behavior in hospital environments [34-36].
Pseudomonas spp. were reduced in number (6.87%), being mainly identified in wound secretions, RT samples (bronchial aspirates and sputum) and internal fluids (biliary and peritoneal). Approximately ¼ of these strains were MDR, with high frequencies of CR and FQ resistance phenotypes (almost 20% of them). Samples were from ED-CAI patients with transfer diagnoses for GE, SUR and NEF+URO (30.07%, 30.18%, 18.86%).
Acinetobacter spp. were present in low numbers, in samples from ED-CAI patients. The majority were identified in wound secretions of burn patients and patients with lower limb pathology (ischemia, gangrene), and infected traumatic injuries, 81.25% were transferred to surgical wards (VS, PS) and FBU. The problem raised by these isolates, however, is the high degree of antibiotic resistance, with a frequency of almost ¼ of their number, of XDR strains. The high resistance to carbapenems, the only effective beta-lactams on Acinetobacter species (52.94%), together with resistance to AG and FQ (52.94%, 47.05%) made infections caused by these strains extremely difficult to treat, due to the lack of effective therapy.
The current study brings more information about CAI hospitalized by ED, a topic that is not often addressed in the literature, at least in our geographical area, there is little data in this regard. However, the current study has some limitations: it addresses infectious pathology hospitalized through a single ED, in a particular pandemic period, burdened by restrictions imposed by the health system, but also by the decreasing addressability of the population. The descriptive design did not allow clear evidence of these differences.
5. Conclusions
During the period under study, the proportion of ED hospitalizations increased, with a significant percentage of patients with infectious diagnoses, mostly elderly, with associated pathologies, hospitalized mainly in surgical wards. It was found that the disease state and the need for hospital care in men was earlier than in women.
Of the identified pathogens, GNB was mostly predominant. E. coli was isolated most frequently, but with maintenance of susceptibility to the usual ABs, while non-fermenters were isolated less frequently, but with increased AMR rates. In the case of SA strains, we note the significantly increased percentage of community MRSA strains.
The ongoing study of AMR in ED isolates, as well as the introduction of rapid microbiological diagnostic methods are imperative to timely identify AMR strains and improve therapeutic protocols. We also emphasize the need for ASP in the ED with the identification of interventions to improve patient outcomes and care and reduce the consequences of antimicrobial use in the hospital and community.
Author Contributions
Conceptualization, C.M. and M.L.; methodology, CM. and SM.; software, OI.; validation, M.L., D.M. and S.M.; formal analysis, S.M.; investigation, A.V. and O.I.; resources, L.B. and O.I.; data curation, A.V.; writing—original draft preparation, SM, R.J and OI.; writing—review and editing, D.M., A.V. and R.J.; supervision, M.L. and D.M.; project administration, S.M. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “PIUS BRÎNZEU” EMERGENCY CLINICAL COUNTY HOSPITAL TIMISOARA, ROMANIA (No 306/16 06.2022).
Data Availability Statement
Not applicable.
Acknowledgments
A part of the study was presented in an oral form, at the Interdisciplinary Congress of Emergency Medicine, Cluj Napoca, Romania 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, J.H.; Kasahara, K; Edelstein, P.H.; Bilker, W.B.; Lautenbach, E. Risk factors for infection or colonization with CTX-M extended-spectrum-β-lactamase-positive Escherichia coli. Antimicrob Agents Chemother. 2012, 56(11), 5575–5580. [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B. et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006, 355(7), 666–674. [CrossRef]
- Kroening-Roche, J.C.; Soroudi, A.; Castillo, E.M.; Vilke, G.M. Antibiotic and bronchodilator prescribing for acute bronchitis in the emergency department. J Emerg Med. 2012. 43(2), 221–227. [CrossRef]
- Gonzales, R.; Camargo, C.AJ.; MacKenzie, T.; Kersey, A.S.; Maselli, J.; Levin, S.K. et al. Antibiotic treatment of acute respiratory infections in acute care settings. Acad Emerg Med Off J Soc Acad Emerg Med. 2006, 13(3), 288–294. [CrossRef]
- Metlay, J.P.; Camargo, C.AJ.; MacKenzie, T.; McCulloch, C.; Maselli, J.; Levin, S.K. et al. Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments. Ann Emerg Med. 2007, 50(3), 221–230. [CrossRef]
- May, L.; Cosgrove, S.; L’Archeveque, M.; Talan, D.A.; Payne, P.; Jordan, J. et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. 2013, 62(1), 69-77. [CrossRef]
- May, L.; Martin -Quirós, A.; Ten Oever, J.; Hoogerwerf, J.; Schoffelen, T.; Schouten, J. Antimicrobial stewardship in the emergency department: characteristics and evidence for effectiveness of interventions. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021, 27(2), 204–209. [CrossRef]
- Lawton, R.M.; Fridkin, S.K.; Gaynes, R.P.; McGowan, J.E. Practices to improve antimicrobial use at 47 US hospitals: the status of the 1997 SHEA/IDSA position paper recommendations. Society for Healthcare Epidemiology of America/Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2000, 21(4), 256–259. [CrossRef]
- Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012, 33(4), 322–327. [CrossRef]
- Bishop, B.M. Antimicrobial Stewardship in the Emergency Department: Challenges, Opportunities, and a Call to Action for Pharmacists. J Pharm Pract. 2016, 29(6),556–563. [CrossRef]
- Yi, S.; Ramachandran, A.; Epps, L.; Mayah, A.; Burkholder, T.W.; Jaung, M.S. et al. Emergency department antimicrobial use in a low-resource setting: results from a retrospective observational study at a referral hospital in Liberia. BMJ Open. 2022, 12(4). [CrossRef]
- Shankar, P.R. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006, 2009, Bulletin of the World Health Organization. vol. 87, 804. [CrossRef]
- Hong, S-I.; Kim, J-S.; Kim, Y-J.; Seo, D-W.; Kang, H.; Kim, S.J. et al. Characteristics of Patients Who Visited Emergency Department: A Nationwide Population-Based Study in South Korea (2016-2018). Int J Environ Res Public Health. 2022, 19(14). [CrossRef]
- Lee, J.H.; Park, G.J.; Kim, S.C.; Kim, H.; Lee, S.W. Characteristics of frequent adult emergency department users: A Korean tertiary hospital observational study. Medicine. 2020, 99(18). [CrossRef]
- Licker, M.; Musuroi, C.; Muntean, D.; Crainiceanu, Z. Updates in the management of multidrug-resistant bacterial infections in burn patients. Paper presented at 16th National Conference on Microbiology and Epidemiology, Bucharest, 9-11 nov. 2023. https://www.srm.ro/media/2023/11/volum-rezumate-cnme-1.pdf.
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv wound care. 2015, 4(7), 373–81. [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A,; Leaper, D.J.; McBain, A.J.; Malone, M. et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound repair Regen Off Publ Wound Heal Soc [and] Eur Tissue Repair Soc. 2017, 25(5), 744–757. [CrossRef]
- Di Lodovico, S.; Bacchetti, T.; D’Ercole, S.; Covone, S.; Petrini, M.; Di Giulio, M. et al. Complex Chronic Wound Biofilms Are Inhibited in vitro by the Natural Extract of Capparis spinose. Front Microbiol. 2022, 13, 1–11. [CrossRef]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S. et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann Surg. 2020, 271(6), 1174–1185. [CrossRef]
- Moreau-Marquis, S.; Stanton, B.A.; O’Toole, G.A. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther. 2008, 21(4), 595–599. https://doi.org/https://doi.org/10.1016/j.pupt.2007.12.001. [CrossRef]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M,; Secor, P.R.; Wozniak, D.J. et al. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 2021, 34(8). https://doi.org/https://doi.org/10.1016/j.celrep.2021.108782. [CrossRef]
- Nauclér, P.; Huttner, A.; van Werkhoven, C.H.; Singer, M.; Tattevin, P.; Einav, S. et al. Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021, 27(2),175–81. [CrossRef]
- Pulia, M.; Redwood, R.; May, L. Antimicrobial Stewardship in the Emergency Department. Emerg Med Clin North Am. 2018, 36(4), 853–872. https://doi.org/https://doi.org/10.1016/j.emc.2018.06.012. [CrossRef]
- Barlam, T.;F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J. et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016, 62(10), e51–77. [CrossRef]
- Mathioudakis, A.G.; Chatzimavridou-Grigoriadou, V.; Corlateanu, A.; Vestbo, J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017, 26(143). [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M. et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane database Syst Rev. 2017, 10(10), CD007498. [CrossRef]
- Sun, L.; Li, L.; Du, S.; Liu, Y.; Cao, B. An evaluation of the Unyvero pneumonia system for rapid detection of microorganisms and resistance markers of lower respiratory infections-a multicenter prospective study on ICU patients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2021, 40(10), 2113–2121. [CrossRef]
- Collins, M.E.; Popowitch, E.B,; Miller, M.B. Evaluation of a Novel Multiplex PCR Panel Compared to Quantitative Bacterial Culture for Diagnosis of Lower Respiratory Tract Infections. J Clin Microbiol. 2020, 58(5). [CrossRef]
- Pickens, C.; Wunderink, R.G.; Qi, C.; Mopuru, H.; Donnelly, H.; Powell, K. et al. A multiplex polymerase chain reaction assay for antibiotic stewardship in suspected pneumonia. Diagn Microbiol Infect Dis. 2020. 98(4). https://doi.org/https://doi.org/10.1016/j.diagmicrobio.2020.115179. [CrossRef]
- Klein, M.; Bacher, J.; Barth, S.; Atrzadeh, F.; Siebenhaller, K.; Ferreira, I. et al. Multicenter Evaluation of the Unyvero Platform for Testing Bronchoalveolar Lavage Fluid. J Clin Microbiol. 2021, 59 (3). [CrossRef]
- Benkő, R.; Gajdács, M.; Matuz, M.; Bodó, G.; Lázár, A.; Hajdú, E. et al. Prevalence and Antibiotic Resistance of ESKAPE Pathogens Isolated in the Emergency Department of a Tertiary Care Teaching Hospital in Hungary: A 5-Year Retrospective Survey. Antibiot (Basel, Switzerland). 2020, 9(9). [CrossRef]
- Grignon, O.; Montassier, E.; Corvec, S.; Lepelletier, D.; Hardouin, J-B.; Caillon, J. et al. Escherichia coli antibiotic resistance in emergency departments. Do local resistance rates matter? Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015, 34(3), 571–577. [CrossRef]
- Rothe K, Wantia N, Spinner CD, Schneider J, Lahmer T, Waschulzik B, et al. Antimicrobial resistance of bacteraemia in the emergency department of a German university hospital (2013-2018): potential carbapenem-sparing empiric treatment options in light of the new EUCAST recommendations. BMC Infect Dis. 2019, 19(1), 1091. [CrossRef]
- El-Sokkary, R.; Uysal, S.; Erdem, H.; Kullar, R.; Pekok, A.; Amer, F. et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: an international ID-IRI survey. Eur J Clin Microbiol Infect Dis. 2021, 40. [CrossRef]
- Baditoiu, L.; Axente, C.; Lungeanu, D.; Muntean, D.; Horhat, F.; Moldovan, R. et al. Intensive care antibiotic consumption and resistance patterns: a cross-correlation analysis. Ann Clin Microbiol Antimicrob. 2017, 16(1):71. [CrossRef]
- Axente C, Licker M, Moldovan R, Hogea E, Muntean D, Horhat F, et al. Antimicrobial consumption, costs and resistance patterns: a two year prospective study in a Romanian intensive care unit. BMC Infect Dis. 2017, 17(1):358. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).