1. Introduction
Prostate cancer (Pca) is one of the most common malignant tumors in men. According to the American Cancer Society, prostate cancer is the second most common cancer in men and the second leading cause of cancer death in men, diagnosed at a distant stage increased from 3.9 % to 8.2% over the past decade1. The pathogenesis of PCa is still not fully understood, but age, genetic factors, and chronic inflammation have been confirmed to be related to the development of prostate cancer2–4.However, there are various adverse reactions to androgen deprivation therapy (ADT), an important treatment for prostate cancer5,6. At present, a large number of studies have confirmed that immune cells play a key role in anti-tumor immunity in prostate cancer7-9. But research results on the causal relationship between immune cells and prostate cancer are inconsistent. Related research shows that dysfunction of natural immune cells and adaptive immune cells may lead to the occurrence and development of prostate cancer10,11. There are also studies indicating indicate that immune cell activation and proliferation may be closely related to the progression of prostate cancer12,13.
Mendelian randomization analysis can use genetic variants that are strongly correlated with exposure factors as instrumental variables (IV) to evaluate the causal relationship between exposure factors and outcomes14. So far, although there are few studies on the causal relationship between immune cells and disease levels, the experimental results are valuable. Zhang et al. 15and Cao et al. 16 studied the causal effects of immune cells on Schizophrenia (SCZ) and bone mineral densit respectively, but there is no research on Pca yet. Therefore, this study performed a comprehensive two-sample MR analysis to explore the causal role between immune cells and Pca
2. Materials and methods
2.1. Study design
This study utilized a two-sample Mendelian randomization analysis to assess the causal relationship between 731 immune cell characteristics (divided into 7 groups) and prostate health. Mendelian randomization employs genetic variation as a proxy for risk factors, with instrumental variables meeting specific conditions for causal inference. These conditions include a direct association between genetic variation and the exposure factor, no relationship between genetic variation and potential confounding factors between the exposure factor and outcome, and genetic variation not influencing the exposure factor via other pathways. As the analyses relied on publicly available aggregate statistics rather than individual-level data, ethical approval from an institutional review board was not required.
2.2. Immunity-wide GWAS data sources
GWAS summary statistics for each immune trait are available from the GWAS public catalog (accession numbers from GCST90001391 to GCST90002121)17. A total of 731 immunophenotypes were analyzed, including various parameters such as absolute cell counts (n = 118), median fluorescence intensities (MFI) reflecting surface antigen levels (n = 389), morphological parameters (MP) (n = 32), and relative cell counts (n = 192). The MFI, AC, and RC features encompass B cells, CDCs, mature stages of T cells, monocytes, myeloid cells, TBNK (T cells, B cells, natural killer cells), and Treg panels. The SNPs were imputed using a Sardinian sequence-based reference panel18.
2.3. Genome-wide association study (GWAS) data sources for Pca
GWAS summary statistics of cellular immunity were obtained from the UK Biobank. Additionally, genome-wide association study summary statistics for prostate cancer were obtained from UK Biobank. The study involved 173,493 prostate cancer patients and 9,132 controls (a total of 182,625 Europeans) and analyzed 12,097,504 single nucleotide polymorphisms (SNPs).
2.4. Selection of instrumental variables(IVs)
The significance level of IVs for each immune trait was set to 1 × 10−5. To prune these SNPs, the clumping procedure in the 'TwoSampleMR' packages (version 0.5.7) was used, with a linkage disequilibrium (LD) r2 threshold of < 0.001 within a 100 kb distance. LD r2 was calculated based on the 1000 Genomes Projects as a reference panel. For Pca, the significance level was also set to 1 × 10−5. Subsequently, the MR pleiotropy residual sum and outlier (MR-PRESSO) test was applied to detect potential horizontal pleiotropy and remove outliers to eliminate the effects of pleiotropy. Finally, the proportion of phenotypic variation explained (PVE) and F statistic were calculated for each IV to evaluate IV strength and avoid weak instrumental bias.
2.5. Statistical analysis
All analyses were performed using R 4.3.1 software (
http://www.Rproject.org) .We used the "Mendelian Randomization"
19 and "TwoSampleMR" packages (version 0.5.7) to evaluate the causal relationship between immune cells and prostate cancer. The causal effect was calculated by dividing the SNP-outcome effect estimated by the SNP-exposure effect estimate. For cell immune phenotypes containing multiple SNPs, multiple tests, including fixed-/random-effects inverse variance weighted (IVW) test
20, weighted median method
21and MR-Egger were performed. Cochran's Q statistic and its corresponding p-value were utilized to assess heterogeneity among the selected IVs. In cases where heterogeneity was observed, we opted for random-effects IVW instead of fixed-effect IVW. Odds ratios (OR) and their 95% confidence intervals (CI) were employed to demonstrate whether immune cells can predict prostate cancer risk. The MR-Egger
22 regression and MR-PRESSO
23 tests were utilized to identify horizontal pleiotropy and potential outliers of SNPs, and corrected association results were obtained after removing outliers.
2.6. Reverse Mendelian Randomization Analysis
All statistical analyses were conducted using R (version 4.3.1). The IVW, weighted median, and MR-Egger regression methods were performed using the 'TwoSampleMR' package (version 0.5.7). The MR-PRESSO test was performed using the 'MRPRESSO' package. The statistical significance of the MR effect estimates was defined as a false discovery rate (FDR) of less than 0.05 to adjust for multiple testing.
3. Result
3.1. Exploration of the causal effect of immunophenotypes on Pca
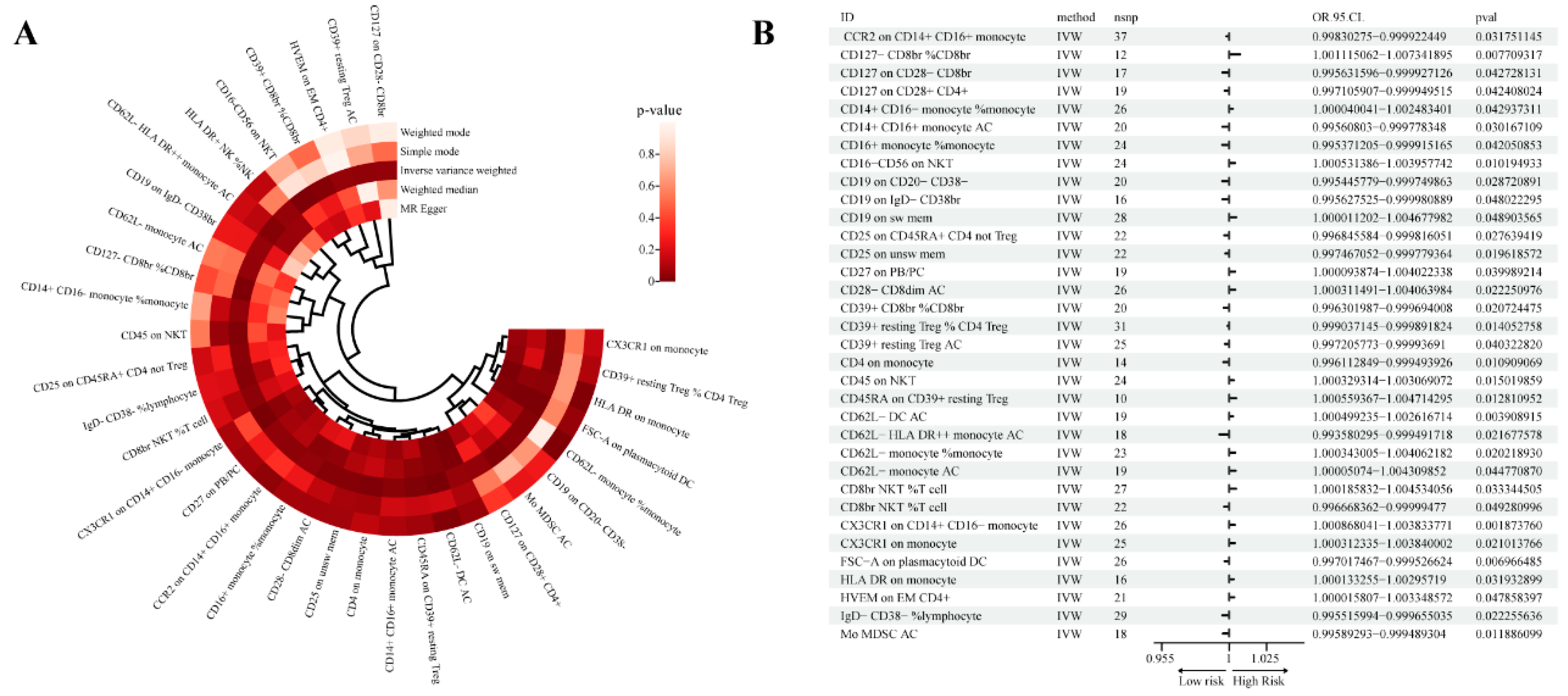
We detected a total of 35 immune phenotypes related to prostate cancer, including IgD- CD38- %lymphocyte, IgD+ CD38br %lymphocyte, CD62L- monocyte AC, CD62L- monocyte %monocyte , etc., each immune phenotype Type includes at least 10 SNPs as IVs. Then, we evaluated the association between these 34 immune phenotypes and Pca risk using five methods: MR Egger, Weighted median, Inverse variance weighted, Simple mode and Weighted mode (
Supplementary Table S1).
Our analysis is mainly based on Inverse variance weighted (IVW), in which there are 16 immunophenotypes for prostate cancer risk factors and 18 immunophenotypes for protection (
Figure 1).
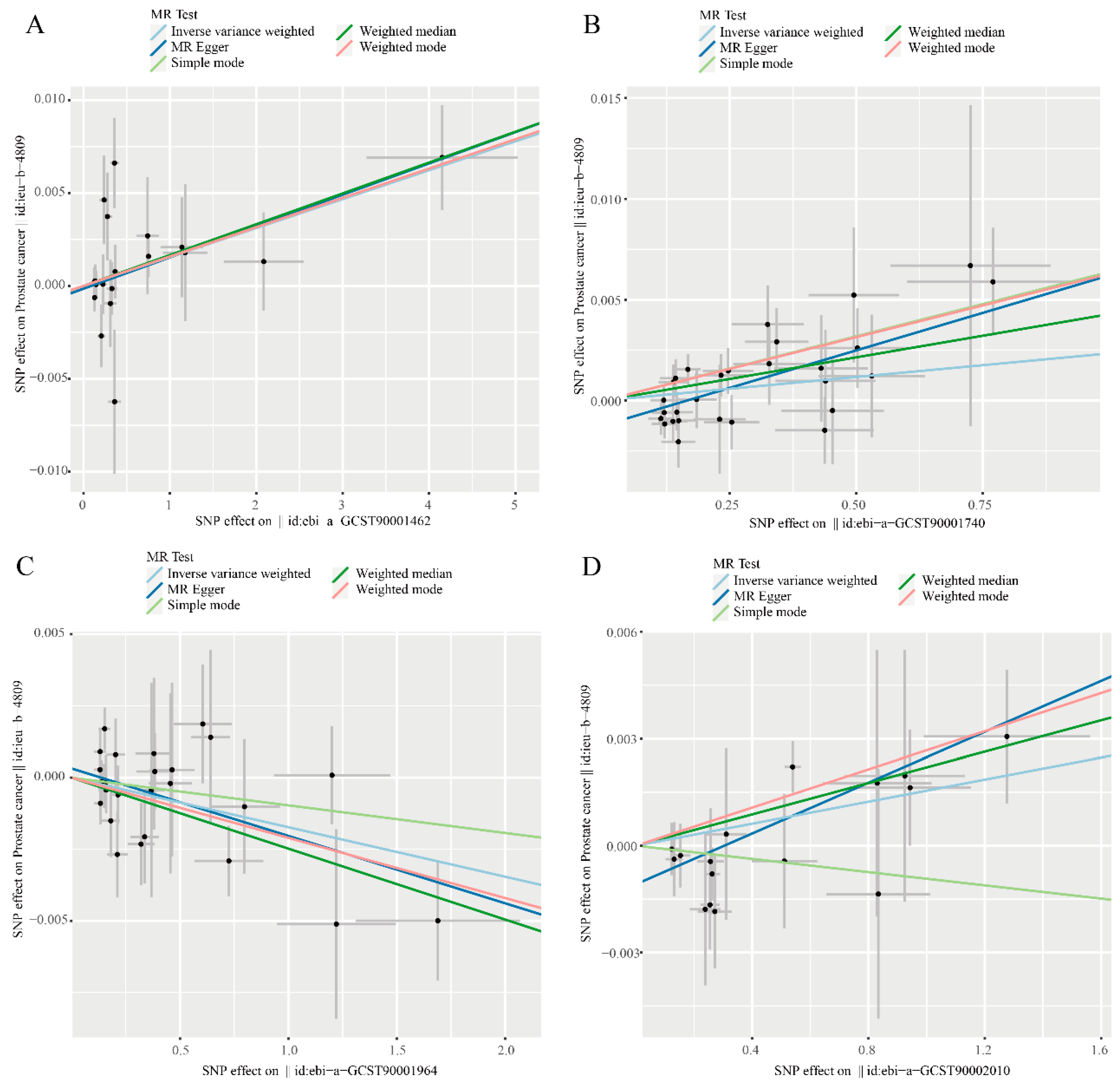
Through further analysis of the results, we found that there are 4 immune phenotypes that meet Inverse variance weighted (P < 0.05), Weighted median (P < 0.05) and MR Egger (P < 0.05) at the same time, namely CD62L- DC AC, CD19 on sw mem, FSC-A on plasmacytoid DC and HLA DR on monocyte.The genomic information covered by SNPs in these cells can be found in
Supplementary Table S3.Scatter plot showing the relationship between immune cells and prostate cancer risk (
Figure 2).
Specific analysis, Inverse variance weighted (IVW) suggested that CD62L- DC AC has an adverse effec1 P=0.019) also had similar results. In addition, Weighted mode (OR=1.002, 95%CI=1.000~1.003, P=0.017) also supports this statement. Inverse variance weighted (IVW) suggested that CD19 on sw mem has an adverse effect on Pca risk (OR=1.002, 95%CI=1.000~1.005, P=0.049), Weighted median (OR=1.004, 95%CI=1.001~1.007, P=0.007) and MR Egger (OR=1.007, 95%CI=1.003~1.012, P=0.002). Inverse variance weighted (IVW) suggested that HLA DR on monocyte has an adverse effect on the risk of Pca (OR=1.002, 95%CI=1.000~1.003, P=0.032), Weighted median (OR=1.002, 95%CI=1.000~1.004, P=0.041) and MR Egger (OR=1.004, 95%CI=1.001~1.006, P=0.009). Inverse variance weighted (IVW) suggested that FSC-A on plasmacytoid DC has a protective effect on Pca (OR=0.998, 95%CI=0.997~1.000, P=0.007), Weighted median (OR=0.998, 95%CI=0.996~0.999, P=0.008) and MR Egger(OR=0.998, 95%CI=0.996~0.999, P=0.014) (
Supplementary Table S1).
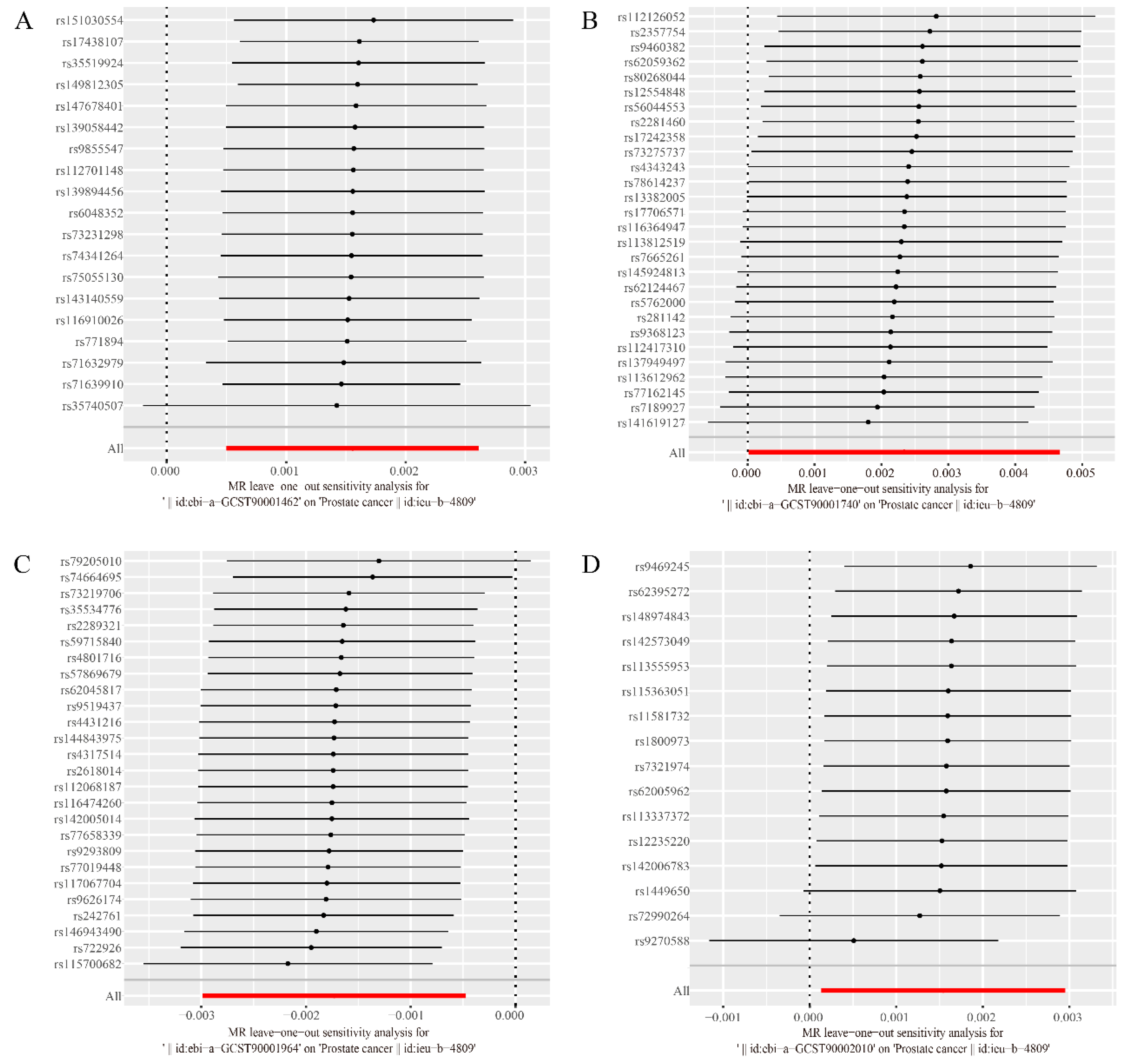
Furthermore, both the intercept of MR-Egger, the global test of MR-PRESSO and leave-one-out test (
Figure 3) ruled out the possibility of horizontal pleiotropy for these four associations. Details of the sensitivity analysis demonstrate the robustness of the observed causal associations. (
Supplementary Table S2, S4 and S5)
3.2. Exploration of the causal effect of Pca onset on immunophenotypes
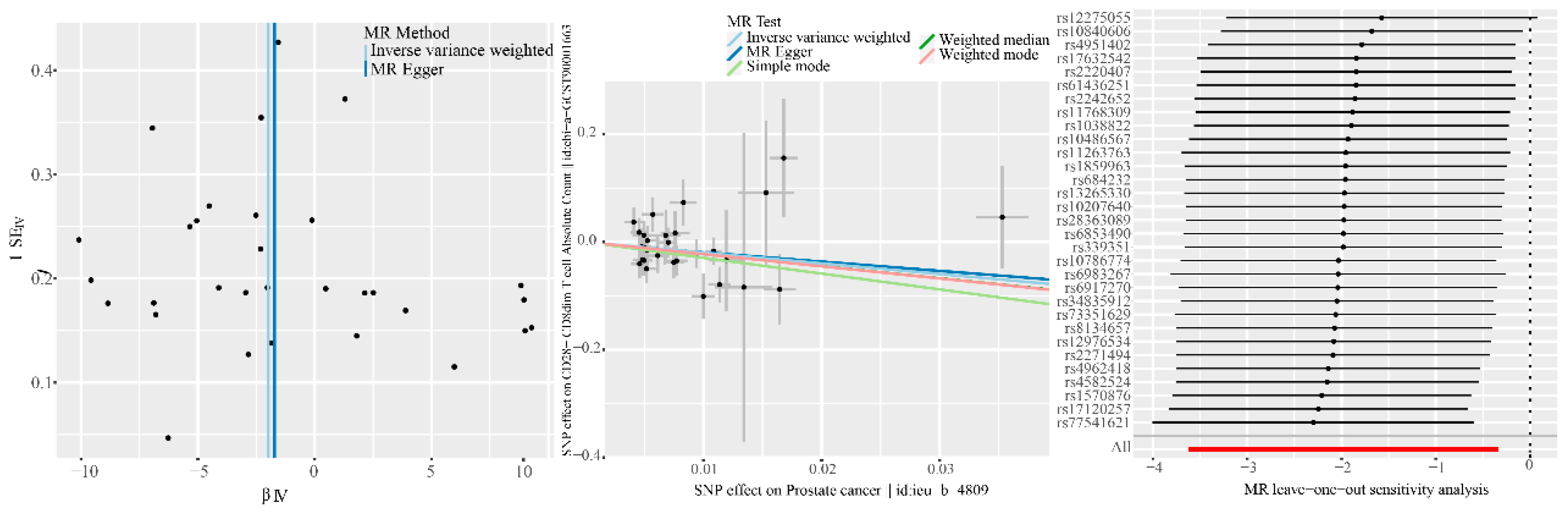
When we performed reverse MR analysis and tried to explore the causal effect of Pca onset on immune phenotype, we also used double-sample MR analysis, using IVW as the main analysis method. A statistically significant association was found between Pca and CD28- CD8dim AC (b: -1.981, OR: 0.138, 95%CI = 0.027–0.714, P = 0.018), although the Weighted median (b: -2.248, OR: 0.106, 95% CI = 0.010–1.123, P = 0.062) and MR Egger (b: -1.718, OR: 0.179, 95% CI = 0.003–11.953, P = 0.429) are no significance was detected, but The b values of other methods are consistent in direction with the IVW method, and we believe that the main effect is valid (
Table 1). Funnel plot, scatter plot, and leave-one-out sensitivity analysis are shown in
Figure 4. In the forward MR analysis, the IVW method suggested that CD28- CD8dim AC had a significant relationship with the risk of Pca (OR: 1.002, 95%CI = 1.000-1.004, P = 0.022), Weighted median (OR: 1.003, 95%CI = 1.000-1.005, P = 0.041) also had similar results (
Supplementary Table S1). Furthermore, both the intercept of MR-Egger and the global test of MR-PRESSO ruled out the possibility of horizontal pleiotropy for these two associations. Details of the sensitivity analysis demonstrate the robustness of the observed causal associations (
Supplementary Table S2).
In addition, gene information corresponding to the above five target immune phenotypes are shown in
Supplementary Table S3.
4. Discussion
In this study, we used the dual-sample MR method to extract and analyze a large amount of genetic data, and explored the potential causal relationship between 731 immune cell traits and Pca. Our results show that a total of 34 types of immune cells are significantly related to Pca, among which CD62L- DC AC, CD19 on sw mem, FSC-A on plasmacytoid DC and HLA DR on monocyte are all positive in at least three testing methods. In addition, CD28- CD8dim AC showed positive results in both forward and reverse two-sample Mendelian randomization.
Our study found that the risk of Pca increased with the number of CD62L-DC ACs. CD62L- DC AC is a subtype of DC that can activate their immune response by ingesting, processing, and presenting foreign antigens to other immune cells, such as T cells and B cells. This type of cells plays an important immune regulatory role in the body, promoting the body's clearance of pathogens and maintaining immune homeostasis. Studies have shown that the use of dendritic cell (DC) vaccines to treat prostate cancer, especially in advanced prostate patients, has a certain degree of clinically and biochemically significant responses and has certain potential for the treatment of prostate cancer24. In addition, dendritic cells also play an important role in the immunotherapy of prostate cancer25,26.
CD19 on sw mem in the B cell has proven to be associated with elevaed SCZ risk. CD19 is a B cell surface molecule that can participate in the signaling of B cell antigen receptors together with another B cell surface molecule CD20, thereby regulating the immune response of B cells. Aws Saudi et al., while studying the role of B cells in prostate cancer, found that compared to healthy donors, the peripheral blood CD19+ B-cell compartment was significantly decreased in PCa patients27.
In addition, FSC-A on plasmacytoid DC, HLA DR on monocyte and CD28- CD8dim AC are also significantly related to the risk of Pca. When Alessandro Sciarra et al.28 studied whether prostate adenocarcinoma produces specific modifications in DC subsets count, they found that the plasmacytoid DC count in prostate cancer patients was significantly reduced (P=0.002). Even if prostate cancer was divided into localized and metastatic groups, it was compared with the healthy group. Comparatively, the results were statistically significant (P=0.005 and P=0.023, respectively), which is consistent with our findings.
In the study by B. Frey et al.13, a decrease in HLA-DR expression on monocytes was detected in all patients after stereotactic ablative body radiotherapy (SABR). It shows that there is a certain correlation between the increase of HLA DR on monocyte and the occurrence of prostate cancer. Combined with our research, it can be proved that the increase of HLA DR on monocyte may be a risk factor leading to the occurrence or progression of prostate cancer.
CD28- CD8dim AC is a T cell subset of Tregs. There are currently no studies reporting the connection between it and Pca. However, Tregs play an important role in the occurrence and development of prostate cancer. Studies have shown that immune evasion has become a major obstacle to anti-cancer treatment, and inhibiting Treg function can effectively treat prostate cancer29-31,which is consistent with the results of forward Mendelian randomization. When we studied the causal relationship between CD28- CD8dim AC and Pca, we found that they also satisfied the positive reverse Mendelian randomization. This means that they have a bidirectional causal relationship, which has not been covered in previous studies, and may be related to a variety of biological pathways and interactions.
This study conducted a two-sample MR analysis based on the published results of a large GWAS cohort. The large sample size is approximately 180,000 people, so it has high statistical efficiency. The conclusions of this study are based on genetic instrumental variables and use multiple MR analysis methods for causal inference. The results are robust and not confounded by horizontal pleiotropy and other factors. At the same time, our study has several limitations. First, our study population is all European, so the conclusion may not be applicable to other ethnic groups, which also makes our results not generalizable. Secondly, we used a relatively loose GWAS threshold (P < 1×10-5) as the screening criterion, which may lead to false positive results and multiple hypothesis testing problems, but at the same time it can also more comprehensively evaluate the relationship between immune spectrum and Pca. causal connections between. Third, CD28-CD8dim AC, as a special type of Treg, is positive for both forward and reverse Mendelian randomization. In order to gain a deeper understanding of this relationship, more experimental research and data analysis may be needed. However, it was not further explored in this study.
5. Conclusions
Our study used multiple MR methods to comprehensively evaluate the causal relationship between a total of 731 immune phenotypes of 7 major immune cell types in the population and Pca, showing evidence of risk assessment between 34 immune phenotypes and prostate cancer. At the same time, more research is needed to confirm these results so that it can provide more ideas for the prevention and immunotherapy of prostate cancer.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
References
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J Clin 72, 7-33, (2022). [CrossRef]
- Liu, Y. et al. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med 12, 55, (2014). [CrossRef]
- Gronberg, H. Prostate cancer epidemiology. Lancet 361, 859-864, (2003). [CrossRef]
- Giri, V. N. et al. Genetic testing in prostate cancer management: Considerations informing primary care. CA Cancer J Clin 72, 360-371, (2022). [CrossRef]
- Lam, T. et al. The Adverse Effects of Androgen Deprivation Therapy in Prostate Cancer and the Benefits and Potential Anti-oncogenic Mechanisms of Progressive Resistance Training. Sports Med Open 6, 13, (2020). [CrossRef]
- Edmunds, K., Tuffaha, H., Galvao, D. A., Scuffham, P. & Newton, R. U. Incidence of the adverse effects of androgen deprivation therapy for prostate cancer: a systematic literature review. Support Care Cancer 28, 2079-2093, (2020). [CrossRef]
- Wang, C., Zhang, Y. & Gao, W. Q. The evolving role of immune cells in prostate cancer. Cancer Lett 525, 9-21, (2022). [CrossRef]
- Wu, Z. et al. The Landscape of Immune Cells Infiltrating in Prostate Cancer. Front Oncol 10, 517637, (2020). [CrossRef]
- Weiner, A. B. et al. High intratumoral plasma cells content in primary prostate cancer defines a subset of tumors with potential susceptibility to immune-based treatments. Prostate Cancer Prostatic Dis 26, 105-112, (2023). [CrossRef]
- Japp, A. S. et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol Immunother 64, 1487-1494, (2015). [CrossRef]
- Sutherland, S. I. M., Ju, X., Horvath, L. G. & Clark, G. J. Moving on From Sipuleucel-T: New Dendritic Cell Vaccine Strategies for Prostate Cancer. Front Immunol 12, 641307, (2021). [CrossRef]
- Stultz, J. & Fong, L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis 24, 697-717, (2021). [CrossRef]
- Frey, B. et al. Systemic modulation of stress and immune parameters in patients treated for prostate adenocarcinoma by intensity-modulated radiation therapy or stereotactic ablative body radiotherapy. Strahlenther Onkol 196, 1018-1033, (2020). [CrossRef]
- Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian Randomization. JAMA 318, 1925-1926, (2017) . [CrossRef]
- Wang, C. et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry 23, 590, (2023). [CrossRef]
- Cao, R. R. et al. The immune factors have complex causal regulation effects on bone mineral density. Front Immunol 13, 959417, (2022). [CrossRef]
- Orru, V. et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 52, 1036-1045, (2020). [CrossRef]
- Sidore, C. et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet 47, 1272-1281, (2015). [CrossRef]
- Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 46, 1734-1739, (2017). [CrossRef]
- Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 26, 2333-2355, (2017). [CrossRef]
- Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 40, 304-314, (2016). [CrossRef]
- Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32, 377-389, (2017). [CrossRef]
- Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50, 693-698, (2018). [CrossRef]
- Ragde, H., Cavanagh, W. A. & Tjoa, B. A. Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol 172, 2532-2538, (2004). [CrossRef]
- Santegoets, S. J. et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer 2, 31, (2014). [CrossRef]
- Datta, J. et al. Rationale for a Multimodality Strategy to Enhance the Efficacy of Dendritic Cell-Based Cancer Immunotherapy. Front Immunol 6, 271, (2015). [CrossRef]
- Saudi, A. et al. Immune-Activated B Cells Are Dominant in Prostate Cancer. Cancers (Basel) 15, (2023). [CrossRef]
- Sciarra, A. et al. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11C+ (myeloid) in prostate adenocarcinoma patients. Prostate 67, 1-7, (2007). [CrossRef]
- Karpisheh, V. et al. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sci 284, 119132, (2021). [CrossRef]
- Vinay, D. S. et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 35 Suppl, S185-S198, (2015). [CrossRef]
- Silvestri, I. et al. A Perspective of Immunotherapy for Prostate Cancer. Cancers (Basel) 8, (2016). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).