Submitted:
15 November 2023
Posted:
15 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Polymeric materials for solid phase extraction
2.1. Commercially available polymeric materials applied as sorbents
2.1.1. Commercial polymeric anion exchangers
2.1.2. Commercial polymeric cation exchangers
2.1.3. Modified and impregnated commercial resins
2.2. Polymeric sorbents prepared with additional functionalization
2.3. Polymer-supported ionic liquid
2.4. Magnetic polymeric materials for solid phase extraction
2.5. Polymeric composites with nanoparticles
2.6. Polymeric materials for solid phase microextraction
2.7. Ion imprinted polymers as sorbents for speciation analysis
2.7.1. IIPs prepared by chemical immobilization
2.7.2. IIPs prepared by trapping technique
2.7.3. IIPs prepared by surface imprinting
2.8. Polymeric monoliths as sorbents for solid phase extraction
2.9. Metal-organic frameworks
2.10. Covalent organic frameworks
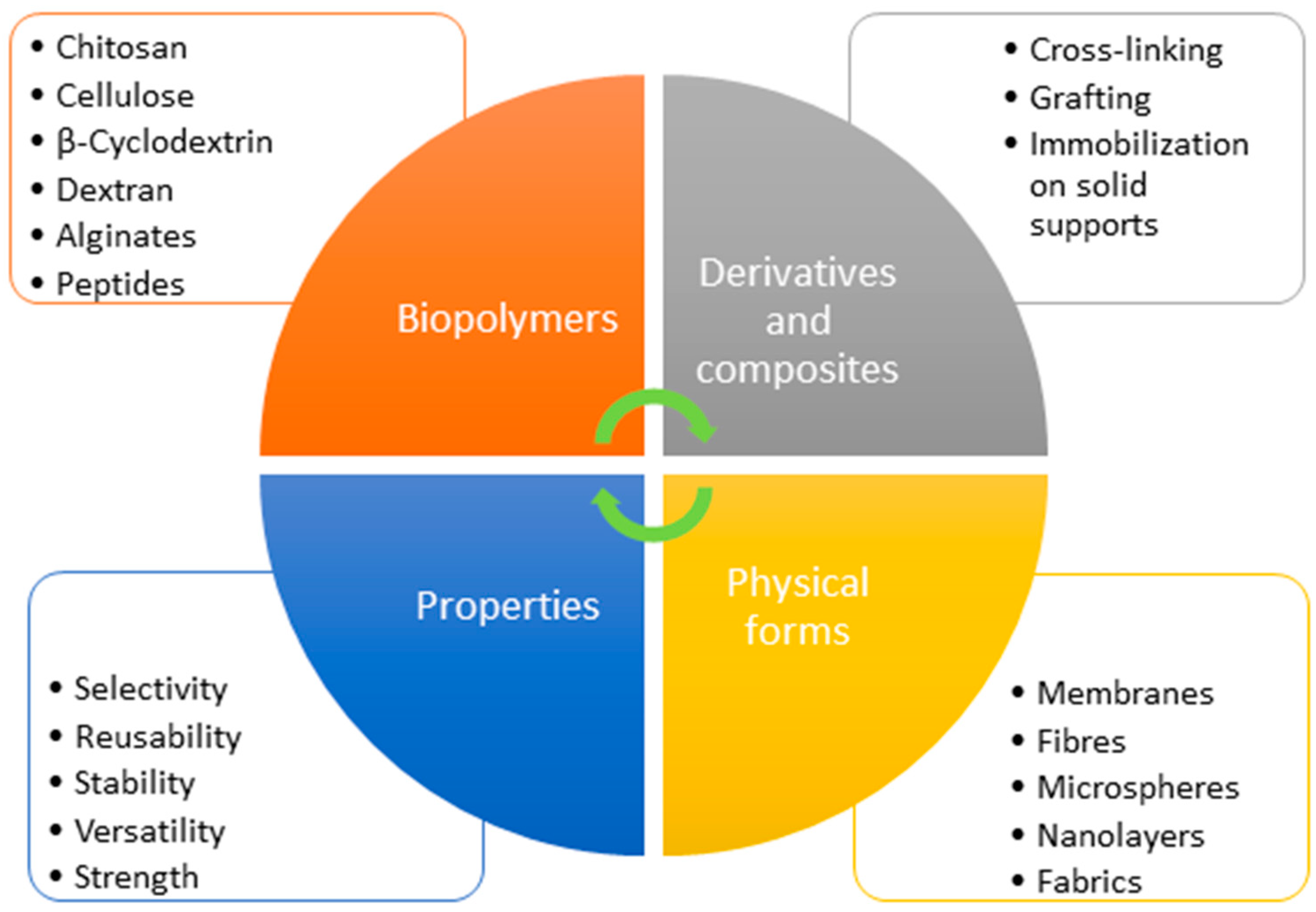
2.11. Biopolymers
2.11.1. Chitosan-Based Materials
2.11.2. Cellulose-Based Materials
2.11.3. β-Cyclodextrin, Dextran and Alginates
2.11.4. Peptides
2.11.5. Analytical aspects of biopolymers
3. Concluding remarks and future trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFS | Atomic fluorescence spectrometry |
| APTMS | 3-aminopropyltriethoxysilane |
| APDC | Ammonium pyrrolidinedithiocarbamate |
| ASA | Arsanilic acid |
| BP | 2,2′-bipyridyl |
| CBA | Carbarsone |
| COF | Covalent organic framework |
| CPAA | Charged particle activation analysis |
| CRM | Certified reference material |
| CVG-AFS | Cold vapour generation-atomic fluorescence spectrometry |
| DBT | Dibutyltin |
| DDTC | Diethyl dithiocarbamate |
| DGT | Diffusive gradients in thin films |
| DHBPT | 3,6-bis (3,5-dimethyl-1-H-pyrzol-1-yl)-1,2-dihydro-1,2,4,5-tetrazine |
| DMAs | Dimethyl arsenic acid |
| DVB | Divinylbenzene |
| EDTA | Ethylendiaminetetraacetic acid |
| EDXRF | Energy-dispersive X-ray fluorescence |
| EGDMA | Ethylene glycol dimethacrylate |
| EF | Enrichment factor |
| ETAAS | Electrothermal-atomic absorption spectrometry |
| FAAS | Flame-atomic absorption spectrometry |
| GFAAS | Graphite furnace-atomic absorption spectrometry |
| HEMA | Hydroxyethyl methacrylate |
| HGAAS | Hydride generation atomic absorption spectrometry |
| HG-AFS | Hydride generation atomic fluorescence spectrometry |
| 8-HQ | 8-hydroxyquinoline |
| HPLC | High performance liquid chromatography |
| iAs | Inrganic arsenic compounds |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-AES | Inductively coupled plasma-atomic emission spectrometry |
| ICP-ОES | Inductively coupled plasma-optical emission spectrometry |
| IIP | Ion imprinted polymer |
| IIP-SPE | Solid phase extraction with ion imprinted polymer |
| INAA | Instrumental neutron activation analysis |
| ITA | Itaconic acid |
| LAICP-MS | Laser ablation inductively coupled plasma mass spectrometry |
| LC | Liquid chromatography |
| LEP-OES | Liquid electrode plasma-optical emission spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MAA | Methacrylic acid |
| MAC | N-methacryloyl-l-cysteine |
| γ-MAPS | Methacryloxypropyltrimethoxysilane |
| MBT | Monobutyltin |
| MMAs | Monomethyl arsenic acid |
| MOF | Metal-organic framework |
| NATU | N-allylthiourea |
| NOBE | N, O-bismethacryl ethanolamine |
| NPA | Nitarsone |
| NPs | Nanoparticles |
| oAs | Organoarsenic compounds |
| PAR | 4-(2-pyridylazo)resorcinol |
| PDC | Pyrrolidinedithiocarbamate |
| PEG | Poly(ethylene glycol) |
| PVA | Poly(vinyl alcohol) |
| PVP | Polyvinyl pyrrolidone |
| RAFT | Reversible addition-chain fragmentation polymerization |
| ROX | Oxarsone |
| SA | Sodium alginate |
| SBAE | Strong base anion exchange |
| SPE | Solid phase extraction |
| SPME | Solid phase microextraction |
| ST | Styrene |
| TAN | 1-(2-thiazolylazo)-2-naphthol |
| TBT | Tributyltin |
| TLC | Thin layer chromatography |
| TMPTMA | Trimethylolpropane trimethacrylate |
| 1-VIA | 1-vinyl imidazole |
| 4-VP | 4-vinylphiridine |
References
- The IUPAC Compendium of Chemical Terminology; Gold, V., Ed.; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, 2019;
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for Terms Related to Chemical Speciation and Fractionation of Elements. Definitions, Structural Aspects, and Methodological Approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [CrossRef]
- Marcinkowska, M.; Barałkiewicz, D. Multielemental Speciation Analysis by Advanced Hyphenated Technique – HPLC/ICP-MS: A Review. Talanta 2016, 161, 177–204. [CrossRef]
- Yu, X.; Liu, C.; Guo, Y.; Deng, T. Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. Molecules 2019, 24, 926. [CrossRef]
- Gonzalvez, A.; Armenta, S.; Cervera, M.L.; de la Guardia, M. Non-Chromatographic Speciation. TrAC Trends Anal. Chem. 2010, 29, 260–268. [CrossRef]
- Das, D.; Gupta, U.; Das, A.K. Recent Developments in Solid Phase Extraction in Elemental Speciation of Environmental Samples with Special Reference to Aqueous Solutions. TrAC Trends Anal. Chem. 2012, 38, 163–171. [CrossRef]
- Viana, J.L.M.; Menegário, A.A.; Fostier, A.H. Preparation of Environmental Samples for Chemical Speciation of Metal/Metalloids: A Review of Extraction Techniques. Talanta 2021, 226. [CrossRef]
- García-Bellido, J.; Freije-Carrelo, L.; Moldovan, M.; Encinar, J.R.; Das, D.; Gupta, U.; Das, A.K. Recent Advances in GC-ICP-MS: Focus on the Current and Future Impact of MS/MS Technology. TrAC - Trends Anal. Chem. 2020, 130, 163–171. [CrossRef]
- Wang, T. Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry (LC-ICP-MS). J. Liq. Chromatogr. Relat. Technol. 2007, 30, 807 – 831. [CrossRef]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Arsenic and Antimony Speciation Analysis in the Environment Using Hyphenated Techniques to Inductively Coupled Plasma Mass Spectrometry: A Review. Int. J. Environ. Waste Manag. 2010, 5, 4 – 63. [CrossRef]
- Miravet, R.; Hernández-Nataren, E.; Sahuquillo, A.; Rubio, R.; López-Sánchez, J.F. Speciation of Antimony in Environmental Matrices by Coupled Techniques. TrAC - Trends Anal. Chem. 2010, 29, 28 – 39. [CrossRef]
- Komorowicz, I.; Barałkiewicz, D. Arsenic and Its Speciation in Water Samples by High Performance Liquid Chromatography Inductively Coupled Plasma Mass Spectrometry - Last Decade Review. Talanta 2011, 84, 247 – 261. [CrossRef]
- Delafiori, J.; Ring, G.; Furey, A. Clinical Applications of HPLC-ICP-MS Element Speciation: A Review. Talanta 2016, 153, 306 – 331. [CrossRef]
- Yu, X.; Liu, C.; Guo, Y. Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. 2019. [CrossRef]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic Nanoparticle Based Solid-Phase Extraction of Heavy Metal Ions: A Review on Recent Advances. Microchim. Acta 2018, 185. [CrossRef]
- He, Y.; He, M.; Nan, K.; Cao, R.; Chen, B.; Hu, B. Magnetic Solid-Phase Extraction Using Sulfur-Containing Functional Magnetic Polymer for High-Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometric Speciation of Mercury in Environmental Samples. J. Chromatogr. A 2019, 1595, 19–27. [CrossRef]
- Díez, S.; Bayona, J.M. Trace Element Determination by Combining Solid-Phase Microextraction Hyphenated to Elemental and Molecular Detection Techniques. J. Chromatogr. Sci. 2006, 44, 458 – 471. [CrossRef]
- Mei, M.; Huang, X.; Chen, L. Recent Development and Applications of Poly (Ionic Liquid)s in Microextraction Techniques. TrAC Trends Anal. Chem. 2019, 112, 123–134. [CrossRef]
- Li, Y.-K.; Yang, T.; Chen, M.-L.; Wang, J.-H. Recent Advances in Nanomaterials for Analysis of Trace Heavy Metals. Crit. Rev. Anal. Chem. 2021, 51, 353 – 372. [CrossRef]
- Hu, B.; He, M.; Chen, B. Nanometer-Sized Materials for Solid-Phase Extraction of Trace Elements. Anal. Bioanal. Chem. 2015, 407, 2685–2710. [CrossRef]
- Ahmad, A.; Siddique, J.A.; Laskar, M.A.; Kumar, R.; Mohd-Setapar, S.H.; Khatoon, A.; Shiekh, R.A. New Generation Amberlite XAD Resin for the Removal of Metal Ions: A Review. J. Environ. Sci. 2015, 31, 104–123. [CrossRef]
- Tunçeli, A.; Ocak, G.; Acar, O.; Türker, A.R. Development of a Method for Speciation of Inorganic Arsenic in Waters Using Solid Phase Extraction and Electrothermal Atomic Absorption Spectrometry. Int. J. Environ. Anal. Chem. 2015, 95, 1395–1411. [CrossRef]
- Mihucz, V.G.; Enesei, D.; Veszely, Á.; Bencs, L.; Pap-Balázs, T.; Óvári, M.; Streli, C.; Záray, G. A Simple Method for Monitoring of Removal of Arsenic Species from Drinking Water Applying On-Site Separation with Solid Phase Extraction and Detection by Atomic Absorption and X-Ray Fluorescence Based Techniques. Microchem. J. 2017, 135, 105–113. [CrossRef]
- Mihucz, V.G.; Bencs, L.; Koncz, K.; Tatár, E.; Weiszburg, T.; Záray, G. Fast Arsenic Speciation in Water by On-Site Solid Phase Extraction and High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry. Spectrochim. Acta - Part B At. Spectrosc. 2017, 128, 30–35. [CrossRef]
- Hosseini, M.S.; Nazemi, S. Preconcentration Determination of Arsenic Species by Sorption of As(v) on Amberlite IRA-410 Coupled with Fluorescence Quenching of l-Cysteine Capped CdS Nanoparticles. Analyst 2013, 138, 5769. [CrossRef]
- CHEN, M.; HUO, Y.; WANG, J. Speciation of Inorganic Arsenic in a Sequential Injection Dual Mini-Column System Coupled with Hydride Generation Atomic Fluorescence Spectrometry. Talanta 2009, 78, 88–93. [CrossRef]
- Acharya, R.; Nair, A.G.C.; Reddy, A.V.R. Speciation and Instrumental Neutron Activation Analysis for Arsenic in Water Samples. J. Radioanal. Nucl. Chem. 2009, 281, 279–282. [CrossRef]
- Datta, J.; Dasgupta, S.; Guin, R.; Venkatesh, M.; Suvarna, S.; Chowdhury, D.P. Determination of Total Arsenic and Speciation of As(III) and As(V) in Ground Water by Charged Particle Activation Analysis. J. Radioanal. Nucl. Chem. 2016, 308, 927–933. [CrossRef]
- Wang, N.; Tyson, J. Non-Chromatographic Speciation of Inorganic Arsenic by Atomic Fluorescence Spectrometry with Flow Injection Hydride Generation with a Tetrahydroborate-Form Anion-Exchanger. J. Anal. At. Spectrom. 2014, 29, 665–673. [CrossRef]
- Issa, N. Ben; Rajaković-Ognjanović, V.N.; Marinković, A.D.; Rajaković, L. V. Separation and Determination of Arsenic Species in Water by Selective Exchange and Hybrid Resins. Anal. Chim. Acta 2011, 706, 191–198. [CrossRef]
- Issa, N. Ben; Rajaković-Ognjanović, V.N.; Jovanović, B.M.; Rajaković, L. V. Determination of Inorganic Arsenic Species in Natural Waters—Benefits of Separation and Preconcentration on Ion Exchange and Hybrid Resins. Anal. Chim. Acta 2010, 673, 185–193. [CrossRef]
- Guerrero, M.M.L.; Alonso, E.V.; Pavón, J.M.C.; Cordero, M.T.S.; De Torres, A.G. On-Line Preconcentration Using Chelating and Ion-Exchange Minicolumns for the Speciation of Chromium(Iii) and Chromium(vi) and Their Quantitative Determination in Natural Waters by Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 2012, 27, 682–688. [CrossRef]
- Saçmaci, Ş.; Kartal, Ş.; Kumsuz, S. Chromium Speciation in Environmental Samples by Solid-Phase Extraction Using Lewatit Ionac SR-7 Resin and Flame Atomic Absorption Spectrometry. J. AOAC Int. 2014, 97, 1719–1724. [CrossRef]
- Mamatha, P.; Venkateswarlu, G.; Swamy, A.V.N.; Sahayam, A.C. Microwave Assisted Extraction of Cr( <scp>iii</Scp> ) and Cr( <scp>vi</Scp> ) from Soil/Sediments Combined with Ion Exchange Separation and Inductively Coupled Plasma Optical Emission Spectrometry Detection. Anal. Methods 2014, 6, 9653–9657. [CrossRef]
- Mamatha, P.; Venkateswarlu, G.; Thangavel, S.; Sahayam, A.C. Methodology for the Speciation of Cr(III) and Cr(VI) in Leaf Powders Using Sulfate Form of Dowex-1 After Microwave Extraction and ICP-OES Detection. At. Spectrosc. 2019, 40, 31–35. [CrossRef]
- Gredilla, A.; Larreta, J.; Martínez-Arkarazo, I.; Vallejuelo, S.; Arana, G.; Raposo, J.; Diego, A.; Madariaga, J. Inexpensive Low-Cost Mercury Speciation by Hydride Generation Atomic Absorption Spectrometry after Ion Exchange Separation in a FIA System (FIA-IE-HG-AAS). Methylmercury Form. Sources Heal. Eff. 2011, 61–80.
- Łukaszczyk, L.; Żyrnicki, W. Speciation Analysis of Sb(III) and Sb(V) in Antileishmaniotic Drug Using Dowex 1×4 Resin from Hydrochloric Acid Solution. J. Pharm. Biomed. Anal. 2010, 52, 747–751. [CrossRef]
- Hagiwara, K.; Inui, T.; Koike, Y.; Aizawa, M.; Nakamura, T. Speciation of Inorganic Arsenic in Drinking Water by Wavelength-Dispersive X-Ray Fluorescence Spectrometry after in Situ Preconcentration with Miniature Solid-Phase Extraction Disks. Talanta 2015, 134, 739–744. [CrossRef]
- Sarenqiqige; ASHITOMI, M.; YOSHIMURA, K. Speciation Analysis of Ultratrace Chromium in Water by On-Line Reaction/Concentration/Separation Method Using a Cation-Exchange Column. Anal. Sci. 2013, 29, 823–829. [CrossRef]
- Burcu Kabak; Arslan, Y.; Trak, D.; Kendüzler, E. Speciation, Preconcentration and Determination of Inorganic Chromium Species in Spring, Drinking, and Waste Water Samples. J. Water Chem. Technol. 2021, 43, 46–52. [CrossRef]
- Çaylak, O.; Elçi, Ş.G.; Höl, A.; Akdoğan, A.; Divrikli, Ü.; Elçi, L. Use of an Aminated Amberlite XAD-4 Column Coupled to Flow Injection Cold Vapour Generation Atomic Absorption Spectrometry for Mercury Speciation in Water and Fish Tissue Samples. Food Chem. 2019, 274, 487–493. [CrossRef]
- Aksoy, E.; Elçi, Ş.G.; Siyal, A.N.; Elçi, L. Chromium Speciation Using an Aminated Amberlite XAD-4 Resin Column Combined with Microsample Injection-Flame Atomic Absorption Spectrometry. Acta Chim. Slov. 2018, 65, 512–520. [CrossRef]
- Sharma, N.; Tiwari, S.; Saxena, R. On-Line Solid Phase Extraction Method Based on Flow Injection-FAAS Using 1,10-Phenanthroline Modified Chelating Resin for Chromium Speciation in Industrial Water Samples. RSC Adv. 2016, 6, 10775–10782. [CrossRef]
- Saxena, R.; Sharma, N.; Tiwari, S. Chromium Speciation Using Flow-Injection Preconcentration on Xylenol Orange Functionalized Amberlite XAD-16 and Determination in Industrial Water Samples by Flame Atomic Absorption Spectrometry. Anal. Sci. 2015, 31, 1303–1308. [CrossRef]
- Tiwari, S.; Sharma, N.; Saxena, R. On-Line Speciation of Chromium Using a Modified Chelating Resin and Determination in Industrial Water Samples by Flame Atomic Absorption Spectrometry. New J. Chem. 2016, 40, 1412–1419. [CrossRef]
- Tiwari, S.; Sharma, N.; Saxena, R. Online Preconcentration Procedure for Chromium Speciation and Determination in Industrial Water Samples Using Flame Atomic Absorption Spectrometry. Anal. Sci. 2016, 32, 1321–1325. [CrossRef]
- Saxena, R.; Tiwari, S.; Sharma, N. Flow-Injection Solid Phase Extraction Using Dowex Optipore L493 Loaded with Dithizone for Preconcentration of Chromium Species from Industrial Waters and Determination by FAAS. RSC Adv. 2015, 5, 69196–69204. [CrossRef]
- Narin, I.; Kars, A.; Soylak, M. A Novel Solid Phase Extraction Procedure on Amberlite XAD-1180 for Speciation of Cr(III), Cr(VI) and Total Chromium in Environmental and Pharmaceutical Samples. J. Hazard. Mater. 2008, 150, 453–458. [CrossRef]
- Saygi, K.O.; Tuzen, M.; Soylak, M.; Elci, L. Chromium Speciation by Solid Phase Extraction on Dowex M 4195 Chelating Resin and Determination by Atomic Absorption Spectrometry. J. Hazard. Mater. 2008, 153, 1009–1014. [CrossRef]
- Yayayürük, A.E.; Eroğlub, A.E. Use of Amberlite XAD-7HP for the Separation of Mn(II) and Mn(VII) in Waters. J. Anal. Chem. 2017, 72, 63–69. [CrossRef]
- Giacomino, A.; Ruo Redda, A.; Caligiuri, R.; Inaudi, P.; Squadrone, S.; Abete, M.C.; Abollino, O.; Morandi, S.; Conca, E.; Malandrino, M. Development of an Easy Portable Procedure for On-Site Determination of Mercury and Methylmercury. Food Chem. 2021, 342, 128347. [CrossRef]
- Inaudi, P.; Mondino, E.; Abollino, O.; Malandrino, M.; Argenziano, M.; Favilli, L.; Boschini, R.; Giacomino, A. On-Site Determination of Methylmercury by Coupling Solid-Phase Extraction and Voltammetry. Molecules 2022, 27, 3178. [CrossRef]
- Jia, X.; Zhao, J.; Ren, H.; Wang, J.; Hong, Z.; Zhang, X. Zwitterion-Functionalized Polymer Microspheres-Based Solid Phase Extraction Method on-Line Combined with HPLC–ICP-MS for Mercury Speciation. Talanta 2019, 196, 592–599. [CrossRef]
- Moffat, C.D.; Weiss, D.J.; Shivalingam, A.; White, A.J.P.; Salaun, P.; Vilar, R. Molecular Recognition and Scavenging of Arsenate from Aqueous Solution Using Dimetallic Receptors. Chem. - A Eur. J. 2014, 20, 17168–17177. [CrossRef]
- Bullen, J.C.; Torres-Huerta, A.; Salaün, P.; Watson, J.S.; Majumdar, S.; Vilar, R.; Weiss, D.J. Portable and Rapid Arsenic Speciation in Synthetic and Natural Waters by an As(V)-Selective Chemisorbent, Validated against Anodic Stripping Voltammetry. Water Res. 2020, 175. [CrossRef]
- Jia, X.; Gong, D.; Wang, J.; Huang, F.; Duan, T.; Zhang, X. Arsenic Speciation in Environmental Waters by a New Specific Phosphine Modified Polymer Microsphere Preconcentration and HPLC–ICP-MS Determination. Talanta 2016, 160, 437–443. [CrossRef]
- Döker, S.; Uzun, L.; Denizli, A. Arsenic Speciation in Water and Snow Samples by Adsorption onto PHEMA in a Micro-Pipette-Tip and GFAAS Detection Applying Large-Volume Injection. Talanta 2013, 103, 123–129. [CrossRef]
- Sharma, N.; Tiwari, S.; Saxena, R. On-Line Solid Phase Extraction Method Based on Flow Injection-FAAS Using 1,10-Phenanthroline Modified Chelating Resin for Chromium Speciation in Industrial Water Samples. RSC Adv. 2016, 6, 10775–10782. [CrossRef]
- Çimen, G.; Tokalioǧlu, Ş.; Özentürk, I.; Soykan, C. Speciation and Preconcentration of Chromium from Water and Food Samples by Synthesized Chelating Resin. J. Braz. Chem. Soc. 2013, 24, 856–864. [CrossRef]
- Gu, Y.; Zhu, X. Speciation of Cr(III) and Cr(VI) Ions Using a β-Cyclodextrin-Crosslinked Polymer Micro-Column and Graphite Furnace Atomic Absorption Spectrometry. Microchim. Acta 2011, 173, 433–438. [CrossRef]
- Saçmaci, Ş.; Kartal, Ş.; Yilmaz, Y.; Saçmaci, M.; Soykan, C. A New Chelating Resin: Synthesis, Characterization and Application for Speciation of Chromium (III)/(VI) Species. Chem. Eng. J. 2012, 181–182, 746–753. [CrossRef]
- Şahan, S.; Saçmaci, Ş.; Kartal, Ş.; Saçmaci, M.; Şahin, U.; Ülgen, A. Development of a New On-Line System for the Sequential Speciation and Determination of Chromium Species in Various Samples Using a Combination of Chelating and Ion Exchange Resins. Talanta 2014, 120, 391–397. [CrossRef]
- Erdem Yayayürük, A.; Yayayürük, O.; Tukenmez, E.; Karagoz, B. Polystyrene-Divinyl Benzene Microspheres with Amino Methyl Phosphonic Acid Functional Hairy Brushes for the Sorption and Speciation of Chromium Prior to Inductively Coupled Plasma Mass Spectrometric Determination. Microchim. Acta 2019, 186. [CrossRef]
- Hazer, O.; Demir, D. Speciation of Chromium in Water Samples by Solid-Phase Extraction on a New Synthesized Adsorbent. Anal. Sci. 2013, 29, 729–734. [CrossRef]
- Jia, X.; Gong, D.; Xu, B.; Chi, Q.; Zhang, X. Development of a Novel, Fast, Sensitive Method for Chromium Speciation in Wastewater Based on an Organic Polymer as Solid Phase Extraction Material Combined with HPLC-ICP-MS. Talanta 2016, 147, 155–161. [CrossRef]
- Inui, T.; Fujita, K.; Kitano, M.; Nakamura, T. Determination of Cr(III) and Cr(VI) at Sub-Ppb Levels in Water with Solid-Phase Extraction/Metal Furnace Atomic Absorption Spectrometry. Anal. Sci. 2010, 26, 1093–1098. [CrossRef]
- Yayayürük, O.; Henden, E.; Bicak, N. Determination of Mercury(Ii) in the Presence of Methylmercury after Preconcentration Using Poly(Acrylamide) Grafted onto Cross-Linked Poly(4-Vinyl Pyridine): Application to Mercury Speciation. Anal. Sci. 2011, 27, 833–838. [CrossRef]
- Zarco-Fernández, S.; Mancheño, M.J.; Muñoz-Olivas, R.; Cámara, C. A New Specific Polymeric Material for Mercury Speciation: Application to Environmental and Food Samples. Anal. Chim. Acta 2015, 897, 109–115. [CrossRef]
- Vinhal, J.O.; Gonçalves, A.D.; Cruz, G.F.B.; Cassella, R.J. Speciation of Inorganic Antimony (III & V) Employing Polyurethane Foam Loaded with Bromopyrogallol Red. Talanta 2016, 150, 539–545. [CrossRef]
- Fornieles, A.C.; Garc, A.; Torres, D.; Alonso, E.V.; Cordero, M.T.S.; Pav, J.M.C. Speciation of Antimony (III) and Antimony(V) in Seawater by Flow Injection Solid Phase Extraction Coupled with Online Hydride Generation Inductively Coupled Plasma Mass Spectrometry. 2011, 1619–1626. [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in Poly(Ionic Liquid)s: Syntheses and Applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [CrossRef]
- Mecerreyes, D. Polymeric Ionic Liquids: Broadening the Properties and Applications of Polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [CrossRef]
- Chen, M.L.; Zhao, Y.N.; Zhang, D.W.; Tian, Y.; Wang, J.H. The Immobilization of Hydrophilic Ionic Liquid for Cr(vi) Retention and Chromium Speciation. J. Anal. At. Spectrom. 2010, 25, 1688–1694. [CrossRef]
- Thangaraj, V.; Bhaskarapillai, A. Organic Acids Modify the Binding Selectivity of Crosslinked Poly(Ionic Liquid) between Sb(III) and Sb(V). Mater. Today Commun. 2020, 25, 101507. [CrossRef]
- Thangaraj, V.; Bhaskarapillai, A.; Velmurugan, S. Synthesis of a Crosslinked Poly(Ionic Liquid) and Evaluation of Its Antimony Binding Properties. J. Hazard. Mater. 2020, 384, 121481. [CrossRef]
- Escudero, L.B.; Olsina, R.A.; Wuilloud, R.G. Polymer-Supported Ionic Liquid Solid Phase Extraction for Trace Inorganic and Organic Mercury Determination in Water Samples by Flow Injection-Cold Vapor Atomic Absorption Spectrometry. Talanta 2013, 116, 133–140. [CrossRef]
- Wang, J.; Zhang, W.; Zheng, Y.; Zhang, N.; Zhang, C. Multi-Functionalization of Magnetic Graphene by Surface-Initiated ICAR ATRP Mediated by Polydopamine Chemistry for Adsorption and Speciation of Arsenic. Appl. Surf. Sci. 2019, 478, 15–25. [CrossRef]
- Elyas Sodan, N.; Elci, S.G.; Arslan Kartal, A.; Hol, A.; Elci, L. Speciation and Preconcentration of Chromium in Real Samples by Magnetic Polythiophene Nanoparticle Solid-Phase Extraction (SPE) Coupled with Microsampling Injection–Flame Atomic Absorption Spectrometry (FAAS). Instrum. Sci. Technol. 2021, 49, 585–603. [CrossRef]
- Suručić, L.; Janjić, G.; Marković, B.; Tadić, T.; Vuković, Z.; Nastasović, A.; Onjia, A. Speciation of Hexavalent Chromium in Aqueous Solutions Using a Magnetic Silica-Coated Amino-Modified Glycidyl Methacrylate Polymer Nanocomposite. Materials (Basel). 2023, 16. [CrossRef]
- He, Y.; He, M.; Chen, B.; Hu, B. Preparation of Functional Magnetic Porous Organic Polymer as Sorbent for Mercury Speciation Followed by HPLC-ICP-MS Analysis. J. Anal. At. Spectrom. 2021, 36, 1568–1575. [CrossRef]
- Llaver, M.; Casado-Carmona, F.A.; Lucena, R.; Cárdenas, S.; Wuilloud, R.G. Ultra-Trace Tellurium Preconcentration and Speciation Analysis in Environmental Samples with a Novel Magnetic Polymeric Ionic Liquid Nanocomposite and Magnetic Dispersive Micro-Solid Phase Extraction with Flow-Injection Hydride Generation Atomic Fluoresce. Spectrochim. Acta - Part B At. Spectrosc. 2019, 162. [CrossRef]
- He, M.; Su, S.; Chen, B.; Hu, B. Simultaneous Speciation of Inorganic Selenium and Tellurium in Environmental Water Samples by Polyaniline Functionalized Magnetic Solid Phase Extraction Coupled with ICP-MS Detection. Talanta 2020, 207, 120314. [CrossRef]
- Song, X.; Luo, Q.; Huang, X. Speciation of Organotin Compounds in Water and Seafood Samples by Online Hyphenation of Porous Polymer-Based Magnetism-Enhanced in-Tube Solid Phase Microextraction and HPLC. Anal. Chim. Acta 2022, 1223, 340175. [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent Advances in Nanomaterials for Analysis of Trace Heavy Metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372. [CrossRef]
- Karadjova, I.; Dakova, I.; Yordanova, T.; Vasileva, P. Nanomaterials for Elemental Speciation. J. Anal. At. Spectrom. 2016, 31, 1949–1973. [CrossRef]
- Chen, M.; Lin, Y.; Gu, C.; Wang, J. Arsenic Sorption and Speciation with Branch-Polyethyleneimine Modified Carbon Nanotubes with Detection by Atomic Fluorescence Spectrometry. Talanta 2013, 104, 53–57. [CrossRef]
- Letsoalo, M.R.; Godeto, T.W.; Magadzu, T.; Ambushe, A.A. Selective Speciation of Inorganic Arsenic in Water Using Nanocomposite Based Solid-Phase Extraction Followed by Inductively Coupled Plasma-Mass Spectrometry Detection. J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng. 2019, 54, 924–932. [CrossRef]
- Silva, F.L.; Scheel, G.L.; Segatelli, M.G.; Tarley, C.R.T. Redox Preconcentration/Speciation of Chromium by Using Nanocomposites Based on Carbon Nanotubes and Functional Polymers. In Composite Nanoadsorbents; Elsevier, 2018; pp. 139–180 ISBN 9780128141328.
- Zare-Moghadam, M.; Shamsipur, M.; Molaabasi, F.; Hajipour-Verdom, B. Chromium Speciation by Isophthalic Acid-Doped Polymer Dots as Sensitive and Selective Fluorescent Probes. Talanta 2020, 209. [CrossRef]
- Ali, J.; Tuzen, M.; Kazi, T.G.; Hazer, B. Inorganic Arsenic Speciation in Water Samples by Miniaturized Solid Phase Microextraction Using a New Polystyrene Polydimethyl Siloxane Polymer in Micropipette Tip of Syringe System. Talanta 2016, 161, 450–458. [CrossRef]
- Ali, J.; Tuzen, M.; Hazer, B.; Kazi, T.G. Chromium Speciation in Water Samples by Loading a New Sulfide-Containing Biodegradable Polymer Adsorbent in Tip of the Syringe System. Water. Air. Soil Pollut. 2019, 230. [CrossRef]
- Panhwar, A.H.; Tuzen, M.; Hazer, B.; Kazi, T.G. Solid Phase Microextraction Method Using a Novel Polystyrene Oleic Acid Imidazole Polymer in Micropipette Tip of Syringe System for Speciation and Determination of Antimony in Environmental and Food Samples. Talanta 2018, 184, 115–121. [CrossRef]
- Branger, C.; Meouche, W.; Margaillan, A. Recent Advances on Ion-Imprinted Polymers. React. Funct. Polym. 2013, 73, 859–875. [CrossRef]
- Rao, T.P.; Kala, R.; Daniel, S. Metal Ion-Imprinted Polymers-Novel Materials for Selective Recognition of Inorganics. Anal. Chim. Acta 2006, 578, 105–116. [CrossRef]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A Critical Review on the Synthesis and Application of Ion-Imprinted Polymers for Selective Preconcentration, Speciation, Removal and Determination of Trace and Essential Metals from Different Matrices. Crit. Rev. Anal. Chem. 2022, 52, 314–326. [CrossRef]
- Lazar, M.M.; Ghiorghita, C.A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28. [CrossRef]
- Fu, J.; Chen, L.; Li, J.; Zhang, Z. Current Status and Challenges of Ion Imprinting. J. Mater. Chem. A 2015, 3, 13598–13627. [CrossRef]
- Chen, L.; Dai, J.; Hu, B.; Wang, J.; Wu, Y.; Dai, J.; Meng, M.; Li, C.; Yan, Y. Recent Progresses on the Adsorption and Separation of Ions by Imprinting Routes. Sep. Purif. Rev. 2020, 49, 265–293. [CrossRef]
- Cajamarca, F.A.; Tarley, C.R.T. Influence of Synthesis Parameters and Polymerization Methods on the Selective and Adsorptive Performance of Bio-Inspired Ion Imprinted Polymers. Separations 2022, 9. [CrossRef]
- Shakerian, F.; Kim, K.-H.; Kwon, E.; Szulejko, J.E.; Kumar, P.; Dadfarnia, S.; Haji Shabani, A.M. Advanced Polymeric Materials: Synthesis and Analytical Application of Ion Imprinted Polymers as Selective Sorbents for Solid Phase Extraction of Metal Ions. TrAC - Trends Anal. Chem. 2016, 83, 55–69. [CrossRef]
- El Ouardi, Y.; Giove, A.; Laatikainen, M.; Branger, C.; Laatikainen, K. Benefit of Ion Imprinting Technique in Solid-Phase Extraction of Heavy Metals, Special Focus on the Last Decade. J. Environ. Chem. Eng. 2021, 9, 106548. [CrossRef]
- Jinadasa, K.K.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ionic Imprinted Polymer Solid-Phase Extraction for Inorganic Arsenic Selective Pre-Concentration in Fishery Products before High-Performance Liquid Chromatography – Inductively Coupled Plasma-Mass Spectrometry Speciation. J. Chromatogr. A 2020, 1619, 460973. [CrossRef]
- Jinadasa, K.K.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ionic Imprinted Polymer – Vortex-Assisted Dispersive Micro-Solid Phase Extraction for Inorganic Arsenic Speciation in Rice by HPLC-ICP-MS. Talanta 2020, 220. [CrossRef]
- Li, Z.; Chen, X.; Zhang, X.; Wang, Y.; Li, D.; Gao, H.; Duan, X. Selective Solid-Phase Extraction of Four Phenylarsonic Compounds from Feeds, Edible Chicken and Pork with Tailoring Imprinted Polymer. Food Chem. 2021, 347, 129054. [CrossRef]
- Gallego-Gallegos, M.; Liva Garrido, M.; Muñoz Olivas, R.; Baravalle, P.; Baggiani, C.; Cámara, C. A New Application of Imprinted Polymers: Speciation of Organotin Compounds. J. Chromatogr. A 2010, 1217, 3400–3407. [CrossRef]
- Kisomi, A.S.; Alizadeh, T.; Shakeri, A.; Nouri, A.; Farsadrooh, M.; Najafi AsliPashaki, S. Application of μ-TLC for Speciation of Inorganic Arsenic by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2020, 159, 105443. [CrossRef]
- Kisomi, A.S.; Alizadeh, T.; Shakeri, A. μ-Thin-Layer Chromatography Coupled with Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry Using Tin(II)-Imprinted Polymer Nanoparticles as a Stationary Phase for Speciation of Tin. Microchim. Acta 2020, 187. [CrossRef]
- Shakerian, F.; Dadfarnia, S.; Haji Shabani, A.M.; Nili Ahmad Abadi, M. Synthesis and Characterisation of Nano-Pore Antimony Imprinted Polymer and Its Use in the Extraction and Determination of Antimony in Water and Fruit Juice Samples. Food Chem. 2014, 145, 571–577. [CrossRef]
- Yordanova, T.; Dakova, I.; Karadjova, I. NOVEL ION-IMPRINTED POLYMER AS A TOOL FOR SELECTIVE DETERMINATION OF SbIII IN NATURAL WATERS. Comptes Rendus L’Academie Bulg. des Sci. 2023, 76, 368–376. [CrossRef]
- Leśniewska, B.; Godlewska-Zyłkiewicz, B.; Wilczewska, A.Z. Separation and Preconcentration of Trace Amounts of Cr(III) Ions on Ion Imprinted Polymer for Atomic Absorption Determinations in Surface Water and Sewage Samples. Microchem. J. 2012, 105, 88–93. [CrossRef]
- Les̈niewska, B.; Trzonkowska, L.; Zambrzycka, E.; Godlewska-Zyłkiewicz, B. Multi-Commutation Flow System with on-Line Solid Phase Extraction Exploiting the Ion-Imprinted Polymer and FAAS Detection for Chromium Speciation Analysis in Sewage Samples. Anal. Methods 2015, 7, 1517 – 1526. [CrossRef]
- Leśniewska, B.; Jakubowska, I.; Zambrzycka, E.; Godlewska-Zyłkiewicz, B. A Novel Ion-Imprinted Polymeric Sorbent for Separation and Determination of Chromium(III) Species in Wastewater. Turkish J. Chem. 2016, 40, 933–943. [CrossRef]
- Trzonkowska, L.; Leśniewska, B.; Godlewska-Żyłkiewicz, B. Development of Solid Phase Extraction Method Based on Ion Imprinted Polymer for Determination of Cr(III) Ions by ETAAS in Waters. Water (Switzerland) 2022, 14. [CrossRef]
- Mitreva, M.; Dakova, I.; Karadjova, I. Iron(II) Ion Imprinted Polymer for Fe(II)/Fe(III) Speciation in Wine. Microchem. J. 2017. [CrossRef]
- Dakova, I.; Mitreva, M.; Karadjova, I. Fe(II)Ion-Imprinted Copolymer Gels − Smart Materials for Fe(II)/Fe(III) Speciation in Surface Waters. Polym. Int. 2022, 71, 706–714. [CrossRef]
- Roushani, M.; Beygi, T.M.; Saedi, Z. Synthesis and Application of Ion-Imprinted Polymer for Extraction and Pre-Concentration of Iron Ions in Environmental Water and Food Samples. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2016, 153, 637–644. [CrossRef]
- Ara, B.; Muhammad, M.; Salman, M.; Ahmad, R.; Islam, N.; Zia, T. ul H. Preparation of Microspheric Fe(III)-Ion Imprinted Polymer for Selective Solid Phase Extraction. Appl. Water Sci. 2018, 8, 1–14. [CrossRef]
- Dakova, I.; Karadjova, I.; Georgieva, V.; Georgiev, G. Ion-Imprinted Polymethacrylic Microbeads as New Sorbent for Preconcentration and Speciation of Mercury. Talanta 2009, 78, 523–529. [CrossRef]
- Yordanova, T.; Dakova, I.; Balashev, K.; Karadjova, I. Polymeric Ion-Imprinted Nanoparticles for Mercury Speciation in Surface Waters. Microchem. J. 2014, 113, 42–47. [CrossRef]
- Dakova, I.G.; Karadjova, I.B. Hg(II)-Imprinted Polymer Gels – Smart Materials for Mercury Determination and SpeciatioN. In Proceedings of the 9th ECCOMAS Thematic Conference on Smart Structures and Materials, SMART 2019; 2019; pp. 1706–1717.
- Rodríguez-Reino, M.P.; Rodríguez-Fernández, R.; Peña-Vázquez, E.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury Speciation in Seawater by Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometry Following Solid Phase Extraction Pre-Concentration by Using an Ionic Imprinted Polymer Based on Methyl-Mercury-Phenobarbital Interaction. J. Chromatogr. A 2015, 1391, 9–17.
- Jinadasa, K.K.; Herbello-Hermelo, P.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury Speciation in Edible Seaweed by Liquid Chromatography - Inductively Coupled Plasma Mass Spectrometry after Ionic Imprinted Polymer-Solid Phase Extraction. Talanta 2021, 224. [CrossRef]
- Abedi, H.; Ebrahimzadeh, H. Imprinted Polymer-Based Extraction for Speciation Analysis of Inorganic Tin in Food and Water Samples. React. Funct. Polym. 2013, 73, 634–640. [CrossRef]
- Biffis, A.; Dvorakova, G.; Falcimaigne-Cordin, A. Molecular Imprinting. In; Haupt, K., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2012; pp. 29–82 ISBN 978-3-642-28421-2.
- Mitreva, M.; Dakova, I.; Yordanova, T.; Karadjova, I. Chromate Surface-Imprinted Silica Gel Sorbent for Speciation of Cr in Surface Waters. Turkish J. Chem. 2016. [CrossRef]
- Vasileva, P.; Dakova, I.; Yordanova, T.; Karadjova, I. New Composite Sorbent for Speciation Analysis of Soluble Chromium in Textiles. Open Chem. 2019, 17, 1095–1104. [CrossRef]
- Dakova, I.; Yordanova, T.; Karadjova, I. Non-Chromatographic Mercury Speciation and Determination in Wine by New Core–Shell Ion-Imprinted Sorbents. J. Hazard. Mater. 2012, 231–232, 49–56. [CrossRef]
- Liu, Y.; Meng, X.; Han, J.; Liu, Z.; Meng, M.; Wang, Y.; Chen, R.; Tian, S. Speciation, Adsorption and Determination of Chromium(III) and Chromium(VI) on a Mesoporous Surface Imprinted Polymer Adsorbent by Combining Inductively Coupled Plasma Atomic Emission Spectrometry and UV Spectrophotometry. J. Sep. Sci. 2013, 36, 3949–3957. [CrossRef]
- Su, Y.; Kang, Y.; Huang, Q.; Zhang, J.; Liu, J.; Hu, Z.; Liu, Z.; Liu, Y. Cr(VI) Anion-Imprinted Polymer Synthesized on Mesoporous Silicon via Synergistic Action of Bifunctional Monomers for Precise Identification and Separation of Cr(VI) from Aqueous Solution by Fixed-Bed Adsorption. Water Sci. Technol. 2023, 87, 2061–2078. [CrossRef]
- Zhang, Z.; Li, J.; Song, X.; Ma, J.; Chen, L. Hg2+ Ion-Imprinted Polymers Sorbents Based on Dithizone-Hg2+ Chelation for Mercury Speciation Analysis in Environmental and Biological Samples. RSC Adv. 2014, 4, 46444–46453. [CrossRef]
- Türkmen, D.; Özkaya Türkmen, M.; Akgönüllü, S.; Denizli, A. Development of Ion Imprinted Based Magnetic Nanoparticles for Selective Removal of Arsenic (III) and Arsenic (V) from Wastewater. Sep. Sci. Technol. 2022, 57, 990–999. [CrossRef]
- Qi, X.; Gao, S.; Ding, G.; Tang, A.N. Synthesis of Surface Cr (VI)-Imprinted Magnetic Nanoparticles for Selective Dispersive Solid-Phase Extraction and Determination of Cr (VI) in Water Samples. Talanta 2017, 162, 345–353. [CrossRef]
- Jiang, W.; Jin, X.; Yu, X.; Wu, W.; Xu, L.; Fu, F. Ion-Imprinted Magnetic Nanoparticles for Specific Separation and Concentration of Ultra-Trace Methyl Mercury from Aqueous Sample. J. Chromatogr. A 2017, 1496, 167–173. [CrossRef]
- Zhang, H.; Davison, W. In Situ Speciation Measurements of Trace Components in Natural Waters Using Thin-Film Gels. Nature 1994, 367, 546–548.
- Fan, H.T.; Lu, Y.; Liu, A.J.; Jiang, B.; Shen, H.; Huang, C.C.; Li, W.X. A Method for Measurement of Free Cadmium Species in Waters Using Diffusive Gradients in Thin Films Technique with an Ion-Imprinted Sorbent. Anal. Chim. Acta 2015, 897, 24–33. [CrossRef]
- Sui, D.-P.; Chen, H.-X.; Liu, L.; Liu, M.-X.; Huang, C.-C.; Fan, H.-T. Ion-Imprinted Silica Adsorbent Modified Diffusive Gradients in Thin Films Technique: Tool for Speciation Analysis of Free Lead Species. Talanta 2016, 148, 285–291. [CrossRef]
- Mitreva, M.; Dakova, I.; Yordanova, T.; Karadjova, I. Chromate Surface-Imprinted Silica Gel Sorbent for Speciation of Cr in Surface Waters. Turkish J. Chem. 2016. [CrossRef]
- Dakova, I.; Yordanova, T.; Karadjova, I. Non-Chromatographic Mercury Speciation and Determination in Wine by New Core-Shell Ion-Imprinted Sorbents. J. Hazard. Mater. 2012, 231–232, 49–56. [CrossRef]
- Zhang, Z.; Li, J.; Song, X.; Ma, J.; Chen, L. Hg2+ Ion-Imprinted Polymers Sorbents Based on Dithizone-Hg2+ Chelation for Mercury Speciation Analysis in Environmental and Biological Samples. RSC Adv. 2014, 4, 46444–46453. [CrossRef]
- Zhao, L. yu; Zhu, Q. yun; Mao, L.; Chen, Y. jun; Lian, H. zhen; Hu, X. Preparation of Thiol- and Amine-Bifunctionalized Hybrid Monolithic Column via “One-Pot” and Applications in Speciation of Inorganic Arsenic. Talanta 2019, 192, 339–346. [CrossRef]
- Sun, Y.; Zhao, L.; Lian, H.; Mao, L.; Cui, X. Carboxyl-Functionalized Hybrid Monolithic Column Prepared by “Thiol-Ene” Click Reaction for Noninvasive Speciation Analysis of Chromium with Inductively Coupled Plasma-Mass Spectrometry. Anal. Chim. Acta 2020, 1137, 85–93. [CrossRef]
- Wang, C.; He, M.; Chen, B.; Hu, B. Polymer Monolithic Capillary Microextraction On-Line Coupled with ICP-MS for Determination of Inorganic Selenium Species in Natural Waters. Talanta 2018, 188, 736–743. [CrossRef]
- Ou, X.; He, M.; Chen, B.; Hu, B. One-Step Synthesis of Mercapto Modified Hierarchical Porous Polymer Capillary Monolithic Column for Chip Based Array Microextraction of Mercury Species in Cells. Chem. Eng. J. 2021, 420. [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-Organic Framework-Based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL Series of Metal Organic Frameworks (MOFs) as Novel Adsorbents for Heavy Metals in Water: A Review. J. Hazard. Mater. 2022, 429, 128271. [CrossRef]
- Pinar Gumus, Z.; Soylak, M. Metal Organic Frameworks as Nanomaterials for Analysis of Toxic Metals in Food and Environmental Applications. TrAC - Trends Anal. Chem. 2021, 143, 116417. [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 1–21. [CrossRef]
- Saboori, A. A Nanoparticle Sorbent Composed of MIL-101(Fe) and Dithiocarbamate-Modified Magnetite Nanoparticles for Speciation of Cr(III) and Cr(VI) Prior to Their Determination by Electrothermal AAS. Microchim. Acta 2017, 184, 1509–1516. [CrossRef]
- Kalantari, H.; Manoochehri, M. A Nanocomposite Consisting of MIL-101(Cr) and Functionalized Magnetite Nanoparticles for Extraction and Determination of Selenium(IV) and Selenium(VI). Microchim. Acta 2018, 185, 2–9. [CrossRef]
- Esmaeilzadeh, M. Ultrasound-Assisted Dispersive Magnetic Solid Phase Extraction Based on Metal-Organic Framework/1-(2-Pyridylazo)-2-Naphthol Modified Magnetite Nanoparticle Composites for Speciation Analysis of Inorganic Tin. New J. Chem. 2019, 43, 4929–4936. [CrossRef]
- Esmaeilzadeh, M. A Composite Prepared from a Metal-Organic Framework of Type MIL-101(Fe) and Morin-Modified Magnetite Nanoparticles for Extraction and Speciation of Vanadium(IV) and Vanadium(V). Microchim. Acta 2019, 186, 2–8. [CrossRef]
- Rezabeyk, S.; Manoochehri, M. Speciation Analysis of Tl(I) and Tl(III) after Magnetic Solid Phase Extraction Using a Magnetite Nanoparticle Composite Modified with Aminodibenzo-18-Crown-6 Functionalized MIL-101(Cr). Microchim. Acta 2018, 185, 3–10. [CrossRef]
- Abbaszadeh, A.; Tadjarodi, A. Speciation Analysis of Inorganic Arsenic in Food and Water Samples by Electrothermal Atomic Absorption Spectrometry after Magnetic Solid Phase Extraction by a Novel MOF-199/Modified Magnetite Nanoparticle Composite. RSC Adv. 2016, 6, 113727–113736. [CrossRef]
- Aslan, F.; Tor, A. Determination and Speciation of Trace Inorganic Arsenic Species in Water Samples by Using Metal Organic Framework Mixed-Matrix Membrane and EDXRF Spectrometry. Chemosphere 2022, 307, 135661. [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [CrossRef]
- Volynkin, S.S.; Demakov, P.A.; Shuvaeva, O. V.; Kovalenko, K.A. Metal-Organic Framework Application for Mercury Speciation Using Solid Phase Extraction Followed by Direct Thermal Release–Electrothermal Atomization Atomic Absorption Spectrophotometric Detection (ETA AAS). Anal. Chim. Acta 2021, 1177, 338795. [CrossRef]
- Chen, Z.; Chen, B.; He, M.; Hu, B. Magnetic Metal-Organic Framework Composites for Dual-Column Solid-Phase Microextraction Combined with ICP-MS for Speciation of Trace Levels of Arsenic. Microchim. Acta 2020, 187. [CrossRef]
- Zhou, D.B.; Xiao, Y.B.; Han, F.; Lv, Y.N.; Ding, L.; Song, W.; Liu, Y.X.; Zheng, P.; Chen, D. Magnetic Solid-Phase Extraction Based on Sulfur-Functionalized Magnetic Metal-Organic Frameworks for the Determination of Methylmercury and Inorganic Mercury in Water and Fish Samples. J. Chromatogr. A 2021, 1654, 462465. [CrossRef]
- Liu, J.M.; Wang, X.Z.; Zhao, C.Y.; Hao, J.L.; Fang, G.Z.; Wang, S. Fabrication of Porous Covalent Organic Frameworks as Selective and Advanced Adsorbents for the On-Line Preconcentration of Trace Elements against the Complex Sample Matrix. J. Hazard. Mater. 2018, 344, 220–229. [CrossRef]
- Kang, B.; Liu, H.; Chen, G.; Lin, H.; Chen, S.; Chen, T. Novel Covalent Organic Frameworks Based Electrospun Composite Nanofiber Membranes as Pipette-Tip Strong Anion Exchange Sorbent for Determination of Inorganic Arsenic in Rice. Food Chem. 2023, 408, 135192. [CrossRef]
- Bi, R.; Li, F.; Chao, J.; Dong, H.; Zhang, X.; Wang, Z.; Li, B.; Zhao, N. Magnetic Solid-Phase Extraction for Speciation of Mercury Based on Thiol and Thioether-Functionalized Magnetic Covalent Organic Frameworks Nanocomposite Synthesized at Room Temperature. J. Chromatogr. A 2021, 1635, 461712. [CrossRef]
- Godage, N.H.; Gionfriddo, E. Use of Natural Sorbents as Alternative and Green Extractive Materials: A Critical Review. Anal. Chim. Acta 2020, 1125, 187–200. [CrossRef]
- Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-da-Silva, A.L. Recent Advances on Magnetic Biosorbents and Their Applications for Water Treatment. Environ. Chem. Lett. 2020, 18, 151–164. [CrossRef]
- Nawaz, S.; Tabassum, A.; Muslim, S.; Nasreen, T.; Baradoke, A.; Kim, T.H.; Boczkaj, G.; Jesionowski, T.; Bilal, M. Effective Assessment of Biopolymer-Based Multifunctional Sorbents for the Remediation of Environmentally Hazardous Contaminants from Aqueous Solutions. Chemosphere 2023, 329, 138552. [CrossRef]
- Zia, Z.; Hartland, A.; Mucalo, M.R. Use of Low-Cost Biopolymers and Biopolymeric Composite Systems for Heavy Metal Removal from Water. Int. J. Environ. Sci. Technol. 2020, 17, 4389–4406. [CrossRef]
- Dai, J.; Ren, F.L.; Tao, C. Adsorption of Cr(VI) and Speciation of Cr(VI) and Cr(III) in Aqueous Solutions Using Chemically Modified Chitosan. Int. J. Environ. Res. Public Health 2012, 9, 1757–1770. [CrossRef]
- Cui, C.; He, M.; Chen, B.; Hu, B. Chitosan Modified Magnetic Nanoparticles Based Solid Phase Extraction Combined with ICP-OES for the Speciation of Cr(Iii) and Cr(Vi). Anal. Methods 2014, 6, 8577–8583. [CrossRef]
- Djerahov, L.; Vasileva, P.; Karadjova, I. Self-Standing Chitosan Film Loaded with Silver Nanoparticles as a Tool for Selective Determination of Cr(VI) by ICP-MS. Microchem. J. 2016, 129, 23–28. [CrossRef]
- Boyaci, E.; Eroǧlu, A.E.; Shahwan, T. Sorption of As(V) from Waters Using Chitosan and Chitosan-Immobilized Sodium Silicate Prior to Atomic Spectrometric Determination. Talanta 2010, 80, 1452–1460. [CrossRef]
- Dai, J.; Ren, F.L.; Tao, C.Y.; Bai, Y. Synthesis of Cross-Linked Chitosan and Application to Adsorption and Speciation of Se (VI) and Se (IV) in Environmental Water Samples by Inductively Coupled Plasma Optical Emission Spectrometry. Int. J. Mol. Sci. 2011, 12, 4009–4020. [CrossRef]
- Sabarudin, A.; Motomizu, S. Functionalization of Chitosan with 3,4,5-Trihydroxy Benzoic Acid Moiety for The Uptake of Chromium Species. J. Pure Appl. Chem. Res. 2013, 2, 48–54. [CrossRef]
- Tolessa, T.; Zhou, X.X.; Amde, M.; Liu, J.F. Development of Reusable Magnetic Chitosan Microspheres Adsorbent for Selective Extraction of Trace Level Silver Nanoparticles in Environmental Waters Prior to ICP-MS Analysis. Talanta 2017, 169, 91–97. [CrossRef]
- Chen, M.L.; Ma, H.J.; Zhang, S.Q.; Wang, J.H. Mercury Speciation with L-Cysteine Functionalized Cellulose Fibre as Adsorbent by Atomic Fluorescence Spectrometry. J. Anal. At. Spectrom. 2011, 26, 613–617. [CrossRef]
- Nakakubo, K.; Nishimura, T.; Biswas, F.B.; Endo, M.; Wong, K.H.; Mashio, A.S.; Taniguchi, T.; Nishimura, T.; Maeda, K.; Hasegawa, H. Speciation Analysis of Inorganic Selenium in Wastewater Using a Highly Selective Cellulose-Based Adsorbent via Liquid Electrode Plasma Optical Emission Spectrometry. J. Hazard. Mater. 2022, 424, 127250. [CrossRef]
- Jamroz, E.; Kocot, K.; Zawisza, B.; Talik, E.; Gagor, A.; Sitko, R. A Green Analytical Method for Ultratrace Determination of Hexavalent Chromium Ions Based on Micro-Solid Phase Extraction Using Amino-Silanized Cellulose Membranes. Microchem. J. 2019, 149, 104060. [CrossRef]
- Zawisza, B.; Sitko, R.; Queralt, I.; Margui, E.; Gagor, A. Cellulose Mini-Membranes Modified with TiO2 for Separation, Determination, and Speciation of Arsenates and Selenites. Microchim. Acta 2020, 187. [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.H.; Sobhy, M.E. Engineered Nano-Zirconium Oxide-Crosslinked-Nanolayer of Carboxymethyl Cellulose for Speciation and Adsorptive Removal of Cr(III) and Cr(VI). Powder Technol. 2017, 321, 444–453. [CrossRef]
- Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Speciation of Cr (III) and Cr (VI) Ions via Fabric Phase Sorptive Extraction for Their Quantification via HPLC with UV Detection. J. Chromatogr. Sep. Tech. 2016, 7. [CrossRef]
- López-García, I.; Marín-Hernández, J.J.; Hernández-Córdoba, M. Microcrystalline Cellulose for the Dispersive Solid-Phase Microextraction and Sensitive Determination of Chromium in Water Using Electrothermal Atomic Absorption Spectrometry. J. Anal. At. Spectrom. 2018, 33, 1529–1535. [CrossRef]
- Lukojko, E.; Talik, E.; Gagor, A.; Sitko, R. Highly Selective Determination of Ultratrace Inorganic Arsenic Species Using Novel Functionalized Miniaturized Membranes. Anal. Chim. Acta 2018, 1008, 57–65. [CrossRef]
- Chen, S.; Qin, X.; Gu, W.; Zhu, X. Speciation Analysis of Mn(II)/Mn(VII) Using Fe3O4@ionic Liquids-β-Cyclodextrin Polymer Magnetic Solid Phase Extraction Coupled with ICP-OES. Talanta 2016, 161, 325 – 332. [CrossRef]
- Gu, Y.; Zhu, X. Speciation of Cr(III) and Cr(VI) Ions Using a β-Cyclodextrin-Crosslinked Polymer Micro-Column and Graphite Furnace Atomic Absorption Spectrometry. Microchim. Acta 2011, 173, 433–438. [CrossRef]
- Filik, H.; Avan, A.A. Dextran Modified Magnetic Nanoparticles Based Solid Phase Extraction Coupled with Linear Sweep Voltammetry for the Speciation of Cr(VI) and Cr(III) in Tea, Coffee, and Mineral Water Samples. Food Chem. 2019, 292, 151–159. [CrossRef]
- Yang, T.; Zhang, X.-Y.; Zhang, X.-X.; Chen, M.-L.; Wang, J.-H. Chromium(III) Binding Phage Screening for the Selective Adsorption of Cr(III) and Chromium Speciation. ACS Appl. Mater. Interfaces 2015, 7, 21287–21294. [CrossRef]
- Yang, T.C.; Zall, R.R. Absorption of Metals by Natural Polymers Generated from Seafood Processing Wastes. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 168–172. [CrossRef]
- Onsosyen, E.; Skaugrud, O. Metal Recovery Using Chitosan. J. Chem. Technol. Biotechnol. 1990, 49, 395–404. [CrossRef]
- Dakova, I.; Vasileva, P.; Karadjova, I. Cr(III) Ion-Imprinted Hydrogel Membrane for Chromium Speciation Analysis in Water Samples. Gels 2022, 8. [CrossRef]
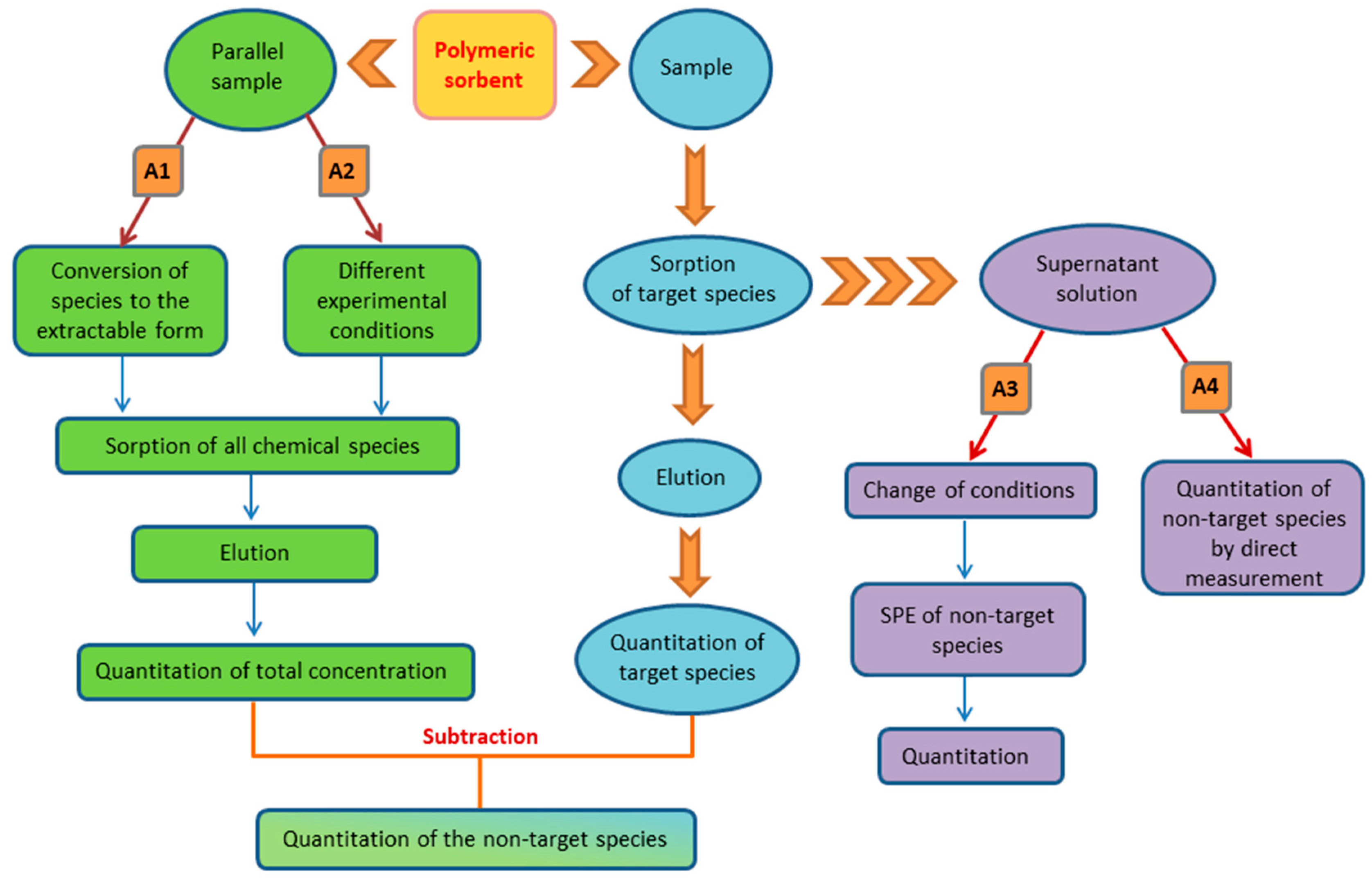
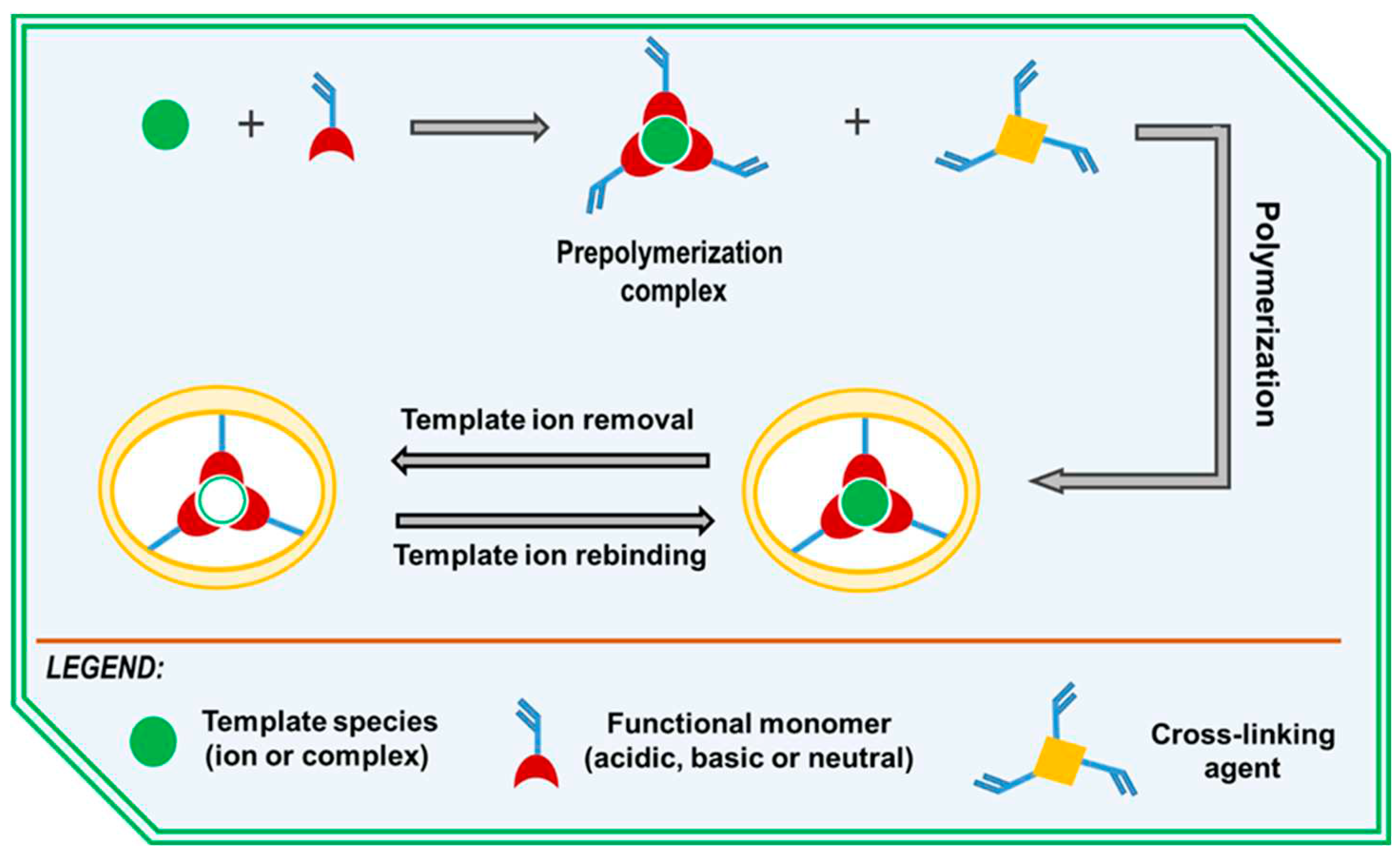
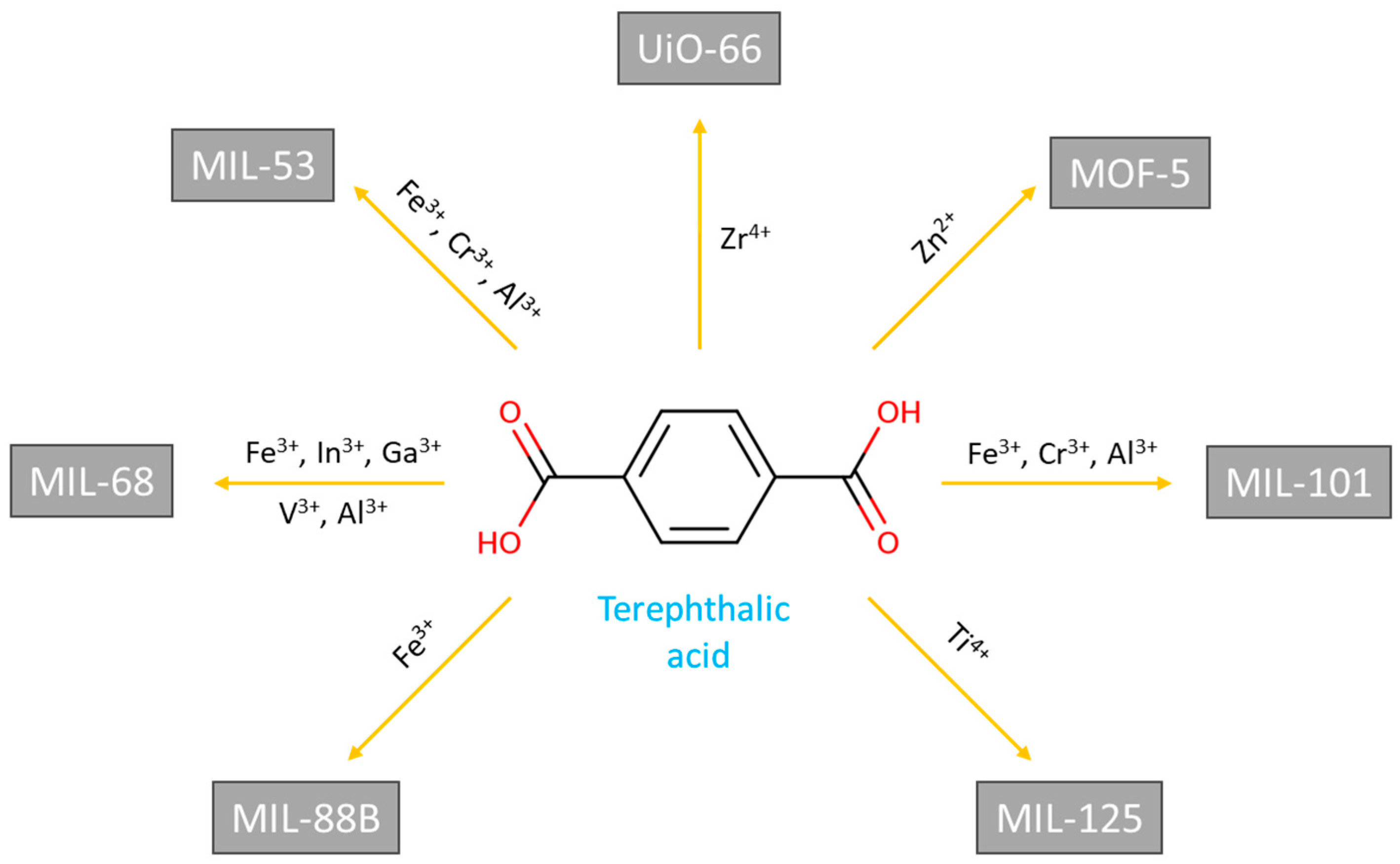
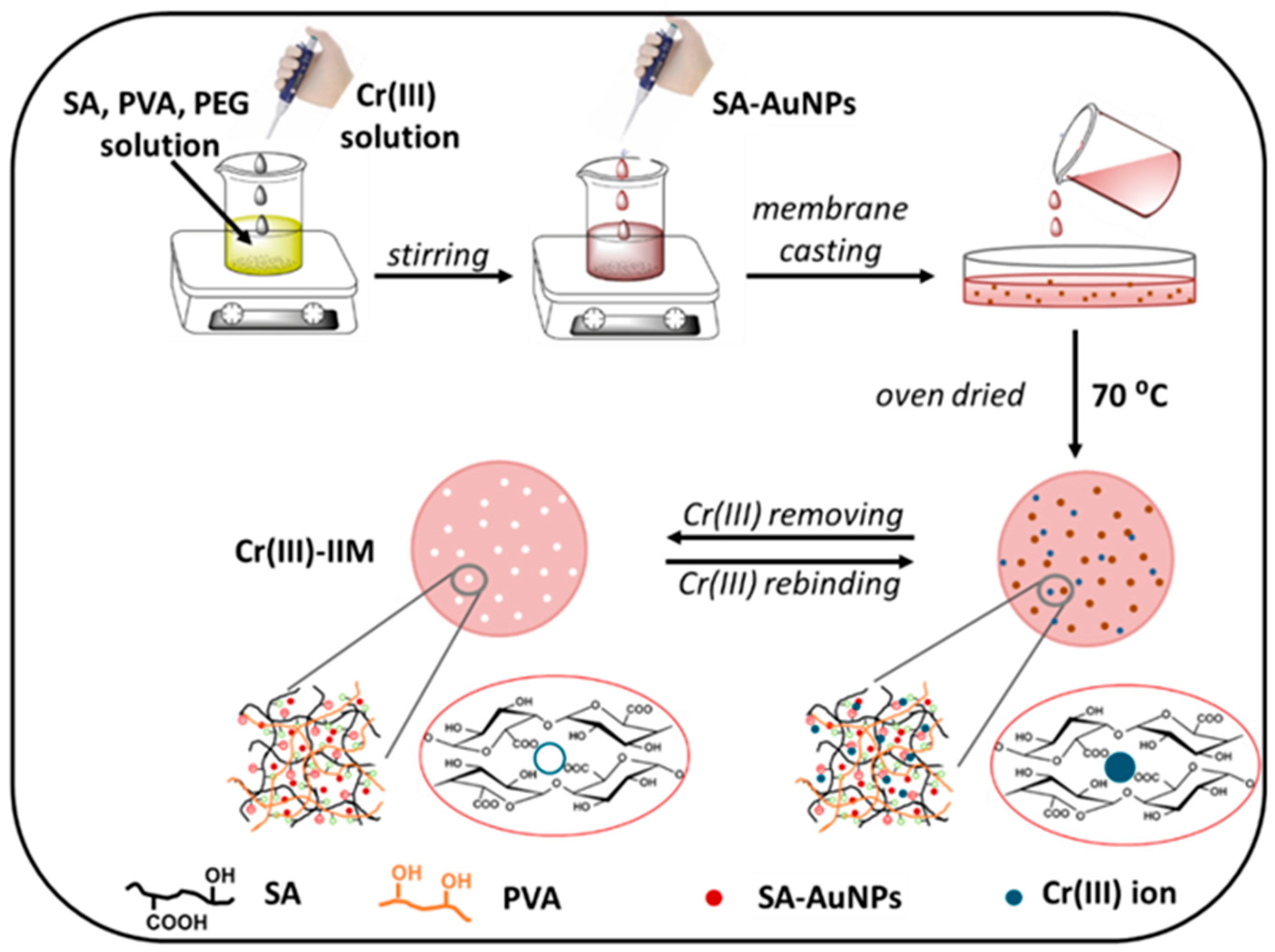
| Sorbent | Separated species | pH or acidity | Sorption capacity, mg/g |
Sample | Analytical technique | LOD, µg/L |
Ref. |
|---|---|---|---|---|---|---|---|
| Amberlite IRA 900 | As(III), As(V) |
4.0 | 229.9 | underground hot waters, tap water | ETAAS | - 0.126 |
[22] |
| Amberlite IRA 410 | As(III), As(V) |
7–7.5 | 13.2 | natural and drinking waters | spectrofluorometric | - 0.75 |
[25] |
| Dowex 1-X8 SAX resin | As(III), As(V) |
6.0-8.0 | Groundwater, waterworks | HR-CS-GFAAS and TXRF | 0.5 | [23] | |
| Dowex 1X8 resin | As(III), As(V) |
8 M HCl 8 M acetic acid |
NRCC CRM DORM-2, ground water | INAA | 20–45 | [27] | |
| Dowex 1X8 resin | As(III), As(V) |
1 M acetic acid | ground water | CPAA | 10 | [28] | |
| 717 anion exchange resin | As(III), As(V) |
CRM of riverine water (SLRS-4), lake water |
HG-AFS | 0.02 0.3 |
[26] | ||
| tetrahydroborate immobilized on Amberlite IRA-400 | As(III), As(V) |
0.1 M HCl | tap, well, pond and sea waters | FI-HG-AFS | 131 151 |
[29] | |
| Lewatit MonoPlus M 500 HY resin |
As(III), As(V) |
7.50 | drinking water | ICP-MS | 0.24 | [31] | |
| Lewatit MonoPlus M 500 HY-Fe resin HY-Ag resin |
As(III), As(V), DMAs(V), MMAs(V)V |
9 | drinking, natural and wastewater | ICP-MS or HGAAS |
0.2 | [30] | |
| Amberlite IRA 910 | Cr(III), Cr(VI) |
4.5 | seawaters | FI-ICP-MS | 0.03 0.009 |
[32] | |
| Lewatit Ionac SR-7 | Cr(III), Cr(VI) |
3 | 17 | Wastewater, tap water | FAAS | - 0.003 |
[33] |
| Dowex-1 | Cr(III), Cr(VI) |
4.5 | Soils, sediments | ICP-OES | - 0.02 |
[34] | |
| Dowex-1 | Cr(III), Cr(VI) |
4.5 | black tea, green tea, spinach, fruit trees | ICP-OES | 182 212 |
[35] | |
| TSK IC-Cation | Cr(III), Cr(VI) |
3.4 | water samples | HPLC-UV-Vis | 0.81 Crtotal 0.61 Cr(VI) |
[39] | |
| Dowex 21K and poly 2-(5-methylisoxazol)methacrylamide-co-2- acrylamido-2- methyl-1-propane sulfonic acid-co- divinyl-benzene |
Cr(III), Cr(VI) |
3 | Environmental waters | FAAS | 0.05 0.3 |
[62] | |
| Amberlite CG-120 |
Cr(III), Cr(VI) |
spring, drinking and waste waters | FAAS | 0.3 | [40] | ||
| Dowex M-41 | Hg(II), methylHg |
3% HCl | Estuarine water samples | FIA-HG-QFAAS | 1.93 0.8 |
[36] | |
| Amberlite XAD-7HP |
Sb(III), Sb(V) |
1.5 M HCl | meglumine antimoniate | ICP-OES | 44, 52 |
[37] |
| Sorbent | Separated species | pH or acidity | Sorption capacity, mg/g |
Sample | Analytical technique | LOD, µg/L |
Ref. |
|---|---|---|---|---|---|---|---|
| 1,10-Phenanthroline immobilized on Amberlite XAD-16 | Cr(III), Cr(VI) |
5 | 33.2 | industrial waters |
FI-FAAS | 0.09 | [43] |
| Xylenol Orange Functionalized Amberlite XAD-16 | Cr(III), Cr(VI) |
5 | 26.7 | industrial waters |
FI-FAAS | 0.11 | [44] |
| α-benzoin oxime modified Amberlite XAD-16 | Cr(III), Cr(VI) |
5 | 27.6 | industrial waters |
FI-FAAS | 0.14 | [45] |
| Salicylic acid loaded on Amberlite XAD-16 | Cr(III), Cr(VI) |
5 | 29.4 | industrial waters |
FI-FAAS | 0.1 | [46] |
| Dithizone modified Dowex Optipore L493 | Cr(III), Cr(VI) |
5 | 31.8 | industrial waters |
FI-FAAS | 0.13 | [47] |
| Aminated Amberlite XAD-4 | Cr(III), Cr(VI) |
8 | 67 | Mineral, drinking and waste water | MIS-FAAS | 0.041 | [42] |
| Dowex M 4195 chelating resin with bis-picolylamine functional group | Cr(III), Cr(VI) |
2 | 29.7 | tap, river and electroplating water |
FAAS | - 1.94 |
[49] |
| Amberlite XAD-1180 resin | Cr(III), Cr(VI) |
0.5 M H2SO4 | food, water, pharmaceutical samples | FAAS | 7.7 Cr(VI) 8.6 Crtotal |
[48] | |
| CYPHOS 101 modified Amberlite XAD-1180 | Hg(II), methylHg |
4 M HCl | CRMs (BCR-463 Tuna Fish and Tuna Fish ERM-CE 464); fish samples | ASV |
0.40, 0.50 - |
[51] | |
| CYPHOS 101 modified Amberlite XAD-1180 | Hg(II), methylHg |
4 M HCl | CRMs (Tuna Fish ERM-CE 464); fish samples | SW-ASV |
- 0.45 |
[52] | |
| CYPHOS 101 modified Amberlite XAD-4, XAD-16 and XAD-1180 | Hg(II), methylHg |
3 M HCl | Water samples | CV-AAS | 2.41 | [67] | |
| Aminated Amberlite XAD-4 | Hg(II), methylHg |
4.0 | fish and mussel samples | FI-CVG-AAS | 0.148, 0.157 |
[41] |
| Template species |
Functional monomer / cross-linker | Separated species | pH /EF | Capacity, mg/g |
Sample | Analytical technique | LOD, µg/L |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Chemical immobilization | ||||||||
| As(III) | 1-VIA / DVB | As(III), As(V) |
8.5/50 | - | Fish; CRM (dogfish muscle), dog- fish liver, mussel tissue | HPLC-ICP-MS | 0.321 0.391 |
[102] |
| As(III) | 1-VIA / DVB | As(III), As(V) |
8/10 | - | Rice | HPLC-ICP-MS | 0.201 0.411 |
[103] |
| As(V) | ITA / EGDMA | As(III), As(V) |
3-4/- | - | water | TLC/LA-ICP-MS | - 0.06 |
[106] |
| ROX |
NOBE / TMPTMA | ASA, ROX, NPA, CBA |
-/- | 1.708 2.040 1.580 1.504 |
feeds, edible chicken and pork | HPLC | 21.31 41.41 31.71 32.31 |
[104] |
| Sn(II) | NATU / EGDMA | Sn(II), Sn(IV) |
6/- | - | Waters, plasma samples | LA-ICP-MS | 0.3, 0.4 |
[107] |
| Trapping technique | ||||||||
| Sb(III)–APDC complex, | ST / EGDMA | Sb(III), Sb(V) |
5/232 | 6.7 | Waters fruit juices | ETAAS | 3.92 | [108] |
| Sb(III) – thionalide complex | MAA / TMPTMA | Sb(III), Sb(V) |
8/25 | - | Surface waters | ICP-OES | 13 | [109] |
| Cr(III) – PDC complex | AA / EGDMA | Cr(III), Cr(VI) |
3.50–4.75/10 | 1.34 | tap and river water, municipal sewage | ETAAS | 0.018 | [112] |
| Cr(III)– 8-HQ | ST / DVB | Cr(III), Cr(VI) |
9/33 | 8.5 | CRM of waste water | FAAS | 2.1 | [111] |
| Cr(III) – nicotinate complex | AA / EGDMA | Cr(III), Cr(VI) |
9–10/- | - | wastewater | FAAS | 0.08 | [112] |
| Cr(III)– 1,10-phenanthroline complex | ST or ST+4-VP / EGDMA | Cr(III), Cr(VI) |
4.5/20 | 1.186 | tap water and infusion of a green tea | ETAAS | 0.018 | [113] |
| Fe(II) – BP complex | 4-VP / TMPTMA | Fe(II), Fe(III) |
7/- | 28.017 | wine | ETAAS | 0.03 Fetotal, 0.1 Fe(III) |
[114] |
| Fe(II) – BP or PAR complex | 4-VP or HEMA / TMPTMA | Fe(II), Fe(III) |
7/- | 1.797 | Surface waters | ETAAS | 0.5, 0.2 |
[115] |
| Fe(III) – DHBPT complex | MAA / EGDMA | Fe(II), Fe(III) |
4.5/- | 40.41 | Foods, waters | FAAS | - 1.56 Fe(III) |
[116] |
| Fe(III) – 8-HQ complex | MAA / DVB | Fe(II), Fe(III) |
2.5/240 | 1707 | drinking water | FAAS | - | [117] |
| Hg(II) –TAN complex | MAA / TMPTMA | Hg(II), methylHg |
7/− | 32.07 | seawater, mineral waters | CV-AAS | 0.006 |
[118] |
| Hg(II) – PDC complex | MAA / TMPTMA | 7/25 | 64.07 | river water | CV-AAS | 0.0153, 0.023 |
[119] | |
| Hg(II) – TAN complex | MAA or VIA or HEMA / TMPTMA | Hg(II), methylHg |
7/− | 73.77 (MAA), 78.07 (VIA) | surface waters | CV-AAS | 0.005, 0.006 |
|
| MeHg- phenobarbital complex | MAA / EGDMA | Hg(II), methylHg, ethylHg |
8/50 | - | seawater | HPLC-ICP-MS | 0.003, 0.002, 0.002 |
[121] |
| MeHg- phenobarbital complex | MAA / EGDMA | methylHg, Hg(II) |
9/50 | - | seaweed | LC-ICP-MS | 0.0071 0.021 |
[122] |
| Sn(IV) – PAR complex | MAA / EGDMA | Sn(IV), Sn(II) |
8/- | tap water, river water, food samples |
GF-AAS | 1.3 | [123] | |
| Surface imprinting technique | ||||||||
| As(III)–MAC complex As(V)–MAC complex |
HEMA / EGDMA on Fe3O4 HEMA / EGDMA on Fe3O4 |
As(III), As(V) |
5/- | 76.83 85.57 |
water | ICP-MS | - | [131] |
| Cr(III) | 3-aminopropyl-triethoxysilane on SBA-15 | Cr(III), Cr(VI) |
6/− | 38.50 | plating and leather wastewater | ICP-AES and UV-vis | 0.53 |
[128] |
| Cr(VI) | 1-(trimethoxysilylpro-pyl)-3-methylimid-azolium on silica gel | Cr(III), Cr(VI) |
2–3/- |
6.427 |
Surface waters | ETAAS | - 0.02 |
[137] |
| Cr(VI) | 1-(trimethoxysilyl-propyl)-3-methyl-imidazolium on µSiO2 | Cr(III), Cr(VI) |
3/- |
9.47 |
textiles | ETAAS | - 0.0156 |
[126] |
| Cr(VI) | MAA and 4-VP / MPTMS on SBA-15 | Cr(III), Cr(VI) |
2/- |
96.32 |
water | ICP-AES | - | [129] |
| Cr(VI) | VIA, APTMS / MPTMS on Fe3O4 | Cr(III), Cr(VI) |
3/98 |
2.49 |
water | FAAS | - 0.29 |
[132] |
| Hg(II) – PDC complex | MAA / TMPTMA on silica gel | Hg(II), methylHg |
7/− | wine | CV-AAS. | 0.02 | [138] | |
| Hg(II) – dithizone complex | 3-aminopropyltriethoxy-silane | Hg(II), methylHg |
6/− | human hair, fish meat, seawater | AFS | 0.015 0.02 |
[139] | |
| MeHg-PDC complex | MAA / TMPTMA on Fe3O4@SiO2 |
methylHg, ethylHg, Hg(II) |
5/25 | 25 | Natural water |
CE-ICP-MS | 0.0842 | [133] |
| MOF / Modification | Chemical species | Sample | EF | Analytical technique | LOD, ng/L |
Ref. |
|---|---|---|---|---|---|---|
| MIL-101(Fe) / dithiocarbamate-modified magnetite nanoparticles | Cr(VI) | Water, tea | 470 | ETAAS | 1 | [148] |
| MIL-101(Cr) / amino dithiocarbamate modified magnetite nanoparticles | Se(IV) | Waters | 220 | ETAAS | 10 |
[149] |
| MIL-101(Cr)/1-(2-pyridylazo)-2-naphthol-modified magnetite nanoparticles | Sn(IV) | Waters | 400 | ETAAS | 5 | [150] |
| MIL-101(Fe) / morin-modified magnetite nanoparticles | V(V) | Waters | 400 | ETAAS | 3 | [151] |
| MIL-101(Cr) / aminodibenzo-18-crown-6 magnetite nanoparticles | Tl(I) | Waters | 857 | ETAAS | 1.5 | [152] |
| MOF-199 / dithiocarbamate modified magnetite nanoparticles | As(III) | Waters | 240 | ETAAS | 1.2 | [153] |
| MIL-101(Fe) mixed-matrix membrane | As(V) | Waters | - | EDXRF | 0.0941 | [154] |
| UiO-66 [Zr6O4(OH)4(bdc)6] UiO-66 / cysteine-modified |
Hg2+ phenylHg+ methylHg+ |
Waters | - | TR-ETAAS | 0.061 0.061 0.061 |
[156] |
| Magnetic Zr-terephtalate MOF composite Magnetic Zr-terephtalate MOF composite / mercapto-functionalized |
As(V) MMA DMA As(III) |
Squamous carcinoma cells | 23 25 24 24 |
Online MSPME-ICP-MS | 4.8 3.8 6.3 7.1 |
[157] |
| UiO-66-SH / magnetite nanoparticles | Hg2+ methylHg+ |
Waters, fish | 45.7 47.6 |
MSPE-HPLC-ICP-MS | 1.4 2.6 |
[158] |
| Material / Modification | Chemical species | Sample | Analytical technique | LOD, ng/L |
Ref. |
|---|---|---|---|---|---|
| Chitosan grafted with 2-hydroxyethyltrimethyl ammonium chloride | Cr(VI) | Pond, lake, tap water | FAAS | 20 | [166] |
| Chitosan modified magnetic nanoparticles cross-linked with AgNPs | Cr(III) Total Cr |
Environmental waters | ICP-OES | 0.021 0.031 |
[167] |
| Chitosan film / loaded with silver nanoparticles | Cr(VI) Cr(III) |
Surface waters | ICP-MS | 0.022 | [168] |
| Chitin, Chitosan-immobilized sodium silicate | As(V) | Waters | HGAAS ICP-MS |
- | [169] |
| Diethylene Triamine Cross-Linked Chitosan | Se(VI) | Environmental waters | ICP-OES | 12 | [170] |
| Chitosan based chelating resin / modified with 3,4,5-trihydroxy benzoic acid | Cr(VI) Cr(III) |
- | ICP-AES | - | [171] |
| Magnetic chitosan microspheres | PVP-AgNPs Cit-AgNPs PVA-AgNPs |
Environmental water, waste water | ICP-MS | 0.0232 0.0162 0.0212 |
[172] |
| Cellulose fibers / modified with L-cysteine | Hg(II) MeHg |
Waters Cosmetics |
CVG-AFS |
1 3 |
[173] |
| Cellulose / dithiocarbamate-modified | Se(IV) Total Se |
Wastewater |
LEP-OES | [174] | |
| Cellulose membranes / coated with amorphous silica / amino-silanized | Cr(VI) | Waters | EDXRF | 0.161 | [175] |
| Cellulose membranes / grafted with TiO2 | Se(IV) As(V) |
Waters | EDXRF | 0.41 0.251 |
[176] |
| Carboxymethyl cellulose nanolayer / cross-linked using glutaraldehyde onto surface of nano-ZrO2 | Cr(VI) Cr(III) |
Tap and sea water, industrial wastewater | AAS | - | [177] |
| Cellulose fabric / modified with a sol-gel/polytetrahydrofuran composite | Cr(III) Cr(VI) |
Ground water Drinking water Waste water |
HPLC-UV | 0.0011 0.0031 |
[178] |
| Microcrystalline cellulose | Cr(III) | Tap and bottled waters | ETAAS | 6 | [179] |
| Cellulose membrane / coated with silica / modified with (3-mercaptopropyl)-trimethoxysilane | As(III) | Tap, sea, ground and waste water | EDXRF | 0.0451 | [180] |
| β-cyclodextrin / modified with ionic liquid / attached on Fe3O4 nanoparticles | Mn(II) Mn(VII) |
Tap, lake and mineral water | ICP-OES | 0.152 0.272 |
[181] |
| β-cyclodextrin cross-linked polymer | Cr(III) | Tap water | GFAAS | 0.0562 | [182] |
| Magnetic dextran (Sephadex G-150) |
Cr(VI) Total Cr |
Mineral water, Tea, Coffee |
Voltammetry | 0.013 | [183] |
| Poly(vinyl alcohol)/sodium alginate/AuNPs hydrogel membranes | Cr(III) Cr(VI) |
Waters | ETAAS | 0.0012 0.012 |
[187] |
| Immobilized Cr(III) Binding Phages | Cr(III) | Water | ICP-MS | 15 | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





