Submitted:
15 November 2023
Posted:
16 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Differential expression analysis
- metastatic versus primary PCa groups for GSE3325, GSE3933, GSE68882 datasets;
- mCRPC versus PCa groups for GSE32269, GSE6811, GSE70770, GSE6752, GSE35988 datasets;
- AR-driven versus non AR-driven groups for GSE101607;
- CHGA negative versus CHGA positive/SYP positive/SR negative groups were compared for GSE77930.
2.3. Over-representation analysis
2.4. Statistical analysis
3. Results
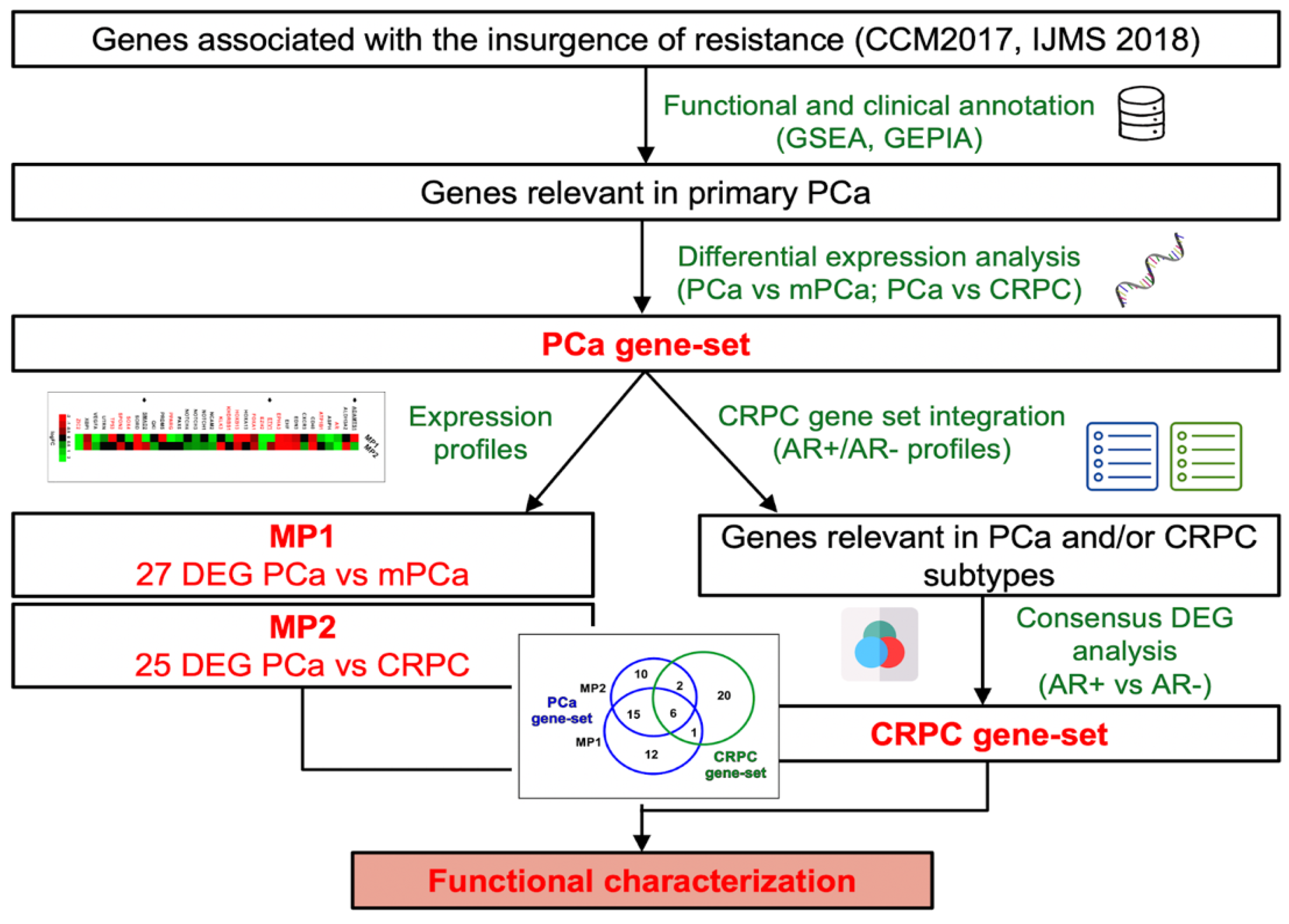
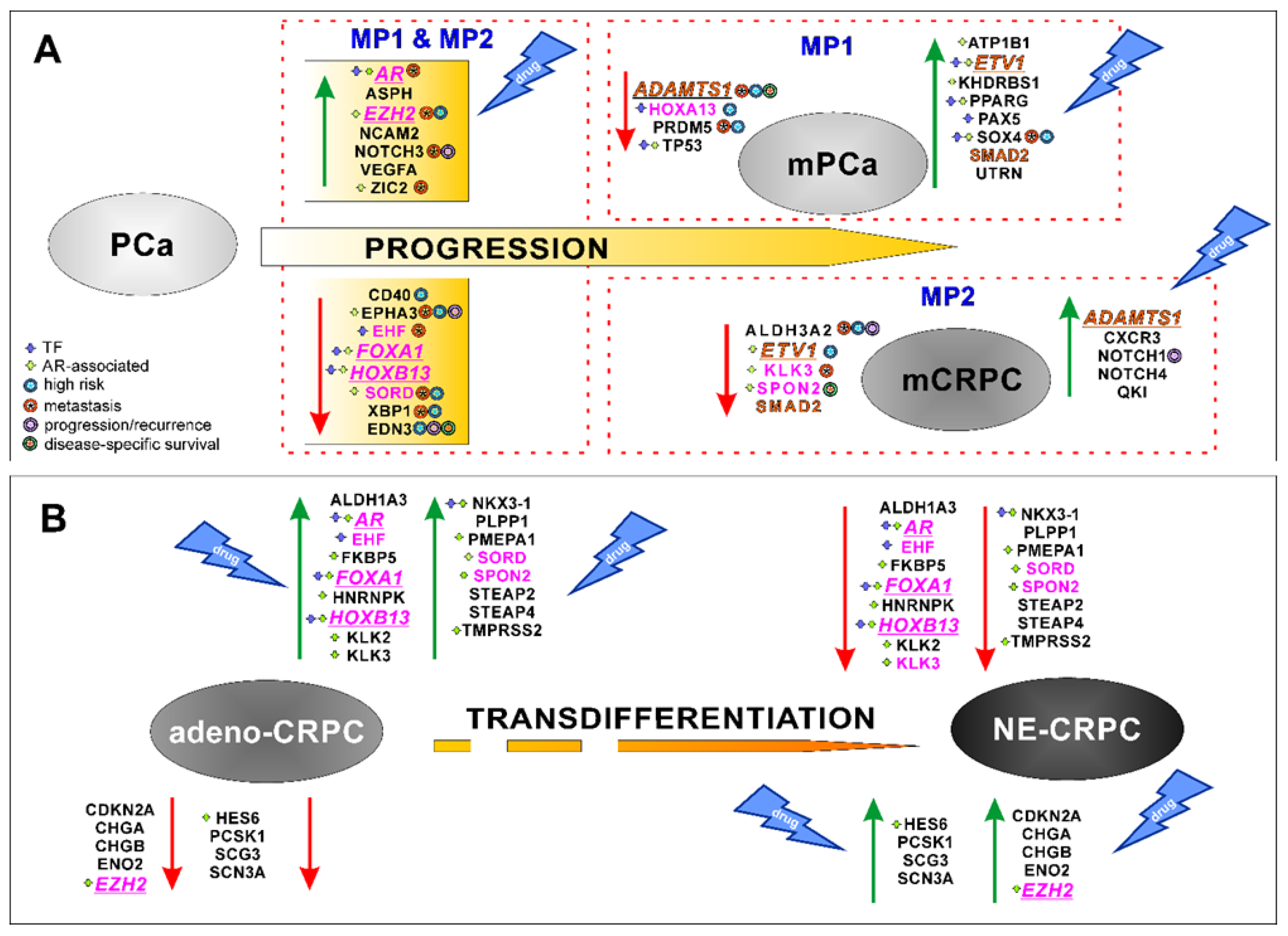
3.1. Identification and characterization of clinically relevant gene sets in PCa progression to metastatic and castration-resistant phenotypes.
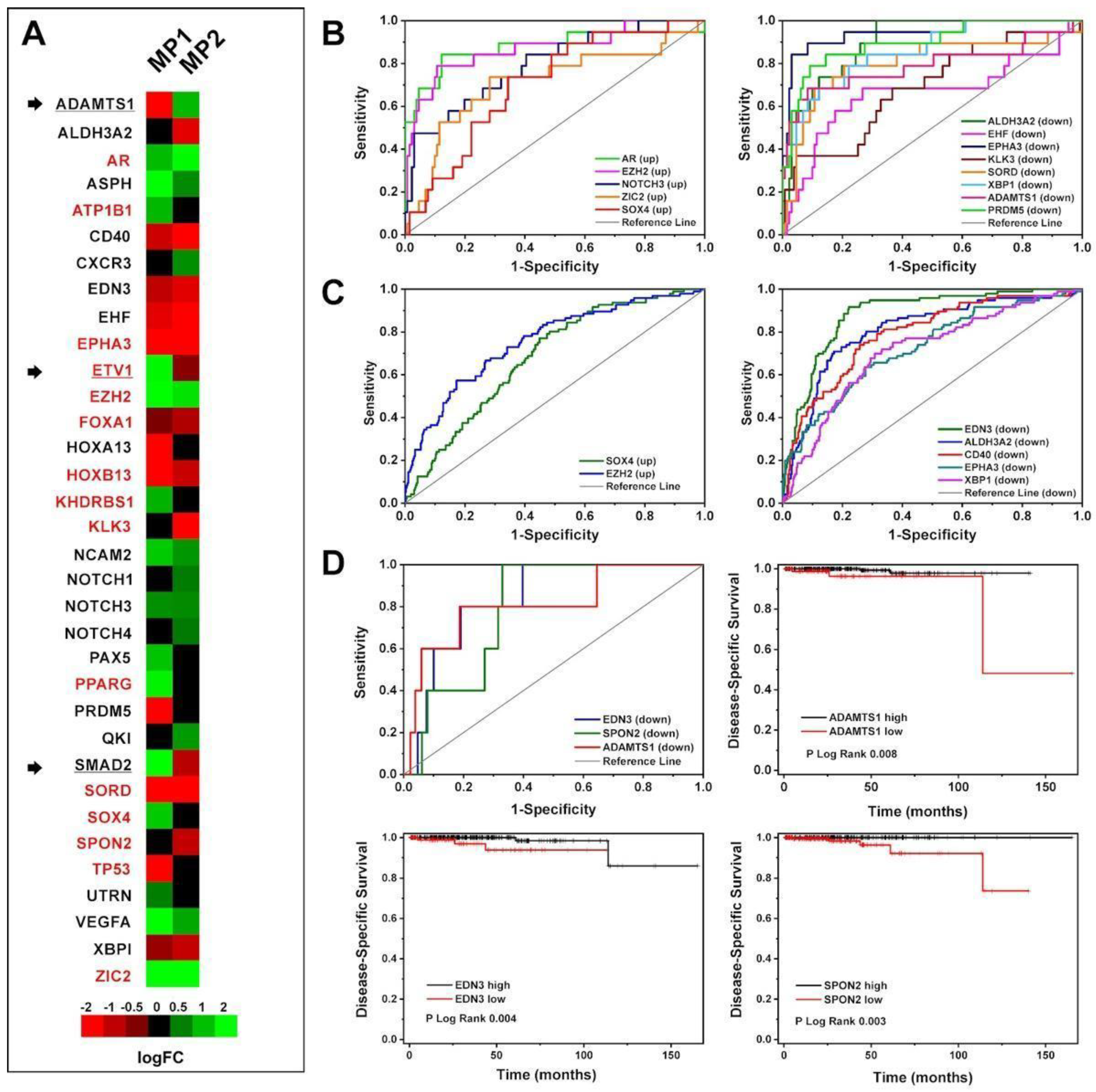
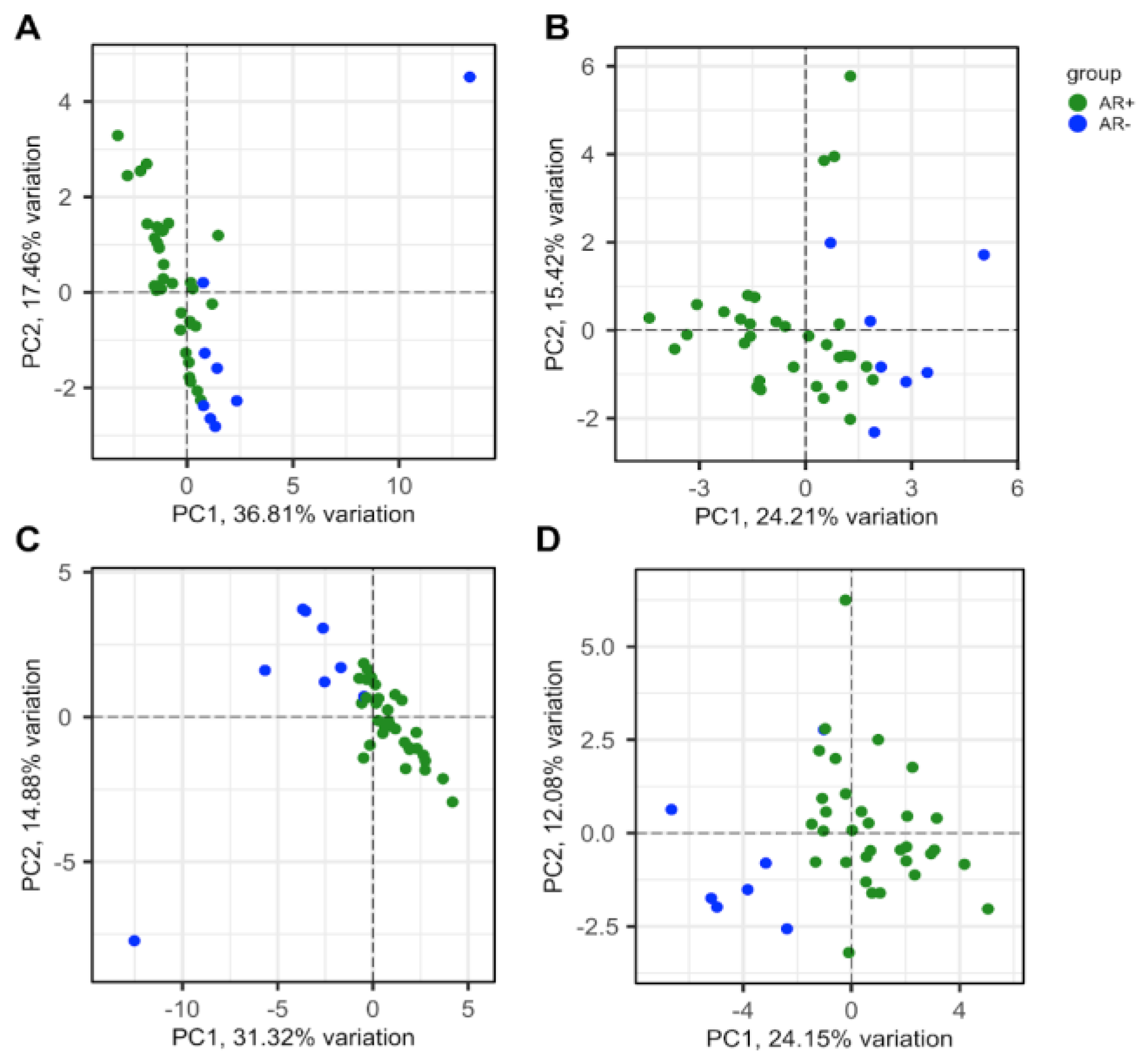
3.2. Evaluation of 50-gene set alterations in PCa tissue samples at different evolutive stages
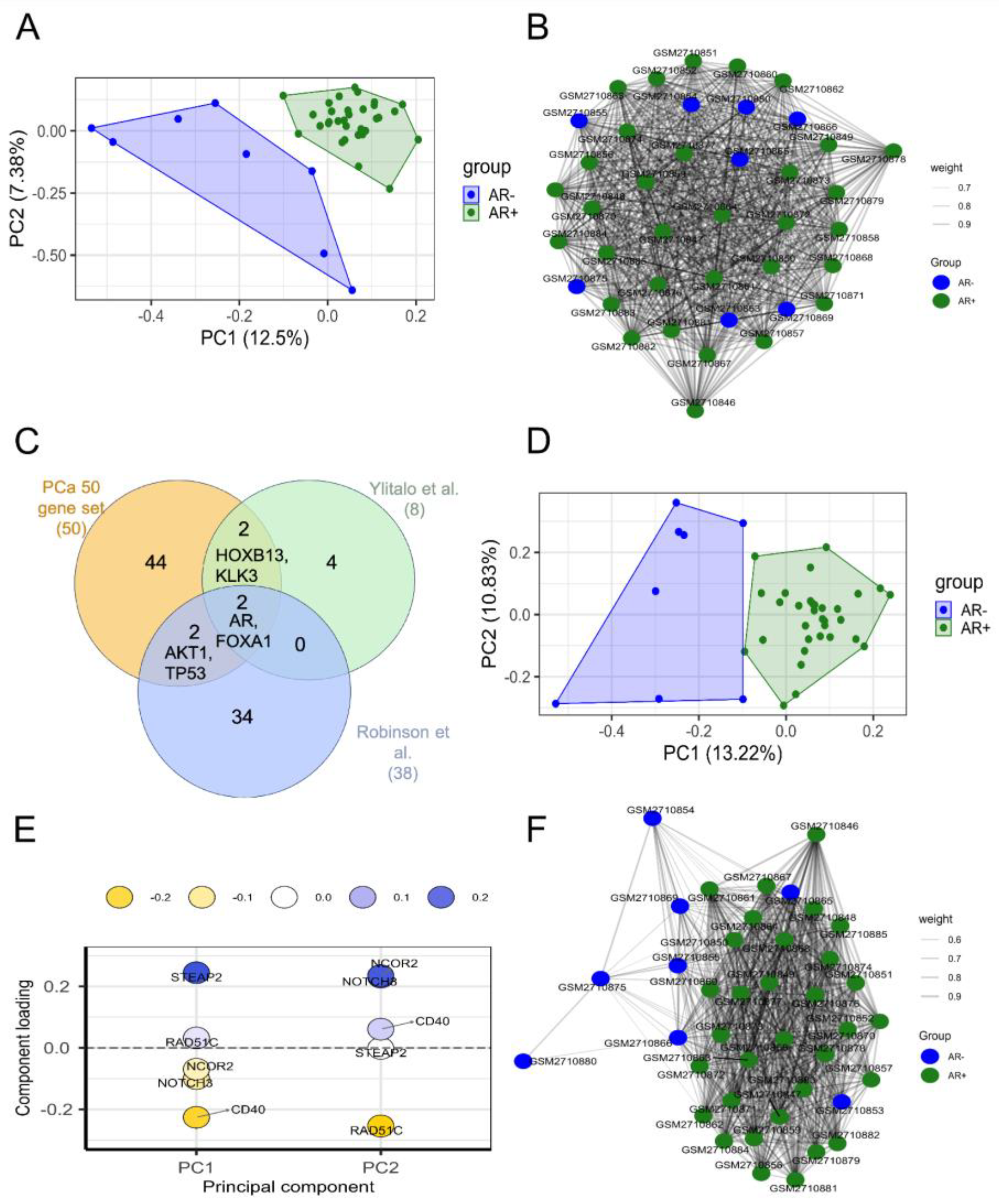
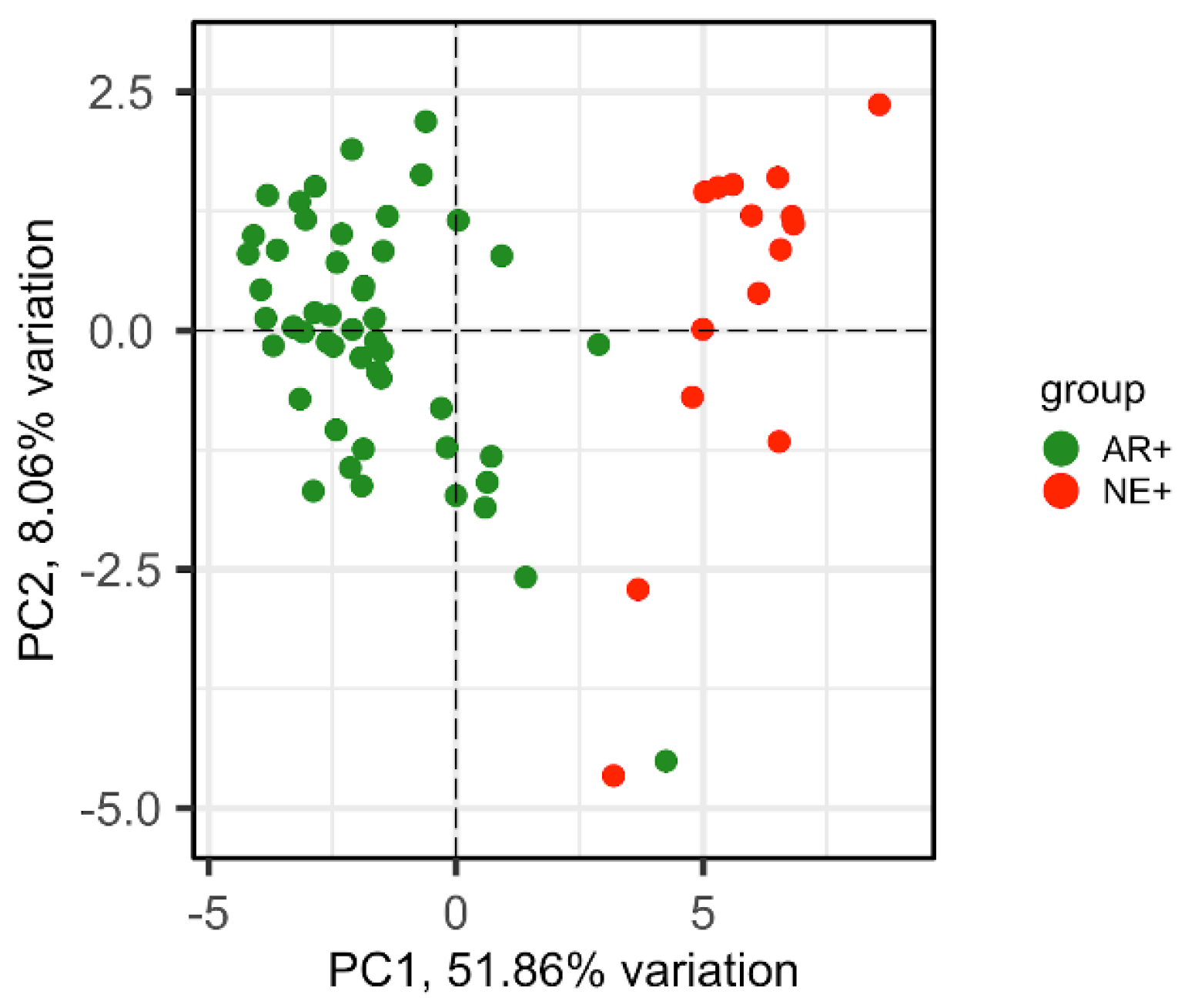
3.3. Identification of genes associated with different CRPC phenotypes
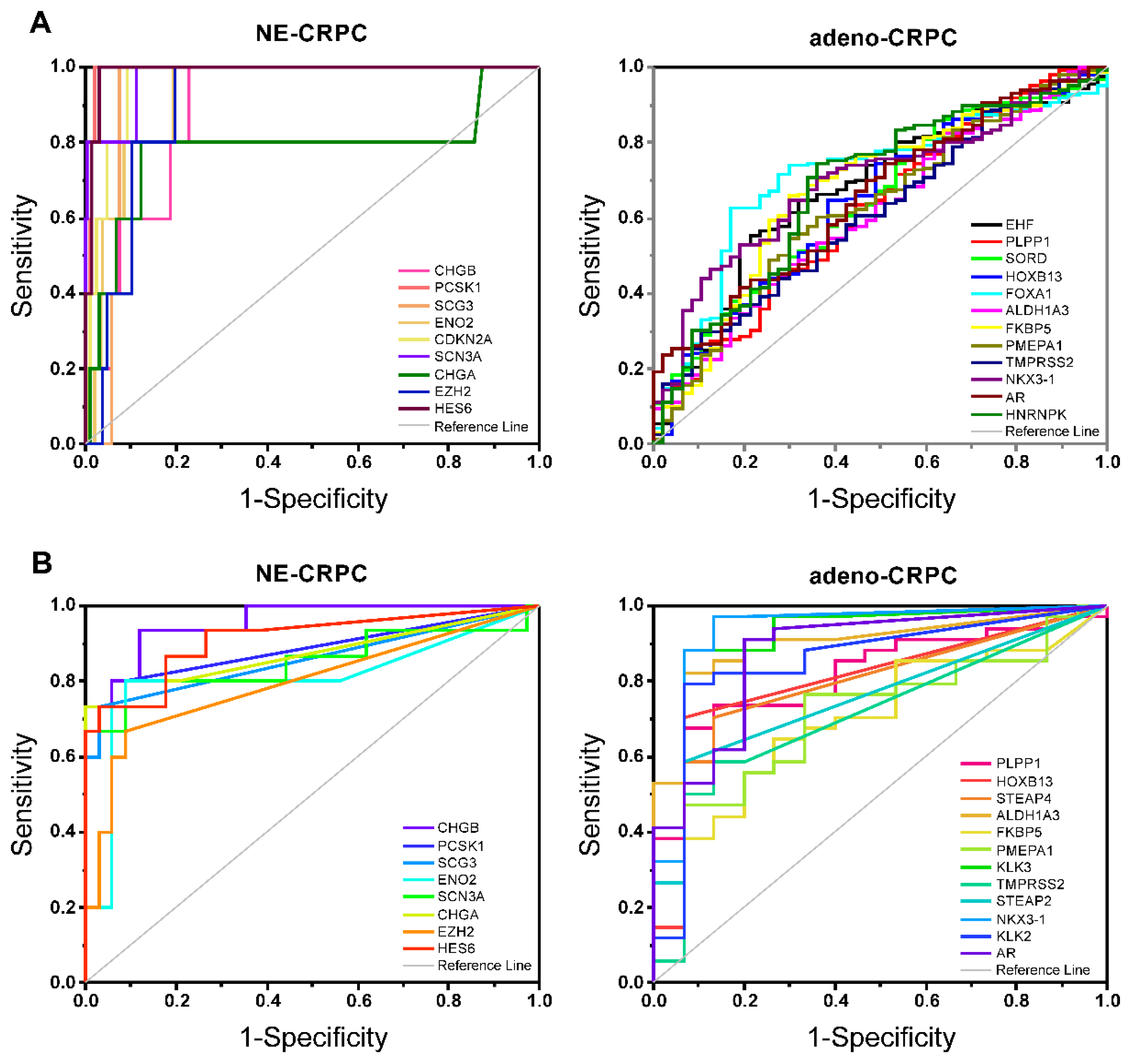
3.4. Validation of CRPC-gene set on independent datasets
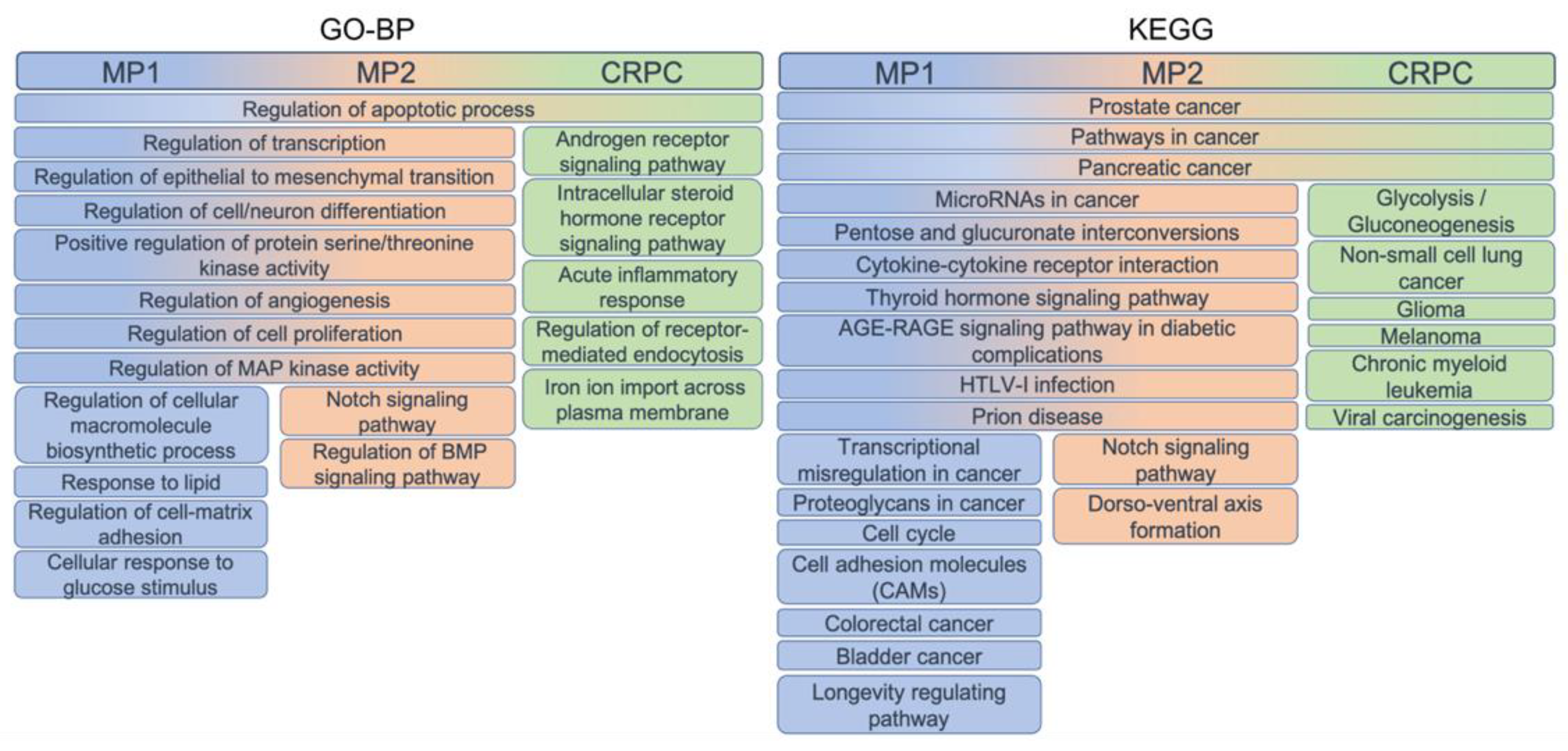
3.5. Functional enrichment of gene-sets
3.6. Investigating the context-specific essentiality
3.7. Computational drug identification based on the PCa- and CRPC-gene sets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global Cancer Incidence in Older Adults, 2012 and 2035: A Population-Based Study: Global Cancer Incidence in Older Adults. Int. J. Cancer 2019, 144(1), 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bungaro, M.; Buttigliero, C.; Tucci, M. Overcoming the Mechanisms of Primary and Acquired Resistance to New Generation Hormonal Therapies in Advanced Prostate Cancer: Focus on Androgen Receptor Independent Pathways. CDR 2020. [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Mizokami, A. Undesirable Status of Prostate Cancer Cells after Intensive Inhibition of AR Signaling: Post-AR Era of CRPC Treatment. Biomedicines 2021, 9(4), 414. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Zanardi, E.; Vallome, G.; Cavo, A.; Cerbone, L.; Di Meglio, A.; Fabbroni, C.; Latocca, M. M.; Rizzo, F.; Messina, C.; Rubagotti, A.; Barboro, P.; Boccardo, F. Targeting Androgen-Independent Pathways: New Chances for Patients with Prostate Cancer? Critical Reviews in Oncology/Hematology 2017, 118, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Conteduca, V.; Zoubeidi, A.; Beltran, H. Biological Evolution of Castration-Resistant Prostate Cancer. European Urology Focus 2019, 5(2), 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Granata, I.; Capaia, M.; Piccirillo, M.; Guarracino, M. R.; Venè, R.; Brizzolara, A.; Petretto, A.; Inglese, E.; Morini, M.; Astigiano, S.; Amaro, A. A.; Boccardo, F.; Balbi, C.; Barboro, P. Adaptive Phenotype Drives Resistance to Androgen Deprivation Therapy in Prostate Cancer. Cell Commun Signal 2017, 15(1), 51. [Google Scholar] [CrossRef]
- Sheahan, A. V.; Ellis, L. Epigenetic Reprogramming: A Key Mechanism Driving Therapeutic Resistance. Urologic Oncology: Seminars and Original Investigations 2018, 36 (8), 375–379. [CrossRef]
- Roubaud, G.; Liaw, B. C.; Oh, W. K.; Mulholland, D. J. Strategies to Avoid Treatment-Induced Lineage Crisis in Advanced Prostate Cancer. Nat Rev Clin Oncol 2017, 14(5), 269–283. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6 (3), 82. [CrossRef]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C. K. Y.; Bedard, P. L.; Tortora, G.; Douillard, J.-Y.; Van Allen, E. M.; Schultz, N.; Swanton, C.; André, F.; Pusztai, L. A Framework to Rank Genomic Alterations as Targets for Cancer Precision Medicine: The ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). Annals of Oncology 2018, 29(9), 1895–1902. [Google Scholar] [CrossRef]
- Ruggero, K.; Farran-Matas, S.; Martinez-Tebar, A.; Aytes, A. Epigenetic Regulation in Prostate Cancer Progression. Curr Mol Bio Rep 2018, 4(2), 101–115. [Google Scholar] [CrossRef]
- Da Silva-Diz, V.; Lorenzo-Sanz, L.; Bernat-Peguera, A.; Lopez-Cerda, M.; Muñoz, P. Cancer Cell Plasticity: Impact on Tumor Progression and Therapy Response. Seminars in Cancer Biology 2018, 53, 48–58. [Google Scholar] [CrossRef]
- Vellano, Christopher P., Michael G. White, Miles C. Andrews, Manoj Chelvanambi, Russell G. Witt, Joseph R. Daniele, Mark Titus, et al. «Androgen Receptor Blockade Promotes Response to BRAF/MEK-Targeted Therapy». Nature 606, fasc. 7915 (23 giugno 2022): 797–803. [CrossRef]
- Varambally, S.; Yu, J.; Laxman, B.; Rhodes, D. R.; Mehra, R.; Tomlins, S. A.; Shah, R. B.; Chandran, U.; Monzon, F. A.; Becich, M. J.; Wei, J. T.; Pienta, K. J.; Ghosh, D.; Rubin, M. A.; Chinnaiyan, A. M. Integrative Genomic and Proteomic Analysis of Prostate Cancer Reveals Signatures of Metastatic Progression. Cancer Cell 2005, 8(5), 393–406. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.; Li, C.; Higgins, J. P.; van de Rijn, M.; Bair, E.; Montgomery, K.; Ferrari, M.; Egevad, L.; Rayford, W.; Bergerheim, U.; Ekman, P.; DeMarzo, A. M.; Tibshirani, R.; Botstein, D.; Brown, P. O.; Brooks, J. D.; Pollack, J. R. Gene Expression Profiling Identifies Clinically Relevant Subtypes of Prostate Cancer. Proc Natl Acad Sci U S A 2004, 101(3), 811–816. [Google Scholar] [CrossRef]
- LaTulippe, E.; Satagopan, J.; Smith, A.; Scher, H.; Scardino, P.; Reuter, V.; Gerald, W. L. Comprehensive Gene Expression Analysis of Prostate Cancer Reveals Distinct Transcriptional Programs Associated with Metastatic Disease. Cancer Res 2002, 62(15), 4499–4506. [Google Scholar] [PubMed]
- Cai, C.; Wang, H.; He, H. H.; Chen, S.; He, L.; Ma, F.; Mucci, L.; Wang, Q.; Fiore, C.; Sowalsky, A. G.; Loda, M.; Liu, X. S.; Brown, M.; Balk, S. P.; Yuan, X. ERG Induces Androgen Receptor-Mediated Regulation of SOX9 in Prostate Cancer. J Clin Invest 2013, 123(3), 1109–1122. [Google Scholar] [CrossRef]
- Tamura, K.; Furihata, M.; Tsunoda, T.; Ashida, S.; Takata, R.; Obara, W.; Yoshioka, H.; Daigo, Y.; Nasu, Y.; Kumon, H.; Konaka, H.; Namiki, M.; Tozawa, K.; Kohri, K.; Tanji, N.; Yokoyama, M.; Shimazui, T.; Akaza, H.; Mizutani, Y.; Miki, T.; Fujioka, T.; Shuin, T.; Nakamura, Y.; Nakagawa, H. Molecular Features of Hormone-Refractory Prostate Cancer Cells by Genome-Wide Gene Expression Profiles. Cancer Res 2007, 67(11), 5117–5125. [Google Scholar] [CrossRef] [PubMed]
- Ross-Adams, H.; Lamb, A. D.; Dunning, M. J.; Halim, S.; Lindberg, J.; Massie, C. M.; Egevad, L. A.; Russell, R.; Ramos-Montoya, A.; Vowler, S. L.; Sharma, N. L.; Kay, J.; Whitaker, H.; Clark, J.; Hurst, R.; Gnanapragasam, V. J.; Shah, N. C.; Warren, A. Y.; Cooper, C. S.; Lynch, A. G.; Stark, R.; Mills, I. G.; Grönberg, H.; Neal, D. E.; CamCaP Study Group. Integration of Copy Number and Transcriptomics Provides Risk Stratification in Prostate Cancer: A Discovery and Validation Cohort Study. EBioMedicine 2015, 2(9), 1133–1144. [Google Scholar] [CrossRef]
- Chandran, U. R.; Ma, C.; Dhir, R.; Bisceglia, M.; Lyons-Weiler, M.; Liang, W.; Michalopoulos, G.; Becich, M.; Monzon, F. A. Gene Expression Profiles of Prostate Cancer Reveal Involvement of Multiple Molecular Pathways in the Metastatic Process. BMC Cancer 2007, 7, 64. [Google Scholar] [CrossRef]
- Grasso, C. S.; Wu, Y.-M.; Robinson, D. R.; Cao, X.; Dhanasekaran, S. M.; Khan, A. P.; Quist, M. J.; Jing, X.; Lonigro, R. J.; Brenner, J. C.; Asangani, I. A.; Ateeq, B.; Chun, S. Y.; Siddiqui, J.; Sam, L.; Anstett, M.; Mehra, R.; Prensner, J. R.; Palanisamy, N.; Ryslik, G. A.; Vandin, F.; Raphael, B. J.; Kunju, L. P.; Rhodes, D. R.; Pienta, K. J.; Chinnaiyan, A. M.; Tomlins, S. A. The Mutational Landscape of Lethal Castration-Resistant Prostate Cancer. Nature 2012, 487(7406), 239–243. [Google Scholar] [CrossRef]
- Ylitalo, E. B.; Thysell, E.; Jernberg, E.; Lundholm, M.; Crnalic, S.; Egevad, L.; Stattin, P.; Widmark, A.; Bergh, A.; Wikström, P. Subgroups of Castration-Resistant Prostate Cancer Bone Metastases Defined Through an Inverse Relationship Between Androgen Receptor Activity and Immune Response. European Urology 2017, 71(5), 776–787. [Google Scholar] [CrossRef]
- Tsai, H. K.; Lehrer, J.; Alshalalfa, M.; Erho, N.; Davicioni, E.; Lotan, T. L. Gene Expression Signatures of Neuroendocrine Prostate Cancer and Primary Small Cell Prostatic Carcinoma. BMC Cancer 2017, 17(1), 759. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L. D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; Lucas, J. M.; Brown, L. G.; Dumpit, R. F.; DeSarkar, N.; Higano, C.; Yu, E. Y.; Coleman, R.; Schultz, N.; Fang, M.; Lange, P. H.; Shendure, J.; Vessella, R. L.; Nelson, P. S. Substantial Interindividual and Limited Intraindividual Genomic Diversity among Tumors from Men with Metastatic Prostate Cancer. Nat Med 2016, 22(4), 369–378. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E. M.; Wu, Y.-M.; Schultz, N.; Lonigro, R. J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C. C.; Attard, G.; Beltran, H.; Abida, W.; Bradley, R. K.; Vinson, J.; Cao, X.; Vats, P.; Kunju, L. P.; Hussain, M.; Feng, F. Y.; Tomlins, S. A.; Cooney, K. A.; Smith, D. C.; Brennan, C.; Siddiqui, J.; Mehra, R.; Chen, Y.; Rathkopf, D. E.; Morris, M. J.; Solomon, S. B.; Durack, J. C.; Reuter, V. E.; Gopalan, A.; Gao, J.; Loda, M.; Lis, R. T.; Bowden, M.; Balk, S. P.; Gaviola, G.; Sougnez, C.; Gupta, M.; Yu, E. Y.; Mostaghel, E. A.; Cheng, H. H.; Mulcahy, H.; True, L. D.; Plymate, S. R.; Dvinge, H.; Ferraldeschi, R.; Flohr, P.; Miranda, S.; Zafeiriou, Z.; Tunariu, N.; Mateo, J.; Perez-Lopez, R.; Demichelis, F.; Robinson, B. D.; Schiffman, M.; Nanus, D. M.; Tagawa, S. T.; Sigaras, A.; Eng, K. W.; Elemento, O.; Sboner, A.; Heath, E. I.; Scher, H. I.; Pienta, K. J.; Kantoff, P.; de Bono, J. S.; Rubin, M. A.; Nelson, P. S.; Garraway, L. A.; Sawyers, C. L.; Chinnaiyan, A. M. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161(5), 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B. S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B. S.; Arora, V. K.; Kaushik, P.; Cerami, E.; Reva, B.; Antipin, Y.; Mitsiades, N.; Landers, T.; Dolgalev, I.; Major, J. E.; Wilson, M.; Socci, N. D.; Lash, A. E.; Heguy, A.; Eastham, J. A.; Scher, H. I.; Reuter, V. E.; Scardino, P. T.; Sander, C.; Sawyers, C. L.; Gerald, W. L. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18(1), 11–22. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E. M.; Sboner, A.; Fedrizzi, T.; Mosquera, J. M.; Robinson, B. D.; De Sarkar, N.; Kunju, L. P.; Tomlins, S.; Wu, Y. M.; Nava Rodrigues, D.; Loda, M.; Gopalan, A.; Reuter, V. E.; Pritchard, C. C.; Mateo, J.; Bianchini, D.; Miranda, S.; Carreira, S.; Rescigno, P.; Filipenko, J.; Vinson, J.; Montgomery, R. B.; Beltran, H.; Heath, E. I.; Scher, H. I.; Kantoff, P. W.; Taplin, M.-E.; Schultz, N.; deBono, J. S.; Demichelis, F.; Nelson, P. S.; Rubin, M. A.; Chinnaiyan, A. M.; Sawyers, C. L. Genomic Correlates of Clinical Outcome in Advanced Prostate Cancer. Proc. Natl. Acad. Sci. U.S.A. 2019, 116(23), 11428–11436. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J. M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B. V. S. K.; Varambally, S.; Tomlins, S. A.; Nanus, D. M.; Tagawa, S. T.; Van Allen, E. M.; Elemento, O.; Sboner, A.; Garraway, L. A.; Rubin, M. A.; Demichelis, F. Divergent Clonal Evolution of Castration-Resistant Neuroendocrine Prostate Cancer. Nat Med 2016, 22(3), 298–305. [Google Scholar] [CrossRef] [PubMed]
- Capaia, M.; Granata, I.; Guarracino, M.; Petretto, A.; Inglese, E.; Cattrini, C.; Ferrari, N.; Boccardo, F.; Barboro, P. A HnRNP K–AR-Related Signature Reflects Progression toward Castration-Resistant Prostate Cancer. IJMS 2018, 19(7), 1920. [Google Scholar] [CrossRef]
- Blighe, K.; Lewis, M. PCAtools: Everything Principal Components Analysis, 2019. https://github.com/kevinblighe/PCAtools.
- Jackson, S.; Cimentada, J.; Ruiz, E. Corrr: Correlations in R. https://github.com/drsimonj/corrr.
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47(4), 458–472. [Google Scholar] [CrossRef]
- Liu, S.; Kumari, S.; Hu, Q.; Senapati, D.; Venkadakrishnan, V. B.; Wang, D.; DePriest, A. D.; Schlanger, S. E.; Ben-Salem, S.; Valenzuela, M. M.; Willard, B.; Mudambi, S.; Swetzig, W. M.; Das, G. M.; Shourideh, M.; Koochekpour, S.; Falzarano, S. M.; Magi-Galluzzi, C.; Yadav, N.; Chen, X.; Lao, C.; Wang, J.; Billaud, J.-N.; Heemers, H. V. A Comprehensive Analysis of Coregulator Recruitment, Androgen Receptor Function and Gene Expression in Prostate Cancer. eLife 2017, 6, e28482. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Keller, J. M.; Yeung, K.; Keller, E. T.; Fu, Z. Transcriptional Regulation of RKIP Expression by Androgen in Prostate Cells. Cell Physiol Biochem 2012, 30(6), 1340–1350. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; He, H.; Dai, Q.; Qin, G.; Chen, J.; Cai, C.; Fu, X.; Bi, X.; Zhu, J.; Liao, D.; Lu, X.; Mo, Z.; Zhu, Y.; Zhong, W. Identification of Novel Serological Tumor Markers for Human Prostate Cancer Using Integrative Transcriptome and Proteome Analysis. Med Oncol 2012, 29(4), 2877–2888. [Google Scholar] [CrossRef]
- Polkinghorn, W. R.; Parker, J. S.; Lee, M. X.; Kass, E. M.; Spratt, D. E.; Iaquinta, P. J.; Arora, V. K.; Yen, W.-F.; Cai, L.; Zheng, D.; Carver, B. S.; Chen, Y.; Watson, P. A.; Shah, N. P.; Fujisawa, S.; Goglia, A. G.; Gopalan, A.; Hieronymus, H.; Wongvipat, J.; Scardino, P. T.; Zelefsky, M. J.; Jasin, M.; Chaudhuri, J.; Powell, S. N.; Sawyers, C. L. Androgen Receptor Signaling Regulates DNA Repair in Prostate Cancers. Cancer Discovery 2013, 3(11), 1245–1253. [Google Scholar] [CrossRef]
- Mendiratta, P.; Mostaghel, E.; Guinney, J.; Tewari, A. K.; Porrello, A.; Barry, W. T.; Nelson, P. S.; Febbo, P. G. Genomic Strategy for Targeting Therapy in Castration-Resistant Prostate Cancer. JCO 2009, 27(12), 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Tien, A. H.; Sadar, M. D. Androgen-Responsive Gene Expression in Prostate Cancer Progression. In Androgen-Responsive Genes in Prostate Cancer; Wang, Z., Ed.; Springer New York: New York, NY, 2013; pp. 135–153. [Google Scholar] [CrossRef]
- Gottlieb, B.; Beitel, L. K.; Nadarajah, A.; Paliouras, M.; Trifiro, M. The Androgen Receptor Gene Mutations Database: 2012 Update. Hum. Mutat. 2012, 33(5), 887–894. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S. E.; Zeidan, B.; Taylor, M. G.; Bickers, B.; Al-Ruwaili, J.; Aukim-Hastie, C.; Townsend, P. A. Proteomics in Prostate Cancer Biomarker Discovery. Expert Review of Proteomics 2010, 7(1), 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Favero, F.; Risch, T.; Simon, R.; Feuerbach, L.; Assenov, Y.; Heckmann, D.; Sidiropoulos, N.; Waszak, S. M.; Hübschmann, D.; Urbanucci, A.; Girma, E. G.; Kuryshev, V.; Klimczak, L. J.; Saini, N.; Stütz, A. M.; Weichenhan, D.; Böttcher, L.-M.; Toth, R.; Hendriksen, J. D.; Koop, C.; Lutsik, P.; Matzk, S.; Warnatz, H.-J.; Amstislavskiy, V.; Feuerstein, C.; Raeder, B.; Bogatyrova, O.; Schmitz, E.-M.; Hube-Magg, C.; Kluth, M.; Huland, H.; Graefen, M.; Lawerenz, C.; Henry, G. H.; Yamaguchi, T. N.; Malewska, A.; Meiners, J.; Schilling, D.; Reisinger, E.; Eils, R.; Schlesner, M.; Strand, D. W.; Bristow, R. G.; Boutros, P. C.; Von Kalle, C.; Gordenin, D.; Sültmann, H.; Brors, B.; Sauter, G.; Plass, C.; Yaspo, M.-L.; Korbel, J. O.; Schlomm, T.; Weischenfeldt, J. Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell 2018, 34(6), 996–1011.e8. [Google Scholar] [CrossRef]
- Sailer, V.; Von Amsberg, G.; Duensing, S.; Kirfel, J.; Lieb, V.; Metzger, E.; Offermann, A.; Pantel, K.; Schuele, R.; Taubert, H.; Wach, S.; Perner, S.; Werner, S.; Aigner, A. Experimental in Vitro, Ex Vivo and in Vivo Models in Prostate Cancer Research. Nat Rev Urol 2023, 20(3), 158–178. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Marampon, F. ; Giusti,I.; Carosa, E.; Di Sante, S.; Ricevuto,E.; Dolo, V.; Tombolini, V.; Jannini, E.A.; Festuccia, C. Differential Effects of PXD101 (Belinostat) on Androgen-Dependent and Androgen-Independent Prostate Cancer Models. Int J Oncol. [CrossRef]
- Ferrari, A. C.; Alumkal, J. J.; Stein, M. N.; Taplin, M.-E.; Babb, J.; Barnett, E. S.; Gomez-Pinillos, A.; Liu, X.; Moore, D.; DiPaola, R.; Beer, T. M. Epigenetic Therapy with Panobinostat Combined with Bicalutamide Rechallenge in Castration-Resistant Prostate Cancer. Clinical Cancer Research 2019, 25(1), 52–63. [Google Scholar] [CrossRef]
- Welsbie, D. S.; Xu, J.; Chen, Y.; Borsu, L.; Scher, H. I.; Rosen, N.; Sawyers, C. L. Histone Deacetylases Are Required for Androgen Receptor Function in Hormone-Sensitive and Castrate-Resistant Prostate Cancer. Cancer Research 2009, 69(3), 958–966. [Google Scholar] [CrossRef]
- He, Y.; Xu, W.; Xiao, Y.-T.; Huang, H.; Gu, D.; Ren, S. Targeting Signaling Pathways in Prostate Cancer: Mechanisms and Clinical Trials. Sig Transduct Target Ther 2022, 7(1), 198. [Google Scholar] [CrossRef]
- Majera, D.; Skrott, Z.; Bouchal, J.; Bartkova, J.; Simkova, D.; Gachechiladze, M.; Steigerova, J.; Kurfurstova, D.; Gursky, J.; Korinkova, G.; Cwiertka, K.; Hodny, Z.; Mistrik, M.; Bartek, J. Targeting Genotoxic and Proteotoxic Stress-Response Pathways in Human Prostate Cancer by Clinically Available PARP Inhibitors, Vorinostat and Disulfiram. Prostate 2019, 79(4), 352–362. [Google Scholar] [CrossRef] [PubMed]
- Mitra Ghosh, T.; White, J.; Davis, J.; Mazumder, S.; Kansom, T.; Skarupa, E.; Barnett, G. S.; Piazza, G. A.; Bird, R. C.; Mitra, A. K.; Yates, C.; Cummings, B. S.; Arnold, R. D. Identification and Characterization of Key Differentially Expressed Genes Associated With Metronomic Dosing of Topotecan in Human Prostate Cancer. Front. Pharmacol. 2021, 12, 736951. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Capaia, M.; Boccardo, F.; Barboro, P. Etoposide and Topoisomerase II Inhibition for Aggressive Prostate Cancer: Data from a Translational Study. Cancer Treatment and Research Communications 2020, 25, 100221. [Google Scholar] [CrossRef] [PubMed]
- Alabi, B. R.; Liu, S.; Stoyanova, T. Current and Emerging Therapies for Neuroendocrine Prostate Cancer. Pharmacology & Therapeutics 2022, 238, 108255. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, C.; Xu, K.; Su, W.; Mao, X.; Zou, Y.; Li, B. The Effect of Prostate Cancer-Targeting Doxorubicin Nanomicelles Combined with Photothermal Therapy on Castration-Resistant Prostate Cancer. j biomed nanotechnol 2022, 18(5), 1276–1288. [Google Scholar] [CrossRef]
- Li, K.; Zhan, W.; Chen, Y.; Jha, R. K.; Chen, X. Docetaxel and Doxorubicin Codelivery by Nanocarriers for Synergistic Treatment of Prostate Cancer. Front. Pharmacol. 2019, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Dhani, N. C.; Emmenegger, U.; Adams, L.; Jongstra, J.; Tannock, I. F.; Sridhar, S. S.; Knox, J. J.; Day, J. R.; Groskopf, J.; Joshua, A. M. Phase II Study of Cytarabine in Men with Docetaxel-Refractory, Castration-Resistant Prostate Cancer with Evaluation of TMPRSS2-ERG and SPINK1 as Serum Biomarkers: PHASE II STUDY OF CYTARABINE IN CRPC. BJU International 2012, 110(6), 840–845. [Google Scholar] [CrossRef]
- Ueki, T.; Uemura, H.; Nagashima, Y.; Ohta, S.; Ishiguro, H.; Kubota, Y. Antitumour Effect of Electrochemotherapy with Bleomycin on Human Prostate Cancer Xenograft. BJU Int 2008, 080612012630360. [Google Scholar] [CrossRef]
- Iannantuono, G. M.; Torino, F.; Rosenfeld, R.; Guerriero, S.; Carlucci, M.; Sganga, S.; Capotondi, B.; Riondino, S.; Roselli, M. The Role of Histology-Agnostic Drugs in the Treatment of Metastatic Castration-Resistant Prostate Cancer. IJMS 2022, 23(15), 8535. [Google Scholar] [CrossRef]
- Jianhua Li, J. L.; Huanxian Wu, H. W.; Shidong Lv, S. L.; Dongling Quan, D. Q.; Danni Yang, D. Y.; Jiahuan Xu, J. X.; Boyu Chen, B. C.; Baofang Ou, B. O.; Shaoyu Wu, S. W.; Qiang Wei, Q. W. Enhanced Antitumor Efficacy by Combining Afatinib with MDV3100 in Castration-Resistant Prostate Cancer. Pharmazie 2022, No. 2, 59–66. [Google Scholar] [CrossRef]
- Matheux, A.; Gassiot, M.; Fromont, G.; Leenhardt, F.; Boulahtouf, A.; Fabbrizio, E.; Marchive, C.; Garcin, A.; Agherbi, H.; Combès, E.; Evrard, A.; Houédé, N.; Balaguer, P.; Gongora, C.; Mbatchi, L. C.; Pourquier, P. PXR Modulates the Prostate Cancer Cell Response to Afatinib by Regulating the Expression of the Monocarboxylate Transporter SLC16A1. Cancers 2021, 13(14), 3635. [Google Scholar] [CrossRef] [PubMed]
- Vignani, F.; Bertaglia, V.; Buttigliero, C.; Tucci, M.; Scagliotti, G. V.; Di Maio, M. Skeletal Metastases and Impact of Anticancer and Bone-Targeted Agents in Patients with Castration-Resistant Prostate Cancer. Cancer Treatment Reviews 2016, 44, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, A.; Ocio, E. M.; Crusoe, E.; Santamaria, C.; Hernández-Campo, P.; Blanco, J. F.; Sanchez-Guijo, F. M.; Hernández-Iglesias, T.; Briñón, J. G.; Fisac-Herrero, R. M.; Lee, F. Y.; Pandiella, A.; San Miguel, J. F.; Garayoa, M. Dasatinib as a Bone-Modifying Agent: Anabolic and Anti-Resorptive Effects. PLoS ONE 2012, 7(4), e34914. [Google Scholar] [CrossRef]
- Tong, D. Unravelling the Molecular Mechanisms of Prostate Cancer Evolution from Genotype to Phenotype. Critical Reviews in Oncology/Hematology 2021, 163, 103370. [Google Scholar] [CrossRef] [PubMed]
- Nickols, N. G.; Nazarian, R.; Zhao, S. G.; Tan, V.; Uzunangelov, V.; Xia, Z.; Baertsch, R.; Neeman, E.; Gao, A. C.; Thomas, G. V.; Howard, L.; De Hoedt, A. M.; Stuart, J.; Goldstein, T.; Chi, K.; Gleave, M. E.; Graff, J. N.; Beer, T. M.; Drake, J. M.; Evans, C. P.; Aggarwal, R.; Foye, A.; Feng, F. Y.; Small, E. J.; Aronson, W. J.; Freedland, S. J.; Witte, O. N.; Huang, J.; Alumkal, J. J.; Reiter, R. E.; Rettig, M. B. MEK-ERK Signaling Is a Therapeutic Target in Metastatic Castration Resistant Prostate Cancer. Prostate Cancer Prostatic Dis 2019, 22(4), 531–538. [Google Scholar] [CrossRef] [PubMed]
- Inamura, S.; Ito, H.; Taga, M.; Tsuchiyama, K.; Hoshino, H.; Kobayashi, M.; Yokoyama, O. Low-Dose Docetaxel Enhanced the Anticancer Effect of Temsirolimus by Overcoming Autophagy in Prostate Cancer Cells. Anticancer Res 2019, 39(10), 5417–5425. [Google Scholar] [CrossRef] [PubMed]
- Shariatifar, H.; Ranjbarian, F.; Hajiahmadi, F.; Farasat, A. A Comprehensive Review on Methotrexate Containing Nanoparticles; an Appropriate Tool for Cancer Treatment. Mol Biol Rep 2022, 49(11), 11049–11060. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Leu, W.-J.; Hsu, L.-C.; Ho, C.-H.; Liu, S.-P.; Guh, J.-H. Phosphodiesterase Type 5 Inhibitors Synergize Vincristine in Killing Castration-Resistant Prostate Cancer Through Amplifying Mitotic Arrest Signaling. Front. Oncol. 2020, 10, 1274. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, S. Reposition of the Fungicide Ciclopirox for Cancer Treatment. PRA 2021, 16(2), 122–135. [Google Scholar] [CrossRef]
- Shi, Z.-D.; Pang, K.; Wu, Z.-X.; Dong, Y.; Hao, L.; Qin, J.-X.; Wang, W.; Chen, Z.-S.; Han, C.-H. Tumor Cell Plasticity in Targeted Therapy-Induced Resistance: Mechanisms and New Strategies. Sig Transduct Target Ther 2023, 8(1), 113. [Google Scholar] [CrossRef]
- Li, Q.; Deng, Q.; Chao, H.-P.; Liu, X.; Lu, Y.; Lin, K.; Liu, B.; Tang, G. W.; Zhang, D.; Tracz, A.; Jeter, C.; Rycaj, K.; Calhoun-Davis, T.; Huang, J.; Rubin, M. A.; Beltran, H.; Shen, J.; Chatta, G.; Puzanov, I.; Mohler, J. L.; Wang, J.; Zhao, R.; Kirk, J.; Chen, X.; Tang, D. G. Linking Prostate Cancer Cell AR Heterogeneity to Distinct Castration and Enzalutamide Responses. Nat Commun 2018, 9(1), 3600. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.G.; Hostetter, G.; Tang, L.; Frank, S.B.; Saboda, K.; Mehra, R; Wang, L. ; Li, X.; Keller, E.T.; Miranti, C. K. Notch3 Promotes Prostate Cancer-Induced Bone Lesion Development via MMP-3. Oncogene, 2020, 39, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Deng, L.; Li, C.; He, Q.; He, Y.; Hu, X.; Cai, Y.; Gan, Y. Loss of EHF Facilitates the Development of Treatment-Induced Neuroendocrine Prostate Cancer. Cell Death Dis 2021, 12(1), 46. [Google Scholar] [CrossRef] [PubMed]
- Püschel, J.; Dubrovska, A.; Gorodetska, I. The Multifaceted Role of Aldehyde Dehydrogenases in Prostate Cancer Stem Cells. Cancers 2021, 13(18), 4703. [Google Scholar] [CrossRef]
- Farah, E.; Li, C.; Cheng, l.; Kong, Y.; Lanman, N.A.; Pascuzzi, P.; Lorenz, G.R.; Zhang, Y.; Ahmad, N.; Li, L.; Ratliff, T.; Liu, X. NOTCH Signaling is Activated in and Contributes to Resistance in Enzalutamide-Resistant Prostate Cancer Cells. J Biol Chem 2019, 294(21), 8543–8554. [Google Scholar] [CrossRef]
- Han, H.; Park, C. K.; Choi, Y.-D.; Cho, N. H.; Lee, J.; Cho, K. S. Androgen-Independent Prostate Cancer Is Sensitive to CDC42-PAK7 Kinase Inhibition. Biomedicines 2022, 11, 101. [Google Scholar] [CrossRef]
- De Arao Tan, I.; Ricciardelli, C.; Russell, D. L. The Metalloproteinase ADAMTS1: A Comprehensive Review of Its Role in Tumorigenic and Metastatic Pathways: The Metalloproteinase ADAMTS1. Int. J. Cancer 2013, 133(10), 2263–2276. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garrido, O.; Peris-Torres, C.; Redondo-García, S.; Asenjo, H. G.; Plaza-Calonge, M. D. C.; Fernandez-Luna, J. L.; Rodríguez-Manzaneque, J. C. ADAMTS1 Supports Endothelial Plasticity of Glioblastoma Cells with Relevance for Glioma Progression. Biomolecules 2020, 11(1), 44. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Zabala-Letona, A.; Cortazar, A. R.; Astobiza, I.; Dominguez-Herrera, A.; Ercilla, A.; Crespo, J.; Viera, C.; Fernández-Ruiz, S.; Martinez-Gonzalez, A.; Torrano, V.; Martín-Martín, N.; Gomez-Muñoz, A.; Carracedo, A. Identification of Androgen Receptor Metabolic Correlome Reveals the Repression of Ceramide Kinase by Androgens. Cancers 2021, 13(17), 4307. [Google Scholar] [CrossRef]
- Ramalingam, S.; Eisenberg, A.; Foo, W. C.; Freedman, J.; Armstrong, A. J.; Moss, L. G.; Harrison, M. R. Treatment-Related Neuroendocrine Prostate Cancer Resulting in Cushing’s Syndrome. Int. J. Urol. 2016, 23(12), 1038–1041. [Google Scholar] [CrossRef]
- Ramos-Montoya, A.; Lamb, A. D.; Russell, R.; Carroll, T.; Jurmeister, S.; Galeano-Dalmau, N.; Massie, C. E.; Boren, J.; Bon, H.; Theodorou, V.; Vias, M.; Shaw, G. L.; Sharma, N. L.; Ross-Adams, H.; Scott, H. E.; Vowler, S. L.; Howat, W. J.; Warren, A. Y.; Wooster, R. F.; Mills, I. G.; Neal, D. E. HES6 Drives a Critical AR Transcriptional Programme to Induce Castration-resistant Prostate Cancer through Activation of an E 2 F 1-mediated Cell Cycle Network. EMBO Mol Med 2014, 6(5), 651–661. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, A.; Saini, S. Role of Transcription Factors and Chromatin Modifiers in Driving Lineage Reprogramming in Treatment-Induced Neuroendocrine Prostate Cancer. Front. Cell Dev. Biol. 2023, 11, 1075707. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Alabi, B. R.; Yin, Q.; Stoyanova, T. Molecular Mechanisms Underlying the Development of Neuroendocrine Prostate Cancer. Seminars in Cancer Biology 2022, 86, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Blee, A.; Huang, H. Lineage Plasticity-Mediated Therapy Resistance in Prostate Cancer. Asian J Androl 2019, 21(3), 241. [Google Scholar] [CrossRef] [PubMed]
- Hankey, W.; Zhong, C.; Qianben, W. Shaping Chromatin States in Prostate Cancer by Pioneer Transcription Factors. Cancer Research 2020, 80(12), 2427–36. [Google Scholar] [CrossRef] [PubMed]
- Baena, E.; Shao, Z.; Linn, D. E.; Glass, K.; Hamblen, M. J.; Fujiwara, Y.; Kim, J.; Nguyen, M.; Zhang, X.; Godinho, F. J.; Bronson, R. T.; Mucci, L. A.; Loda, M.; Yuan, G.-C.; Orkin, S. H.; Li, Z. ETV1 Directs Androgen Metabolism and Confers Aggressive Prostate Cancer in Targeted Mice and Patients. Genes Dev. 2013, 27(6), 683–698. [Google Scholar] [CrossRef] [PubMed]
- Sangphil, O.; Shin, S.; Song, H. ,. Grande, J.P.; Janknecht, R. Relationship between ETS Transcription Factor ETV1 and TGF-β-Regulated SMAD Proteins in Prostate Cancer. Scientific Reports, 2019, 9(1), 8186. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Elliott, B.; Johnson, R.; Khan, S. A. Alterations in TGFβ Signaling during Prostate Cancer Progression. Am J Clin Exp Urol 2021, 9(4), 318–328. [Google Scholar]
- Blanco Calvo, M.; Bolós Fernández, V.; Medina Villaamil, V.; Aparicio Gallego, G.; Díaz Prado, S.; Grande Pulido, E. Biology of BMP Signalling and Cancer. Clin Transl Oncol 2009, 11(3), 126–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




