Submitted:
15 November 2023
Posted:
16 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
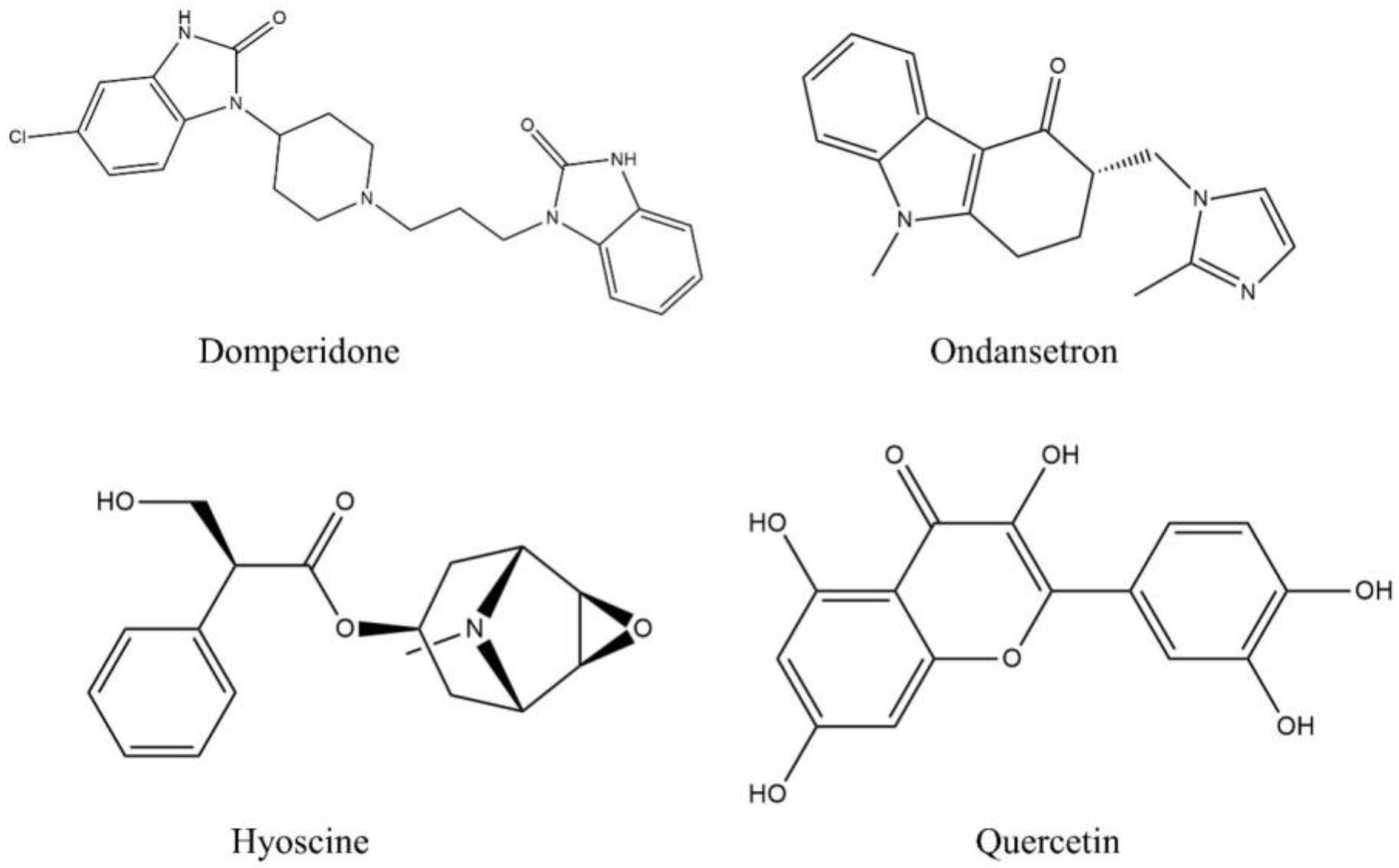
2.1. Chemical reagents and standards
2.2. Animals
2.3. In vivo investigation
2.3.1. CuSO4.5H2O-induced emesis model in broiler chicks
2.4. Statistical analysis
2.5. In silico investigation
2.5.1. Receptors selection and preparation
2.5.2. Ligand collection and preparation
2.5.3. Molecular docking protocol
2.5.4. Toxicity prediction
3. Results
3.1. In vivo investigation
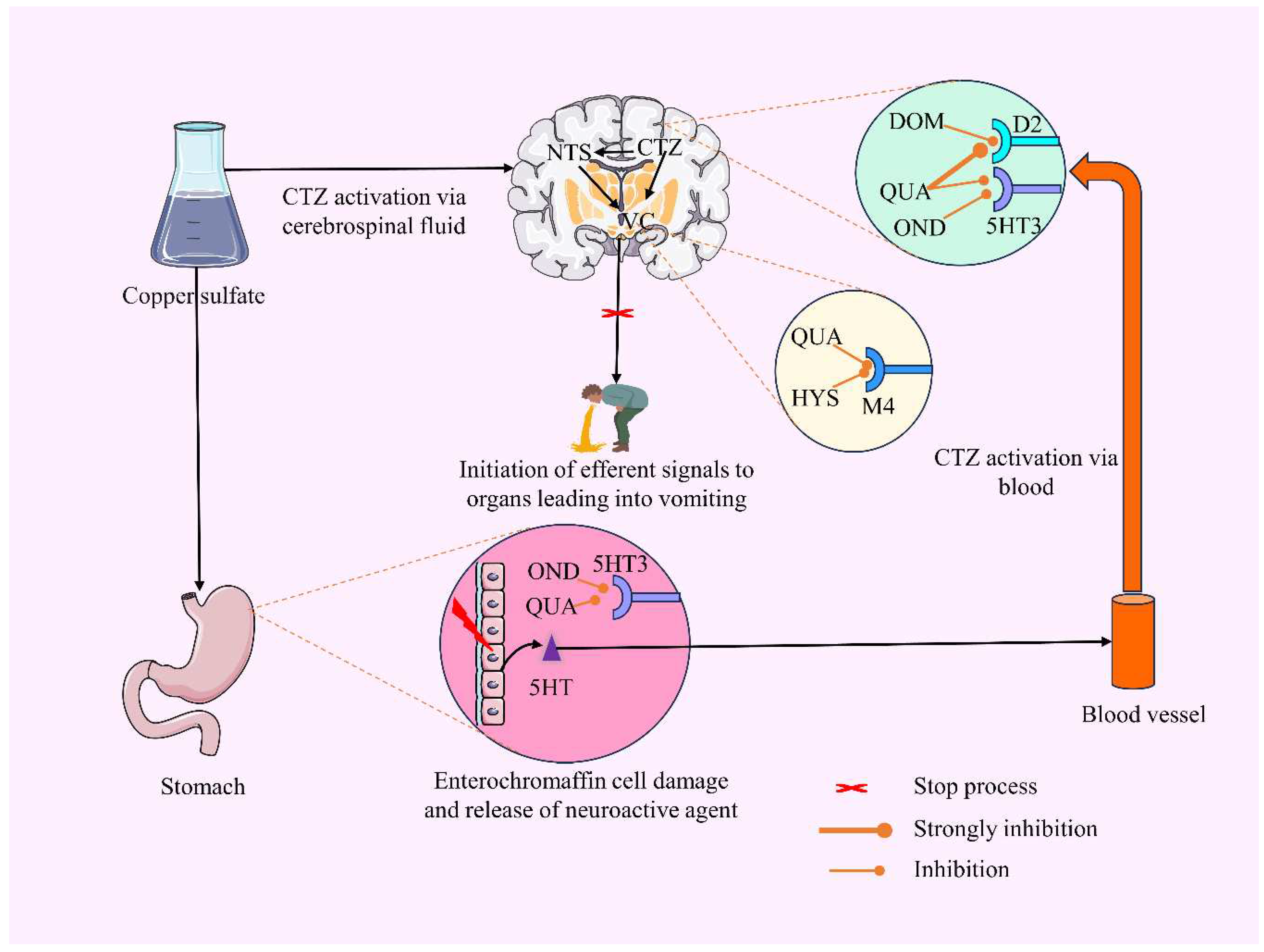
3.2. CuSO4.5H2O-induced emesis test
3.3. In silico investigation
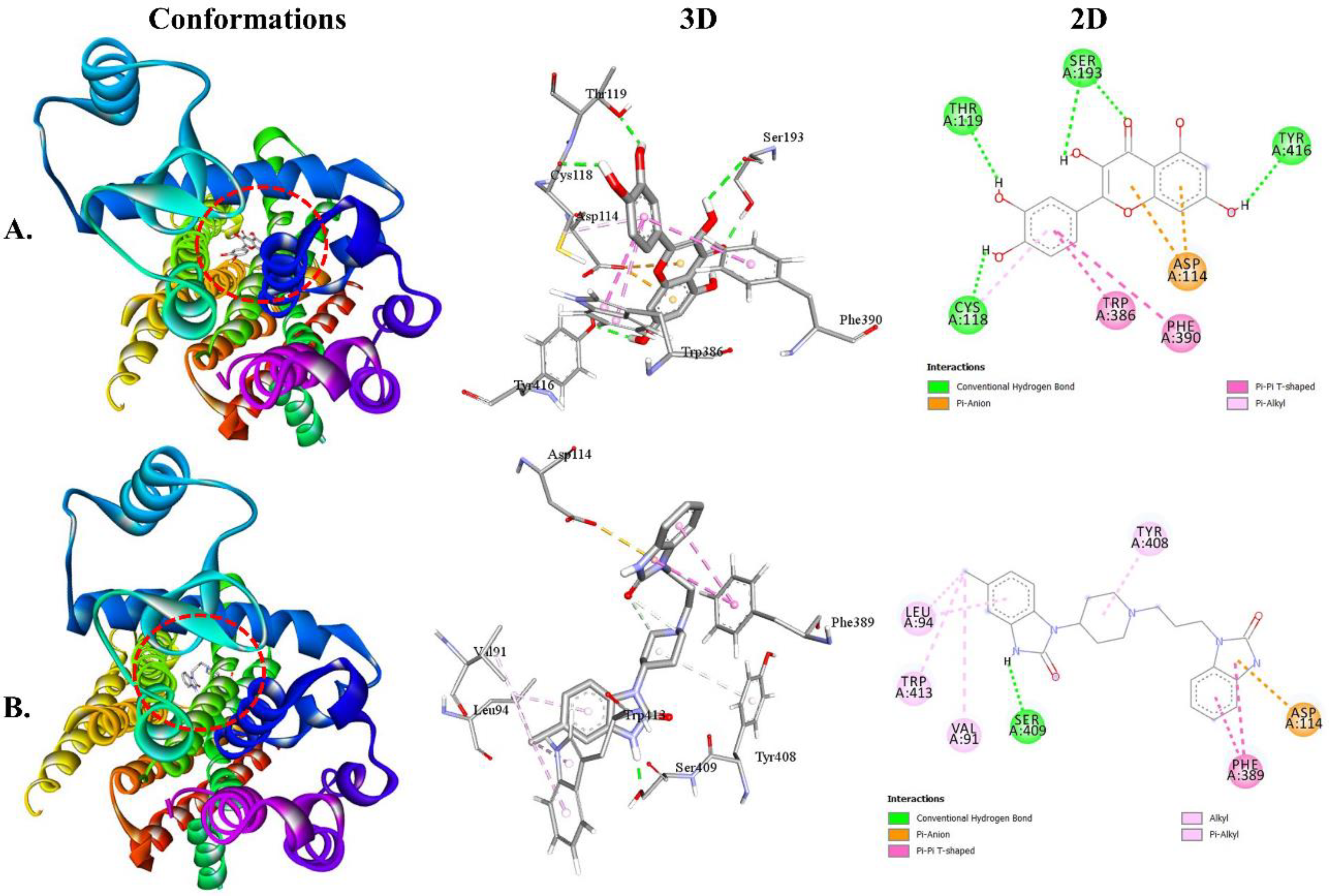
3.3.1. QUA and DOM with dopaminergic (D2 and D3) receptor interaction
3.3.2. QUA and OND with serotoninergic (5HT3) receptor interaction
3.3.3. QUA and HYS with muscarinic (M1, M2, M3, M4, and M5) receptor interaction
3.3.4. In silico toxicity predictions
| Properties | Parameters | QUA | DOM | OND | HYS |
|---|---|---|---|---|---|
| Toxicity | LD50 | 159 mg/kg | 715 mg/kg | 95 mg/kg | 1,275 mg/kg |
| Toxicity class | 3 | 4 | 3 | 4 | |
| Hepatotoxicity | Inactive | Inactive | Inactive | Inactive | |
| Carcinogenicity | Active | Inactive | Inactive | Inactive | |
| Immunotoxicity | Inactive | Active | Inactive | Inactive | |
| Mutagenicity | Active | Inactive | Active | Inactive | |
| Cytotoxicity | Inactive | Inactive | Inactive | Inactive |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, W.; Shahbaz, O.; Teskey, G.; Beever, A.; Kachour, N.; Venketaraman, V.; Darmani, N.A. Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems. Int. J. Mol. Sci. 2021, 22, 5797. [Google Scholar] [CrossRef]
- de la Puente-Redondo, V.; TINGLEY III, F.D.; Schneider, R.P.; Hickman, M.A. The Neurokinin-1 Antagonist Activity of Maropitant, an Antiemetic Drug for Dogs, in a Gerbil Model. J. Vet. Pharmacol. Ther. 2007, 30, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; Yates, B.J. What Is Nausea? A Historical Analysis of Changing Views. Auton. Neurosci. 2017, 202, 5–17. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, M.R.; Sharma, S. Physiology, Chemoreceptor Trigger Zone. In StatPearls [Internet]; StatPearls Publishing, 2022. [Google Scholar]
- Iqbal, I.M.; Spencer, R. Postoperative Nausea and Vomiting. Anaesth. Intensive Care Med. 2012, 13, 613–616. [Google Scholar] [CrossRef]
- Kobrinsky, N.L. Regulation of Nausea and Vomiting in Cancer Chemotherapy. A Review with Emphasis on Opiate Mediators. Am. J. Pediatr. Hematol. Oncol. 1988, 10, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Dodds, L.J. The Control of Cancer Chemotherapy-induced Nausea and Vomiting. J. Clin. Pharm. Ther. 1985, 10, 143–166. [Google Scholar] [CrossRef]
- Salunkhe, S.S.; Bhatia, N.M.; Kawade, V.S.; Bhatia, M.S. Development of Lipid Based Nanoparticulate Drug Delivery Systems and Drug Carrier Complexes for Delivery to Brain. J. Appl. Pharm. Sci. 2015, 5, 110–129. [Google Scholar] [CrossRef]
- Becker, D.E. Nausea, Vomiting, and Hiccups: A Review of Mechanisms and Treatment. Anesth. Prog. 2010, 57, 150–157. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Kamli, H.; Islam, T.; Sonia, F.A.; Kazi, M.A.; Siam, M.S.H.; Rahman, N.; Bappi, M.H.; Mia, M.N.; Hossen, M.M. Antiemetic Activity of Trans-Ferulic Acid Possibly through Muscarinic Receptors Interaction Pathway: In Vivo and in Silico Study. Results Chem. 2023, 101014. [Google Scholar] [CrossRef]
- Gan, T.J. Mechanisms Underlying Postoperative Nausea and Vomiting and Neurotransmitter Receptor Antagonist-Based Pharmacotherapy. CNS Drugs 2007, 21, 813–833. [Google Scholar] [CrossRef]
- Shames, B. Anatomy and Physiology of the Duodenum. In Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set; Elsevier, 2019; pp. 786–803. [Google Scholar] [CrossRef]
- 1Khan, I.A.; Aziz, A.; Sarwar, H.S.; Munawar, S.H.; Manzoor, Z.; Anwar, H. Evaluation of Antiemetic Potential of Aqueous Bark Extract of Cinnamon Loureiroi. Can. J. App. Sci 2014, 1, 26–32. [Google Scholar]
- Chang, T. Nausea and Vomiting. In Supportive Care in Pediatric Oncology: A Practical Evidence-Based Approach; Springer, 2014; pp. 159–175. [Google Scholar]
- Bhargava, K.P.; Dixit, K.S.; Gupta, Y.K. Enkephalin Receptors in the Emetic Chemoreceptor Trigger Zone of the Dog. Br. J. Pharmacol. 1981, 72, 471–475. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Sanger, G.J. Nausea and the Quest for the Perfect Anti-Emetic. Eur. J. Pharmacol. 2014, 722, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hasan, M.M.; Ahmed, S.W. Natural Antiemetics: An Overview. Pak. J. Pharm. Sci 2014, 27, 1583–1598. [Google Scholar]
- Denholm, L.; Gallagher, G. Physiology and Pharmacology of Nausea and Vomiting. Anaesth. Intensive Care Med. 2018, 19, 513–516. [Google Scholar] [CrossRef]
- McKarns, S.C. A Review of Neuroreceptors for Clinical and Experimental Neuropharmacology in Central Nervous System Disorders. Curr. Rev. Clin. Exp. Pharmacol. Former. Curr. Clin. Pharmacol. 2023, 18, 192–241. [Google Scholar] [CrossRef]
- Navari, R.M. Pharmacological Management of Chemotherapy-Induced Nausea and Vomiting: Focus on Recent Developments. Drugs 2009, 69, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.J.; Inall, F.C. The Physiology and Pharmacology of Postoperative Nausea and Vomiting. Anaesthesia 1994, 49, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Leslie, R.A. The Area Postrema and Vomiting. Front. Neuroendocrinol. 1994, 15, 301–320. [Google Scholar] [CrossRef]
- Hornby, P.J. Central Neurocircuitry Associated with Emesis. Am. J. Med. 2001, 111, 106–112. [Google Scholar] [CrossRef]
- Holmes, A.M.; Rudd, J.A.; Tattersall, F.D.; Aziz, Q.; Andrews, P.L.R. Opportunities for the Replacement of Animals in the Study of Nausea and Vomiting. Br. J. Pharmacol. 2009, 157, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Andrews, P.L.R. The Ferret in Nausea and Vomiting Research: Lessons in Translation of Basic Science to the Clinic. Biol. Dis. Ferret 2014, 735–778. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Krumm, B. E.; Zhuang, Y.; Mao, C.; Zhang, Y.; Wang, Y.; Huang, X. P.; Liu, Y. F.; He, X.; Li, H.; Yin, W.; Jiang, Y.; Zhang, Y.; Roth, B. L.; Xu, H. E. Structural genomics of the human dopamine receptor system. Cell res. 2023, 33, 604–616. [Google Scholar] [CrossRef]
- Haubrich, J.; Hagena, H.; Tsanov, M.; Manahan-Vaughan, D. Editorial: Dopaminergic control of experience encoding, memory and cognition. Front. Behave. Neurosci. 2023, 17, 1230576. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M. S.; Islam, T.; Rokonuzzman, M.; Shamsh Prottay, A. A.; Akter, F.; Hossain, M. I.; Chowdhury, R.; Kazi, M. A.; Khalipha, A. B. R.; Coutinho, H. D. M.; Islam, M. T. Modulatory effects of phytol on the antiemetic property of domperidone, possibly through the D2 receptor interaction pathway: in vivo and in silico studies. 3 Biotech 2023, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Darmani, N. A. Dopamine receptors in emesis: Molecular mechanisms and potential therapeutic function. Pharmacol. res. 2020, 161, 105124. [Google Scholar] [CrossRef] [PubMed]
- Mannoury la Cour, C.; Salles, M. J.; Pasteau, V.; Millan, M. J. Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D₂and D₃ receptors. Molecu. Pharmacol. 2011, 79, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hasan, M.M.; Ahmed, S.W.; Mahmood, Z.A.; Azhar, I.; Habtemariam, S. Anti-Emetic Effects of Bioactive Natural Products. Phytopharmacology 2013, 4, 390–433. [Google Scholar]
- Horn, C.C. The Physiology of Vomiting. Nausea vomiting diagnosis Treat. 2017, 15–25. [Google Scholar] [CrossRef]
- McNicol, E.; Horowicz-Mehler, N.; Fisk, R.A.; Bennett, K.; Gialeli-Goudas, M.; Chew, P.W.; Lau, J.; Carr, D. Management of Opioid Side Effects in Cancer-Related and Chronic Noncancer Pain: A Systematic Review. J. Pain 2003, 4, 231–256. [Google Scholar] [CrossRef]
- Athavale, A.; Athavale, T.; Roberts, D.M. Antiemetic Drugs: What to Prescribe and When. Aust. Prescr. 2020, 43, 49. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. Compr. Pharmacol. 2022, 408. [Google Scholar] [CrossRef]
- Muschietti, L. V; Ulloa, J.L.; Redko, F.D.C. The Role of Flavonoids as Modulators of Inflammation and on Cell Signaling Pathways. Nat. Prod. as Source Mol. with Ther. Potential Res. Dev. Challenges Perspect. 2018, 159–208. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Bhimanwar, R.; Kothapalli, L.; Khawshi, A. Quercetin as Natural Bioavailability Modulator: An Overview. Res. J. Pharm. Technol. 2020, 13, 2045–2052. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Updat. 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The Anti-Obesity Effect of Quercetin Is Mediated by the AMPK and MAPK Signaling Pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Hu, D.; Gu, X.; Wang, G.; Zhou, Z.; Sun, L.; Pei, J. Performance and Mechanism of Lignin and Quercetin as Bio-Based Anti-Aging Agents for Asphalt Binder: A Combined Experimental and Ab Initio Study. J. Mol. Liq. 2022, 359, 119310. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V. V; Watanabe, K. Molecular Targets of Quercetin with Anti-Inflammatory Properties in Atopic Dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic Effects of Quercetin in Streptozocin-Induced Diabetic Rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and Its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Jung, S.-M.; ngo Lee, J.-H.; Kim, J.-H.; Yoon, I.-S.; Lee, J.-H.; Choi, S.-H.; Lee, S.-M.; Chang, C.-G.; Kim, H.-C. Quercetin Inhibits the 5-Hydroxytryptamine Type 3 Receptormediated Ion Current by Interacting with Pre-Transmembrane Domain I. Mol. Cells (Springer Sci. Bus. Media BV) 2005, 20. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational Methods in Drug Discovery. Pharmacol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Singh, S.S. Preclinical Pharmacokinetics: An Approach towards Safer and Efficacious Drugs. Curr. Drug Metab. 2006, 7, 165–182. [Google Scholar] [CrossRef]
- Akita, Y.; Yang, Y.; Kawai, T.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Watanabe, K. New Assay Method for Surveying Anti-Emetic Compounds from Natural Sources. Nat. Prod. Sci. 1998, 4, 72–77. [Google Scholar]
- El-Mageed, H.R.A.; Abdelrheem, D.A.; Ahmed, S.A.; Rahman, A.A.; Elsayed, K.N.M.; Ahmed, S.A.; El-Bassuony, A.A.; Mohamed, H.S. Combination and Tricombination Therapy to Destabilize the Structural Integrity of COVID-19 by Some Bioactive Compounds with Antiviral Drugs: Insights from Molecular Docking Study. Struct. Chem. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.U.; Zoete, V.; Michielin, O.; Guex, N. Defining and Searching for Structural Motifs Using DeepView/Swiss-PdbViewer. BMC Bioinformatics 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ali, M.T.; Shawan, M.M.A.K.; Sarwar, M.G.; Khan, M.A.K.; Halim, M.A. Halogen-Directed Drug Design for Alzheimer’s Disease: A Combined Density Functional and Molecular Docking Study. Springerplus 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Sarkar, C.; Hassan, S.M.H.; Khan, R.A.; Arman, M.; Ray, P.; Islam, M.T.; Daştan, S.D.; Sharifi-Rad, J.; Almarhoon, Z.M. In Silico Screening of Natural Products as Potential Inhibitors of SARS-CoV-2 Using Molecular Docking Simulation. Chin. J. Integr. Med. 2022, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Badr, E.A.A.; Almansour, N.M.; Alzahrani, O.R.; Ahmed, M.N.; Soliman, M.E.S.; Naeem, M.A.; Shawky, A.M.; Sidhom, P.A. Naturally Occurring Plant-Based Anticancerous Candidates as Prospective ABCG2 Inhibitors: An in Silico Drug Discovery Study. Mol. Divers. 2022, 26, 3255–3277. [Google Scholar] [CrossRef] [PubMed]
- AVS, S.K.; Sinha, S.; Donakonda, S. Virus-Host Interaction Analysis in Colorectal Cancer Identifies Core Virus Network Signature and Small Molecules. Comput. Struct. Biotechnol. J. 2022, 20, 4025–4039. [Google Scholar] [CrossRef]
- Dey, D.; Hossain, R.; Biswas, P.; Paul, P.; Islam, M.A.; Ema, T.I.; Gain, B.K.; Hasan, M.M.; Bibi, S.; Islam, M.T. Amentoflavone Derivatives Significantly Act towards the Main Protease (3CLPRO/MPRO) of SARS-CoV-2: In Silico Admet Profiling, Molecular Docking, Molecular Dynamics Simulation, Network Pharmacology. Mol. Divers. 2023, 27, 857–871. [Google Scholar] [CrossRef]
- Hossain, R.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Islam, M.S.; Dey, D.; Jain, D.; Faria, F.; Akbor, R.; Atolani, O. Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through Gabaa and Gabab Receptor Interaction Pathway. Pharmaceuticals 2021, 14, 721. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Adane, F.; Assefa, W.; Alem, M.B.; Dessalegn, M. Sub-Chronic Toxicity of the Aqueous Leaf Extract of Ocimum Lamiifolium Hochst. Ex Benth on Biochemical Parameters and Histopathology of Liver and Kidney in Rats: In Vivo and in-Silico Toxicity Studies. BMC Complement. Med. Ther. 2023, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Brianna; Lee, S. H. Chemotherapy: How to Reduce Its Adverse Effects While Maintaining the Potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Gelberg, H. Pathophysiological Mechanisms of Gastrointestinal Toxicity. Compr. Toxicol. 2018, 139. [Google Scholar] [CrossRef]
- Wallig, M.A. Digestive System. In Fundamentals of Toxicologic Pathology; Elsevier, 2018; pp. 395–442. [Google Scholar]
- Horn, C.C.; Meyers, K.; Lim, A.; Dye, M.; Pak, D.; Rinaman, L.; Yates, B.J. Delineation of Vagal Emetic Pathways: Intragastric Copper Sulfate-Induced Emesis and Viral Tract Tracing in Musk Shrews. Am. J. Physiol. Integr. Comp. Physiol. 2014, 306, R341–R351. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.; Camilleri, M.; Sifrim, D.; Houghton, L.A.; Elsenbruch, S.; Lindberg, G.; Azpiroz, F.; Parkman, H.P. Fundamentals of Neurogastroenterology: Physiology/Motility–Sensation. Gastroenterology 2016, 150, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Rocco, A.; Mandatori, I.; Mattia, C. Non-Analgesic Effects of Opioids: Opioid-Induced Nausea and Vomiting: Mechanisms and Strategies for Their Limitation. Curr. Pharm. Des. 2012, 18, 6043–6052. [Google Scholar] [CrossRef]
- Babic, T.; Browning, K.N. The Role of Vagal Neurocircuits in the Regulation of Nausea and Vomiting. Eur. J. Pharmacol. 2014, 722, 38–47. [Google Scholar] [CrossRef]
- Sanger, G.J.; Andrews, P.L.R. An Analysis of the Pharmacological Rationale for Selecting Drugs to Inhibit Vomiting or Increase Gastric Emptying during Treatment of Gastroparesis. Aliment. Pharmacol. Ther. 2023, 57, 962–978. [Google Scholar] [CrossRef]
- Khani, A.; Oskuyi, A.E.; Asghari, R.; Khalkhli, H.R.; Sharifi, H. Olanzapine Enhances the Effect of Conventional Drugs in Chemotherapy Inducing Nausea and Vomiting: A Randomized Clinical Trial. Casp. J. Intern. Med. 2022, 13, 356. [Google Scholar] [CrossRef]
- Mitchelson, F. Pharmacological Agents Affecting Emesis: A Review (Part I). Drugs 1992, 43, 295–315. [Google Scholar] [CrossRef]
- Jacoby, H.I. Safety Pharmacology and the GI Tract. Toxicol. Gastrointest. Tract 2018, 53–81. [Google Scholar]
- Kumar, D.; Jain, V.; Rai, B. Capturing the Synergistic Effects between Corrosion Inhibitor Molecules Using Density Functional Theory and ReaxFF Simulations-A Case for Benzyl Azide and Butyn-1-Ol on Cu Surface. Corros. Sci. 2022, 195, 109960. [Google Scholar] [CrossRef]
- Perwitasari, D.A.; Gelderblom, H.; Atthobari, J.; Mustofa, M.; Dwiprahasto, I.; Nortier, J.W.R.; Guchelaar, H.-J. Anti-Emetic Drugs in Oncology: Pharmacology and Individualization by Pharmacogenetics. Int. J. Clin. Pharm. 2011, 33, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Borison, H.L. Copper Sulphate Emesis: A Study of Afferent Pathways from the Gastrointestinal Tract. Am. J. Physiol. Content 1951, 164, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Niijima, A.; Jiang, Z.-Y.; Daunton, N.G.; Fox, R.A. Effect of Copper Sulphate on the Rate of Afferent Discharge in the Gastric Branch of the Vagus Nerve in the Rat. Neurosci. Lett. 1987, 80, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Krovat, E.M.; Steindl, T.; Langer, T. Recent Advances in Docking and Scoring. Curr. Comput. Aided. Drug Des. 2005, 1, 93–102. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Rokonuzzman, M.; Hossain, M.I.; Ansari, S.A.; Ansari, I.A.; Islam, T.; Al Hasan, M.S.; Mubarak, M.S.; Islam, M.T. Anxiolytic-like Effects by trans-Ferulic Acid Possibly Occur through GABAergic Interaction Pathways. Pharmaceuticals 2023, 16, 1271. [Google Scholar] [CrossRef] [PubMed]
- Plewczynski, D.; Łaźniewski, M.; Augustyniak, R.; Ginalski, K. Can We Trust Docking Results? Evaluation of Seven Commonly Used Programs on PDBbind Database. J. Comput. Chem. 2011, 32, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Briggs, D.B.; Strominger, N.L. Mechanisms of Radiation-Induced Emesis in the Dog. Pharmacol. Ther. 1988, 39, 367–371. [Google Scholar] [CrossRef]
- Belkacemi, L.; Darmani, N.A. Dopamine Receptors in Emesis: Molecular Mechanisms and Potential Therapeutic Function. Pharmacol. Res. 2020, 161, 105124. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Morita, M.; Horii, A.; Nishiike, S.; Kitahara, T.; Uno, A. Neural Mechanisms of Motion Sickness. J. Med. Investig. 2001, 48, 44–59. [Google Scholar]
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, E.A.; Joseph, P.F. The Economics of Biosimilars. Am. Heal. drug benefits 2013, 6, 469. [Google Scholar]
- Ruggeri, B.A.; Camp, F.; Miknyoczki, S. Animal Models of Disease: Pre-Clinical Animal Models of Cancer and Their Applications and Utility in Drug Discovery. Biochem. Pharmacol. 2014, 87, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.; Laggner, C.; Langer, T. Why Drugs Fail-a Study on Side Effects in New Chemical Entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Aktar, M.A.; Chowdhury, R.; Ferdous, J.; Rahman, M.A.; Hasan, M.S.A.; Islam, M.T. Therapeutic potentials of ononin with mechanistic insights: A comprehensive review. Fo. Biosci. 2023, 2023, 103302. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Medina-Franco, J.L.; Kuca, K.; Valko, M. IN SILICO METHODS FOR DRUG DESIGN AND DISCOVERY. [CrossRef]
- White, H.S. Clinical Significance of Animal Seizure Models and Mechanism of Action Studies of Potential Antiepileptic Drugs. Epilepsia 1997, 38, S9–S17. [Google Scholar] [CrossRef]
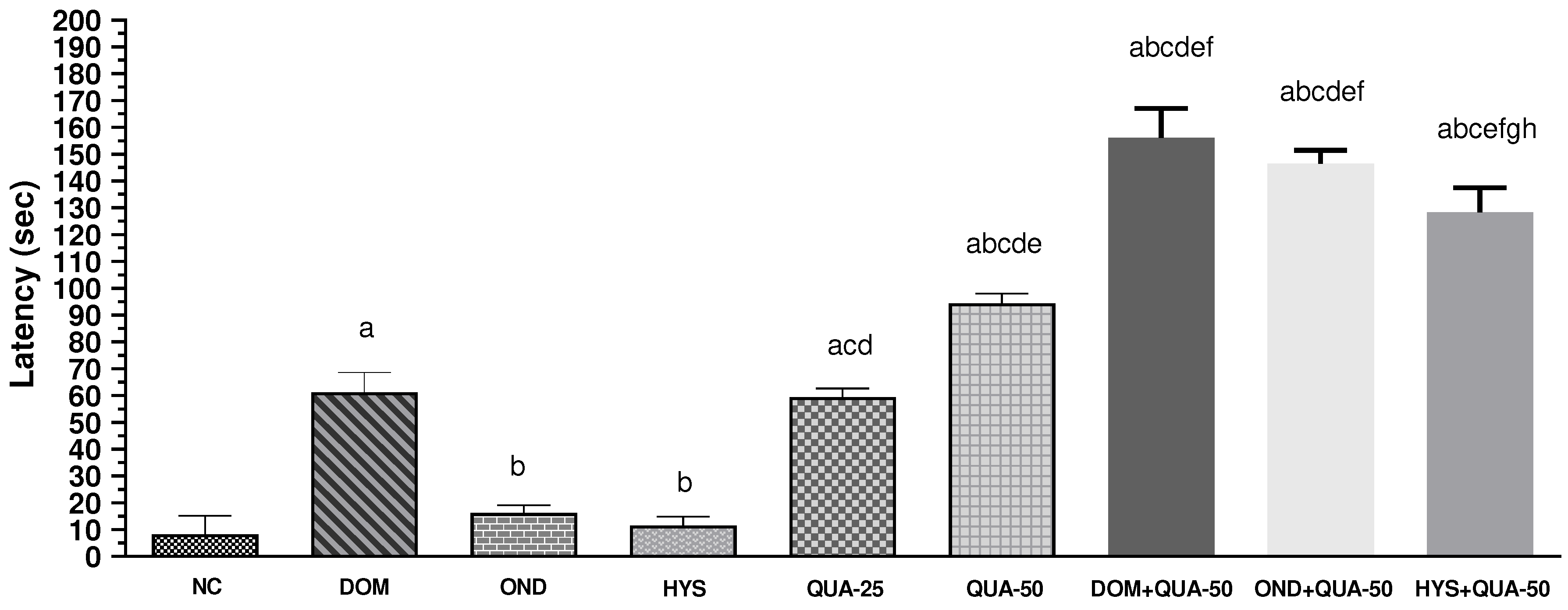
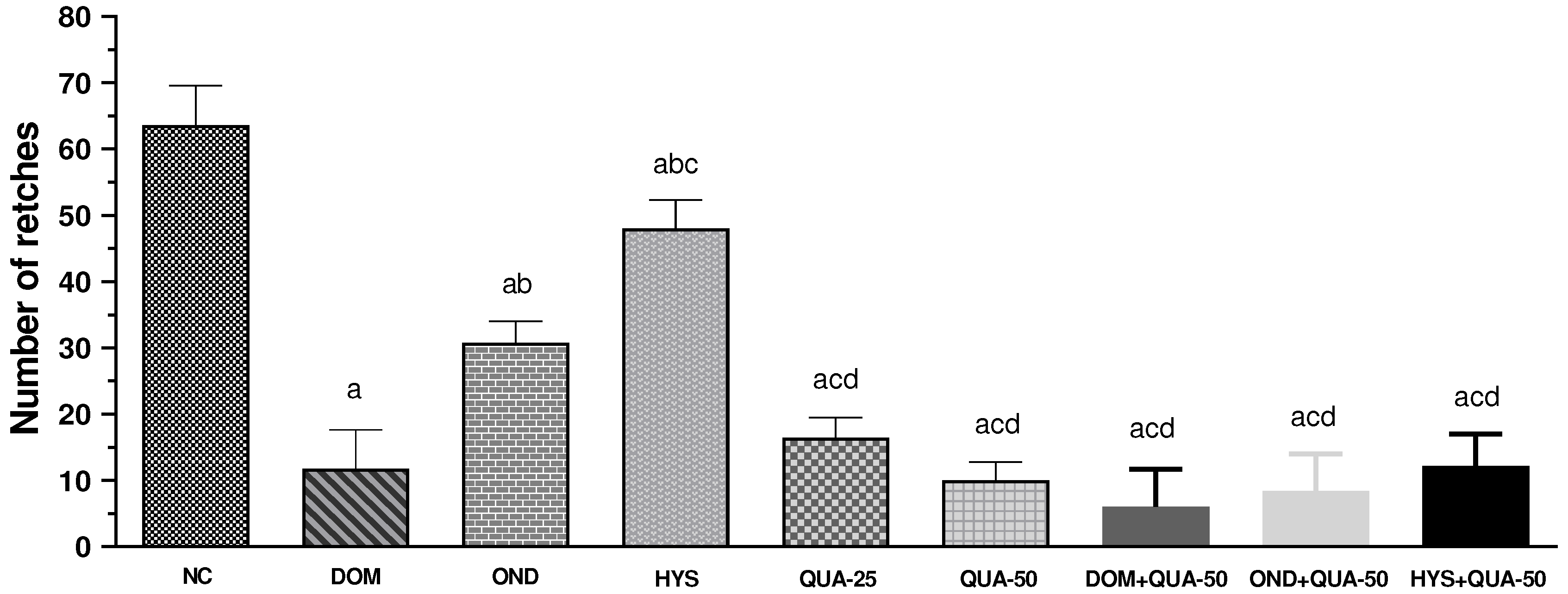
| Name of group | %Increase in latency | %Decrease in retches |
| NC (vehicle) | - | - |
| DOM | 86.48 | 81.63 |
| OND | 48.98 | 52.06 |
| HYS | 28.26 | 24.00 |
| QUA-50 | 91.25 | 84.43 |
| QUA-25 | 86.08 | 74.32 |
| DOM+QUA-50 | 94.73 | 90.66 |
| OND+QUA-50 | 94.36 | 87.04 |
| HYS+QUA-50 | 93.56 | 81.01 |
| Receptors | Binding Affinity | No. of HB | HB residues | HB length (Å) | Other bond residues |
| D2-QUA | -9.7 | 4 | SER193 TYR416 THR119 CYS118 |
1.85 2.20 2.14 2.18 |
ASP114 TRP386 PHE390 |
| D3-QUA | -8.5 | 3 | VAL189 TYR365 VAL111 |
2.36 2.09 3.56 |
ASP110 ILE183 PHE345 HIS349 VAL107 ILE183 |
| D2-DOM | -9.8 | 1 | SER409 | 1.89 |
ASP114 PHE389 VAL91 LEU94 TYR408 TRP413 |
| D3-DOM | -9.4 | 3 | GLY1107 VAL1103 PHE1104 |
2.83 1.77 3.53 |
GLU1011 ASP1020 LEU1032 ALA1074 |
| Receptors | Binding Affinity | No. of HB | HB residues | HB length (Å) | Other bond residues |
|---|---|---|---|---|---|
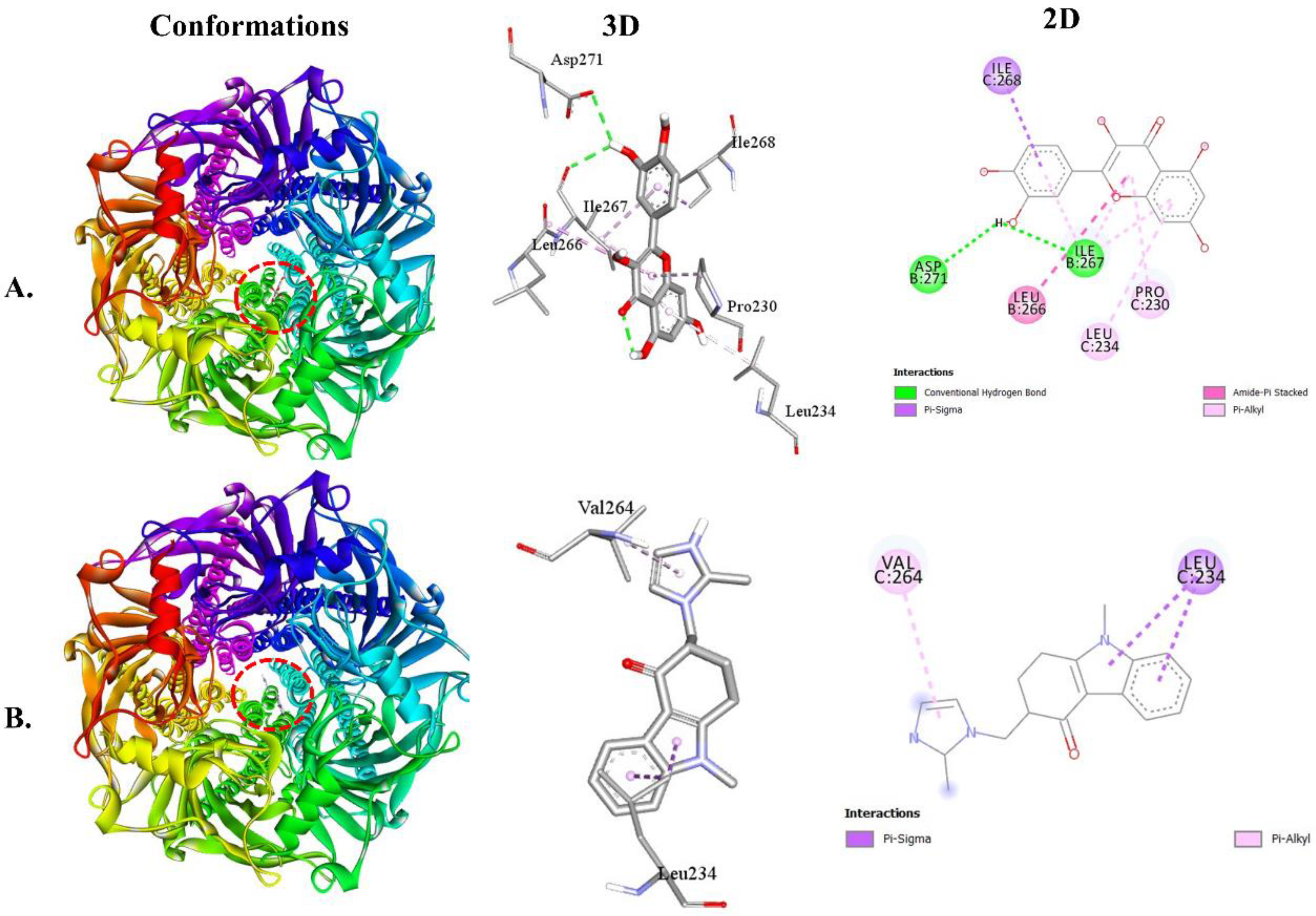
| 5HT3-QUA | -7.9 | 2 | ILE267 ASP271 |
2.51 2.95 |
ILE268 LEU266 ILE267 LEU234 PRO230 |
| 5HT3-OND | -8.1 |
- | - | - | LEU234 VAL264 |
| Receptors | Binding Affinity | No. of HB | HB residues | HB length (Å) | Other bond residues |
|---|---|---|---|---|---|
| M1-QUA | -7.8 |
2 | TYR106 ILE180 |
2.03 2.77 |
TYR404 |
| M2-QUA | -8.2 |
2 | PHE181 TYR104 |
2.43 1.96 |
TRP422 |
| M3-QUA | -9 |
2 | TRP206 ARG183 |
2.08 3.22 |
ARG171 MET187 VAL210 TYR175 |
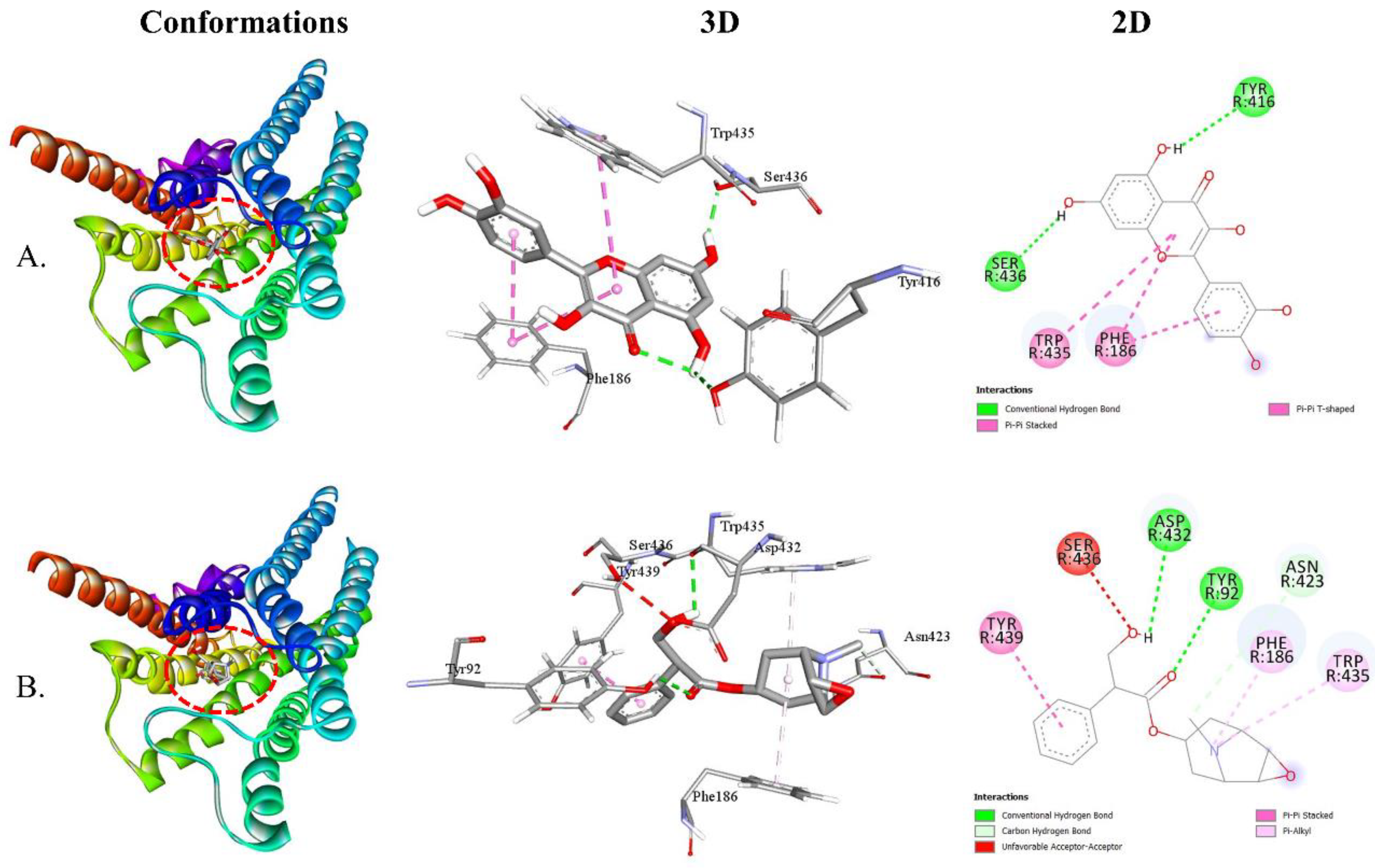
| M4-QUA | -9.2 |
2 | TYR416 SER436 |
2.54 2.06 |
PHE186 TRP435 |
| M5-QUA | -8 |
2 | ILE185 HIS478 |
2.76 2.19 |
TYR481 TRP477 |
| M1-HYS | -7.1 | 4 | THR189 CYS178 TYR404 LEU183 |
2.01 2.33 3.62 3.41 |
TYR179 |
| M2-HYS | -7.5 | - | - | - | TYR426 |
| M3-HYS | -8.6 |
2 | ASN507 TYR529 |
2.26 3.47 |
CYS532 TYR148 TRP503 TYR506 ALA235 |
| M4-HYS | -8.9 |
3 | TYR92 ASP432 ASN423 |
2.81 2.71 3.70 |
TYR439 PHE186 TRP435 |
| M5-HYS | -7.7 |
ILE185 HIS478 SER189 |
1.93 2.62 2.95 |
TRP477 TYR481 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





