Submitted:
15 November 2023
Posted:
16 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
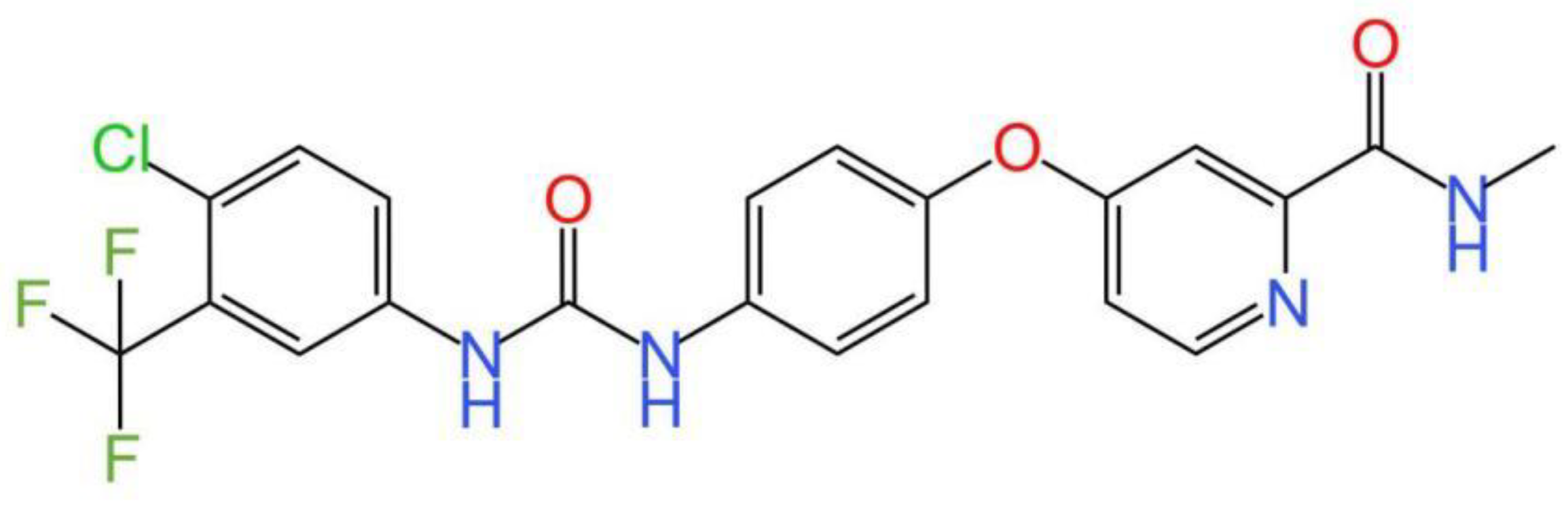
2. Experimental Section
2.1. Materials and Apparatus
2.2. Polymorphs Screening Experiments
2.2.1. Preparations of form A
2.2.2. Preparations of form C
2.2.3. Preparations of form D
2.2.4. Preparations of form X
2.2.5. Preparations of amorphous
2.3. Co-crystal Screening Experiments
2.3.1. Preparations of Co-crystals
2.3.2. Preparations of Physical Mixtures
2.3.3. Stoichiometry Studies of SF Co-crystals
2.4. Single Crystal Growth
2.5. Solid characterization
2.5.1. Powder X-ray Diffraction (PXRD)
2.5.2. Single-Crystal X-ray Diffraction (SCXRD).
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Thermogravimetric Analysis (TGA)
2.5.5. Polarizing Light Microscopy and Hot-Stage Polarizing Light Microscopy (PLM and HSPLM)
2.5.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5.7. High-Performance Liquid Chromatography (HPLC)
2.6. Solubility and In-vitro Dissolution Experiments
2.6.1. Solubility and Kinetic Solubility Experiments
2.6.2. In-vitro Dissolution Experiments
2.7. Stability Test
3. Results and Discussion
3.1. Experimental Results
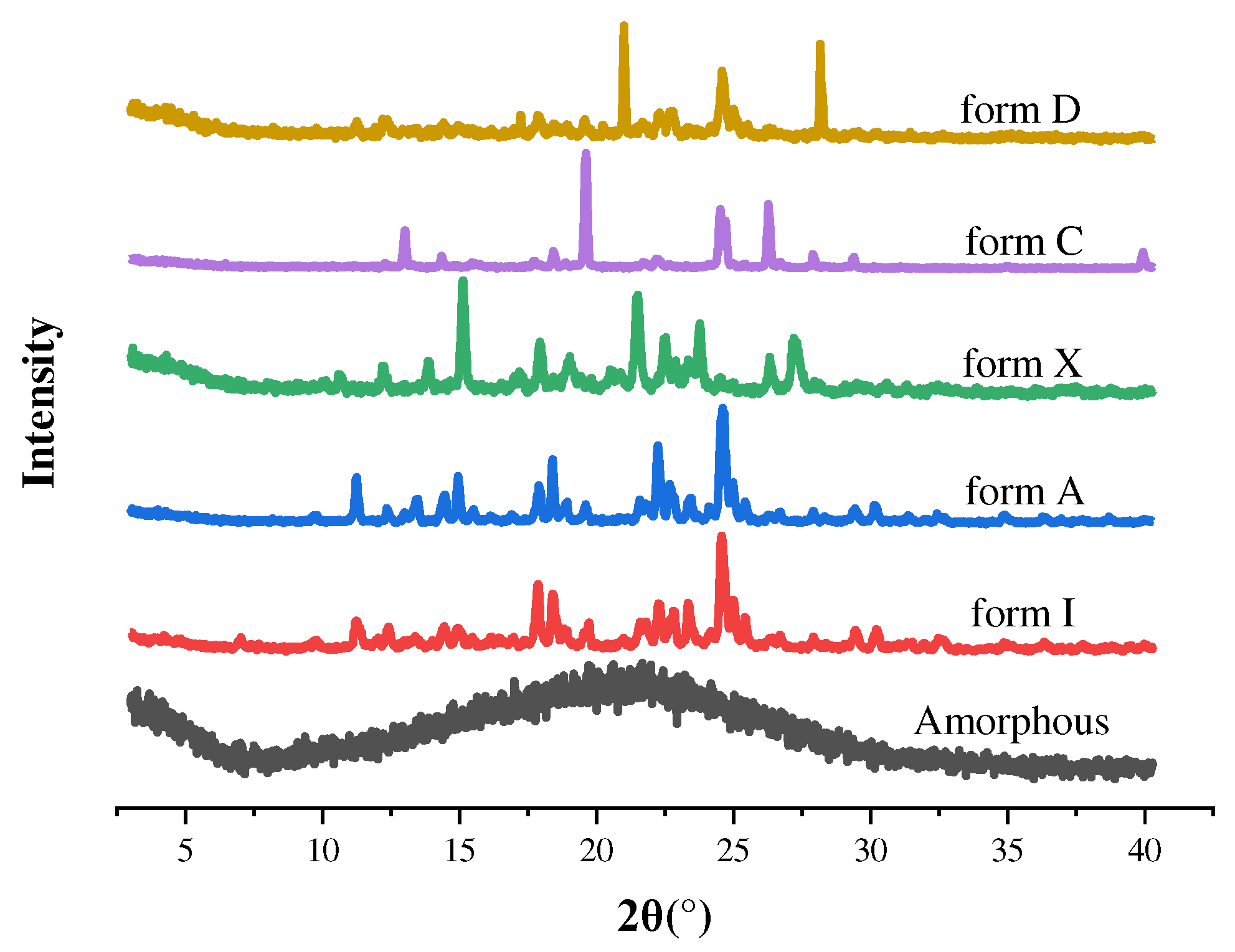
3.1.1. Solid-state Form Screening Results
3.1.2. Stoichiometry Studies of SF Co-crystals
3.2. Results of Characterization
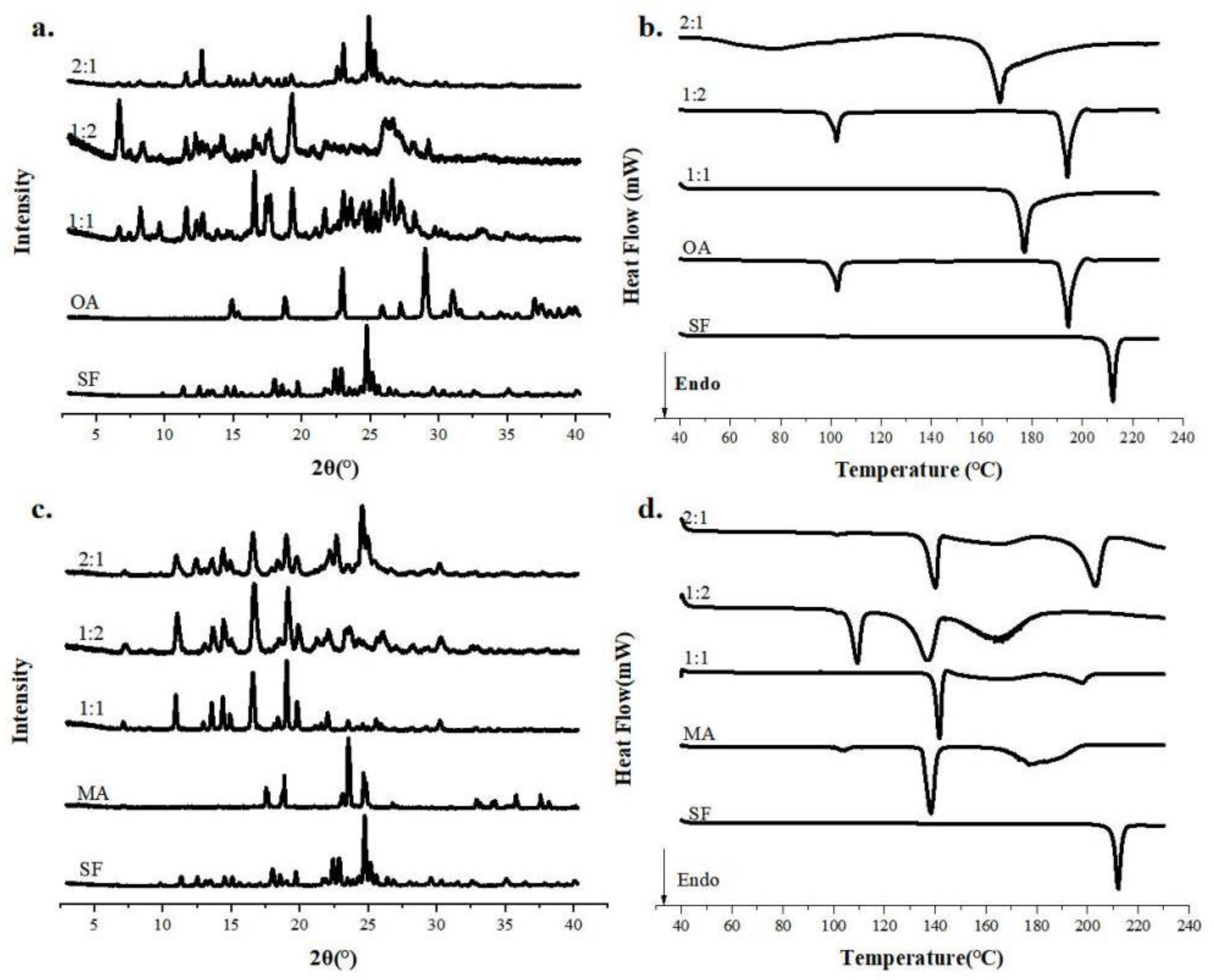
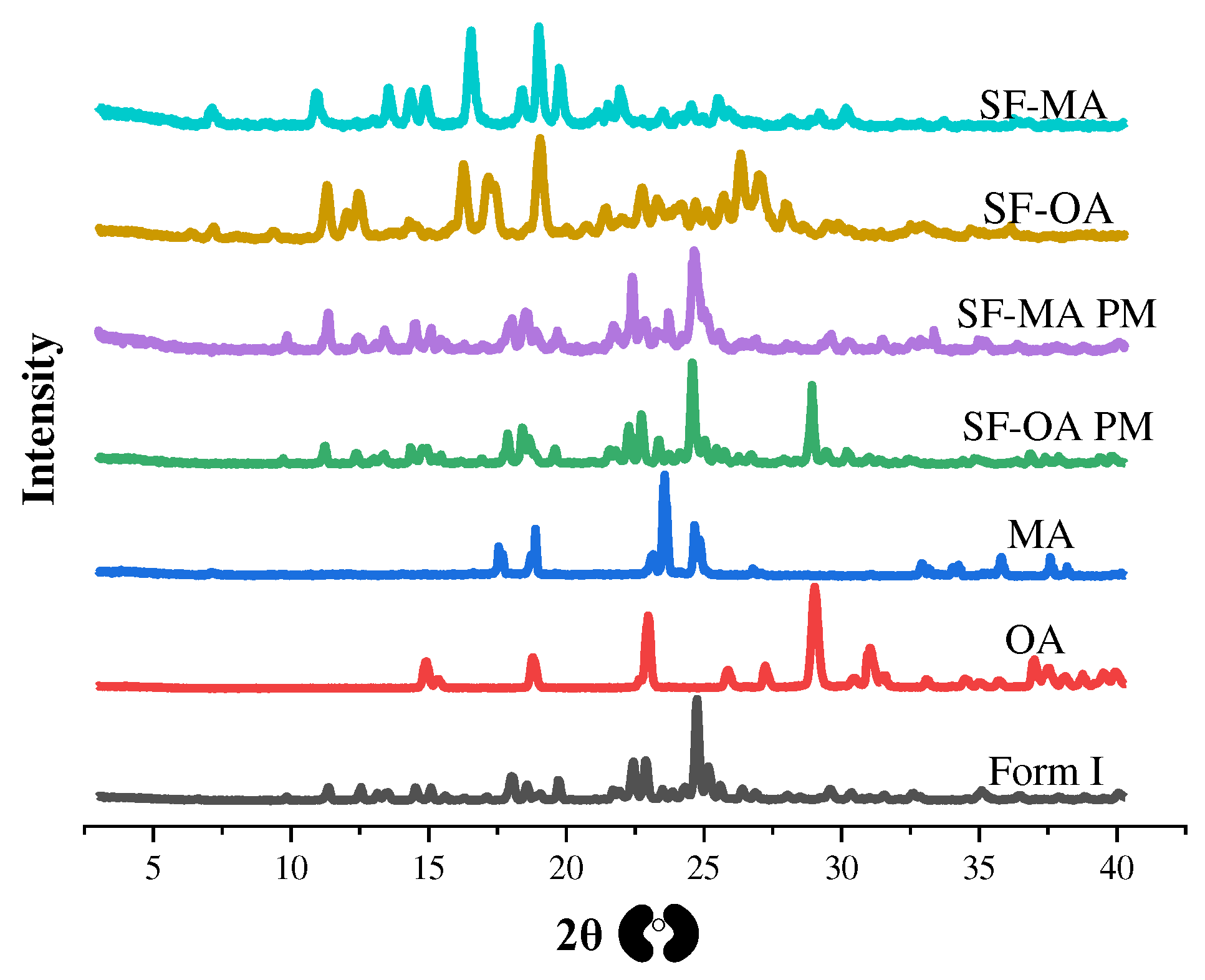
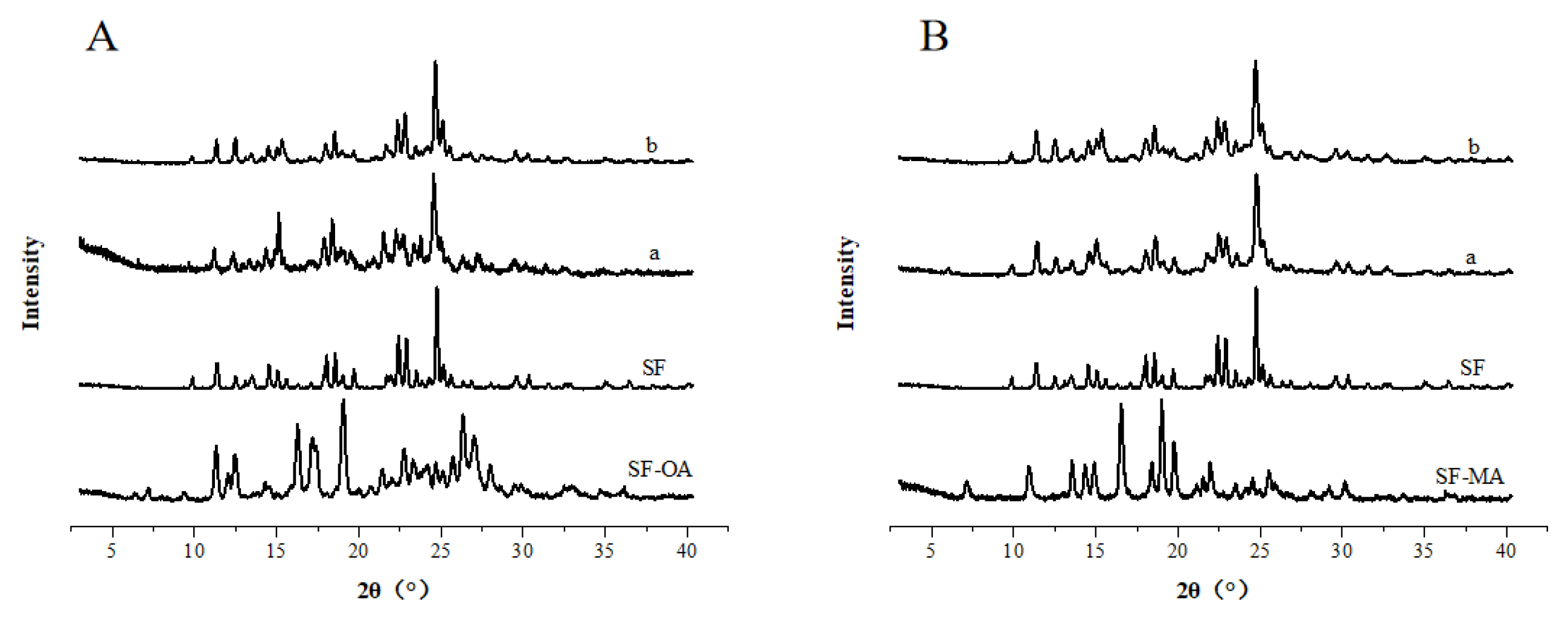
3.2.1. PXRD Patterns
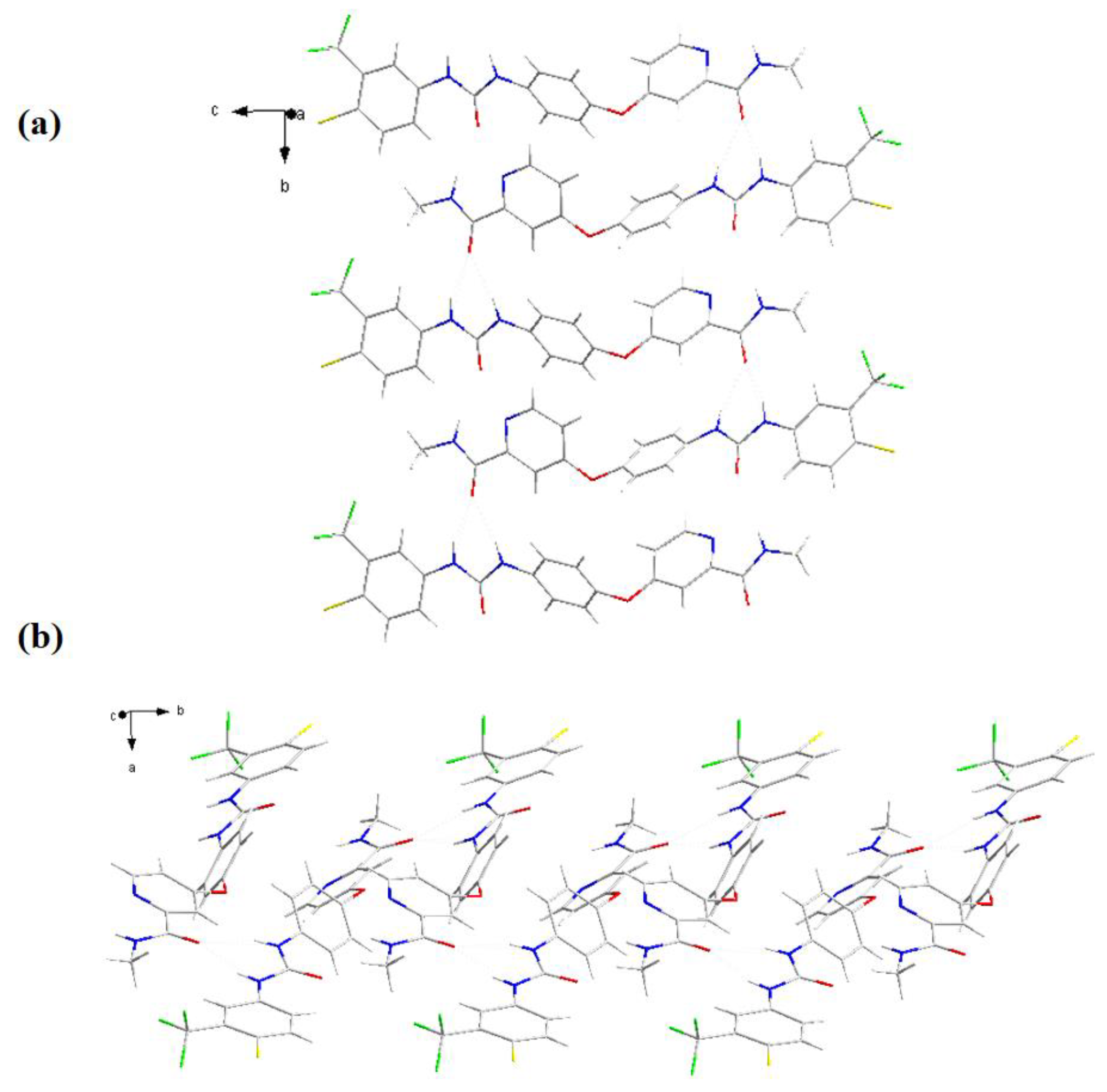
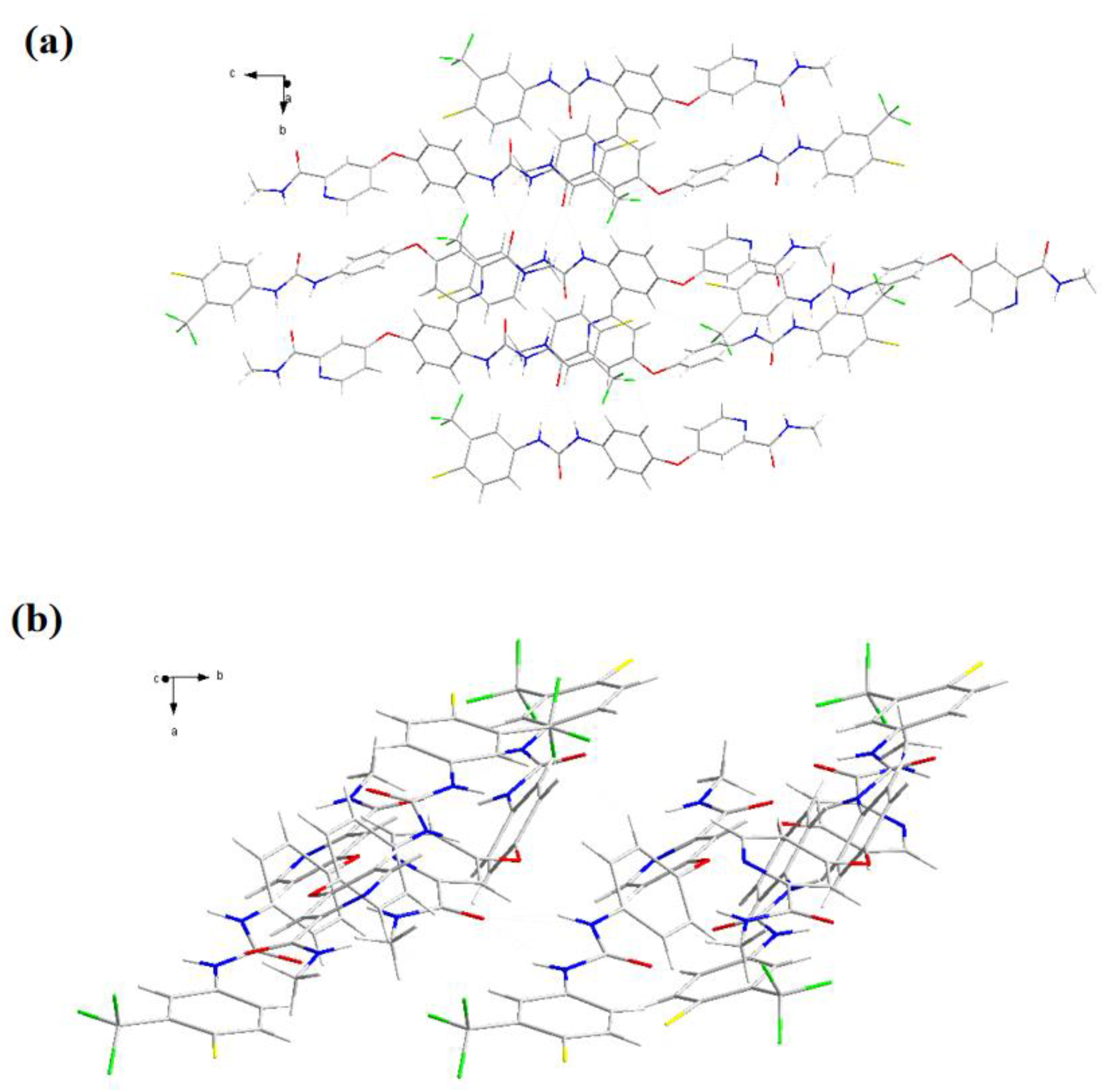
3.2.2. SCXRD
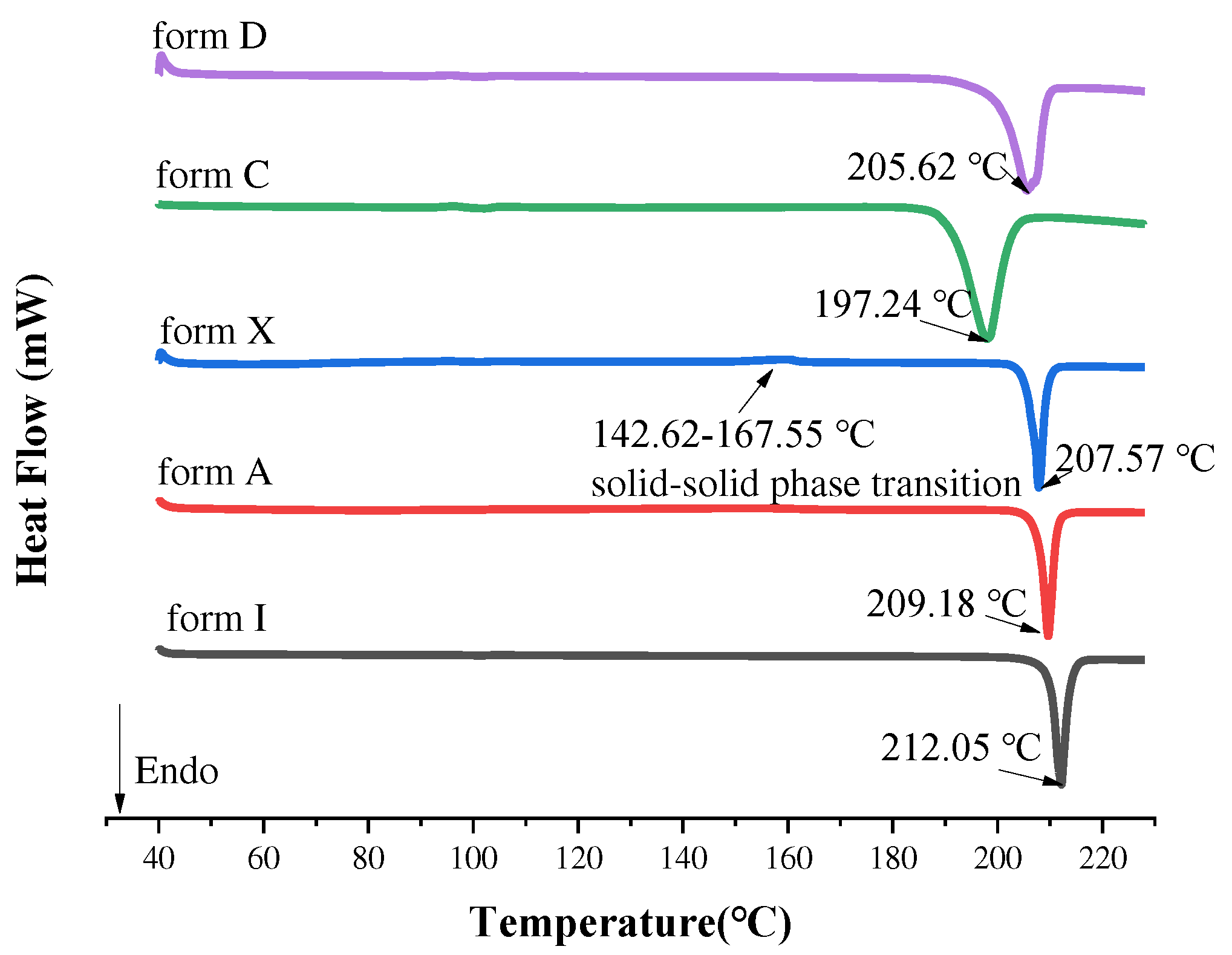
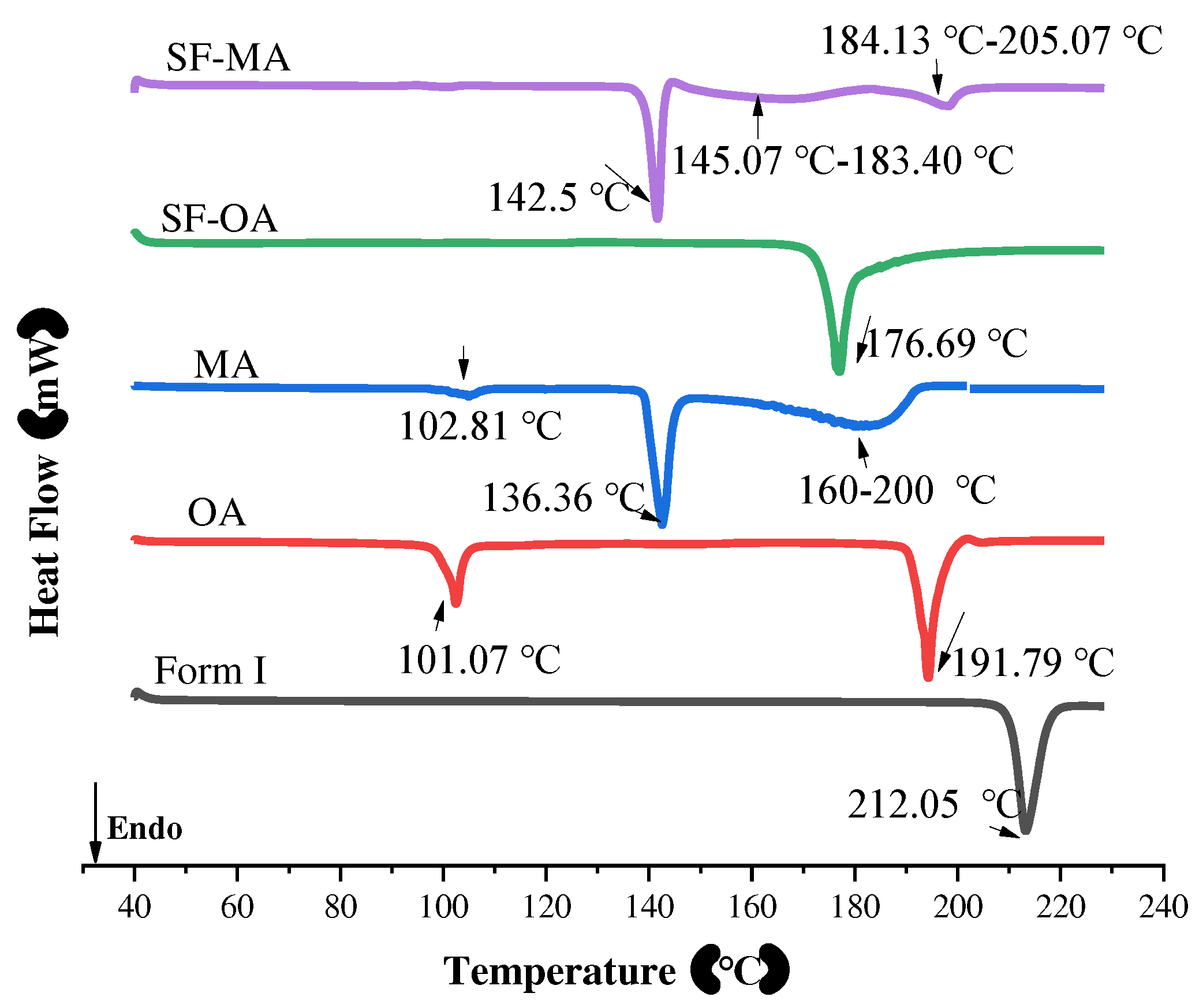
3.2.3. DSC
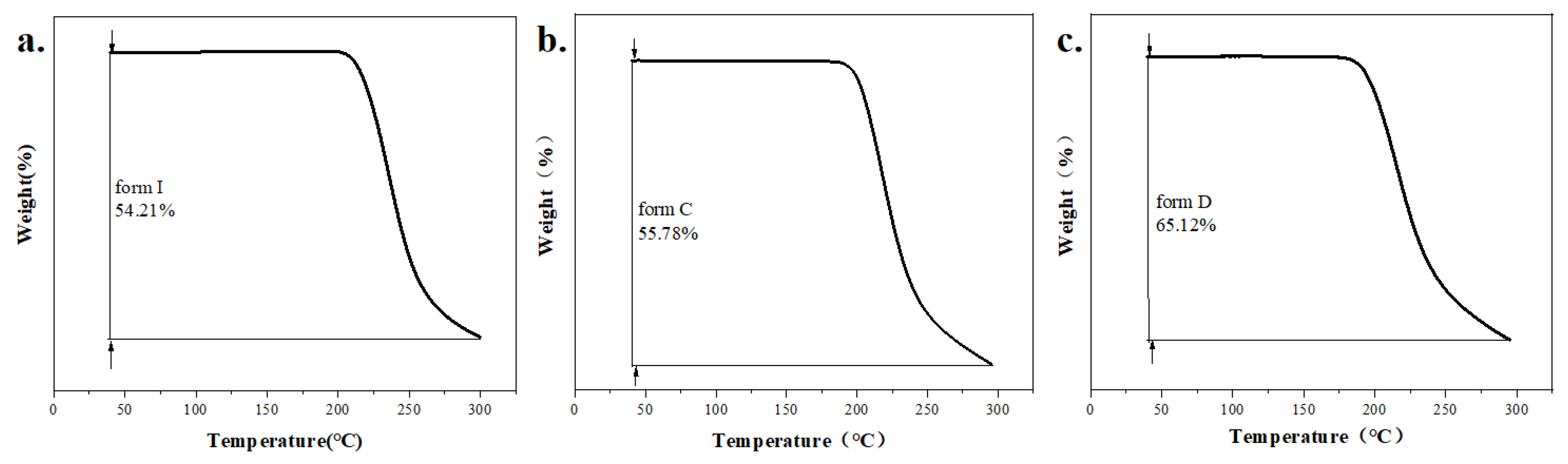
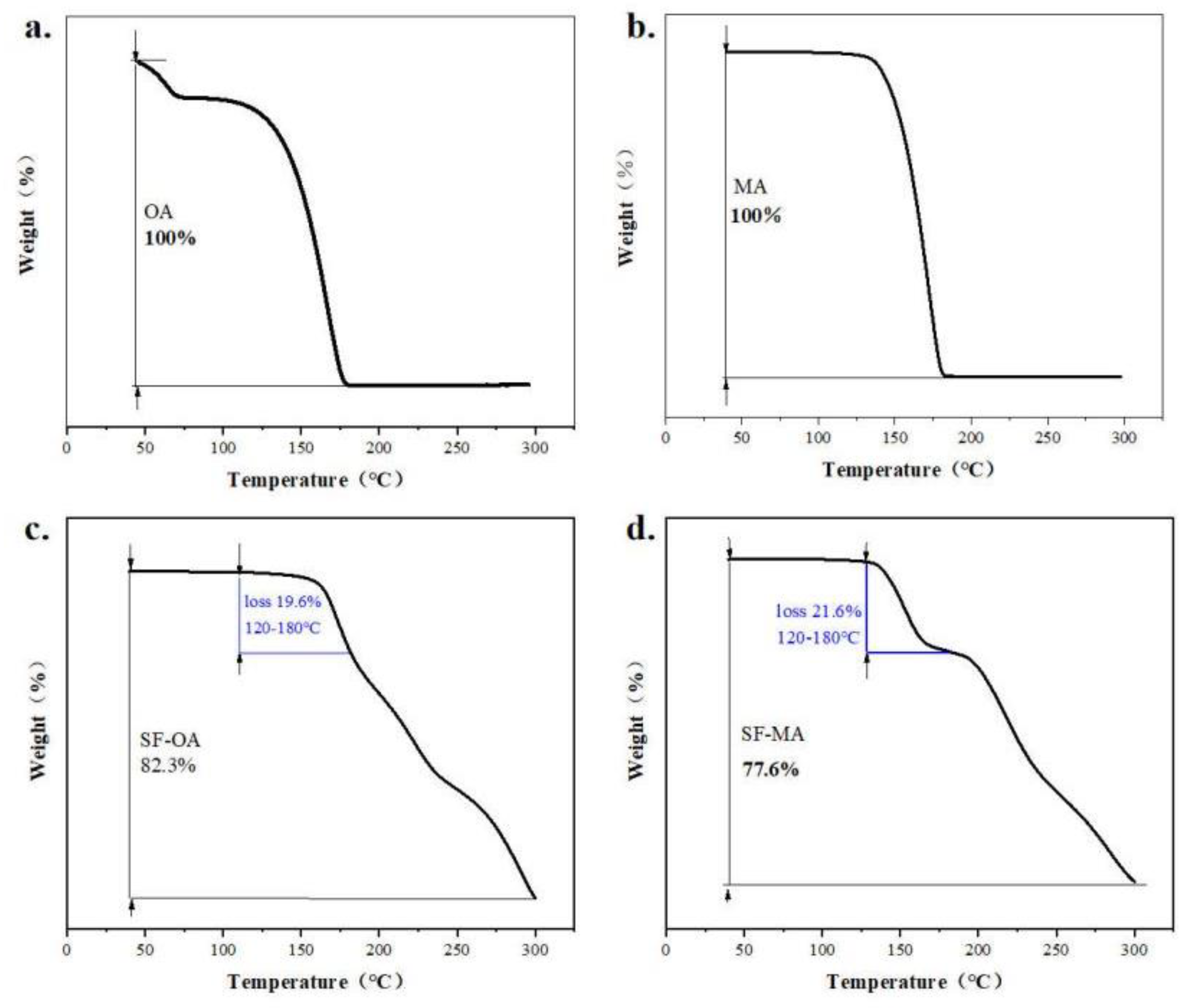
3.2.4. TGA
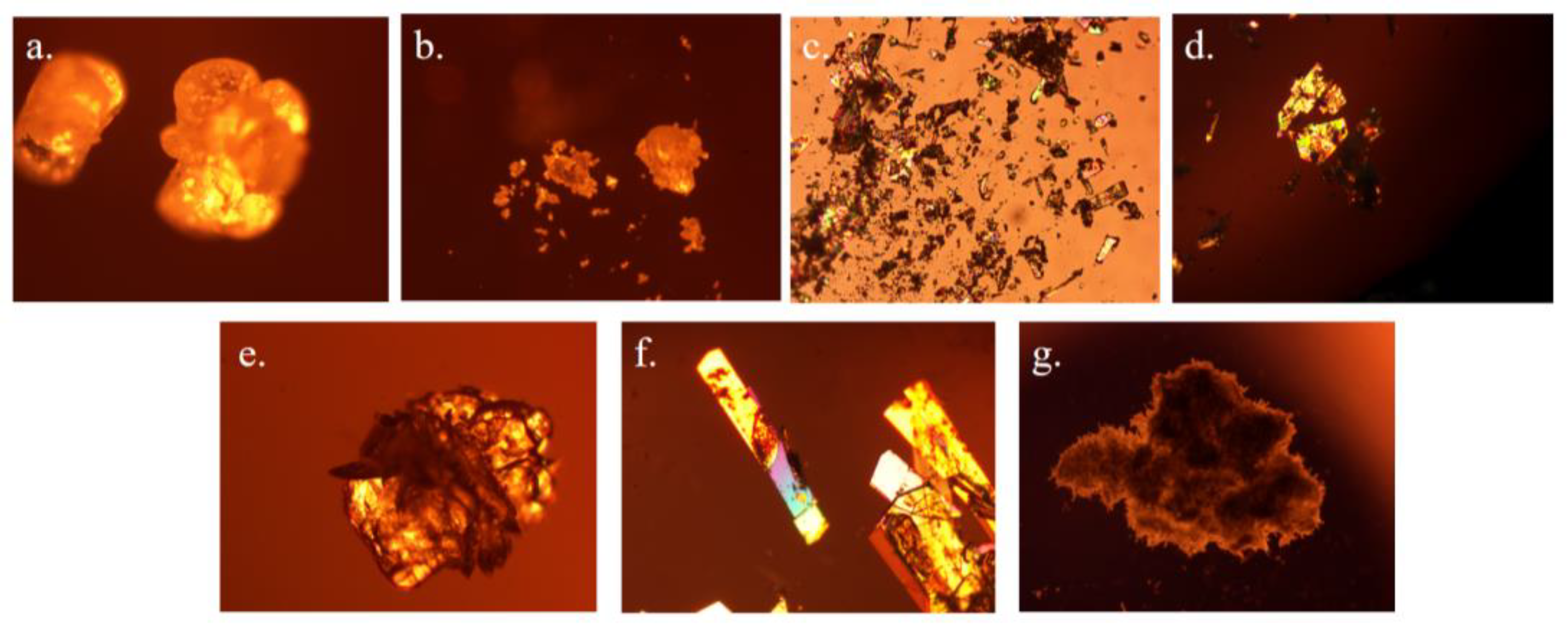
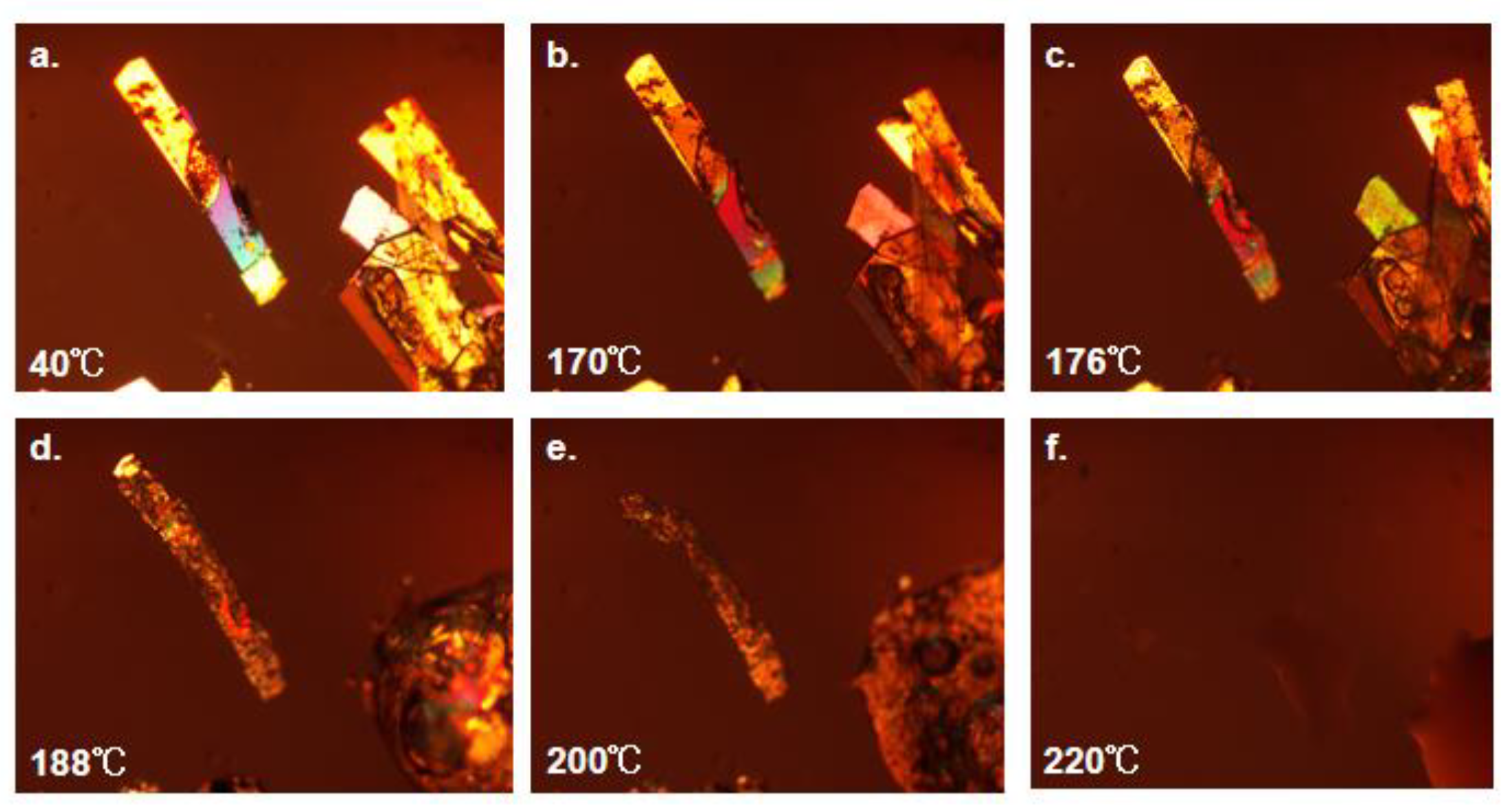
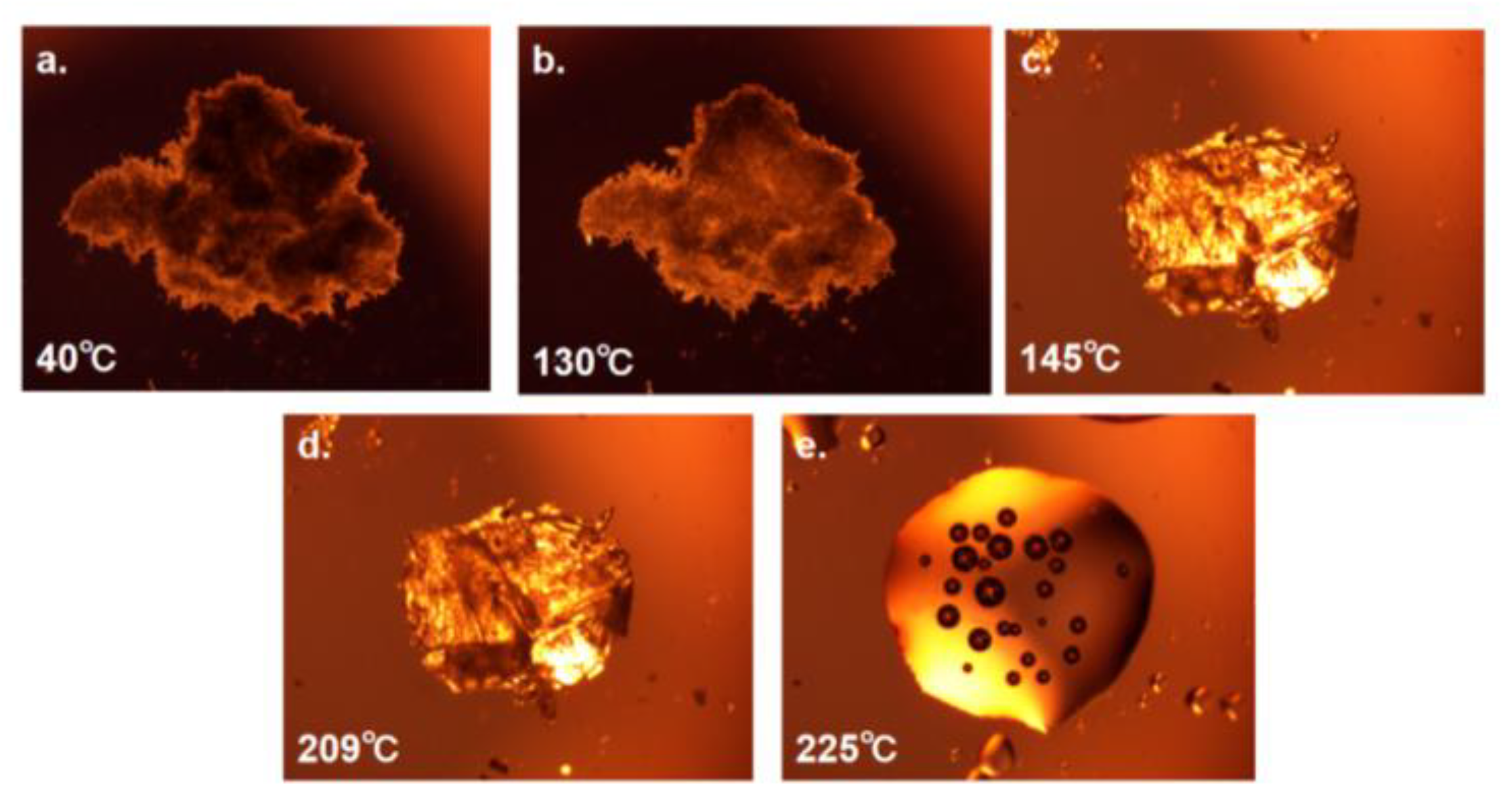
3.2.5. PLM and HSPLM
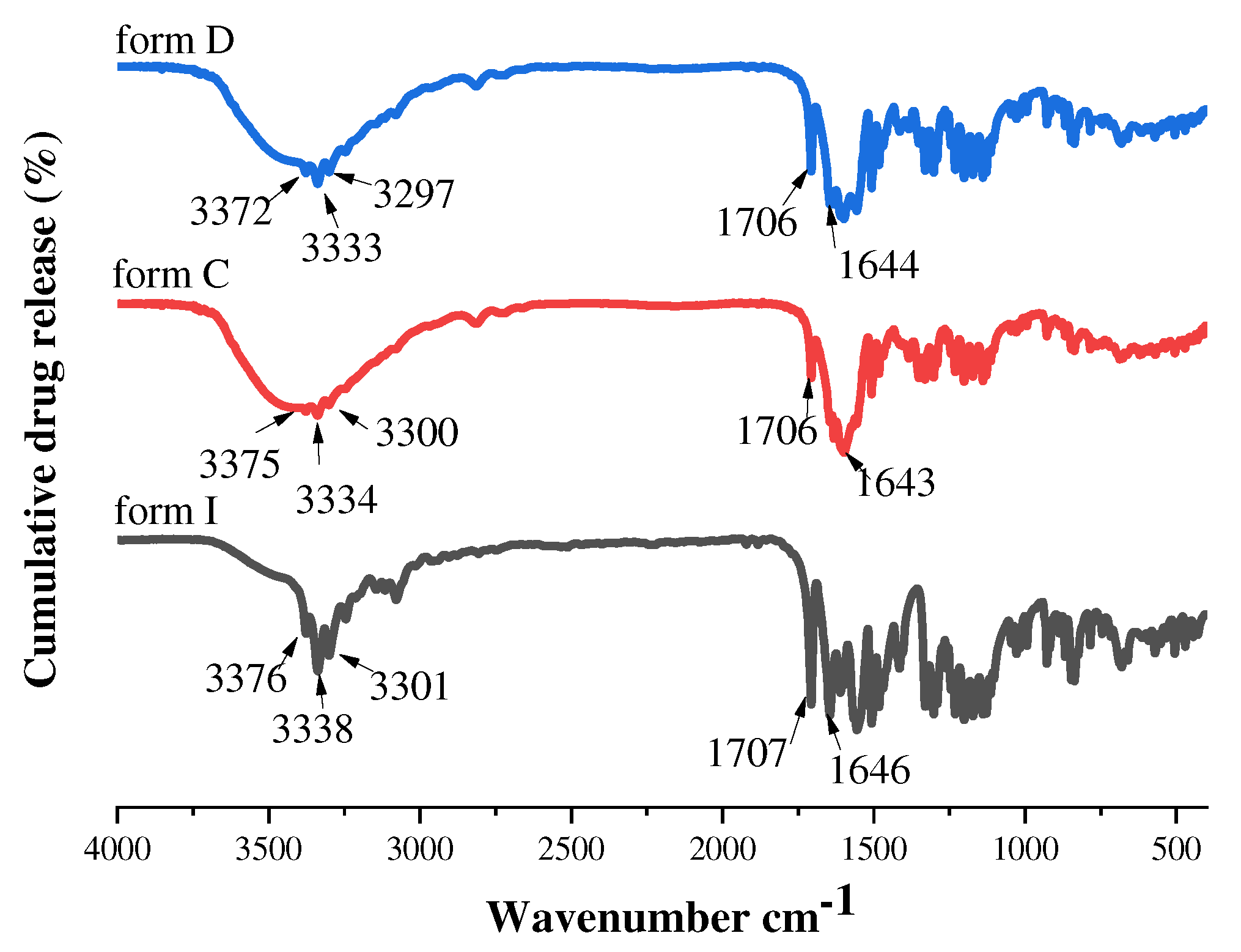
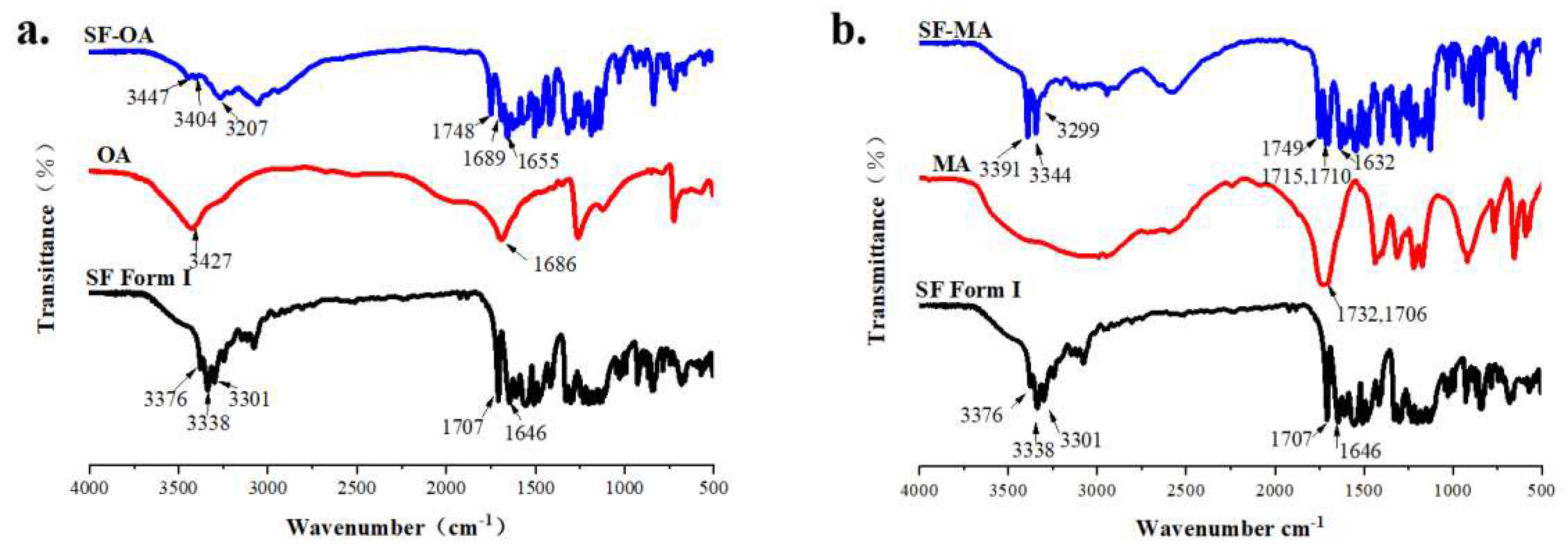
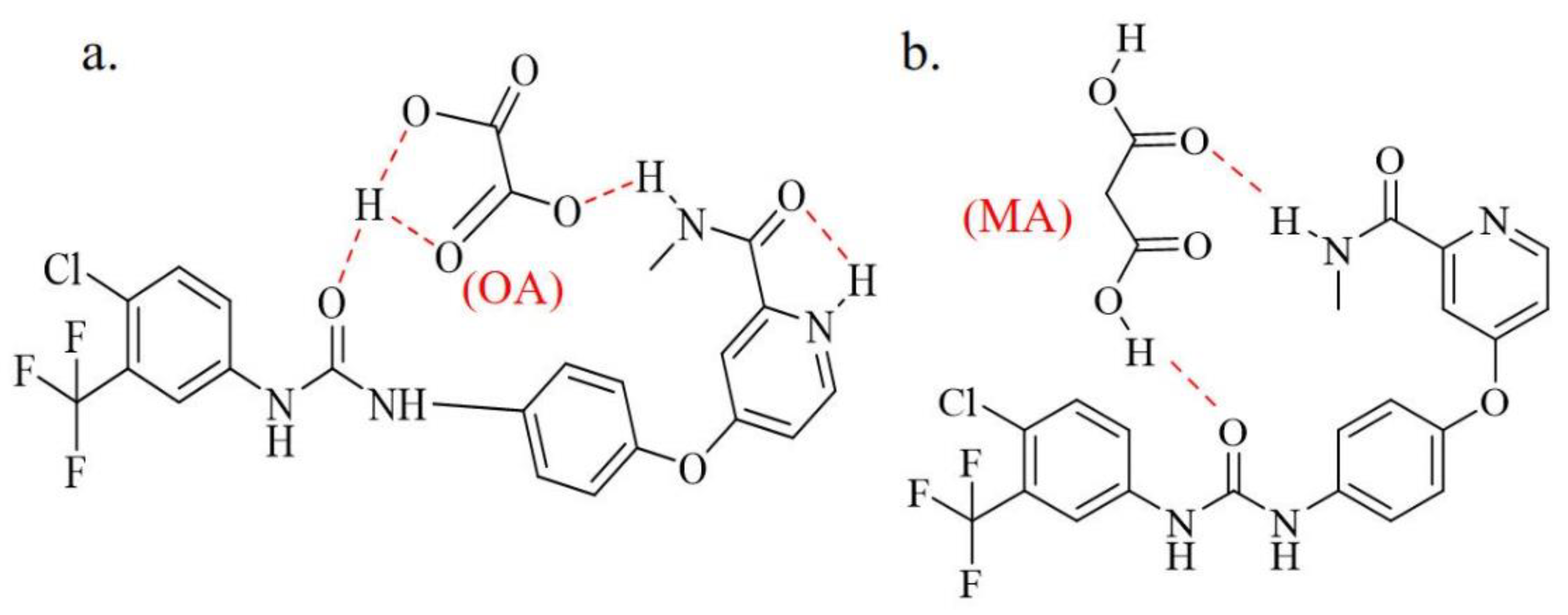
3.2.6. FT-IR
3.3. Solution and In-Vitro Dissolution Experiments
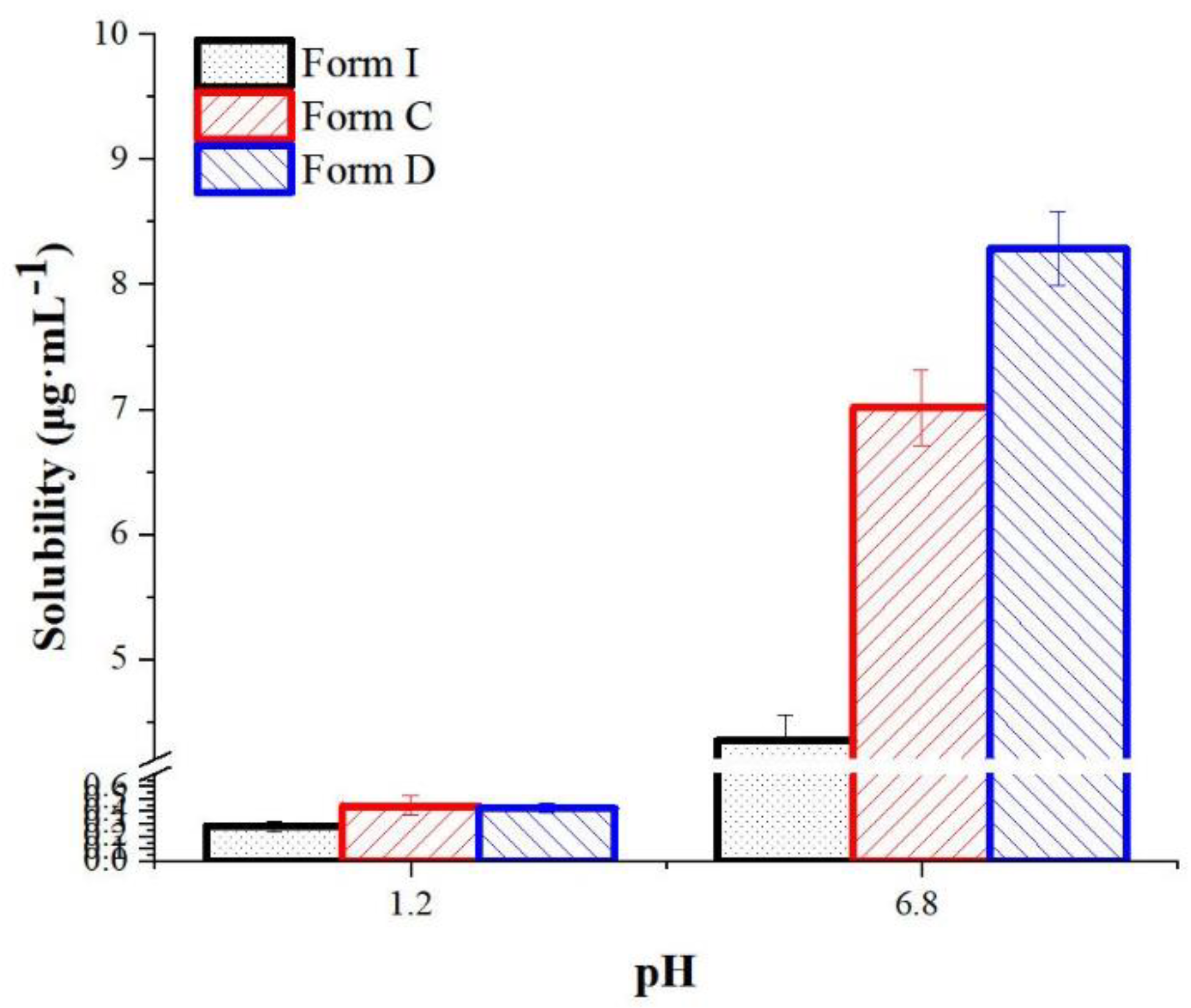
3.3.1. Equilibrium Solubility of form C and form D
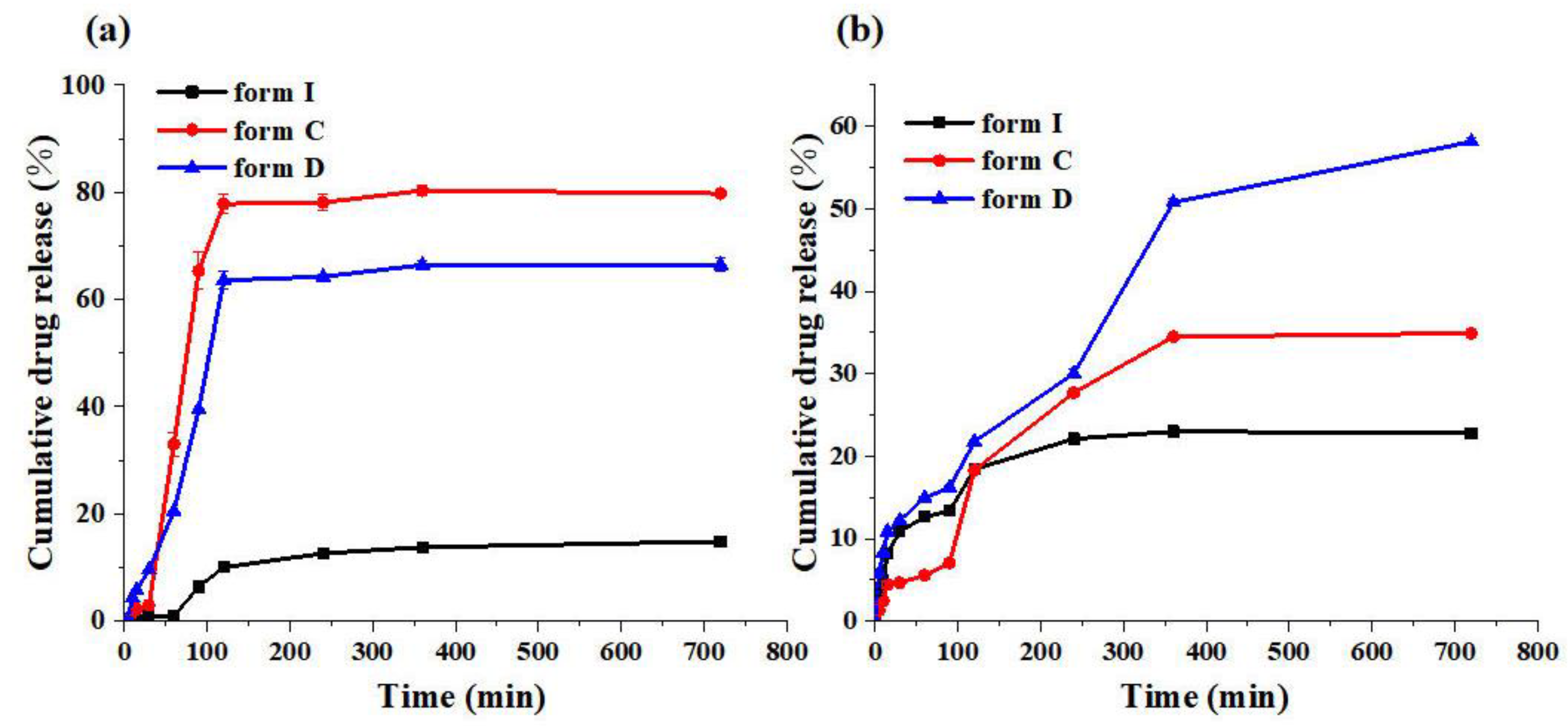
3.3.2. In-Vitro Dissolution Experiments
3.3.3. Kinetic Solubility of Co-crystals
3.3.4. The Discussion of Co-crystals Solubility Mechanism
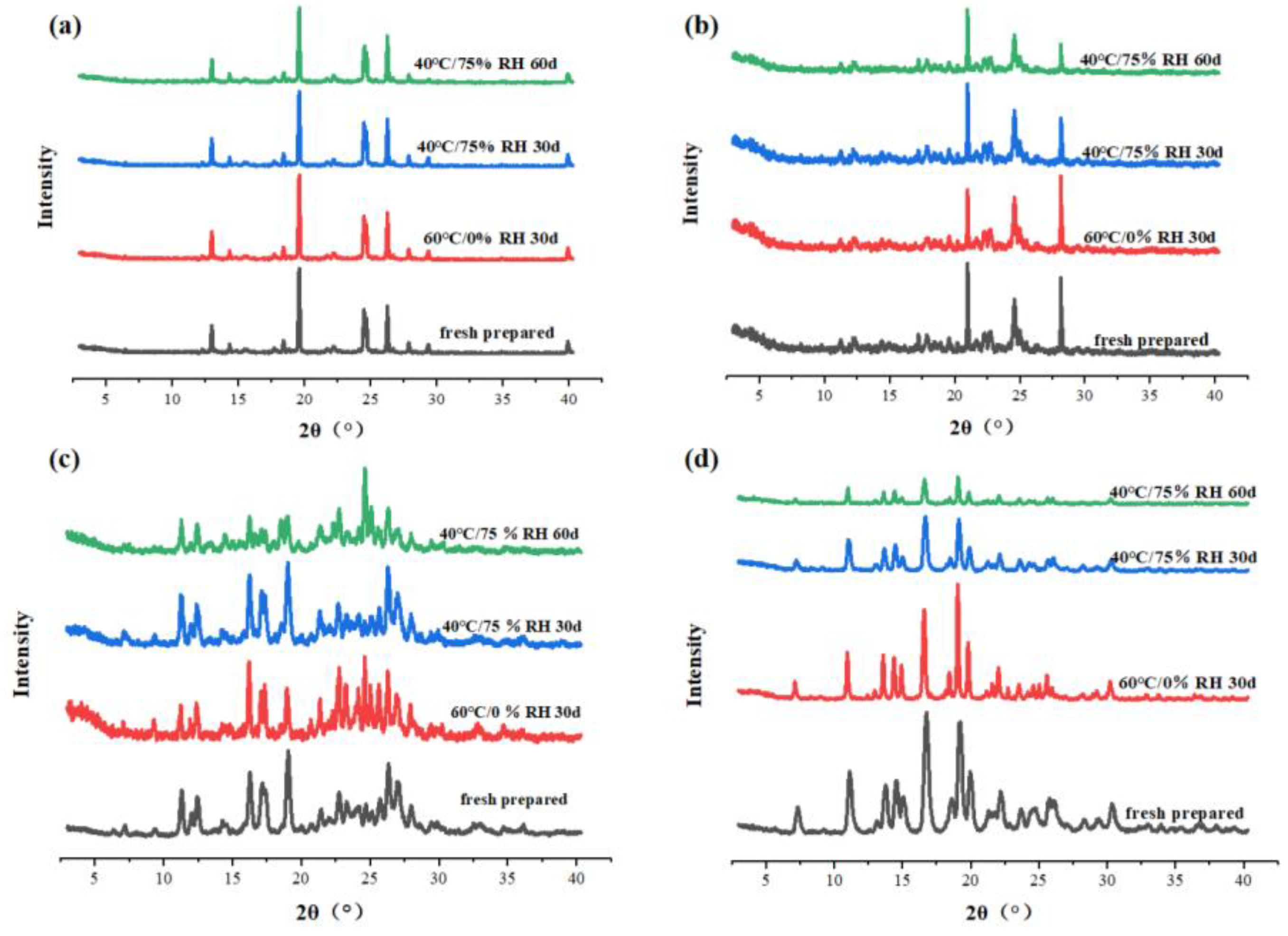
3.5. Stability Tests
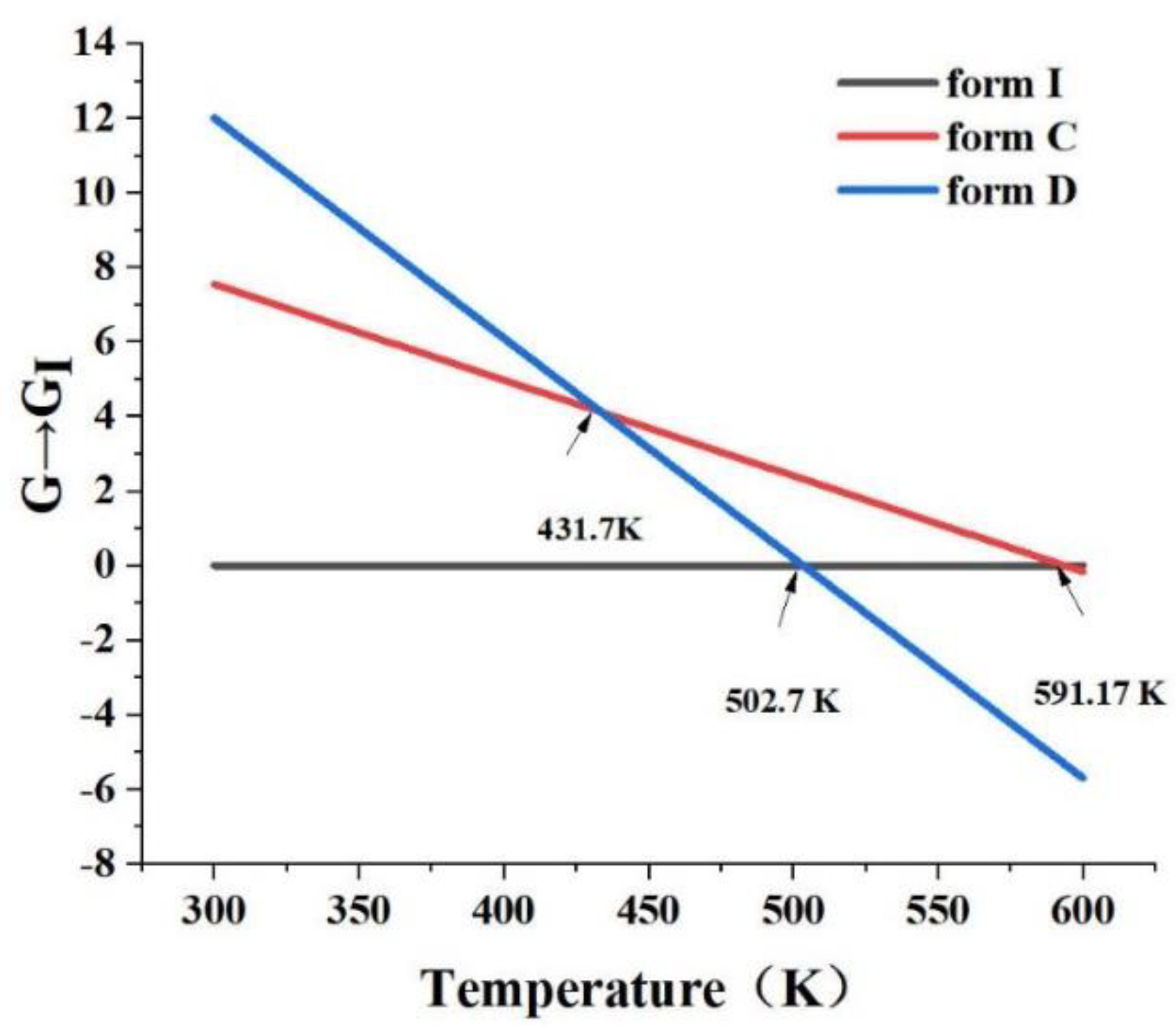
3.6. Relative Stability between Polymorphs.
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- KUMARI L, CHOUDHARI Y, PATEL P, et al. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life. 2023, 13(5): 1099; [CrossRef]
- OFTSSON T, BREWSTER M E. Pharmaceutical applications of cyclodextrins: basic science and product development. Journal of Pharmacy and Pharmacology. 2010, 62(11): 1607-21; [CrossRef]
- Cruz-Cabeza AJ. Acid–base crystalline complexes and the pKa rule. CrystEngComm, 2012, 14(20):6362-5; [CrossRef]
- Gong W, Mondal PK, Ahmadi S, Wu Y, Rohani S. Cocrystals, Salts, and Salt-Solvates of olanzapine; selection of coformers and improved solubility. Int J Pharm. 2021, 608:121063-72. [CrossRef]
- Domingos S, Andre V, Quaresma S, Martins IC, Minas da Piedade MF, Duarte MT. New forms of old drugs: improving without changing. J Pharm Pharmacol. 2015, 67(6):830-46. [CrossRef]
- JAMBHEKAR S S, BREEN P J. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discovery Today. 2013, 18(23): 1173-84. [CrossRef]
- Butreddy A, Almutairi M, Komanduri N, Bandari S, Zhang F, Repka MA. Multicomponent crystalline solid forms of aripiprazole produced via hot melt extrusion techniques: An exploratory study. J Drug Deliv Sci Technol. 2021, 63:102529-39. [CrossRef]
- Moisescu-Goia C, Muresan-Pop M, Simon V. New solid state forms of antineoplastic 5-fluorouracil with anthelmintic piperazine. Journal of Molecular Structure. 2017;1150:37-43. [CrossRef]
- Bueno MS, Minambres GG, Bongioanni A et al. Exploring solid forms of oxytetracycline hydrochloride. Int J Pharm. 2020, 585:119496-504. [CrossRef]
- MANIRUZZAMAN M, LAM M, MOLINA C, et al. RETRACTED ARTICLE: Study of the Transformations of Micro/Nano-crystalline Acetaminophen Polymorphs in Drug-Polymer Binary Mixtures. AAPS PharmSciTech. 2017, 18(5): 1428-37. [CrossRef]
- Drozd K V, Manin A N, Boycov D E, et al. Simultaneous Improvement of Dissolution Behavior and Oral Bioavailability of Antifungal Miconazole via Cocrystal and Salt Formation. Pharmaceutics, 2022, 14(5): 1107. [CrossRef]
- Zhu Y J, Zheng B, Wang H Y, et al. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacologica Sinica, 2017, 38(5): 614-622. [CrossRef]
- Cao M, Xu Y, Youn JI, Cabrera R, Zhang X, Gabrilovich D et al. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest. 2011, 91(4):598-608. [CrossRef]
- IYER R, FETTERLY G, LUGADE A, et al. Sorafenib: a clinical and pharmacologic review. Expert Opinion on Pharmacotherapy. 2010, 11(11): 1943-55. [CrossRef]
- Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008, 61(4):535-48. [CrossRef]
- Takeuchi A, Eto M, Tatsugami K, et al. Mechanism of synergistic antitumor effect of sorafenib and interferon-α on treatment of renal cell carcinoma. The Journal of urology. 2010, 184(6): 2549-2556. [CrossRef]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66(24):11851-8. [CrossRef]
- Kong FH, Ye QF, Miao XY, Liu X, Huang SQ, Xiong L et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics. 2021, 11(11):5464-90. [CrossRef]
- Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018, 50(10):1-9. [CrossRef]
- Kim A, Balis FM, Widemann BC. Sorafenib and sunitinib. Oncologist. 2009, 14(8):800-5. [CrossRef]
- Wiergowska G, Stasilowicz A, Miklaszewski A, Lewandowska K, Cielecka-Piontek J. Structural Polymorphism of Sorafenib Tosylate as a Key Factor in Its Solubility Differentiation. Pharmaceutics. 2021, 13(3):384-93. [CrossRef]
- Zhang Z, Niu B, Chen J, He X, Bao X, Zhu J et al. The use of lipid-coated nanodiamond to improve bioavailability and efficacy of sorafenib in resisting metastasis of gastric cancer. Biomaterials. 2014, 35(15):4565-72. [CrossRef]
- Shi Y, Zhao Z, Gao Y, Pan DC et al. Oral delivery of sorafenib through spontaneous formation of ionic liquid nanocomplexes. Jounaral of Control Release. 2020, 322:602-9. [CrossRef]
- Wiergowska G, Stasilowicz A, Miklaszewski A, Lewandowska K, Cielecka-Piontek J. Structural Polymorphism of Sorafenib Tosylate as a Key Factor in Its Solubility Differentiation. Pharmaceutics. 2021, 13(3):384-93. [CrossRef]
- Martins Santos OM, Jacon Freitas JT, Bitencourt M, Martins FT, Doriguetto AC. Three new orbifloxacin multicomponent crystal forms towards pharmaceutical improvement. Journal of MolecularS tructure. 2020, 1217:128371-8. [CrossRef]
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012, 2012:195727-85736. [CrossRef]
- Wang XQ, Fan JM, Liu YO, Zhao B, Jia ZR, Zhang Q. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int J Pharm. 2011, 419(1-2):339-46. [CrossRef]
- EBADI M, BUSKARAN K, BULLO S, et al. Drug delivery system based on magnetic iron oxide nanoparticles coated with (polyvinyl alcohol-zinc/aluminium-layered double hydroxide-sorafenib). Alexandria Engineering Journal, 2021, 60(1): 733-47. [CrossRef]
- GAO W, JIA X, WU J, et al. Preparation and evaluation of folate-decorated human serum albumin nanoparticles for the targeted delivery of sorafenib to enhance antihepatocarcinoma efficacy. Journal of Drug Delivery Science and Technology, 2019, 54: 101349. [CrossRef]
- Sun W, Wang Y, Cai M, Lin L, Chen X, Cao Z et al. Codelivery of sorafenib and GPC3 siRNA with PEI-modified liposomes for hepatoma therapy. Biomater Sci. 2017, 5(12):2468-79. [CrossRef]
- Xiao Y, Liu Y, Yang S, et al. Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids and Surfaces B: Biointerfaces, 2016, 141: 83-92. [CrossRef]
- Yang P, Qin C, Du S, Jia L, Qin Y, Gong J et al. Crystal Structure, Stability and Desolvation of the Solvates of Sorafenib Tosylate. Crystals. 2019;9(7):367-80. [CrossRef]
- Li C, Zhong J, Liu B, Yang T, Lv B, Luo Y. Study on Typical Diarylurea Drugs or Derivatives in Cocrystallizing with Strong H-Bond Acceptor DMSO. ACS Omega. 2021, 6(8):5532-47. [CrossRef]
- Phan C, Shen J, Yu K, Liu J, Tang G. Hydrogen Bonds, Topologies, Energy Frameworks and Solubilities of Five Sorafenib Salts. Int J Mol Sci. 2021, 22(13):6682-93. [CrossRef]
- Liu C, Wang C, Wan S, Liu L, Sun C, Qian F. An Elusive Drug–Drug Cocrystal Prepared Using a Heteroseeding Strategy. Crystal Growth & Design., 2021, 21(10): 5659-68. [CrossRef]
- CHEN Y, ZHU W, LIANG J. New-type sorafenib tosylate polymorphic form has differential scanning calorimetry pattern in high transition temperature and specified melting decomposition peak, CN102850267-A [P/OL]. <Go to ISI>://DIIDW:2013F00136.
- WANG B, LIN D, ZHANG J. Crystalline form of sorafenib for drug used for treating cancer, has specific characteristic peaks at preset Bragg angle measured by X-ray powder diffraction,CN103664771-A;CN103664771-B[P/OL]. <Go to ISI>://DIIDW:2014J37488.
- HUANG X, ZHU S, WANG J, et al. New sorafenib crystalline form B shows specific characteristic absorption peaks in X-ray powder diffraction pattern, CN109422676-A [P/OL]. <Go to ISI>://DIIDW:201923360A.
- SHAO Q, WEI W, XIA Y, et al. New Sorafenib free base crystal form, prepared by adding sorafenib free base into alcohol, heating to dissolve, and filtering while hot, adding the filtrate dropwise into water, stirring, filtering, and drying, CN113773249-A [P/OL]. <Go to ISI>://DIIDW:2021E96191.
- Lemmerer A, Govindraju S, Johnston M, Motloung X, Savig KL. Co-crystals and molecular salts of carboxylic acid/pyridine complexes: can calculated pKa's predict proton transfer? A case study of nine complexes. CrystEngComm. 2015,17(19):3591-5. [CrossRef]
- Douroumis D, Ross SA, Nokhodchi A. Advanced methodologies for cocrystal synthesis. Adv Drug Deliv Rev. 2017,117:178-95. [CrossRef]
- Dolomanov, O.V., Bourhis, L.J., Gildea, R.J, Howard, J.A.K. & Puschmann, H. J. Appl. Cryst. 2009, 42, 339-341.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284.
- W. T. Pennington, J. Appl. Crystallogr., 1999, 32, 1028–1029.
- CHIKHALIKAR S, GHAGARE M, KANKAN R N, et al. New sorafenib in amorphous form useful in pharmaceutical composition and medicine for treating cancer, WO2009106825-A1; IN200800402-I3 [P/OL]. <Go to ISI>://DIIDW:2009N22664.
- Shimpi MR, Alhayali A, Cavanagh KL, Rodríguez-Hornedo N, Velaga SP. Tadalafil–Malonic Acid Cocrystal: Physicochemical Characterization, pH-Solubility, and Supersaturation Studies. Crystal Growth & Design. 2018, 18(8):4378-87. [CrossRef]
- GUO M, SUN X, CHEN J, et al. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm Sin B. 2021, 11(8):2537-2564. [CrossRef]
- MA Q, HE H, LIU C. Hygroscopic properties of oxalic acid and atmospherically relevant oxalates. Atmospheric Environment,2013,69:281-8. [CrossRef]
- Shevchenko A, Bimbo L M, Miroshnyk I, et al. A new cocrystal and salts of itraconazole: Comparison of solid-state properties, stability and dissolution behavior. International Journal of Pharmaceutics. 2012, 436(1-2): 403-409. [CrossRef]
- Perlovich G. Melting points of one-and two-component molecular crystals as effective characteristics for rational design of pharmaceutical systems. Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials. 2020, 76(4): 696-706. [CrossRef]
- Al-Obaidi H, Lawrence M J, Al-Saden N, et al. Investigation of griseofulvin and hydroxypropylmethyl cellulose acetate succinate miscibility in ball milled solid dispersions. International journal of pharmaceutics. 2013, 443(1-2): 95-102. [CrossRef]
- Caires F J, Lima L S, Carvalho C T, et al. Thermal behaviour of malonic acid, sodium malonate and its compounds with some bivalent transition metal ions. Thermochimica acta. 2010, 497(1-2): 35-40. [CrossRef]
- Okur N Ü, Siafaka P I, Gökçe E H. Challenges in oral drug delivery and applications of lipid nanoparticles as potent oral drug carriers for managing cardiovascular risk factors. Current Pharmaceutical Biotechnology. 2021, 22(7): 892-905. [CrossRef]
- Lee T, Wang P Y. Screening, manufacturing, photoluminescence, and molecular recognition of co-crystals: cytosine with dicarboxylic acids. Crystal growth & design. 2010, 10(3): 1419-1434. [CrossRef]
- YAMAMOTO K, IKEDA Y. Kinetic solubility and lipophilicity evaluation connecting formulation technology strategy perspective. Journal of Drug Delivery Science and Technology. 2016, 33: 13-8. [CrossRef]
- SAAL C, PETEREIT A C. Optimizing solubility: Kinetic versus thermodynamic solubility temptations and risks. European Journal of Pharmaceutical Sciences. 2012, 47(3): 589-95. [CrossRef]
- Berry DJ, Steed JW. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv Drug Deliv Rev. 2017, 117:3-24. [CrossRef]
- Babu NJ, Nangia A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Crystal Growth & Design. 2011, 11(7):2662-79. [CrossRef]
- REGGANE M, WIEST J, SAEDTLER M, et al. Bioinspired co-crystals of Imatinib providing enhanced kinetic solubility. European Journal of Pharmaceutics and Biopharmaceutics. 2018, 128: 290-9. [CrossRef]
- Gugoasa A L, Staden S R. Advanced Methods for the Analysis of Testosterone, Current Medicinal Chemistry. 2018, 25(33). [CrossRef]
- WILSON V, LOU X, OSTERLING D J, et al. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: phase behavior during dissolution, speciation, and membrane mass transport. Journal of Controlled Release. 2018, 292: 172-82. [CrossRef]
- Yan Y, Chen J-M, Lu T-B. Simultaneously enhancing the solubility and permeability of acyclovir by crystal engineering approach. CrystEngComm. 2013,15(33). [CrossRef]
- SETO Y, SATO H, MASUDA Y. Effect of water vapor pressure on thermal dehydration of lithium sulfate monohydrate. Thermochimica Acta. 2002, 388(1): 21-5. [CrossRef]
- SHI X, DENG Y, WANG Z, et al. Two new nilotinib polymorphs with solubility advantages prepared by the melt crystallization process. Journal of Drug Delivery Science and Technology. 2023, 84: 104511. [CrossRef]
- Braga D, Casali L, Grepioni F. The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools?. International Journal of Molecular Sciences, 2022, 23(16): 9013. [CrossRef]



| forms | Materials | Methods | Solvents |
|---|---|---|---|
| form C | form I | Evaporation | Methanol/H2O (6:1, v:v) |
| form D | form I | Evaporation | Acetic Acid |
| SF-OA | form I / Oxalic Acid | Slurry | Dichloromethane |
| SF-MA | form I / Malonic Acid | Slurry | Dichloromethane |
| form A | form I | Cooling | Dichloromethane/Methanol (6:1, v:v) |
| form X | form I | Antisolvent | Methanol/ H2O (1:4, v:v) |
| Amorphous | form I | Melting | - |
| Polymorphs | Angular positions of characteristic diffraction peaks (2θ) |
| form C | 6.44°,13.01°,14.34°,18.43°,19.60°,24.57°,26.27°,27.90°. |
| form D | 11.24°,17.22°,20.99°,24.99°,28.17°. |
| SF-OA | 7.22°, 9.41°, 11.32°, 12.05°, 16.28°, 17.22°, 18.98°, 20.73°, 22.72°, 24.12°, 26.33° |
| SF-MA | 7.26°, 8.40°, 9.22°, 11.11°, 15.04°, 16.71°, 19.19°, 22.17°, 23.68°. |
| form I | 7.02°,11.23°,12.39°,14.01 ° ,14.43°,14.93°,17.86°,18.41°,18.82°,19.71°,21.66°,21.80°,22.27°,22.79°,23.34°,24.56°,24.98°,25.42°,29.44°. |
| form A | 9.72°,11.23°,13.42°,14.42°,14.94°,17.89°,18.39°,18.91°,19.59°,21.57°,22.23°,22.72°,23.42°,24.58°,24.97°,27.89 °. |
| form X | 10.64°,12.19°,13.88°,15.11°,17.93°,21.49°,23.75°,26.32°,27.21°. |
| Form I | Form C | SF-OA | |
| Empirical formula | C21H16ClF3N4O3 | C21H16ClF3N4O3 | C21H16ClF3N4O3·C2H2O4 |
| Formula weight | 464.83 | 464.83 | 554.82 |
| Temperature/K | 294 | 170.00 | 170.00 |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/c | P21/c | P21/n |
| a/Å | 8.1587(16) | 7.8479(9) | 11.6364(4) |
| b/Å | 9.8055(19) | 9.5480(11) | 8.5629(3) |
| c/Å | 27.758(5) | 27.142(3) | 28.4389(9) |
| α/° | 90 | 90 | 90 |
| β/° | 94.358(3) | 93.606(5) | 98.5960(10) |
| γ/° | 90 | 90 | 90 |
| Volume/Å3 | 2214.2(7) | 2029.8(4) | 2801.86(16) |
| Z | 4 | 4 | 4 |
| ρcalcg/cm3 | - | 1.521 | 1.486 |
| μ/mm-1 | - | 1.447 | 0.214 |
| F(000) | - | 952.0 | 1296.0 |
| Radiation | MoKα(λ = 0.71073) | MoKα(λ = 0.71073) | MoKα(λ = 0.71073) |
| Rint | - | 0.1052 | 0.0569 |
| wR2 | 0.102 | 0.1335 | 0.1405 |
| CCDC number | 813502 | 2290456 | 2290468 |
| Torsion Angle (°) | form I | form C | SF-OA |
|---|---|---|---|
| C12-O2-C15-C16 | -9.8 | 169.7 | 8.8 |
| C6-N1-C8-N2 | -170.62 | -170.1 | -175.9 |
| C6-N1-C8-O1 | 9.3 | 9.9 | 4.1 |
| C9-N2-C8-N1 | 6.8 | 8.4 | -6.3 |
| C17-N4-C20-C21 | 179.51 | -179.9 | -175.3 |
| C16-C17-C20-O3 | 18.5 | 19.5 | -179.5 |
| C15-O2-C11-C12 | -9.8 | 109.9 | 83.4 |
| C1-C2-C3-Cl1 | -4.2 | 175.2 | -0.9 |
| C1-C2-C3-C4 | 177.05 | -3.4 | 178.6 |
| C15-O2-C12-C13 | 170.80 | -73.6 | 101.1 |
| C16-C17-C20-N4 | -161.45 | 161.0 | 0.7 |
| Form I | Form A | Form C | Form D | Form X* | |
| Tm (℃) | 212.09 | 209.18 | 197.24 | 205.62 | - |
| Tm (K) | 485.24 | 482.33 | 470.39 | 478.77 | - |
| △Hm (kJ/mol) | 103.68 | 95.04 | 88.427 | 73.979 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




