1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common malignant pancreatic tumor, still characterized by a poor prognosis with 5-year survival rates of around 12% and an still ongoing increase in death rate [
1,
2]. Due to the lack of early and specific symptoms, the majority of patients are diagnosed at an advanced or even metastasized stage leaving only palliative treatment. The only curative option is surgery which, however, is only eligible for about 20% of patients [
3,
4]. PDAC metastasizes predominantly in the liver, but also in lung and peritoneum [
4,
5,
6]. PDAC patients with liver metastases or peritoneal exhibit a significantly shorter disease-free survival and overall poorer prognosis than patients with solitary lung metastases [
7].
The multistep process of metastasis starts with dissemination of tumor cells from the primary tumor and ends with proliferation and outgrowth of macroscopic metastases at secondary sites [
8,
9,
10]. As a prerequisite for dissemination, carcinoma cells have to acquire a motile and invasive phenotype, which is commonly described to be achieved by Epithelial-Mesenchymal-Transition (EMT) [
11,
12]. EMT is associated with the downregulation of adhesion molecules and loss of epithelial proteins like E-cadherin and conversely, a gain of mesenchymal characteristics like increased expression of mesenchymal markers such as Vimentin, L1CAM, or the transcription factors ZEB1, ZEB2 and OVOL2 (ZNF339) [
11,
12,
13,
14,
15,
16,
17]. Furthermore, this loss of cell differentiation of carcinoma cells has been associated with the acquisition of cancer stem cell (CSC) properties [
18,
19,
20]. Mani et al. were the first who demonstrated that breast cancer cells that have undergone EMT acquire a cancer stem cell-like phenotype, and likewise, stem cell-like cells resemble cells that have undergone EMT [
18].
CSCs are a small group of cancer cells within a cancer cell population with the unique ability to both self-renewal and generation of more differentiated cells. Owing to these unique features, CSCs are regarded as essential for tumor initiation at primary and secondary sites, including PDAC [
21,
22,
23]. Recent studies indicate that CSC-properties can be gained and lost depending on the microenvironment [
20,
24,
25,
26,
27], indicating that CSCs are not a stable, but highly plastic cell population. Several markers, e.g. ABCG2, CD133, CD24, CD44, Nanog, Nestin and SOX2, have been proposed for the identification of CSCs in PDAC already indicating a high heterogeneity within the CSC population [
28,
29,
30,
31,
32,
33,
34]. The intermediate filament Nestin and the stem cell (SC)-transcription factor SOX2 seem to play a role in the maintenance of CSC properties [
35,
36]. Nestin impacts cell motility and EMT properties in PDAC cells and its knockdown in PDAC cells led to reduced tumor incidence and volume, as well as formation of liver metastases in a murine PDAC model [
37,
38]. Elevated SOX2 expression has been rarely detected in pancreatic intraepithelial neoplasia rather than poorly differentiated and neurally invasive tumors supporting a role of this factor in later stages of tumorigenesis and metastasis [
39]. In line with these findings it has been shown that SOX2 is involved in Mesenchymal-Epithelial-Transition (MET), the reversion of EMT, as knockdown of SOX2 in colorectal cancer (CRC) cells altered expression of key genes involved in the EMT process including E-cadherin and Vimentin [
40]. Furthermore,
de novo SOX2 expression in pancreatic cancer cells is sufficient to promote self-renewal, de-differentiation and impacting stemness characteristics via modulating specific cell cycle regulatory genes and EMT-driver genes [
32].
In vitro, self-renewal abilities are assessed by colony formation and here, different colony types exhibiting distinct morphologies and different CSC potentials have been described [
41]. While Holoclones are considered to be the colony type with the highest amount of CSCs, Paraclones are characterized by more differentiated cells [
41,
42]. Meroclones form an intermediate stage of these two colony types and differ from Holoclones mainly in their lower proportion of CSCs [
41,
42]. Our group previously demonstrated that Panc1 Holoclone cells derived by single-cell cloning from the parental PDAC cell line Panc1 showed elevated and exclusive expression of the CSC-marker Nestin compared to Paraclones [
41]. Of note, experimental evidence has been provided that also carcinoma cells with an epithelial phenotype can contribute to metastasis, using a cluster-like migration pattern, which has also been described for PDAC cells [
43]. Moreover, recent studies indicate that so-called hybrid cells concomitantly showing epithelial and mesenchymal characteristics exhibit the highest plasticity regarding cancer stemness, tumor initiation capacity as well as adaptation capability, which gives these cells the highest probability to metastasize [
43]. Still, it is poorly understood whether different CSC-phenotypes exist along the EMT/MET axis and how these impact malignancy associated properties. Thus, to explore whether and how different EMT states are associated with CSC properties in PDAC and how this impacts tumor cell growth and metastasis, Holo- and Paraclone cells were isolated and expanded from another PDAC cell line (Panc89). In contrast to mesenchymal Panc1 cells which have presumably already undergone EMT but still are derived from a primary PDAC, Panc89 cells originate from a lymph node metastasis and have presumably undergone EMT and MET during the metastatic process. Using these well-defined cell models the present study aimed at a comprehensive in vitro and in vivo analysis of isolated Holo- and Paraclone cell variants from Panc1 and Panc89 cells concerning EMT and CSC properties as well as tumorigenicity and metastasis. Overall, our data support the view of great plasticity and heterogeneity within cancer (stem) cells in PDAC, differentially impacting tumor growth and metastatic behavior.
2. Materials and Methods
Cell lines and cell culture
As a model for mesenchymal PDAC cells, the human cell line Panc1 was used (purchased from ATCC, Manassas, Virginia US) originating from a primary tumor of a PDAC patient. As a model for epithelial PDAC cells, the human cell line Panc89 (kindly provided by Prof. T. Okabe, University of Tokyo, JP) originating from a lymph node metastasis of PDAC was used. Holo- and Paraclone cells of both cell lines were isolated and expanded via single-cell cloning (see below). All cell lines were cultivated in Panc-medium (RPMI 1640 supplemented with 10% FCS, 1% L-glutamine, and 1% sodium pyruvate (Biochrom, Berlin, DE)).
For analysis of PDAC cell adhesion behavior to endothelial cells of different metastatic sites, lung (HuLEC-5a) and liver (TMNK-1) endothelial cell lines as well as the mesothelial cell line Met-5a were used. HuLEC-5a cells (purchased from ATCC, Manassas, Virginia US) are human microvascular endothelial cells that are derived from the lungs of a male patient [
44]. They were cultured in MCDB 131 (Biochrom, Berlin, DE) medium containing 10% FCS, 1% L-glutamine, 10 ng/ml Epithelial Growth Factor and 1 µg/ml hydrocortisone. TMNK-1 (purchased from NIBIOHN JCRB cell bank, Osaka, JP) are human liver sinusoidal endothelial cells [
45], originating from the liver of a female patient [
45]. They were cultured in DMEM (Biochrom, Berlin, DE) containing 10% FCS, 1% L-glutamine, and 1% penicillin-streptomycin. Met-5a cells (purchased from ATCC, Manassas, Virginia US) originating from mesothelium, were isolated from the pleural fluids of non-cancerous individuals [
46,
47] and cultured in Medium199 (Sigma Aldrich/Merck KGaA, Darmstadt, DE) containing 10% FCS, 400 nM hydrocortisone, 870 nM porcine insulin, and 20nM HEPES.
Single-cell cloning and clone expansion
Single-cell cloning and expansion was performed with parental PDAC cell lines to isolate and expand single-cell derived Holo- and Paraclone cells. Single-cell cloning of Panc1 cells to isolate Panc1 Holo- and Paraclone cells, respectively, was performed previously [
41]. To isolate Holo- and Paraclone cells from Panc89 cells, the parental cells were pre-diluted to a cell count of 1000 cells per ml Panc-medium. From this cell suspension, 100 µl (corresponding to 100 cells) were added to 20 ml of Panc-medium to obtain a concentration of one cell per 200 µl of cell medium. Cells were seeded 1 cell/well in a transparent, flat 96-well plate. The plate was centrifuged and the first screening was performed directly after centrifugation by imaging the plate in the brightfield channel of a NYONE® Scientific Imager (SynenTec GmbH, Elmshorn, DE) and the brightfield channel of EVOS microscope (AMG, Bothell, US). Only wells containing exactly one cell were considered for further monitoring and expansion. Microscopical screening was performed twice per week. After 12-20 days, the colony shape was determined and the colonies were monitored until a cell confluence of about 80% was reached. Cell clones with a stable colony morphology [
41,
42] were detached from one well and transferred to one well of a 6-well plate. The morphology of expanded cell clones was checked regularly. Only clones with a clear CSC- or non-CSC morphology [
41,
42] and stable phenotype were further cultivated, expanded, and used for phenotypic and functional characterization. Phenotypes were regularly checked by marker analysis via qPCR.
RNA isolation and RT-qPCR
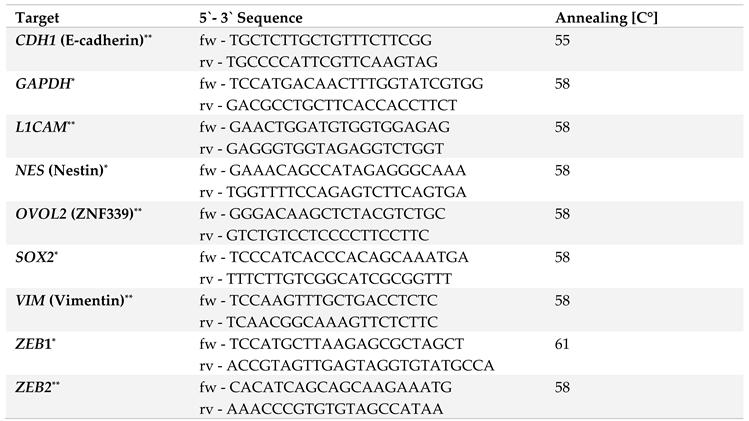
Total RNA was isolated using the total RNA kit peqGOLD (PeqLab, Erlangen, DE) and subjected to reverse transcription according to manufacturer’s instructions (Fermentas via Thermo Fisher Scientific, Darmstadt, DE). The qPCR analysis was performed in duplicates on a LightCycler 480 (Roche, Basel, CH) for a maximum of 50-60 cycles ending with a melting curve analysis for primer quality control. Primers used (Eurofins, Ebersberg DE; RealTime Primers via Biomol, Hamburg, DE), primer sequences, and annealing-temperatures are listed in
Table 1. For relative quantification of RNA levels, C
T-values of genes of interest were normalized to the respective C
T-value for the reference gene GAPDH.
Analysis of CSC- and EMT- marker via immunofluorescence staining
For the analysis of different CSC- and EMT-marker on protein level in Panc1 and Panc89 cell variants, an immunofluorescent staining (IFS) was performed. Panc1 cell variants were stained with anti-Nestin (clone 10C2; Thermo Scientific, Schwerte, DE) and anti-ZEB2 (polyclonal; Novus Biologicals, Wiesbaden Nordenstadt, DE) antibodies, while all Panc89 cell variants were stained with anti-SOX2 (clone D6D9; Cell Signaling Technology, Danvers, MA - US) and anti-L1CAM (clone UJ127.11; Gerd Moldenhauer, German Cancer Research Center, Heidelberg, DE) antibodies.
First, glass coverslips were placed in each well of a 12-well plate. Then, 0.5 × 104 cells/well were seeded. After 48h, cell culture medium was removed and the cells were washed with PBS. Fixation of the cells was performed by incubation with 4% PFA for 15 min at RT. Next, the coverslips were washed 3 × 5 min with PBS and incubated with ice-cold methanol (MetOH) for 10 min at -20°C for permeabilization of the cells. Afterward, the coverslips were washed with PBS and cells were blocked with 4% bovine serum albumin (BSA)-0.3% TritonX-100 in PBS for 1h at RT. After a washing step with 0.3% TritonX-100/PBS, incubation with the primary antibodies was performed (see below).
Concomitant double IFS staining of Nestin and ZEB2 in Panc1 cells:
Incubation of the antibodies and Hoechst 33258 (Merck Millipore, Darmstadt, DE) was carried out in 1% BSA-0.3% TritonX-100/PBS and in a humidity chamber. Incubation of the primary antibodies was performed at 4°C overnight (ON). Anti-Nestin antibody (Thermo Scientific, Schwerte, DE) was diluted 1:200 (= 5 µg/ml), anti-ZEB2 antibody (Novus Biologicals, Wiesbaden-Nordenstadt, DE) was diluted 1:50 (= 2 µg/ml), mouse IgG1 isotype control (R&D Systems GmbH, Wiesbaden, DE) was diluted 1:100 (= 5 µg/ml) and rabbit IgG isotype control (Bio-Techne, Minnesota, US) was diluted 1:500 (= 2 µg/ml). Incubation of primary antibodies was performed as a concomitant double staining incubating with both primary antibodies at the same time. After ON incubation, cells were washed with 0.3% TritonX-100/PBS. Afterward, anti-mouse antibodies conjugated with AlexaFluor 488 (Invitrogen, Carlsbad, US), anti-rabbit antibodies conjugated with AlexaFluor 647 (Invitrogen, Carlsbad, US) and Hoechst 33258 were each diluted 1:500 in 1% BSA-0.3% TritonX-100/PBS and added to the cells for 1h at RT. After washing with PBS and dH2O, coverslips were sealed with Fluor Safe Reagent (Electron Microscopy Sciences, Hatfield, PA) and transparent nail polish.
Sequential IFS staining of SOX2 and L1CAM in Panc89:
Incubation of anti-SOX2 (Cell Signaling Technology, Danvers, MA - US) and related secondary antibody was carried out in 1% BSA-0.3% TritonX-100/PBS and in a humidity chamber. Incubation of anti-L1CAM and related secondary antibody, as well as Hoechst 33258 staining was carried out in 1%BSA/PBS. Anti-SOX2 antibody was diluted 1:200 (= 25 µg/ml), anti-L1CAM antibody was diluted 1:100 (= 10 µg/ml), mouse IgG1 isotype control was diluted 1:50 (= 10 µg/ml) and rabbit IgG isotype control was diluted 1:40 (= 25 µg/ml). Incubation of primary antibodies was performed sequentially, starting with the ON staining of SOX2 at 4°C. After incubation with an anti-SOX2 antibody, cells were washed with 0.3% TritonX-100/ PBS and incubation with anti-rabbit antibody conjugated with AlexaFluor 647 diluted 1:500 in 1% BSA-0.3% TritonX-100/PBS was performed for 1h at RT. After washing with PBS, incubation with anti-L1CAM antibody was carried out for 1h at RT. After washing with PBS, cells were incubated with the anti-mouse antibody conjugated with AlexaFluor 488 and Hoechst 33258, both diluted 1:500 in 1% BSA/PBS for 1h at RT. After washing with PBS and dH2O, coverslips were sealed with Fluor Safe Reagent and transparent nail polish. Image acquisition and staining evaluation of all cell lines were performed with the Lionheart FX Automated Microscope and related software (Gen5 Data Analysis Software (BioTek, Bad Friedrichshall, DE).
RNA sequencing and transcriptomic analysis
Total high-purity RNA of all cell variants was isolated using the total RNA kit peqGOLD (PeqLab, Erlangen, DE) with an additional DNase digestion step according to manufacturer's specifications. Afterward, RNA-triplicates (3 biological replicates) of each cell line were placed in a 96-well plate in a concentration of at least 150 ng/15 µl RNase-free water. RNA sequencing was performed at the Institute of Clinical Molecular Biology (Kiel, DE) under the supervision of Sören Franzenburg. Input total RNA was quantified using the Quant-it RNA Assay (Thermo Fisher, Waltham, US), and RIN scores were determined using the Agilent Tape Station (Agilent, Santa Clara, US). All RIN scores were > 8. 200 ng input total RNA were processed using the Illumina stranded mRNA kit (Illumina, San Diego, US). Libraries were sequenced on the Illumina NovaSeq 6000 SP Flowcell using 100bp paired-end reads. Transcriptomic analysis of mRNA sequencing data was performed by Hauke Busch and Axel Künstner (Medical Systems Biology Group, Institute of Experimental Dermatology, University of Lübeck, DE). Raw sequencing data (fastq format) was mapped against the human transcriptome (Ensembl GRCh38.106) using kallisto (v0.46.1) [
49], and gene expression was summarized from the scaledTPM values of the transcripts using tximport [
50]. Differential gene expression was performed using DESeq2 [
51]. Gene set enrichment analysis (GSEA) on the shrunken log2 fold changes from apeglm [
52] was performed using GAGE (v2.48.0) [
53] against HALLMARK, REACTOME and Gene Ontology Biological Processes (GOBP) gene sets extracted from the msigdbr R package (v7.5.1).
Cell growth analysis
Panc1 and Panc89 cell variants were seeded at 5 × 103 cells/well in triplicates in 96-well plates for seven days (168h). Cells were imaged every day with the brightfield channel (confluence operator) and the UV channel (nuclei count operator) of the NYONE® Scientific Imager (SynenTec GmbH, Elmshorn, DE). To analyze the number of nuclei, every day three wells per each cell variant were dyed using Hoechst 33342 staining (1:5000; Thermo Fisher, Carlsbad/California, US). The examined data were analyzed via nonlinear regression (curve fit) to maintain the growth rate (κ) for logistic growth for the nuclei count analysis (kNuclei count) (YT®-Software (SYNENTEC GmbH, Elmshorn, DE) in GraphPad Prism 9.5.0 (GraphPad Software, California, US).
Treatment response analysis
Panc1 and Panc89 cell variants were seeded at 5 × 104 cells/well in duplicates in 96-well plates. After 24h, cells were left untreated for control or treated with 0.0038 µM of the standard cytostatic drug Gemcitabine. After 72h, cells were stained with Hoechst 33342 (1:5000; Thermo Fisher, Carlsbad/California, US) and nuclei were counted using the NYONE® Imager (YT®-Software (SYNENTEC GmbH, Elmshorn, DE). Nuclei count data of treated cells were normalized to untreated cells.
Migration assay
To analyze cell migration of Panc1 and Panc89 cell variants, two-chambered silicone inserts by Ibidi® (Ibidi GmbH, Martinsried, DE) with a defined cell-free gap were used. Before use, inserts were pre-warmed at 37°C for about 30 min and then placed into wells of a 24-well plate. Per each chamber of an Ibidi® insert, 5 × 104 PDAC cells were seeded in a volume of 75 - 100 µl Panc-medium and incubated for at least 24h or to a cell confluence of approximately 100%. Afterward, inserts were removed resulting in a cell-free gap between the cell layer of both chambers and wells were refilled with 1 ml of FCS-free Panc-medium.
Migration behavior was determined by measuring cell confluence on the cell-free gap over a period of 32h using the CELLAVISTA®/NYONE® Imager and the YT® Wound healing-operator (both SynenTec GmbH, Elmshorn, DE). Measuring intervals were 8h and the plate was stored in the incubator at 37°C, 5% CO2 and 85% relative humidity during the time between measurements. Migration rate was determined by measuring the cell confluence within the gap. Initially, the operator measured opposing areas at the edge of the gap and reported the cell confluence at this starting point as being between 10-20%. Throughout the experiment, the cell confluence was evaluated to this initial value with an increase in cell confluence within the gap defined as migration. If no migration occurred, the cell confluence within the gap remained unchanged.
Invasion assay
The invasive behavior of Panc1 and Panc89 cell variants was analyzed in spheroid invasion assays. For this purpose, 5 × 103 cells/well were seeded in 96-well ultra-low-attachment plates (Corning, Kennebunk ME, US; faCellitate, Mannheim, DE) in 200 µl Panc-medium enabling spheroid formation. Directly after seeding, the plate was centrifuged. After spheroid formation for 48h, 150 µl of cell Panc-medium was removed from each well and replaced by 50 µl Matrigel (Corning, New York, US) to achieve a protein concentration of 6.25 mg/l. After addition of Matrigel, the plate was centrifuged and incubated at 37°C, 5% CO2 and 85% humidity. After 30 min, the first imaging was performed using NYONE® Scientific imaging device and the YT® Spheroid invasion-operator. Duration for monitoring cell invasion was chosen according to the invasive behavior of the PDAC cell variants. To account for these differences, Panc1 cell variants were imaged at time point 0, 24, 48, 72h, while Panc89 cell variants were measured at time point 0h as well as after 5 and 7 days. Determination of invasive fronts was carried by counting the number of protruding cells or cell clusters in each spheroid in the image data generated by the NYONE® Scientific Imager (YT®-Software, SynenTec GmbH, Elmshorn, DE). Invasive distances were measured using a digital measuring instrument integrated into the YT®-Software. Specifically, the invasive distance was determined by utilizing a green-marked circle corresponding to the spheroid diameter as the starting point, while the distal edge of the cells facing away from the spheroid was designated as the end point of maximum invasion.
Adhesion assay
To analyze cell adhesion of Panc1 and Panc89 cell variants, 1× 105/well HuLEC-5a, Met-5a or TMNK-1 cells, each diluted in 200 µl of their respective medium, were seeded in a 96-well plate. After 24h, Panc1 and Panc89 cell variants were added. Before, 5 × 105 cells/ml PDAC cells were suspended in FCS-free Panc-medium and stained with CellTracker Green CMFDA (5-chloromethylfluorescein diacetate, diluted 1:2000, Thermo Scientific, Schwerte, DE) for 30 min at 37° C under consistent agitation.
After staining, cells were washed with Panc-medium and seeded at 1 × 104 cells/well in 200 µl Panc-medium onto HuLEC-5a, Met-5a or TMNK-1 cells. Afterward, plates were centrifuged at 300× g for 5 min at RT to ensure subsequent contact of all PDAC cells to endothelial or mesothelial cells. Immediately thereafter, the plate was measured in the NYONE® Imager (SynenTec GmbH, Elmshorn, DE), determining the maximal fluorescence count of PDAC cells (t = 0h). To determine the fluorescence of PDAC cells adhered to the endothelial or mesenchymal cells, measurement was performed after 4h. Before measuring, medium containing non-adhered PDAC cells was removed from the wells and replaced with fresh Panc-medium. The number of fluorescent cells at 4h was compared to the number of fluorescent cells at time 0 h to determine the adhesion rate. The adhesion rate was calculated as percentage of adherent cells by dividing the number of fluorescent cells at each time point by the number of fluorescent cells at time 0h.
Tumorigenicity assay in vivo
All animal studies were executed in compliance with the European guidelines for care and use of laboratory animals and approved by local authorities (V242-44598/2018 (80-8/18)). The tumorigenicity assay for Panc1 Holo- and Paraclone cells was already performed [
41]. The analysis of tumorigenicity and metastatic behavior of Panc89 Holo- and Paraclone cells was performed accordingly [
41]. For this purpose, 1 × 10
4 cells of either Panc89 Holo- or Paraclone cells were diluted in 75 µl PBS and intrasplenically inoculated in 8-week-old, female SCID-beige mice (each group n = 10) (Charles River, Sulzfeld, DE). Progression of tumor formation was monitored regularly by palpation and abdominal ultrasound examination using Vevo 770 (FUJIFILM VisualSonics Inc., Toronto, CA). Mice were sacrificed depending on tumor burden and general health condition. Organs and tumors were fixed in 4% PBS-buffered formalin and embedded in Paraffin for sectioning and immunohistological examination.
Immunohistochemical staining of Paraffin-embedded tissue sections
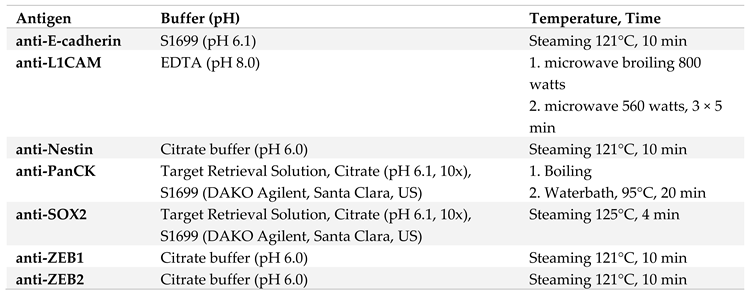
Formalin-fixed and Paraffin-embedded (FFPE) organs and tumors from Panc1 or Panc89 Holo- or Paraclone cell inoculated mice were used for immunohistochemical (IHC) analysis. Antigen retrieval was performed as listed in
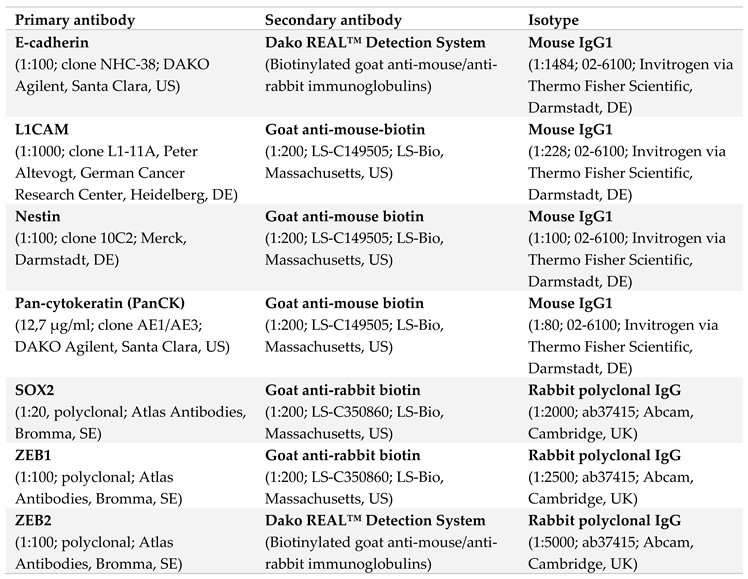
Table 2 and tissue sections were stained with antibodies listed in
Table 3. Incubation of anti-PanCK, anti-L1CAM, anti-ZEB1, anti-Nestin and anti-SOX2 was performed 1h at RT (30 min for anti-E-cadherin), followed by 30 min incubation at RT for all secondary antibodies (15 min for ZEB2 secondary antibody).
IHC sections were digitalized (Axioscan Z1, Carl Zeiss AG, Jena, DE) and stainings were analyzed using ZEN3.3 software (Carl Zeiss AG, Jena, DE). To quantify IHC staining of tumors, a tumor score was defined to characterize tumors in terms of percentage of positive stained cells (Frequency) and staining intensity (Intensity). The Frequency score was set from 1-4, Intensity score was set from 1-3 (
Table 4).
Statistical analysis
The statistical evaluation was carried out using GraphPad Prism (Version 9.5.0 (GraphPad Software by Docmatics, San Diego, California). All data sets were tested for normal distribution using Shapiro-Wilk test.
Parametric data including multiple groups were tested by one-way analysis of variance (one-way ANOVA) for statistical significance. Non-parametrical datasets of multiple groups were analyzed with Kruskal-Wallis one-way ANOVA on ranks test. Statistically significant differences between the groups were assumed at p-values ≤ 0.05 according to Student-Newman-Keuls method (parametric data) and Dunn’s method (non-Parametric data), respectively.
Graphs of parametric data were presented by mean with standard deviation or mean with standard error of means (depending on technical and biological replicates), while graphs of non-parametric data were presented by median with (interquartile) range. Students t-test was used to examine two samples of normally distributed data.
Columned data sets were analyzed using one-way-ANOVA plus Tukey´s multiple comparisons test for parametric data and Kruskal-Wallis test plus Dunn´s multiple comparisons test for non-parametric data. Grouped data sets were analyzed using two-way-ANOVA and Tukey´s multiple comparisons test. Growth curve experiments were analyzed via nonlinear regression (curve fit) to maintain the growth rate (κ) for logistic growth.
Survival analysis was performed by Kaplan-Meier-Survival analysis with curve comparison and Mantel-Cox log-rank test, resulting in significantly different survival curves with a p-value of ≤ 0.001 (***).
Statistical significance was defined at a p-value of ≤ 0.033. Significances are indicated by asterisks: p ≤ 0.033= *, p ≤ 0.002= **, p ≤ 0.001= ***.
4. Discussion
Cancer cell plasticity and tumor heterogeneity, e.g., manifested by the presence and interplay of CSC and non-CSC populations, along with the pronounced tumor stroma are associated with poor prognosis and increased resistance to chemotherapy of PDAC [
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71]. Besides, CSCs and EMT are both linked to cancer progression and early metastasis, which is also applies to PDAC [
18,
19,
20,
72]. CSCs are characterized by the unique ability to self-renewal, initiation of tumoral lesions at primary and secondary sites, the capability to resist cell death induction and to give rise to plastic, highly proliferative transit-amplifying progenitor cell populations [
21,
22,
23,
73,
74,
75,
76,
77]. As they also generate more or less differentiated cancer cells, CSCs are one important origin of tumor cell heterogeneity [
20,
24,
25,
26,
27,
78]. The link between EMT and MET processes and CSCs has been already shown [
18,
19,
20,
72]s. Yet, it is still poorly understood whether CSC- and EMT-phenotypes are always interconnected, how CSC-phenotypes of epithelial and mesenchymal tumor cells are characterized, and whether these might be related to different functional malignant outcomes. To gain a better understanding of plasticity in PDAC with a particular focus on CSCs and EMT and its contribution to malignancy associated properties, two different PDAC cell models were comparatively analyzed. Mesenchymal Panc1 cells derived from a primary PDAC have presumably already undergone EMT, while Panc89 cells originate from a lymph node metastasis and have most likely undergone EMT and MET leading to metastatic outgrowth in the lymph node [
79]. Isolated Panc1 and Panc89 Holo- and Paraclone cells, as well as the two related parental cell populations, were comprehensively analyzed by expression and functional analysis in vitro and
in vivo. First, CFAs and expression analysis of CSC-markers revealed different CSC phenotypes in the two PDAC cell lines. While Panc1 Holoclone cells were characterized by high Nestin expression and absent SOX2 expression, Panc89 Holoclone cells exhibited an inverse expression pattern showing a SOX2 dominated phenotype. Moreover, the CSC-phenotype of Panc89 Holoclones was associated with a higher colony formation ability compared to Panc1 Holoclones, indicating a more pronounced self-renewal capacity of these cells. Of note, pathways involved in the activity of cell cycle checkpoints, transcriptional regulation of TP53 and Mueller Plurinet seemed to generally play a role in CSC populations of PDAC, as these genes were upregulated in both Panc1 and Panc89 Holoclone cells compared to related Paraclones. Mueller Plurinet is a protein-protein network shared by pluripotent cells and has become a prominent classifying system for pluripotency and self-renewal properties [
55]. Activation of TP53 and cell cycle checkpoints are interconnected since TP53 enables dividing cells to repair anomalies before proceeding through cell cycle checkpoints, acting as a tumor suppressor [
80,
81,
82,
83]. Therefore, mutations in TP53 are strongly associated with tumor progression in various human cancers, also PDAC, and recent reports have shown its impact on stemness properties in cancer cells [
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90]. Overall, these data support the existence of distinct CSC-phenotypes in PDAC cells.
Furthermore, clear differences with respect to the EMT-phenotype were noted. Parental Panc89 and Panc89 Holoclone cells were predominantly characterized by an epithelial phenotype exemplified by high E-cadherin expression and concomitantly low expression of mesenchymal markers (Vimentin, L1CAM, ZEB1 and ZEB2). In contrast, all Panc1 cell variants were characterized by low E-cadherin expression and marked expression of mesenchymal markers underscoring the mesenchymal phenotype. As Panc89 Paraclone cells showed high E-cadherin expression, low Vimentin expression but also considerable expression of L1CAM, ZEB1 and ZEB2, which was higher compared to the other Panc89 cell variants but still lower compared to Panc1 cell variants (despite ZEB2 expression), these cells seemed to exhibit a partial/hybrid EMT-phenotype as described in other tumor cell models [
91,
92,
93,
94]. Moreover, these phenotypes were also widely confirmed by IHC staining of the tumors formed in vivo by the inoculated Holo- and Paraclone cell populations. However, in Panc89 Holoclone tumors areas with high L1CAM expression and low SOX2 expression but also with ZEB1 or ZEB2 expression were noted, and Panc1 Holoclone tumors showed elevated SOX2 expression which was absent in Panc1 Holoclone cells
in vitro. This might be due to the fact that SOX2 functions as a stability factor in the control of PDAC cell proliferation, being required for growth of PDAC cells. Apparently, SOX2 needs to be stably expressed, as increased as well as decreased levels of SOX2 reduces tumor growth of PDAC in vivo and in vivo [
95]. These findings support our data showing that a gain in SOX2 expression in Panc89 and Panc1 Holoclone tumors might be crucial to ensure tumor outgrowth. Altogether, these data indicate a robust phenotype stability of Holo- and Paraclones of either PDAC cell line under constant environmental conditions. Albeit when the latter are changing this might lead to a phenotypic switching of the tumor cells, presumably forced by altering environmental factors the tumor cells are exposed to, as seen in the in vivo tumors. Hence, myofibroblasts and macrophages, both important stroma cell populations in pancreatic tumors and metastases [
96,
97], were already shown to induce L1CAM expression in PDAC cells [
98,
99].
Even though the transcriptomic profiling revealed that both Panc1 and Panc89 Holoclone cells show similarities in TP53 and cell cycle checkpoint regulation as well as stemness associated Mueller Plurinet activity, we could identify two distinct EMT-CSC-phenotypes: a mesenchymal Nestin-dominated (Panc1 Holoclone cells) and an epithelial SOX2-dominated CSC-phenotype (Panc89 Holoclone cells). Of note, both phenotypes were associated with clearly different functional properties. Besides the more pronounced colony formation, Panc89 Holoclone cells showed a clearly faster growth behavior compared to Panc89 Paraclone cells but also to Panc1 Holoclone cells, being in line with the fact that proliferative activity of epithelial cells is generally higher than those of mesenchymal cells [
100,
101]. In line with a higher cell turnover of epithelial Panc89 cell variants, parental Panc89 cells as well as their Holoclone and Paraclone cells showed a better response towards Gemcitabine compared to mesenchymal Panc1 cell variants. These differences regarding the response to chemotherapeutic drugs were in accordance with those having already assigned to the classical and basal-like phenotype of PDAC cells [
56,
102]. Of note, mesenchymal CSC populations (Panc1 Holoclone cells) showed an even more resistant phenotype compared to their parental and non-CSC cells, while epithelial CSC (Panc89 Holoclone cells) showed the strongest treatment response. These data support the rationale for using chemotherapy as an effective treatment for fast proliferating tumor cells. However, this strategy fails to eliminate tumor cells with decreased growth rates [
103,
104,
105]. Furthermore, these findings underscore the heterogeneity of CSC which manifests in different treatment sensitivities.
Showing a slower cell growth, Panc1 cells exhibited a faster cell invasion in a typical mesenchymal cell manner with increased numbers of invasive fronts and longer invasion distances, underscored by the activity of EMT and invasion associated genes shown by the transcriptomic analysis. In contrast, Panc89 Holoclone cells used a cluster mode of slow cell invasion, again fitting together with the epithelial phenotype of these cells [
79,
100,
101]. Also in line with these findings, expression of
VIM [
106,
107,
108,
109,
110,
111] and
L1CAM [
98,
112,
113,
114] have been associated with an increased invasive potential in a variety of cancers, also in PDAC cells.
Moreover, these distinct growth and invasive properties were linked to different metastasis patterns in vivo. Accordingly, Panc1 Holoclone cells exhibiting the strongest invasive potential using a mesenchymal invasion mode led to the highest number of metastatic lesions predominantly in the pancreas compared Panc1 Paraclones as well as epithelial Panc89 cell variants which formed less but larger tumors. The fact that Panc1 Holoclone cells led to formation of more and larger tumors compared to Panc1 Paraclones underscores that the presence of CSC properties in mesenchymal PDAC cells is prerequisite for metastatic tumor formation.
However, inoculation of Panc89 Holoclones led the most pronounced macroscopic tumor burden with the highest number of large macroscopic tumors compared to all other cell variants. Albeit the differences between Panc89 Holo- and Paraclones were not that pronounced, also with respect to survival times, pointing again to a microenvironment mediated phenotypic switch of the tumor cells (see above). Of note, tumor formation of Panc1 and Panc89 Paraclone cells suggested that non-CSC variants can gain CSC properties, since both Paraclone variants were able to induce tumor formation, although not as pronounced as Panc1 and Panc89 Holoclone cells. Despite this gain of CSC properties in Panc1 and Panc89 Paraclone cells, expression of CSC-markers in vivo remained low in these tumors.
In contrast to Panc1 tumors, tumors formed by Panc89 cell variants predominantly manifested in the peritoneum. Adhesion assays to organ specific endothelial cells did not reveal any considerable differences between Holo- and Paraclone populations of either cell line but indicated that Panc89 cell variants better adhere to lung endothelium than Panc1 cell variants. However, these results were not in line with tumor manifestation patterns in vivo. In contrast, this might indicate that the different organ colonization and metastatic outgrowth of the inoculated cell populations rely more on other cell properties and the local microenvironment in the organ and not so much on adhesion to the organ-specific endothelium. Moreover, CSC properties do not seem to determine homing to secondary sites rather than dissemination mode and outgrowth of the tumor cells.
As outlined before, both Nestin and SOX2 have been associated with CSC-phenotypes and were linked to malignancy associated properties. Thus, in a murine PDAC model, shRNA mediated reduction of Nestin expression led to decreased tumor volume and hepatic metastases [
37,
38], being in line with our results demonstrating that Nestin expressing Panc1 Holoclone cells showed higher self-renewal capacity and invasive properties in vitro and formed a higher number of tumors in vivo compared to Panc1 Paraclone cells. In contrast, Panc89 Holoclones lacked Nestin expression but were characterized by high SOX2 expression compared to Panc89 Paraclone cells, as well as compared to all Panc1 cell variants. However, in vivo Panc1 and Panc89 Holoclone tumors both exhibited similar amounts of SOX2, supporting the role of the microenvironment as a determining factor of CSC properties [
20,
24,
25,
26,
27,
115]. Of note, elevated SOX2 expression in Panc89 Holoclones was associated with the formation of the highest number of colonies and the fastest growth rate in vitro and the formation of the largest tumors
in vivo. Furthermore, it can be speculated that elevation of SOX2 might also be involved in the outgrowth of Panc1 Holoclone tumors [
32,
95,
116].
PDAC cells are known to predominantly metastasize to the liver, lung and the peritoneum [
4,
5,
6]. As described above, the highest number of Panc1 Holoclone tumors was predominantly found in the pancreas, while Panc89 Holoclone cells formed most tumors in the peritoneum.
The fact that mesenchymal Panc1 cells are derived from the primary tumor, together with their gene activity associated with EMT and invasion, suggests that these cells have undergone EMT to leave the primary tumor. However, whether these cells would ever have been able to form metastases in this patient remains unsolved. Furthermore, it may explain, why most Panc1 Holoclone tumors were found in the pancreas, indicating that these cells are still optimally adapted to their original tissue. In contrast, epithelial Panc89 cells originate from a lymph node metastasis, thus these cells have proven their disseminating potential to leave the primary tumor (either after EMT using a mesenchymal invasion mode or while maintaining an epithelial/hybrid cell stage using a cluster-like mode) and to grow out as metastasis at a secondary site (e.g., after MET).
Author Contributions
Conceptualization: L-MP and SSe; Investigation: L-MP, U-UY, AS, AK, A-SM, CH, J-PG, OW, PH, LS, SF, HK, US, HB; Resources: US, SF, HB, SSe; Writing—original draft preparation: L-MP, SSe; Writing—review and editing: all authors; Visualization: L-MP; Funding acquisition: SF, SSe. L-MP, U-UY, AS, AK, A-SM, CH, J-PG, OW, PH, LS, SF, HK, US, HB and SSe contributed to the article and approved the submitted version.
Figure 1.
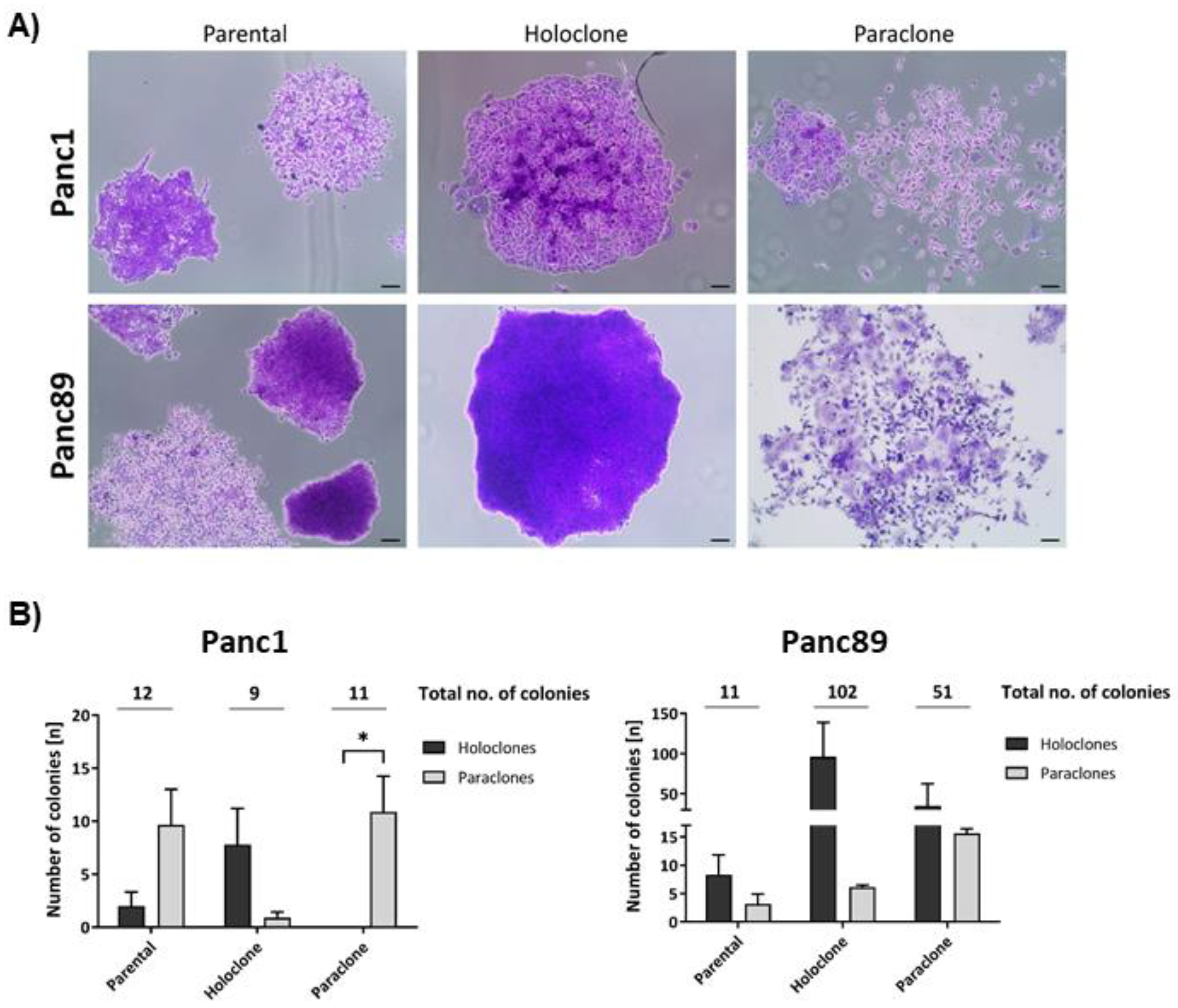
Panc1 and Panc89 cell variants exhibit differences in their colony formation ability. Parental Panc1 and Panc89 as well as Holo- and Paraclone cells were analyzed regarding their colony formation ability via CFA. 400 cells of each cell line were seeded in duplicates/triplicates in 6-well plates and after eight to eleven days, colonies were fixed with paraformaldehyde and stained with crystal violet. The colonies formed were morphologically characterized regarding Holo- and Paraclones. A) Typical colony morphologies generated by parental cells as well as the respective Holo- and Paraclones. B) Number of Holo- and Paraclones per well formed by parental Panc1, Holo- and Paraclone cells (left) as well as parental Panc89, Holo- and Paraclone cells (right). Y-axis in B (right) is segmented with 0-18 for the bottom part and 30-200 for the upper part. Independent experiments n = 3, scale bar = 100 µm.
Figure 1.
Panc1 and Panc89 cell variants exhibit differences in their colony formation ability. Parental Panc1 and Panc89 as well as Holo- and Paraclone cells were analyzed regarding their colony formation ability via CFA. 400 cells of each cell line were seeded in duplicates/triplicates in 6-well plates and after eight to eleven days, colonies were fixed with paraformaldehyde and stained with crystal violet. The colonies formed were morphologically characterized regarding Holo- and Paraclones. A) Typical colony morphologies generated by parental cells as well as the respective Holo- and Paraclones. B) Number of Holo- and Paraclones per well formed by parental Panc1, Holo- and Paraclone cells (left) as well as parental Panc89, Holo- and Paraclone cells (right). Y-axis in B (right) is segmented with 0-18 for the bottom part and 30-200 for the upper part. Independent experiments n = 3, scale bar = 100 µm.
Figure 2.
Panc1 and Panc89 cell variants show distinct transcriptional and gene expression CSC and EMT signatures. A) Principal Component Analysis (PCA) based on the 10% most varying transcripts across all of parental Panc1 and Panc89, Holo- and Paraclone cell samples using the R package PCAtools (v2.10.0). PCA was performed for the highest variation in the first principal component (PC1) on the X-axis, and for the lower variations by the second principal component (PC2) on the Y-axis. All cell variants are denoted by color (red = Panc1 cell variants, blue = Panc89 cell variants) and symbol, respectively. B) Gene Set Variation Analysis (GSVA) of gene sets associated with positive or negative regulation on the EMT program and invasion properties in parental Panc1 and Panc89, Holo- and Paraclone cells. The numbering of all cell variants represents the number of the independent replicate. C) Transcriptomic analysis of CSC- and EMT- associated marker genes in parental Panc1 and Panc89 as well as Holo- and Paraclone cells. CDH1 (epithelial), VIM and L1CAM (mesenchymal) represented EMT-marker genes, ZEB1 and ZEB2 represented marker genes for EMT-induction and NES, OVOL2 and SOX2 represented CSC-marker genes. Data are represented as means with SD. Gene expression analysis by qPCR was performed for the CSC-marker D) NES, SOX2 and OVOL2, for the EMT-marker E) CDH1, L1CAM and VIM as well as for EMT-inducers F) ZEB1 and ZEB1. G) Immunofluorescence staining of EMT- and CSC-markers was performed for Panc1 cell variants with the CSC-markers NESTIN and the EMT-inducer ZEB2, while the CSC-markers SOX2 and the mesenchymal EMT-marker L1CAM were stained in Panc89 cell lines. All cell nuclei were stained with Hoechst 33342. Representative images from n = 3 independent experiments are shown (scale bar = 200 µm). Every analysis was performed with independent experiments n = 3.
Figure 2.
Panc1 and Panc89 cell variants show distinct transcriptional and gene expression CSC and EMT signatures. A) Principal Component Analysis (PCA) based on the 10% most varying transcripts across all of parental Panc1 and Panc89, Holo- and Paraclone cell samples using the R package PCAtools (v2.10.0). PCA was performed for the highest variation in the first principal component (PC1) on the X-axis, and for the lower variations by the second principal component (PC2) on the Y-axis. All cell variants are denoted by color (red = Panc1 cell variants, blue = Panc89 cell variants) and symbol, respectively. B) Gene Set Variation Analysis (GSVA) of gene sets associated with positive or negative regulation on the EMT program and invasion properties in parental Panc1 and Panc89, Holo- and Paraclone cells. The numbering of all cell variants represents the number of the independent replicate. C) Transcriptomic analysis of CSC- and EMT- associated marker genes in parental Panc1 and Panc89 as well as Holo- and Paraclone cells. CDH1 (epithelial), VIM and L1CAM (mesenchymal) represented EMT-marker genes, ZEB1 and ZEB2 represented marker genes for EMT-induction and NES, OVOL2 and SOX2 represented CSC-marker genes. Data are represented as means with SD. Gene expression analysis by qPCR was performed for the CSC-marker D) NES, SOX2 and OVOL2, for the EMT-marker E) CDH1, L1CAM and VIM as well as for EMT-inducers F) ZEB1 and ZEB1. G) Immunofluorescence staining of EMT- and CSC-markers was performed for Panc1 cell variants with the CSC-markers NESTIN and the EMT-inducer ZEB2, while the CSC-markers SOX2 and the mesenchymal EMT-marker L1CAM were stained in Panc89 cell lines. All cell nuclei were stained with Hoechst 33342. Representative images from n = 3 independent experiments are shown (scale bar = 200 µm). Every analysis was performed with independent experiments n = 3.
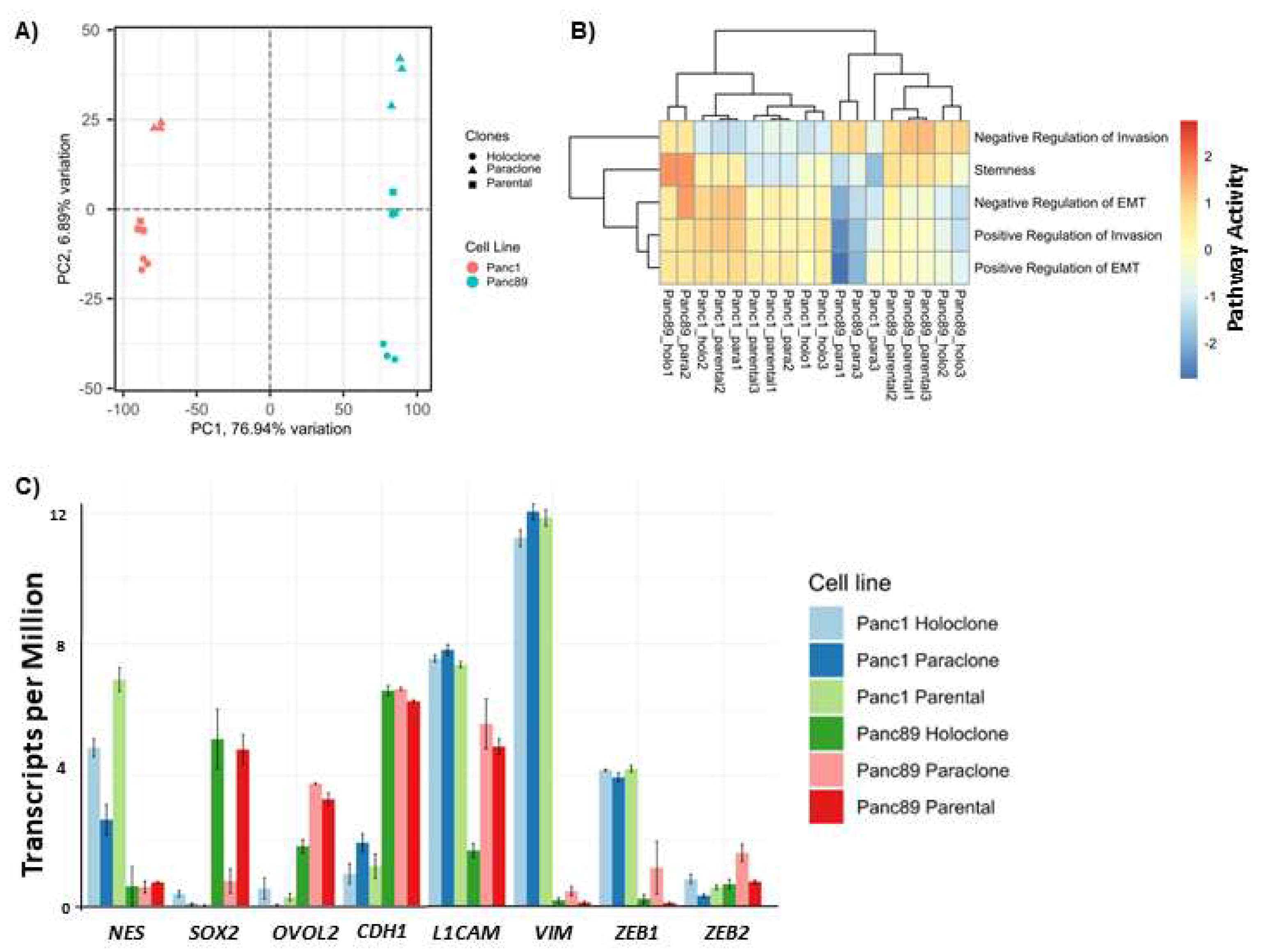
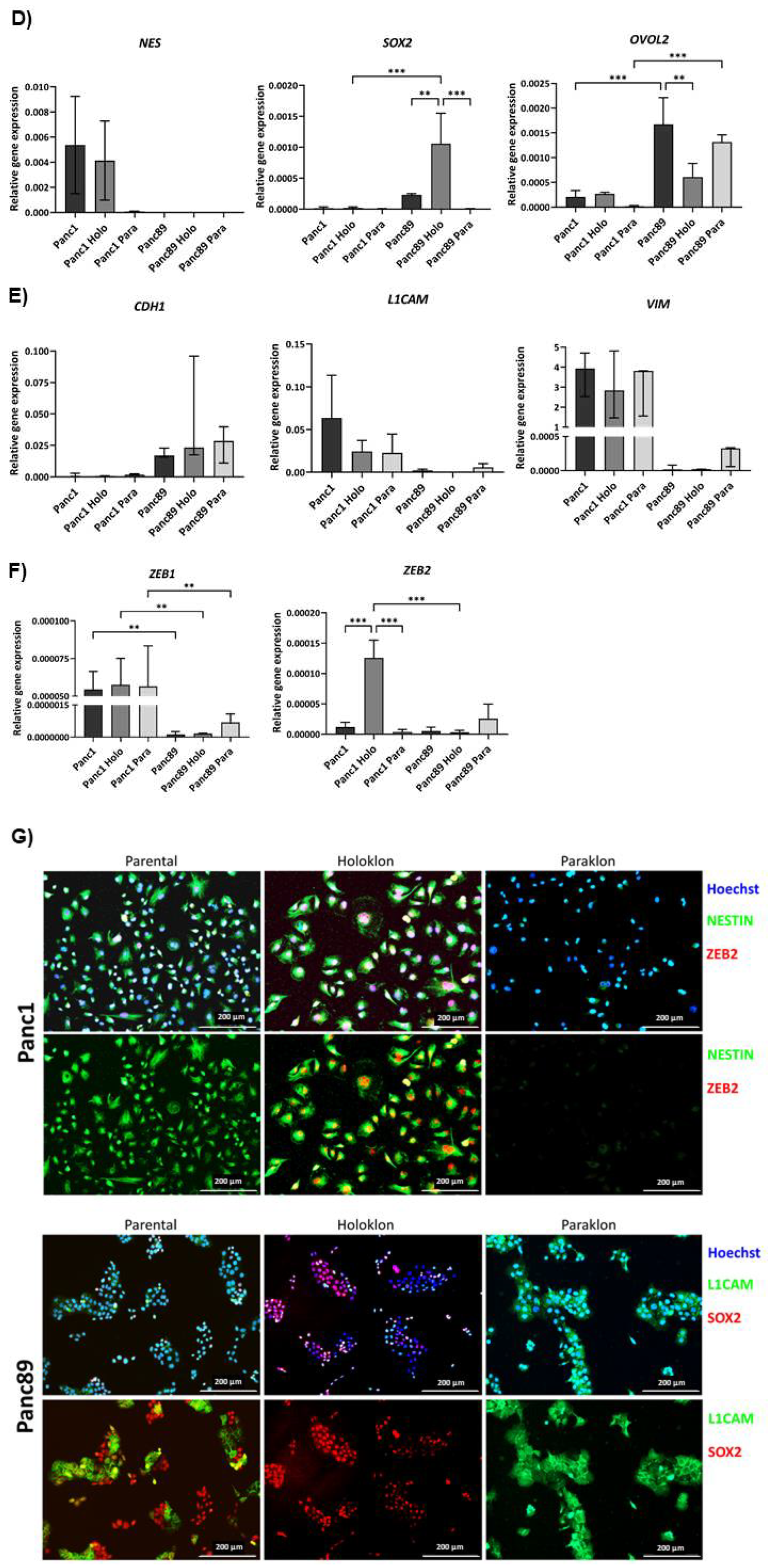
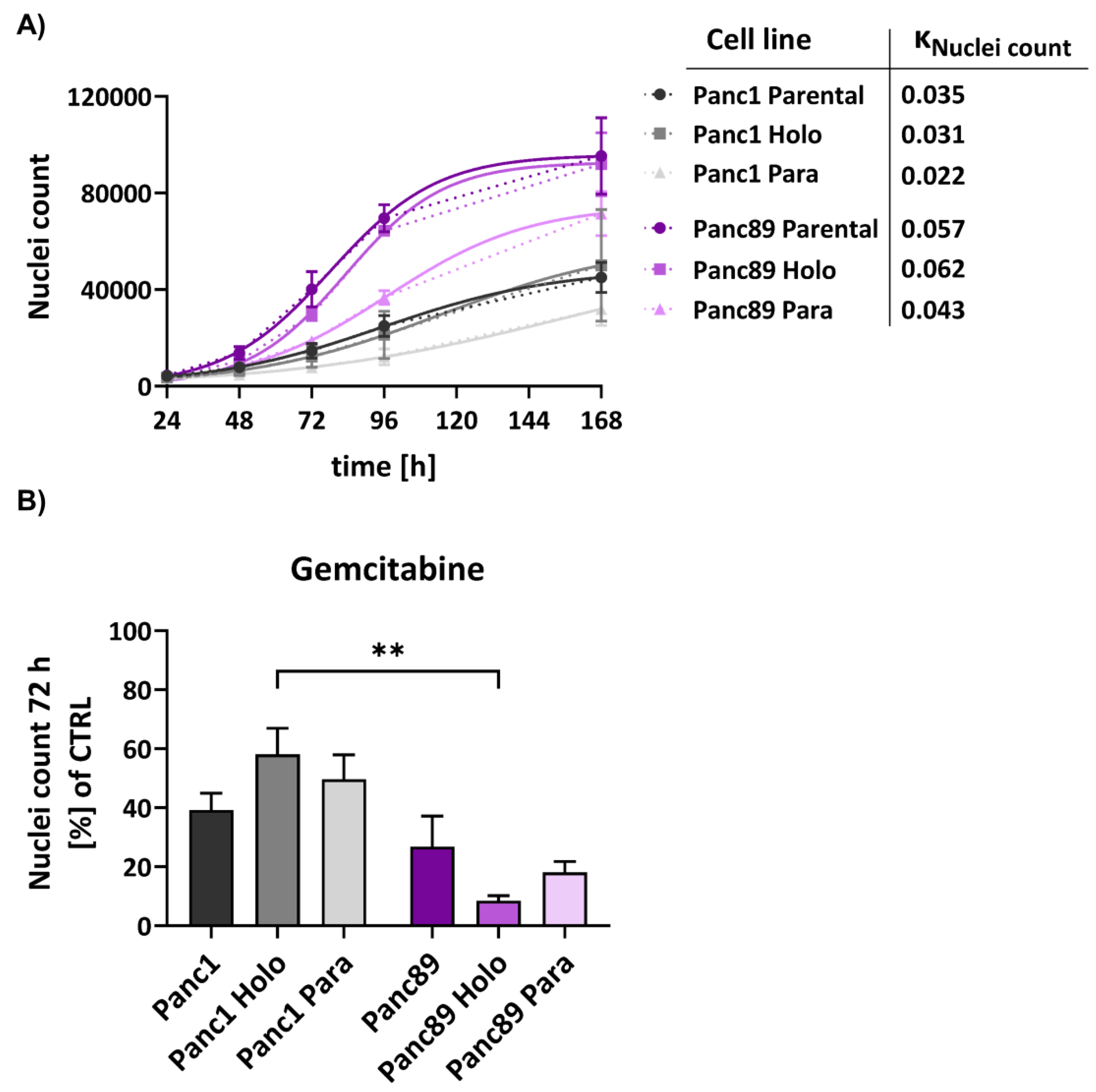
Figure 3.
Panc1 and Panc89 cell variants show different characteristics regarding cell growth rates and response to chemotherapy. A) Growth rate analysis of parental Panc1 and Panc89, as well as Holo- and Paraclone cells revealed Panc89 cell lines to show faster cell growth compared to Panc1 cell lines. 5×103 cells of each cell line were seeded in a 96-well plate in triplicates and nuclei number was monitored via Hoechst 33342 staining for 168 h. Data are depicted as total number of counted cell nuclei. B) Panc1 and Panc89 cell variants were seeded at 5×104 cells in duplicates in 96-well plates. After 24h, cells were left untreated or treated with 0.0038 µM Gemcitabine. After 72h, cells were stained with Hoechst 33342 for nuclei count analysis and nuclei number of treated cells was normalized to nuclei number of untreated cells. Every analysis was performed with independent experiments n = 3. (kNuclei count = growth rate).
Figure 3.
Panc1 and Panc89 cell variants show different characteristics regarding cell growth rates and response to chemotherapy. A) Growth rate analysis of parental Panc1 and Panc89, as well as Holo- and Paraclone cells revealed Panc89 cell lines to show faster cell growth compared to Panc1 cell lines. 5×103 cells of each cell line were seeded in a 96-well plate in triplicates and nuclei number was monitored via Hoechst 33342 staining for 168 h. Data are depicted as total number of counted cell nuclei. B) Panc1 and Panc89 cell variants were seeded at 5×104 cells in duplicates in 96-well plates. After 24h, cells were left untreated or treated with 0.0038 µM Gemcitabine. After 72h, cells were stained with Hoechst 33342 for nuclei count analysis and nuclei number of treated cells was normalized to nuclei number of untreated cells. Every analysis was performed with independent experiments n = 3. (kNuclei count = growth rate).
Figure 4.
Panc1 and Panc89 cell lines show variances in their migration, invasion and adhesion abilities. A) Migration ability of parental Panc1 and Panc89 as well as Holo- and Paraclone cells was determined by monitoring the increase of cell confluence on a cell-free gap for 8h, revealing Panc89 cell lines to show increased cell migration compared to Panc1 cell lines. B) Representative images of cell migration of Panc1 and Panc89 cell variants after 8h. D) Representative images of spheroid formation and invasion ability of Panc1 and Panc89 cell variants. E) Spheroid size for Panc1 cell variants on day 0 and day 3 and Panc89 cell variants on day 0 and day 7. F) Analysis of the number of invasive fronts of Panc1 cell variants (day 1, day 3) and Panc89 cell variants (day 5, day 7). F) Analysis of invasion distance of Panc1 cell variants (day 1, day 3) and Panc89 cell variants (day 5, day 7). H) Adhesion assays for Panc1 and Panc89 cell variants were performed with liver endothelial cells, lung endothelial cells and mesothelial cells for 4h. Data of n = 3 independent experiments are shown.
Figure 4.
Panc1 and Panc89 cell lines show variances in their migration, invasion and adhesion abilities. A) Migration ability of parental Panc1 and Panc89 as well as Holo- and Paraclone cells was determined by monitoring the increase of cell confluence on a cell-free gap for 8h, revealing Panc89 cell lines to show increased cell migration compared to Panc1 cell lines. B) Representative images of cell migration of Panc1 and Panc89 cell variants after 8h. D) Representative images of spheroid formation and invasion ability of Panc1 and Panc89 cell variants. E) Spheroid size for Panc1 cell variants on day 0 and day 3 and Panc89 cell variants on day 0 and day 7. F) Analysis of the number of invasive fronts of Panc1 cell variants (day 1, day 3) and Panc89 cell variants (day 5, day 7). F) Analysis of invasion distance of Panc1 cell variants (day 1, day 3) and Panc89 cell variants (day 5, day 7). H) Adhesion assays for Panc1 and Panc89 cell variants were performed with liver endothelial cells, lung endothelial cells and mesothelial cells for 4h. Data of n = 3 independent experiments are shown.
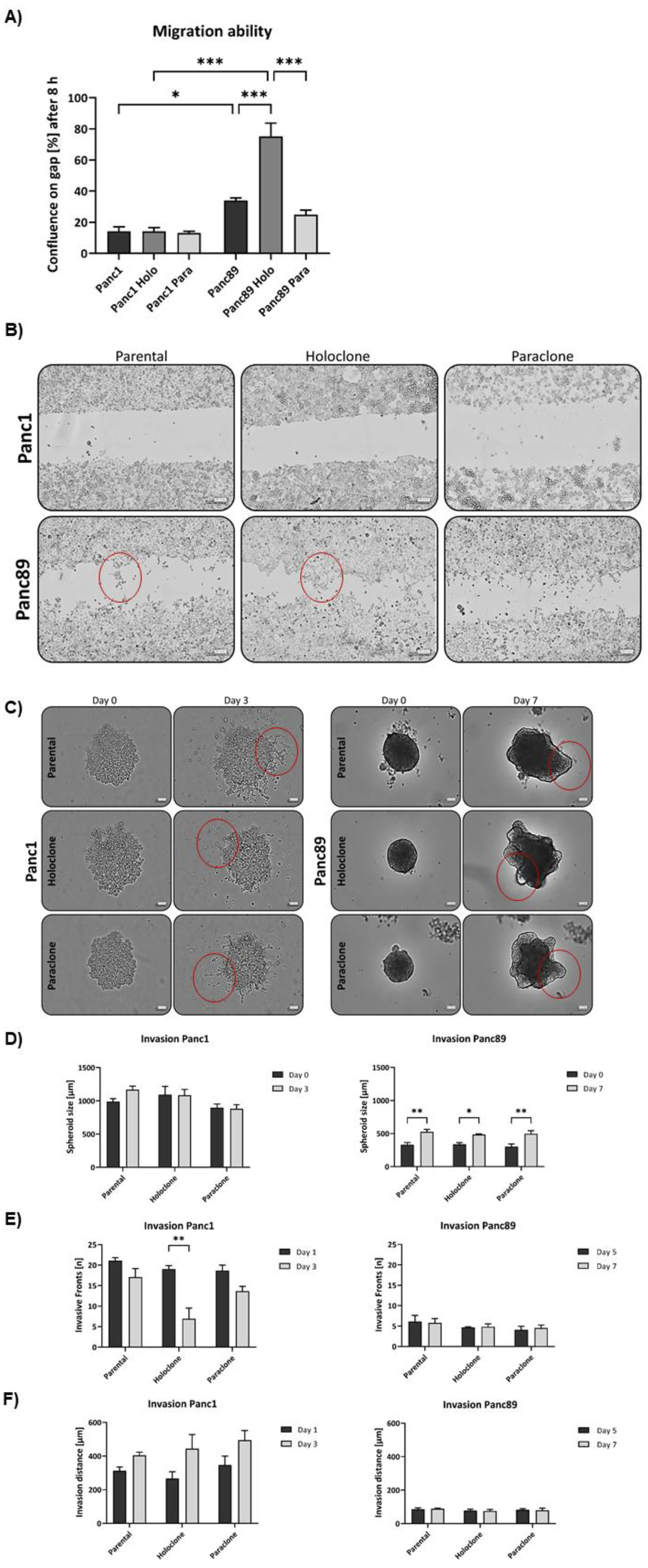
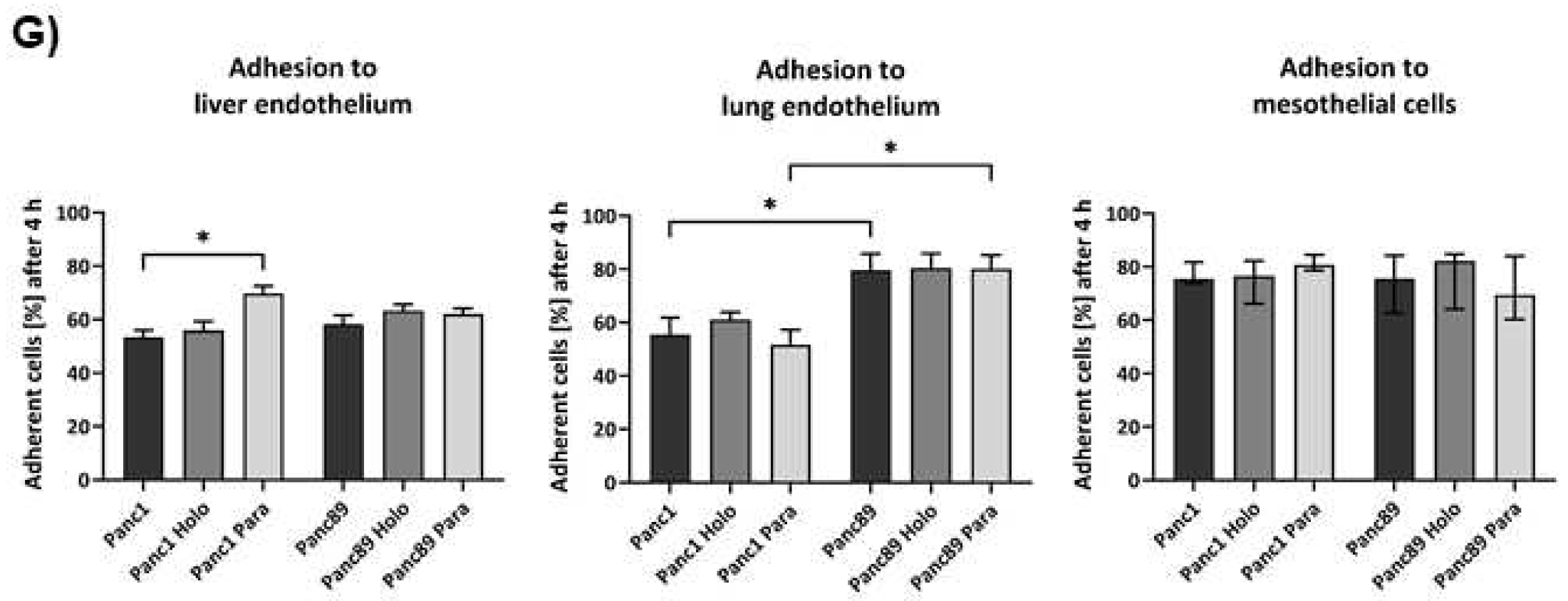
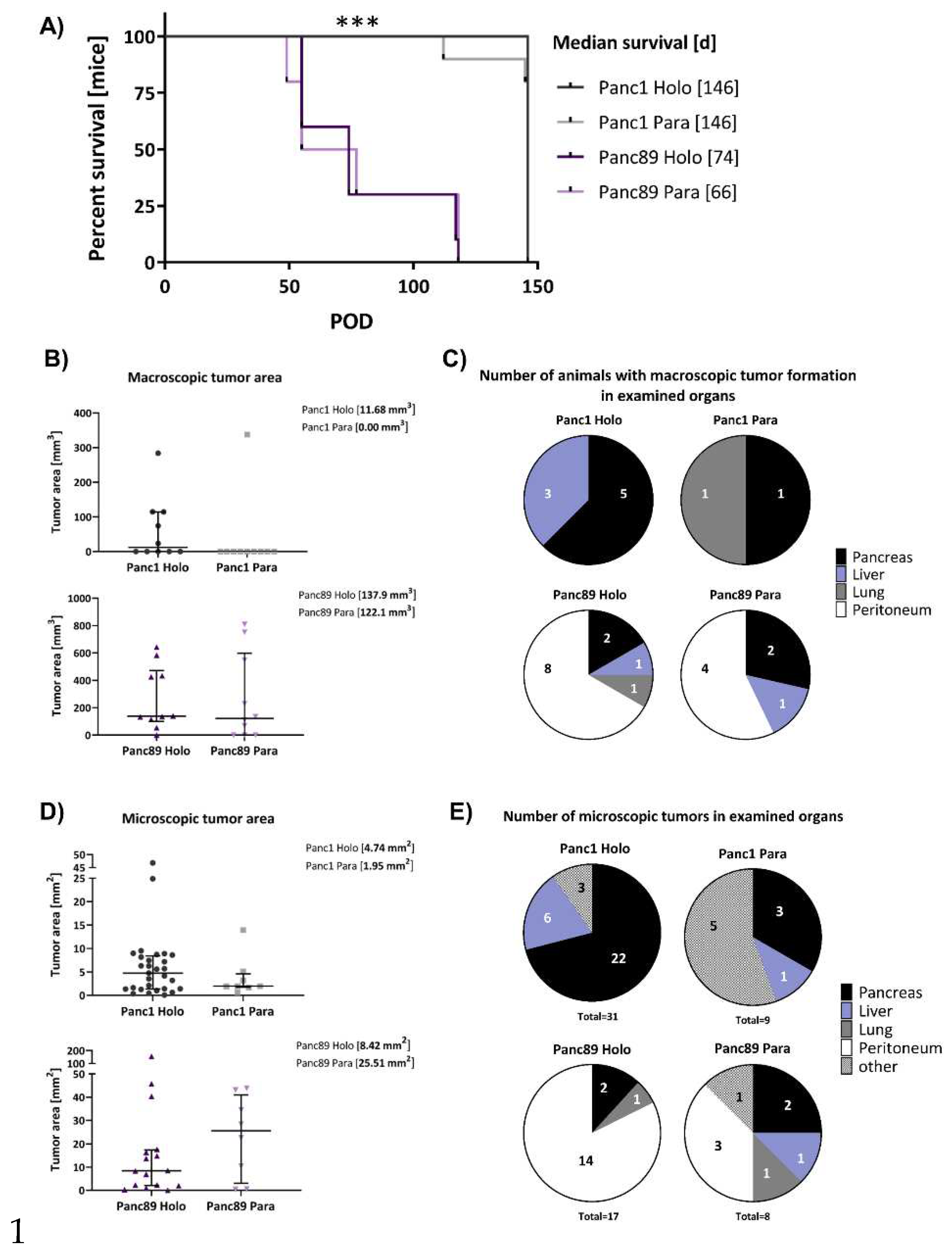
Figure 5.
Panc1 and Panc89 cell clones differ with respect to their metastatic capacity in vivo. Mice were inoculated intrasplenically with 1×104 Panc1 or Panc89 Holo- or Paraclone cells (group of ten mice per cell line). A) Kaplan-Meier-Survival analysis of animals inoculated with Panc1 or Panc89 Holo- and Paraclone cells. B, C) Macroscopic tumor areas [mm3] and organs of macroscopic tumor manifestation in Panc1 and Panc89 Holo- or Paraclone cell inoculated mice. D, E) Microscopic tumor areas [mm2] as well as number and location of microscopic tumors after staining of tissue sections. Statistical analysis was performed with unpaired t-test for non-parametric data and Mann-Whitney test. (Holo = Holoclone cells, Para = Paraclone cells, POD = post-operative day).
Figure 5.
Panc1 and Panc89 cell clones differ with respect to their metastatic capacity in vivo. Mice were inoculated intrasplenically with 1×104 Panc1 or Panc89 Holo- or Paraclone cells (group of ten mice per cell line). A) Kaplan-Meier-Survival analysis of animals inoculated with Panc1 or Panc89 Holo- and Paraclone cells. B, C) Macroscopic tumor areas [mm3] and organs of macroscopic tumor manifestation in Panc1 and Panc89 Holo- or Paraclone cell inoculated mice. D, E) Microscopic tumor areas [mm2] as well as number and location of microscopic tumors after staining of tissue sections. Statistical analysis was performed with unpaired t-test for non-parametric data and Mann-Whitney test. (Holo = Holoclone cells, Para = Paraclone cells, POD = post-operative day).
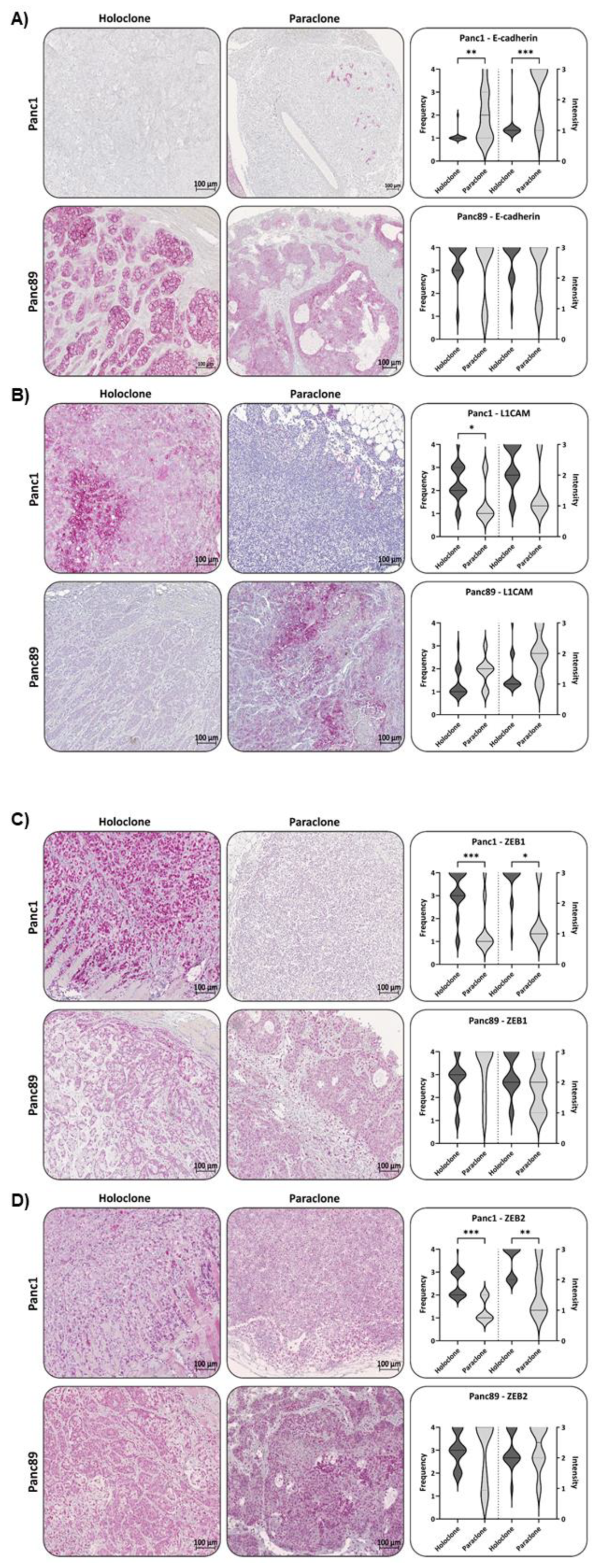
Figure 6.
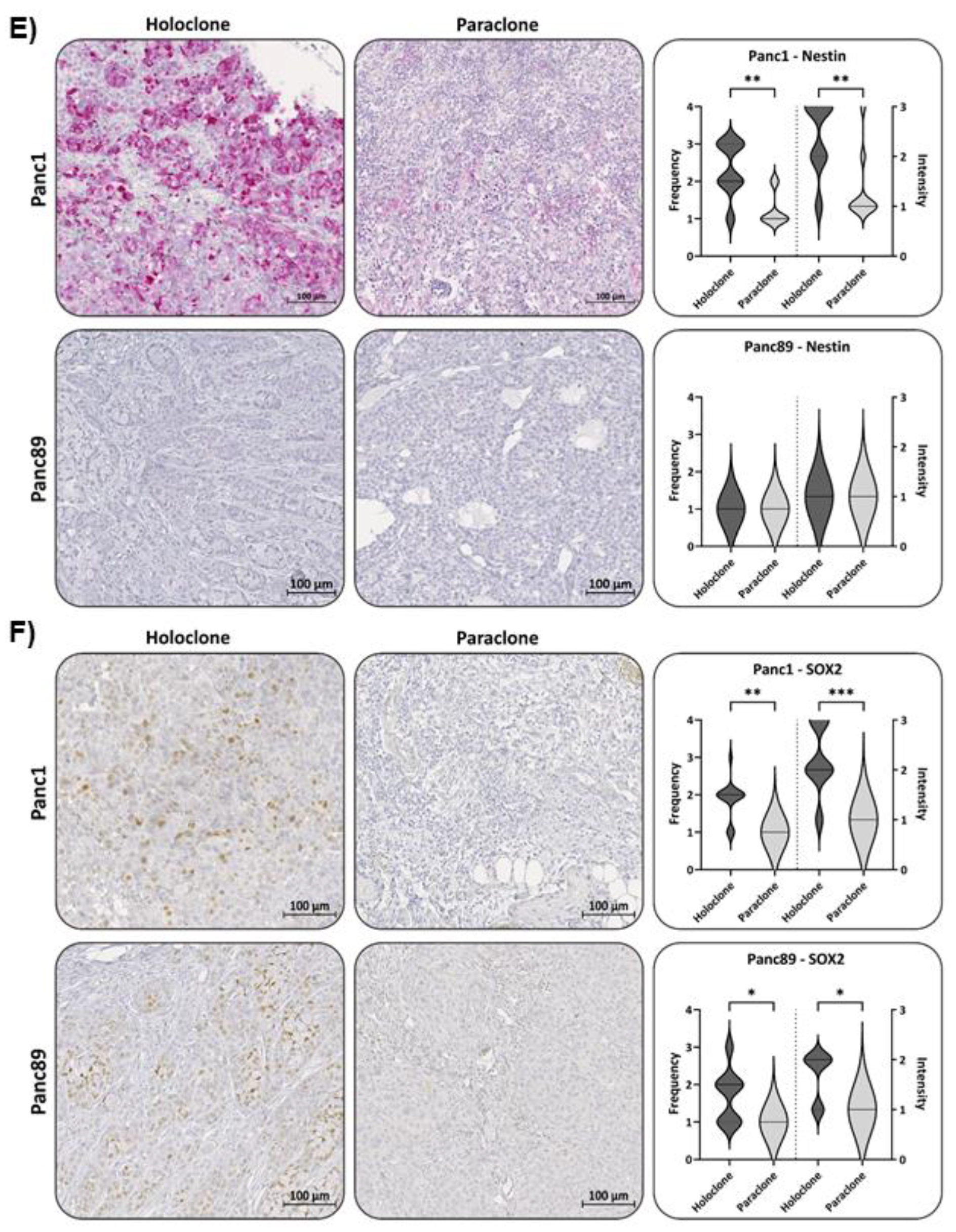
Panc1 and Panc89 Holo- and Paraclone tumors exhibit differences in EMT- and CSC- marker expression. Mice were inoculated intrasplenically with 1×104 Panc1 or Panc89 Holo- or Paraclone cells (group of ten mice per cell line) and extracted tissues and tumor lesions were stained for the epithelial marker A) E-cadherin, the mesenchymal marker B) L1CAM, the EMT-inducers C) ZEB1 and D) ZEB2 and the CSC-markers E) Nestin and F) SOX2. Representative pictures of stained tissues of Panc1 and Panc89 Holo- or Paraclone tumors are presented in the left and middle picture panels, the right panel shows the statistical analysis of frequency and intensity score of positively stained cells. The upper row shows the data of analyzed Panc1 Holo- and Paraclone lesions, the lower panel shows the data of Panc89 Holo- and Paraclone lesions. Scale bar = 100 µm.
Figure 6.
Panc1 and Panc89 Holo- and Paraclone tumors exhibit differences in EMT- and CSC- marker expression. Mice were inoculated intrasplenically with 1×104 Panc1 or Panc89 Holo- or Paraclone cells (group of ten mice per cell line) and extracted tissues and tumor lesions were stained for the epithelial marker A) E-cadherin, the mesenchymal marker B) L1CAM, the EMT-inducers C) ZEB1 and D) ZEB2 and the CSC-markers E) Nestin and F) SOX2. Representative pictures of stained tissues of Panc1 and Panc89 Holo- or Paraclone tumors are presented in the left and middle picture panels, the right panel shows the statistical analysis of frequency and intensity score of positively stained cells. The upper row shows the data of analyzed Panc1 Holo- and Paraclone lesions, the lower panel shows the data of Panc89 Holo- and Paraclone lesions. Scale bar = 100 µm.
Table 1.
Target genes, primer sequences, and annealing temperatures used for quantitative polymerase chain reaction.
Table 1.
Target genes, primer sequences, and annealing temperatures used for quantitative polymerase chain reaction.
Table 2.
List of antigen retrieval conditions.
Table 2.
List of antigen retrieval conditions.
Table 3.
List of antibodies used for IHC of FFPE tissue sections from Panc1 and Panc89 Holo- or Paraclone cell inoculated animals.
Table 3.
List of antibodies used for IHC of FFPE tissue sections from Panc1 and Panc89 Holo- or Paraclone cell inoculated animals.
Table 4.
Frequency Score (= % positively stained cells) and Intensity score (= intensity of staining) used for quantification of IHC analysis.
Table 4.
Frequency Score (= % positively stained cells) and Intensity score (= intensity of staining) used for quantification of IHC analysis.