Submitted:
16 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Coating Preparation
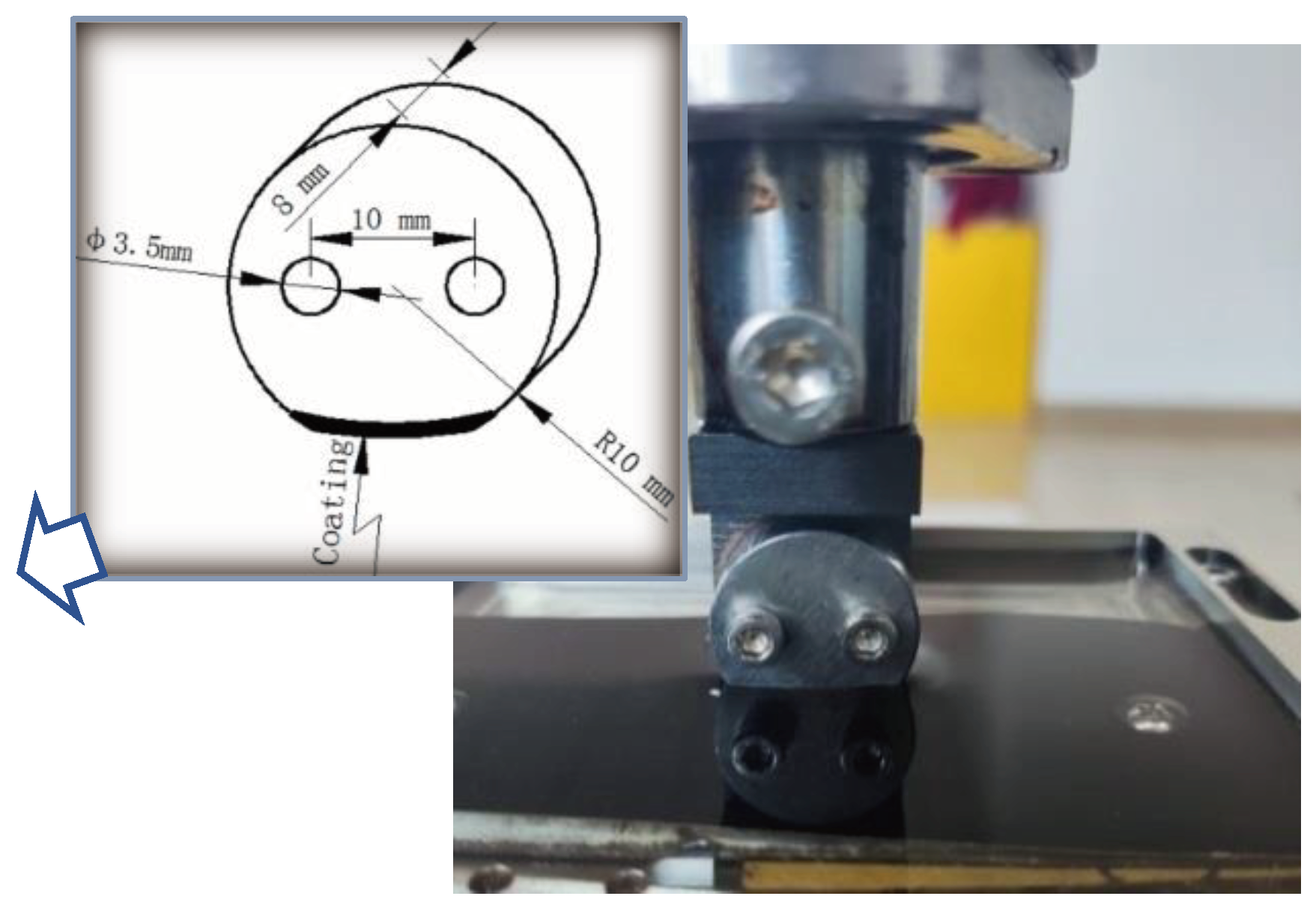
2.2. Wear Testing
| No. | Material | Preparation method | Particle size (μm) |
EDS (wt%) | |||||||||||
| Ni | Cr | Mo | Si | Fe | Nb | Ti | V | Zr | Al | C | B | ||||
| 1 | IN625 | Gas-water atomization | 30-70 | 55.46 | 22.85 | 9.94 | 0.24 | 4.11 | 4.96 | 1.21 | 0.32 | 0.28 | 0.32 | 0.10 | 0.21 |
| 2 | TA15 | PREP | 15-53 | 3.21 | 0.22 | 0.52 | 0 | 0 | 0.1 | 82.86 | 2.1 | 1.89 | 7.8 | 0.51 | 0.79 |
| 3 | B4C | Carbothermal reduction | 1-5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0.05 | 0.02 | 2.26 | 18.5 | 79.1 |
| Laser power P / w |
Linear velocity vL / (m.min-1) |
Axial offset d / (mm.r-1) |
Powder-feeding rate vP / (g.min-1) |
Protective airflow g / (L.min-1) |
| 4400 | 5 | 1.6 | 28 | 7 |
2.3. Microstructure Characterization
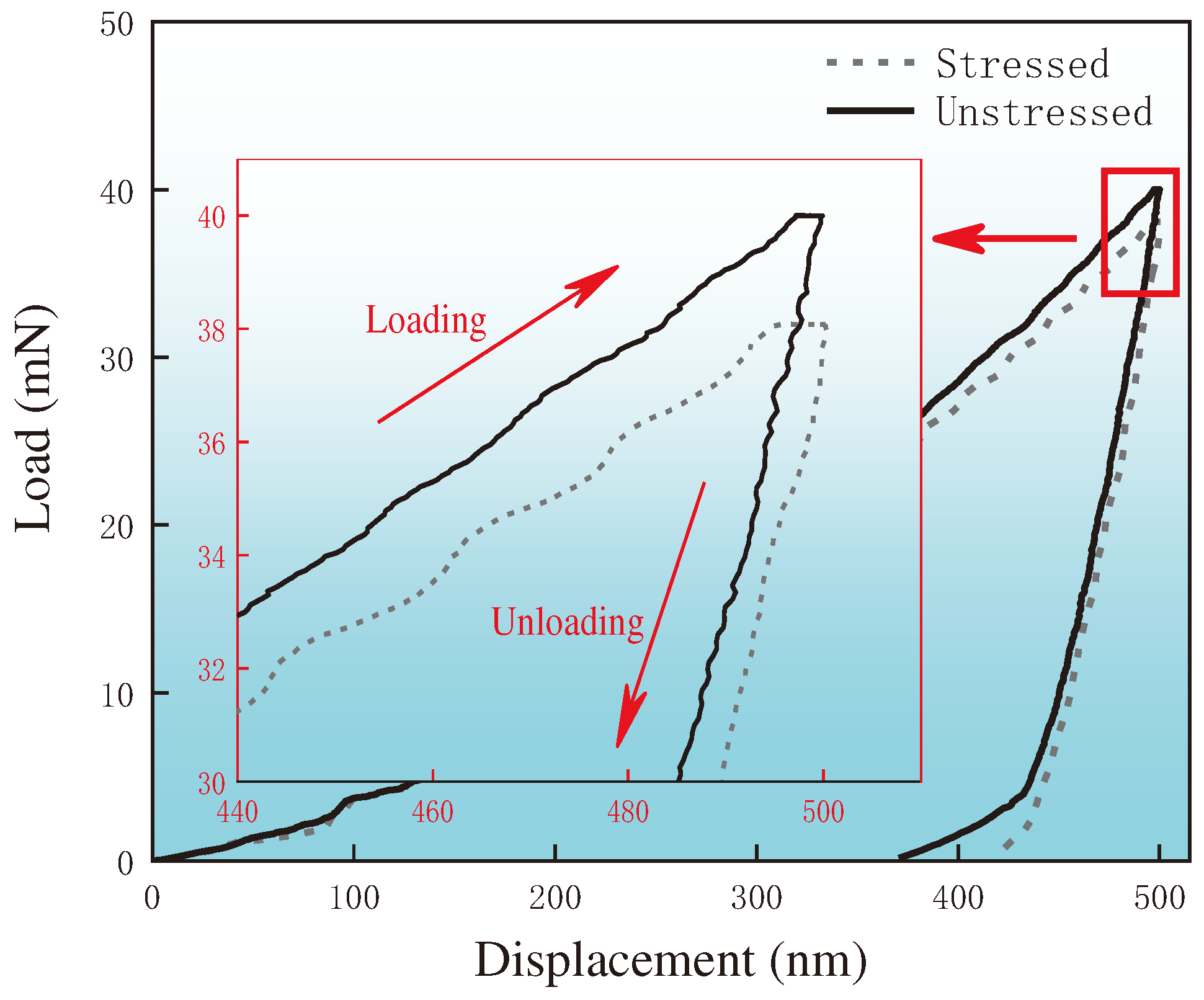
2.4. Nanoindentation Testing
3. Results
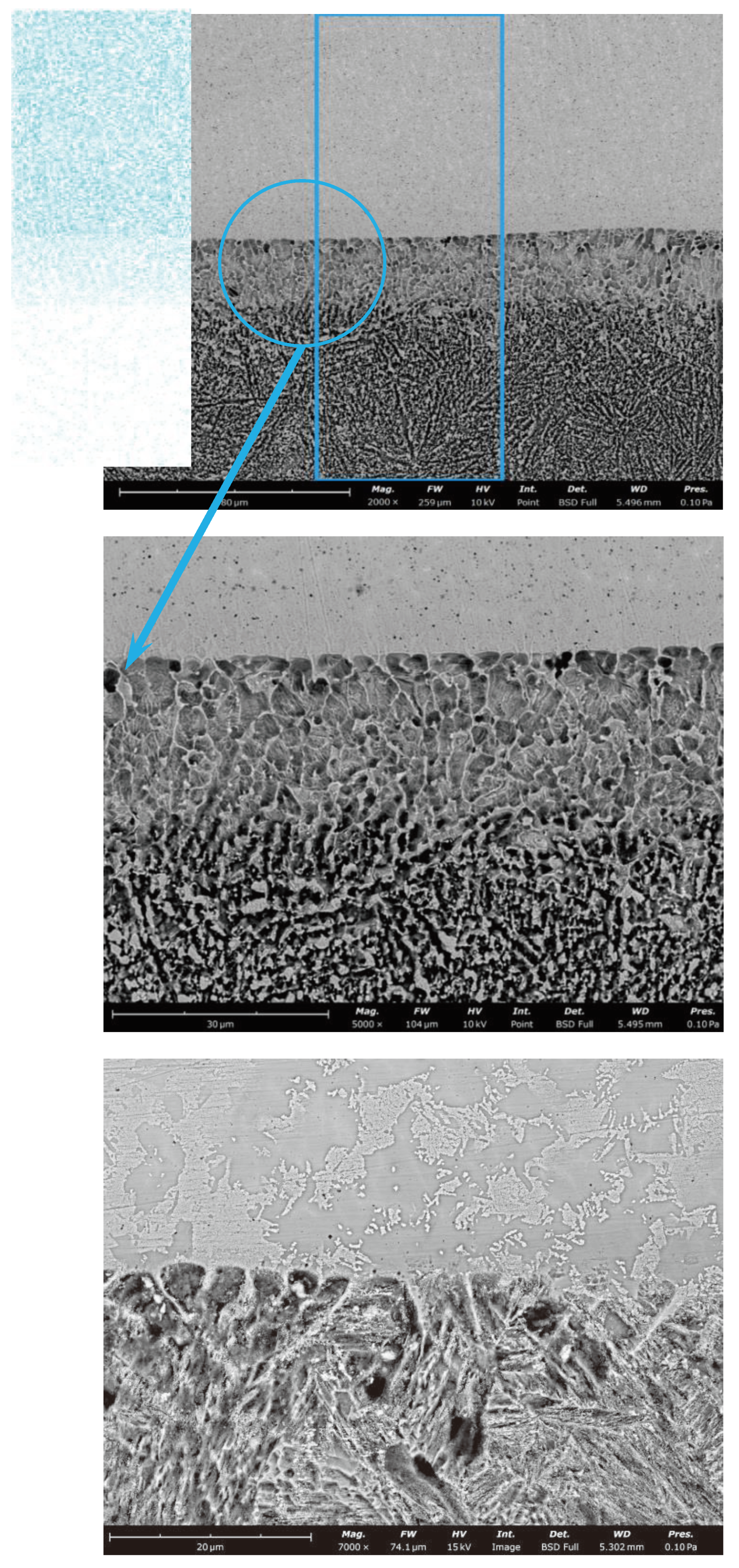
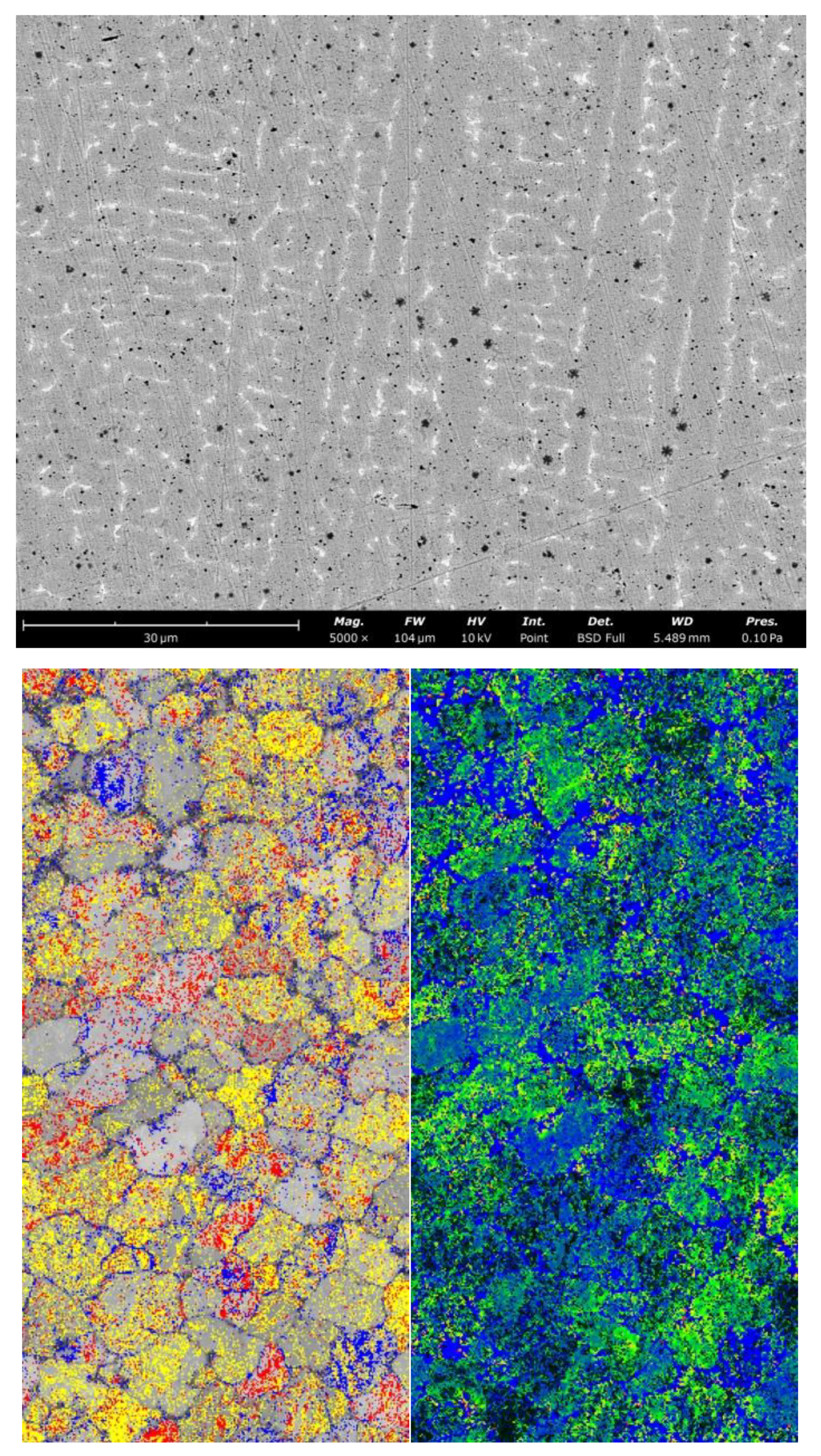
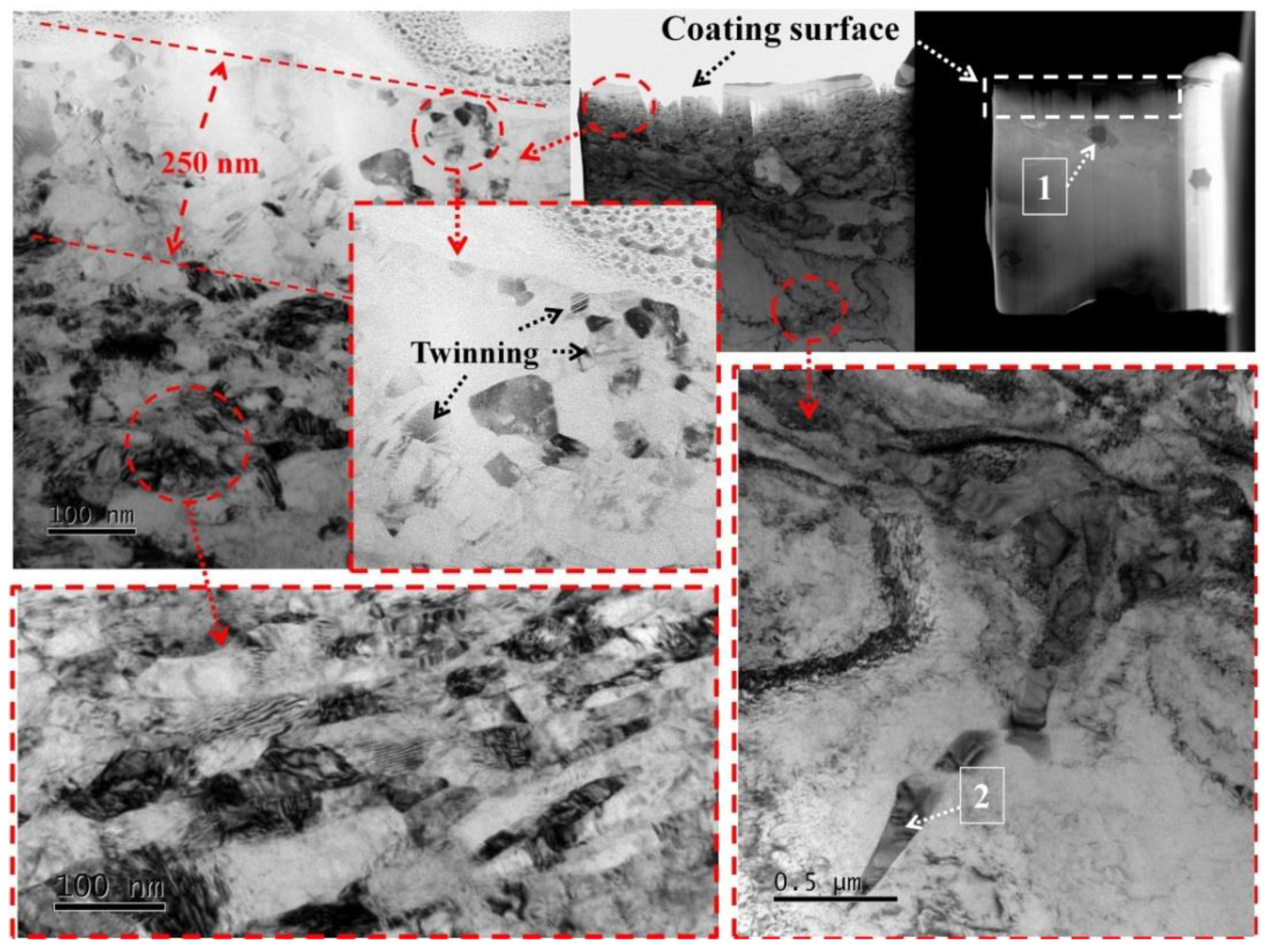
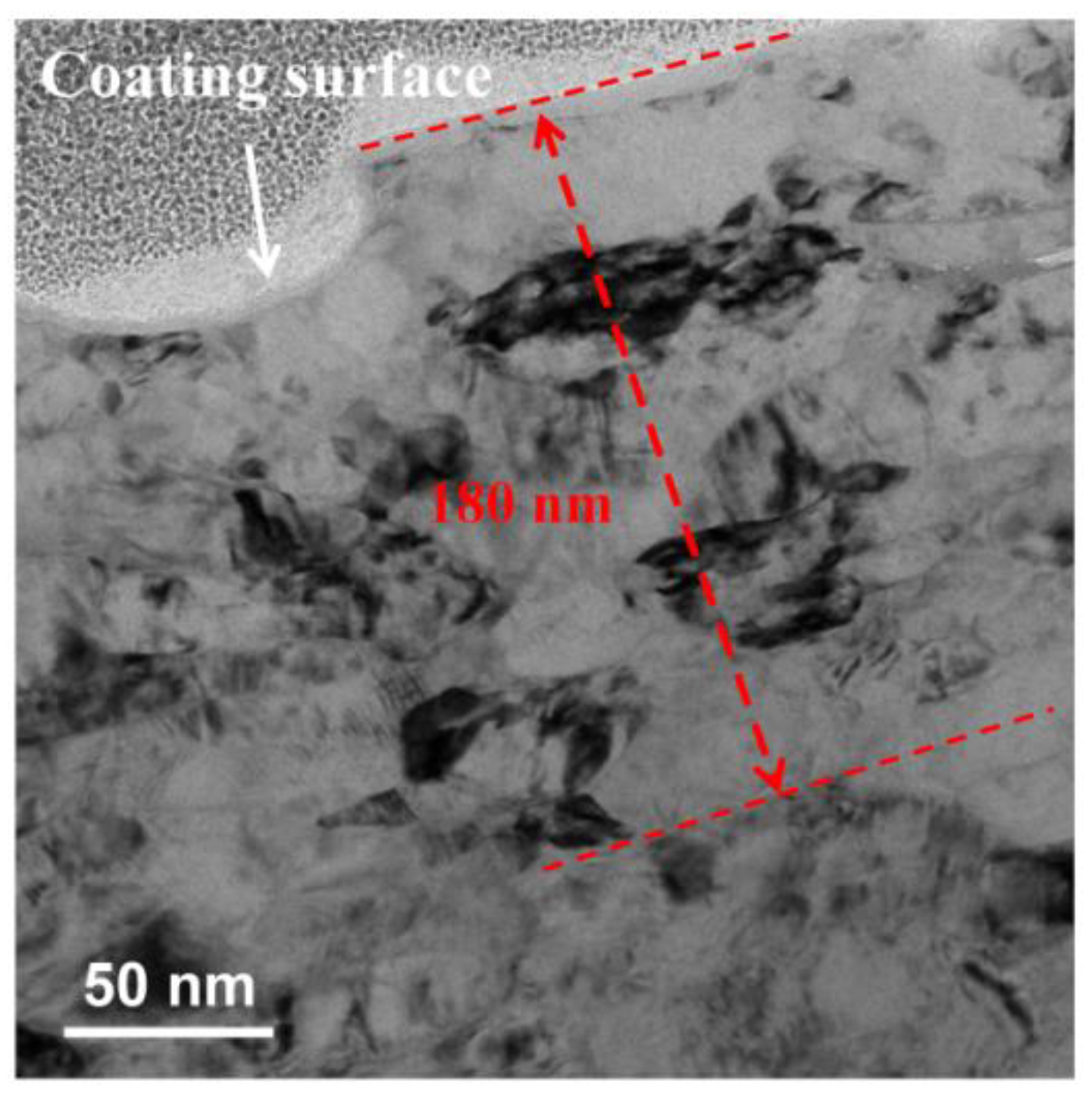
3.1. Microstructures of Fusion Interface
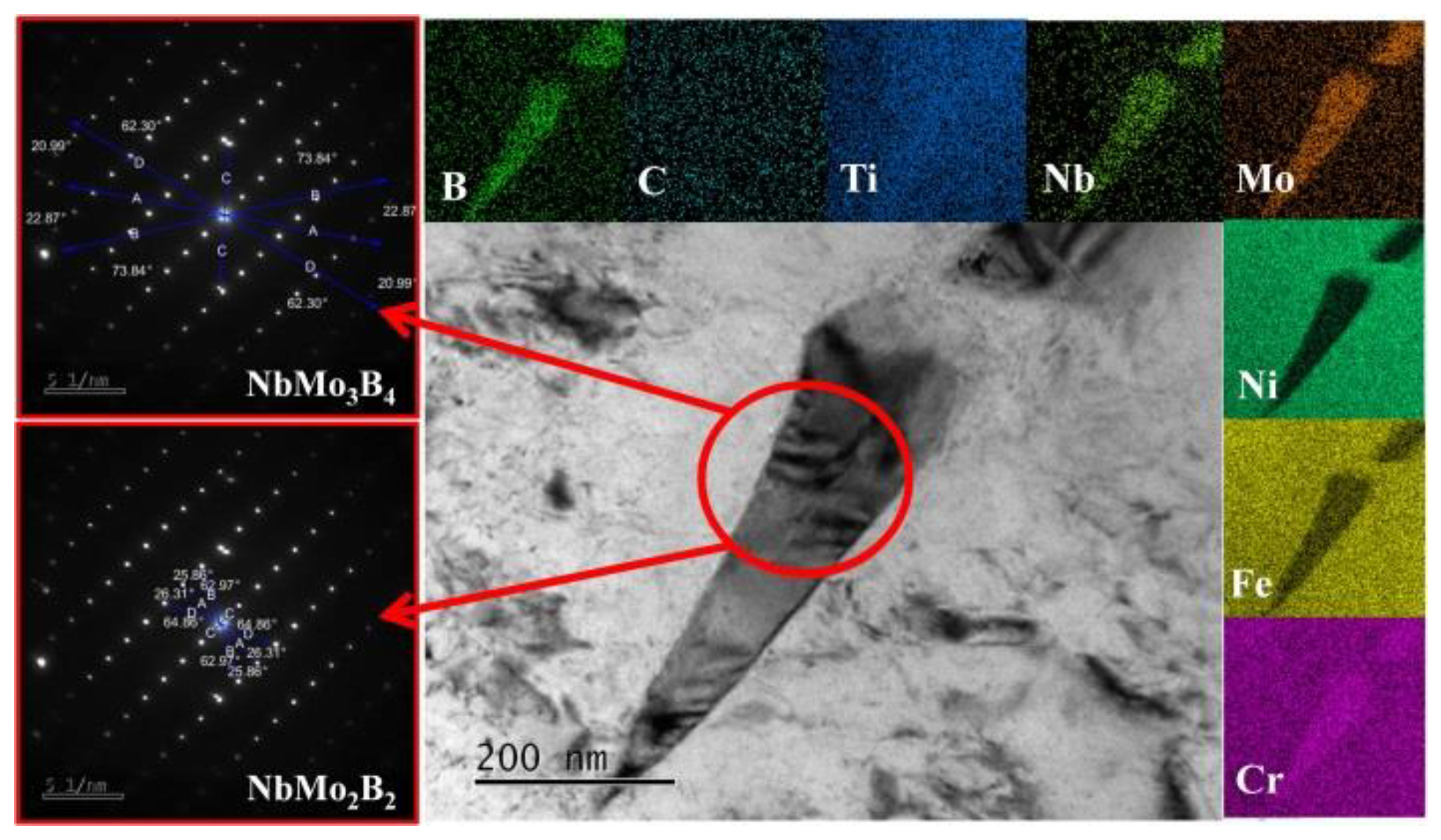
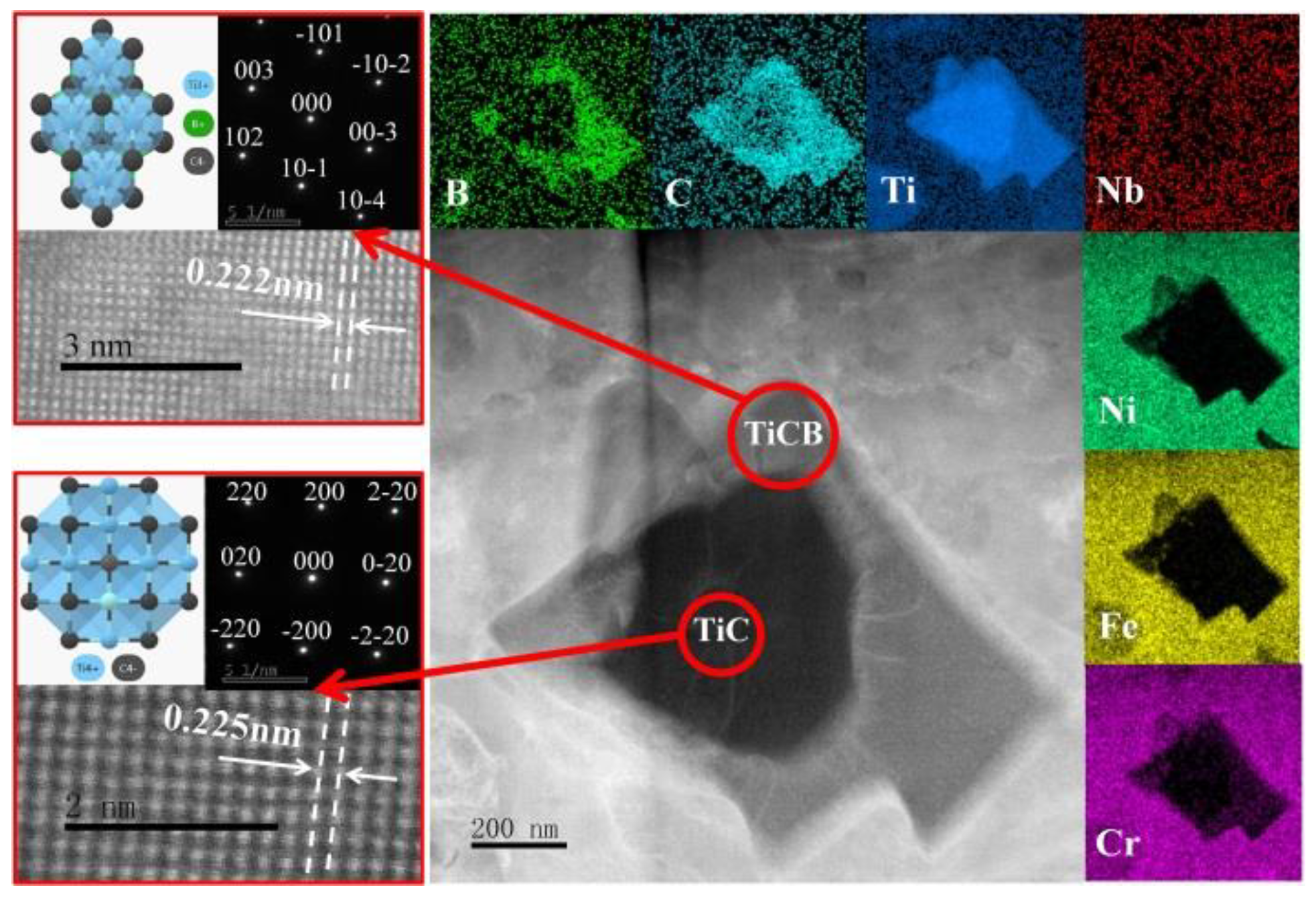
3.2. Morphologies of (Ti,Nb)(C,B)/IN625 Composite Coating
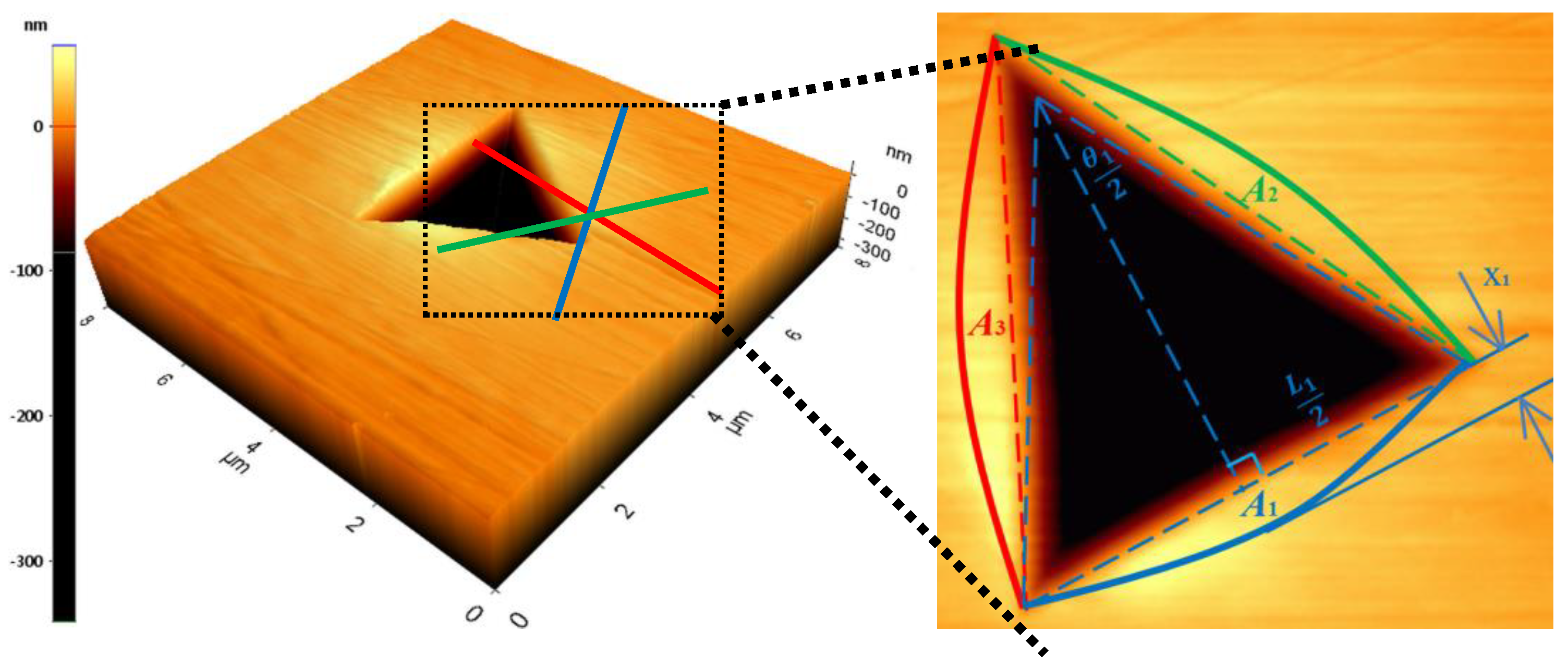
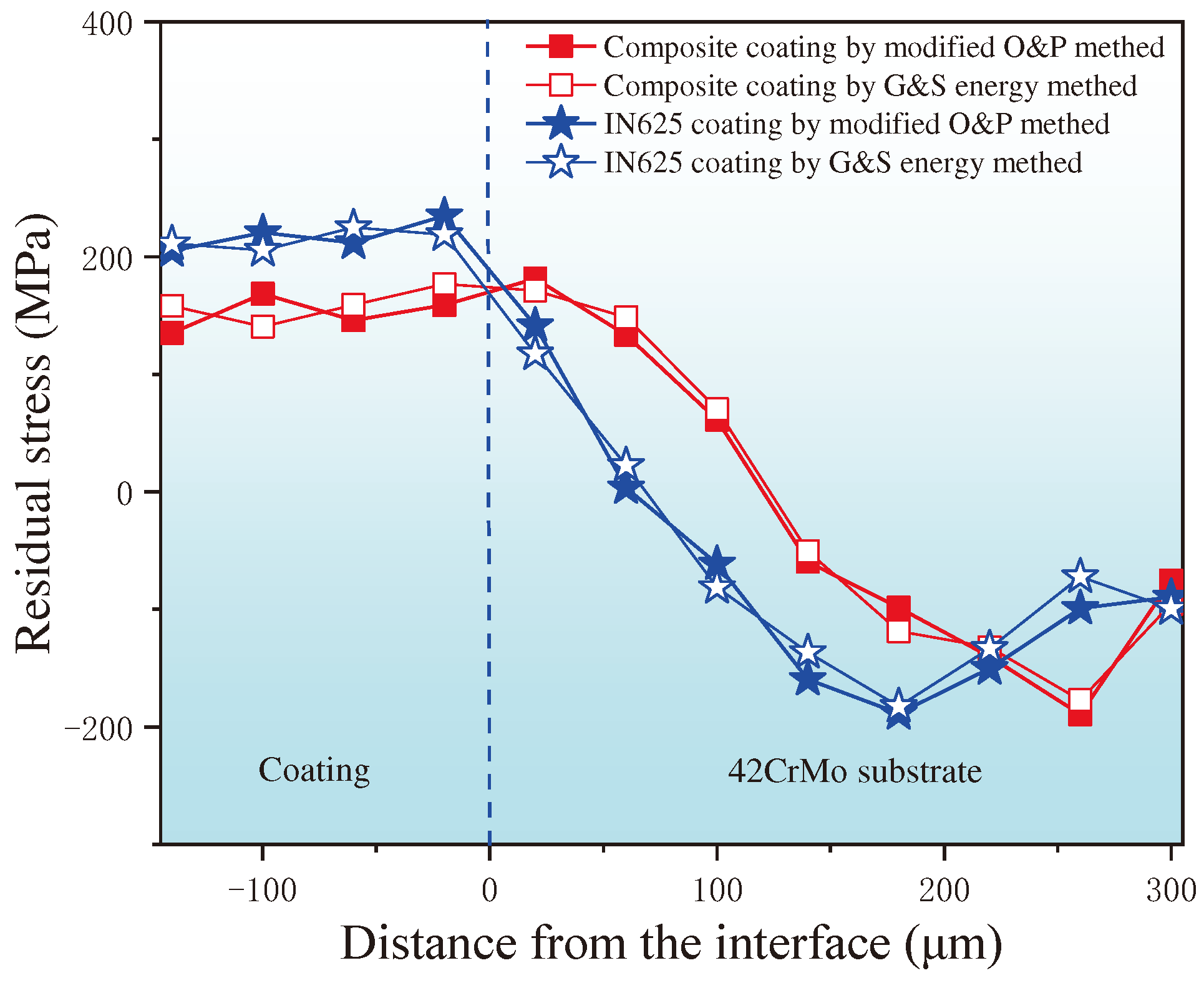
3.3. Stress Distribution at the Interface of (Ti,Nb)(C,B)/IN625 Coating
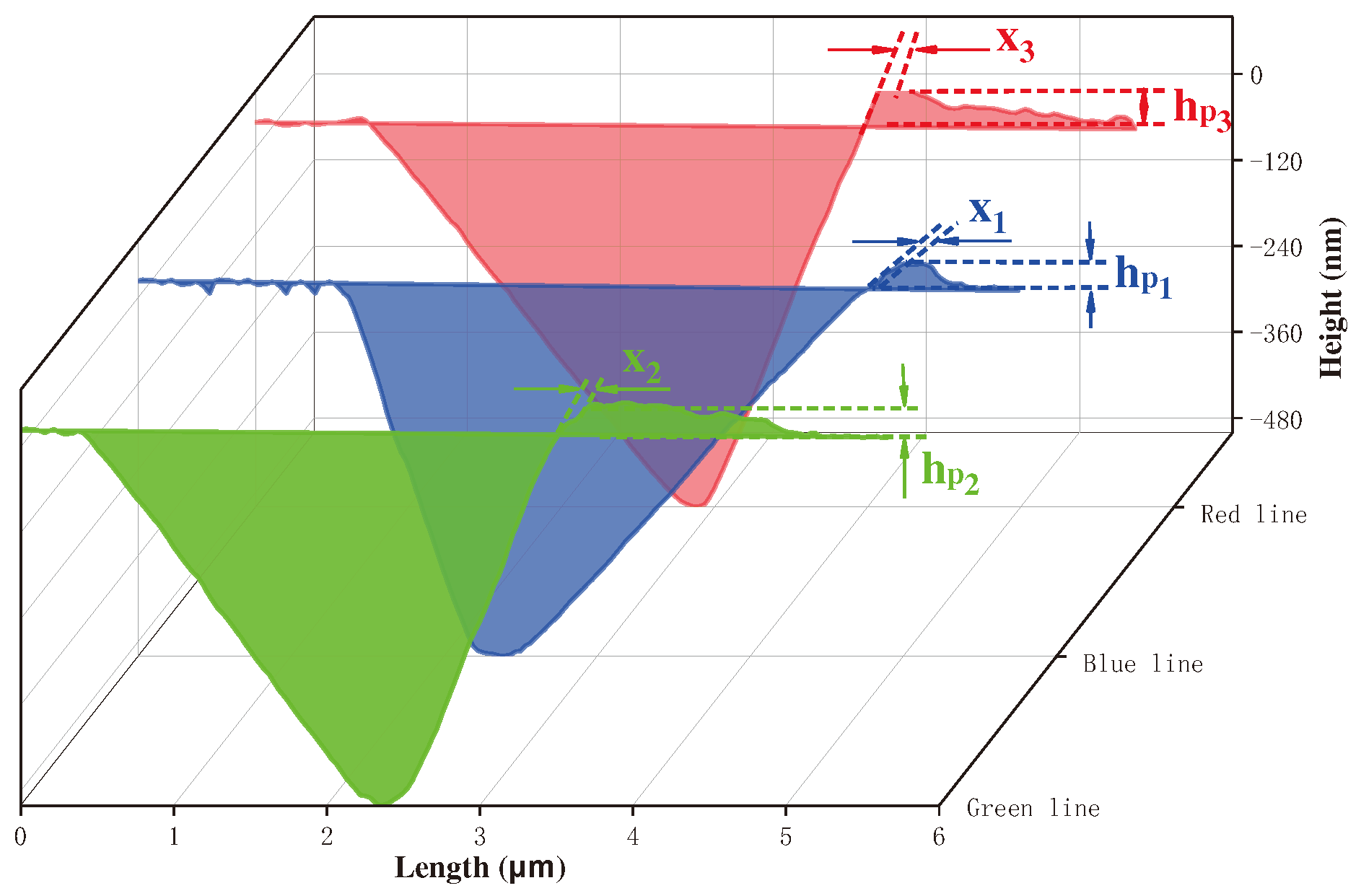
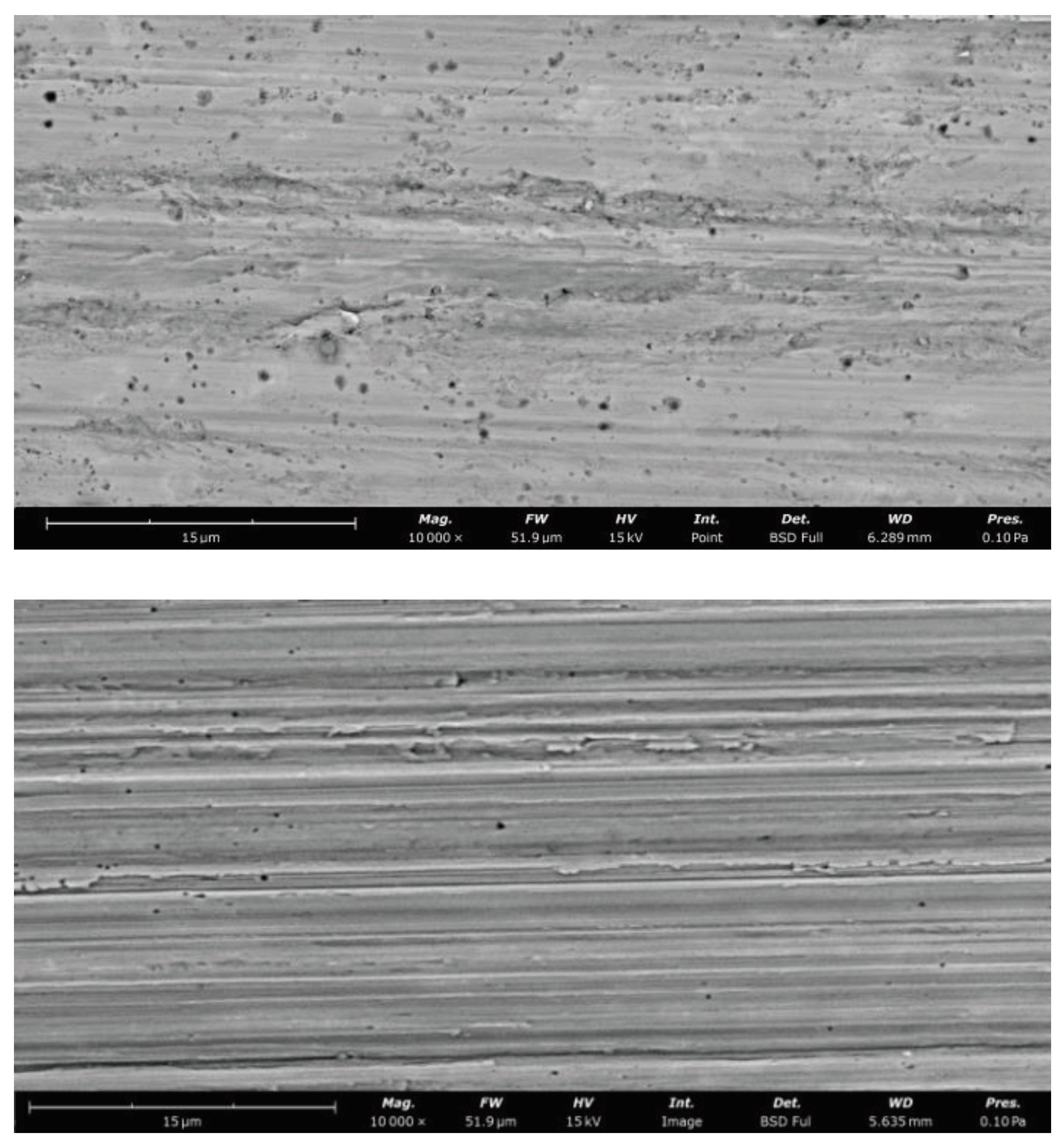
3.4. Characteristics and Properties of Wear on Coating Surfaces
4. Discussion
5. Conclusions
CRediT authorship contribution statement
Declaration of Competing Interest
Data Availability
Acknowledgements
Conflict of Interest Statement
References
- Schopphoven T, Gasser A, Wissenbach K, et al. Investigations on ultra-high-speed laser material deposition as alternative for hard chrome plating and thermal spraying[J]. Journal of Laser Applications, 2016, 28(2): 022501. [CrossRef]
- Lampa C, Smirnov I. High speed laser cladding of an iron based alloy developed for hard chrome replacement [J]. Journal of Laser Applications, 2019, 31(2): 022511. [CrossRef]
- Raykia O. Alternative with a future: High-speed laser metal deposition replaces hard chrome plating[J]. Laser Technik Journal, 2017, 14(1): 28-30. [CrossRef]
- Shen B W, Du B R, Wang M H, et al. Comparison on microstructure and properties of stainless steel layer formed by extreme high-speed and conventional laser melting deposition [J], Frontiers in Materials, 2019, 6: 248-256. [CrossRef]
- Schopphoven T, Gasser A, Backes G. EHLA: extreme high speed laser material deposition[J]. Laser Technik Journal, 2017, 14(4): 26-29. [CrossRef]
- LI Zhaohui, LI Meiyan, HAN Bin, et al. High-pressure plunger high-speed laser cladding nickel-based alloy coating structure and wear resistance[J]. Surface technology, 2020, 49(10): 45-54. (in Chinese).
- Guo Y M, Ye F X, Qi H, et al. Research Status and Development of Ultra-high Speed Laser Cladding[J]. China Surface Engineering, 2022, 35(06): 39-50. (in Chinese).
- Yuan W Y, Li R F, Chen Z H, et al. A comparative study on microstructure and properties of traditional laser cladding and high-speed laser cladding of Ni45 alloy coatings[J]. Surface and Coatings Technology, 2020, 405: 126582. [CrossRef]
- Asghar O, Lou L Y, Yasir M, et al. Enhanced tribological properties of LA43M magnesium alloy by Ni60 coating via ultra-high-speed laser cladding[J]. Coatings, 2020, 10: 638-651. [CrossRef]
- Dong H, Han Y, Fu A Q, et al. Microstructure and corrosion resistance of Ni/stainless steel surfacing layer deposited via high-speed laser cladding[J]. Surface Technology, 2019, 48(5): 21-27. (in Chinese).
- Qiao Y X, Huang J, Huang D, et al. Effects of laser scanning speed on microstructure, microhardness, and corrosion behavior of laser cladding Ni45 coatings[J]. Journal of Chemistry, 2020, 10: 1438473. [CrossRef]
- Yang J X, Bai B, Ke H, et al. Effect of metallurgical behavior on microstructure and properties of FeCrMoMn coatings prepared by high-speed laser cladding[J]. Optics and Laser Technology, 2021, 144: 107431. [CrossRef]
- Zhang N. Study on the Microstructure and Properties of Functionally Gradient Materials Prepared by FAPACS [D]. Taiyuan university of Technology, 2020. (in Chinese).
- Zhang N, Xu Y F, Wang M H, et al. M2 coating prepared by ultra-high speed laser cladding: Microstructure and interfacial residual stress[J]. Materials Today Communications, 2023, 35: 105638. [CrossRef]
- Eddine B S, Mokhtar B, Ali D. Influences of TiC Impurities on Dry-sliding Wear of polycrystalline Ceramic[J]. Journal of Wuhan University of Technology(Materials Science), 2022, 37(04): 570-575. [CrossRef]
- Fan J Z, Shen W X, Zhang Z F, et al. Properties of B4C-TiB2 ceramics prepared by spark plasma sintering[J]. Chinese Physics B, 2021, 30(03): 579-584. [CrossRef]
- Chen L, Sun Y Z, Li L, et al. Improvement of high temperature oxidation resistance of additively manufactured TiC/Inconel 625 nanocomposites by laser shock peening treatment[J]. Additive Manufacturing, 2020, 34: 101276. [CrossRef]
- Chen L, Zhang X Z, Wu Y, et al. Effect of surface morphology and microstructure on the hot corrosion behavior of TiC/IN625 coatings prepared by extreme high-speed laser cladding[J]. Corrosion Science, 2022, 201: 110271. [CrossRef]
- Ge T. Microstructure and Properties of TiC/IN625 Composite Coatings by Extreme High Speed Laser Cladding [D]. Jiangsu university, 2022. (in Chinese).
- Zhang N, Meng Q S, Chen S P, et al. TiC-TiB2-Ni/TiAl/Ti gradient functionally materials synthesized by in-situ synthesis via field-activated and pressure-assisted synthesis [J]. Journal of Functional Materials, 2010, 41(09): 1497-1500. (in Chinese).
- Zhang E L, Yang B, Zeng S Y, et al. Formation mechanism of TiC in Al/TiC composites prepared by direct reaction synthesis[J]. Transactions of Nonferrous Metals Society of China, 1998, 8(1): 92-96.
- Zhu L N, Xu B S, Wang H D, et al. Determination of hardness of plasma-sprayed FeCrBSi coating on steel substrate by nanoindentation[J]. Materials Science and Engineering: A, 2010, 528(1): 425–428. [CrossRef]
- Lee Y H, Kwon D. Measurement of residual-stress effect by nanoindentatio on elastically strained (100) W [J]. Scripta Materialia, 2003, 49: 459-465. [CrossRef]
- Munir Z A, Anselmi-Tamburini U. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method [J]. Journal of Mateirals Scinece, 2006, 41: 763-777. [CrossRef]
- Chen S P. Diffusion bonding mechanism and properties of the joints between gradient cermets and metals bonding by the FAPAS process [D]. Taiyuan university of technology, 2010. (in Chinese).
- Li L Q, Shen F M, Zhou Y D, et al. Comparative study of stainless steel AISI 431 coatings prepared by extreme-high-speed and conventional laser cladding[J]. Journal of Laser Applications, 2019, 31: 042009. [CrossRef]
- Zhou J L, Shen F, Liu J, et al. Thermoelastic rotating contact of an FGM coating with temperature-dependent and arbitrary varying properties[J]. Science China Technological Sciences, 2023, 66(04): 1038. [CrossRef]
- Aziz S B, Dewan M W, Huggett D J, et al. Impact of Friction Stir Welding (FSW) Process Parameters on Thermal Modeling and Heat Generation of Aluminum Alloy Joints[J]. Acta Metallurgica Sinica (English Letters), 2016, 29(09): 869. [CrossRef]
- Zuo L, Zhao X, Li Z, et al. A review of friction stir joining of SiCp/Al composites[J]. Chin. J. Aero., 2020, 33(03): 792. [CrossRef]
- Hu G X, Cai X, Rong Y H. Fundamental Materials Science (3rd Edition) [M]. Shanghai: Shanghai Jiao Tong University Press, 2010: 213.
- Chen S P, Meng Q S, Munir Z A. Graded Materials of (TiB2)pNi with Nickel Substrate Prepared by Field-Activated Pressure-Assisted Synthesis Process[J]. J. Wuhan University. of Technology. (Mater. Sci. Ed.), 2010, 25(01): 39-43. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





